Abstract
Proprotein convertase-subtilisin/kexin type 9 (PCSK9) monoclonal antibody is a new therapy to reduce low-density lipoprotein cholesterol (LDL-C) level in patients with familial hypercholesterolemia (FH). This pooled analysis aimed to estimate the efficacy and safety of PCSK9 antibody therapy in FH. Reports of randomized controlled trials (RCTs) comparing PCSK9 antibody to placebo were retrieved by a search of MEDLINE via PubMed, EMBASE, the Cochrane Library databases, ClinicalTrials.gov and Clinical Trial Results (up to November 30, 2015) with no language restriction. Data were abstracted by a standardized protocol. We found eight RCTs (1,879 patients with FH) for the pooled analysis. As compared with placebo, PCSK9 antibody therapy remarkably reduced LDL-C level (mean reduction: -48.54 %, 95 % CI: -53.19 to -43.88), total cholesterol (mean reduction: -31.08%, 95 % CI: -35.20 to -26.95), lipoprotein (a) (mean reduction: -20.44%, 95 % CI: -25.21 to -15.66), and apolipoprotein B (mean reduction: -36.32%, 95 % CI: -40.75 to -31.90) and elevated the level of high-density lipoprotein cholesterol (mean change: 6.29 %, 95 % CI: 5.12 to 7.46) and apolipoprotein A1(mean change: 4.86%, 95 % CI: 3.77 to 5.95). Therapy with and without PCSK9 antibodies did not differ in rate of adverse events (pooled rate: 50.86 % vs. 48.63%; RR: 1.03; 95 % CI: 0.92 to 1.15; P = 0.64; heterogeneity P = 0.13; I2= 40%) or serious adverse events (pooled rate: 7.14% vs. 6.74%; RR: 1.05; 95 % CI: 0.70 to 1.58; P = 0.80; heterogeneity P = 0.69; I2= 0%). PCSK9 antibody may be an effective and safe treatment for FH.
Keywords: efficacy, safety, proprotein convertase subtilisin/kexin type 9 monoclonal antibody, familial hypercholesterolemia
INTRODUCTION
Familial hypercholesterolemia (FH) is a genetic disease involved in lipid metabolism caused by mutations in low-density lipoprotein receptor (LDLR), apolipoprotein B (ApoB) and proprotein convertase subtilisin/kexin type 9 (PCSK9) [1]. FH is clinically classified as heterozygous familial hypercholesterolemia (HeFH) and homozygous familial hypercholesterolemia (HoFH) [2]. The characteristics of patients with FH are elevated plasma level of low-density lipoprotein cholesterol (LDL-C) and increased risk of premature coronary heart disease [3, 4]. Statins are the first-line drugs for treatment of FH [5], but the guidelines recommending LDL-C goals are not achieved despite high-intensity statin therapy [6]. Combined treatment with high-strength statins and ezetimibe or other drugs may help lower LDL-C levels [7, 8], but achieving the treatment targets is difficult [3, 9–11]. As well, some patients fail to adhere to statins treatment because of its side effects [12].
PCSK9 is a kind of serine protease that is synthesized and secreted by the liver; it is expressed in the liver, small intestine, kidney and nervous system [13, 14]. PCSK9 binds to LDLR for LDLR degradation in lysosomes, which eventually elevates the plasma level of LDL-C [15, 16]. PCSK9 is connected to dyslipidemia, especially LDL-C metabolism [17], and is closely related to risk of coronary heart disease.
The FH phenotype is caused by gain-of-function mutations in PCSK9 [3, 15]. Inhibiting PCSK9 has led to potential therapeutic agents for FH [15, 18–20]. The use of PCSK9 monoclonal antibodies can reduce circulating LDL-C level in patients with FH and could be synergistic with statins [21].
The efficiency and safety of PCSK9 inhibitor therapy for hypercholesterolemia has been evaluated [22–24], but a pooled analysis of the therapy for FH is lacking. In addition, the efficacy outcomes for lipids in FH are inconsistent. Thus, we conducted a pooled analysis of randomized controlled trials (RCTs) to systemically evaluate the efficiency and safety of PCSK9 antibody therapy for FH.
RESULTS
Study selection and patient characteristics
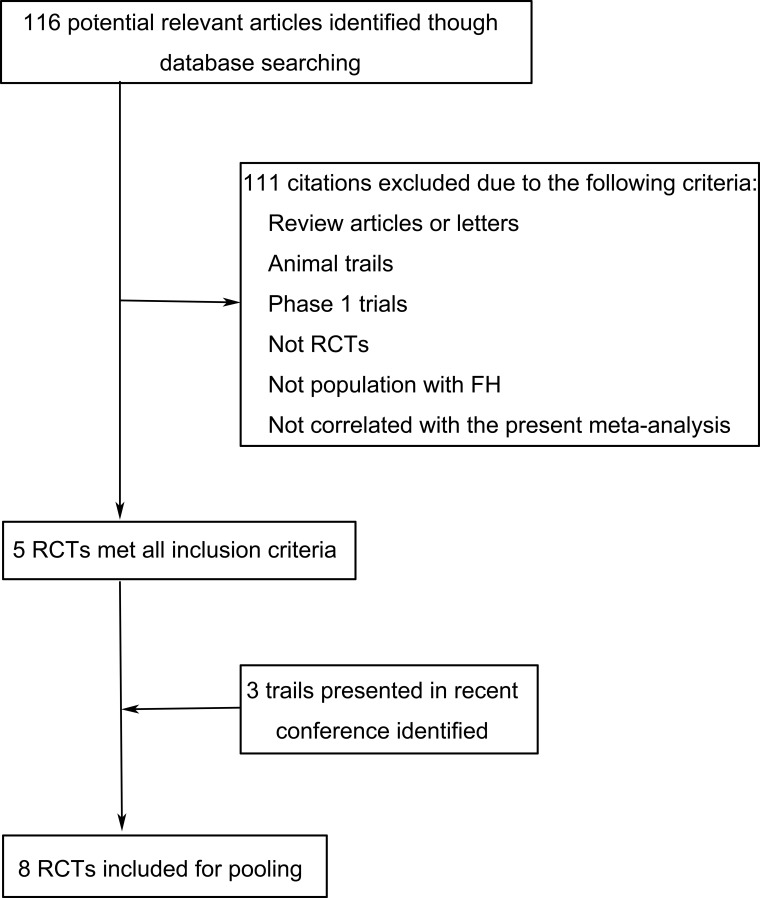
Our search retrieved 116 related studies in total; 111 were excluded because they were review articles, letters, animal trials, phase 1 trials, not RCTs, not population with FH, or were not correlated with the present pooled analysis. We included unpublished reports for three clinical trials (ODYSSEY FHI, ODYSSEY FHII and ODYSSEY HIGH FH) (Figure 1). Our final sample included reports for eight studies including 1,879 patients with FH. All eight studies were of good quality (Jadad score≥3).
Figure 1. Flow chart for study selection.
RCT, randomized controlled trial; FH, familial hypercholesterolemia.
Characteristics of the eight studies are in the Table 1. One study was of HoFH patients and seven were of HeFH patients. Two reports were of phase 2 trials and six were of phase 3 trials; Alirocumab was subcutaneously injected as PCSK9 antibody in five studies and evolocumab in three others; Four trials were 12 weeks and four were > 12 weeks long.
Table 1. Baseline characteristics of clinical trials.
| Study | Journal, Year | Phase | Patients, n | Mean age (y) | Women, n (%) | Duration (w) | Investigational drug and dose | Control | Population | LLT background |
|---|---|---|---|---|---|---|---|---|---|---|
| RUTHERFORD | Circulation, 2012 |
2 | 167 | 50 (13) | 79 (47) | 12 | Evolocumab 350 mg Q4W and 420 mg Q4W | Placebo | HeFH | Statin ± ezetimibe |
| TESLA Part B | Lancet, 2014 | 3 | 49 | 31 (13) | 24 (49) | 12 | Evolocumab 420 mg Q4W | Placebo | HoFH | Statin ± ezetimibe |
| RUTHERFORD-2 | Lancet, 2014 | 3 | 329 | 51 (14) | 139 (42) | 12 | Evolocumab 140 mg Q2W and 420 mg Q4W | Placebo | HeFH | Statin ± ezetimibe |
| Stein et al. | Lancet, 2012 | 2 | 77 | 53 (10) | 30 (39) | 12 | Alirocumab 150, 200, or 300 mg Q4W and 150 mg Q2W | Placebo | HeFH | Statin ± ezetimibe |
| ODYSSEY FH I | ESC Congress 2014 |
3 | 486 | 52 (12) | 212 (55) | 24 | Alirocumab 75 mg with potential up-titration to 150 mg Q2W | Placebo | HeFH | Statin ± other LLT |
| ODYSSEY FH II | ESC Congress 2014 |
3 | 249 | 53 (13) | 118 (47) | 24 | Alirocumab 75 mg with potential up-titration to 150 mg Q2W | Placebo | HeFH | Statin ± other LLT |
| ODYSSEY HIGH FH | AHA Scientific Sessions 2014 |
3 | 107 | 52 (11) | 50 (47) | 24 | Alirocumab 150 mg Q2W | Placebo | HeFH | Statin ± other LLT |
| ODYSSEY LONG TERM | NEJM, 2015 | 3 | 2341 | 61 (10) | 884 (38) | 24 | Alirocumab 150 mg Q2W | Placebo | HeFH + HC | Statin ± other LLT |
Data are mean (SD), number (%); Q2W, every 2 weeks; Q4W, every 4 weeks; HeFH, heterozygous familial hypercholesterolemia; HoFH, homozygous familial hypercholesterolemia; HC, hypercholesterolemia; LLT, lipid-lowering therapy.
Clinical end points
Efficacy outcomes
We used all eight reports for the analysis of LDL-C with a random-effects model because of significant heterogeneity (P < 0.00001, I2 = 100%). Level of LDL-C were reduced almost 50% with than without PCSK9 antibody treatment (mean reduction: −48.54%, 95% confidence interval [CI]: −53.19 to −43.88) (Table 2). On subgroup analysis, LDL-C level was reduced more in patients with HeFH than HoFH (mean reduction: −51.03%, 95% CI: −55.59 to −46.48 vs. -31.00%, 95 %CI: -33.96 to −28.04). Heterogeneity tests for subgroups showed a striking difference between HeFH and HoFH groups (P < 0.00001), so the heterogeneity was caused in part by the different populations. However, analyses by type of PCSK9 antibody or duration of treatment did not reveal heterogeneity (Table 3).
Table 2. Pooled-analysis results of the percentage change in level of serum lipid and the incidence of adverse events.
| Outcomes | Patients, n | WMD/RR (95% CI) | P value | I2, % | Heterogeneity P value |
|---|---|---|---|---|---|
| LDL-C | 1875 | −48.54 %[-53.19, -43.88] | P < 0.00001 | 100% | P < 0.00001 |
| HDL-C | 1460 | 6.29 %[5.12, 7.46] | P < 0.00001 | 97% | P < 0.00001 |
| TC | 1082 | −31.08%[-35.20, -26.95] | P < 0.00001 | 99% | P < 0.00001 |
| Lp(a) | 1383 | −20.44%[-25.21, -15.66] | P < 0.00001 | 100% | P < 0.00001 |
| ApoA1 | 1392 | 4.86%[3.77, 5.95] | P < 0.00001 | 97% | P < 0.00001 |
| ApoB | 1438 | −36.32%[-40.75, -31.90] | P < 0.00001 | 100% | P < 0.00001 |
| TG | 1383 | −7.92%[-19.19, 3.36] | P = 0.17 | 100% | P < 0.00001 |
| Adverse events | 1462 | 1.03[0.92, 1.15] | P = 0.64 | 40% | P = 0.13 |
| Serious adverse events | 1385 | 1.05[0.70, 1.58] | P = 0.80 | 0% | P = 0.69 |
| Discontinuation | 545 | 1.01[0.09, 10.89] | P = 0.99 | NA | NA |
| Death | 545 | NE | NA | NA | NA |
| Headache | 1301 | 0.83[0.49, 1.38] | P = 0.46 | 0% | P = 0.86 |
| Injection site reactions | 1421 | 1.43[0.93, 2.21] | P = 0.10 | 0% | P = 0.66 |
| Nasopharyngitis | 1385 | 1.09[0.78, 1.54] | P = 0.61 | 31% | P = 0.20 |
| Gastroenteritis | 571 | 1.15[0.49, 2.66] | P = 0.75 | 31% | P = 0.22 |
| Nausea | 652 | 0.67[0.28, 1.62] | P = 0.37 | 47% | P = 0.13 |
| Upper respiratory tract infections | 701 | 1.03[0.53, 1.99] | P = 0.93 | 0% | P = 0.37 |
| AST or ALT>3ULN | 622 | 1.49[0.24, 9.10] | P = 0.67 | 0% | P = 0.62 |
| CK>5ULN | 622 | 0.63[0.17, 2.29] | P = 0.48 | 28% | P = 0.25 |
WMD, weighted mean difference; RR, risk ratio; CI, confidence interval; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TC, total cholesterol; Lp(a), lipoprotein(a); ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; TG, triglycerides; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CK, creatine kinase; ULN, upper limit of normal; NA, not applicable; NE, not estimable.
Table 3. Subgroup analyses with regard to the percentage change in plasma level of LDL-C.
| Subgroup | Patients, n | WMD (95% CI) | P value | I2,% | Heterogeneity P value | P value for subgroup differences |
|---|---|---|---|---|---|---|
| Adjustment for type of FH | P < 0.00001 | |||||
| HeFH | 1826 | −51.03%[-55.59, -46.48] | P < 0.00001 | 100% | P < 0.00001 | |
| HoFH | 49 | −31.00%[-33.96, -28.04] | P < 0.00001 | NA | NA | |
| Adjustment for type of PCSK9 antibody | P = 0.78 | |||||
| Alirocumab | 1330 | −49.28%[-54.95, -43.60] | P < 0.00001 | 100% | P < 0.00001 | |
| Evolocumab | 545 | −47.21%[-60.28, -34.15] | P < 0.00001 | 99% | P < 0.00001 | |
| Adjustment for duration of treatment | P = 0.17 | |||||
| ≤12 weeks | 622 | −43.54%[-55.57, -31.51] | P < 0.00001 | 99% | P < 0.00001 | |
| >12 weeks | 1253 | −53.02%[-59.05, -47.00] | P < 0.00001 | 100% | P < 0.00001 |
LDL-C, low-density lipoprotein cholesterol; WMD, weighted mean difference; CI, confidence interval; FH, familial hypercholesterolemia; HeFH, heterozygous familial hypercholesterolemia; HoFH, homozygous familial hypercholesterolemia; PCSK9, proprotein convertase subtilisin/kexin type 9; NA, not applicable.
Seven trials assessed high-density lipoprotein cholesterol (HDL-C), five total cholesterol (TC), six lipoprotein (a) (Lp(a)), six apolipoprotein A1 (ApoA1), seven ApoB and six triglycerides (TG) (Table 2). HDL-C level was significantly increased with PCSK9 antibodies (mean change: 6.29%, 95% CI: 5.12 to 7.46). However, the mean changes in TC, Lp(a), ApoA1, ApoB and TG were -31.08% (95% CI: -35.20 to -26.95), -20.44% (95% CI: -25.21 to -15.66), 4.86% (95% CI: 3.77 to 5.95), -36.32% (95% CI: -40.75 to -31.90) and -7.92% (95% CI: -19.19 to 3.36), respectively. We used a random-effects model to analyze HDL-C, TC, Lp(a), ApoA1, ApoB and TG because of the significant heterogeneity (all P < 0.00001, I2 = 97% to 100%). The changes in lipid levels with and without PCSK9 antibodies were significant, except for a decrease in TG level (P = 0.17).
Safety outcomes
We evaluated the adverse events for the eight trials and compared the data for clinical safety outcomes (Table 2). PCSK9 antibody treatment for FH did not increase the rate of adverse events (pooled rate: 50.86 % vs. 48.63%; pooled relative risk [RR]: 1.03; 95% CI: 0.92 to 1.15; P = 0.64; heterogeneity P = 0.13; I2 = 40%) or serious adverse events (pooled rate: 7.14% vs. 6.74%; RR: 1.05; 95% CI: 0.70 to 1.58; P = 0.80; heterogeneity P = 0.69; I2 = 0%) as compared with placebo. The incidence of increased aspartate aminotransferase or alanine aminotransferase (AST or ALT) level greater than three times the upper limit of normal (ULN) did not differ with and without PCSK9 antibody (pooled rate: 0.94% vs. 0.51%; RR: 1.49; 95% CI: 0.24 to 9.10; P = 0.67; heterogeneity P = 0.62; I2 = 0%). The pooled incidence of increased creatine kinase (CK) level greater than five times the ULN was similar with the two treatments(pooled rate: 0.94% vs. 1.53%; RR: 0.63; 95% CI: 0.17 to 2.29; P = 0.48; heterogeneity P = 0.25; I2 = 28%). In addition, for other adverse events, the rates of nasopharyngitis, headache, gastroenteritis, upper respiratory tract infections and injection-site reactions were greater but not significantly with than without PCSK9 antibodies.
Sensitivity/subgroup analyses
Sensitivity analysis was used to determine whether exclusion of any single study altered pooled RRs or weighted mean differences (WMDs). We found no heterogeneity for safety outcomes but found heterogeneity for efficacy outcomes, which was not addressed well by sensitivity analysis. Then, we performed subgroup analysis of changes in lipid and apolipoprotein levels after PCSK9 antibody treatment by different PCSK9 antibodies, types of FH and duration of treatment and found that the heterogeneity was caused in part by the different types of FH.
Publication bias
We calculated Nfs0.05 to estimate the publication bias for each comparison and found Nfs0.05 values were greater than the number of studies except for incidence of serious adverse events, death, discontinuation, headache, upper respiratory tract infections and increased AST/ALT and CK included in the pooled analysis. The Nfs0.05 value for several safety outcomes was smaller than the number of included studies, which may be consistent with “small study” bias.
DISCUSSION
To our knowledge, this is the first pooled analysis of studies comparing the efficiency and safety of PCSK9 antibodies to no anti-PCSK9 antibodies for FH. Treatment with PCSK9 antibodies was associated with significantly reduced levels of LDL-C, TC, ApoB, and Lp(a) and elevated levels of HDL-C and ApoA1 in FH patients, with no difference in adverse events or serious adverse events with and without treatment.
In eight phase 2 and phase 3 trials that were eligible for the pooled analysis (1,879 FH patients) [25–29], the clinical adverse events with PCSK9 antibody treatment mainly concerned headache, injection-site reactions, nasopharyngitis, gastroenteritis, nausea, and upper respiratory tract infections. The total rate of adverse events or serious adverse events with treatment did not differ from the control rate. As well, laboratory analyses, including increased ALT/AST (> 3ULN) or CK (> 5ULN) levels, did not reveal a significant difference in safety issues between the two treatments. Therefore, on the strength of available data, PCSK9 antibody therapy for FH seems safe and tolerated, but more standardized trials and clinical trials are needed to further verify the safety.
We found great heterogeneity in lipid profile analyses of patients with PCSK9 antibody treatment. On sensitivity and subgroup analyses, the heterogeneity was partly caused by the different types of FH (HoFH or HeFH). Usually, patients with HeFH at least have one normal LDLR allele [3], but in HoFH patients, two LDLR alleles are abnormal [30]. Most HoFH patients are compound heterozygotes with defective LDLR-alleles [31] and others are LDLR-negative. PCSK9 antibodies might be more efficacious in reducing LDL-C level in FH patients with residual LDLR function.
FH is caused by loss-of-function mutations in the LDLR gene, leading to cell uptake of plasma LDL-C blocked by the liver and highly increased serum LDL-C level [3, 32]. Elevated LDL-C level, which is associated with atherosclerosis in affected arteries, is a major risk factor for the occurrence and development of coronary artery disease [33, 34]. Statins reduce the plasma concentration of LDL-C by increasing the hepatic expression of LDLR and removing LDL in circulation. At present, intensive statin therapy is widely indicated as first-line therapy in FH [3] to reduce serum LDL-C level and the risk of coronary artery disease [35]. However, despite high-intensity statin therapy, achieving the recommended treatment targets of LDL-C to prevent cardiovascular events is difficult in most patients with FH [3, 11, 36]. About 10% of patients are unable to tolerate high-intensity statins because of the side effects [37–39].
In recent years, combined treatment with statins and other lipid-lowering drugs has been a good therapeutic strategy to further reduce LDL-C levels for patients with FH [40]; the monoclonal antibody against PCSK9 is an innovative lipid-lowering drug. In our pooled-analysis of phase 2 and phase 3 clinical trials, treatment with PCSK9 antibodies combined with statins for FH, was effective in reducing LDL-C level, with few side effects [25–29]. In addition, statin therapy upregulates serum PCSK9 levels [15, 41, 42], and treatment with PCSK9 antibodies might strengthen statins to lower LDL-C level. So combined treatment with PCSK9 antibodies and statins may have a synergistic effect in lowering LDL-C level.
The RUTHERFORD-2 trial involved 329 HeFH patients with statins, with or without ezetimibe, randomly assigned to receive evolocumab 140 mg subcutaneously every 2 weeks or 420 mg every 4 weeks or placebo [26]. Compared with placebo, treatment with evolocumab biweekly or monthly led to 59.2% and 61.3% reduction in mean LDL-C level, respectively, after 12 weeks.
In the trial of Stein and colleagues, the efficacy and tolerability of alirocumab were evaluated in 77 patients with HeFH in the United States and Canada [27]. Alirocumab at 150 to 300 mg was found generally safe and efficacious. As well, alirocumab dose-dependently reduced LDL-C level by 28.9% to 67.9% versus 10.7% in the placebo group.
The TESLA Part B trial included 50 patients with HoFH who received evolocumab 420 mg or placebo every 4 weeks for 12 weeks; 49 patients actually received the study drug and completed the study [28]. Treatment with evolocumab significantly reduced LDL-C level by 30.9% as compared with placebo.
Moreover, in our analysis, other lipid levels were modified by PCSK9 antibody, including significant decreases in Lp(a), TC and ApoB levels and increase in HDL-C and ApoA1 levels. In addition, TG level was changed, although not significantly. The change in lipid profile is not conducive to the occurrence and development of atherosclerosis [43].
In patients with FH, PCSK9 antibody therapy satisfactorily regulates lipid levels, especially reducing serum level of LDL-C. Our pooled analysis revealed the good safety and tolerant profile with short-term administration of PCSK9 antibodies for FH. Results of ongoing trials of PCSK9 antibodies for FH, to evaluate the efficiency, safety and clinical outcomes with long-term treatment, are awaited.
MATERIALS AND METHODS
This pooled analysis was conducted following the preferred reporting items of the systematic reviews and meta-analysis (PRISMA) statement. [44]
Selection criteria
Studies were eligible for the pooled analysis if they 1) were RCTs, 2) involved human subjects with FH, and 3) compared PCSK9 antibody to no PCSK9 antibody regardless of other lipid-lowering therapy. Studies not meeting these criteria, non-clinical studies, non-RCTs and studies without complete data were excluded.
Search sources and strategy
We performed a literature search of MEDLINE via PubMed, EMBASE, the Cochrane Library databases, ClinicalTrials.gov and Clinical Trial Results (www.clinicaltrialresults.org) for reports of clinical trials and RCTs published in any language up to November 30, 2015, by using the following keywords: “PCSK9” or “proprotein convertase subtilisin/kexin type 9” or “bococizumab” or “AMG 145” or “evolocumab” or ‘REGN727” or “SAR236553” or “alirocumab” and “familial hypercholesterolemia”. Reference lists of relevant trials and reviews were manually checked for additional reports.
Data management and quality assessment
Abstracted data included first author's name, year of publication, study design, number of enrolled patients, follow-up duration, baseline characteristics of patients, drug interventions, clinical outcomes and adverse events. We recorded percentage change in lipid and apolipoprotein levels after treatment with PCSK9 antibody as the primary end point. The incidence of adverse events was a secondary end point.
Two reviewers (BL and PPH) assessed report eligibility and abstracted data independently by using a standardized report form and evaluated the quality of reports independently following the Jadad scale [45]. Any discrepancies were resolved by consensus.
Statistical analysis
The pooled analysis involved use of REVMAN 5.3. Heterogeneity among studies was tested by the Cochran Q test and I2 test. A fixed-effects or random-effects model was applied depending on the heterogeneity results [46]: with lack of heterogeneity (P > 0.10 or I2 < 50%), the fixed-effects model was used, and with significant heterogeneity (P < 0.10 or I2 > 50%), the random-effects model was used. Two-tailed P < 0.05 was considered statistically significant, and RR or WMD was reported with 95% CIs. Furthermore, we performed sensitivity and subgroup analyses to lessen the influence of heterogeneity by removing an individual trial or classifying the studies based on similar features. Finally, publication bias was assessed by the fail-safe number (Nfs): risk of publication bias was suggested if the calculated Nfs was less than the number of observed studies. The Nfs0.05 was calculated as Nfs0.05 = (∑Z/1.64)2-k, where “k” is the number of studies in the pooled analysis.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [81170087], the National Natural Science Foundation of China [81400284], the Natural Science Foundation of Shandong Province [ZR2014HM044, ZR2014HP045], the Promotive research fund for excellent young and middle-aged scientists of Shandong Province [BS2014YY037] and the ischemic heart disease research and innovation team of Jinan City.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Maxwell KN, Breslow JL. Proprotein convertase subtilisin kexin 9: the third locus implicated in autosomal dominant hypercholesterolemia. Curr Opin Lipidol. 2005;16:167–72. doi: 10.1097/01.mol.0000162321.31925.a3. [DOI] [PubMed] [Google Scholar]
- 2.Khachadurian AK. The Inheritance of Essential Familial Hypercholesterolemia. Am J Med. 1964;37:402–7. doi: 10.1016/0002-9343(64)90196-2. [DOI] [PubMed] [Google Scholar]
- 3.Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, Wiegman A, Santos RD, Watts GF, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478–90a. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benn M, Watts GF, Tybjaerg-Hansen A, Nordestgaard BG. Familial hypercholesterolemia in the danish general population: prevalence, coronary artery disease, and cholesterol-lowering medication. J Clin Endocrinol Metab. 2012;97:3956–64. doi: 10.1210/jc.2012-1563. [DOI] [PubMed] [Google Scholar]
- 5.Alonso R, Mata P, Zambon D, Mata N, Fuentes-Jimenez F. Early diagnosis and treatment of familial hypercholesterolemia: improving patient outcomes. Expert Rev Cardiovasc Ther. 2013;11:327–42. doi: 10.1586/erc.13.7. [DOI] [PubMed] [Google Scholar]
- 6.Waters DD, Brotons C, Chiang CW, Ferrieres J, Foody J, Jukema JW, Santos RD, Verdejo J, Messig M, Mcpherson R, Seung KB, Tarasenko L. Lipid treatment assessment project 2: a multinational survey to evaluate the proportion of patients achieving low-density lipoprotein cholesterol goals. Circulation. 2009;120:28–34. doi: 10.1161/CIRCULATIONAHA.108.838466. [DOI] [PubMed] [Google Scholar]
- 7.Williams RR, Hunt SC, Schumacher MC, Hegele RA, Leppert MF, Ludwig EH, Hopkins PN. Diagnosing heterozygous familial hypercholesterolemia using new practical criteria validated by molecular genetics. Am J Cardiol. 1993;72:171–6. doi: 10.1016/0002-9149(93)90155-6. [DOI] [PubMed] [Google Scholar]
- 8.Raal FJ, Pilcher GJ, Panz VR, Van Deventer HE, Brice BC, Blom DJ, Marais AD. Reduction in mortality in subjects with homozygous familial hypercholesterolemia associated with advances in lipid-lowering therapy. Circulation. 2011;124:2202–7. doi: 10.1161/CIRCULATIONAHA.111.042523. [DOI] [PubMed] [Google Scholar]
- 9.Pijlman AH, Huijgen R, Verhagen SN, Imholz BP, Liem AH, Kastelein JJ, Abbink EJ, Stalenhoef AF, Visseren FL. Evaluation of cholesterol lowering treatment of patients with familial hypercholesterolemia: a large cross-sectional study in The Netherlands. Atherosclerosis. 2010;209:189–94. doi: 10.1016/j.atherosclerosis.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg AC, Hopkins PN, Toth PP, Ballantyne CM, Rader DJ, Robinson JG, Daniels SR, Gidding SS, De Ferranti SD, Ito MK, Mcgowan MP, Moriarty PM, Cromwell WC, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:133–40. doi: 10.1016/j.jacl.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Watts GF, Gidding S, Wierzbicki AS, Toth PP, Alonso R, Brown WV, Bruckert E, Defesche J, Lin KK, Livingston M, Mata P, Parhofer KG, Raal FJ, et al. Integrated guidance on the care of familial hypercholesterolaemia from the International FH Foundation. Int J Cardiol. 2014;171:309–25. doi: 10.1016/j.ijcard.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 12.Dadu RT, Ballantyne CM. Lipid lowering with PCSK9 inhibitors. Nat Rev Cardiol. 2014;11:563–75. doi: 10.1038/nrcardio.2014.84. [DOI] [PubMed] [Google Scholar]
- 13.Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, Basak A, Prat A, Chretien M. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci U S A. 2003;100:928–33. doi: 10.1073/pnas.0335507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naureckiene S, Ma L, Sreekumar K, Purandare U, Lo CF, Huang Y, Chiang LW, Grenier JM, Ozenberger BA, Jacobsen JS, Kennedy JD, Distefano PS, Wood A, et al. Functional characterization of Narc 1, a novel proteinase related to proteinase K. Arch Biochem Biophys. 2003;420:55–67. doi: 10.1016/j.abb.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res. 2009;50(Suppl):S172–7. doi: 10.1194/jlr.R800091-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian YW, Schmidt RJ, Zhang Y, Chu S, Lin A, Wang H, Wang X, Beyer TP, Bensch WR, Li W, Ehsani ME, Lu D, Konrad RJ, et al. Secreted PCSK9 downregulates low density lipoprotein receptor through receptor-mediated endocytosis. J Lipid Res. 2007;48:1488–98. doi: 10.1194/jlr.M700071-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Welder G, Zineh I, Pacanowski MA, Troutt JS, Cao G, Konrad RJ. High-dose atorvastatin causes a rapid sustained increase in human serum PCSK9 and disrupts its correlation with LDL cholesterol. J Lipid Res. 2010;51:2714–21. doi: 10.1194/jlr.M008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–72. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 19.Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, Derre A, Villeger L, Farnier M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–6. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 20.Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, Heath SC, Timpson NJ, Najjar SS, Stringham HM, Strait J, Duren WL, Maschio A, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40:161–9. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubuc G, Chamberland A, Wassef H, Davignon J, Seidah NG, Bernier L, Prat A. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2004;24:1454–9. doi: 10.1161/01.ATV.0000134621.14315.43. [DOI] [PubMed] [Google Scholar]
- 22.Zhang XL, Zhu QQ, Zhu L, Chen JZ, Chen QH, Li GN, Xie J, Kang LN, Xu B. Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials. BMC Med. 2015;13:123. doi: 10.1186/s12916-015-0358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Lin L, Zhang W, Zhou L, Wang H, Luo X, Luo H, Cai Y, Zeng C. Efficiency and safety of proprotein convertase subtilisin/kexin 9 monoclonal antibody on hypercholesterolemia: a meta-analysis of 20 randomized controlled trials. J Am Heart Assoc. 2015;4:e001937. doi: 10.1161/JAHA.115.001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navarese EP, Kolodziejczak M, Schulze V, Gurbel PA, Tantry U, Lin Y, Brockmeyer M, Kandzari DE, Kubica JM, D’agostino RB, Sr, Kubica J, Volpe M, Agewall S, et al. Effects of Proprotein Convertase Subtilisin/Kexin Type 9 Antibodies in Adults With Hypercholesterolemia: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;163:40–51. doi: 10.7326/M14-2957. [DOI] [PubMed] [Google Scholar]
- 25.Raal F, Scott R, Somaratne R, Bridges I, Li G, Wasserman SM, Stein EA. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation. 2012;126:2408–17. doi: 10.1161/CIRCULATIONAHA.112.144055. [DOI] [PubMed] [Google Scholar]
- 26.Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, Langslet G, Scott R, Olsson AG, Sullivan D, Hovingh GK, Cariou B, Gouni-Berthold I, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:331–40. doi: 10.1016/S0140-6736(14)61399-4. [DOI] [PubMed] [Google Scholar]
- 27.Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, Wu R, Pordy R. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380:29–36. doi: 10.1016/S0140-6736(12)60771-5. [DOI] [PubMed] [Google Scholar]
- 28.Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, Wasserman SM, Stein EA. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:341–50. doi: 10.1016/S0140-6736(14)61374-X. [DOI] [PubMed] [Google Scholar]
- 29.Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–99. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 30.Raal FJ, Santos RD. Homozygous familial hypercholesterolemia: current perspectives on diagnosis and treatment. Atherosclerosis. 2012;223:262–8. doi: 10.1016/j.atherosclerosis.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 31.Usifo E, Leigh SE, Whittall RA, Lench N, Taylor A, Yeats C, Orengo CA, Martin AC, Celli J, Humphries SE. Low-density lipoprotein receptor gene familial hypercholesterolemia variant database: update and pathological assessment. Ann Hum Genet. 2012;76:387–401. doi: 10.1111/j.1469-1809.2012.00724.x. [DOI] [PubMed] [Google Scholar]
- 32.Austin MA, Hutter CM, Zimmern RL, Humphries SE. Genetic causes of monogenic heterozygous familial hypercholesterolemia: a HuGE prevalence review. Am J Epidemiol. 2004;160:407–20. doi: 10.1093/aje/kwh236. [DOI] [PubMed] [Google Scholar]
- 33.Stone NJ, Levy RI, Fredrickson DS, Verter J. Coronary artery disease in 116 kindred with familial type II hyperlipoproteinemia. Circulation. 1974;49:476–88. doi: 10.1161/01.cir.49.3.476. [DOI] [PubMed] [Google Scholar]
- 34.Risk of fatal coronary heart disease in familial hypercholesterolaemia. Scientific Steering Committee on behalf of the Simon Broome Register Group. BMJ. 1991;303:893–6. doi: 10.1136/bmj.303.6807.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Versmissen J, Oosterveer DM, Yazdanpanah M, Defesche JC, Basart DC, Liem AH, Heeringa J, Witteman JC, Lansberg PJ, Kastelein JJ, Sijbrands EJ. Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ. 2008;337:a2423. doi: 10.1136/bmj.a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huijgen R, Kindt I, Verhoeven SB, Sijbrands EJ, Vissers MN, Kastelein JJ, Hutten BA. Two years after molecular diagnosis of familial hypercholesterolemia: majority on cholesterol-lowering treatment but a minority reaches treatment goal. PLoS One. 2010;5:e9220. doi: 10.1371/journal.pone.0009220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larosa JC, Pedersen TR, Somaratne R, Wasserman SM. Safety and effect of very low levels of low-density lipoprotein cholesterol on cardiovascular events. Am J Cardiol. 2013;111:1221–9. doi: 10.1016/j.amjcard.2012.12.052. [DOI] [PubMed] [Google Scholar]
- 38.Rosenbaum D, Dallongeville J, Sabouret P, Bruckert E. Discontinuation of statin therapy due to muscular side effects: a survey in real life. Nutr Metab Cardiovasc Dis. 2013;23:871–5. doi: 10.1016/j.numecd.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Banach M, Rizzo M, Toth PP, Farnier M, Davidson MH, Al-Rasadi K, Aronow WS, Athyros V, Djuric DM, Ezhov MV, Greenfield RS, Hovingh GK, Kostner K, et al. Statin intolerance - an attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch Med Sci. 2015;11:1–23. doi: 10.5114/aoms.2015.49807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davidson MH, Mcgarry T, Bettis R, Melani L, Lipka LJ, Lebeaut AP, Suresh R, Sun S, Veltri EP. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol. 2002;40:2125–34. doi: 10.1016/s0735-1097(02)02610-4. [DOI] [PubMed] [Google Scholar]
- 41.Awan Z, Seidah NG, Macfadyen JG, Benjannet S, Chasman DI, Ridker PM, Genest J. Rosuvastatin, proprotein convertase subtilisin/kexin type 9 concentrations, and LDL cholesterol response: the JUPITER trial. Clin Chem. 2012;58:183–9. doi: 10.1373/clinchem.2011.172932. [DOI] [PubMed] [Google Scholar]
- 42.Careskey HE, Davis RA, Alborn WE, Troutt JS, Cao G, Konrad RJ. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J Lipid Res. 2008;49:394–8. doi: 10.1194/jlr.M700437-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Willeit P, Kiechl S, Kronenberg F, Witztum JL, Santer P, Mayr M, Xu Q, Mayr A, Willeit J, Tsimikas S. Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): prospective 15-year outcomes in the Bruneck Study. J Am Coll Cardiol. 2014;64:851–60. doi: 10.1016/j.jacc.2014.03.061. [DOI] [PubMed] [Google Scholar]
- 44.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, Mcquay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 46.Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]