Abstract
Understanding the molecular events that regulate osteoblast differentiation is essential for the development of effective approaches to bone regeneration. In this study, we analysed the osteoinductive properties of extracellular calcium in bone marrow-derived mesenchymal stem cell (BM-MSC) differentiation. We cultured BM-MSCs in 3D gelatin scaffolds with Ca2+ and BMP-2 as osteoinductive agents. Early and late osteogenic gene expression and bone regeneration in a calvarial critical-size defect model demonstrate that extracellular Ca2+ enhances the effects of BMP-2 on Osteocalcin, Runx2 and Osterix expression and promotes bone regeneration in vivo. Moreover, we analysed the molecular mechanisms involved and observed an antagonistic effect between Ca2+ and BMP-2 on SMAD1/5, ERK and S6K signalling after 24 hours. More importantly, a cooperative effect between Ca2+ and BMP-2 on the phosphorylation of SMAD1/5, S6, GSK3 and total levels of β-CATENIN was observed at a later differentiation time (10 days). Furthermore, Ca2+ alone favoured the phosphorylation of SMAD1, which correlates with the induction of Bmp2 and Bmp4 gene expression. These data suggest that Ca2+ and BMP-2 cooperate and promote an autocrine/paracrine osteogenic feed-forward loop. On the whole, these results demonstrate the usefulness of calcium-based bone grafts or the addition of exogenous Ca2+ in bone tissue engineering.
Introduction
The combination of calcium-containing biomaterials and mesenchymal progenitors is regarded as a potential bone graft substitute in tissue engineering. In fact, bone is a heterogeneous biocomposite tissue that consists of an inorganic phase (essentially hydroxyapatite), an organic phase (mainly type I collagen) and cells [1] Physiological bone remodelling occurs when osteoblast precursors attach, differentiate and replace the bone previously resorbed by osteoclasts. Thus, osteoblast progenitors differentiate after exposure to multiple degradation products released by osteoclasts. The bone components released presented high extracellular calcium concentrations, collagen fragments and growth factors.
Functional type I collagen has a unique triple helical structure that uncoils during degradation to generate gelatin. This uncoiling exposes its RGD sequences, which promote integrin binding and cell attachment [2]. Unlike collagen, gelatin does not present antigenicity in physiological conditions [3]. These properties make gelatin an appropriate scaffold for use in 3D cell cultures [3]
Ca2+ levels differ significantly in different bone niches. Calcium from bone matrix is released in its ionic form into the remodelling microenvironment [4]. For instance, in the hemivacuole, studies have reported values in the range of 8–40 mM, in contrast to the non-resorbing surface of the osteoclast, where values were < 2 mM during resorption [5]. This local increment of Ca2+ concentration has a strong impact on the chemotaxis, proliferation and differentiation of osteoblasts [6]. Mechanistically, extracellular Ca2+ activates calcium-sensing receptors (CaSR). CaSRs are G protein-coupled receptors that are expressed in mesenchymal stem cells and osteoblasts [7], although their functional role in bone homeostasis remains unclear. Furthermore, voltage-gated Ca2+ channels increase intracellular Ca2+ levels in osteoblasts [8]. The maximum effects of extracellular Ca2+ on osteoprogenitor migration and differentiation are achieved at concentrations in the range of 2–10 mM [6]. However, although the osteoconductive or osteoinductive properties of Ca2+-containing scaffolds have been thoroughly exploited [9], little is known about their intracellular signal transduction in osteoblasts [10].
Osteogenic growth factors might be secreted by bone resident cells or released from bone matrix during bone remodelling. These factors that are released after resorption include several members of the TGFβ/BMP superfamily of osteogenic signals. TGFβ/BMP signals at the cell surface are transduced by the Smad family of transcription factors, which directly regulate gene expression and additional non-canonical pathways such as p38 MAP-kinase and phosphoinositide 3-kinase (PI3K)/AKT [11]. BMP target genes include a growing number of osteoblast-determining transcription factors such as Dlx5, Runx2 and Osterix [12,13]. More importantly, the efficacy of BMPs in bone regeneration is well known in both animal models and clinical applications such as bone fracture healing [14], alveolar cleft defects [15], spinal fusion [16] and craniofacial bone defects [17,18]. As a result, BMP-2 and BMP-7 have been approved for medical use in specific osteoinductive applications.
Tissue engineered scaffolds for bone regeneration should lead to the attachment, propagation, and differentiation of the transplanted cells. Thus, there are three main components in the field of bone tissue engineering: a scaffold that provides structure and substrate for tissue growth, a source of progenitor cells and stimuli to direct growth and differentiation within the scaffold [9]. Despite early successes, many challenges are still faced. For instance, beside improvement of individual components, understanding the interactions between them is key for success [19]. We combined bone marrow-derived mesenchymal stem cells (BM-MSCs) and 3D gelatin scaffolds with Ca2+ and BMP-2 as osteoinductive agents. In an attempt to define the mechanisms of osteoinduction by Ca2+, we first assessed the potential effects of Ca2+ on osteoblast differentiation and bone formation and its cooperation with BMP-2. More importantly, we identified the molecular mechanisms involved in this cooperative osteogenic interaction. We propose that knowledge of these signalling events induced by Ca2+ could benefit the field of bone tissue engineering.
Materials and methods
Isolation and culture of bone marrow-derived mesenchymal stem cells (BM-MSCs)
BM-MSCs were isolated from the femurs of 6–8 week old BALB/c mice, as previously described [20,21]. Mice were euthanised and the femurs dissected. Next, the soft tissues were cleaned and the femurs kept in complete media (DMEM supplemented with 10% FBS, penicillin/streptomycin, 1 mM pyruvate and 2 mM glutamine). The femur ends were cut using a rongeur and the bone marrow was flushed and collected. Cell suspension was filtered with a 70 μm cell strainer (Falcon, USA), transferred to a 100 mm cell culture plate and incubated at 37°C. The media was changed after 24 hours and then every eight hours for two to three days to discard non-adherent cells. After five to seven days, when the adherent cells reached 75%-80% of confluence, the cells were washed three times with warmed PBS and trypsinised for three minutes at room temperature. The lifted cells were cultured and expanded for future experiments.
2D gelatin coating and 3D gelatin scaffold preparation
Twelve-well plate surfaces were coated with a thin layer of 0.1% gelatin/PBS solution. Plates were left for one hour inside a laminar flow hood to dry the treated surfaces and then stored until use. To prepare 3D scaffolds, a 1 mm3 gelatin sponge (Gelita, B. Braun) was used. Sponges were cut into 1 mm thick slices for in vitro assays, whereas 2 mm slices were used for the in vivo experiments. Scaffolds were soaked in complete media and incubated for 12–24 hours at 37°C. The BM-MSCs (2x 105 cells in 20 μl of media per scaffold) were then seeded under sterile conditions into the gelatin scaffold, and everything was introduced into a microcentrifuge tube and incubated in a vertical position for four to six hours at 37°C. This seeding allowed efficient and equal attachment of the cells to the scaffold. To assess the effect of extracellular calcium on the osteoblast differentiation of BM-MSCs, CaSO4 or CaCl2 (Sigma-Aldrich) was used as source of Ca2+. BM-MSCs seeded on 3D gelatin scaffolds were cultured in the presence of different CaCl2 or CaSO4 concentrations in the culture media, which ranged from 3 mM to 10 mM, throughout the whole experimental period. Fresh media (100 μl) was added to the corresponding condition every three days.
Gene expression analysis by RT-qPCR
RNA isolation was performed using Trisure (Bioline Reagents, UK) in accordance with the manufacturer’s protocol. The purified RNA was quantified using a spectrophotometer (Nanodrop). Two micrograms of RNA were retrotranscribed to cDNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) in accordance with the manufacturer’s protocol. The gene expression of Alpl (Mm00475834.m1), Osteocalcin (Bglap2) (Mm03413826.m1), Runx2 (Mm03003491.m1), Osterix(Sp7) (Mm00504574.m1), Bmp2 (Mm01340178.m1), Bmp4 (Mm00432087.m1), Fgf21 (Mm00840165.g1) and Axin2 (Mm00443610.m1) was analysed using Taqman probes (Applied Biosystems) and normalised to Gapdh (Mm9999915.g1) expression by the 2ΔΔCt method.
Western blot assay
BM-MSCs seeded in 3D gelatin scaffolds were cultured for 24 hours or 10 days. Cells were lysed with 75 μl of lysis buffer (PBS, 1% Triton X-100, 100 mM PMSF, 1 μg/ml pepstatin, 1 μg/ml leupeptin, 1 mM of sodium orthovanadate, 10 mM NaF and 10 mM β-glycerophosphate) for one hour at 4°C. Thirty micrograms of protein samples were subjected to SDS-PAGE and immunoblotting. Membranes were incubated with different antibodies: pGSK3α/β Ser9/21 (9331S), pSMAD1/5/8 Ser465/467 (9511S) and pS6 Ser235/236 (2211) and pp38 Thr180/Tyr182 (9211S) from Cell Signaling Technology, pErk1/2 (M5670) from Sigma, β-catenin (610154) from BD Transduction Laboratories and α-tubulin (T6199) from Sigma, all diluted to a ratio of 1:1000. Horseradish peroxidase-conjugated secondary antibodies were used, followed by incubation with EZ-ECL reagent (Biological Industries). A chemiluminescent image of the immunoblots was captured with a Fujifilm LAS 3000 device.
Calvarial critical-size bone defects and in vivo bone regeneration
Procedures for animal experimentation were approved by the Animal Research Ethics Committee of the University of Barcelona and Generalitat de Catalunya. All methods in animal experiments were performed according to the approved institutional guidelines. A surgical procedure was performed in 10-week old male BALB/c mice. Animals were housed individually and fed ad libitum. Animals were anesthetised with isoflurane inhalation and an intraperitoneal injection of buprenorphine (0.05 mg/kg) was administered to provide intraoperative analgesia. To expose the parietal bones, a longitudinal midline incision was made and the tissues retracted. A circular critical-size bone defect with an outer diameter of 5 mm was carried out with a trephine bur on the left parietal. We cultured 4x 105 BM-MSCs per scaffold in accordance with the protocol described above for 3D gelatin scaffold preparation. After cells in the 3D gelatin scaffolds had been exposed to the respective conditions for 48 hours, a 1% final concentration solution of low melting agarose at 36°C was added as a gelling agent. Scaffolds were implanted to fill the bone defects, depending on the respective experimental group. The incised tissues were sutured and the animals monitored daily during the recovery phase. Five weeks after surgery, the animals were euthanised and the calvariae dissected.
Histological analysis
After skull fixation with 4% paraformaldehyde for 48 hours, the calvariae were decalcified with a Decalcifier II (Leica) for two days. They were then dehydrated, embedded in paraffin and cut into 6 μm sections to examine bone formation. Slides were stained using hematoxylin/eosin and Masson’s trichrome technique. Histological sections were evaluated using a light microscope (Nikon Eclipse E800). Quantification of the trichrome images was performed from the images at 4x magnification using ImageJ software.
Statistical analysis
Data were was obtained from at least three independent experiments and presented as mean ± SEM. Data was analyzed for normality of the distribution by the Kolmogorov-Smirnov test. ANOVA (Bonferroni’s test) analysis was performed when more than two groups were compared. Differences were considered significant at p values of less than 0.05: *p < .05, **p < .01, and ***p < .001.
Results
Extracellular calcium increases osteogenic gene expression in BM-MSCs cultured in 3D gelatin scaffolds
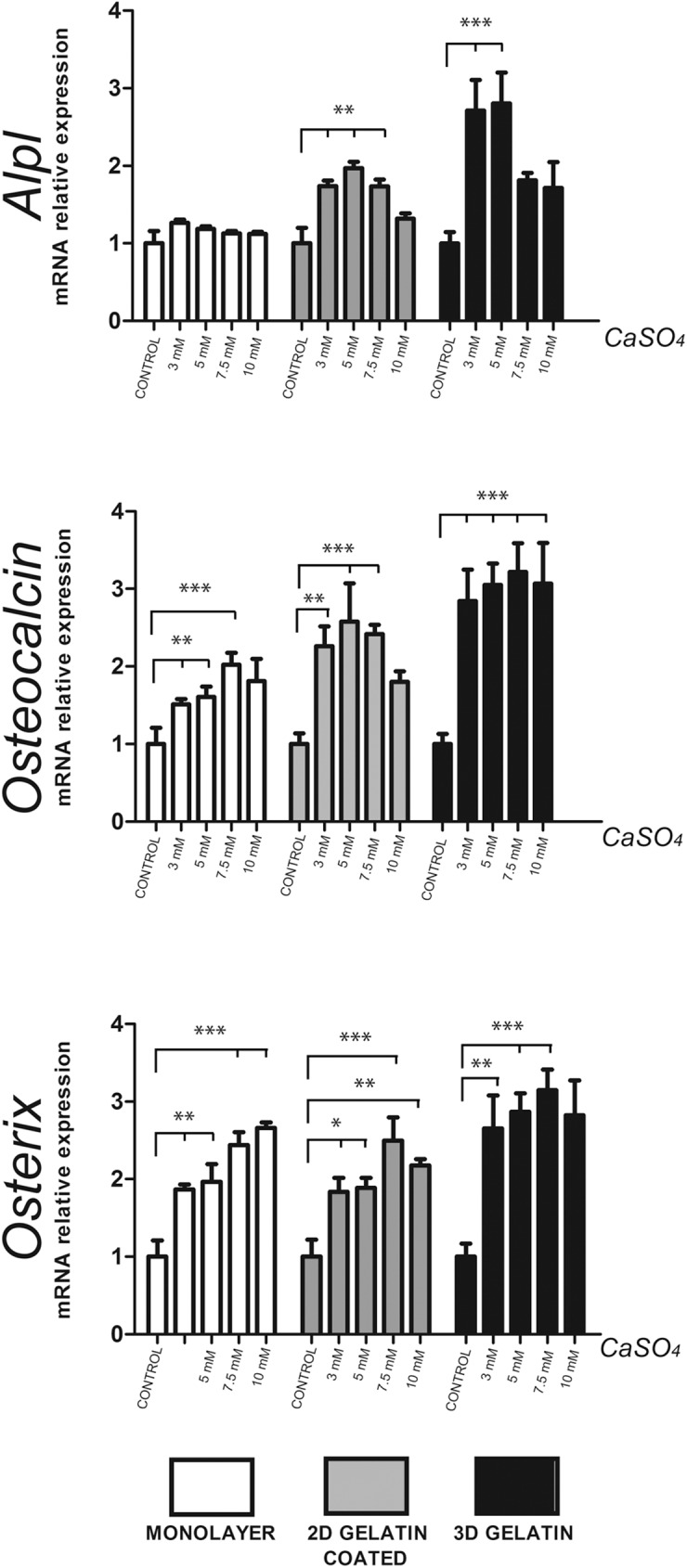
In order to evaluate the influence of the culture system on the osteoblast differentiation of BM-MSCs, we compared three different culture models: untreated plastic surface, 2D gelatin-coated surface and 3D gelatin scaffold. Cells seeded on 3D gelatin scaffolds showed greater upregulation of all osteogenic markers evaluated (Alpl p< 0.001; Osteocalcin p< 0.001 and Osterix p< 0.001) than monolayers on plastic surfaces or 2D gelatin coated plates (S1 Fig). This result suggests that 3D gelatin scaffolds promote higher osteoblast differentiation than plastic or 2D gelatin-coated surfaces.
We then assessed whether extracellular Ca2+ could have a beneficial effect on osteoblast differentiation. We evaluated the effects of different Ca2+ concentrations on BM-MSCs using the three culture systems described above. Higher expressions of Alpl, Osteocalcin and Osterix were obtained using Ca2+ concentrations from 3 mM to 10 mM (Fig 1). A concentration of 7.5 mM was optimal for the late osteogenic differentiation markers Osteocalcin and Osterix. Taken together, these results suggest that extracellular Ca2+ concentrations of between 3 mM and 10 mM produce a beneficial effect on the expression of Osteocalcin and Osterix, regardless of the culture model used. We confirmed the specificity of these effects by comparing CaSO4 and CaCl2 as calcium ion sources or by chelating Ca2+ with EDTA. Both CaSO4 and CaCl2 stimulated the expression of osteogenic markers. Moreover, the addition of EDTA completely blocked the positive effects of CaSO4 and CaCl2 on gene expression (S2 Fig).
Fig 1. Extracellular calcium induces expression of osteogenic markers on bone marrow mesenchymal stem cells.
Three different culture models were compared (cells in monolayer in plastic surface, cells in monolayer in gelatin-coated dishes and cells in 3D gelatin scaffolds). After 10 days, the mRNA expression of Alpl, Osteocalcin (Bglap2) and Osterix was analysed and normalised to Gapdh levels (n = 3). Differences were considered significant at p values: * p < 0.05,** p < 0.01, and ***p < 0.001.
Cooperation of calcium and BMP-2 in osteoblast differentiation and bone regeneration of calvarial critical-size defects in mice
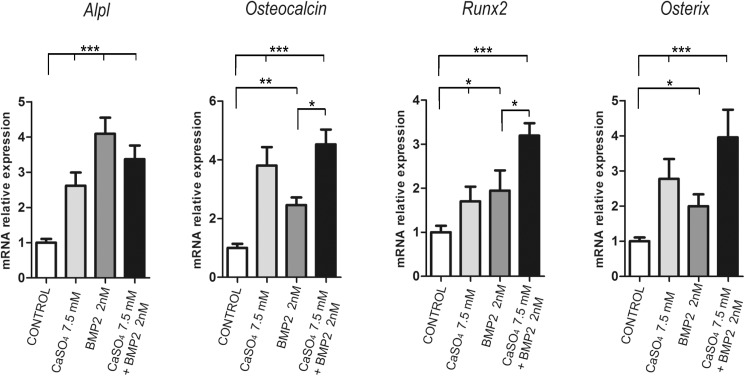
We further evaluated whether Ca2+ would cooperate with osteoinductive cytokines such as BMPs. BM-MSCs were cultured in a 3D gelatin scaffold and stimulated with 7.5 mM CaSO4 and/or BMP-2 (2 nM). Incubation for 10 days with CaSO4 or BMP-2 alone promoted expression of all the bone markers analysed (Fig 2). More importantly, although a combination of Ca2+ and BMP-2 did not present any additive effects on the expression of the early osteogenic marker Alpl, they produced a significant additive effect on the expression of Osteocalcin, Runx2 and Osterix (Fig 2). Thus, when added to culture media, Ca2+ exerts a cooperative action with BMP-2 on late osteogenic marker expression.
Fig 2. Extracellular calcium increases the effects of BMP-2 on osteogenic marker expression.
Primary BM-MSCs were cultured on 3D gelatin scaffolds with 7.5 mM of CaSO4 and/or 2 nM of BMP-2 for 10 days. The mRNA expression of Alpl, Osteocalcin (Bglap2), Runx2 and Osterix was analysed and normalised to Gapdh levels (n = 3). Differences were considered significant at p values: * p < 0.05,** p < 0.01, and ***p < 0.001.
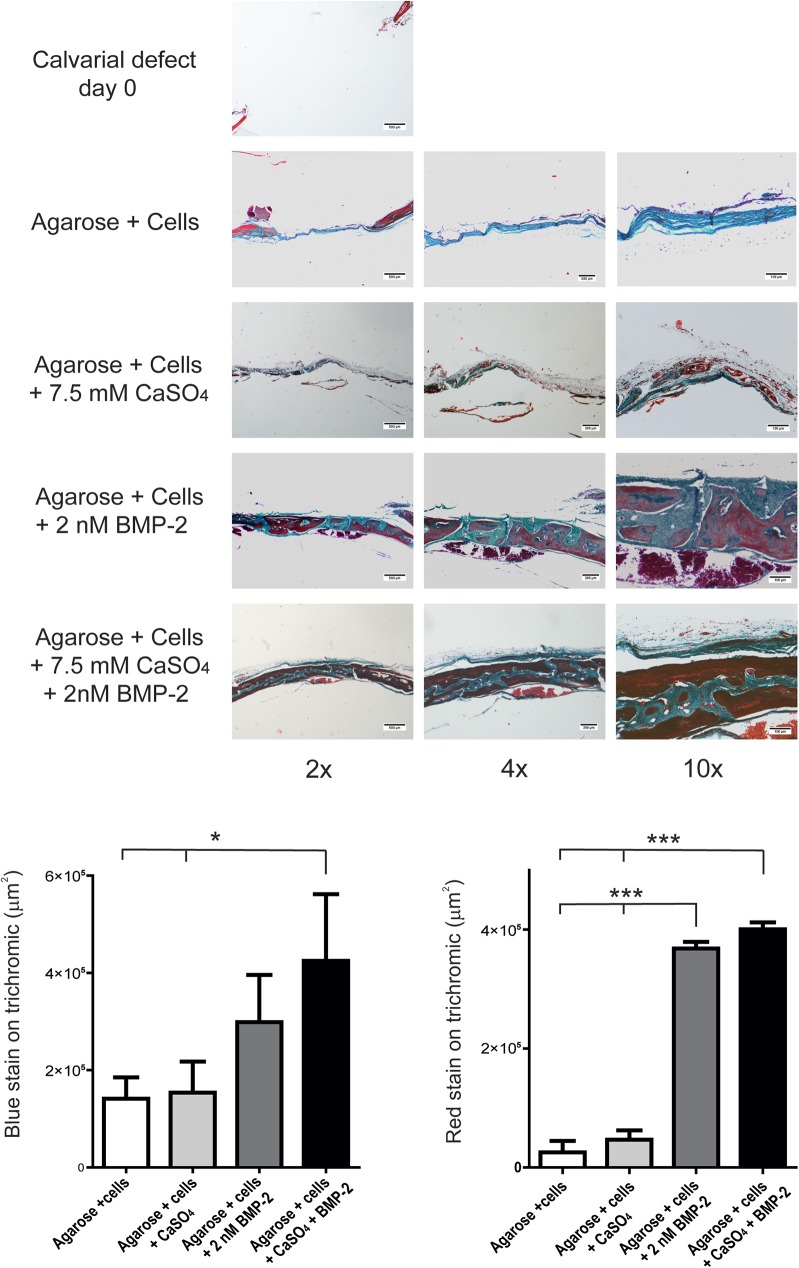
To extend our in vitro results on the cooperation between Ca2+ and BMPs to an in vivo context, we analysed bone formation in calvarial critical-size bone defects in mice. Five-millimetre defects were performed in parietal bones and further implanted with BM-MSCs previously seeded in 3D gelatin scaffolds and pre-treated for 48 hours with either 7.5 mM CaSO4 or 2 nM BMP-2 alone or combined. After five weeks, skulls were retrieved and analysed for bone formation in the defect. Masson’s trichrome stains showed dense connective tissue but no major bone formation in the control group. Higher levels of mineralisation and bone maturation were found in those implants treated with BMP-2. Similarly, higher bone formation took place when implants were pre-treated by combination of CaSO4 and BMP-2 (Fig 3). Moreover, a combination of CaSO4 and BMP-2 led to a more mature bone structure. Both osteoblast and osteocytes can be observed in these bone regeneration areas (Fig 3).
Fig 3. Extracellular calcium increases the effects of BMP-2 on bone regeneration in vivo.
Calvarial critical-size bone defects were generated in mice and implanted with cells cultured in 3D scaffolds pre-treated with 7.5 mM of CaSO4 and/or 2 nM of BMP-2 for 48 hours. After five weeks, the implanted constructs were retrieved and processed for Masson’s trichrome staining. Scale bars are shown for the different magnifications. Lower panels show the relative quantification of the areas of new bone formation from the 4x magnification stained by thrichrome in red or blue (n = 3).
Signalling pathways involved in the cooperation of calcium and BMP-2 during osteogenesis of BM-MSCs
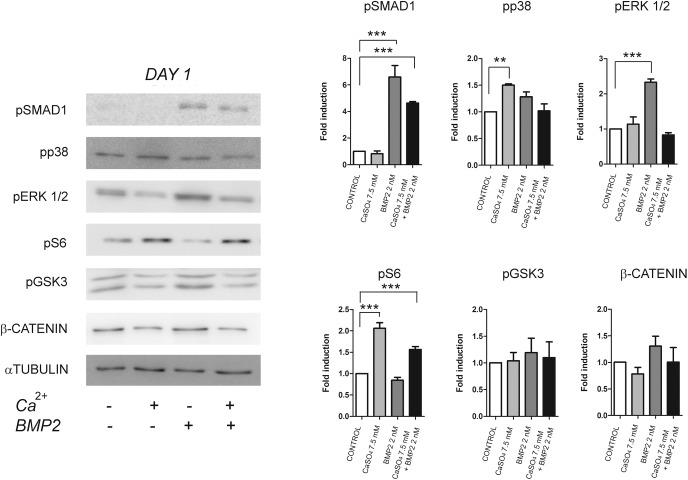
To determine the mechanisms of cooperation between extracellular calcium and BMP-2 in BM-MSCs differentiation, we analysed intracellular signalling triggered by both signalling molecules at early and late differentiation points. Early analysis was performed 24 hours after CaSO4 and/or BMP-2 stimulation. As expected, BMP-2 promoted phosphorylation of SMAD1/5 and increased the levels of phosphorylated ERK1/2 (Fig 4). By contrast, p38 and S6-kinase (S6K) signalling pathways were activated when cells were treated with Ca2+ alone. It is worth noting that, at this initial differentiation stage, an antagonistic effect on each signalling pathways was obtained when Ca2+ was added together with BMP-2 (Fig 4).
Fig 4. Early effects of extracellular calcium on cell signalling.
Cells were cultured in 3D gelatin scaffolds with Ca2+ (7.5 mM) and/or BMP-2 (2nM) for 24 hours and extracts analysed by Western blot. Data was quantified relative to the levels of α-TUBULIN (n = 3). Differences were considered significant at p values: * p < 0.05,** p < 0.01, and ***p < 0.001.
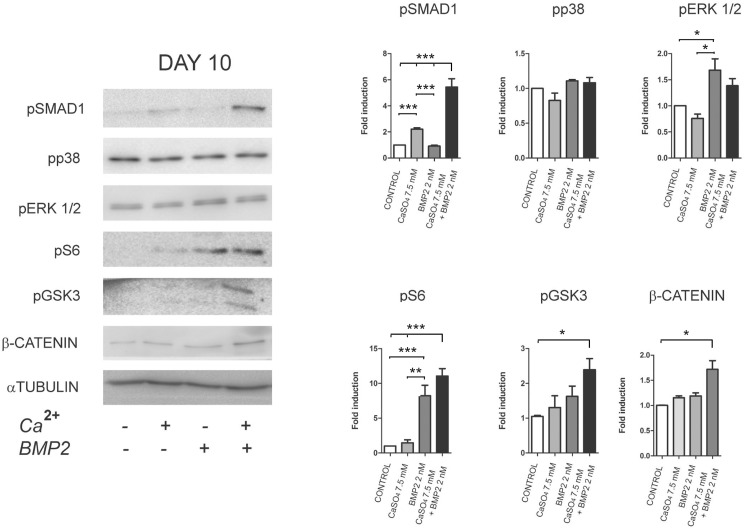
The same intracellular components were subsequently assessed after treatment with a combination of CaSO4 and/or BMP-2 for 10 days. A significant additive or cooperative effect between Ca2+ and BMP-2 was observed on the phosphorylation levels of SMAD1/5 (Ser463-465), S6 (Ser235-236), GSK3β (Ser9) and the total levels of β-CATENIN (Fig 5). Phosphorylation at Ser9 of GSK3β is mediated by AKT and results in the inhibition of its β-CATENIN repression action. Thus, these results suggest that extracellular calcium produces a differential time-dependent effect on BMP-2 and AKT signalling. A signalling network antagonistic to BMP-2 is activated early on, whereas Ca2+ promotes a cooperative effect on several intracellular signalling events later on.
Fig 5. Late effects of extracellular calcium on cell signalling.
Cells were cultured in 3D gelatin scaffolds with Ca2+ (7.5 mM) and/or BMP-2 (2nM) for 10 days. Data was quantified relative to the levels of α-TUBULIN (n = 3). Differences were considered significant at p values: * p < 0.05, ** p < 0.01, and ***p < 0.001.
Extracellular calcium promotes endogenous secreted BMP-2 and BMP-4 mRNA expression
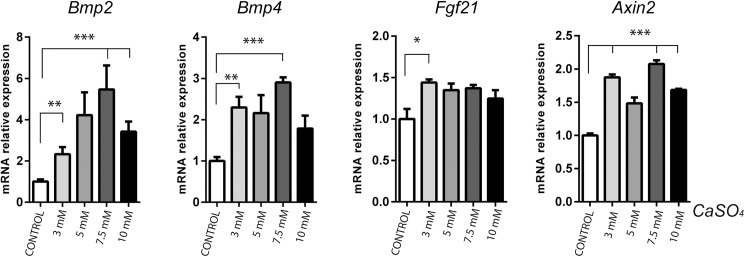
After 10 days of differentiation, BM-MSCs stimulated with calcium alone showed activation of the SMAD1/5 pathway (Fig 5). Since there is no evidence that calcium activates BMP receptors directly, it could be suggested that the increased availability of BMP receptor ligands was responsible. We therefore hypothesised that, once these cells are committed to the osteoblast lineage, Ca2+ induces cells to secrete endogenous factors that reinforce differentiation through an autocrine/paracrine mechanism. We assayed whether Ca2+ induced BMP-2 or BMP-4 expression. BM-MSCs cultured in 3D gelatin scaffolds were exposed to CaSO4 concentrations (from 3 mM to 10 mM) for 10 days. An increase, which reached its maximum at 7.5 mM, was obtained for both Bmp2 and Bmp4 mRNA expression (Fig 6). In addition, we also determined the mRNA levels of Fgf21 (a tyrosine kinase receptor ligand that inhibits osteoblastogenesis[22] and Axin2 (a target of the Wnt/β-CATENIN pathway downstream of GSK3). A significant increase in Axin2 expression was found, in line with Bmp2 and Bmp4 mRNA expression. By contrast, Fgf21 mRNA expression was only slightly elevated, without any dose-response effect (Fig 6). Taken together, these results demonstrate that BM-MSCs stimulated with Ca2+ secrete higher levels of multiple critical cytokines that amplify their osteoblastic differentiation response.
Fig 6. Extracellular calcium induces Bmp2, Bmp4, Fgf21 and Axin2 mRNA expression.
BM-MSCs were cultured on 3D gelatin scaffolds with different extracellular calcium concentrations for 10 days. The mRNA expression of Bmp2, Bmp4, Fgf21 and Axin2 was analysed and normalised to the levels of Gapdh (n = 3). Differences were considered significant at p values: * p < 0.05,** p < 0.01, and ***p < 0.001.
Discussion
Different anatomical zones are used to obtain autogenous bone grafts to reconstruct bone defects. These sources include the iliac crest, cranial bone, mandibular symphysis, rib and tibia [23]. However, drawbacks include limited availability and morbidity at the donor site. To overcome these disadvantages, numerous tissue engineering approaches have been developed to take advantage of physiological osteoinductive signals [24]. Both Ca2+ and BMP-2 are known to be co-released into the extracellular space by osteoclasts after bone matrix resorption. Our hypothesis was that extracellular Ca2+ signals interact with BMP and lead to higher osteoblast differentiation and bone formation from BM-MSCs. Here, early and late osteogenic marker expression and histological assessment of bone regeneration in a calvarial critical-size defect model demonstrated that extracellular Ca2+ enhances the effects of BMP-2 on Osteocalcin, Runx2 and Osterix expression and promotes bone regeneration in vivo. More importantly, mechanistically, both osteoinductors combined cooperate to increase long-term activation of SMAD and AKT signalling.
Osteogenic gene expression was significantly higher when extracellular Ca2+ was added, regardless of the cell culture system used. MG63 osteoblastic cells, both in monolayer culture and 3D gelatin hydrogels, have been reported to show significant mineralisation when cultured with 8 mM Ca2+ [25]. Moreover, BM-MSCs treated simultaneously with two different sources of Ca2+, CaSO4 and CaCl2, with or without EDTA, demonstrated that the osteogenic effect was specific for Ca2+. This outcome is consistent with the EGTA inhibition of osteocalcin secretion [26] and BAPTA, an intracellular Ca2+ chelator, in the response of osteoblasts to extracellular calcium [27].
Osteoinductive factors released from resident cells or after osteoclast bone resorption regulate the recruitment and differentiation of osteoblastic progenitor cells. The binding of BMPs to their cognate receptors triggers canonical Smad and Smad-independent pathways, including ERK, p38 and PI3K/AKT signalling [11,28]. Several authors have reported that CaSR activation by extracellular Ca2+ also activates these same pathways [4,27,29]. We found that both calcium and BMP-2 induce activation of common signalling components, but in a differential time-dependent response. An early antagonistic effect between Ca2+ and BMP-2 signalling was demonstrated. This contrasting effect is consistent with previous reports showing a crosstalk between calcium signalling and BMP pathway in which high intracellular calcium inhibits BMP signalling [30]. Furthermore, Ca2+/calmodulin dependent kinase II (CAMKII), a primary transducer of calcium ions, directly interacts with SMADs and antagonises their function [31]. This reverse effect could be reinforced when we also consider that MAPK signalling inhibits BMP signals at the level of SMAD1 [32]. Osteoblast differentiation is a multistep cascade of gene expression that initially supports proliferation and survival [33]. The early prominent proliferative and pro-survival role of the MAPK and AKT/S6K network could induce the enlargement of the osteogenic progenitor pool[4].
However, when late differentiation events were analysed, Ca2+ and BMP-2 were found to cooperatively stimulate osteoblast differentiation through the strengthening of specific osteogenic signalling pathways. Increases in the phosphorylation of SMAD1/5, S6, GSK3β and expression of β-CATENIN were consistent with the significantly higher expression of Osteocalcin, Runx2 and Osterix and the greater bone formation in vivo [34,35]. Cooperative crosstalk between Ca2+ and BMP-2 in osteoblasts through the induction of the calcium-dependent transcription factor NFATc1 by BMP-2 has also been described recently [36]. NFAT transcription factors have proved necessary for osteoblast differentiation and bone formation [37]. Mechanistically, NFAT transcription factors activate osteogenesis through their interaction with OSX and their ability to stimulate Wnt/β-CATENIN signalling [37,38]. Unexpectedly, cell cultures exposed to Ca2+ alone for 10 days displayed significantly higher SMAD signalling. This observation correlated with the increase in Bmp2, Bmp4 and Axin2 gene expression. BM-MSCs, periodontal ligament cells and dental pulp cells exposed to calcium-derived biomaterials have been reported to induce the upregulation of Bmp2 mRNA expression [8,39,40]. These studies implicated MAPK activity and AP-1 transcription factors in such effects [8,41]. Thus, our data support the suggestion that calcium induces an autocrine/paracrine loop by endogenous BMP upregulation. Furthermore, Axin2 (a target gene of Wnt/β-CATENIN signalling downstream of GSK3β) was also expressed in long-term cell cultures. Our group recently showed that PI3K/AKT activity is relevant in bone formation in vivo and leads to the activation of SMAD1/5 and GSK3β/β-CATENIN signalling [35]. Several reports have demonstrated the synergistic interaction and the significance between BMP and Wnt during osteoblast differentiation and bone formation in vitro and in vivo [21,34]. Taken together, our results demonstrate a delayed calcium signalling effect that likely integrates and reinforces an osteogenic programme from multiple inputs.
Supporting information
Three different culture models were compared (cells cultured in monolayer in plastic surface, cells cultured in monolayer in gelatin-coated dishes and cells cultured in 3D gelatin scaffolds). Primary BM-MSCs were cultured in each system for 10 days. The mRNA expression of Alpl, Osteocalcin (Bglap2) and Osterix was analysed and normalised to the levels of Gapdh (n = 3).
(PDF)
BM-MSCs cultured in 3D gelatin scaffolds were exposed to CaSO4 and CaCl2 as a source of extracellular calcium (7.5 mM) with or without EDTA (7.5 mM). After 10 days, the mRNA expression of Osteocalcin (Bglap2), Runx2 and Osterix was analysed and normalised to the levels of Gapdh (n = 3).
(PDF)
Acknowledgments
The authors thank A. Angelo for her assistance with animal handling and P. Barenblit and B. Torrejón for his technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the Ministry of Education and Science of Spain (BFU2014-56313P) and Fundació La Marató de TV3 (120330). N. Artigas received a fellowship from the University of Barcelona. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Boskey AL (2013) Bone composition: relationship to bone fragility and antiosteoporotic drug effects. Bonekey Rep 2: 447 doi: 10.1038/bonekey.2013.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez RA, Kim HW, Ginebra MP (2012) Polymeric additives to enhance the functional properties of calcium phosphate cements. J Tissue Eng 3: 2041731412439555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rohanizadeh R, Swain MV, Mason RS (2008) Gelatin sponges (Gelfoam) as a scaffold for osteoblasts. J Mater Sci Mater Med 19: 1173–1182. doi: 10.1007/s10856-007-3154-y [DOI] [PubMed] [Google Scholar]
- 4.Dvorak MM, Riccardi D (2004) Ca2+ as an extracellular signal in bone. Cell Calcium 35: 249–255. [DOI] [PubMed] [Google Scholar]
- 5.Berger CE, Rathod H, Gillespie JI, Horrocks BR, Datta HK (2001) Scanning electrochemical microscopy at the surface of bone-resorbing osteoclasts: evidence for steady-state disposal and intracellular functional compartmentalization of calcium. J Bone Miner Res 16: 2092–2102. doi: 10.1359/jbmr.2001.16.11.2092 [DOI] [PubMed] [Google Scholar]
- 6.Chai YC, Carlier A, Bolander J, Roberts SJ, Geris L, et al. (2012) Current views on calcium phosphate osteogenicity and the translation into effective bone regeneration strategies. Acta Biomater 8: 3876–3887. doi: 10.1016/j.actbio.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 7.Chattopadhyay N, Yano S, Tfelt-Hansen J, Rooney P, Kanuparthi D, et al. (2004) Mitogenic action of calcium-sensing receptor on rat calvarial osteoblasts. Endocrinology 145: 3451–3462. doi: 10.1210/en.2003-1127 [DOI] [PubMed] [Google Scholar]
- 8.Barradas AM, Fernandes HA, Groen N, Chai YC, Schrooten J, et al. (2012) A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials 33: 3205–3215. doi: 10.1016/j.biomaterials.2012.01.020 [DOI] [PubMed] [Google Scholar]
- 9.Murphy CM, O'Brien, Little DG, Schindeler A (2013) Cell-scaffold interactions in the bone tissue engineering triad. Eur Cell Mater 26: 120–132. [DOI] [PubMed] [Google Scholar]
- 10.Szpalski C, Wetterau M, Barr J, Warren SM (2012) Bone tissue engineering: current strategies and techniques—part I: Scaffolds. Tissue Eng Part B Rev 18: 246–257. doi: 10.1089/ten.TEB.2011.0427 [DOI] [PubMed] [Google Scholar]
- 11.Gamez B, Rodriguez-Carballo E, Ventura F (2014) MicroRNAs and post-transcriptional regulation of skeletal development. J Mol Endocrinol 52: R179–197. doi: 10.1530/JME-13-0294 [DOI] [PubMed] [Google Scholar]
- 12.Ulsamer A, Ortuno MJ, Ruiz S, Susperregui AR, Osses N, et al. (2008) BMP-2 induces Osterix expression through up-regulation of Dlx5 and its phosphorylation by p38. J Biol Chem 283: 3816–3826. doi: 10.1074/jbc.M704724200 [DOI] [PubMed] [Google Scholar]
- 13.Ortuno MJ, Ruiz-Gaspa S, Rodriguez-Carballo E, Susperregui AR, Bartrons R, et al. (2010) p38 regulates expression of osteoblast-specific genes by phosphorylation of osterix. J Biol Chem 285: 31985–31994. doi: 10.1074/jbc.M110.123612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Axelrad TW, Einhorn TA (2009) Bone morphogenetic proteins in orthopaedic surgery. Cytokine Growth Factor Rev 20: 481–488. doi: 10.1016/j.cytogfr.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 15.Carstens MH, Chin M, Ng T, Tom WK (2005) Reconstruction of #7 facial cleft with distraction-assisted in situ osteogenesis (DISO): role of recombinant human bone morphogenetic protein-2 with Helistat-activated collagen implant. J Craniofac Surg 16: 1023–1032. [DOI] [PubMed] [Google Scholar]
- 16.Vaccaro AR, Chiba K, Heller JG, Patel T, Thalgott JS, et al. (2002) Bone grafting alternatives in spinal surgery. Spine J 2: 206–215. [DOI] [PubMed] [Google Scholar]
- 17.Hassan AH, Hosny KM, Murshid ZA, Alhadlaq A, Yamani A, et al. (2015) Controlled release of injectable liposomal in situ gel loaded with recombinant human bone morphogenetic protein-2 for the repair of alveolar bone clefts in rabbits. J Liposome Res: 1–8. [DOI] [PubMed] [Google Scholar]
- 18.Chenard KE, Teven CM, He TC, Reid RR (2012) Bone morphogenetic proteins in craniofacial surgery: current techniques, clinical experiences, and the future of personalized stem cell therapy. J Biomed Biotechnol 2012: 601549 doi: 10.1155/2012/601549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanovici J, Le Nail LR, Brennan MA, Vidal L, Trichet V, et al. (2016) Bone regeneration strategies with bone marrow stromal cells in orthopaedic surgery. Curr Res Transl Med 64: 83–90. doi: 10.1016/j.retram.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 20.Soleimani M, Nadri S (2009) A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc 4: 102–106. doi: 10.1038/nprot.2008.221 [DOI] [PubMed] [Google Scholar]
- 21.Aquino-Martinez R, Rodriguez-Carballo E, Gamez B, Artigas N, Carvalho-Lobato P, et al. (2016) Mesenchymal Stem Cells Within Gelatin/CaSO4 Scaffolds Treated Ex Vivo with Low Doses of BMP-2 and Wnt3a Increase Bone Regeneration. Tissue Eng Part A 22: 41–52. doi: 10.1089/ten.TEA.2015.0181 [DOI] [PubMed] [Google Scholar]
- 22.Wei W, Dutchak PA, Wang X, Ding X, Bookout AL, et al. (2012) Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proc Natl Acad Sci U S A 109: 3143–3148. doi: 10.1073/pnas.1200797109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rawashdeh MA, Telfah H (2008) Secondary alveolar bone grafting: the dilemma of donor site selection and morbidity. Br J Oral Maxillofac Surg 46: 665–670. doi: 10.1016/j.bjoms.2008.07.184 [DOI] [PubMed] [Google Scholar]
- 24.Henkel J, Woodruff MA, Epari DR, Steck R, Glatt V, et al. (2013) Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res 1: 216–248. doi: 10.4248/BR201303002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takagishi Y, Kawakami T, Hara Y, Shinkai M, Takezawa T, et al. (2006) Bone-like tissue formation by three-dimensional culture of MG63 osteosarcoma cells in gelatin hydrogels using calcium-enriched medium. Tissue Eng 12: 927–937. doi: 10.1089/ten.2006.12.927 [DOI] [PubMed] [Google Scholar]
- 26.Moreau R, Aubin R, Lapointe JY, Lajeunesse D (1997) Pharmacological and biochemical evidence for the regulation of osteocalcin secretion by potassium channels in human osteoblast-like MG-63 cells. J Bone Miner Res 12: 1984–1992. doi: 10.1359/jbmr.1997.12.12.1984 [DOI] [PubMed] [Google Scholar]
- 27.Danciu TE, Adam RM, Naruse K, Freeman MR, Hauschka PV (2003) Calcium regulates the PI3K-Akt pathway in stretched osteoblasts. FEBS Lett 536: 193–197. [DOI] [PubMed] [Google Scholar]
- 28.Sieber C, Kopf J, Hiepen C, Knaus P (2009) Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev 20: 343–355. doi: 10.1016/j.cytogfr.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 29.Riccardi D, Finney BA, Wilkinson WJ, Kemp PJ (2009) Novel regulatory aspects of the extracellular Ca2+-sensing receptor, CaR. Pflugers Arch 458: 1007–1022. doi: 10.1007/s00424-009-0681-z [DOI] [PubMed] [Google Scholar]
- 30.Leclerc C, Neant I, Moreau M (2011) Early neural development in vertebrates is also a matter of calcium. Biochimie 93: 2102–2111. doi: 10.1016/j.biochi.2011.06.032 [DOI] [PubMed] [Google Scholar]
- 31.Wicks SJ, Lui S, Abdel-Wahab N, Mason RM, Chantry A (2000) Inactivation of smad-transforming growth factor beta signaling by Ca(2+)-calmodulin-dependent protein kinase II. Mol Cell Biol 20: 8103–8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kretzschmar M, Doody J, Timokhina I, Massague J (1999) A mechanism of repression of TGFbeta/ Smad signaling by oncogenic Ras. Genes Dev 13: 804–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lefebvre V, Bhattaram P (2010) Vertebrate skeletogenesis. Curr Top Dev Biol 90: 291–317. doi: 10.1016/S0070-2153(10)90008-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez-Carballo E, Ulsamer A, Susperregui AR, Manzanares-Cespedes C, Sanchez-Garcia E, et al. (2011) Conserved regulatory motifs in osteogenic gene promoters integrate cooperative effects of canonical Wnt and BMP pathways. J Bone Miner Res 26: 718–729. doi: 10.1002/jbmr.260 [DOI] [PubMed] [Google Scholar]
- 35.Gamez B, Rodriguez-Carballo E, Graupera M, Rosa JL, Ventura F (2016) Class I PI-3-Kinase Signaling Is Critical for Bone Formation through Regulation of SMAD1 Activity in Osteoblasts. J Bone Miner Res. [DOI] [PubMed] [Google Scholar]
- 36.Mandal CC, Das F, Ganapathy S, Harris SE, Ghosh Choudhury G, et al. (2016) Bone Morphogenetic Protein-2 (BMP-2) Activates NFATc1 Transcription Factor via an Autoregulatory Loop Involving Smad/Akt/Ca2+ Signaling. J Biol Chem 291: 1148–1161. doi: 10.1074/jbc.M115.668939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koga T, Matsui Y, Asagiri M, Kodama T, de Crombrugghe B, et al. (2005) NFAT and Osterix cooperatively regulate bone formation. Nat Med 11: 880–885. doi: 10.1038/nm1270 [DOI] [PubMed] [Google Scholar]
- 38.Fromigue O, Hay E, Barbara A, Marie PJ (2010) Essential role of nuclear factor of activated T cells (NFAT)-mediated Wnt signaling in osteoblast differentiation induced by strontium ranelate. J Biol Chem 285: 25251–25258. doi: 10.1074/jbc.M110.110502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang M, Peng Z, Mai Z, Chen L, Mao Q, et al. (2014) Fluid shear stress stimulates osteogenic differentiation of human periodontal ligament cells via the extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase signaling pathways. J Periodontol 85: 1806–1813. doi: 10.1902/jop.2014.140244 [DOI] [PubMed] [Google Scholar]
- 40.Maeda H, Nakano T, Tomokiyo A, Fujii S, Wada N, et al. (2010) Mineral trioxide aggregate induces bone morphogenetic protein-2 expression and calcification in human periodontal ligament cells. J Endod 36: 647–652. doi: 10.1016/j.joen.2009.12.024 [DOI] [PubMed] [Google Scholar]
- 41.Tada H, Nemoto E, Kanaya S, Hamaji N, Sato H, et al. (2010) Elevated extracellular calcium increases expression of bone morphogenetic protein-2 gene via a calcium channel and ERK pathway in human dental pulp cells. Biochem Biophys Res Commun 394: 1093–1097. doi: 10.1016/j.bbrc.2010.03.135 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Three different culture models were compared (cells cultured in monolayer in plastic surface, cells cultured in monolayer in gelatin-coated dishes and cells cultured in 3D gelatin scaffolds). Primary BM-MSCs were cultured in each system for 10 days. The mRNA expression of Alpl, Osteocalcin (Bglap2) and Osterix was analysed and normalised to the levels of Gapdh (n = 3).
(PDF)
BM-MSCs cultured in 3D gelatin scaffolds were exposed to CaSO4 and CaCl2 as a source of extracellular calcium (7.5 mM) with or without EDTA (7.5 mM). After 10 days, the mRNA expression of Osteocalcin (Bglap2), Runx2 and Osterix was analysed and normalised to the levels of Gapdh (n = 3).
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.