Abstract
Background
Although gastric adenoma is widely accepted as a precursor of gastric cancer, pre-existing adenoma is not always detected in gastric cancer patients.
Objective
To investigate the clinical characteristics of early gastric cancer (EGC) arising from adenoma, compared with those of EGC without pre-existing adenoma.
Methods
Patients who underwent endoscopic resection for EGC at a single tertiary hospital were divided into two groups based on the presence (ex-adenoma group) or absence (de novo group) of pre-existing adenoma on pathologic specimens. Clinicopathologic characteristics, endoscopic features and long-term outcomes were analyzed.
Results
Of 1,509 patients, 236 (15.6%) were included in the ex-adenoma group. Mean age (P = 0.003) and Helicobacter pylori infection rate (P = 0.040) were significantly higher in the ex-adenoma than in the de novo group. Mean endoscopic size was significantly larger, elevated lesions were more prevalent (both P < 0.001), and carcinomas were more differentiated in the ex-adenoma group than in the de novo group (P = 0.037). The degree of atrophy (P = 0.025) or intestinal metaplasia (P < 0.001) was more advanced in the ex-adenoma group. Synchronous gastric neoplasia was significantly more prevalent in the ex-adenoma group (P < 0.001), whereas metachronous cancer recurrence rate was not significantly different between the two groups.
Conclusions
EGCs with pre-existing adenoma show a greater association with H. pylori–related chronic inflammation than those without, which could explain the differences in the characteristics between groups. Potential differences in carcinogenic mechanisms between the groups were explored.
Introduction
Gastric cancer is the fourth most common cancer and the second leading cause of cancer-related death in the world [1, 2]. Although the incidence and mortality of gastric cancer have been decreasing worldwide, it remains one of the most common cancers in East Asia, especially in Korea and Japan [3]. Improvements in endoscopic techniques and instruments have made endoscopic submucosal dissection (ESD) the curative treatment modality of choice for early gastric cancer (EGC) without lymph node metastasis. Although ESD has shown many advantages over conventional surgery, including reduced invasiveness, lower cost, and shorter hospital stay, metachronous cancer may subsequently occur in the residual gastric mucosa [4]. Thus, regular examination for metachronous gastric cancer is necessary in patients who undergo ESD.
Although the precise mechanisms underlying gastric tumorigenesis remain unclear, it is thought to be a multifactorial and multistep process. Chronic Helicobacter pylori infection, together with genetic or environmental factors are major determinants of the risk of gastric cancer [5]. Since Correa et al. proposed the hypothesis of gastric carcinogenesis, it has been widely accepted that chronic inflammation develops into atrophic gastritis, intestinal metaplasia, gastric adenoma, and eventually gastric adenocarcinoma [5]. H. pylori infection plays an important role in the initiation of this sequential process. Gastric adenoma is considered as a significant precancerous lesion, and the annual incidence of gastric cancer is 0.6% for patients with mild to moderate dysplasia and 6% for those with severe dysplasia [6].
Carcinogenesis from adenoma to carcinoma, which is known as the adenoma-carcinoma sequence, is a well-established theory in colorectal cancer [7]. The adenoma-carcinoma sequence has also been recognized in the field of gastric cancer, although it is found less frequently than in colorectal cancer [8, 9]. Differentiated or intestinal-type carcinoma, rather than diffuse-type carcinoma, is mainly associated with this carcinogenic pathway [9, 10]. Gastric cancer derived from the adenoma-carcinoma sequence might arise through a stepwise accumulation of genetic alterations similar to that of colorectal cancer; however, other clinical characteristics of gastric cancer developed from adenoma have not been well described to date [11].
The aim of the present study was to examine the clinical characteristics of EGC arising from adenoma compared with that without pre-existing adenoma. Baseline demographics, endoscopic and pathologic features, and long-term outcomes were analyzed in detail.
Methods
Patients and study design
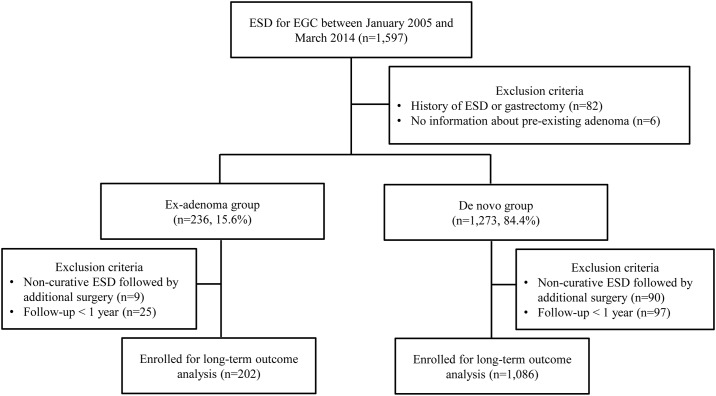
A schematic protocol of the study design is provided in Fig 1. Between January 2005 and March 2014, patients who underwent ESD for EGCs at Seoul National University Hospital (Seoul, Republic of Korea) were screened for this retrospective cohort study. Patients with a prior history of ESD or gastrectomy or those who had insufficient information about the presence of pre-existing adenoma were excluded from the analysis. The remaining patients were assigned to one of two groups according to the presence or absence of pre-existing adenoma. Patients with adenomatous components at the margin of EGCs were defined as the ex-adenoma group, and those without were defined as the de novo group (Fig 2). The medical records of the patients were reviewed with regard to age, gender, comorbidities, H. pylori positivity, and endoscopic features including size, location, macroscopic type, and gross morphology of tumors. Pathologic characteristics such as lesion size on the resected specimen, depth of invasion, cancer differentiation, Lauren’s classification, severity of atrophy, and intestinal metaplasia in the adjacent mucosa were also examined. Long-term outcomes including metachronous cancer recurrence were recorded. The study was approved by the Ethics Committee of the Seoul National University Hospital (IRB no. H-1404-106-572) and was conducted in accordance with the Declaration of Helsinki.
Fig 1. Schematic protocol of this study.
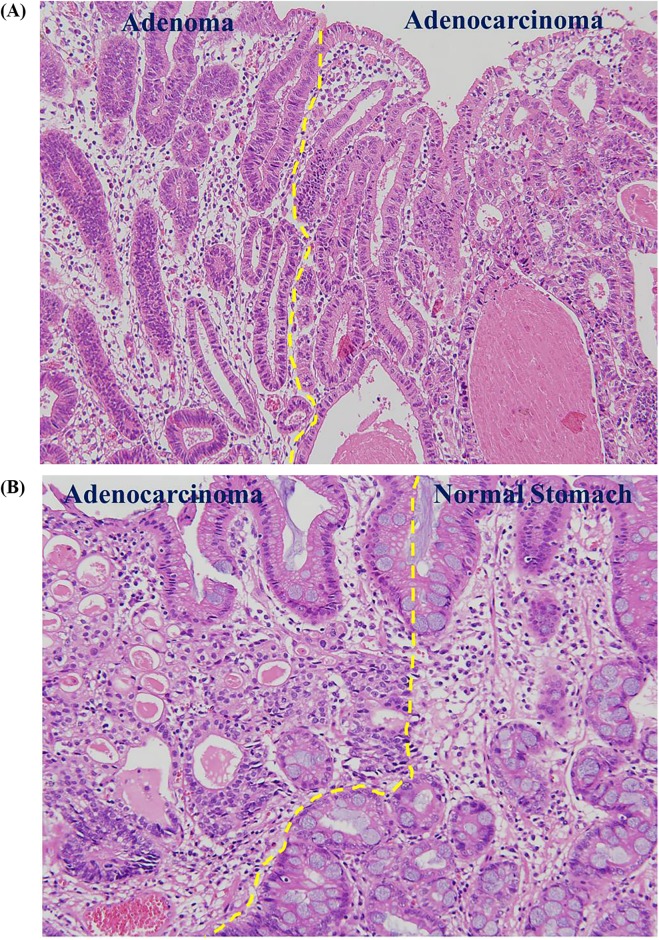
Fig 2. Histopathology of early gastric cancers with or without pre-existing adenoma.
(A) Representative image of the ex-adenoma group. Adenomatous components (left) were detected at the margin of the tubular adenocarcinoma (right) (Hematoxylin and eosin stain; original magnification, 200×). (B) Representative image of the de novo group. A sharp transition from the normal gastric mucosa (right) to tubular adenocarcinoma (left) was observed. No evidence of gastric adenoma was found in its vicinity.
ESD procedures
The indications for ESD in the treatment of EGC were defined as follows: (1) differentiated adenocarcinoma, (2) lesions ≤2 cm in diameter upon endoscopic estimation, and (3) no evidence of submucosal invasion or lymph node/distant metastasis on endoscopic ultrasonography and/or abdominal computed tomography. The ESD procedure was performed as described elsewhere [12, 13]. Intravenous midazolam (0.06 mg/kg) was administered for conscious sedation with cardiorespiratory monitoring. ESD was performed using a standard single-channel endoscope (Olympus H260; Olympus Optical Co, Tokyo, Japan) with an insulation-tipped knife (Helmet Snare; Kachu Technology Co., Seoul, Republic of Korea). After completion of ESD, biopsy samples of noncancerous gastric mucosae were obtained from two sites in the lesser curvature side of the antrum and two sites in the lesser curvature side of the body after obtaining informed consent [14].
Evaluation of endoscopic characteristics
Endoscopic still images and reports were retrospectively reviewed to investigate the size, location, macroscopic type, and gross morphology of the lesion. Indigo carmine chromoendoscopy was used to estimate lesion size more precisely during ESD. The location of the lesion was divided into three parts: upper, middle, and lower [15]. According to the Paris endoscopic classification, lesions were classified into three macroscopic types: elevated, flat, or depressed [16]. Gross morphological features such as erythema, ulcer, erosion, fold convergence, exudate, whitish discoloration, spontaneous bleeding, or nodularity were also evaluated. An ulcer was defined as a loss of mucosal integrity (>5 mm in diameter) with a well-defined crater, whereas an erosion was defined as a flat or slightly depressed mucosal break <5 mm in diameter [17, 18]. The presence of fold convergence was characterized by abrupt cutting, clubbing, and fusion of adjacent folds. Nodularity was defined as the presence of an irregularly raised or nodular mucosa without a dominant mass [19].
Evaluation of pathologic characteristics
Pathological diagnosis was made on the basis of the third edition of the Japanese Classification of Gastric Carcinoma: size, histologic type, and depth of invasion were evaluated [15]. In addition, histologic subtyping of gastric carcinoma was performed according to Lauren’s classification into intestinal, diffuse, and mixed types [20]. Noncancerous gastric tissues were analyzed for the severity of mucosal atrophy and intestinal metaplasia and histologically evaluated for the presence of H. pylori [21]. The severity of mucosal atrophy and intestinal metaplasia were classified as negative (absent to mild) or positive (moderate to severe). H. pylori infection status was considered positive when either rapid urease test (CLO test; Delta West, Bently, Australia) or histology was positive.
Follow-up strategies and long-term outcome measurements
Endoscopic examinations were performed at 3, 6, and 12 months after ESD and annually thereafter to detect any residual lesion or metachronous cancer of the stomach. Metachronous gastric cancer was defined as a new carcinoma developed at a previously uninvolved site in the remnant stomach at least 1 year after ESD. Gastric neoplasms detected within 1 year of ESD were regarded as missed lesions at the initial evaluation and categorized separately as synchronous gastric neoplasias. For long-term outcome analysis, patients who underwent additional surgery immediately after ESD or those whose follow-up period was less than 1 year were excluded.
Statistical analysis
Data were analyzed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA). All data were expressed as the mean ± standard deviation (range) or as numbers (percentages). The means of continuous variables were compared using the Student t-test, and categorical variables were analyzed with the chi-square test or Fisher’s exact test. Metachronous cancer recurrence-free survival was calculated using the Kaplan-Meier method. All unknown values were excluded from the analysis. A P-value <0.05 was considered statistically significant.
Results
Baseline characteristics of patients
Between January 2005 and March 2014, 1,597 patients with EGC were treated by ESD. Of these, 1,509 patients were included in the study and allocated to one of two groups based on the presence of adenomatous components at the edge of EGCs, namely the ex-adenoma group (n = 236) and the de novo group (n = 1,273). The baseline characteristics of the two groups are shown in Table 1. The mean age was significantly higher in the ex-adenoma group than in the de novo group (65.61 ± 9.22 vs. 63.62 ± 9.62 years, P = 0.003). The proportion of male patients, body mass index, and medical comorbidities were not significantly different between the two groups. H. pylori infection rate was higher in the ex-adenoma group than in the de novo group (64.8% vs. 57.2%, P = 0.040).
Table 1. Baseline characteristics of patients with early gastric cancers treated with endoscopic submucosal dissection.
| Ex-adenoma group (n = 236) |
De novo group (n = 1273) |
P value | |
|---|---|---|---|
| Mean age, year | 65.61 ± 9.22 | 63.62 ± 9.62 | 0.003 |
| Gender, male | 157 (66.5) | 875 (68.7) | 0.502 |
| Body mass index, mean | 24.04 ± 3.23 | 24.44 ± 8.96 | 0.500 |
| Comorbidities | |||
| Diabetes mellitus | 34 (14.4) | 160 (12.6) | 0.438 |
| Hypertension | 69 (29.2) | 353 (27.7) | 0.636 |
| Lung disease | 9 (3.8) | 28 (2.2) | 0.141 |
| Chronic liver disease | 15 (6.4) | 66 (5.2) | 0.463 |
| Chronic kidney disease | 3 (1.3) | 15 (1.2) | 0.904 |
| Stroke | 3 (1.3) | 38 (3.0) | 0.137 |
| Coronary heart disease | 8 (3.4) | 70 (5.5) | 0.179 |
| Other malignancy | 17 (7.2) | 87 (6.8) | 0.837 |
| H. pylori infectiona | 0.040 | ||
| Positive | 138 (64.8) | 680 (57.2) | |
| Negative | 75 (35.2) | 508 (42.8) | |
| Unknownb | 23 (—) | 85 (—) |
Values are presented as mean ± standard deviation or n (%)
H. pylori, Helicobacter pylori
aPatients were classified as H. pylori positive when either rapid urease test or histology was positive
bExcluded from the analysis
Additional analysis of H. pylori infection status was performed to minimize false-negative results (Table 2). As the infection progressed, the gastric mucosa showed severe atrophic changes and the bacterial load could be reduced in the stomach [22]. Patients were therefore divided into four groups based on the combination of the results of H. pylori infection and the severity of mucosal atrophy as follows: group A [H. pylori (-) and negative atrophy], group B [H. pylori (+) and negative atrophy], group C [H. pylori (+) and positive atrophy], and group D [H. pylori (-) and positive atrophy]. In this second analysis, group A patients were negative for H. pylori infection, whereas B, C, and D were considered H. pylori confirmed or expected. Group D was included as H. pylori confirmed or expected because atrophy detected in this group was considered as H. pylori–related. The H. pylori infection rate was significantly higher in the ex-adenoma group (83.4% vs. 74.2%, P = 0.005).
Table 2. Further analysis of H. pylori infection considering the severity of atrophic gastritis.
| Ex-adenoma group (n = 236) |
De novo group (n = 1,273) |
P value | |
|---|---|---|---|
| H. pylori infectiona | 0.005 | ||
| H. pylori negative (Group A) | 34 (16.6) | 298 (25.8) | |
| H. pylori confirmed or expected (Group B, C, D) | 171 (83.4) | 856 (74.2) | |
| Unknownb | 31 (—) | 119 (—) |
Values are presented as n (%)
H. pylori, Helicobacter pylori
aPatients were divided into 4 groups according to the results of H. pylori infection (rapid urease test or histology) and the severity of mucosal atrophy: group A [H. pylori (-) and negative atrophy], group B [H. pylori (+) and negative atrophy], group C [H. pylori (+) and positive atrophy], and group D [H. pylori (-) and positive atrophy].
bExcluded from the analysis
Endoscopic features of the two groups
Table 3 shows the endoscopic features of EGCs in the two groups. The lesion size estimated by endoscopy was significantly greater (19.25 ± 14.42 vs. 12.06 ± 7.49, P < 0.001) and the elevated type was more common in the ex-adenoma group (60.2% vs. 29.8%, P < 0.001) than in the de novo group. Erythema and erosion were significantly more frequent in the de novo group, whereas exudate and nodularity were more frequently observed in the ex-adenoma group. The location of the lesions did not differ between the groups.
Table 3. Comparison of endoscopic characteristics of early gastric cancers between the two groups.
| Ex-adenoma group (n = 236) |
De novo group (n = 1,273) |
P value | |
|---|---|---|---|
| Endoscopic size, mm | 19.25 ± 14.42 | 12.06 ± 7.49 | < 0.001 |
| Location | 0.130 | ||
| Upper | 10 (4.2) | 62 (4.9) | |
| Middle | 88 (37.3) | 390 (30.6) | |
| Lower | 138 (58.5) | 821 (64.5) | |
| Macroscopic type | < 0.001 | ||
| Elevated | 142 (60.2) | 379 (29.8) | |
| Flat | 30 (12.7) | 262 (20.6) | |
| Depressed | 64 (27.1) | 632 (49.5) | |
| Gross morphology | |||
| Erythema | 41 (17.4) | 464 (36.4) | < 0.001 |
| Ulcer | 3 (1.9) | 30 (3.3) | 0.050 |
| Erosion | 53 (22.5) | 463 (36.4) | < 0.001 |
| Fold convergence | 2 (0.8) | 14 (1.1) | 1.000 |
| Exudate | 14 (5.9) | 34 (2.7) | 0.009 |
| Whitish discoloration | 4 (1.7) | 15 (1.2) | 0.522 |
| Spontaneous bleeding | 15 (6.4) | 98 (7.7) | 0.472 |
| Nodularity | 67 (28.4) | 153 (12.0) | < 0.001 |
Values are presented as mean ± standard deviation or n (%)
Pathologic characteristics of the two groups
Table 4 shows the pathologic findings of the two groups. Similar to the endoscopic size, pathologic size was significantly greater in the ex-adenoma group (23.99 ± 15.57 vs. 17.50 ± 10.88, P < 0.001). The presence of atrophy (47.7% vs. 39.3%, P = 0.025) and intestinal metaplasia (84.0% vs. 65.4%, P < 0.001) were significantly more frequent in the ex-adenoma group. In addition, carcinomas showed better differentiation in the ex-adenoma group (97.9% vs. 94.7%, P = 0.037). The depth of the lesions and Lauren’s classification did not differ significantly between the two groups.
Table 4. Comparison of pathologic features between the two groups.
| Ex-adenoma group (n = 236) |
De novo group (n = 1,273) |
P value | |
|---|---|---|---|
| Pathologic size, mm | 23.99 ± 15.57 | 17.50 ± 10.88 | < 0.001 |
| Depth of invasiona | 0.088 | ||
| Mucosa | 209 (88.6) | 1,055 (82.9) | |
| Sm1 | 15 (6.4) | 112 (8.8) | |
| Sm2 | 12 (5.1) | 106 (8.3) | |
| Atrophyb | 0.025 | ||
| Negative | 104 (52.3) | 684 (60.7) | |
| Positive | 95 (47.7) | 442 (39.3) | |
| Not applicablec | 37 (—) | 147 (—) | |
| Intestinal metaplasiab | < 0.001 | ||
| Negative | 34 (16.0) | 409 (34.6) | |
| Positive | 178 (84.0) | 772 (65.4) | |
| Not applicablec | 23 (—) | 85 (—) | |
| Differentiation | 0.037 | ||
| Differentiated | 231 (97.9) | 1,206 (94.7) | |
| Undifferentiated | 5 (2.1) | 67 (5.3) | |
| Lauren’s classification | 0.132 | ||
| Intestinal | 231 (97.9) | 1,212 (95.5) | |
| Diffuse | 4 (1.7) | 26 (2.0) | |
| Mixed | 1 (0.4) | 31 (2.4) | |
| Unknownc | 0 (—) | 4 (—) | |
Values are presented as mean ± standard deviation or n (%)
aSm1, tumor invasion <0.5 mm of the muscularis mucosae; Sm2, tumor invasion ≥0.5 mm into the muscularis mucosae
bThe severity of mucosal atrophy and intestinal metaplasia were classified as negative (absent to mild) or positive (moderate to severe)
cExcluded from the analysis
Long-term outcomes
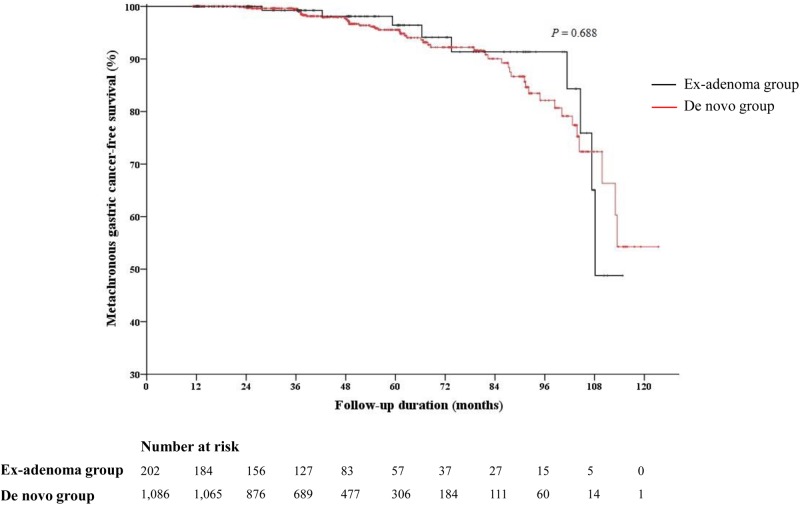
The median follow-up duration was 45.1 months in the ex-adenoma group and 45.4 months in the de novo group, which were not statistically different (Table 5). Synchronous gastric neoplasia was significantly more frequent in the ex-adenoma group than in the de novo group (21.8% vs. 12.2%, P < 0.001), which was mostly due to the significantly higher rate of synchronous gastric adenoma in the ex-adenoma group (14.9% vs. 7.8%, P = 0.001), while synchronous gastric carcinoma rates did not differ significantly between the two groups. There was no significant difference in the metachronous cancer recurrence rate during the follow-up period (Fig 3). In the subsequent analysis, there was no statistically significant difference in the characteristics of metachronous cancer (Table 6).
Table 5. Follow-up results of the two groups.
| Ex-adenoma group (n = 202) |
De novo group (n = 1,086) |
P value | |
|---|---|---|---|
| Follow-up duration, median (IQR) | 45.1 (24.3–60.9) | 45.4 (24.3–61.0) | 0.913 |
| Synchronous gastric neoplasiaa | 44 (21.8) | 133 (12.2) | < 0.001 |
| Synchronous gastric adenoma | 30 (14.9) | 85 (7.8) | 0.001 |
| Synchronous gastric carcinoma | 14 (6.9) | 48 (4.4) | 0.126 |
Values are presented as median (interquartile range) or n (%)
IQR, interquartile range
aSynchronous gastric neoplasia was defined as a gastric adenoma or cancer developed in areas other than the site of primary gastric cancer within 1 year of endoscopic submucosal dissection
Fig 3. Kaplan-Meier curve of metachronous gastric cancer recurrence.
There were no statistically significant differences in the metachronous cancer recurrence rate between the ex-adenoma group and the de novo group (P = 0.688).
Table 6. Characteristics of metachronous gastric cancer in two groups.
| Ex-adenoma group (n = 11) |
De novo group (n = 65) |
P value | |
|---|---|---|---|
| Pathologic size, mm | 14.20 ± 5.92 | 19.31 ± 14.64 | 0.259 |
| Differentiationa | 0.212 | ||
| Differentiated | 7 (63.6) | 54 (83.1) | |
| Undifferentiated | 4 (36.4) | 11 (16.9) | |
| Depth of invasiona | 0.210 | ||
| Mucosa | 8 (72.7) | 55 (87.3) | |
| Beyond mucosa | 3 (27.3) | 8 (12.7) |
Values are presented as mean ± standard deviation or n (%)
aTwo patients in the de novo group were excluded from the analysis due to insufficient information
Discussion
The present study is the first to describe the clinical characteristics of EGC arising from adenoma in comparison with that without pre-existing adenoma. Patients in the ex-adenoma group were older and had a higher rate of H. pylori infection than those in the de novo group. Endoscopic and pathologic size of lesions was greater in the ex-adenoma group than that in the de novo group. In addition, atrophy or intestinal metaplasia was more frequent in the adjacent mucosa in the ex-adenoma group. Similar to gastric adenoma, the elevated type lesion was predominant in the ex-adenoma group. The differentiated carcinoma rate was higher in the ex-adenoma group than in the de novo group. Considering that EGC arising from adenoma develops from H. pylori–induced gastritis and progresses through a multistep process over a long period, these characteristics are well-matched with each step of the carcinogenic process.
Premalignant lesions have been identified in various human cancers, including colon, liver, pancreas, breast, uterine cervix, and skin cancers. Among these, the adenoma-carcinoma sequence is a well-established carcinogenic pathway in most colorectal cancers and in specific types of hepatocellular carcinoma [23]. Previous molecular studies showed that alterations in specific genes play crucial roles in the progression of colorectal adenoma to carcinoma [7]. In addition, ex-adenoma colorectal cancers show distinctive clinicopathologic characteristics compared with de novo colorectal cancers [24]. Little information is available on the characteristics of gastric cancer developed from adenoma, with only one study showing that EGC with a high microsatellite instability (MSI) mutation rate is associated with co-existing underlying adenoma [25].
In our study, pre-existing adenoma was detected in 15.6% (236/1,509) of endoscopically resected EGCs. These results are difficult to compare with those of previous studies because of the limited amount of data available. A recent study by Jahng et al. analyzed the incidence of co-existing underlying adenoma in surgically treated EGCs and showed a rate of 39.7% (29/73) in the MSI-high EGC group vs. 19.9% (29/146) in the non-MSI-high group [25]. A brief retrospective review of medical records of surgically resected EGCs in our institute showed that 6.45% of EGCs were accompanied by pre-existing adenoma. To the best of our knowledge, this is the first study to examine the rate of pre-existing adenoma in EGCs eligible for endoscopic resection. Further study is needed to clarify this issue.
In the present study, H. pylori infection rates were compared using two methods. H. pylori infection rates showed marginal differences between the two groups when comparisons were made based on the results of either rapid urease test or histology (Table 1). The establishment of H. pylori infection induced serial changes in the gastric mucosa resulting from the chronic infection. The spread of the infection led to severe atrophic changes in the gastric mucosa. The development of intestinal metaplasia resulted in a reduction in the bacterial load in the stomach, which could have reduced the yield for H. pylori infection. Since H. pylori infection was evaluated only by biopsy and without the use of the urea breath test or serology, which could have increased the risk of false-negative results, a second analysis was performed that included an evaluation of the status of the surrounding mucosa. This analysis showed significant differences between the two groups. The combined results indicated that EGCs that arise from gastric adenomas are more frequently associated with H. pylori–related chronic inflammation than de novo EGCs.
The results of the present study indicated that the rate of metachronous cancer recurrence did not differ significantly between two groups, despite the higher rate of concurrent gastric neoplasms in the ex-adenoma group. H. pylori infection triggers chronic inflammation of the gastric mucosa, and the normal mucosa adjacent to H. pylori–infected gastric cancer is also susceptible to the development of a second gastric neoplasm as a result of chronic mucosal damage. This concept of “field cancerization” may explain the high incidence of synchronous or metachronous gastric neoplasms in gastric cancer patients [26]. Our results showing a higher rate of synchronous gastric adenoma in the ex-adenoma group can be explained by the higher possibility of field formation in this group. However, the prevalence of synchronous or metachronous gastric carcinoma did not differ between the two groups, despite a lower number of premalignant lesions expected in the de novo group. These results indicate that gastric cancer may occur through different mechanisms in the two groups. Further studies are necessary to elucidate the mechanisms underlying gastric carcinogenesis.
The strength of this study lies in the large number of patients included in the analysis, which makes the results relatively robust. Furthermore, to the best of our knowledge this is the first study to demonstrate the clinicopathologic characteristics and long-term outcomes of EGCs with pre-existing adenoma compared with those of de novo EGCs.
The present study had several limitations. First, only EGCs treated with endoscopic resection were included in the analysis, which may have introduced selection bias. Diffuse type gastric cancer accounts for approximately 30–50% of all gastric cancers, whereas the rate was 2% in the present study [27, 28]. In addition, the proportion of intestinal-type carcinomas did not significantly differ between the two groups, although it was higher in the ex-adenoma group. This result is inconsistent with those of previous studies suggesting that intestinal-type carcinoma is associated with adenoma-carcinoma sequence, as indicated by a higher rate of intestinal-type carcinoma in H. pylori-related gastric carcinogenesis. For the same reason, the depth of invasion and the degree of differentiation in gastric cancer in general may be somewhat different from our results. Careful interpretation is required to avoid selection bias, and a large cohort study targeting all types of gastric cancer is needed to verify our novel findings. Second, this was a retrospective study based on the analysis of medical records and a molecular analysis was not included. In the present study, we were unable to determine whether EGCs in the de novo group developed directly from the normal mucosa without an intermediate adenoma step or, if a pre-existing adenomatous component was entirely converted into a carcinoma. The prevalence of de novo EGCs may be overestimated.
In conclusion, EGCs arising from adenoma were more closely associated with chronic inflammation caused by H. pylori infection than EGCs without pre-existing adenoma. Despite differences in the carcinogenic mechanism, both groups showed a high incidence of synchronous and metachronous gastric cancer, underscoring the importance of careful surveillance in all endoscopically resected EGCs.
Acknowledgments
This work was supported by Promising-Pioneering Researcher Program through Seoul National University in 2015.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by Promising-Pioneering Researcher Program through Seoul National University in 2015. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700–13. 10.1158/1055-9965.EPI-13-1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. [DOI] [PubMed] [Google Scholar]
- 3.Shin A, Kim J, Park S. Gastric cancer epidemiology in Korea. J Gastric Cancer. 2011;11(3):135–40. 10.5230/jgc.2011.11.3.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10(1):1–11. 10.1007/s10120-006-0408-1 [DOI] [PubMed] [Google Scholar]
- 5.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52(24):6735–40. [PubMed] [Google Scholar]
- 6.de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. [DOI] [PubMed] [Google Scholar]
- 7.Walther A, Johnstone E, Swanton C, Midgley R, Tomlinson I, Kerr D. Genetic prognostic and predictive markers in colorectal cancer. Nat Rev Cancer. 2009;9(7):489–99. 10.1038/nrc2645 [DOI] [PubMed] [Google Scholar]
- 8.Lee S, Bang S, Song K, Lee I. Differential expression in normal-adenoma-carcinoma sequence suggests complex molecular carcinogenesis in colon. Oncol Rep. 2006;16(4):747–54. [PubMed] [Google Scholar]
- 9.Tamura G, Sakata K, Nishizuka S, Maesawa C, Suzuki Y, Terashima M, et al. Allelotype of adenoma and differentiated adenocarcinoma of the stomach. J Pathol. 1996;180(4):371–7. [DOI] [PubMed] [Google Scholar]
- 10.Tahara E. Genetic pathways of two types of gastric cancer. IARC Sci Publ. 2004;(157):327–49. [PubMed] [Google Scholar]
- 11.Tahara E. Molecular biology of gastric cancer. World J Surg. 1995;19(4):484–8; discussion 9–90. [DOI] [PubMed] [Google Scholar]
- 12.Yang HJ, Kim SG, Lim JH, Choi J, Im JP, Kim JS, et al. Predictors of lymph node metastasis in patients with non-curative endoscopic resection of early gastric cancer. Surg Endosc. 2015;29(5):1145–55. 10.1007/s00464-014-3780-7 [DOI] [PubMed] [Google Scholar]
- 13.Choi JM, Kim SG, Yang HJ, Lim JH, Choi J, Im JP, et al. Endoscopic predictors for undifferentiated histology in differentiated gastric neoplasms prior to endoscopic resection. Surg Endosc. 2016;30(1):89–98. 10.1007/s00464-015-4165-2 [DOI] [PubMed] [Google Scholar]
- 14.Choi J, Kim SG, Yoon H, Im JP, Kim JS, Kim WH, et al. Eradication of Helicobacter pylori after endoscopic resection of gastric tumors does not reduce incidence of metachronous gastric carcinoma. Clin Gastroenterol Hepatol. 2014;12(5):793–800 10.1016/j.cgh.2013.09.057 [DOI] [PubMed] [Google Scholar]
- 15.Japanese Gastric Cancer A. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14(2):101–12. 10.1007/s10120-011-0041-5 [DOI] [PubMed] [Google Scholar]
- 16.The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58(6 Suppl):S3–43. [DOI] [PubMed] [Google Scholar]
- 17.Kanno T, Iijima K, Abe Y, Yagi M, Asonuma S, Ohyauchi M, et al. A multicenter prospective study on the prevalence of Helicobacter pylori-negative and nonsteroidal anti-inflammatory drugs-negative idiopathic peptic ulcers in Japan. J Gastroenterol Hepatol. 2015;30(5):842–8. 10.1111/jgh.12876 [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto S, Watabe K, Tsutsui S, Kiso S, Hamasaki T, Kato M, et al. Lower serum level of adiponectin is associated with increased risk of endoscopic erosive gastritis. Dig Dis Sci. 2011;56(8):2354–60. 10.1007/s10620-011-1681-3 [DOI] [PubMed] [Google Scholar]
- 19.Jung SJ, Cho SJ, Choi IJ, Kook MC, Kim CG, Lee JY, et al. Argon plasma coagulation is safe and effective for treating smaller gastric lesions with low-grade dysplasia: a comparison with endoscopic submucosal dissection. Surg Endosc. 2013;27(4):1211–8. 10.1007/s00464-012-2577-9 [DOI] [PubMed] [Google Scholar]
- 20.Lauren P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31–49. [DOI] [PubMed] [Google Scholar]
- 21.Price AB. The Sydney System: histological division. J Gastroenterol Hepatol. 1991;6(3):209–22. [DOI] [PubMed] [Google Scholar]
- 22.Kang HY, Kim N, Park YS, Hwang JH, Kim JW, Jeong SH, et al. Progression of atrophic gastritis and intestinal metaplasia drives Helicobacter pylori out of the gastric mucosa. Dig Dis Sci. 2006;51(12):2310–5. 10.1007/s10620-006-9276-0 [DOI] [PubMed] [Google Scholar]
- 23.Kudo M. Hepatocellular adenoma in type Ia glycogen storage disease. J Gastroenterol. 2001;36(1):65–6. [DOI] [PubMed] [Google Scholar]
- 24.Mueller JD, Bethke B, Stolte M. Colorectal de novo carcinoma: a review of its diagnosis, histopathology, molecular biology, and clinical relevance. Virchows Arch. 2002;440(5):453–60. 10.1007/s00428-002-0623-z [DOI] [PubMed] [Google Scholar]
- 25.Jahng J, Youn YH, Kim KH, Yu J, Lee YC, Hyung WJ, et al. Endoscopic and clinicopathologic characteristics of early gastric cancer with high microsatellite instability. World J Gastroenterol. 2012;18(27):3571–7. 10.3748/wjg.v18.i27.3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ushijima T. Epigenetic field for cancerization. J Biochem Mol Biol. 2007;40(2):142–50. [DOI] [PubMed] [Google Scholar]
- 27.Chen YC, Fang WL, Wang RF, Liu CA, Yang MH, Lo SS, et al. Clinicopathological Variation of Lauren Classification in Gastric Cancer. Pathol Oncol Res. 2016;22(1):197–202. 10.1007/s12253-015-9996-6 [DOI] [PubMed] [Google Scholar]
- 28.Kushima R, Vieth M, Borchard F, Stolte M, Mukaisho K, Hattori T. Gastric-type well-differentiated adenocarcinoma and pyloric gland adenoma of the stomach. Gastric Cancer. 2006;9(3):177–84. 10.1007/s10120-006-0381-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.