Abstract
The genetic control of apomixis was studied in numerous segregating progenies originated from intercrossing and selfing of obligate sexual and facultative apomictic parents in Poa pratensis by means of the flow cytometric seed screen. The data support a novel model with five major genes required to control asexual seed formation: the Apospory initiator (Ait) gene, the Apospory preventer (Apv) gene, a Megaspore development (Mdv) gene, the Parthenogenesis initiator (Pit) gene, and the Parthenogenesis preventer (Ppv) gene. Differences in expressivity and interactions of these genes are responsible for the wide variation of the mode of reproduction. Apospory and parthenogenesis as well as the initiator and preventer genes of these components segregate independently. The genotypes with the highest expressivity of apospory and parthenogenesis were assigned as Ait-/apvapv/Pit-/ppvppv, those with intermediate expressivity as Ait-/Apv-/Pit-/Ppv-, and those with low expressivity as aitait/apvapv/pitpit/ppvppv. Among the self progenies of obligate sexual individuals, plants with a low capacity for apospory and/or parthenogenesis occurred, indicating that the sexual parents were heterozygous for the preventer genes and homozygous for the recessive initiator alleles (aitait/Apv-/pitpit/Ppv-). The dominant allele Ait exhibits incomplete penetrance. The degree of expressivity of apospory and parthenogenesis was constant among several harvest years of F1 plants.
INTRODUCTION
Asexual seed formation is a complex trait resulting from several modifications of the sexual life cycle. The most important elements are the circumvention of meiotic reduction and the fertilization-independent embryo formation. Although apomixis excludes recombination and segregation, a surprisingly high variability can be observed in some apomictic species and populations. Most plants of apomictic species are facultative apomicts that form seeds via the sexual and the apomictic pathways to different extents. Obligate apomictic and obligate sexual individuals occur at a much lower frequency within such species. Apomixis and sexuality are not mutually exclusive (Koltunow, 1993; Nogler, 1994; Tucker et al., 2003).
The knowledge about the genetic basis of gametophytic apomixis is limited (reviewed in Grossniklaus et al., 2001; Bicknell and Koltunow, 2004). In contrast with former assumptions of monogenic control (Savidan, 1980; Voigt and Burson, 1983; Leblanc et al., 1995; Bicknell and Borst, 1996), new results support an independent control of the components apomeiosis and parthenogenesis by dominant Mendelian factors (Van Dijk et al., 1999; Noyes and Rieseberg, 2000; Matzk et al., 2001). However, this does not exclude that even each component of apomixis could be controlled by multiple genes. Moreover, there is no knowledge of factors conditioning the penetrance or expressivity of apomixis in different genetic backgrounds (Spillane et al., 2001).
Studies of the genetic control of any traits are complicated in apomicts because of the lack of recombination, irregular segregation, and polyploidy. But more difficult are analyses of apomictic seed formation itself because the processes of interest take place deeply embedded within the ovule, and the gametophytic generation is represented by only a few cells during a short time of the life cycle. As a result, most studies of the genetic control of apomixis suffer from drawbacks, such as small sample sizes, estimation of apomixis by correlated characteristics, insufficient discrimination between the individual components, and impossibility to distinguish between several genes responsible for one component. Most of these shortcomings can be overcome applying the flow cytometric seed screen (FCSS) (Matzk et al., 2000). In any plant material, the different events of sporogenesis and embryo and endosperm formation can be analyzed simultaneously by this approach.
Kentucky bluegrass (Poa pratensis) is an important fodder and turf grass. Considerable effort is being expended to breed improved cultivars, particularly in Europe and North America. P. pratensis is an aposporous pseudogamous facultative apomict and highly variable as to reproductive, chromosomal, and phenotypic features. Apomixis rates from 0 to 100%, and chromosome numbers from 2n = 18 to 150 are known. The causes of this variability are still poorly understood, leading to the conclusion: the intricate reproductive anomalies of Poa could not be sufficiently clarified despite many efforts (Nogler, 1994). High degrees of heterozygosity and polyploidy, chromosomal instabilities, aneuploidy, and other factors hamper comprehensive studies of the genetic control of the mode of reproduction in P. pratensis. Nevertheless, the potential of the FCSS and previously established obligate sexual genotypes (Matzk, 1991a, 1991b) encouraged us to conduct such analyses. A new hypothesis for the regulation of asexual seed formation is presented. Four questions concerning the inheritance of apomixis will be answered in this article. (1) How many major genes regulate apomixis? (2) Do the individual components and genes segregate independently? (3) Is the inheritance of the individual components dominant or recessive? (4) Are penetrance and expressivity varying in dependence of gene/allele combinations?
Hypothesis for Inheritance of Apomixis
The following hypothesis for the genetic control of apomixis in P. pratensis will be tested in this article. The hypothesis is based on the assumptions that (1) apomixis has evolved out of sexual ancestors (Holsinger, 2000), and (2) apomixis is a short circuit of the sexual pathway resulting from deletion or deregulation of key stages of the sexual development program as well as from newly evolved genes that were not involved in the sexual propagation (Spillane et al., 2001). Only the inheritance of the mode of embryo development will be considered. The endosperm formation depends on fertilization in sexuals as well as in apomicts of P. pratensis. Whereas the hypothesis will be exemplified for a diploid constitution, the analyses of the inheritance in P. pratensis will consider the actual ploidy of plants.
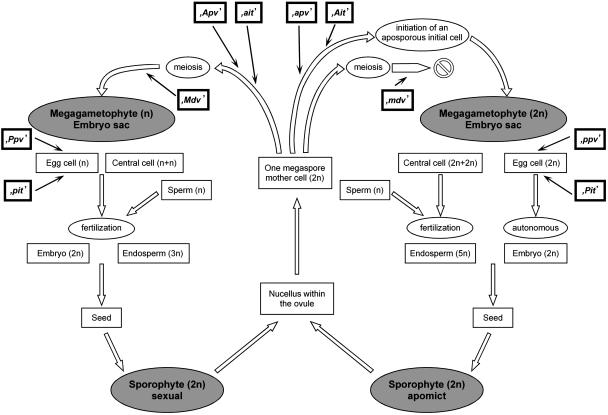
As a consequence of the selective advantages of sexuality, genes have evolved to stabilize the sexual life cycle. We postulate two such genes (Figure 1, left side), one that prevents aposporous embryo sac formation (the Apospory preventer [Apv] gene) and another that prevents fertilization-independent embryo formation (the Parthenogenesis preventer [Ppv] gene). The penetrance and expressivity of the dominant preventer alleles Apv and Ppv should be complete in sexual plants. The homozygous recessive alleles apv and ppv tolerate but do not induce apomeiosis or parthenogenesis, respectively (low expressivity). In sexual species, selection for homozygous dominant alleles is to be expected because in heterozygous constitution (Apv apv and Ppv ppv) apomeiosis and parthenogenesis would segregate and individually lead to sterility or reduced fitness in the resulting triploid and haploid plants. Even in the case that the recessive alleles of both preventer genes occur homozygously within plants of sexual species (apv apv/ppv ppv), apomictic seed formation should occur only sporadically.
Figure 1.
Life Cycles via the Sexual and the Aposporous-Pseudogamous Reproduction.
Five major genes (boxes with wide black borders) control the sexual (left side) and apomictic (right side) pathway: Apospory prevention dominant alleles in sexuals (Apv) and recessive alleles in apomicts (apv); Apospory initiation dominant alleles in apomicts (Ait) and null or recessive alleles in sexuals (ait); Parthenogenesis prevention dominant alleles in sexuals (Ppv) and recessive alleles in apomicts (ppv); Parthenogenesis initiation dominant alleles in apomicts (Pit) and null or recessive alleles in sexuals (pit); Megaspore development dominant alleles in sexuals (Mdv) and recessive alleles in apomicts (mdv).
For a high degree of asexual seed formation, two additional genes are postulated (the Apospory initiator [Ait] gene and the Parthenogensis initiator [Pit] gene). The dominant alleles induce apospory and parthenogenesis, respectively (Figure 1, right side). In contrast with the preventer genes Apv and Ppv, which are involved in the regular program of sexual seed formation, the initiator genes Ait and Pit should not have functional homologs in sexual ancestors. Therefore, hemizygosity may be expected for these loci in interspecific hybrids between sexual and apomictic species (Ozias-Akins et al., 1998). Contrary to sexual species, the sexual individuals of apomictic species may bear recessive or null alleles of the initiator loci (ait and pit). In the case that an aposporous initial cell is selected or an autonomous embryo development is initiated, the cascade of gene regulation may continue as for sexual seed formation. In polyploid apomictic accessions, the dominant alleles of the initiator genes will be present in simplex or after fertilization of unreduced egg cells in duplex but not in homozygous constitution. Homozygosity of dominant apomixis factors is not easily to be realized during evolution or by breeding because recombination is excluded already in the heterozygous constitution; moreover, it does not have a selective advantage against the heterozygous constitution.
The postulated preventer and initiator genes obviously are counteracting each other. This gene interaction results in an intermediate expressivity of the apomixis components in genotypes with dominant alleles of both loci (Apv and Ait or Ppv and Pit). The highest level of apospory and parthenogenesis may be expected from plants with dominant initiator and homozygous recessive preventer alleles (Ait-/apv apv and Pit-/ppv ppv/). Obligate sexual individuals of apomictic species should have the genetic constitution ait ait/Apv-/pit pit/Ppv-/.
Finally, an additional gene may stabilize the apomictic pathway and increase the degree of asexual seed formation in facultative aposporous apomicts by inducing a selective abortion of megaspores or megagametophytes originating from the legitimate megaspore mother cell. Such genetic control may be achieved through a recessive mutation of a gene integrated primarily in the regulation of sexual developmental processes (the Megaspore development [Mdv] gene). A stable and high apomixis expression may be expected from the genotype Ait-/apv apv/mdv mdv/Pit-/ppv ppv/. Seeds with multiple embryos occur frequently in aposporous apomicts. Up to four seedlings per seed were observed in P. pratensis. The polyembryonic seeds may originate from one reduced plus one or more unreduced (aposporous) embryo sacs or from several unreduced embryo sacs. Apomictic plants with the dominant allele Mdv may form multiple embryos derived from reduced and unreduced embryo sacs. The homozygous recessive alleles mdv mdv allow the formation of polyembryos exclusively from multiple aposporous embryo sacs because of abortion of the legitimate embryo sacs. In addition, sterility as observed occasionally within aposporous apomictic populations (Koltunow et al., 2000) may be a consequence of the specific allele constitution ait ait/Apv-/mdv mdv/ (without aposporous capacity and with megaspore abortion). Therefore, alleles such as mdv have no chance to survive in sexual species or in diplosporous apomicts.
Apospory and parthenogenesis should segregate independently. There is no evolutionary advantage for tight linkage of both traits because recombination is prevented, even if the genes are localized on different chromosomes. Detailed results supporting the genetic control of abortion in legitimate megaspores by mdv, as well as additional factors responsible for the high variability in reproductive mode, will be presented elsewhere.
RESULTS
Low, Intermediate, and High Expressivity of Apospory and Parthenogenesis Represent Different Genetic Constitutions
The studies using FCSS demonstrate extensive variation in the expression of parthenogenesis and apospory, confirming the previous results on parthenogenesis using the auxin test (Matzk, 1991b). An almost continuous variation from 0 to 100% was observed for both traits. However, examination of the exact distribution of the apospory and parthenogenesis frequencies determined by single seed analyses from >300 individual plants out of various populations (Table 1) revealed gaps from 16 to 25 and 70 to 79% for apospory and from 19 to 28 and 67 to 75% for parthenogenesis. Considering 0% as a separate class, the discrimination between four classes of expressivity is possible. The different expressivity may be the result of interactions between the initiator and preventer genes both for apospory and for parthenogenesis.
Table 1.
Classes of Expressivity Observed for Apospory and Parthenogenesis and the Corresponding Assigned Genotypes (Pooled Data from Several Hundred Plants Out of ∼20 F1 and I1 Populations)
| Expressivity
|
Assigned Genotypesa
|
||
|---|---|---|---|
| Classes | Variation (%) | Gap (%) | |
| Apospory: | |||
| High | 80–100 | 70–79 | Ait-/apv apv/ |
| Intermediate | 26–69 | 16–25 | Ait-/Apv-/ |
| Low | 1–16 | ait ait/apv apv/ | |
| Zero | 0 | ait ait/Apv-/ | |
| Parthenogenesis: | |||
| High | 76–100 | 67–75 | Pit-/ppv ppv/ |
| Intermediate | 29–66 | 19–28 | Pit-/Ppv-/ |
| Low | 1–19 | pit pit/ppv ppv/ | |
| Zero | 0 | pit pit/Ppv-/ | |
A diploid constitution (which does not occur in P. pratensis) was chosen to simplify the discrimination between homozygous recessive and heterozygous or homozygous dominant genotypes; the number of dominant or recessive alleles may vary depending on the actual ploidy level.
The classes of high expressivity of apospory and parthenogenesis should represent the carriers of dominant initiator alleles together with homozygous recessive preventer alleles. An intermediate expressivity may correspond with the presence of dominant alleles of both genes that control apospory and parthenogenesis, respectively. The homozygous recessive constitution of both loci may result in low expressivity of apospory and parthenogenesis. Obligate sexuals (0% apospory and 0% parthenogenesis) should bear the homozygous recessive initiator and the heterozygous dominant preventer alleles (Table 1).
Self Progenies of Obligate Sexual Plants Segregate for Apospory and/or Parthenogenesis
Among the self progenies of obligate sexual parents (for their origin and characterization, see Table 8), plants with a capability for apospory and/or parthenogenesis occurred (Table 2), but the expressivity of both traits was low in all cases except for apospory in the clone S3. These results support the hypothesis that most of the obligate sexual parents are heterozygous for the postulated preventer genes of apospory and/or parthenogenesis and homozygous recessive for the respective initiator genes. The segregation ratios for the apospory and parthenogenesis preventer genes observed as total numbers from all self progenies were not significantly different from the expected segregation of a dominant allele in simplex constitution (Table 2). The highest degree of apospory or parthenogenesis observed in such segregating self progenies from obligate sexual individuals should represent the maximum expressivity caused by the homozygous recessive alleles of the preventer genes apv and ppv, respectively.
Table 8.
Characterization of the Sexual and Apomictic Parents Used for the First Cycle of Crosses and of the F1 Plants Used for the Second Cycle
| Number | Origin | Mode of Reproductiona | Chromosome Number (2n) | Polyembryos (%) | ATb |
|---|---|---|---|---|---|
| S1 | Clone out of cv Berbi, Germany | Obligate sexual | 30 | 0.0 | − |
| S2 | Clone out of cv Berbi, Germany | Obligate sexual | 30 | 0.0 | − |
| S3 | Clone cv Roznovska, Czech Republic | Obligate sexual | 60 | 0.0 | − |
| S4 | Clone cv Roznovska, Czech Republic | Obligate sexual | 30 | 0.0 | − |
| S5 | Clone cv Prato, The Netherlands | Obligate sexual | 49 | 0.3 | − |
| SJ | Population cv Jori, Germany | Obligate sexual | ∼30 | 0.0 | − |
| SL | Population cv Lato, Germany | Obligate sexual | ∼32 | 0.0 | − |
| A15 | cv Beata, Poland | Facultative apomictic | 42–90 | 0.5 | + |
| A17 | cv Erte, Denmark | Facultative apomictic | ∼54 | 4.2 | + |
| A21 | cv Union, Germany | Facultative apomictic | ∼62 | 1.6 | + |
| A23 | cv Ligrotta, Germany | Facultative apomictic | ∼66 | 6.1 | + |
| A24 | cv Limousine, Germany | Facultative apomictic | ∼50 | 3.0 | + |
| A25 | cv Ottos, Germany | Facultative apomictic | ∼62 | 3.2 | + |
| A31 | cv Blacksburg, USA | Facultative apomictic | ∼51 | 12.8 | + |
| A32 | cv Monopoly, The Netherlands | Facultative apomictic | ∼52 | 3.4 | + |
| F11 | F1 from 1(S1 × A15) | Obligate sexual | ∼60 | 0.0 | − |
| F12 | F1 from 6(S3 × F11) | Low aposporous | ∼56 | 0.0 | |
| F13 | F1 from 2(S1 × A17) | Intermediate apospor | ∼40 | 1.0 | |
| F14 | F1 from 2(F12 × F13) | Obligate sexual | ∼47 | 0.0 | |
| F15 | F1 from 2(F12 × F13) | Low apomictic | ∼52 | 12.0 | |
| F16 | F1 from 2(F12 × F13) | Obligate sexual | ∼47 | 0.0 | |
| F17 | F1 from 4(F14 × F15) | Low apomictic | ∼56 | 14.3 |
Facultative apomictic: intermediate to high expressivity of apospory and parthenogenesis.
AT, auxin test; + and − with or without parthenogenesis capacity.
Table 2.
Segregation for Apospory and Parthenogenesis within Self Progenies from Obligate Sexual Plants
| Number of Plants Belonging to Four Classes of Expressivityb
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of I1 Plants | Apospory
|
Parthenogenesis
|
||||||||||
| Self Progenya | High | Intermediate | Low | Zero | High | Intermediate | Low | Zero | ||||
| I1 S1 | 11 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 11 | |||
| I1 S2 | 14 | 0 | 0 | 0 | 4 | 0 | 0 | 5 | 9 | |||
| I1 S3 | 97 | 1 | 2 | 24 | 55 | 0 | 0 | 26 | 71 | |||
| I1 S5 | 12 | 0 | 0 | 3 | 9 | 0 | 0 | 3 | 9 | |||
| I1 SJ | 7 | 0 | 0 | 4 | 3 | 0 | 0 | 5 | 2 | |||
| I1 SL | 7 | 0 | 0 | 3 | 4 | 0 | 0 | 2 | 5 | |||
| I1 F14 | 9 | 0 | 0 | 4 | 5 | 0 | 0 | 2 | 7 | |||
| I1 F16 | 10 | 0 | 0 | 4 | 6 | 0 | 0 | 1 | 9 | |||
| Observed in total | 167 | 1 | 2 | 42 | 90 | 0 | 0 | 44 | 123 | |||
| Expected ratios of the classes low:zero (apv:Apv and ppv:Ppv) | 1:3 | 1:3 | ||||||||||
| χ2/P valuesc | 3.27/0.07 | 0.16/0.69 | ||||||||||
I1, first inbreeding (or self) generation; in I1 S2 and I1 S3, not all plants analyzed for apospory.
The genetic constitution of plants with high, intermediate, low, and zero expressivity is assigned Ait/apv, Ait/Apv, ait/apv, and ait/Apv for apospory and Pit/ppv, Pit/Ppv, pit/ppv, and pit/Ppv for parthenogenesis, respectively.
χ2 > 3.84 and P < 0.05 (in bold) are significant.
The clone S1 did not segregate for apospory or parthenogenesis, indicating a homozygous constitution (dominant for preventer and recessive for initiator genes) or a higher than duplex dosage of the dominant alleles of the preventer genes. The occurrence of a few plants with high apospory capacity among the self progenies of the obligate sexual clone S3 indicates that the dominant allele of the initiator gene Ait was present but not expressed in the mother.
In contrast with obligate sexual plants, self progenies from a plant with a slight expressivity of apospory and parthenogenesis (I1F17) did not segregate for the traits, but female sterility occurred with a ratio of ∼30%. This finding confirms the postulated homozygous recessive constitution (ait ait/apv apv/pit pit/ppv ppv/) for plants of this class of expressivity. The sterility may be a consequence of Mdv mdv allele segregation. The plants with low expressivity of apospory and parthenogenesis (each <15%) formed <1% apomictic embryos.
Segregation Analyses of F1 Families Support the Postulated Model of Inheritance of Apomixis
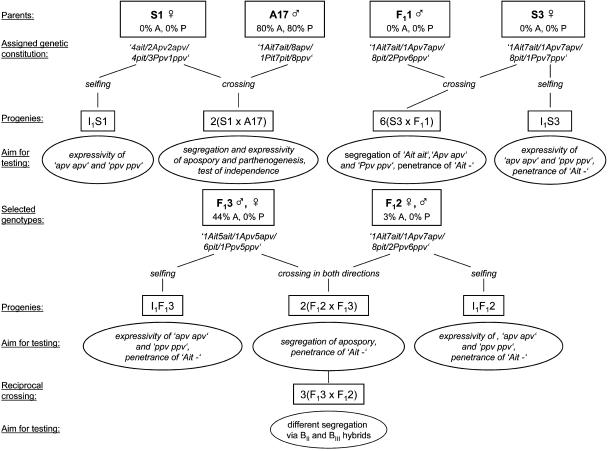
Genotypes with a defined mode of reproduction were used as parents to elucidate the genetic control of apomixis (Figure 2). On the basis of the expressivity of apospory and parthenogenesis and their segregation, the genotypes of the parents were assigned. For the expected segregation ratios, the ploidy level and the dosage of the dominant alleles were considered (according to Haldane, 1930).
Figure 2.
Flow Chart Demonstrating the Most Important Segregating Populations and the Claims of Analyses.
In all combinations, the observed segregation of the genes Apv, Pit, and Ppv was consistent with the expectation derived from genotypes assigned to the parents (Table 3). In the 2(S1 × A17) family, only progeny plants having a dominant allele at the Ppv locus were expected (3Ppv1ppv × 8ppv = ∞:0); however, two homozygous recessive individuals were identified. This could be a consequence of chromatid segregation (double reduction) in the first meiotic division of the mother. The nulliplex, simplex, duplex, and triplex constitution of the parents could be verified by the χ2 test.
Table 3.
Observed and Expected Allele Fequencies of the Postulated Genes in Cross and Self Progenies (Pure Chromosome Segregation Assumed)
| Assigned Genotypes of the Parents
|
Segregation Observed and (Expected)a
|
χ2 and P Valuesb
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Family | Female | Male | Ait:ait | Apv:apv | Pit:pit | Ppv:ppv | a | b | c | d |
| Crossing: | ||||||||||
| 2(S1 × A17) | 4ait/2Apv2apv/4pit/3Ppv1ppv | 1Ait7ait/8apv/1Pit7pit/8ppv | 37:21 | 44:14 | 27:31 | 56:2 | 4.41 | 2.33 | 0.28 | |
| (1:1) | (5:1) | (1:1) | (∞:0) | 0.04 | 0.13 | 0.60 | ||||
| 6(S3 × F11) | 1Ait7ait/1Apv7apv/8pit/1Ppv7ppvc | 1Ait7ait/1Apv7apv/8pit/2Ppv6ppv | 8:18 | 18:8 | 0:26 | 23:3 | 27.13 | 0.46 | 0.01 | |
| (3:1) | (3:1) | (0:∞) | (25:3) | <0.01 | 0.50 | 0.89 | ||||
| 2(F12 × F13) | 1Ait7ait/1Apv7apv/8pit/2Ppv6ppvc | 1Ait5ait/1Apv5apv/6pit/1Ppv5ppv | 17:21 | 27:11 | 0:38 | 32:6 | 18.56 | 0.32 | 1.02 | |
| (3:1) | (3:1) | (0:∞) | (25:3) | <0.01 | 0.57 | 0.31 | ||||
| 3(F13 × F12)d | 1Ait5ait/1Apv5apv/6pit/1Ppv5ppv | 1Ait7ait/1Apv7apv/8pit/2Ppv6ppvc | 2:20 | 17:5 | 1:21 | 20:2 | 50.97 | 0.06 | 0.06 | |
| (3:1) | (3:1) | (0:∞) | (25:3) | <0.01 | 0.81 | 0.81 | ||||
| Selfing: | ||||||||||
| I1 S1 | 4ait/2Apv2apv/4pit/3Ppv1ppv | 0:4 | 4:0 | 0:11 | 11:0 | 0.11 | ||||
| (0:∞) | (35:1) | (0:∞) | (∞:0) | 0.74 | ||||||
| I1 S3 | 1Ait7ait/1Apv7apv/8pit/1Ppv7ppvc | 3:76 | 57:22 | 0:97 | 71:26 | 213.6 | 0.34 | 0.17 | ||
| (3:1) | (3:1) | (0:∞) | (3:1) | <0.01 | 0.56 | 0.68 | ||||
| I1 F12 | 1Ait7ait/1Apv7apv/8pit/2Ppv6ppvc | 9:29 | 29:9 | 0:38 | 35:3 | 53.37 | 0.04 | 0.95 | ||
| (3:1) | (3:1) | (0:∞) | (187:9) | <0.01 | 0.85 | 0.33 | ||||
| I1 F13 | 1Ait5ait/1Apv5apv/6pit/1Ppv5ppv | 4:12 | 11:5 | 0:16 | 15:1 | 21.33 | 0.33 | 3.00 | ||
| (3:1) | (3:1) | (0:∞) | (3:1) | <0.01 | 0.56 | 0.08 | ||||
Expected ratios according to Haldane (1930).
a, b, c, and d correspond with Ait:ait, Apv:apv, Pit:pit, and Ppv:ppv, respectively. χ2 > 3.84 (top) and P < 0.05 (bottom, in bold) are significant (no value where expected segregation is 0:00).
Phenotype obligate sexual (lacking penetrance of Ait).
Reciprocally to 2(F12 × F13).
The segregation of the Ait gene, however, did not altogether fit with the expectations. The dominant allele seemed to be underrepresented in most families (Table 3, compare P values in column a), most likely a result of incomplete penetrance of the gene.
In the cross 3(F13 × F12), both parents were assigned as homozygous recessive for the Parthenogenesis initiator (Pit) gene, but one F1 with the dominant allele occurred among 22 plants. In this case, accidental selfing or seed contamination (one seed) cannot be excluded.
Reciprocal crosses were made (Figure 2) with parents having both no capacity for parthenogenesis but differing in expressivity of apospory (3 and 44%). In the case that the plant with the high capacity of apospory was the mother, the portion of dominant alleles was increased as a consequence of the BIII hybrids. But the observed and expected ratios were not significantly different in the 2(F12 × F13) and in the 3(F13 × F12) family (Table 3).
Neither Apospory and Parthenogenesis nor Initiator and Preventer Genes for Both Components Revealed Tight Linkage
Several F1 families were analyzed just by bulked seed samples that allow only a classification in lacking, complete, or incomplete expression of apospory and parthenogenesis but not quantification of the expressivity or any statistical analyses as presented by the single seed analyses described above. Apospory and parthenogenesis segregated independently. Between 326 F1 plants out of 15 F1 families, facultative apomicts (97 plants) and obligate sexuals (94 plants) occurred most frequently in addition to plants with incomplete apospory but without parthenogenesis (69 plants) or vice versa without apospory but with incomplete parthenogenesis (51 plants). Even three obligate apomicts and 12 obligate aposporous plants without any parthenogenetic capacity were identified. Obligate parthenogenetic plants without aposporous capacity were not found, maybe because of the small sample size or the genetic constitution of the random sample of parents.
Considering the segregation data obtained by single seed analyses (Table 3), 12 out of 16 possible dominant and recessive allele combinations were identified (Table 4). The random pattern of parents (most without Pit) in addition to a small number of progeny plants may explain the absence of the four assigned genotypes AitapvPitPpv, AitApvPitppv, aitApvPitppv, and aitapvPitppv from our sample. For complex linkage analyses with four genes in polyploids, large plant numbers of the segregating populations are necessary. We preferred for the family 2(S1 × A17) with 58 F1 plants a pairwise comparison for the four genes (Table 5). The observed segregation ratios of the six gene pairs each in the four possible combinations of dominant and recessive alleles also did not indicate a tight linkage. Only in one case (Ait/Pit) the differences between observed and expected ratios were a little higher than the confidence value of χ2.
Table 4.
Segregation of Various Populations for 16 Possible Allele Combinations of the Four Apospory/Parthenogenesis Controlling Genes
| Assigned Genotypesa | 2(S1 × A17) | 6(S3 × F11) | 2(F12 × F13) | 3(F13 × F12) | I1 F12 | I1 F13 | Total |
|---|---|---|---|---|---|---|---|
| AitApvPitPpv | 19 | 0 | 0 | 0 | 0 | 0 | 19 |
| aitApvPitPpv | 2 | 0 | 0 | 0 | 0 | 0 | 2 |
| AitapvPitPpv | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AitApvpitPpv | 11 | 5 | 11 | 1 | 3 | 2 | 33 |
| AitApvPitppv | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| aitapvPitPpv | 4 | 0 | 0 | 1 | 0 | 0 | 5 |
| AitapvpitPpv | 5 | 2 | 2 | 1 | 3 | 2 | 15 |
| AitApvpitppv | 0 | 1 | 3 | 0 | 1 | 0 | 5 |
| aitApvpitPpv | 12 | 10 | 12 | 16 | 14 | 6 | 70 |
| aitApvPitppv | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AitapvPitppv | 2 | 0 | 0 | 0 | 0 | 0 | 2 |
| Aitapvpitppv | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| aitApvpitppv | 0 | 1 | 1 | 0 | 0 | 0 | 2 |
| aitapvPitppv | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| aitapvpitPpv | 3 | 4 | 7 | 3 | 2 | 5 | 24 |
| aitapvpitppv | 0 | 3 | 1 | 1 | 0 | 1 | 6 |
| Total | 58 | 26 | 38 | 23 | 23 | 16 | 184 |
Only the dominant versus homozygous recessive constitutions are considered, independent of the ploidy level of the plants. Uppercase letters of gene symbols describe dominant alleles in heterozygous or homozygous constitution; lowercase letters of gene symbols represent homozygous recessive constitution.
Table 5.
Test for Free versus Linked Recombination of Ait, Apv, Pit, and Ppv within the 2(S1 × A17) Family by Comparison of the Observed and Expected (in Parenthesis) Segregation Ratios for the Six Combinations of Gene Pairs (for Genetic Constitution of the Parents, See Table 3 or Figure 2)
| Genetic Constitution on the Two Locia
|
||||||
|---|---|---|---|---|---|---|
| Gene Pairs | d/d | d/r | r/d | r/r | χ2 | Significanceb |
| Ait/Apv | 30 (5) | 7 (1) | 14 (5) | 7 (1) | 7.63 | ns |
| Ait/Pit | 21 (1) | 16 (1) | 6 (1) | 15 (1) | 8.07 | * |
| Ait/Ppv | 35 (1) | 2 (0) | 21 (1) | 0 (0) | ∞/3.50 | ***/nsc |
| Apv/Pit | 21 (5) | 23 (5) | 6 (1) | 8 (1) | 2.83 | ns |
| Apv/Ppv | 44 (5) | 0 (0) | 12 (1) | 2 (0) | ∞/0.91 | ***/nsc |
| Pit/Ppv | 25 (1) | 2 (0) | 31 (1) | 0 (0) | ∞/0.64 | ***/nsc |
d, dominant; r, recessive alleles.
ns, *, and ***, not significant, P < 0.05, and P < 0.001.
The significant deviation of the observed from the expected segregation 2 (0) may be caused by chromatid segregation of the postulated 3Ppv1ppv genetic constitution; excluding these cases, the segregation does not differ significantly.
The Dominant Allele of the Initiator Gene of Apospory (Ait) Shows Incomplete Penetrance
Incomplete penetrance of Ait was the reason for the originally erroneous classification of the obligate sexual clone S3, as could be demonstrated later by several progeny analyses (Table 6). Two plants with ∼50% and one with >80% apospory were identified in the self progeny. Within the cross progeny 6(S3 × F11), which was generated in the greenhouse by hand-emasculation of the mother and controlled pollination with a sexual father, eight out of 26 F1 plants exhibited an at least intermediate apospory capacity. This means that the dominant allele Ait was present but masked in the sexual mother S3 as well as in some of the F1 and I1 progenies.
Table 6.
Examples of Incomplete Penetrance of the Ait Gene
| Reproductive Origin
|
Expressivity of Apospory
|
Genotype Assigned from Own Phenotypea
|
Genotype Revised from Progeny Phenotypes
|
|||
|---|---|---|---|---|---|---|
| Mother | Progeny | Mother | Progenya | |||
| S3 | (n + n) | 0% | ait ait - -/Apv - - -/ | Ait - - -/Apv - - -/ | ||
| I1 S3 | all (n + n) | 79 × 0 or low, | ait ait - -/ | |||
| 2 × intermediate, | Ait - - -/Apv - - -/ | |||||
| 1 × high | Ait - - -/apv apv - -/ | |||||
| 6(S3 × F11) | all (n + n) | 18 × 0 or low, | ait ait - -/ | |||
| 8 × intermediate or high | Ait - - -/ | |||||
| F12 | (n + n) | 3% | ait ait - -/apv apv - -/ | Ait - - -/Apv - - -/ | ||
| I1 F12 | all (n + n) | 29 × 0 or low, | ait ait - -/ | |||
| 9 × intermediate or high | Ait - - -/ | |||||
| F13 | (n + n) | 44% | Ait - - -/Apv - - -/ | Ait - - -/Apv - - -/ | ||
| I1 F13 | 7 × (n + n) | 6 × 0 or low, | ait ait - -/Apv - - -/ | |||
| 1 × intermediate | Ait - - -/Apv - - -/ | |||||
| 9 × (2n + n) | 4 × 0, | ait ait - -/Apv - - -/ | Ait - - -/Apv - - -/ | |||
| 2 × low, | ait ait - -/apv apv - -/ | Ait - - -/Apv - - -/ | ||||
| 3 × intermediate or high | Ait - - -/ | |||||
Discordance is in bold.
One of the F1 plants (F12) of the family 6(S3 × F11) was characterized by a low capacity (3%) of apospory, based on the analyses of 100 single seeds originating from three harvest years. The genotype derived from this phenotype would be ait ait/apv apv/pit pit/Ppv-/. Among 38 self progenies, nine plants exhibited a high or intermediate expressivity of apospory, indicating the presence of the dominant allele of the Ait gene. Hence, the genotype of the mother was misclassified as a result of lacking Ait expression (Table 6).
In one other case (F13), an intermediate expressivity of apospory (44%) was determined (again 100 seeds from three harvest years analyzed), and the genotype was classified as Ait-/Apv-/. However, some BIII plants that occurred within the self progeny were, unexpectedly, obligate sexuals (Table 6). This indicates again an absence of Ait expression because BIII plants are carriers of the complete chromosome complement of the mother (and a reduced chromosome set of the father).
Environmental effects (see three harvest years of F12 and F13 in Table 7), spontaneous mutations (too high frequencies), and contamination by uncontrolled pollination (repeated in successive generations under controlled conditions) can be excluded as reasons for the reduced recovery of the Ait allele. Additionally, the erroneous determination of apospory by BIII embryos originating from fertilization of reduced egg cells by unreduced male gametes was excluded because the FCSS enables the discrimination between BIII embryos resulting from unreduced female or male gametes. The parental plant F11, as well as several plants from the family 6(S3 × F11), frequently generated BIII embryos via unreduced pollen as indicated by seeds with 3C embryos and 4C endosperm. In our data sets of apospory capacity, only BIII embryos originating from unreduced female gametes (3C embryo + 5C endosperm) were included.
Table 7.
Differences in Expressivity of Apospory and Parthenogenesis in Different Harvest Years
| Apospory (%) in Harvest Year
|
Parthenogenesis (%) in Harvest Year
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Plant No. | 1 | 2 | 3 | Mean | P Valuesa | 1 | 2 | 3 | Mean | P Valuesa |
| F12 | 4 | 0 | 5 | 3.2 | 0.19–1.00 ns | 0 | 0 | 0 | 0.0 | 1.00 ns |
| F13 | 44 | 46 | 44 | 44.6 | 1.00 ns | 0 | 0 | 0 | 0.0 | 1.00 ns |
| F18 | 58 | 54 | 56.0 | 1.00 ns | 0 | 0 | 0.0 | 1.00 ns | ||
| F19 | 13 | 10 | 11.5 | 0.71 ns | 36 | 41 | 38.5 | 0.64 ns | ||
| F110 | 60 | 100 | 80.0 | 0.005 ** | 67 | 100 | 83.5 | 0.014 * | ||
| F111 | 100 | 97 | 98.5 | 1.00 ns | 100 | 97 | 98.5 | 1.00 ns | ||
ns, *, and **, not significant, P < 0.05, and P < 0.01.
Seed samples from six F1 plants with zero, low, intermediate, or high expressivity of apospory and/or parthenogenesis indicated only for one plant (F110) significantly different expressivities of apospory and parthenogenesis between harvest years (Table 7). In another case (F12, harvest year 2), a misclassification of expressivity for apospory (zero instead of low) was possible in spite of nonsignificant differences.
DISCUSSION
Confirmation of the Hypothesis for a Complex Inheritance of Apomixis
Our results clearly demonstrate that apomixis in natural systems can be more complex than often reported and that the number of genes controlling apomixis has likely been underestimated in several previous studies (for review, see Bicknell and Koltunow, 2004).
The proposed novel hypothesis for the control of apomixis by five major genes has been confirmed in P. pratensis. The components apomeiosis and parthenogenesis segregate independently from one another. Several obligate aposporous plants without any capacity for parthenogenesis but also facultative parthenogenetic individuals without aposporous capability were identified. In contrast with the independent but monogenic inheritance of apomeiosis and of parthenogenesis reported for Taraxacum officinale (Van Dijk et al., 1999) and Erigeron annuus (Noyes and Rieseberg, 2000), our results support a model of multiple genes for each component. This became possible only via the quantification of the expression of each trait in individual plants from segregating populations. The differing degrees of apospory and parthenogenesis between the plants may result from interactions of the alleles of one initiator and one preventer gene each for apospory and parthenogenesis (Ait/Apv/Pit/Ppv). Already, Nogler (1984) recommended the consideration of differences in the expressivity of the individual components. He found in Ranunculus auricomus that the expressivity of apospory by one dominant allele A was decreasing from diploid to triploid and tetraploid individuals bearing an increased number of the recessive a+ allele. The four classes of expressivity observed in our studies cannot be explained by monogenic control with different dosages of the dominant and recessive alleles because plants with intermediate or high expressivity never occurred after selfing of obligate sexual plants or plants with a low capacity of apospory and/or parthenogenesis. Also, a model assuming multiple alleles at one locus is not well compatible with our findings of segregation of obligate sexual plants, the homozygosity of plants with low expressivity, and the segregation ratios after crossing obligate sexuals with plants of high apomictic capacity. In other studies, modifier genes (Koltunow et al., 2000) or environmental conditions (Knox, 1967; Nogler, 1984; Quarin, 1986) were assumed as potential reasons for the observed quantitative differences in apomixis expression.
The inheritance of apomixis by alleles with incomplete penetrance was hypothesized by Mogie (1992). We present clear evidence for an incomplete penetrance of the Ait gene in P. pratensis. Reduced penetrance appears to depend more on the genetic background than on environmental factors. Contrary to Ait, the dominant alleles of the Pit gene and the Apv and Ppv genes always exhibited complete penetrance.
An alternation in ploidy via MI and BIII plants increases the probability that apomictic genotypes with high levels of apospory and parthenogenesis arise (bearing the preventer genes in homozygous recessive and the initiator genes in simplex or duplex dominant constitution: Ait ait-/apv apv-/Pit Pit pit/ppv ppv-). Both MI and BIII embryos occur frequently in P. pratensis, and several highly apomictic genotypes were recovered in some F1 to F3 families. Also in Dichanthium a high degree of variability as to the apomictic capacity results from alternating ploidy levels by MI and BIII embryos (De Wet, 1968).
Large sample sizes are needed to find out in all cases the low expressivity (1 to 20%) of the homozygous recessive preventer alleles in association with the recessive initiator alleles (ait ait-/apv apv-/pit pit-/ppv ppv-). The class of low expressivity can easily be overlooked in the absence of an efficient screening method; therefore, conclusions that obligate sexual genotypes do not segregate for the mode of reproduction should be considered with care. Huff and Bara (1993) recommended the combination of at least two techniques (they used flow cytometric analyses of leaf nuclei and DNA marker analyses) for the exact determination of the genetic origin of aberrant plants in P. pratensis. Most of the problems and difficulties connected with the traditional cytological and embryological techniques, and with the progeny test, the auxin test, and the application of molecular markers for analyses of apomixes, were overcome by application of FCSS, which is accurate, easy to perform, not time consuming, and yields highly reproducible results (Matzk et al., 2000, 2001, 2003).
Critical Remarks on Genetic Analyses of Apomixis
There are many critical caveats as to analyses of the inheritance of apomixis (Bicknell and Koltunow, 2004), including (1) interspecific or interploidy crosses, (2) lack of reciprocal crosses, (3) indirect measurements of the trait, (4) analyses of only one component but generalization for apomixis as a whole, (5) analysis of apomixis as a qualitative trait (versus sexuality), and (6) no quantification of the degree of expression. Interspecific or interploidy crosses generate hybrids with a disturbed meiosis resulting in an underestimation of the sexual segregants. The most informative genetic data may be expected if the individual elements of gametophytic apomixis are each analyzed separately considering the degree of expression, ideally in segregants derived from intraspecific crosses between several genotypes with different genetic backgrounds, as was done in our studies with P. pratensis.
Our previous conclusion regarding the inheritance of parthenogenesis in P. pratensis (Matzk, 1991b) based upon results obtained with the auxin test (AT) may now be interpreted in light of our FCSS analyses. In the earlier studies, different classes of expression of parthenogenesis (high, middle, and low) were observed, but the one class of low expressivity (<1 to 10%) was ranked together with 0% because inaccuracies of the AT were suspected. The previous conclusion for a dominant inheritance of parthenogenesis can now be specified to be correct for the initiator gene Pit, and the low expressivity has been real, based upon a specific genetic constitution (homozygosity of pit and ppv). The same should hold true for independent studies performed later on the basis of our obligate sexual genotypes and the AT in the group of M. Falcinelli (Barcaccia et al., 1998). The Italian group developed molecular markers (RAPD, AFLP, SCAR, and SAMPL) and is focused now on the mapping and isolation of apomixis gene(s) (Albertini et al., 2001; Porceddu et al., 2002). However, the actual function of the marked gene is not yet clear. Generally speaking, for successful marker-assisted selection in apomixis research, it is essential to know which individual gene for apospory and/or parthenogenesis is actually linked, a point which has not been sufficiently considered in the past.
Similarities of Apomixis Phenotypes Resulting from Genes Identified in P. pratensis and Mutants Described in Sexual Species
The inheritance of parthenogenesis in P. pratensis based on the two genes Pit and Ppv is comparable with the genetic control of autonomous embryo formation in the ‘Salmon’ system of wheat (Triticum aestivum) (Tsunewaki and Mukai, 1990). The parthenogenetic capacity of the primary alloplasmic wheat lines amounted up to 30%, but we were able to generate lines with ∼90% parthenogenesis via doubled haploids (Matzk et al., 1995). In the ‘Salmon’ lines, the short arm of the wheat chromosome 1B is substituted by the short arm of the 1R (rye) chromosome. Two genes located on the translocated fragment (the Parthenogenesis-inducing [Ptg] gene under sporophytic control and the Parthenogenesis-suppressing [Spg] gene under gametophytic control), in interaction with cytoplasmic factors from defined Aegilops species, control the autonomous embryo formation (Tsunewaki and Mukai, 1990). In P. pratensis, we prefer the term “preventer gene” instead of “suppressor gene” because a suppressor is a secondary mutation that restores a function lost because of a primary mutation; therefore, suppressors should evolve in the derivatives (apomicts) and not in the ancestor (sexuals). Whether or not Ptg and Spg of wheat correspond to the Pit and Ppv genes in P. pratensis needs detailed investigations.
The recessive pleiotropic mutation multiple archesporial cells 1 (mac1), which permits the development of several megasporocytes per ovule in maize (Zea mays) (Sheridan et al., 1996), shares similarities to our recessive allele of the Apv gene. The dominant allele Apv prevents the formation of additional (i.e., aposporous) embryo sacs in P. pratensis. It was hypothesized that in sexual species a genetic control exists that restricts the reproductive potential in the nucellus to a single cell comparable with the wild-type gene Mac1 in maize (Koltunow and Grossniklaus, 2003). A high ratio of polyembryonic seeds as found in mutants of rye (Secale cereale) (Linacero and Vázquez, 1994) or pearl millet (Pennisetum glaucum) (Rao et al., 1986) could also be an indication for the formation of multiple embryo sacs per ovule.
The phenotype of the haploidy initiator (hap) mutant of barley (Hordeum vulgare) (Hagberg and Hagberg, 1980) closely resembles that resulting from the recessive allele ppv of the Ppv gene in P. pratensis. In both cases, the homozygous recessive alleles bring about an expressivity of 1 to 20% parthenogensis. The wild types of barley and of other sexual species could be carriers of the homozygous dominant alleles of Hap and/or Ppv. After combination of hap with the tri mutant (forming ∼50% unreduced egg cells; Finch and Bennett, 1979) of barley, the doubled homozygous plants formed <1% embryos from unreduced parthenogenetic egg cells (F. Matzk, unpublished data), a frequency comparable with that observed for homozygous recessive apv/ppv plants in P. pratensis.
Taken together, our model of a complex genetic control of apomixis is consistent with a broad range of observations in various apomictic and sexual plant species. Thus, our model will become a valuable guide for molecular approaches aimed at the isolation of apomixis genes.
METHODS
In a first cycle of crosses, obligate sexual clones or cultivars were used as female parents and facultative apomictic cultivars as pollinators (their most important characteristics are shown in Table 8). In a second cycle, selected individual F1 plants without any capacity for apospory and/or parthenogenesis were intercrossed as the female, with F1 plants having a capacity for apospory and/or parthenogenesis as the male parent. Panicles of the female parents were hand-emasculated and bagged together with panicles of the male parent at the stage of anthesis. Crosses were performed in the spring in the greenhouse, and the progeny plants were grown outside in pots or planted in the field. In total, more than 1000 and 500 F1 plants were generated in the first and second cycles, respectively.
The so-called progeny test was applied in form of an evaluation of the F1 as well as the F2 families originating from selfing or open pollination on the field (∼500 F2 families each with 20 to 25 individuals). The homogenity or heterogenity of the progenies was classified from 1 (completely homogeneous = apomictic origin) to 9 (completely heterogeneous = sexual origin) to estimate the mode of reproduction of the mother plant. Seeds of bagged and open pollinated panicles were harvested during 3 years from the individual plants of segregating progenies to identify the mode of reproduction using FCSS.
To characterize the mode of reproduction of individual plants, two repeats each with 30 or 50 bulked seeds (bs) were initially analyzed by FCSS. After that, 30 to 50, or in some cases 100, single seeds (ss) were used from plants that reproduced facultatively along various pathways to quantify their modes of reproduction. The sexual pathway is characterized by a 2C embryo + 3C endosperm. The capacity of aposporous embryo sac formation of an individual plant can be calculated by adding the percentages of 2C and 3C embryos each joint with 5C endosperm, whereas the capacity of parthenogenesis is calculated by adding the frequencies of 1C embryos joint with 3C endosperm and 2C embryos joint with 5C endosperms. The individual plants were classified according to the adapted and expanded nomenclature system of Rutishauser (1969) as BII hybrid (from fertilization of reduced egg cell with reduced or unreduced pollen), BIII hybrid (from fertilization of unreduced egg cell with reduced pollen), MI plant (maternal plant from autonomous development of reduced egg cell), and MII plant (maternal plant from autonomous development of unreduced egg cell).
For the FCSS analyses (Matzk et al., 2000, 2001), suspensions of nuclei from mature seeds were prepared by crushing dry single or bulked seeds between two pieces of fine granulated sandpaper and rinsing these with 2 mL of staining buffer (100 mM Tris-HCl, 5.3 mM MgCl2, 86 mM NaCl, 0.03 mM sodium citrate, 7.3 mM Triton X-100, 0.003 mM 4′-6-diamidino-2-phenylindole, pH 7.0). The suspensions were then filtered through nylon tissue with 30-μm mesh width. After filtration, 1 mL of staining buffer was added, and the tubes were stored in the dark on ice for 1 to 4 h before measurement. The fluorescence intensity of 4′-6-diamidino-2-phenylindole–stained nuclei was determined using the flow cytometer Ploidy Analyser PA (Partec, Münster, Germany) equipped with an Osram HBO 103 W/2 high-pressure mercury lamp (Munich, Germany) for UV excitation. Two runs, each with ∼1000 (ss) to 4000 (bs) nuclei, were measured per sample. Seed samples of the cv Jori were used as external standards in the FCSS analyses.
The somatic chromosome numbers were determined in root tip cells from 10 plants of the cv Jori and Lato after pretreatment in distilled water at 1°C for 24 h, fixation in 3:1 ethanol:acetic acid for at least 24 h, and carmine staining. The mean chromosome number of ‘Jori’ was used as standard for the flow cytometric estimation of the ploidy in segregating populations.
To estimate the ploidy of individual plants from segregating populations as well as from the parental plants by flow cytometry, suspensions of cell nuclei were prepared by chopping a young leaf with a razor blade in staining buffer (see above). The ploidy was estimated in relation to the DNA content of nuclei from ‘Jori’ plants for which the chromosome number was counted under the light microscope.
The auxin test was used in some cases to confirm the parthenogenesis capacity found by FCSS. The number of embryos from 100 auxin-stimulated seeds per plant was determined (Matzk, 1991a, 1991b).
χ2 tests were performed on the individual self and cross families to test the goodness of fit for the observed and expected segregation ratios for each of the postulated genes for apospory and parthenogenesis. Three dosages of the dominant alleles (simplex, duplex, and triplex) were considered for the expected segregation ratios of gametophytes and sporophytes, taking into account the actual ploidy of the parents according to Haldane (1930). A random pairing and random assortment of chromosomes was assumed. The Fisher's exact probability test was used to estimate the confidence values for the degree of apospory and parthenogenesis in different harvest years.
Supplementary Material
Acknowledgments
The authors thank the coeditor and two anonymous reviewers for helpful suggestions and T. Sharbel for critical reading the manuscript as well as the technician of F.M.'s laboratory for excellent work. This research was supported in part by the European Union project “Natural apomixis as a novel tool in plant breeding (Apo Tool),” contract number QLG2-2000-00603 of the Quality of Life and Management of Living Resources section and by the Gemeinschaft zur Förderung der privaten deutschen Pflanzenzüchtung e.V. (project F56/99).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Fritz Matzk (matzk@ipk-gatersleben.de).
Online version contains Web-only data.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.104.027359.
References
- Albertini, E., Barcaccia, G., Porceddu, A., Sorbolini, S., and Falcinelli, M. (2001). Mode of reproduction is detected by Parth1 and Sex1 SCAR markers in a wide range of facultative apomictic Kentucky bluegrass varieties. Mol. Breed. 7, 293–300. [Google Scholar]
- Barcaccia, G., Mazzucato, A., Albertini, E., Zethof, J., Gerats, A., Pezzotti, M., and Falcinelli, M. (1998). Inheritance of parthenogenesis in Poa pratensis L.: Auxin test and AFLP linkage analyses support monogenic control. Theor. Appl. Genet. 97, 74–82. [Google Scholar]
- Bicknell, R.A., and Borst, N.K. (1996). Isolation of reduced genotypes of Hieracium pilosella using anther culture. Plant Cell Tissue Organ Cult. 45, 37–41. [Google Scholar]
- Bicknell, R.A., and Koltunow, A.M. (2004). Understanding apomixis: Recent advances and remaining conundrums. Plant Cell 16 (suppl.), S228–S245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wet, J.M.J. (1968). Diploid-tetraploid-haploid cycles and the origin of variability in Dichanthium agamospecies. Evolution 22, 394–397. [DOI] [PubMed] [Google Scholar]
- Finch, R.A., and Bennett, M.D. (1979). Action of triploid inducer (tri) on meiosis in barley (Hordeum vulgare L.). Heredity 43, 87–93. [Google Scholar]
- Grossniklaus, U., Nogler, G.A., and van Dijk, P.J. (2001). How to avoid sex: The genetic control of gametophytic apomixis. Plant Cell 13, 1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg, A., and Hagberg, G. (1980). High frequency of spontaneous haploids in the progeny of an induced mutation in barley. Hereditas 93, 341–343. [Google Scholar]
- Haldane, J.B.S. (1930). Theoretical genetics of autopolyploids. J. Genet. 22, 359–372. [Google Scholar]
- Holsinger, K.E. (2000). Reproductive systems and evolution in vascular plants. Proc. Natl. Acad. Sci. USA 97, 7037–7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff, D.R., and Bara, J.M. (1993). Determining genetic origins of aberrant progeny from facultative apomictic Kentucky bluegrass using a combination of flow cytometry and silver-stained RAPD markers. Theor. Appl. Genet. 87, 201–208. [DOI] [PubMed] [Google Scholar]
- Knox, K.B. (1967). Apomixis: Seasonal and population differences in a grass. Science 157, 325–326. [DOI] [PubMed] [Google Scholar]
- Koltunow, A.M. (1993). Apomixis: Embryo sacs and embryos formed without meiosis or fertilization in ovules. Plant Cell 5, 1425–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltunow, A.M., and Grossniklaus, U. (2003). Apomixis: A developmental perspective. Annu. Rev. Plant Biol. 54, 547–574. [DOI] [PubMed] [Google Scholar]
- Koltunow, A.M., Johnson, S.D., and Bicknell, R.A. (2000). Apomixis is not developmentally conserved in related, genetically characterized Hieracium plants of varying ploidy. Sex. Plant Reprod. 12, 253–266. [Google Scholar]
- Leblanc, O., Grimanelli, D., Gonzalez de Leon, D., and Savidan, Y. (1995). Detection of the apomictic mode of reproduction in maize-Tripsacum hybrids using maize RFLP markers. Theor. Appl. Genet. 90, 1198–1203. [DOI] [PubMed] [Google Scholar]
- Linacero, R., and Vázquez, A.M. (1994). Genetic analysis of a polyembryonic mutant in rye. Sex. Plant Reprod. 7, 290–296. [Google Scholar]
- Matzk, F. (1991. a). A novel approach to differentiated embryos in the absence of endosperm. Sex. Plant Reprod. 4, 88–94. [Google Scholar]
- Matzk, F. (1991. b). New efforts to overcome apomixis in Poa pratensis L. Euphytica 55, 65–72. [Google Scholar]
- Matzk, F., Hammer, K., and Schubert, I. (2003). Coevolution of apomixis and genome size within the genus Hypericum. Sex. Plant Reprod. 16, 51–58. [Google Scholar]
- Matzk, F., Meister, A., Brutovska, R., and Schubert, I. (2001). Reconstruction of reproductive diversity in Hypericum perforatum L. opens novel strategies to manage apomixis. Plant J. 26, 275–282. [DOI] [PubMed] [Google Scholar]
- Matzk, F., Meister, A., and Schubert, I. (2000). An efficient screen for reproductive pathways using mature seeds of monocots and dicots. Plant J. 21, 97–108. [DOI] [PubMed] [Google Scholar]
- Matzk, F., Meyer, H.-M., Bäumlein, H., Balzer, H.-J., and Schubert, I. (1995). A novel approach to the analysis of the initiation of embryo development in gramineae. Sex. Plant Reprod. 8, 266–272. [Google Scholar]
- Mogie, M. (1992). The Evolution of Asexual Reproduction in Plants. (London: Chapman & Hall).
- Nogler, G.A. (1984). Gametophytic apomixis. In Embryology of Angiosperms, B.M. Johri, ed (Berlin: Springer-Verlag), pp. 475–518.
- Nogler, G.A. (1994). Genetics of gametophytic apomixis—A historical sketch. Polish Bot. Stud. 8, 5–11. [Google Scholar]
- Noyes, R.D., and Rieseberg, L.H. (2000). Two independent loci control agamospermy (apomixis) in the triploid flowering plant Erigeron annuus. Genetics 155, 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozias-Akins, P., Roche, D., and Hanna, W.W. (1998). Tight clustering and hemizygosity of apomixis-linked molecular markers in Pennisetum squamulatum implies genetic control of apospory by a divergent locus that may have no allelic form in sexual genotypes. Proc. Natl. Acad. Sci. USA 95, 5127–5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porceddu, A., Albertini, E., Barcaccia, G., Falistocco, E., and Falcinelli, M. (2002). Linkage mapping in apomictic and sexual Kentucky bluegrass (Poa pratensis L.) genotypes using a two way pseudo-testcross strategy based on AFLP and SAMPL markers. Theor. Appl. Genet. 104, 273–280. [DOI] [PubMed] [Google Scholar]
- Quarin, C.L. (1986). Seasonal changes in the incidence of apomixis of diploid, triploid, and tetraploid plants of Paspalum cromyorrhizon. Euphytica 35, 515–522. [Google Scholar]
- Rao, P.N., Ranganadham, P., and Nirmala, A. (1986). Twins and triplets in pearl millet: Their cytology and origin. Ann. Bot. 58, 627–631. [Google Scholar]
- Rutishauser, A. (1969). Embryologie und Fortpflanzungsbiologie der Angiospermen. (Wien: Springer-Verlag).
- Savidan, Y. (1980). Chromosomal and embryological analyses in sexual x apomictic hybrids of Panicum maximum Jacq. Theor. Appl. Genet. 57, 153–156. [DOI] [PubMed] [Google Scholar]
- Sheridan, W.F., Avalkina, N.A., Shamrov, I.I., Batygina, T.B., and Golubovskaya, I.N. (1996). The mac1 gene: Controlling the commitment to the meiotic pathway in maize. Genetics 142, 1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane, C., Steimer, A., and Grossniklaus, U. (2001). Apomixis in agriculture: The quest for clonal seeds. Sex. Plant Reprod. 14, 179–187. [DOI] [PubMed] [Google Scholar]
- Tsunewaki, K., and Mukai, Y. (1990). Wheat haploids through the salmon method. In Wheat (Biotechnology in Agriculture and Forestry 13), Y.P.S. Bajaj, ed (Berlin, Heidelberg, New York: Springer-Verlag), pp. 460–478.
- Tucker, M.R., Araujo, A.-C.G., Paech, N.A., Hecht, V., Schmidt, E.D.L., Rossell, J.-B., de Vries, S.C., and Koltunow, A.M.G. (2003). Sexual and apomictic reproduction in Hieracium subgenus pilosella are closely interrelated developmental pathways. Plant Cell 15, 1524–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk, P.J., Tas, I.C.Q., Falque, M., and Barx-Schotman, T. (1999). Crosses between sexual and apomictic dandelions (Taraxacum). II. The breakdown of apomixis. Heredity 83, 715–721. [DOI] [PubMed] [Google Scholar]
- Voigt, P.W., and Burson, B.L. (1983). Breeding of apomictic Eragrostis curvula. In Proceedings of the 14th International Grassland Congress (1981), Lexington, J.A. Smith and V.W. Hayes, eds (Boulder, CO: Westview Press), pp. 160–163.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.