Abstract
Human tissues are intricate ensembles of multiple cell types embedded in complex and well-defined structures of the extracellular matrix (ECM). The organization of ECM is frequently hierarchical from nano to macro, with many proteins forming large scale structures with feature sizes up to several hundred microns. Inspired from these natural designs of ECM, nanotopography-guided approaches have been increasingly investigated for the last several decades. Results demonstrate that the nanotopography itself can activate tissue-specific function in vitro as well as promote tissue regeneration in vivo upon transplantation. In this review, we provide an extensive analysis of recent efforts to mimic functional nanostructures in vitro for improved tissue engineering and regeneration of injured and damaged tissues. We first characterize the role of various nanostructures in human tissues with respect to each tissue-specific function. Then, we describe various fabrication methods in terms of patterning principles and material characteristics. Finally, we summarize the applications of nanotopography to various tissues, which are classified into four types depending on their functions: protective, mechano-sensitive, electro-active, and shear stress-sensitive tissues. Some limitations and future challenges are briefly discussed at the end.
Keywords: Nanotopography, Tissue engineering, Regenerative medicine, Biomaterials, Cell–material interface
1. Introduction
Human organs maintain homeostasis, transfer information, generate force, and adapt to changes in the environment. Organs may lose functions over time due to excessive use and inevitable aging, or unexpectedly through disease or other pathologies. Damaged organs can be regenerated with the aid of various drugs, mechanical supports or transplantation. Here, regenerative medicine refers to technology that could be used for reconstructing damaged tissues or organs with various items and techniques including cells, scaffolds, medical devices or gene therapy [1]. Since the introduction of regenerative medicine in 1980s, the field has rapidly grown at an amazing rate, and it is in the transient stage progressing from basic research toward clinical applications [2]. Additionally, costs associated with healthcare are rising dramatically to meet the demands of an ever increasingly older population. For example, in the USA, the expenditure for healthcare reached 17.3% of the gross domestic product (GDP) and this percentage is estimated to continue to increase in the future [1]. Furthermore, in research fields, the scientific publications on regenerative medicine, number of submitted and accepted patents, and number of companies working on commercializing regenerative medicine products and therapies are also gradually increasing [2].
In regenerative medicine, therapies generally focus on a specific organ and must meet the heterogeneity and demands of the individual organs. Therefore, regenerative medicine has to start from the understanding of underlying cellular behaviors, tissue specific environment, and cause of tissue degradation and injury. In terms of cellular behavior, for example, cells in various tissues respond differently according to diverse factors such as physical topography or rigidity (durotaxis) [3], gradient of cellular adhesion sites (haptotaxis) [4], electric fields (electrotaxis) [5], and chemical factors (chemotaxis) [6,7]. Importantly, different cells may behave oppositely even under identical stimuli: fibroblasts migrate toward the more rigid region while neurons stretch neurite to the direction of softer site. Furthermore, the environmental effects originate from the organ-specific functions such as protection (skin), mechanical maintenance (bone), transmission of force (ligament and tendons), conduction of electrical signal (neuron), blood circulation (heart and vessels), and force generation (skeletal muscle).
Among the various factors described above, physical cues are of great importance since cells reside in a physical microenvironment with tissue specific topography and rigidity. Such physical structures are widely observed in the human body such as the neuronal network in the brain, aligned myofibrils in the heart and skeletal muscle, and collagen fibers in the skin. Since these structures are intended for tissue specific functions, pathology may change the physical nanostructures in these organs, which results in the malfunction or disconnection of signal or force transfer. For example, it is well known that neurons in the human brain have a radially spreading morphology connecting the cerebral cortex and white matter. In the case of physiological malfunctions such as Alzheimer’s or Parkinson’s diseases, neuronal interconnections are severely damaged, thus losing informational pathways [8]. The goals of regenerative medicine with the aid of nanostructures lie in facilitating guided cell migration and proliferation to restore physiological structures and functions and minimizing possible side effects. To fabricate the observed natural structures with distinct physical properties, various materials and fabrication methods have been used over time.
In this review, we provide an extensive overview on recent efforts for constructing in vivo-like microenvironment with nanofabricated structures and results of cell-specific functions dependent on physical cues. In the first section, the tissue-specific nanostructures will be characterized with respect to their specific functions and physiology such as protective, mechano-sensitive, electro-active and shear stress-sensitive characteristics. In the second section, nanofabrication techniques will be summarized in terms of patterning principles and material characteristics. In the third section, examples of tissue-specific cell functions, tissue-mimicking systems and in vivo implantation results of these systems will be described in conjunction with the physiological similarity of these tissues in their functional roles. We believe that this extensive overview on studies examining the relation of cells to their physical microenvironment will present an in-depth understanding on current regenerative medicine strategies.
2. Microenvironment of human tissues: role of nanotopography
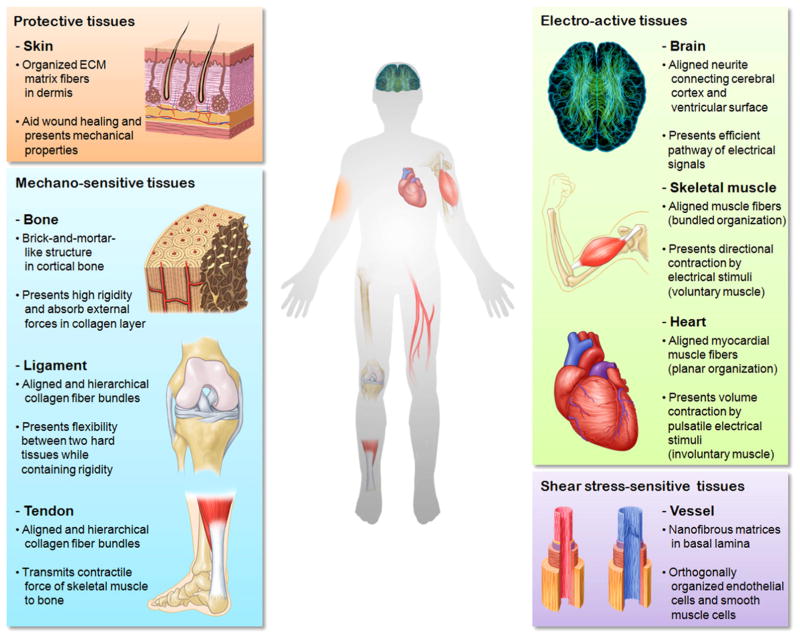
The tissues in the human body contain nanoscale structures which assist tissue-specific functions. As shown in Fig. 1, nanostructures in the human body can be classified depending on their functions and physiological environment into four types, namely protective tissue (skin), mechano-sensitive tissues (bone, ligament/tendon), electro-active tissues (neuron, skeletal muscle, heart) and shear stress-sensitive tissue (blood vessel). Since these tissues are exposed to different stimuli and environments, an understanding of each tissue-specific structure is required.
Fig. 1.
Various nanostructures in the human body. The tissues are classified into four categories, namely protective, mechano-sensitive, electro-active and shear stress-sensitive tissues with respect to the tissue specific environment and functions.
2.1. Protective tissues
The skin protects internal tissues and organs from external irritations such as light, pathogens and chemicals. The skin must thus be sufficiently rigid to withstand physical trauma and act as a physical barrier to the external environment and elastic enough to absorb mechanical impact from outside. For these reasons, the skin has moderate elastic modulus of 7–50 MPa [9,10]. From a structural point of view, the skin is composed of three layers: epidermis, dermis and hypodermis. Among these, well aligned ECM fibers such as collagen, fibronectin and keratin fibers (diameter of few tens of nanometers) are observed in the dermis and are responsible for mechanical support and the regulation of cell functions. These ECM fibers have diameters of 60–120 nm with the length of few micrometers. In the dermis, the diameter of ECM fibers increases as the skin depth increases [11]. Generally, it has been known that the matrix fibers in the unwounded dermis are organized in a basket-weave meshwork [12]. There is also macroscopically directional anisotropy of collagen fibers, which is called Langer’s line [13]. With this alignment of ECM fibers, the skin demonstrates anisotropic mechanical properties [14]. For example, due to the alignment of ECM matrix fibers, skin wound healing following a cesarean section is affected by the incision direction, showing better healing and smaller scar formation in transverse incisions compared to sagittal incisions. The presence of nanotopography and its effects on cell and tissue function has inspired in vitro wound healing studies.
2.2. Mechano-sensitive tissues
Mechano-sensitive tissues refer to those tissues that are continuously exposed to mechanical force while walking or exercising, including bone, ligament and tendon. To endure external forces and support the body’s structure, bone (4.5–20 GPa) [15–17] and ligament/tendon (10 MPa–2 GPa) [9,10,18] have relatively high elastic moduli. Mechano-sensitive tissues also act mechanically as a damper, to absorb or diminish abrupt mechanical changes of the body system. Interestingly, mechano-sensitive tissues have hierarchical structures. As shown in Fig. 1, the bone is composed of two distinct areas: cortical bone (also known as compact bone) which has concentric cylindrical features and cancellous bone (also known as spongy bone) which has a spongy-like structure. Especially, in cortical bone, such high mechanical rigidity originated from brick-and-mortar structures, comprising plate-like mineral crystals stacked within the collagen matrix [19,20]. The mineral platelets (dahllite, also known as carbonated apatite) have average lengths and widths of 50×25 nm2, and thicknesses of 1.5–4 nm [21,22]. The collagen fibers found in bone have a diameter of about 80–100 nm, comprised of triple helixes of collagen molecules with a diameter of ~1.5 nm and a length of ~300 nm which are organized in a staggered way [22,23]. Since the mineral platelets are also stacked in a staggered manner and embedded in a collagen matrix, externally applied stresses can be redistributed and fracture energy can be absorbed or dissipated in daily activities such as walking and running [24]. In contrast, the cancellous bone is less dense, softer, and less stiff, and contains red bone marrow, where hematopoiesis, the production of blood cells, occurs.
Similarly, ligaments and tendons, which are fibrous connective tissues, connecting two tissues together, also contain hierarchical nanostructures for their functions. Ligaments connect bone to bone at joints, with enough flexibility to allow the joint movement while containing sufficient rigidity to prevent dislocation, and are commonly observed in knees and shoulders. Tendons connect skeletal muscle to bone to transfer force for the movement. For ligaments and tendons, it is composed of four levels of hierarchy: collagen molecule, collagen fiber, fascicle, and tendon fiber. The collagen fibers are composed of bundles of collagen molecules in a triple helix (diameter of ~1.3 nm) which are packed in a staggered manner. The bundles of collagen fibers with a diameter of 50–500 nm are embedded in a proteoglycan-rich matrix [24], forming a fascicle (diameter of 50–300 μm). Finally, fascicles form tendon fibers with a diameter of 100–500 μm [25]. Due to multilevel, hierarchical, and staggered organization, ligaments and tendons dissipate abruptly applied mechanical force by slippage between collagen molecules or fibers. These uni-directionally aligned hierarchical nanostructures provide sufficient compliance for dynamic action and mechanical impact by inter-fiber sliding which is aided by deformation of the proteoglycan layer [26,27].
2.3. Electro-active tissues
Unlike the passive structures in mechano-sensitive tissues, electro-active tissues have active structures which respond to electrical stimuli. As can be inferred from the name ‘electro-active’, electro-active tissues respond to pulsatile or abrupt electrical stimuli. Brain tissue is an electro-active tissue in which information and stimulation received from the eyes, ears and skin are processed through the relay of signals by neurons. When these signals are received, the data is transmitted as an electrical pulse which is called action potential. These signals are collected through the central nervous system (CNS) and peripheral nervous system (PNS). Here, the CNS includes the retina, brain and spinal cord, and the PNS refers to the rest of the nervous system such as sensory receptors and effectors in organs. To transmit a great amount of signals to the cerebral cortex of the brain, which is believed to be the brain’s central processing region, neurons are constructed of well-organized structures. The neurons in the brain are guided by radial glial cells (RGCs) which connect the ventricular surface to the cortical surface [28]. The RGCs have two long radial processes which are extended in opposite directions and have relatively low elastic modulus of ~600 Pa (converted from shear modulus measurements of ~200 Pa with the relation between shear and elastic modulus, E=2G (1+ν), where E: elastic modulus, G: shear modulus, and ν: Poisson ratio) [29]. Since the neurite outgrowth is directed by topographical guidance toward the longitudinal direction of RGCs and attracted toward a physically softer environment (RGCs have lower elastic modulus compared to neurons. Elastic modulus of neuron: 0.1–10 kPa [30]), neurons extend radially their structures [31].
As opposed to neurons which are only electrically active, skeletal muscle and heart tissue are active both mechanically and electrically. Skeletal muscle and heart tissue are commonly composed of muscular structures which can contract upon electrical stimuli. Skeletal muscles are composed of hundreds or even thousands of longitudinally aligned cylindrically-shaped muscle fibers. Each muscle fiber is a multinucleated cell having diameters ranging from 5 to 100 μm and lengths from 1 to 2 mm even to few centimeters [32]. In the center of these fibers, protein filaments named myofibrils exist in a tubular morphology, and are composed of contractile elements called sarcomeres, the basic functional unit for muscles. Sarcomeres contract from the inter-fibril sliding of F-actin and myosin filaments [33]. This contractility is mediated by motor neurons which are connected to PNS. Although individual sarcomeres contract ~2.4 μm (sarcomere length can change from 1.25 to 3.65 μm), greater contractions can be achieved by a serial arrangement of sarcomeric units [32,34]. Furthermore, a single muscle fiber can only exert a force of 10.2–14.3 μN FL/s (slow fiber, where FL is fiber length), 43.9–71.4 N FL/s (fast fiber), but this contractile force can be enhanced by parallel organization of fiber bundles up to 1.58 W/l (slow fiber), 7.96–10.64 W/l (fast fiber) [35].
Although cardiac muscle has similar sarcomeres, its organization and morphology is different from that of skeletal muscle. In vivo, cardiomyocytes have two-dimensional rectangular leaflet-like morphology with typical lengths of 100–150 μm and widths of 20–35 μm, unlike the cylindrical morphology of skeletal muscle fibers [36]. Such cardiomyocyte morphology is a result of the planar organization of its sarcomeres as a repeating banded structure which comprises a myofibril, being unidirectionally aligned within the cell. Specifically, for coordinated and synchronized contractions of the heart, the action potentials are conducted unidirectionally through gap junction structures termed intercalated disks (IDs) which are usually located in both of the longitudinal ends of cardiomyocytes. In certain pathologies, the distribution and location of IDs may become random. This leads to a random distribution of the action potentials generated in the sinoatrial (SA) node throughout the myocardium, giving rise to myocardial fibrillation [37]. Thus, for proper mechanical contraction and electrical conduction, unidirectionally aligned sarcomeric structures are important.
2.4. Shear stress-sensitive tissues
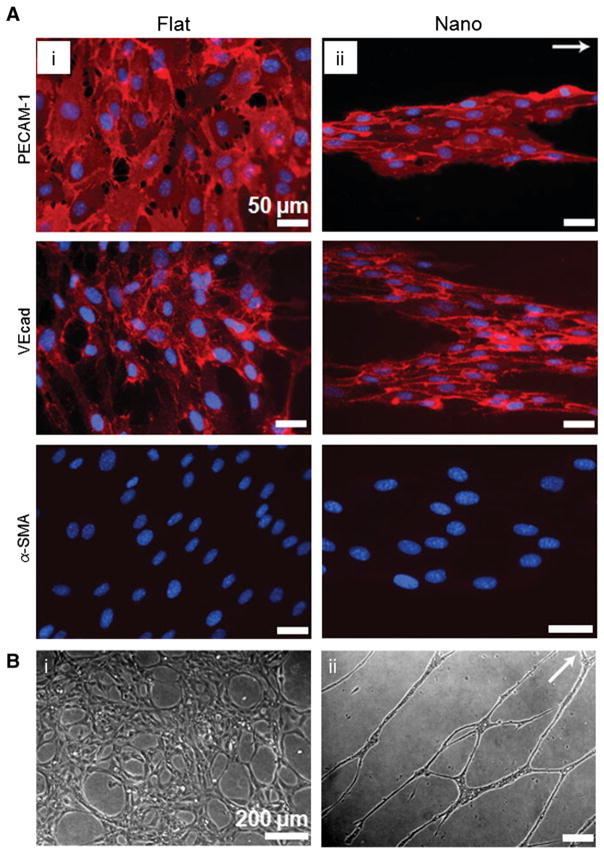
Blood vessels are tissues that are continuously exposed to mechanical stimuli, especially fluidic shear stress and cyclic expansion/contraction due to cardiac contractions. Endothelial cells (ECs) line the interior of blood vessels (endothelium) and show aligned morphology toward the longitudinal direction, responding to flow forces. Vascular tissue can be divided into a number of branches with hierarchical organizations when tissue specific functions are required, such as the filtration in the kidney, gas exchange in the lungs, and extensive branching for coronary circulation. As can be inferred from the classification ‘shear stress-sensitive’, fluidic shear stress is a crucial factor for vascular cell function including proliferation, elongation, and protein secretion. The shear stress applied to the surface of the inner vessel wall ranges from 10 to 70 dyn/cm2 (arteries), or 1 to 6 dyn/cm2 (veins) [38]. In the vascular system, basal lamina guides the endothelial cells in the blood vessel, and is composed of collagen IV and laminin-1 fibers with nanometer scale [39,40]. In addition to the physical nanostructures in natural basal lamina, nanotopographical cues can also be a possible tool for inducing and facilitating cellular functions since the mechano-receptor of shear stress might be the same with that of physical stimuli such as rigidity and topography [41]. Detailed descriptions will be given in Section 4.3. As an additional functional component, smooth muscle cells (SMCs) comprise the medial layer of the blood vessel, covering blood vessels circumferentially guided by basal lamina. The main function of SMCs is to maintain fluidic homeostasis through the interaction with ECs. In order to investigate the function of SMCs as a vascular mediator, the alignment of SMCs as well as the expression of functional markers can be achieved through culturing SMCs on micro- and nanopatterned surfaces.
3. Fabrication methods for nanotopography
3.1. Historical background
The controlled microenvironment is intended for elucidating or guiding the expression of cellular functions. To address this controllability, a great number of fabrication methods have been introduced in the last few decades. Since many reviews are currently available for numerous fabrication methods [42–49], we will focus on the methods that deal with synthetic polymers and their related fabrication techniques. Some fabrication methods described in this section are still valid for the structuring of natural polymers.
According to Moore et al., the first cell culture with topographical cue was reported by R. G. Harrison in 1914 [50]. In his seminal work, he cultured embryonic frog cells on a spider web attached on a cover slip, demonstrating guided elongation of cells along the fibers [50,51]. A few years later, in 1929, P. Weiss reported on the important role of mechanical structures, and in 1934, he further expanded the principle of topographical guidance by culturing neurons on a cover glass covered with oriented, rod-like fibrin, demonstrating aligned nerve fibers in vitro [52]. Although these pioneering works demonstrated that topographical guidance could be an important cue for cellular polarization, the underlying mechanisms could not be elucidated due to the lack of sophisticated fabrication methods.
The intracellular signaling processes responsible for these cellular responses have been revealed rapidly by introducing reproducible abrication techniques with synthetic polymers. Although various fabrication techniques were already established many years ago, the adaptation into the biological field has only been initiated recently. For example, according to Cao et al., although photolithography has been a well-established, commercially available technique in semiconductor industry in the early 1970s, its introduction as a biological platform was first explored in 1988 by Kleinfeld and coworkers [53,54]. By using this method, guided neurite outgrowth and grid formation were demonstrated. As a bottom-up approach, electrospinning was first introduced in 1934 as a patent by A. Formhals [55,56]. However, its adaptation into the biomedical engineering field occurred in the early 2000s with biodegradable poly(lactic acid) (PLA) or poly(lactic-co-glycolic acid) (PLGA) [57,58]. Finally, the introduction of soft lithography in early 1990s by George Whitesides [59,60] has led to an enormous increase in projects and reports investigating this technology for biomedical engineering purposes. Due to the successful utilization of polydimethyl siloxane (PDMS) as a flexible mold, controlled physical topography could now be made in a cost-effective, low-expertise fashion.
3.2. Fabrication methods
Various nanofabrication methods are summarized in Table 1 in terms of the requirement of template and the type of energy sources. In general, the fabrication methods can be classified into two categories: template-assisted and template-free methods. The template-assisted fabrication methods require a certain mold which can be (i) transparent or opaque depending on the utilization of light, (ii) rigid or flexible depending on the pressure requirements, and (iii) compact or permeable to various solvents with respect to the usage of solvents. These template-assisted fabrication techniques can be further categorized with respect to the energy sources required for physical modification. The energy sources can be classified into five types: thermal, optical, physical, chemical and electrical.
Table 1.
Classification of nanofabrication methods in terms of the use of template and type of energy sources. For each method, patterning principle, pattern shapes, and materials used are summarized.
| Template | Energy source | Methods | Mechanism | Resulting patterns | Processable polymers |
|---|---|---|---|---|---|
| Template-assisted | Thermal | Replica molding (RM) | Thermal cross-linking of cavity-filled prepolymer | Negative shape of the mold | Thermocurable |
| Nanoimprint lithography (NIL) | Plastic deformation of polymer resin above Tg | Negative shape of the mold | Thermoplastic, conductive | ||
| Optical | Photolithography | Solubility change upon selective UV exposure | Depends on mask design | Photo-curable | |
| UV-assisted molding | Photo cross-linking of cavity filled prepolymer | Negative shape of the mold | Photo-curable | ||
| Physical | Capillary force lithography (CFL) | Capillary rise of thermoplastic polymer resin above Tg | Partially filled negative shape of the mold | Thermoplastic, solvent soluble | |
| Micromolding in capillaries (MIMIC) | Capillary-driven microchannel filling | Negative shape of the mold | Solvent soluble | ||
| Chemical | Microcontact printing (μCP) | Transfer of materials by using the relative surface energy difference | Extruded patterns of elastomeric stamp | Proteins, SAMs | |
| Electrical | Electrochemical deposition | Electrochemical reduction of the materials on the conductive electrode | Negative shape of the mold (fully or partially filled) | Conductive | |
| Template-free | Thermal | Block copolymer (BCP) lithography | Microphase separation of two immiscible polymers | Nanoscale hole, line and lamellar structure | Block copolymer |
| Optical | E-beam lithography | Solubility change upon selective irradiation of focused electron beam | Arbitrary pattern dependent on the pathway of electron beam | E-beam sensitive | |
| Direct laser writing | Selective cross-linking of polymer by laser irradiation | Arbitrary pattern dependent on the pathway of laser beam | Photo-curable | ||
| Physical | Wrinkle | Mechanical buckling between elastic substrate and rigid film | Random or aligned micro- or nanolines | Elastomer | |
| Crack | Mechanical fracture of the stiff film adhered onto elastic substrate | Aligned or inter-crossing line pattern | Elastomer | ||
| Chemical | Dip-pen lithography | Direct writing of molecules with sharp tip | Arbitrary pattern dependent on the pathway of sharp tip | SAMs | |
| Salt leaching/gas foaming | Selective dissolution of salt particulates/bubble formation in polymer block | Polymer block with numerous voids | Solvent soluble | ||
| Electrical | Electrospinning | Electric field-induced fiber drawing from the polymer solution | Three dimensional nanofibrous mesh | Solvent soluble |
Thermocurable: poly(dimethyl siloxane) (PDMS), and other thermocurable materials.
Photo-curable: photoresist, polyurethane (PU)-based, polyethylene glycol (PEG)-based, poly(N-isopropyl acrylamide) (pNIPAM) and others.
Thermoplastic: polymethyl methacrylate (PMMA), polystyrene (PS), poly(lactic-co-glycolic acid) (PLGA), poly(lactic acid) (PLA), poly(glycolic acid) (PGA), polycaprolactone (PCL) and others.
Conductive: polyaniline (PANi), poly(3,4-ethylenedioxythiophene) poly(styrene sulfonate) (PEDOT:PSS), polypyrrole (PPy) and others.
Block copolymer: polystyrene-block-poly(methyl methacrylate) (PS-b-PMMA), poly(styrene-b-ethylene oxide)(PS-b-PEO), styrene–butadiene–styrene (SBS) and others. Elastomer: usually PDMS.
Solvent soluble: thermoplastic, conductive.
E-beam sensitive: PMMA, e-beam sensitive photoresists and others. SAMs: self-assembled monolayers.
Thermal energy can crosslink prepolymers filled into the cavities of molds (replica molding, RM) [61] or plastically deform thermoplastic polymers by applying heat and mechanical pressure together (nanoimprint lithography, NIL) [62,63]. Usually utilization of thermal energy requires a long process time for crosslinking and heating/cooling. Nonetheless, such technique requires low expertise (in the case of RM) and can make fine structures down to 10 nm (in the case of NIL).
Optical energy utilizes light with the appropriate wavelength for photo crosslinking. The type of template may differ depending on the requirement of selective exposure. Photolithography selectively illuminates light through an optically pre-defined mask. Due to selective exposure, the solubility of the exposed and unexposed polymer regions becomes different, which are subsequently removed selectively by material-specific solvents [64,65]. On the other hand, ultraviolet (UV)-assisted molding utilizes physically pre-defined molds for structuring. In UV-assisted molding, photocurable prepolymers are drop-dispensed onto molds and the whole area is illuminated for a few tens of seconds, resulting in a negative replica of the mold [66]. Since a large portion of UV-curable polymers is susceptible to oxygen-inhibited crosslinking, a sheet of engineering plastics can be used as a supporting blanket to prevent oxygen penetration [67].
Physical energy utilizes capillary force as a driving force. When a liquid drop is in contact with a solid surface, Laplace pressure is generated at the liquid–solid interface. This Laplace pressure can drive liquids to rise into the mold cavity (capillary force lithography, CFL) [68,69], or attract liquid inside the microchannels (micromolding in capillaries, MIMIC) [70]. In both cases, the mold is hydrophilic enough to generate Laplace pressure, and permeable enough to allow air or solvents to dissipate during the molding process. To meet such requirements, PDMS-based materials have been widely used.
With chemical factors, pre-defined patterns can be transferred onto the target substrate similar to stamping. This process is called microcontact printing (μCP) [71]. To be used as a stamp, a material has to meet several requirements such as flexibility for conformal contact, low surface energy for efficient transfer, and inertness for minimized degradation of transferred materials. By considering such requirements, μCP usually utilizes PDMS stamps prepared from RM. Materials such as proteins, self-assembled monolayers (SAMs), or even metals and ceramics can be transferred through μCP. The minimum feature size is determined by the scale of stamp patterns.
Electrical energy utilizes electrochemical oxidation/reduction at the interface between conductive substrates and ionic solutions. When an electric field is applied between a conductive substrate and electrochemically reducible solution, the ions accumulate onto the substrate. The resulting pattern morphology can be guided by the utilization of pre-defined structures on the substrate, or certain types of mold. Although this method can present highly precise structures depending on the structural guidance, it is only applicable to ionic or conductive materials [72,73].
Template-free fabrication techniques do not require pre-defined molds. Similar to the template-assisted fabrication methods, template-free methods can be classified into five categories depending on the energy sources.
Thermal energy can facilitate nanoscale self-assembly through the mobility of the polymer chain. Block copolymer lithography (BCP lithography) utilizes the microphase separation of two immiscible polymer chains in the presence of heat or solvents. The domains of block copolymers, which have two distinctive polymer chains with the total length of few tens of nanometers chemically bound to them, are separated due to the affinity among identical polymers, resulting in regularly ordered nanostructures. With BCP lithography, lines, pillars, and pits can be fabricated with the dimension down to ~15 nm [74,75].
Optically defined structures are the results of selective illumination of light sources. In nanoscale optical patterning, patterns can only be fabricated in a serial manner, which usually requires a long process time, thus being limited to a small area. For example, electron-beam lithography (e-beam lithography, EBL) utilizes a focused electron beam for selective chain scission of photoresists. Since the diameter of the electron beam can be controlled by the current, sub 10 nm structure can be fabricated [76]. Laser direct writing uses laser as a light source. Since sub-micrometer scale structures in the different heights can be made with the point exposure of a femtosecond laser, laser direct writing can be used for fabricating complex three-dimensional nanostructures [77,78].
Physical methods without the aid of template usually utilize self assembly. Wrinkles and cracks are the results of mechanical energy storage (wrinkle) or emission (crack) processes. Wrinkling is a mechanical buckling as a result of mismatch of elastic modulus, which occurs when external forces are applied onto a bi-layered system (e.g., rigid films on top of an elastic substrate). Depending on the ratio of elastic modulus and thickness of rigid films, features with sizes from few tens of nanometers to few tens of micrometers can be fabricated. The degree of ordering or randomness can be modulated from the direction of applied forces [79,80]. Alternatively, cracking is a mechanical fracture occurring on the rigid films adhering onto an elastic substrate. The depth of the cracks can be controlled by modulating the thickness of rigid film, and spacing by applied mechanical stress [81–83].
Chemical forces are used for two-dimensional but highly ordered dip-pen lithography (DPL) and three-dimensional but less ordered salt leaching and gas foaming. Dip-pen lithography directly writes SAMs onto the substrate with a sharp tip as a consequence of chemical diffusion between tip and substrate [84,85]. Although in the first generation of DPL a single atomic force microscopy (AFM) tip was utilized, recently such a long process time has been resolved by introducing an array of sharp tips writing in parallel simultaneously [86]. Salt leaching and gas foaming similarly generate three dimensional porous polymer blocks. In salt leaching, water-soluble salt particulates are mixed with polymer solution, and then dissolved after curing, leaving behind innumerous pores [87]. Similarly, gas foaming uses gas bubbles to make voids in a polymer block [88]. However, the uniform control of gas bubbles is relatively difficult compared to that of salt particles.
An electrical energy source can be used for electrospinning. Electrospinning is a result of electric-field induced solution being pulled onto the substrate. As a consequence, three-dimensionally stacked fibril structures can be made. The diameter of fibers can be controlled by various parameters such as polymer molecular weight, concentration of solution, electric field potential, flow rate of the polymer solution and distance between needle and collector. In terms of fiber orientation, the aligned fiber mats can be fabricated with a rotating mandrel, whereas the random matrices can be fabricated with a stationary collecting plate. One of the most important features of electrospinning is that it can present three-dimensional meshes with high porosity (~90%) into which oxygen and soluble factors can penetrate [57,89,90].
4. Applications of nanotopography to tissue engineering and regenerative medicine
As described in many pioneering works, cell shape is a crucial mediator for growth and differentiation [91,92]. Recently, with the help of μCP patterning method, McBeath et al. demonstrated selective differentiation of MSCs depending on the size of micro islands [93]. Kilian et al. also showed differentiation of MSCs on contact printed topography with various geometric cues such as aspect ratio of rectangles and sharpness of polygons [94]. With unprecedented revolution of nanotechnology and bioengineering, it has been well established that the planar or three-dimensional synthetic ECM could induce morphological change through (i) the control of the number and distribution of focal adhesions and (ii) the modulation of effective modulus or protein adsorption [95]. Undoubtedly, such a generation of synthetic topography has to reflect tissue-specific natures such as protective, mechano-sensitive, electro-active and shear stress-sensitive functions.
In this section, representative examples of reconstructed tissue functions in vitro and tissue generation in vivo are described based on the tissue-specific environment. Although further studies are required for the better emulation of a sophisticated physiological environment, it is believed that the results below demonstrate the potential of creating nanotopographical guidance cues to regulate tissue functions.
4.1. Protective tissues
The main purpose of protective tissues lies in protecting intra-body organs from external damages such as mechanical impact, pathogens, and harmful chemicals. In the human body various protective tissues exist in the skin and dura mater in the brain and spinal cord. The thin membrane in the brain absorbs an external impact with mechanical damping through the cerebrospinal fluid filled beneath the arachnoid membrane, while the dura mater protects the nervous system as a rigid shield. Among these tissues, the skin has distinctive functions, such as wound healing by progressive closing of the wound with the assistance of ECM, as well as inhibition of invasion of pathogens with a sieving mechanism of ECM. Although a handful of studies has demonstrated nanotopography-guided wound healing with dura mater [96,97], our understanding is generally limited as to detailed mechanisms on directed cell migration and the role of ECM.
4.1.1. Skin
Although great advances have been made for skin replacement therapy, strategies to engineer skin must focus not only on replicating its physiology and biological stability, but also preserving the original esthetic nature of uninjured skin. Skin tissue engineering strategies focus on epidermal, dermal or composite tissue generation or regeneration. The epidermis is the outermost layer of the skin, being comprised of stratified epithelial cells and resides on the basal lamina. Since the basal lamina is comprised of an ECM fiber layer, including type IV collagen, laminin, fibronectin and heparin sulfate proteoglycan [98], it has a well-defined micro- and nanotopography with a series of ridges and invaginations termed rete ridges and papillary projections [99]. This topography has been shown to modulate cell function [100]. The dermis is the collagen-rich skin layer underneath the basal lamina and is composed of a meshwork of ECM fibers with diameters in the nanoscale level (30–130 nm). These nanostructures are also responsible for modulating cell proliferation, migration, differentiation and apoptosis as well as providing mechanical support and a network that can facilitate nutrient transport and diffusion [101]. Micro- and nanofabrication techniques thus can be used to generate scaffolds which can emulate native tissue properties (such as ECM architecture), promote cell attachment and clinically assist in the healing and regeneration process.
For the efficient infiltration of host tissues in the dermis, attachment and spreading are important factors to estimate the capability of scaffold. As the dermis is comprised of a meshwork of ECM fibers, electrospinning has been employed as a tool for generating nanopatterned scaffolds for dermal regeneration. Venugopal et al. used electrospinning to create mesh fiber scaffolds of poly(caprolactone) (PCL), collagen, and PCL–collagen composite. The scaffolds could be fabricated on a large scale (11×7 cm2 scaffolds) with diameters ranging from 170 to 250 nm and with high (>90%) porosity, allowing for nutrient diffusion and cell migration. Although the human dermal fibroblasts were shown to attach to all these scaffolds, the cells exhibited a different morphology on the PCL scaffolds from that of the PCL–collagen scaffolds. The PCL–collagen blended scaffolds also were mechanically more stable than the collagen scaffolds. Moreover, PCL–collagen and collagen both demonstrated higher cell proliferation, indicating increased biocompatibility [102,103]. In parallel, individual PCL fibers were coated with collagen to improve proliferation [104]. A similar experiment using dextran/PLGA electrospun fiber scaffolds and mouse dermal fibroblasts also demonstrated cell attachment without the need for ECM precoating or treatment, with enhanced cell viability (up to 10 days) and penetration into the fiber scaffolds [105].
Natural biopolymers have also been explored as dermal scaffold materials. Min et al. used electrospun silk fibroin nanofibers (average diameter: 90 nm) and found higher adhesion and spreading area of human keratinocytes and fibroblasts when cultured on collagen-coated scaffolds, as compared to untreated polystyrene scaffolds and scaffolds treated with laminin and fibronectin [106]. Similar results were found in chitin scaffolds, demonstrating an increase in attachment after collagen type-I treatment [107]. Rho et al. electrospun collagen nanofiber scaffolds (average diameter: 460 nm) and crosslinked the scaffolds to improve mechanical integrity (Fig. 2). Keratinocytes adhered well and spread to collagen-coated or laminin coated scaffolds in vitro, and in vivo implantation of acellular scaffolds in an open wound healing test showed faster early-stage healing than gauze-treated controls [108]. These works suggest that for dermal repair, biocompatibility, biodegradation and mechanical stability must be considered together when selecting a scaffold material as well as determining scaffold dimension, porosity, and design.
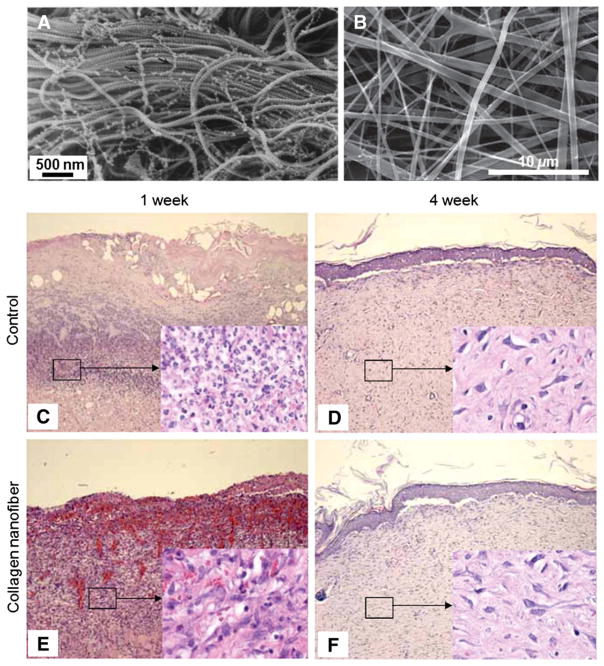
Fig. 2.
(A) Scanning electron microscope images of collagen type VI network observed in human neonatal foreskin. (B) Electrospun type I collagen as a biomimetic nanofibrous extracellular matrix. (C–F) In vivo wound healing test at 1 and 4 weeks. In the 1-week collagen nanofiber group, surface debris disappeared and promoted proliferation of young capillaries and fibroblast was observed (E), while in the control group surface debris exists and dense infiltration of leukocytes and fibroblasts was observed, demonstrating promoted early stage skin wound healing with the assistance of nanofiber matrix (C). However, in the 4-week group, the healing processes were similar (D, F).
A is reprinted with permission from ref. [14]. B–F are reprinted with permission from ref. [108].
For epidermal repair strategies, Rheinwald et al. developed in vitro techniques that could subculture autologous keratinocytes into large sheets of epithelial tissue termed cultured epithelial autografts (CEAs) [109]. Due to the fragility and suboptimal engraftment rates, the necessity of suitable scaffolds that can support CEAs has been increasing. Smith et al. utilized photolithography to create polyvinyl alcohol/polyvinyl acetate copolymer hydrogel sheets of square-shaped micropits and microcolumns (100×100 μm2, 250×250 μm2 and 500×500 μm2 dimensions) of varying depths (5, 10 and 200 μm) [110]. The microtopographical features have to be porous to allow cell migration from the scaffold to host surfaces as well as protective to prevent cells from being sheared off the surface of the scaffold. Cell transfer from micropatterned scaffolds showed a higher grafting efficiency than flat scaffolds and provided more protection from induced shear stress [110].
Additionally, CEA engraftment was more effective on allograft, autologous or engineered dermal substrates [111–113], suggesting the necessity of further investigation of keratinocyte–surface and topography interactions, particularly at the nanoscale level to improve CEA engraftment. Pins et al. used laser machining and soft lithography to microfabricate an analog of the basal lamina with precise topographic features, i.e., channels of varying widths and depths (40 to 200 μm range), on gelatin or type I collagen–glycosaminoglycan (GAG) scaffolds [99]. These lamina analogs were laminated onto dermal substitutes (collagen sponges) to create a bio-inspired model of skin ECM. Cultured keratinocytes were seeded on the surface (lamina analog) of the scaffold and further cultured at the air–liquid interface to induce differentiation and cornification of the epidermis. After 10 days, cells formed a stratified epidermis, and even demonstrated skin-like invaginations of the epidermal surface depending on channel depth [99]. This method of generating stratified epidermis with skin-like topography has also been shown to be compatible with photolithographic techniques [114]. These surface features of skin, such as rete ridges, may also assist appropriate skin function and provide epidermal mechanical stability [115], emphasizing the importance of lamina topography for appropriate skin generation and function.
4.2. Mechano-sensitive tissues
Mechano-sensitive tissues are constantly subjected to external mechanical stresses generated by gravity, muscular contractions, and fluid flows. When the tissues are under mechanical stresses, cells and tissues adapt to their microenvironment by altering the expressions of specific ECM proteins. For example, lack of mechanical stimulus due to microgravity and prolonged bed rest induces atrophy of muscle, tendon, and bone [116,117]. On the other hand, mechanical overload on cardiac myocytes caused by high blood pressure results in pathological cardiac hypertrophy [118]. In addition, mechanically stressed dermal fibroblasts have been shown to differentiate into myofibroblasts expressing-smooth muscle actin [119]. These physiological evidences confirm that various tissues in our body are subjected to mechanical stimulations. Recently, nanotechnology research has been geared toward the fabrication of nanotopographical substrates with physiologically relevant mechanical stimuli to elucidate the role of mechanical forces on cell and tissue behavior [44,120]. These fabricated surfaces are utilized to simulate in vivo environments in a laboratory setting for the elucidation of the effects of specific ECM protein structures that are involved in mechanical stimuli [44].
A key feature of mechanosensitive tissues is that they convert mechanical signals into biochemical responses [121,122]. Many critical mechanosensitive molecules and cellular components, such as cadherins, growth factors, receptors, myosin motors, integrins, cytoskeletal filaments, stretch-activated ion channels, caveolae and other structures and signaling molecules have been identified as key molecules involved in mechanotransduction at the cellular levels. However, little is known about how these different molecules constructively function within tissue structures to produce the orchestrated cellular behavior required for mechanosensation [123]. It is well understood that ECMs function as force conduits in tissues. In addition, integrins have come to be viewed as critical mechanoreceptors within cells to translate mechano-stimulations. The ECM dynamic and matrix stiffness, through integrin receptors, are translated into cytoskeletal tensions. For example, cell traction forces in vivo are transmitted through integrins and they are resisted by the elasticity of the ECM. Several in vitro studies have confirmed the existence of traction forces experienced by cells through the use of elastic substrates that mimic ECM rigidity [124]. The process of integrin-mediated mechanotransduction relies on the ability of the proteins of focal adhesions to change their chemical activity state when physical forces are applied. Several studies have examined cellular focal adhesion behavior using a range of nanometric scale substrates with shallow pits and grooves via photolithography [125], direct laser irradiation [126], and EBL [127]. A key consequence of nanogroove substrates for the study of cell interface is that nanogrooved surfaces may induce enhanced tissue organization and facilitate active self-assembly of ECM molecules to further mediate cell attachment and orientation. Nanogroove features have been shown to modulate protein adsorption and integrin binding. Furthermore, nanogroove substrates also influence the orientation of focal adhesion and fibrinogenesis. However, recent evidences reveal that at the single cell level, cells’ immediate responses to the stiffness of their environment are independent of cell–substrate or cell–ECM forces [128]. Since tissues are interconnected with ECMs and cells, overall interplay and mechanisms of mechanotransduction at the tissue level still need to be elucidated.
4.2.1. Ligament/tendon
Almost all connective tissues are under mechanical tension, even at rest. Ligaments and tendons are fibrous connective tissues that connect bones to other bones and muscles to bones, respectively. They are comprised mainly of hierarchical collagen fibers, and fibroblasts within these ECMs are exposed to dynamic stress, and structural properties of the ligament are influenced by these forces. Ligaments and tendons have an extremely poor intrinsic capacity to regenerate, and even those that do regenerate have significantly inferior biochemical and biomechanical properties in comparison to healthy native tissue [129]. In ligaments, fibroblasts are aligned along the collagen fibers in the longitudinal direction of the tissue and in the principal strain direction. Ligament and tendon cells respond to stretching and other mechanical loads by increasing their level of intracellular calcium, and these cells release ATP in response to mechanical stimulation. Since physiological strains are involved in the maintenance of ligament function, several research projects have been conducted to elucidate mechanical stimulation in the development of an engineered ligament construct [130]. Indeed, several studies show that the rate of collagen synthesis and fibroblast differentiation from mesenchymal stem cells (MSCs) is enhanced by mechanical stresses [131].
For the regeneration of ligament and tendon, several options including autografts, allografts, and xenografts have been utilized for the repair or replacement of damaged tissues [132]. In addition, a variety of synthetic materials has been utilized for ligament replacements, including polytetrafluroethylene (Gore-Tex), polyester (Dacron), carbon fibers, and ligament-augmentation devices (LAD) made of polypropylene [133–135]. For tissue engineering approaches of ligament and tendon regeneration, modified synthetic biomaterial scaffolds have been developed. These scaffolds have been modified to promote cell attachment and orderly tissue repair. For example, Lin et al. showed that when polyglycolic acid (PGA) sutures coated with PCL were unbraided and seeded with anterior cruciate ligament (ACL) cells, they can support fibroblast attachment and proliferation [136]. Additionally, aligned collagen and silk have been used clinically for decades, and they were subsequently adopted in order to provide the necessary mechanical competence of developing ligaments through in vitro cultivation followed by in vivo implantation [137–139]. Altman et al. have developed a processed silk scaffold that has shown promise as ligament replacement solution, and Teh et al. further utilized silk scaffolds system with knitted and aligned components for effective tendon and ligament tissue engineering [140].
Recognizing the importance of microstructure on cell adaptation, several investigators utilized novel fabrication strategies to produce nanofibers as a ligament/tendon tissue engineering solution [141]. Through the process of electrospinning, fibers with diameters at the nanoscale can be constructed with a variety of different mechanical properties [142]. Aligned nanofibers that are longitudinally oriented support fibroblasts to retain a spindle-shaped morphology and secrete more collagen matrix compared to cells seeded on randomly oriented scaffolds when subjected to uniaxial strain [137,143]. These results indicate that orientation and mechanical cues by scaffolds play an important role in ligament and tendon tissue regeneration.
4.2.2. Bone
Bone tissue engineering is an increasing field of research as the treatment of large bone defects remains an unsolved problem. The most usual strategy is to isolate MSCs or osteoblasts from the patients by biopsy and to grow sufficient quantities of tissue in vitro on implantable, three-dimensional scaffolds for use as a bone substitute. For the scaffold design, substrates with varying surface chemistry and topography have been shown to affect osteogenic differentiation. In recent scaffold designs for bone tissue engineering, researchers have included substrate system with micro-nanoscale topographies to enhance the osteogenic efficiency and bone formation [125,144–148]. For example, Ohgushi et al. demonstrated that bone marrow cells embedded in porous hydroxyapatite (HA) ceramics can induce bone formation at 3 weeks upon implantation, with potential applications to bone regeneration [148].
The bone formation depends on the cooperation of several factors such as soluble bioactive factors, physical microenvironment, and specific cell types. Of these factors, mechanical strain plays a crucial role in bone growth, homeostasis, and repair, where osteoblasts and osteocytes, as mechanosensing cells, direct these processes as can be seen from the work of Jagodzinski et al. where the effect of mechanical stretching was more pronounced than that of the chemical stimulus, dexamethasone [122]. During bone formation, bone mass is retained or increased by the activity of these mechanosensing cells upon mechanical load. In a clinical setting, mechanical stimuli are important local regulators of callus and ultimately bone tissue formation during healing of fracture repair. During orthopedic fracture repair, the mechanical loading environment has been controlled by the use of an external fixator or through defined rehabilitation programs. Recently, artificial mechanical environments have been created via fabrication of osteoinductive scaffolds that release a calcium channel agonist to modulate the mechanosensitivity of osteoblasts [149]. In addition, recently Curtin et al. have introduced highly porous collagen nano-hydroxyapatite (nHA) scaffolds with plasmid-DNA delivery platform for stem cell-based bone formation [150]. nHA particles, which are calcium phosphate minerals, are bioresorbable and non-toxic. In addition, nHA particles exhibit a high binding affinity for DNA due to interaction between calcium ions in the apatite and the negatively charged phosphate groups in DNA [150].
The exact mechanism by which bone adapts to its mechanical environment is still unknown. However, the application of mechanical loads has a beneficial effect on the quality and quantity of in vitro generated bone tissues. For example, a study on mechanical loading on osteoblasts in three-dimensional collagen scaffolds under mechanical stress reported that static forces increased cell proliferation and promoted the expression of osteoblastic differentiation markers, such as alkaline phosphatase and osteocalcin [151]. Alternatively, dynamic stresses enhanced the osteocalcin expression but inhibited cell growth and alkaline phosphatase expression [151]. Tanaka et al. also reported that mouse osteoblasts seeded in three-dimensional collagen scaffold under a uniaxial mechanical strain reduced the proliferation rate, while the expression of osteocalcin was increased [152]. Recently, several in vitro studies with microfabrication tools demonstrated that cell shape, independent of soluble factors, has a strong influence on the osteogenic differentiation and commitment of stem cells [93,153,154].
In terms of substrate design and stem cell differentiation, hydrophobic nanopillar structure and 400 nm dot patterns showed enhanced osteogenic differentiation of mesenchymal stem cells [155,156]. Wilkinson et al. have shown that microtopographical features with nanoscale cues mimicking osteoclast resorption pits were used to promote in vitro bone formation without any osteogenic additives in the medium [157]. Similarly, Dalby et al. reported that the osteogenic differentiation and self-renewal of MSCs are mediated by nano patterned substrates [144,145,158] (Fig. 3). When human MSCs (hMSCs) were maintained on dot patterns and displaced randomly by up to 50 nm on box axes from their initial position, the cells showed more osteogenic differentiation while self-renewal of MSCs was evident on the substrates with ordered nano patterns. Recently, it is clearly evident that cellular interactions at the substrate interface and the induction of focal adhesion formation play central roles in the process of osteospecific differentiation and subsequent osteogenesis. The fabrication of complex three-dimensional biomedical devices to include nanoscale feature, however, is a complicated process associated with low reproducibility. Nevertheless, these nanoscale features have provided valuable information regarding specific ECM-mediated osteogenic differentiation and bone formation.
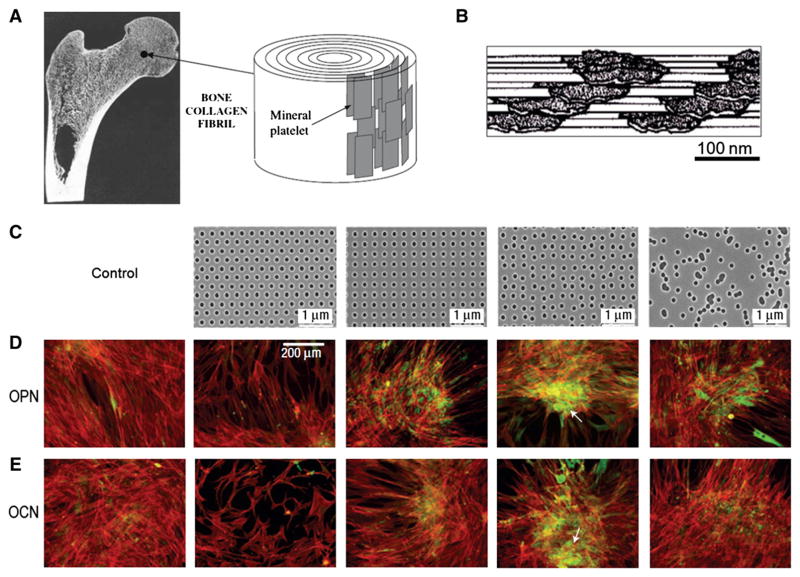
Fig. 3.
(A) The nanostructures of cortical bone composed of plate-like mineral crystals with a dimension of 2–4 nm-thick and up to 100 nm in length. (B) Cross-sectional view of bone structure. The plate-like crystals are embedded in collagen matrix with the volume ratio of mineral to matrix on the order of 1:2, demonstrating stratified stacks with slightly dislocated centers. (C) Nanopit arrays fabricated by nanoimprint lithography with poly(methyl methacrylate) (PMMA). The feature size is 120 nm in diameter, 100 nm in depth, 300 nm center-to-center spacing with arrangement of hexagonal, square, displaced square (±50 nm from true center) and random. (D–E) Osteopontin (OPN) and osteocalcin (OCN) of osteoprogenitors after 21 days of culture. Dense aggregates and enhanced OPN and OCN levels were observed in displaced square 50 (±50 nm from true center ) substrate (fourth column of C, D and E).
A is reprinted with permission from ref. [20]. B is reprinted with permission from ref. [19]. C, D and E are reprinted with permission from ref. [127].
4.3. Electro-active tissues
Electro-active tissues refer to those that can receive, transfer and even react with electrical signals. The electro-receiving tissues include various perception organs such as ganglion cells in the retina, mechano receptors such as the Meissner corpuscle and Ruffini endings in skin, and the cochlear cilia in inner ear. In these tissues, external stimuli are converted into electrical signals called ‘action potentials’ which are electrochemical pulses that propagate within neural and motor cells. When the action potential is generated, it is progressively transmitted along the electro-transferring nerve network which includes the PNS and CNS. Finally, the transferred information to the brain may activate certain organs such as skeletal muscle (voluntary) or heart, and the gastro intestinal tract (involuntary).
Due to the inherent environment in vivo, some of these tissues have shown distinctive responses to the applied electrical signals in vitro. For example, inspired from the inherent electric field existing in the corneal epithelial layer, Song and Zhao demonstrated that an externally applied electric field could accelerate wound closing or opening depending on the field direction [5,159]. Furthermore, Langer and coworkers demonstrated longer neurite length and increased proliferation on electrically conductive substrate [160]. In the case of skeletal muscles, myocyte showed rapid myotube formation on electrically conductive substrates even in the absence of electrical stimuli [161]. Cardiomyocytes cultured under electrical stimulus demonstrated higher ultrastructural formation and cell–cell coupling at 8 days of culture [162,163]. As can be seen from these results, electrical cues have demonstrated a significant role in tissue regeneration. Since the electrical functions of previously described tissues are subjected to the physical nanostructures in them, they must have well organized structures for effective electrical signal transfer such as unidirectional contraction of muscular tissues.
Although some electro-active tissues contain well organized nano-structures, the clinical or engineering approaches were not guided by nanotopography. For example, as the retina, responsible for receiving optical stimuli and transforming it into electrical signals, has well aligned cellular morphologies guided by RGCs, it has been widely studied with the addition of electronic devices such as imaging sensors [164] or cell sheet engineering without introduction of nanotopography [165]. The cochlear cilia in the inner ear detects motion as relative ciliary motions which may be regulated by the opening and closing of ion channels and its nanostructures are not easily replicated with topography. Furthermore, some other tissues have been treated in a macro-scale manner due to the large overall feature size. For instance, the PNS can play a critical role in signal transfer from and toward the spine, and has a relatively large size compared to the CNS (saphenous nerves have diameters of up to 2–3 cm and a length of ~40 cm) [166]. Therefore, it could be usually reconnected end-to-end with surgery when damaged. For these reasons, in this section the electro-active tissues will be confined into three organs: the heart, skeletal muscle and neurons in the brain.
4.3.1. Heart
Critical factor to heart function is the structural organization of heart tissue, specifically the elongated and anisotropic nature of cardiomyocytes which allows for anisotropic heart contraction and action potential propagation [167]. Consequently, cardiac tissue engineering seeks to engineer physiological heart tissue, which demonstrates elongated and well aligned cardiomyocytes, cell-dense monolayers, matured structural organizations such as striated sarcomeres, aligned actin cytoskeleton, and connexin43 expression at cell–cell contacts, and synchronous, anisotropic contraction as well as anisotropic action potential propagation [168]. Although many approaches have been taken to generate physiological cardiac tissue, topographical effects have increasingly come into focus as a critical aspect of the cardiac microenvironment which can modulate a variety of cell functions. As a result, micro- and nanofabrication techniques have emerged as robust and inexpensive methods for recapitulating the topography of the cardiac microenvironment to control cardiomyocyte morphology and function.
Early microfabrication-based cardiac tissue engineering approaches utilized photolithographic techniques to create anisotropic cardiac tissue. Rohr et al. used photolithography to pattern lines of photoresist on glass which prevented cell attachment, thus generating lanes of neonatal rat ventricular myocytes (NRVMs) of varying widths, down to 65 μm on glass. The NRVMs demonstrated a more elongated morphology in the narrowest lanes [169]. Thomas et al. used the same techniques and found that NRVMs grown in narrower lanes also demonstrated greater elongation (elongation ratio: ~4) and cardiomyocytes grown in lanes generated faster propagation of action potential than NRVMs cultured on flat polystyrene surfaces [170]. McDevitt et al. used μCP of laminin lines varying from 5 to 50 μm in width which were printed on non-adhesive polystyrene surfaces (preincubated with 1% bovine serum albumin, BSA) or spin-coated on PLGA biodegradable surfaces [171]. Cultured NRVMs also demonstrated elongated myocyte morphology, upregulation of connexin43 at cell–cell borders and intercalated disks, and synchronous contraction if the distance between the NRVM lanes was small enough [171]. From these initial studies, it has become apparent that certain microfabrication techniques could be used to control the morphology and organization of myocytes.
Despite these initial advances, as cardiac tissue is extremely cell-dense, methods of controlling cardiomyocyte morphology and organization without restricting tissue formation are more desirable. Bursac et al. used microabraded polyvinyl chloride (PVC) cover slips with microgrooves ranging from 0.5 to 5 μm-wide and 0.2 to 2 μm-deep as well as microcontact printing with 12 or 25 μm-equally-spaced fibronectin lanes to culture NRVMs. Both techniques demonstrated elongated, well aligned cardiac tissue, as well as anisotropic electrical properties. Specifically, longitudinal to transverse conduction velocity ratio, a measure of the anisotropy of the action potential, increased for patterned surfaces [172]. Confluent, cell-dense tissue was best achieved by the abraded surfaces. Other microgrooved surfaces have been used to affect cardiomyocyte size as well as connexin43 and n-cadherin expression in addition to cell orientation and alignment [173–175]. Functionally, microgrooved surfaces have also been shown to alter intracellular calcium dynamics as well as expression and regulatory properties of voltage-gated calcium channels [176,177]. Au et al. combined microgrooved surfaces with electrical field stimulation in parallel and perpendicular to the groove directions. When the orientation of the microtopography and the electric field is orthogonal, NRVMs aligned to the topographical cues rather than the electrical field direction. On the other hand, cells cultured on microgrooves parallel to electrical stimulation demonstrated increased elongation, alignment, and some increased electrical functionality [178]. These approaches have sought to combine multiple aspects of the cardiac microenvironment, but still suffer from scaffold non-uniformity and lack of topographical stimuli which are more biomimetic and thus on the nanoscale level.
Another technique which has been used to generate biomimetic scaffolds for cardiac tissue engineering is electrospinning. This approach is versatile as it offers the advantage of porosity for increased nutrient and waste exchange, smaller and tunable feature sizes that are more similar to ECM fiber dimensions, and the ability to generate aligned fiber scaffolds similar to the native ECM structure of the heart [179]. Shin et al. first used electrospun PCL scaffolds with fiber diameters ranging from 100 nm to 5 μm and found excellent NRVM attachment. Generated cardiac tissue was cell-dense, showing synchronized contractions [180]. Due to the porosity of the scaffolds, these cardiac monolayers could be stacked up to 5 layers thick without core ischemia [181]. However, although these scaffolds incorporated ECM-like structures and formed cell-dense tissues, they still lacked the anisotropic nature of native heart tissue. Zong et al. used a post-processing of uniaxial stretching to align and orient electrospun biodegradable fibers with an average diameter of 1 μm. NRVMs cultured on aligned fiber scaffolds demonstrated elongation and alignment along the orientation of the fibers [182]. Other techniques, such as rotary jet spinning, have also been used to create aligned fiber scaffolds which could demonstrate similar effects on cardiomyocytes [183–185].
Rockwood et al. used aligned and random electrospun polyurethane scaffolds with an average fiber diameter of 2.0 μm and showed a decrease of atrial natriuretic peptide (ANP) expression of NRVMs cultured on electrospun scaffolds versus tissue culture polystyrene (TCPS) controls [186]. Here, a decrease of ANP is indicative of a more mature cardiomyocyte phenotype. More interestingly, aligned scaffolds showed a lower level of ANP relative to both random electrospun scaffolds and TCPS [186]. Aligned fiber scaffolds also have anisotropic mechanical properties with differential stiffness in the radial and circumferential directions [187]. This is similar to the mechanics of native heart tissue [188]. More recent advances of electrospun scaffolds include incorporation of nanofibers into collagen gels to increase cardiomyocyte attachment and mechanical properties of construct [189]. Furthermore, incorporation of bioactive [190,191] and electroconductive materials in the fiber polymers could modulate cell function and connexin43 expression [192]. To overcome diffusion limitations of 3D cardiac tissue generation, stacked, aligned fiber mats with a macroscopic biodegradable nutrient delivery system were also reported [193]. These advances are exciting but still suffer from certain limitations, namely insufficient cell characterization, reproducibility, scalability and a limited amount of materials compatible with the spinning process.
Other nanoscale approaches have examined alternatives to electrospinning to generate nanostructured scaffolds with various materials or utilize alternative techniques with greater controllability over scaffold design. Feingberg et al. used surface-initiated assembly on poly(N-isopropylacrylamide) [p(NIPAAm)] surfaces to create nanofabrics of ECM proteins, such as fibronectin, laminin and collagen. Depending on the protein, fabrics could form nanofibers<100 nm in diameter. Cardiomyocytes seeded onto generated nanofabrics adhered well, elongated and aligned to the underlying matrix [194]. Luna et al. used highly anisotropic wrinkles as a cell culture platform with periods ranged from 400 nm to 50 μm. Cultured NRVMs and embryonic stem cell-derived cardiomyocytes (eSC-CMs) showed alignment, elongation and upregulation of connexin43 at cell–cell contacts [195].
Kim et al. has used CFL to fabricate a number of well-defined nano-structured polymer substrates. They first demonstrated that NRVMs adhered more robustly to nanopillar polyethylene glycol (PEG) scaffolds having nanopillars (diameter of 150 nm, height of 400 nm) than flat PEG surfaces [196]. These nanopillars also promoted self-assembly of three dimensional aggregates of NRVMs. Kim et al. also demonstrated that using UV-assisted CFL, they could fabricate well-defined nanogrooved PEG substrates which could mimic ECM fiber dimensions (PEG scaffolds containing well-defined arrays nanogrooves with various sizes from a width of 150 nm, a spacing of 50 nm and a height of 200 nm to a width of 800 nm, a spacing of 800 nm and a depth of 500 nm) (Fig. 4). NRVMs cultured on these surfaces not only demonstrated elongation and alignment to the nanocues and formed a cell-dense monolayer, but also had anisotropic contractions and action potential generation as well as connexin43 upregulation. Further, the cells also demonstrated different sensitivities to the nanogroove dimensions. For example, the NRVMs cultured on 800 nm-wide and 800 nm-spaced scaffolds showed larger size, highest connexin43 expression upregulation and the fastest longitudinal conduction velocity [197] than other nanopatterned and flat PEG scaffolds. As a versatile nanofabrication technique, CFL has shown high controllability and reproducibility in a wide range of studies examining the precise relationship between nanotopography and cardiomyocyte function and development [69].
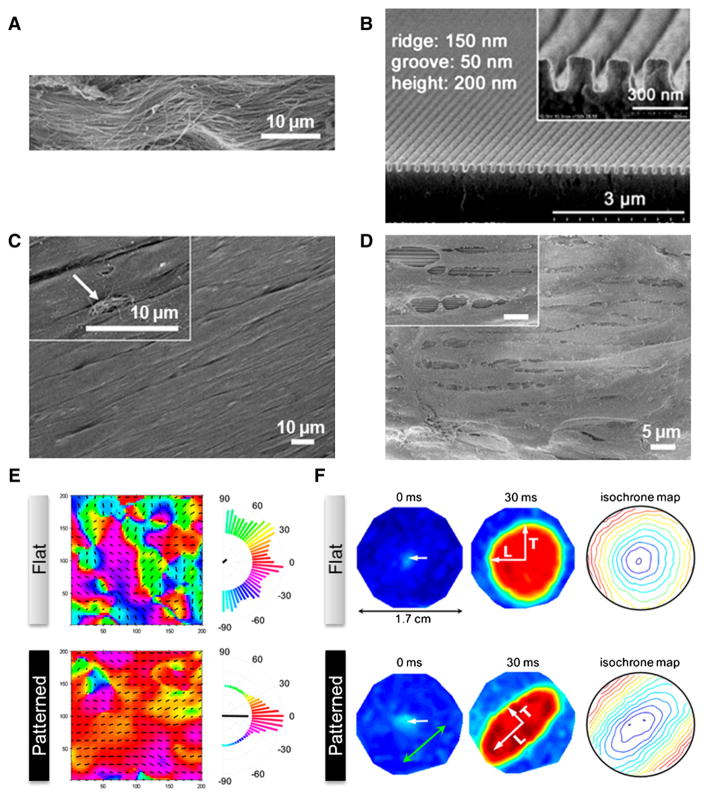
Fig. 4.
(A) Scanning electron microscopy image of ex vivo myocardium of adult rat heart. Unidirectionally organized matrix fibers were observed. (B) Heart-inspired nanopatterned substratum made of polyethylene glycol (PEG) hydrogels. (C) Magnified view of well-aligned rat myocardium. (D) Scanning electron microscopy image of NRVMs cultured on anisotropically nanofabricated substratum, showing similar morphology to (C). (E) Contraction map of NRVM monolayers cultured on flat and nanopatterns. Unlike the random contraction on flat substratum, NRVMs cultured on anisotropic nanopattern demonstrated unidirectional contraction, which is similar to real heart. (F) Action potential propagation analysis of NRVMs cultured on flat and anisotropic nanopatterns. Point stimulation of 3 Hz in 0 ms (indicated by white arrows) propagated anisotropically on cells cultured on nanopatterns, while isotropic propagation was observed in the flat case.
Reprinted with permission from ref. [167].
4.3.2. Skeletal muscle
Skeletal muscle generates force through contraction of the striated multinucleate myotubes that span the length of the muscle. These myotubes have a distinct directionality and are arranged in a parallel array, allowing for force propagation along a common axis [198]. In the mature skeletal muscle tissue, myotubes mature into myofibers, which are arranged in bundles. These fibers are surrounded by a thick ECM layer termed the perimysium [199]. The perimysium consists of collagen fibers that are organized into discrete populations which can extend along and across muscle fibers. Transverse collagen fibers interconnect muscle fibers at discrete points termed perimysial plates, and thick bands of collagen have been observed to run longitudinally along the muscle fibers, although the precise relationship between ECM–cell interactions are still poorly understood [199]. Tissue-engineered skeletal muscle must mimic the organization and physiological function of skeletal muscle. To this aim, previous studies directed the fusion of muscle precursor cells (myoblasts) into myotubes. Although myoblasts can be cultured and differentiated under typical culture conditions in vitro, the resulting myotubes lacked morphological organization, resulting in less functional tissues [198]. Thus, micro and nanofabrication strategies have been employed to direct the formation of organized, functional myotubes and skeletal muscle in vitro.
Parker et al. used μCP of ECM islands to determine whether constraining cell shape and cell traction forces could alter cell migration characteristics. C2C12 mouse myoblasts cultured on square 50×50 μm2 ECM islands formed microspikes (a cell process which helps drive cellular motion) at the corners of the ECM island, whereas cells cultured on symmetrical 50 μm diameter circle ECM islands extended cell processes at random [200]. This study showed that the resulting traction forces from a cell’s microenvironment could be used to direct cell migration. Clark et al. used photolithography and ECM precoating to create lanes of varying widths (5–100 μm) of laminin on glass. Although cell density also plays a role in myotube formation and fusion, H2Kb myogenic cells plated at high density responded to the laminin pattern and aligned themselves to the lanes. Alignment was enhanced when conditions were switched to induce differentiation and after myotube formation, supporting the hypothesis that the prealignment of myoblasts can enhance aligned myotube formation [201]. Interestingly, wider lanes did not result in wider myotube formation, but instead multiple well-aligned myotubes of a fixed width [201].
Other studies to examine the importance of contact guidance in myoblast alignment and myotube formation utilized traditional microfabrication techniques, such as photolithography, hot embossing and soft lithography to create microtopographical cues. Evans et al. used photolithography to pattern 5–100 μm wide channels of varying depths (deep: 2–6 μm, shallow: 0.04–0.14 μm) on glass and cultured fetal and neonatal murine myoblasts on these surfaces. Both neonatal and fetal myoblasts demonstrated a high degree of alignment across all groove widths in the deep microchannels. However, in shallow microchannels only fetal myoblasts responded to the topographical cues, demonstrating that structural elements in the cellular microenvironment play a role in cell positioning and alignment as well the stage of cell maturity [202]. Many subsequent studies sought to explore the sensitivity of myoblast to microenvironment dimension. Clark et al. found that nanogratings (width of 130 nm, spacing of 130 nm and depth of 210 nm) could induce alignment of H2Kb myoblasts, while myotube formation was inhibited [203]. Along with earlier results on microtopography, such nanogratings may actually inhibit myoblast reorganization as the substrate’s topography inhibits lateral migration of the cells and prevents end-to-end coupling of myoblasts [201,203]. Conversely, channels too wide (400 μm) have also only demonstrated short-range ordering of C2C12 myoblasts [204], due to perhaps features too large to restrict random orientation of myoblasts. Altomare et al. compared the effects of microcontact printed ECM with equally sized microgrooves, showing no significant difference in the alignment of C2C12 cells [205].
Further studies have shown enhanced alignment of both primary and C2C12 mouse myoblasts in deeper microgrooves of narrower widths (5 μm depth, 5–10 μm width) [206], C2C12 myoblasts and myotubes in rougher microabraded surfaces [207], in shallow wavy microgrooves (3–6 μm width, 0.4–0.7 μm depth) [208], and in deep narrow microchannels (2 μm width, 7 μm depth) [198]. Other micropatterns, such as square and diamond posts, did not inhibit the alignment of myoblast and myotube. Instead, myotubes were aligned at differing angles to the lattice pattern depending on feature size for myoblasts maximizing the contact area between the cell and the topographical surface [209]. In the study examining C2C12 cells cultured on microabraded surfaces, the expression levels of skeletal muscle specific proteins did not significantly differ between the culture conditions, suggesting that the differentiation of C2C12 myoblasts was not influenced by topographical features [207]. These studies all indicate that a specific range of topographical dimensions can profoundly affect myoblast alignment, and myotube formation and alignment.
More recent works investigating the effects of submicron and nanoscale topography on skeletal muscle have helped to elucidate cell–topography interactions. Huber et al. used electrospinning to fabricate aligned nylon 6/6 fiber scaffolds consisting of ribbons 1.5 μm×400 nm in size. C2C12s cultured on the aligned fiber scaffolds were organized accordingly to the fiber direction forming a continuous sheath, and differentiated into myotubes [210]. This approach is unique in that aligned myotubes were formed without the need for confinement of the cells, resulting in a continuous tissue. Wang et al. cultured C2C12 cells on nanogrooved substrates (450 and 900 nm width) of varying depths (100, 350 and 550 nm) and observed elongation and alignment of C2C12 to topographical cues across all substrates. Further, proliferation of C2C12 cells was inhibited and differentiation into myotubes was enhanced on grooved substrates, and C2C12s responded differentially to pattern depth, exhibiting more elongated structures in deeper grooved substrates [211]. The elongation and alignment of C2C12 myoblasts and myotubes have also been shown in oriented small diameter (10–15 nm) carbon nanowhiskers [212]. Huang et al. used uniaxial stretching of electrospun poly-L-lactic acid (PLLA) scaffolds to produce oriented 500 nm diameter fiber scaffolds and compared the topographical effects on the nanoscale (electrospun PLGA nanofiber with diameter of 500 nm) to microgrooved (patterned PDMS with width/space of 10 μm width and depth of 2.8 μm) scaffolds. Although both scaffolds demonstrated elongation and alignment to the topographical cues, the nanofibers promoted longer myotube formation and inhibited cell proliferation compared to flat controls and micropatterned substrates [213]. Further exploration of micro- to nanotopographical effects on skeletal muscle cells may elucidate the crucial process in the ECM–cell interactions found in native perimysium.
4.3.3. Neurons
On the cellular scale, the nervous system consists of neurons which transmit electrochemical signals, and glial cells which support neurons. Neurons are comprised of a cell body (or soma), an axon (extended long structure to transmit signals to the synapse, the junction between neurons), and dendrites (branched structure around the cell body for receiving signal from other neurons at synapse), and axon and dendrites in vitro called neurites collectively. Some axons are covered by a myelin sheath, an insulator helping fast action potential transfer.
The center of the nervous system, the brain, takes charge of perception and information processing, controls most of motions and behaviors in body, and maintains homeostasis. The significant functions of the brain require electrochemical signal transfer mediated by neurons; therefore, the specific connections between neurons are very important. For the efficient connection and thus effective neuronal process, neurite outgrowth is a fundamental phase in which undifferentiated neurons extend to neurites before differentiating to axons and dendrites subsequently. During neurite outgrowth process, neurons extend leading tips called growth cones, and the growth cone senses the extracellular chemical, mechanical, and topographical environments, which guide the directional structure and movement of an axon and dendrites [31].
Precise understanding of extracellular cues influencing neuronal growth is not only crucial for research into development of neurons but also for designing scaffold for nerve tissue regeneration with knowledge about alignment and migration of neuronal cells. Traditionally, chemical cues for guidance of growth cones have received much attention. There are two types of chemical cues: soluble and bounded factor. Gradients of netrins (a class of proteins related to axon guidance), growth factors, and neurotransmitters are representative soluble factors which work changeably at long distance, while proteins fixed on ECM or neighbor cell membrane are bounded factors which work stably at short distance [31,214].
The bounded factors are important to maintain the orientation of neurons due to their fixed and stable properties. Additionally, some bounded factors such as ECM molecules or cell membrane forms highly organized structures at the nano or microscale level in vivo [50,215–221]. These topographical stimuli have been shown to guide neuronal growth independent of chemical cues. For example, Ono and Kawamura observed migratory streams of neuronal cells associated with tangentially oriented fiber in the fetal mouse in vivo [215]. Assembled and polymerized structures of laminin, an ECM protein, are also related to neuronal growth [31,50,216,217]. In addition, topography of RGC [218], and pioneer axons [219] also guides alignment, migration, and directed process of neurons in vivo. Stepien et al. reported that axons extended on the compact layer of underlying aligned skin fibroblasts more clearly than flat collagen coated surface [220]. Neurite outgrowth on topography which mimics the cell membrane surface has been reported [221]. The stiffness of ECM which can be regulated by topography has also been related to neuronal differentiation [222] and neurite branching [223,224]. To mimic the anisotropic structures of brain cells, various synthetic nanostructures, mainly unidirectionally aligned fibers or grating patterns, have been fabricated by self-assembly [225–228], electrospinning [229–239], nanolithography [240–247], and other techniques [248,249]. On these artificial structures, unique and remarkable behaviors of nerve cells, such as neuronal differentiation, neurite outgrowth, alignment, and migration have been reported.
Neuronal differentiation is a representative example that shows the effect of topography on stem cells. Silva et al. reported differentiation of neural progenitor cells on self-assembled laminin-mimicking nanofiber [225]. The neuronal differentiation of stem cells on other self-assembled peptide nanofibers or aligned electrospun nanofibers made from various materials have also been investigated [228–230,236, 238,248]. Further, on specific nanostructures, stem cells differentiate into nerve cells even in the absence of inducing agents [229,240,241].
Yim et al. demonstrated higher up-regulation of neuronal markers [microtubule-associated protein 2 (MAP2)] on the hMSCs cultured on 350 nm-wide nanogrooves compared to flat and micropatterns. Such expression of MAP2 neuronal marker showed increasing tendency as the feature size decreases (300 nm>1 μm>10 μm>flat). Interestingly, when comparing the relative importance, nanotopography alone showed a stronger neuronal marker expression compared to retinoic acid alone on flat substrate [240]. Lee et al. also reported the directed neuronal differentiation of hESCs for the culture period of 5 days on a gelatin coated PUA nanograting patterns (depth: 350 nm, width: 350 nm, pitch: 500 nm) in the absence of differentiation-inducing agents [241]. Xie et al. showed differentiation of RW4 embryonic bodies (EBs) into neuronal lineage cultured on electrospun PCL nanofibers. Tuj1 (for neurons) and O4 markers (for oligodendrocytes) were more expressed on aligned fibers compared to random fibers, while GFAPs (for astrocytes) were less expressed in the aligned case than in the random case. This result suggests that the alignment of nanofibers could promote differentiation into neuronal lineage while suppressing the differentiation into astrocytes. Since the spinal cord injury is frequently not regenerated due to the glial scar formation, these results are potentially useful for suppressing glial cell differentiation while promoting neuronal regeneration [229].
Unlike the nanotopography, large microtopography itself does not seem to be sufficiently effective for neuronal differentiation. Recknor et al. reported that neuronal differentiation of rat hippocampal progenitor cells (AHPCs) showed no significant difference both on microgrooved (width: 13 μm, spacing: 16 μm, depth: 4 μm) and flat PS substrates [250]. A subsequent study by Oh and Recknor also reported no significant difference of Tuj1 expression between micropatterned and flat surfaces [251]. Beduer et al. also compared various micropatterns (width-spacing: 5 μm–5 μm, 10 μm–10 μm, 20 μm–20 μm, 10 μm–60 μm and control), showing decreased neuronal differentiation of human neural stem cells with respect to the decrease of feature size and even lower differentiation on micropatterns compared to controls. The authors suggested that the micropatterns similar to the diameter of soma might potentially confine the cellular behavior such as neurite extension, and thus reduced differentiation [252]. Based on the observation by Yim et al. [240] that no significant neuronal differentiation was reported on 10 μm patterns as compared to flat controls, micropatterns over 10 μm may not be effective for the neuronal differentiation.
Interestingly, when co-cultured with astrocytes on micropatterns larger than 10 μm, the neuronal differentiation was enhanced presumably due to the astrocyte-alignment-induced soluble factors [250,251]. Such astrocyte-induced effect was also reported in nanoscale topography. For example, Delgado-Rivera et al. co-cultured neurons and astrocytes on polyamide electrospun nanofiber and reported enhanced neuronal outgrowth by fibroblast growth factor-2 (FGF-2) secreted by astrocytes [234].
As described above, synthetic topography has been utilized for aligned growth of neurons, which is potentially important for neural regeneration [227,242–246,249]. Clark et al. investigated the behavior of growth cone guidance on various sizes of microscale laminin lines [(i) isolated single 2 μm line, (ii) repeated lines with 1:1 width and spacing ratio, size of 2–25 μm] fabricated by photolithography [242]. Neurite extension of neurons was highest on an isolated single 2 μm line, while showing no extensions on flat surfaces and 2 μm-wide repeated line patterns. As the width and spacing of line increases, the alignment of neurite outgrowth became more bipolar, in contrast to the multipolar morphologies on the control surface. These results suggest that not only laminin itself but also the patterns of laminin affect the growth cone guidance. Additionally, Dowell-Mesfin et al. observed orientation and growth of hippocampal neurons with immunostaining of tubulin and MAP-2 protein on poly-L-lysine coated silicon wafer with a 1 μm-high pillar pattern with widths of 0.5 and 2 μm and spacing of 1–5 μm [243]. Jang et al. cultured neurons on laminin coated PUA nanoline pattern (pitch: 350 nm and width: 1, 2, 3, 5 times of 350 nm, depth: 350 nm) fabricated by CFL for revealing the cellular mechanism about neurite outgrowth on nanotopography [244]. They observed nanopattern-induced stabilization of filopodia at growth cones with F-actin-rich cytoskeleton and steady neurite extension, while only unstable motions of filopodia were observed on the flat surface. The authors suggest a model of neurite guidance with two distinct filopodia populations stochastically sensing ECM nanotopography.
In combination with various biochemical cues, nanotopography can exert a synergic effect to nerve cells [229,231,232,235,240]. As mentioned previously on the neuronal differentiation, differentiation-inducing agents, when combined with nanotopography, can give higher differentiation efficiency [229,240]. In the case of neuronal growth, such combination of topographical and chemical cues turned out to be promising. For example, Wittmer et al. entrapped brain-derived neurotrophic factor (BDFN) and ciliary neurotrophic factor (CNTF) to the electrospun silk fibers. Cultured rat retinal ganglion cells (RGCs) showed 2-fold enhancement of neurite length on BDFN-containing nanofibers, 2.5-fold on CNTF-containing nanofibers, and 3-fold on containing both factors [235]. Unlike the mixing of growth factors, Ahmed et al. covalently modified polyamide nanofibers with neuroactive peptides derived from human tenascin-C. These modified nanofibers showed enhanced nerve cell attachment, neurite generation, and extension [231]. Some studies compared the immobilization effect on neurite growth and neural differentiation. Patel et al. immobilized extracellular matrix protein (laminin) and growth factor (basic fibroblast growth factor, bFGF) on electrospun PLLA nanofibers. Although neurite extensions were increased both in the aligned and random cases, the neurites were much longer on the aligned matrix fibers. Interestingly, when comparing the immobilized bFGF with that added to the media, neurite extension was higher in the bFGF-immobilized nanofibers [232]. Such immobilization effect also can be observed in neuronal differentiation. Cho et al. utilized nerve growth factor (NGF)-conjugated electrospun PCL-PEG nanofibers for the rat MSCs. According to the relative gene expression, neuronal differentiation was higher on growth factor-conjugated nanofibers compared to the growth factor-soaked fibers, with a higher alignment factor [239].
Since the neuron is electrically active, the effect of electrical stimulation has long been of interest. To examine the effect of electrical stimuli in synergy with topography, several studies have been reported. Lee et al. cultured PC12 cells on polypyrrole (PPy, typical conducting polymer)-coated PLGA nanofibers with aligned and random morphologies. Cells not only showed longer neurite extension on aligned nanofibers compared to random fibers, but they also demonstrated much longer neurite outgrowth when electrically stimulated at 10 mV/cm. Also the portion of neurite-bearing cells showed a similar trend, being higher in the stimulated and aligned case [233]. Lee et al. electrospun piezoelectric polymer polyvinylidene fluoride–trifluoroethylene (PVDF–TrFE) at the nano- and microscale, which were thermally annealed. Since piezoelectric properties were enhanced after annealing, longer average neurite length was shown, both at nano- and microscale levels [237].
Unlike the PNS system, the repair of the nerve system of the brain is challenging due to the complex structure and isolated injuries in the brain environment. To overcome several problems in the neural regeneration such as scar tissue formation, gap formation during phagocytosis, and failure of initial neurite extension, nanofibrous scaffolds can be used as an efficient tool [226]. Park et al. utilized PGA-based scaffold containing small PGA fibers with diameter of 10–15 μm in a woven geometry. When implanting neuronal stem cell (NSC) seeded PGA-based scaffold into the infarction cavities of mouse brain, reduced parenchymal loss, connection of meshwork of host– and donor–donor derived neurons and anatomical connections were shown [253]. Ellis-Behnke et al. transplanted self-assembling peptide nanofiber scaffold in a severed optic tract in the hamster, and demonstrated functional return of vision through the axon reconnection [226].
4.4. Shear stress-sensitive tissues
In the human body, certain tissues are continuously exposed to fluidic conditions such as the kidney, lung and vascular systems. Since these tissues actively react to changes of fluidic shear stress, they can be classified as shear stress-sensitive tissues. The main purpose of shear stress-sensitive tissues is exchanging various byproducts with nutrients and oxygen by diffusion, transporting them through or out of the circulatory system, and actively absorbing diffused ions by active transportation. Usually, the shear stress-sensitive tissues have a large surface area of tissue–vascular interface for efficient material transport. Since these shear stress systems influence overall transport of various factors, degradation of such tissues may severely threaten health. For example, renal failure which is described as a decreased glomerular filtration rate can affect blood acidity, ion concentration and even the healing process of other tissues [254]. In the case of renal failure, some nanoporous membranes have been transplanted into human or animal bodies for the treatment of acute or chronic renal failure [255,256]. However, the number of reports is limited and the majority of studies were mainly focused on in vitro hemofiltration system utilizing nanoporous tubes. In the case of the vascular system, numerous studies have been reported utilizing micro- to nanotopography. Here, the blood vessels will be mainly described as a shear stress-sensitive tissue.
4.4.1. Blood vessels
The blood vessels of the cardiovascular system play an important role in exchanging materials such as oxygen, nutrients and metabolites with other tissues. The blood vessels are commonly composed of three layers: intima, media and adventitia. The intima, the innermost layer of the blood vessel, is a monolayer of ECs which is covered with elastic fibers and directly exposed to blood flow. The media is composed of SMCs and elastic fibers, modulating the diameter of blood vessels through communication with ECs. The adventitia is a connective tissue which is responsible for connecting blood vessels and neighboring tissues. The structures of the blood vessel are different depending on the location and role of the blood vessel. For example, the media of arteries has well-developed elastic fibers so that they can handle the pressure created by cardiac contraction. In contrast, veins have less-developed media and well-developed adventitia, which results in low elasticity of the venous wall to store the blood by extension of the lumen.
4.4.1.1. Endothelial cells (ECs)
Because of their anatomic location, ECs are exposed to various hemodynamic forces such as pressure, circumferential stretch and shear stress [41,257]. These forces are created by cyclic cardiac contraction, thus affecting the modulation of the blood vessel structure. Increased circumferential stretch induced by the enhanced blood pressure can result in an increase of wall thickness [258,259]. On the other hand, the diameter of the blood vessels can also be increased by increasing the shear stress [260,261]. Additionally, shear stress plays a crucial role not only in the remodeling of preexisting blood vessels but also in the stabilization of the new blood vessels [262,263]. For example, Meeson et al. demonstrated that low shear stress led to blood vessel regression caused by apoptosis [263].
The mechanism of shear stress sensing has long been of interest to the researchers. To explain the mechano-transduction mechanism of shear stress sensing, presumed shear-sensitive mechanoreceptors have been suggested. Mechanoreceptors are categorized into two types according to location. One is categorized as membrane structures on the apical surface of the ECs such as ion channels [264–267], caveolae [268,269] and G proteins [270]. The other is categorized as proteins and molecules existing throughout the cells such as integrins [271], cell–cell adhesion molecules [272] and adherens junctions [273]. During mechanotransduction, cytoskeletons of the ECs play a role as a medium for transmitting shear stress exerted on apical surface to the mechanoreceptors throughout the cells [274]. However, despite these efforts, mechanotransduction mechanism of the ECs has not been fully understood until recently. Although in-depth mechanisms are not fully revealed, previous studies have shown that the topographical cue may be interpreted into a similar stimulus to ECs since the cellular responses are also mediated by the focal adhesion protein, integrin. It appears that ECs show similar cellular behaviors in terms of alignment, adhesion, and expression of endothelial markers without the assistance of fluidic cues. Some representative examples of topographical effects are as follows.
The performance of synthetic vascular grafts is estimated by their ability to maintain the cellular behaviors in vitro similar to those in vivo. Many projects have investigated the effects of micro/nanotopographical substrates on behaviors of the ECs and feasibility by using them as the vascular grafts. Topography of surfaces is a major factor which affects morphology of the cells by mediating integrin-mediated adhesion through the cytoskeletal reorganization. Most of the ECs on micro/nanogrooves, in particular, are aligned in parallel to the line direction than flat surfaces and formed aligned focal adhesions and cytoskeletons similar to in vivo [275–277]. Recent research by Liliensiek et al. demonstrated the enhanced alignment of four types of the human vascular ECs grown on various nanopatterned surfaces without treatment of ECM proteins [276]. Furthermore, appropriate tuning of surface chemistry, elastic modulus and topography of the line-patterned substrates results in interdependent cellular alignment of the ECs each with the three factors [278].
Prior to further investigation on functions of ECs, adhesion strength of ECs to the synthetic vascular graft must be considered. Initial adhesion of ECs to the cell substrate is determined by the adsorption capability of the ECM proteins such as collagen and fibronectin. Enhanced surface free energy through nanotopography results in enhanced ECM protein adsorption and initial adhesion of ECs [279]. Furthermore, studies on retention ability of ECs against flow based on microfluidic devices including micro [277] and nanogrooves [275,280] confirmed that adhesion strength could be enhanced by topographical influence.
Proliferation and migration are other vital functions which may affect development and wound healing of blood vessels. The effects of micro/nanotopography on proliferation of ECs have been demonstrated by previous studies [276,281–285]. Lu et al. demonstrated that proliferation of rat aortic ECs (RAECs) on titanium micro/nanogrooves with widths ranging from 750 nm to 100 μm was increased with the decrease of the width of groove [281]. In contrast, human vascular ECs grown on polyurethane films with various nanotopographies (400–4000 nm) showed decreased proliferation than flat surface [276]. The migratory behavior of the ECs showed a tendency to be guided by the orientation of micro- and nanogrooves [276,277]. In addition, Uttayarat et al. demonstrated that guided migration of ECs was enhanced under the flow in parallel with orientation of ECs [277].
As well maintained cellular functions, synthesized vascular grafts need to be capable of maintaining the appropriate phenotype of ECs in vitro. Phenotypic maintenance of ECs are typically evaluated by expression of representative endothelial markers such as platelet endothelial cell adhesion molecule-1 (PECAM-1), vascular-endothelial cadherin (VE-cadherin), vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1). Briefly, PECAM-1 plays a role in controlling adhesion between ECs and leukocytes or to adjacent ECs. VE-cadherin mediates intercellular junctions which assist in maintaining the integrity of the endothelial layer. VCAM-1 and ICAM-1 are up-regulated during inflammation. Studies have demonstrated that nanotopography has the ability to maintain phenotypes of ECs [39,286,287]. Ma et al. studied maintenance of phenotype of human coronary artery ECs (HCAECs) grown on random or aligned PCL nanofiber meshes with grafted gelatin on their surface [39]. ECs on aligned PCL nanofiber meshes showed an aligned morphology in parallel with nanofibers and expression of three endothelial markers (PECAM-1, ICAM-1 and VCAM-1) which represented phenotypic maintenance in vitro. He et al. demonstrated phenotypic maintenance as well as ECM remodeling capacity by examining the HCAECs grown on poly(L-lactic acid)-co-poly(ε-caprolactone) [P(LLA-CL)] nanofiber meshes [287,288].
Capillary formation of ECs is another important behavior because it is the initial process for formation of new blood vessels. Pioneering research by Bettinger et al. demonstrated that nanotopography had an ability to promote natural capillary formation of endothelial progenitor cells (EPCs) (Fig. 5) [289]. Compared to short and less organized capillary tubes on flat substrates [Fig. 5B(i)], long and extensive networks were observed on nanopatterns [Fig. 5B(ii)]. There is an alternative approach to induce capillary formation by using micrometric structures as templates. Lu et al. showed that starch-poly(caprolactone) (SPCL) microfiber meshes could be used as templates for capillary formation of human umbilical vein ECs (HUVECs) [290]. Also, Lee et al. fabricated circular microchannels and cultured calf pulmonary ECs (CPAEs) on them [291]. After 6 days, ECs showed alignment along the microchannel and formed capillary without flow.
Fig. 5.
Supercellular band formation on flat and nanopatterned substrates after 6-day culture of endothelial cells (ECs). (A) Immunostaining images of typical endothelial markers including PECAM-1, VEcad and α-SMA. The direction of the arrow indicates topographical orientation. (B) In vitro capillary formation assay. Endothelial progenitor cells (EPCs) cultured on nanopatterns showed long and extensive network of capillary tube (ii), while on flat substrates unorganized and low density of capillary tubes were observed (i). Scale bar: 50 μm (A), 200 μm (B).
Reprinted with permission from ref. [289].
Recent research for in vivo implantation of the electrospun fiber matrixes has confirmed feasibility for the use of the clinical vascular grafts [285,292]. Wise et al. fabricated electrospun elastin/poly(caprolactone) nanofiber matrix and showed enhanced viability, adhesion, and proliferation of ECs in vitro. After 1 month of in vivo implantation in a rabbit model, vascular grafts maintained their in vitro mechanical properties and showed high biocompatibility without notable problems such as dilatation, anastomotic dehiscence and seroma [285].
4.4.1.2. Smooth muscle cells (SMCs)
The media of blood vessel is composed of multiple layers of SMCs embedded in collagen and elastin lamellae [293–295]. The collagen and SMCs are organized in a helical pattern around the circumference of the blood vessel, while the orientation angle of SMCs between layers is slightly alternating [296,297]. The helically aligned arrangement of SMCs presents three major crucial roles in blood vessel functions: (i) enhancing load-bearing properties, (ii) presenting torsional stability, and (iii) mediating lumen diameter for the control of blood pressure and shear stress [297].
To mimic the aligned nature of SMCs in vitro, various studies have utilized micro- or nanostructured surfaces in the form of nano-[298–300] or microlines [301–304]. These studies demonstrated that the cells were aligned and elongated along the line orientation. Especially, Hu et al. reported increased alignment of cells with the decrease of the feature size of nanopatterns [298], Sarkar et al. reported similar trends on micropatterns [301]. Isenberg et al. have demonstrated transfer of an aligned cell sheet by combining cell detachment characteristic of p(NIPAAm) and topographical cell alignment [305]. When adult human aortic SMCs (AoSMCs) were cultured on a PIPAAm coated microgroove substrate, cells were aligned along the orientation of microgrooves. By decreasing the temperature below the lower critical solution temperature (LCST) the cell sheet could be detached from the culture substrate, ultimately being transferred to the other substrate with aligned morphology.
In parallel, such topography-induced alignment of cellular morphology could increase the mechanical properties of vascular grafts. Zorlutuna et al. cultured vascular smooth muscle cells (VSMCs) on collagen films having 332.5 nm, 500 nm, and 650 nm-wide nanogrooves [306]. When VSMCs and supporting collagen scaffold were under uniaxial tension, a maximum ultimate tensile stress (UTS) appeared on the cells cultured on 650 nm (UTS=~1.63 MPa), showing increased UTS compared to the unpatterned case of ~0.43 MPa. Interestingly, such topographically enhanced UTS value corresponded to that of natural arteries and veins. Isenberg et al. have demonstrated similar anisotropic mechanical properties with bovine aortic SMCs cultured on micropatterned PDMS [307]. Upon stretching, the failure stress and stiffness were more than 50% greater in stretching to the parallel orientation as compared to that of the perpendicular orientation. These results revealed that anisotropic topography itself could increase mechanical properties and induce mechanical anisotropy.
Several studies have demonstrated layer-by-layer assembly approaches to mimic the three-layered structures of blood vessels. Sarkar et al. demonstrated a three-dimensional tissue construct by stacking cell-seeded three layers of micropatterned PCL thin films. For 48 h, cells were polarized along the line direction in each layer, and proliferated regardless of the layer location (top, middle and bottom) [308]. Tan et al. showed layer-by-layer stacking in microchannels by using three vascular cell types within heterogeneous types of biopolymers. Since the collagen matrices are contracted over time, multiple layers could be fabricated by injecting other cell–matrix assemblies inside the microchannels after the shrinkage [309]. Feng et al. also demonstrated multilayers of vascular SMCs in deep microchannels by using layer-by-layer assembly. They introduced the appropriate concentration of cells and collagen type I gel in an alternative fashion after the underlying cells reached near confluency. Due to the topographical guidance of channel walls, SMCs in each layer had aligned morphologies along the channel direction [310].
More recently, to ensure the possibilities of in vivo adaptation, cell-seeded polymer scaffold transplantation was tested. According to Hu et al., SMCs seeded on nanofibrous PLLA showed enhanced level of SMC contractile markers including smooth muscle myosin heavy chain, smoothelin, and myocardin. Furthermore, when implanted in the subcutaneous layer of mice, host tissue infiltration and SMC differentiation were observed [311]. From this observation, it is believed that nanostructured scaffolds may be used as a promising tool for vascular regeneration.
5. Perspectives
Despite great potential of the nanotopography-guided approach presented here, there are a number of limitations for clinical applications. The most significant issue is a lack of ability to create three-dimensional, highly organized structures for ECMs, while recognizing that some techniques such as direct laser writing and electrospinning could create a 3D network. Recent studies have demonstrated that the cells cultured on flat surface behave differently from those in native tissues. For example, the cells on 2D substrates can experience de-differentiation and exhibit increased growth [312–315]. Furthermore, some cells such as T cells take different modes of migration in 3D (so called ‘amoeboid’ migration), which are different from mesenchymal and epithelial cells [316]. To deal with this issue, a simple, direct molding method that can generate hydrogels with controlled features has recently been demonstrated [317–319]. Such a direct molding method would be useful for microengineered hydrogels for tissue engineering as well as for controlled drug delivery, but still suffers from difficulty to fabricate fine nanoscale features due to low mechanical modulus of the hydrogels. For the tissues that require a highly organized 3D environment, an alternative fabrication method would be needed with materials having tunable rigidity and topography.
Another issue is related to the complexity and hierarchy of the ECM structure. Even with the existing advanced micro- and nanofabrication techniques, it is difficult and challenging to prepare highly ordered nanoscale features over a large area. For example, hierarchical packing of collagen proteins leads to the formation of fibrils, long (tens of microns) cylindrical structures, which have varying diameters from 20 to 200 nm. Fabrication of simple geometries in the form of lines, pits, or dots may not be sufficient sometimes to recapitulate the essential components of the ECM architecture.
Finally, the current nanotopography-guided approach is on a trial-and-error basis. It is frequently observed that an optimal nanostructure for a certain cell type is not necessarily optimal for another cell type. A simple guideline would exist for a range of specific organs; i.e., a parallel line is preferred for selective differentiation into neuronal lineage and a dot pattern would be more effective for osteogenic differentiation [47,127,320,321]. Nonetheless, more systematic approaches are needed to elucidate the detailed role of nanotopography in conjunction with material chemistries.
6. Conclusions
In this review, various tissue-specific nanostructures in human organs have been described with respect to their tissue functions. For convenience, the tissues covered in this review have been classified into four categories: protective, mechano-sensitive, electro-active, and shear stress-sensitive tissues. To mimic complex and well-organized ECM structures in vitro, numerous nanofabrication methods have been introduced, which have been classified in terms of templates and energy sources used for the fabrication. With tissue-inspired scaffolds, many studies have demonstrated that cell-specific functions in vitro or tissue regeneration in vivo upon implantation was much improved presumably due to nanotopographical guidance and better cell-to-cell communications. It is believed that this review may enlighten readers with the importance of nanostructures in vivo organ function, and the effectiveness of nanotopographical cues and nanofabrication-based strategies for regenerative medicine.
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant (NRF-2011-220-D00035), the WCU (World Class University) Program (R31-2008-000-10083-0) and the Basic Science Research Program (2010-0027955). D. H. Kim thanks the Department of Bioengineering at the University of Washington for the new faculty startup fund. D. H. Kim is also supported by a Perkins Coie Award for Discovery. N.S. Hwang was supported by the Research Settlement Fund for the new faculty of SNU.
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “Bionics — biologically inspired smart materials”.
References
- 1.Messenger MP, Tomlins PE. Regenerative medicine: a snapshot of the current regulatory environment and standards. Adv Mater. 2011;23:H10–H17. doi: 10.1002/adma.201100254. [DOI] [PubMed] [Google Scholar]
- 2.Mason C. Regenerative medicine 2.0. Regen Med. 2007;2:11–18. doi: 10.2217/17460751.2.1.11. [DOI] [PubMed] [Google Scholar]
- 3.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter SB. Principles of cell motility: the direction of cell movement and cancer invasion. Nature. 1965;208:1183–1187. doi: 10.1038/2081183a0. [DOI] [PubMed] [Google Scholar]
- 5.Zhao M, Song B, Pu J, Wada T, Reid B, Tai GP, Wang F, Guo AH, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- 6.Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10:538–549. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zigmond SH, Hirsch JG. Leukocyte locomotion and chemotaxis — new methods for evaluation and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973;137:387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT. Neurodegenerative disease — amyloid pores from pathogenic mutations. Nature. 2002;418:291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 9.Nahum AM, Melvin J. Accidental Injury: Biomechanics and Prevention. New York: Springer; 2002. [Google Scholar]
- 10.Silver FH, Kato YP, Ohno M, Wasserman AJ. Analysis of mammalian connective-tissue — relationship between hierarchical structures and mechanical-properties. J Long-Term Eff Med. 1992;2:165–198. [PubMed] [Google Scholar]
- 11.Craig AS, Eikenberry EF, Parry DAD. Ultrastructural organization of skin — classification on the basis of mechanical role. Connect Tissue Res. 1987;16:213–223. doi: 10.3109/03008208709006977. [DOI] [PubMed] [Google Scholar]
- 12.Martin P. Wound healing — aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 13.Reihsner R, Balogh B, Menzel EJ. Two-dimensional elastic properties of human skin in terms of an incremental model at the in vivo configuration. Med Eng Phys. 1995;17:304–313. doi: 10.1016/1350-4533(95)90856-7. [DOI] [PubMed] [Google Scholar]
- 14.Keene DR, Engvall E, Glanville RW. Ultrastructure of type-VI collagen in human-skin and cartilage suggests an anchoring function for this filamentous network. J Cell Biol. 1988;107:1995–2006. doi: 10.1083/jcb.107.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abe H, Hayashi K, Sato M. Data Book on Mechanical Properties of Living Cells, Tissues, and Organs. Springer; Tokyo: 1996. [Google Scholar]
- 16.Mente PL, Lewis JL. Experimental-method for the measurement of the elastic-modulus of trabecular bone tissue. J Orthop Res. 1989;7:456–461. doi: 10.1002/jor.1100070320. [DOI] [PubMed] [Google Scholar]
- 17.Rho JY, Ashman RB, Turner CH. Young’s modulus of trabecular and cortical bone material: ultrasonic and microtensile measurements. J Biomech. 1993;26:111–119. doi: 10.1016/0021-9290(93)90042-d. [DOI] [PubMed] [Google Scholar]
- 18.Kadler KE, Hulmes DJS, Hojima Y, Prockop DJ. Assembly of type-I collagen fibrils de novo by the specific enzymatic cleavage of pC collagen — the fibrils formed at about 37 °C are similar in diameter, roundness and apparent flexibility to the collagen fibrils seen in connective-tissue. Ann N Y Acad Sci. 1990;580:214–224. doi: 10.1111/j.1749-6632.1990.tb17930.x. [DOI] [PubMed] [Google Scholar]
- 19.Gao HJ, Ji BH, Jager IL, Arzt E, Fratzl P. Materials become insensitive to flaws at nanoscale: lessons from nature. Proc Natl Acad Sci. 2003;100:5597–5600. doi: 10.1073/pnas.0631609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao HJ. Application of fracture mechanics concepts to hierarchical biomechanics of bone and bone-like materials. Int J Fract. 2006;138:101–137. [Google Scholar]
- 21.Lowenstam HA, Weiner S. On Biomineralization. New York: Oxford University Press; 1989. [Google Scholar]
- 22.Weiner S, Wagner HD. The material bone: structure mechanical function relations. Annu Rev Mater Sci. 1998;28:271–298. [Google Scholar]
- 23.Hay ED. Cell Biology of Extracellular Matrix. New York: Plenum Press; 1991. [Google Scholar]
- 24.Ji BH, Gao HJ. Mechanical principles of biological nanocomposites. Annu Rev Mater Res. 2010;40:77–100. [Google Scholar]
- 25.Fratzl P. Cellulose and collagen: from fibres to tissues. Curr Opin Colloid Interface Sci. 2003;8:32–39. [Google Scholar]
- 26.Fratzl P, Misof K, Zizak I, Rapp G, Amenitsch H, Bernstorff S. Fibrillar structure and mechanical properties of collagen. J Struct Biol. 1998;122:119–122. doi: 10.1006/jsbi.1998.3966. [DOI] [PubMed] [Google Scholar]
- 27.Cribb AM, Scott JE. Tendon response to tensile-stress — an ultrastructural investigation of collagen–proteoglycan interactions in stressed tendon. J Anat. 1995;187:423–428. [PMC free article] [PubMed] [Google Scholar]
- 28.Rakic P. Elusive radial glial cells: historical and evolutionary perspective. Glia. 2003;43:19–32. doi: 10.1002/glia.10244. [DOI] [PubMed] [Google Scholar]
- 29.Lu YB, Franze K, Seifert G, Steinhauser C, Kirchhoff F, Wolburg H, Guck J, Janmey P, Wei EQ, Kas J, Reichenbach A. Viscoelastic properties of individual glial cells and neurons in the CNS. Proc Natl Acad Sci. 2006;103:17759–17764. doi: 10.1073/pnas.0606150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nemir S, West JL. Synthetic materials in the study of cell response to substrate rigidity. Ann Biomed Eng. 2010;38:2–20. doi: 10.1007/s10439-009-9811-1. [DOI] [PubMed] [Google Scholar]
- 31.Franze K, Guck J. The biophysics of neuronal growth. Rep Prog Phys. 2010;73:094601. [Google Scholar]
- 32.Sciote JJ, Morris TJ. Skeletal muscle function and fibre types: the relationship between occlusal function and the phenotype of jaw-closing muscles in human. J Orthod. 2000;27:15–30. doi: 10.1093/ortho/27.1.15. [DOI] [PubMed] [Google Scholar]
- 33.Huxley AF, Niedergerke R. Structural changes in muscle during contraction —interference microscopy of living muscle fibres. Nature. 1954;173:971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- 34.Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966;184:170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol. 2003;552:47–58. doi: 10.1113/jphysiol.2003.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Severs NJ. The cardiac muscle cell. Bioessays. 2000;22:188–199. doi: 10.1002/(SICI)1521-1878(200002)22:2<188::AID-BIES10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 37.Severs NJ, Coppen SR, Dupont E, Yeh HI, Ko YS, Matsushita T. Gap junction alterations in human cardiac disease. Cardiovasc Res. 2004;62:368–377. doi: 10.1016/j.cardiores.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. J Am Med Assoc. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 39.Ma ZW, He W, Yong T, Ramakrishna S. Grafting of gelatin on electrospun poly(caprolactone) nanofibers to improve endothelial cell spreading and proliferation and to control cell orientation. Tissue Eng. 2005;11:1149–1158. doi: 10.1089/ten.2005.11.1149. [DOI] [PubMed] [Google Scholar]
- 40.Flemming RG, Murphy CJ, Abrams GA, Goodman SL, Nealey PF. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials. 1999;20:573–588. doi: 10.1016/s0142-9612(98)00209-9. [DOI] [PubMed] [Google Scholar]
- 41.Paszkowiak JJ, Dardik A. Arterial wall shear stress: observations from the bench to the bedside. Vasc Endovascular Surg. 2003;37:47–57. doi: 10.1177/153857440303700107. [DOI] [PubMed] [Google Scholar]
- 42.Wan ACA, Ying JY. Nanomaterials for in situ cell delivery and tissue regeneration. Adv Drug Deliv Rev. 2010;62:731–740. doi: 10.1016/j.addr.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Bettinger CJ, Langer R, Borenstein JT. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew Chem Int Edit. 2009;48:5406–5415. doi: 10.1002/anie.200805179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim DH, Wong PK, Park J, Levchenko A, Sun Y. Microengineered platforms for cell mechanobiology. Annu Rev Biomed Eng. 2009;11:203–233. doi: 10.1146/annurev-bioeng-061008-124915. [DOI] [PubMed] [Google Scholar]
- 45.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 46.Orive G, Anitua E, Pedraz JL, Emerich DF. Biomaterials for promoting brain protection, repair and regeneration. Nat Rev Neurosci. 2009;10:682–692. doi: 10.1038/nrn2685. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y, Leong KW. Nanoscale surfacing for regenerative medicine. Wires Nanomed Nanobiotechnol. 2010;2:478–495. doi: 10.1002/wnan.74. [DOI] [PubMed] [Google Scholar]
- 48.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci. 2006;103:2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater. 2006;18:1345–1360. [Google Scholar]
- 50.Moore SW, Sheetz MP. Biophysics of substrate interaction: influence on neural motility, differentiation and repair. Dev Neurobiol. 2011;71:1090–1101. doi: 10.1002/dneu.20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harrison RG. The reaction of embryonic cells to solid structures. J Exp Zool. 1914;17:521–544. [Google Scholar]
- 52.Weiss P. In vitro experiments on the factors determining the course of the outgrowing nerve fiber. J Exp Zool. 1934;68:393–448. [Google Scholar]
- 53.Kleinfeld D, Kahler KH, Hockberger PE. Controlled outgrowth of dissociated neurons on patterned substrates. J Neurosci. 1988;8:4098–4120. doi: 10.1523/JNEUROSCI.08-11-04098.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goubko CA, Cao XD. Patterning multiple cell types in co-cultures: a review. Mater Sci Eng C. 2009;29:1855–1868. [Google Scholar]
- 55.Formhals A, Schreiber-Gastell R. Apparatus for producing artificial filaments from material such as cellulose acetate. U S A Patent. 1934
- 56.Nam J, Huang Y, Agarwal S, Lannutti J. Improved cellular infiltration in electrospun fiber via engineered porosity. Tissue Eng. 2007;13:2249–2257. doi: 10.1089/ten.2006.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 58.Stitzel JD, Pawlowski KJ, Wnek GE, Simpson DG, Bowlin GL. Arterial smooth muscle cell proliferation on a novel biomimicking, biodegradable vascular graft scaffold. J Biomater Appl. 2001;16:22–33. doi: 10.1106/U2UU-M9QH-Y0BB-5GYL. [DOI] [PubMed] [Google Scholar]
- 59.Xia YN, Whitesides GM. Soft lithography. Annu Rev Mater Sci. 1998;28:153–184. [Google Scholar]
- 60.Qin D, Xia YN, Whitesides GM. Soft lithography for micro- and nanoscale patterning. Nat Protoc. 2010;5:491–502. doi: 10.1038/nprot.2009.234. [DOI] [PubMed] [Google Scholar]
- 61.Xia YN, Kim E, Zhao XM, Rogers JA, Prentiss M, Whitesides GM. Complex optical surfaces formed by replica molding against elastomeric masters. Science. 1996;273:347–349. doi: 10.1126/science.273.5273.347. [DOI] [PubMed] [Google Scholar]
- 62.Chou SY, Krauss PR, Renstrom PJ. Nanoimprint lithography. J Vac Sci Technol B. 1996;14:4129–4133. [Google Scholar]
- 63.Guo LJ. Nanoimprint lithography: methods and material requirements. Adv Mater. 2007;19:495–513. [Google Scholar]
- 64.Tsang VL, Bhatia SN. Three-dimensional tissue fabrication. Adv Drug Deliv Rev. 2004;56:1635–1647. doi: 10.1016/j.addr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 65.Voldman J, Gray ML, Schmidt MA. Microfabrication in biology and medicine. Annu Rev Biomed Eng. 1999;1:401–425. doi: 10.1146/annurev.bioeng.1.1.401. [DOI] [PubMed] [Google Scholar]
- 66.Colburn M, Johnson S, Stewart M, Damle S, Bailey T, Choi B, Wedlake M, Michaelson T, Sreenivasan SV, Ekerdt J, Willson CG. Step and Flash Imprint Lithography: A New Approach to High-resolution Patterning. Presented at Emerging Lithographic Technologies III; Santa Clara, CA, USA. 1999; On page(s) [Google Scholar]
- 67.Choi SJ, Kim HN, Bae WG, Suh KY. Modulus- and surface energy-tunable ultraviolet-curable polyurethane acrylate: properties and applications. J Mater Chem. 2011;21:14325–14335. [Google Scholar]
- 68.Suh KY, Kim YS, Lee HH. Capillary force lithography. Adv Mater. 2001;13:1386–1389. [Google Scholar]
- 69.Suh KY, Park MC, Kim P. Capillary force lithography: a versatile tool for structured biomaterials interface towards cell and tissue engineering. Adv Funct Mater. 2009;19:2699–2712. [Google Scholar]
- 70.Kim E, Xia YN, Whitesides GM. Polymer microstructures formed by molding in capillaries. Nature. 1995;376:581–584. [Google Scholar]
- 71.Jackman RJ, Wilbur JL, Whitesides GM. Fabrication of submicrometer features on curved substrates by microcontact printing. Science. 1995;269:664–666. doi: 10.1126/science.7624795. [DOI] [PubMed] [Google Scholar]
- 72.Abidian MR, Kim DH, Martin DC. Conducting-polymer nanotubes for controlled drug release. Adv Mater. 2006;18:405–409. doi: 10.1002/adma.200501726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liang L, Liu J, Windisch CF, Exarhos GJ, Lin YH. Direct assembly of large arrays of oriented conducting polymer nanowires. Angew Chem Int Edit. 2002;41:3665–3668. doi: 10.1002/1521-3773(20021004)41:19<3665::AID-ANIE3665>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 74.Park M, Harrison C, Chaikin PM, Register RA, Adamson DH. Block copolymer lithography: periodic arrays of similar to 10(11) holes in 1 square centimeter. Science. 1997;276:1401–1404. [Google Scholar]
- 75.Tsai IY, Kimura M, Stockton R, Green JA, Puig R, Jacobson B, Russell TP. Fibroblast adhesion to micro- and nano-heterogeneous topography using diblock copolymers and homopolymers. J Biomed Mater Res A. 2004;71A:462–469. doi: 10.1002/jbm.a.30183. [DOI] [PubMed] [Google Scholar]
- 76.Craighead HG. Nanoelectromechanical systems. Science. 2000;290:1532–1535. doi: 10.1126/science.290.5496.1532. [DOI] [PubMed] [Google Scholar]
- 77.Deubel M, Von Freymann G, Wegener M, Pereira S, Busch K, Soukoulis CM. Direct laser writing of three-dimensional photonic-crystal templates for telecommunications. Nat Mater. 2004;3:444–447. doi: 10.1038/nmat1155. [DOI] [PubMed] [Google Scholar]
- 78.Klein F, Striebel T, Fischer J, Jiang ZX, Franz CM, von Freymann G, Wegener M, Bastmeyer M. Elastic fully three-dimensional microstructure scaffolds for cell force measurements. Adv Mater. 2010;22:868–871. doi: 10.1002/adma.200902515. [DOI] [PubMed] [Google Scholar]
- 79.Luna JI, Ciriza J, Garcia-Ojeda ME, Kong M, Herren A, Lieu DK, Li RA, Fowlkes CC, Khine M, McCloskey KE. Multiscale biomimetic topography for the alignment of neonatal and embryonic stem cell-derived heart cells. Tissue Eng Part C. 2011;17:579–588. doi: 10.1089/ten.TEC.2010.0410. [DOI] [PubMed] [Google Scholar]
- 80.Genzer J, Groenewold J. Soft matter with hard skin: from skin wrinkles to templating and material characterization. Soft Matter. 2006;2:310–323. doi: 10.1039/b516741h. [DOI] [PubMed] [Google Scholar]
- 81.Zhu XY, Mills KL, Peters PR, Bahng JH, Liu EH, Shim J, Naruse K, Csete ME, Thouless MD, Takayama S. Fabrication of reconfigurable protein matrices by cracking. Nat Mater. 2005;4:403–406. doi: 10.1038/nmat1365. [DOI] [PubMed] [Google Scholar]
- 82.Huh D, Mills KL, Zhu XY, Burns MA, Thouless MD, Takayama S. Tuneable elastomeric nanochannels for nanofluidic manipulation. Nat Mater. 2007;6:424–428. doi: 10.1038/nmat1907. [DOI] [PubMed] [Google Scholar]
- 83.Kim HN, Lee SH, Suh KY. Controlled mechanical fracture for fabricating microchannels with various size gradients. Lab Chip. 2011;11:717–722. doi: 10.1039/c0lc00277a. [DOI] [PubMed] [Google Scholar]
- 84.Piner RD, Zhu J, Xu F, Hong SH, Mirkin CA. “Dip-pen” nanolithography. Science. 1999;283:661–663. doi: 10.1126/science.283.5402.661. [DOI] [PubMed] [Google Scholar]
- 85.Salaita K, Wang YH, Mirkin CA. Applications of dip-pen nanolithography. Nat Nanotechnol. 2007;2:145–155. doi: 10.1038/nnano.2007.39. [DOI] [PubMed] [Google Scholar]
- 86.Huo FW, Zheng ZJ, Zheng GF, Giam LR, Zhang H, Mirkin CA. Polymer pen lithography. Science. 2008;321:1658–1660. doi: 10.1126/science.1162193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hou QP, Grijpma DW, Feijen J. Porous polymeric structures for tissue engineering prepared by a coagulation, compression moulding and salt leaching technique. Biomaterials. 2003;24:1937–1947. doi: 10.1016/s0142-9612(02)00562-8. [DOI] [PubMed] [Google Scholar]
- 88.Yoon JJ, Song SH, Lee DS, Park TG. Immobilization of cell adhesive RGD peptide onto the surface of highly porous biodegradable polymer scaffolds fabricated by a gas foaming/salt leaching method. Biomaterials. 2004;25:5613–5620. doi: 10.1016/j.biomaterials.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 89.Lim SH, Mao HQ. Electrospun scaffolds for stem cell engineering. Adv Drug Deliv Rev. 2009;61:1084–1096. doi: 10.1016/j.addr.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 90.Greiner A, Wendorff JH. Electrospinning: a fascinating method for the preparation of ultrathin fibres. Angew Chem Int Edit. 2007;46:5670–5703. doi: 10.1002/anie.200604646. [DOI] [PubMed] [Google Scholar]
- 91.Folkman J, Moscona A. Role of cell-shape in growth-control. Nature. 1978;273:345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 92.Ingber DE. Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proc Natl Acad Sci. 1990;87:3579–3583. doi: 10.1073/pnas.87.9.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 94.Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xie JW, MacEwan MR, Ray WZ, Liu WY, Siewe DY, Xia YN. Radially aligned, electrospun nanofibers as dural substitutes for wound closure and tissue regeneration applications. ACS Nano. 2010;4:5027–5036. doi: 10.1021/nn101554u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kubinova S, Sykova E. Nanotechnologies in regenerative medicine. Minim Invasive Ther Allied Technol. 2010;19:144–156. doi: 10.3109/13645706.2010.481398. [DOI] [PubMed] [Google Scholar]
- 98.Burgeson R. Dermatology in General Medicine. McGraw-Hill; New York: 1987. pp. 288–303. [Google Scholar]
- 99.PINS GD, TONER M, MORGAN JR. Microfabrication of an analog of the basal lamina: biocompatible membranes with complex topographies. FASEB J. 2000;14:593–602. doi: 10.1096/fasebj.14.3.593. [DOI] [PubMed] [Google Scholar]
- 100.Karuri NW, Liliensiek S, Teixeira AI, Abrams G, Campbell S, Nealey PF, Murphy CJ. Biological length scale topography enhances cell-substratum adhesion of human corneal epithelial cells. J Cell Sci. 2004;117:3153–3164. doi: 10.1242/jcs.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure: a review. Am J Pathol. 1974;77:313–346. [PMC free article] [PubMed] [Google Scholar]
- 102.Venugopal JR, Zhang Y, Ramakrishna S. In vitro culture of human dermal fibroblasts on electrospun polycaprolactone collagen nanofibrous membrane. Artif Organs. 2006;30:440–446. doi: 10.1111/j.1525-1594.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- 103.Venugopal J, Ramakrishna S. Biocompatible nanofiber matrices for the engineering of a dermal substitute for skin regeneration. Tissue Eng. 2005;11:847–854. doi: 10.1089/ten.2005.11.847. [DOI] [PubMed] [Google Scholar]
- 104.Zhang YZ, Venugopal J, Huang ZM, Lim CT, Ramakrishna S. Characterization of the surface biocompatibility of the electrospun PCL–collagen nanofibers using fibroblasts. Biomacromolecules. 2005;6:2583–2589. doi: 10.1021/bm050314k. [DOI] [PubMed] [Google Scholar]
- 105.Pan H, Jiang H, Chen W. Interaction of dermal fibroblasts with electrospun composite polymer scaffolds prepared from dextran and poly lactide-co-glycolide. Biomaterials. 2006;27:3209–3220. doi: 10.1016/j.biomaterials.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 106.Min B-M, Lee G, Kim SH, Nam YS, Lee TS, Park WH. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials. 2004;25:1289–1297. doi: 10.1016/j.biomaterials.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 107.Noh HK, Lee SW, Kim J-M, Oh J-E, Kim K-H, Chung C-P, Choi S-C, Park WH, Min B-M. Electrospinning of chitin nanofibers: degradation behavior and cellular response to normal human keratinocytes and fibroblasts. Biomaterials. 2006;27:3934–3944. doi: 10.1016/j.biomaterials.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 108.Rho KS, Jeong L, Lee G, Seo B-M, Park YJ, Hong S-D, Roh S, Cho JJ, Park WH, Min B-M. Electrospinning of collagen nanofibers: effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials. 2006;27:1452–1461. doi: 10.1016/j.biomaterials.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 109.Rheinwald JG, Green H. Formation of a keratinizing epithelium in culture by a cloned cell line derived from a teratoma. Cell. 1975;6:317–330. doi: 10.1016/0092-8674(75)90183-x. [DOI] [PubMed] [Google Scholar]
- 110.Smith AG, Din A, Denyer M, Crowther NJ, Eagland D, Vowden K, Vowden P, Britland ST. Microengineered surface topography facilitates cell grafting from a prototype hydrogel wound dressing with antibacterial capability. Biotechnol Prog. 2006;22:1407–1415. doi: 10.1021/bp060192n. [DOI] [PubMed] [Google Scholar]
- 111.Cuono C, Langdon R, McGuire J. Use of cultured epidermal autografts and dermal allografts as skin replacement after burn injury. Lancet. 1986;327:1123–1124. doi: 10.1016/s0140-6736(86)91838-6. [DOI] [PubMed] [Google Scholar]
- 112.Orgill DP, Butler C, Regan JF, Barlow MS, Yanna IV, Compton CC. Vascularized collagen–glycosaminoglycan matrix provides a dermal substrate and improves take of cultured epithelial autografts. Plast Reconstr Surg. 1998;102:423–429. doi: 10.1097/00006534-199808000-00020. [DOI] [PubMed] [Google Scholar]
- 113.Compton CC, Hickerson W, Nadire K, Press W. Acceleration of skin regeneration from cultured epithelial autografts by transplantation to homograft dermis. J Burn Care Rehabil. 1993;14:653–662. doi: 10.1097/00004630-199311000-00010. [DOI] [PubMed] [Google Scholar]
- 114.Downing BR, Cornwell K, Toner M, Pins GD. The influence of microtextured basal lamina analog topography on keratinocyte function and epidermal organization. J Biomed Mater Res A. 2005;72A:47–56. doi: 10.1002/jbm.a.30210. [DOI] [PubMed] [Google Scholar]
- 115.OGF The morphology of the attachment between the dermis and the epidermis. Anat Rec. 1950;108:399–413. doi: 10.1002/ar.1091080305. [DOI] [PubMed] [Google Scholar]
- 116.Yamakuchi M, Higuchi I, Masuda S, Ohira Y, Kubo T, Kato Y, Maruyama I, Kitajima I. Type I muscle atrophy caused by microgravity-induced decrease of myocyte enhancer factor 2C (MEF2C) protein expression. FEBS Lett. 2000;477:135–140. doi: 10.1016/s0014-5793(00)01715-4. [DOI] [PubMed] [Google Scholar]
- 117.Rittweger J, Felsenberg D. Recovery of muscle atrophy and bone loss from 90 days bed rest: results from a one-year follow-up. Bone. 2009;44:214–224. doi: 10.1016/j.bone.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 118.Leychenko A, Konorev E, Jijiwa M, Matter ML. Stretch-induced hypertrophy activates NFkB-mediated VEGF secretion in adult cardiomyocytes. PLoS One. 2011;6:e29055. doi: 10.1371/journal.pone.0029055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 120.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2009;10:34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 122.Jagodzinski M, Drescher M, Zeichen J, Hankemeier S, Krettek C, Bosch U, van Griensven M. Effects of cyclic longitudinal mechanical strain and dexamethasone on osteogenic differentiation of human bone marrow stromal cells. Eur Cell Mater. 2004;7:35–41. doi: 10.22203/ecm.v007a04. [DOI] [PubMed] [Google Scholar]
- 123.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 124.Legant WR, Miller JS, Blakely BL, Cohen DM, Genin GM, Chen CS. Measurement of mechanical tractions exerted by cells in three-dimensional matrices. Nat Methods. 2010;7:969–971. doi: 10.1038/nmeth.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dalby MJ, McCloy D, Robertson M, Wilkinson CD, Oreffo RO. Osteoprogenitor response to defined topographies with nanoscale depths. Biomaterials. 2006;27:1306–1315. doi: 10.1016/j.biomaterials.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 126.Zhu B, Lu Q, Yin J, Hu J, Wang Z. Alignment of osteoblast-like cells and cell-produced collagen matrix induced by nanogrooves. Tissue Eng. 2005;11:825–834. doi: 10.1089/ten.2005.11.825. [DOI] [PubMed] [Google Scholar]
- 127.Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CD, Oreffo RO. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007;6:997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 128.Mitrossilis D, Fouchard J, Pereira D, Postic F, Richert A, Saint-Jean M, Asnacios A. Real-time single-cell response to stiffness. Proc Natl Acad Sci. 2010;107:16518–16523. doi: 10.1073/pnas.1007940107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Woo SL, Jia F, Zou L, Gabriel MT. Functional tissue engineering for ligament healing: potential of antisense gene therapy. Ann Biomed Eng. 2004;32:342–351. doi: 10.1023/b:abme.0000017551.93144.1a. [DOI] [PubMed] [Google Scholar]
- 130.Jones BF, Wall ME, Carroll RL, Washburn S, Banes AJ. Ligament cells stretch-adapted on a microgrooved substrate increase intercellular communication in response to a mechanical stimulus. J Biomech. 2005;38:1653–1664. doi: 10.1016/j.jbiomech.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 131.Xu B, Song G, Ju Y, Li X, Song Y, Watanabe S. RhoA/ROCK, cytoskeletal dynamics and focal adhesion kinase are required for mechanical stretch-induced tenogenic differentiation of human mesenchymal stem cells. J Cell Physiol. 2012;227:2722–2729. doi: 10.1002/jcp.23016. [DOI] [PubMed] [Google Scholar]
- 132.Basile P, Dadali T, Jacobson J, Hasslund S, Ulrich-Vinther M, Soballe K, Nishio Y, Drissi MH, Langstein HN, Mitten DJ, O’Keefe RJ, Schwarz EM, Awad HA. Freeze-dried tendon allografts as tissue-engineering scaffolds for Gdf5 gene delivery. Mol Ther. 2008;16:466–473. doi: 10.1038/sj.mt.6300395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Richmond JC, Manseau CJ, Patz R, McConville O. Anterior cruciate reconstruction using a Dacron ligament prosthesis. A long-term study. Am J Sports Med. 1992;20:24–28. doi: 10.1177/036354659202000107. [DOI] [PubMed] [Google Scholar]
- 134.Moyen BJ, Jenny JY, Mandrino AH, Lerat JL. Comparison of reconstruction of the anterior cruciate ligament with, without a Kennedy ligament-augmentation device A randomized, prospective study. J Bone Joint Surg Am. 1992;74:1313–1319. [PubMed] [Google Scholar]
- 135.Vunjak-Novakovic G, Altman G, Horan R, Kaplan DL. Tissue engineering of ligaments. Annu Rev Biomed Eng. 2004;6:131–156. doi: 10.1146/annurev.bioeng.6.040803.140037. [DOI] [PubMed] [Google Scholar]
- 136.Lin VS, Lee MC, O’Neal S, McKean J, Sung KL. Ligament tissue engineering using synthetic biodegradable fiber scaffolds. Tissue Eng. 1999;5:443–452. doi: 10.1089/ten.1999.5.443. [DOI] [PubMed] [Google Scholar]
- 137.Sahoo S, Toh SL, Goh JC. PLGA nanofiber-coated silk microfibrous scaffold for connective tissue engineering. J Biomed Mater Res B Appl Biomater. 2010;95:19–28. doi: 10.1002/jbm.b.31678. [DOI] [PubMed] [Google Scholar]
- 138.Butler DL, Gooch C, Kinneberg KR, Boivin GP, Galloway MT, Nirmalanandhan VS, Shearn JT, Dyment NA, Juncosa-Melvin N. The use of mesenchymal stem cells in collagen-based scaffolds for tissue-engineered repair of tendons. Nat Protoc. 2010;5:849–863. doi: 10.1038/nprot.2010.14. [DOI] [PubMed] [Google Scholar]
- 139.Ouyang HW, Goh JC, Mo XM, Teoh SH, Lee EH. The efficacy of bone marrow stromal cell-seeded knitted PLGA fiber scaffold for Achilles tendon repair. Ann N Y Acad Sci. 2002;961:126–129. doi: 10.1111/j.1749-6632.2002.tb03064.x. [DOI] [PubMed] [Google Scholar]
- 140.Teh TK, Toh SL, Goh JC. Aligned hybrid silk scaffold for enhanced differentiation of mesenchymal stem cells into ligament fibroblasts. Tissue Eng Part C. 2011;17:687–703. doi: 10.1089/ten.tec.2010.0513. [DOI] [PubMed] [Google Scholar]
- 141.Dai X, Xu Q. Nanostructured substrate fabricated by sectioning tendon using a microtome for tissue engineering. Nanotechnology. 2011;22:494008. doi: 10.1088/0957-4484/22/49/494008. [DOI] [PubMed] [Google Scholar]
- 142.Barber JG, Handorf AM, Allee TJ, Li WJ. Braided nanofibrous scaffold for tendon and ligament tissue engineering. Tissue Eng Part A. 2011 doi: 10.1089/ten.tea.2010.0538. http://dx.doi.org/10.1089/ten.tea.2010.0538. [DOI] [PubMed]
- 143.Sahoo S, Ouyang H, Goh JC, Tay TE, Toh SL. Characterization of a novel polymeric scaffold for potential application in tendon/ligament tissue engineering. Tissue Eng. 2006;12:91–99. doi: 10.1089/ten.2006.12.91. [DOI] [PubMed] [Google Scholar]
- 144.Dalby MJ, Gadegaard N, Curtis AS, Oreffo RO. Nanotopographical control of human osteoprogenitor differentiation. Curr Stem Cell Res Ther. 2007;2:129–138. doi: 10.2174/157488807780599220. [DOI] [PubMed] [Google Scholar]
- 145.McMurray RJ, Gadegaard N, Tsimbouri PM, Burgess KV, McNamara LE, Tare R, Murawski K, Kingham E, Oreffo RO, Dalby MJ. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater. 2011;10:637–644. doi: 10.1038/nmat3058. [DOI] [PubMed] [Google Scholar]
- 146.McNamara LE, McMurray RJ, Biggs MJ, Kantawong F, Oreffo RO, Dalby MJ. Nanotopographical control of stem cell differentiation. J Tissue Eng. 2010;2010:120623. doi: 10.4061/2010/120623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Oh S, Brammer KS, Li YS, Teng D, Engler AJ, Chien S, Jin S. Stem cell fate dictated solely by altered nanotube dimension. Proc Natl Acad Sci. 2009;106:2130–2135. doi: 10.1073/pnas.0813200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ohgushi H, Dohi Y, Tamai S, Tabata S. Osteogenic differentiation of marrow stromal stem-cells in porous hydroxyapatite ceramics. J Biomed Mater Res. 1993;27:1401–1407. doi: 10.1002/jbm.820271107. [DOI] [PubMed] [Google Scholar]
- 149.Yang Y, Magnay JL, Cooling L, El HA. Development of a ‘mechano-active’ scaffold for tissue engineering. Biomaterials. 2002;23:2119–2126. doi: 10.1016/s0142-9612(01)00342-8. [DOI] [PubMed] [Google Scholar]
- 150.Curtin CM, Cunniffe GM, Lyons FG, Bessho K, Dickson GR, Duffy GP, O’Brien FJ. Innovative collagen nano-hydroxyapatite scaffolds offer a highly efficient non-viral gene delivery platform for stem cell-mediated bone formation. Adv Mater. 2012;24:749–754. doi: 10.1002/adma.201103828. [DOI] [PubMed] [Google Scholar]
- 151.Akhouayri O, Lafage-Proust MH, Rattner A, Laroche N, Caillot-Augusseau A, Alexandre C, Vico L. Effects of static or dynamic mechanical stresses on osteoblast phenotype expression in three-dimensional contractile collagen gels. J Cell Biochem. 1999;76:217–230. doi: 10.1002/(sici)1097-4644(20000201)76:2<217::aid-jcb6>3.3.co;2-b. [DOI] [PubMed] [Google Scholar]
- 152.Tanaka SM, Sun HB, Roeder RK, Burr DB, Turner CH, Yokota H. Osteoblast responses one hour after load-induced fluid flow in a three-dimensional porous matrix. Calcif Tissue Int. 2005;76:261–271. doi: 10.1007/s00223-004-0238-2. [DOI] [PubMed] [Google Scholar]
- 153.Gao L, McBeath R, Chen CS. Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells. 2010;28:564–572. doi: 10.1002/stem.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Brammer KS, Choi C, Frandsen CJ, Oh S, Jin S. Hydrophobic nanopillars initiate mesenchymal stem cell aggregation and osteo-differentiation. Acta Biomater. 2011;7:683–690. doi: 10.1016/j.actbio.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 156.You MH, Kwak MK, Kim DH, Kim K, Levchenko A, Kim DY, Suh KY. Synergistically enhanced osteogenic differentiation of human mesenchymal stem cells by culture on nanostructured surfaces with induction media. Biomacromolecules. 2010;11:1856–1862. doi: 10.1021/bm100374n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wilkinson A, Hewitt RN, McNamara LE, McCloy D, Dominic Meek RM, Dalby MJ. Biomimetic microtopography to enhance osteogenesis in vitro. Acta Biomater. 2011;7:2919–2925. doi: 10.1016/j.actbio.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 158.Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CDW, Oreffo ROC. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007;6:997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 159.Song B, Zhao M, Forrester JV, McCaig CD. Electrical cues regulate the orientation and frequency of cell division and the rate of wound healing in vivo. Proc Natl Acad Sci. 2002;99:13577–13582. doi: 10.1073/pnas.202235299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schmidt CE, Shastri VR, Vacanti JP, Langer R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc Natl Acad Sci. 1997;94:8948–8953. doi: 10.1073/pnas.94.17.8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jun I, Jeong S, Shin H. The stimulation of myoblast differentiation by electrically conductive sub-micron fibers. Biomaterials. 2009;30:2038–2047. doi: 10.1016/j.biomaterials.2008.12.063. [DOI] [PubMed] [Google Scholar]
- 162.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci. 2004;101:18129–18134. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tandon N, Cannizzaro C, Chao PHG, Maidhof R, Marsano A, Au HTH, Radisic M, Vunjak-Novakovic G. Electrical stimulation systems for cardiac tissue engineering. Nat Protoc. 2009;4:155–173. doi: 10.1038/nprot.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kaiho Y, Ohara Y, Takeshita H, Kiyoyama K, Lee KW, Tanaka T, Koyanagi M. 3D integration technology for 3D stacked retinal chip. presented at 3D System Integration, 2009. 3DIC 2009: IEEE International Conference; San Francisco, CA. 2009; pp. 1–4. [Google Scholar]
- 165.Yang J, Yamato M, Shimizu T, Sekine H, Ohashi K, Kanzaki M, Ohki T, Nishida K, Okano T. Reconstruction of functional tissues with cell sheet engineering. Biomaterials. 2007;28:5033–5043. doi: 10.1016/j.biomaterials.2007.07.052. [DOI] [PubMed] [Google Scholar]
- 166.Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- 167.Kim DH, Lipke EA, Kim P, Cheong R, Thompson S, Delannoy M, Suh KY, Tung L, Levchenko A. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc Natl Acad Sci USA. 2010;107:565–570. doi: 10.1073/pnas.0906504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rohr S, Scholly D, Kleber A. Patterned growth of neonatal rat heart cells in culture. Morphological and electrophysiological characterization. Circ Res. 1991;68:114–130. doi: 10.1161/01.res.68.1.114. [DOI] [PubMed] [Google Scholar]
- 170.Thomas SP, Bircher-Lehmann L, Thomas SA, Zhuang J, Saffitz JE, Kléber AG. Synthetic strands of neonatal mouse cardiac myocytes: structural and electro-physiological properties. Circ Res. 2000;87:467–473. doi: 10.1161/01.res.87.6.467. [DOI] [PubMed] [Google Scholar]
- 171.McDevitt TC, Angello JC, Whitney ML, Reinecke H, Hauschka SD, Murry CE, Stayton PS. In vitro generation of differentiated cardiac myofibers on micropatterned laminin surfaces. J Biomed Mater Res. 2002;60:472–479. doi: 10.1002/jbm.1292. [DOI] [PubMed] [Google Scholar]
- 172.Bursac N, Parker KK, Iravanian S, Tung L. Cardiomyocyte cultures with controlled macroscopic anisotropy. Circ Res. 2002;91:e45–e54. doi: 10.1161/01.res.0000047530.88338.eb. [DOI] [PubMed] [Google Scholar]
- 173.Deutsch J, Motlagh D, Russell B, Desai TA. Fabrication of microtextured membranes for cardiac myocyte attachment and orientation. J Biomed Mater Res. 2000;53:267–275. doi: 10.1002/(sici)1097-4636(2000)53:3<267::aid-jbm12>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 174.Motlagh D, Hartman TJ, Desai TA, Russell B. Microfabricated grooves recapitulate neonatal myocyte connexin43 and N-cadherin expression and localization. J Biomed Mater Res A. 2003;67A:148–157. doi: 10.1002/jbm.a.10083. [DOI] [PubMed] [Google Scholar]
- 175.Motlagh D, Senyo SE, Desai TA, Russell B. Microtextured substrata alter gene expression, protein localization and the shape of cardiac myocytes. Biomaterials. 2003;24:2463–2476. doi: 10.1016/s0142-9612(02)00644-0. [DOI] [PubMed] [Google Scholar]
- 176.Walsh KB, Parks GE. Changes in cardiac myocyte morphology alter the properties of voltage-gated ion channels. Cardiovasc Res. 2002;55:64–75. doi: 10.1016/s0008-6363(02)00403-0. [DOI] [PubMed] [Google Scholar]
- 177.Yin L, Bien H, Entcheva E. Scaffold topography alters intracellular calcium dynamics in cultured cardiomyocyte networks. Am J Physiol Heart Circ Physiol. 2004;287:H1276–H1285. doi: 10.1152/ajpheart.01120.2003. [DOI] [PubMed] [Google Scholar]
- 178.Au HTH, Cheng I, Chowdhury MF, Radisic M. Interactive effects of surface topography and pulsatile electrical field stimulation on orientation and elongation of fibroblasts and cardiomyocytes. Biomaterials. 2007;28:4277–4293. doi: 10.1016/j.biomaterials.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kumbar SG, et al. Electrospun nanofiber scaffolds: engineering soft tissues. Biomed Mater. 2008;3:034002. doi: 10.1088/1748-6041/3/3/034002. [DOI] [PubMed] [Google Scholar]
- 180.Shin M, Ishii O, Sueda T, Vacanti JP. Contractile cardiac grafts using a novel nanofibrous mesh. Biomaterials. 2004;25:3717–3723. doi: 10.1016/j.biomaterials.2003.10.055. [DOI] [PubMed] [Google Scholar]
- 181.Ishii O, Shin M, Sueda T, Vacanti JP. In vitro tissue engineering of a cardiac graft using a degradable scaffold with an extracellular matrix-like topography. J Thorac Cardiovasc Surg. 2005;130:1358–1363. doi: 10.1016/j.jtcvs.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 182.Zong X, Bien H, Chung C-Y, Yin L, Fang D, Hsiao BS, Chu B, Entcheva E. Electrospun fine-textured scaffolds for heart tissue constructs. Biomaterials. 2005;26:5330–5338. doi: 10.1016/j.biomaterials.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 183.Badrossamay MR, McIlwee HA, Goss JA, Parker KK. Nanofiber assembly by rotary jet-spinning. Nano Lett. 2010;10:2257–2261. doi: 10.1021/nl101355x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kenar H, Kose G, Hasirci V. Design of a 3D aligned myocardial tissue construct from biodegradable polyesters. J Mater Sci Mater Med. 2010;21:989–997. doi: 10.1007/s10856-009-3917-8. [DOI] [PubMed] [Google Scholar]
- 185.Orlova Y, Magome N, Liu L, Chen Y, Agladze K. Electrospun nanofibers as a tool for architecture control in engineered cardiac tissue. Biomaterials. 2011;32:5615–5624. doi: 10.1016/j.biomaterials.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 186.Rockwood DN, Akins RE, Jr, Parrag IC, Woodhouse KA, Rabolt JF. Culture on electrospun polyurethane scaffolds decreases atrial natriuretic peptide expression by cardiomyocytes in vitro. Biomaterials. 2008;29:4783–4791. doi: 10.1016/j.biomaterials.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Courtney T, Sacks MS, Stankus J, Guan J, Wagner WR. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials. 2006;27:3631–3638. doi: 10.1016/j.biomaterials.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 188.Ott HC, Matthiesen TS, Goh S-K, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 189.Hosseinkhani H, Hosseinkhani M, Hattori S, Matsuoka R, Kawaguchi N. Micro and nano-scale in vitro 3D culture system for cardiac stem cells. J Biomed Mater Res A. 2010;94A:1–8. doi: 10.1002/jbm.a.32676. [DOI] [PubMed] [Google Scholar]
- 190.Ji W, Sun Y, Yang F, van den Beucken J, Fan M, Chen Z, Jansen J. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm Res. 2011;28:1259–1272. doi: 10.1007/s11095-010-0320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ker EDF, Nain AS, Weiss LE, Wang J, Suhan J, Amon CH, Campbell PG. Bioprinting of growth factors onto aligned sub-micron fibrous scaffolds for simultaneous control of cell differentiation and alignment. Biomaterials. 2011;32:8097–8107. doi: 10.1016/j.biomaterials.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 192.Kai D, Prabhakaran MP, Jin G, Ramakrishna S. Polypyrrole-contained electrospun conductive nanofibrous membranes for cardiac tissue engineering. J Biomed Mater Res A. 2011;99A:376–385. doi: 10.1002/jbm.a.33200. [DOI] [PubMed] [Google Scholar]
- 193.Kenar H, Kose GT, Toner M, Kaplan DL, Hasirci V. A 3D aligned microfibrous myocardial tissue construct cultured under transient perfusion. Biomaterials. 2011;32:5320–5329. doi: 10.1016/j.biomaterials.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Feinberg AW, Parker KK. Surface-initiated assembly of protein nanofabrics. Nano Lett. 2010;10:2184–2191. doi: 10.1021/nl100998p. [DOI] [PubMed] [Google Scholar]
- 195.Luna JI, Ciriza J, Garcia-Ojeda ME, Kong M, Herren A, Lieu DK-T, Li R, Fowlkes CC, Khine M, McCloskey KE. Multi-scale biomimetic topography for the alignment of neonatal and embryonic stem cell-derived heart cells. Tissue Eng Part C. 2011;17:579–588. doi: 10.1089/ten.TEC.2010.0410. [DOI] [PubMed] [Google Scholar]
- 196.Kim D-H, Kim P, Song I, Cha JM, Lee SH, Kim B, Suh KY. Guided three-dimensional growth of functional cardiomyocytes on polyethylene glycol nanostructures. Langmuir. 2006;22:5419–5426. doi: 10.1021/la060283u. [DOI] [PubMed] [Google Scholar]
- 197.Kim D-H, Lipke EA, Kim P, Cheong R, Thompson S, Delannoy M, Suh K-Y, Tung L, Levchenko A. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc Natl Acad Sci. 2010;107:565–570. doi: 10.1073/pnas.0906504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhao Y, Zeng H, Nam J, Agarwal S. Fabrication of skeletal muscle constructs by topographic activation of cell alignment. Biotechnol Bioeng. 2009;102:624–631. doi: 10.1002/bit.22080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011;44:318–331. doi: 10.1002/mus.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, Geisse NA, Adams JC, Whitesides GM, Ingber DE. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. FASEB J. 2002;16:1195–1204. doi: 10.1096/fj.02-0038com. [DOI] [PubMed] [Google Scholar]
- 201.Clark P, Coles D, Peckham M. Preferential adhesion to and survival on patterned laminin organizes myogenesis in vitro. Exp Cell Res. 1997;230:275–283. doi: 10.1006/excr.1996.3429. [DOI] [PubMed] [Google Scholar]
- 202.Evans DJR, Britland S, Wigmore PM. Differential response of fetal and neonatal myoblasts to topographical guidance cues in vitro. Dev Genes Evol. 1999;209:438–442. doi: 10.1007/s004270050275. [DOI] [PubMed] [Google Scholar]
- 203.Clark P, Dunn GA, Knibbs A, Peckham M. Alignment of myoblasts on ultrafine gratings inhibits fusion in vitro. Int J Biochem Cell Biol. 2002;34:816–825. doi: 10.1016/s1357-2725(01)00180-7. [DOI] [PubMed] [Google Scholar]
- 204.Patz TM, Doraiswamy A, Narayan RJ, Modi R, Chrisey DB. Two-dimensional differential adherence and alignment of C2C12 myoblasts. Mater Sci Eng B. 2005;123:242–247. [Google Scholar]
- 205.Riechle M, Altomare L, Gadegaard N, Tanzi M, Fare S. Microcontact printing of fibronectin on a biodegradable polymeric surface fo skeletal muscle cell orientation. Int J Artif Organs. 2010;33:535–543. doi: 10.1177/039139881003300804. [DOI] [PubMed] [Google Scholar]
- 206.Charest JL, García AJ, King WP. Myoblast alignment and differentiation on cell culture substrates with microscale topography and model chemistries. Biomaterials. 2007;28:2202–2210. doi: 10.1016/j.biomaterials.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 207.Shimizu K, Fujita H, Nagamori E. Alignment of skeletal muscle myoblasts and myotubes using linear micropatterned surfaces ground with abrasives. Biotechnol Bioeng. 2009;103:631–638. doi: 10.1002/bit.22268. [DOI] [PubMed] [Google Scholar]
- 208.Lam MT, Sim S, Zhu X, Takayama S. The effect of continuous wavy micropatterns on silicone substrates on the alignment of skeletal muscle myoblasts and myotubes. Biomaterials. 2006;27:4340–4347. doi: 10.1016/j.biomaterials.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 209.Gingras J, Rioux RM, Cuvelier D, Geisse NA, Lichtman JW, Whitesides GM, Mahadevan L, Sanes JR. Controlling the orientation and synaptic differentiation of myotubes with micropatterned substrates. Biophys J. 2009;97:2771–2779. doi: 10.1016/j.bpj.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Huber A, Pickett A, Shakesheff KM. Reconstruction of spatially oriented myotubes in vitro using electrospun, parallel, microfibre arrays. Eur Cell Mater. 2007;14:56–63. doi: 10.22203/ecm.v014a06. [DOI] [PubMed] [Google Scholar]
- 211.Wang P-Y, Yu H-T, Tsai W-B. Modulation of alignment and differentiation of skeletal myoblasts by submicron ridges/grooves surface structure. Biotechnol Bioeng. 2010;106:285–294. doi: 10.1002/bit.22697. [DOI] [PubMed] [Google Scholar]
- 212.Dugan JM, Gough JE, Eichhorn SJ. Directing the morphology and differentiation of skeletal muscle cells using oriented cellulose nanowhiskers. Biomacromolecules. 2010;11:2498–2504. doi: 10.1021/bm100684k. [DOI] [PubMed] [Google Scholar]
- 213.Huang NF, Patel S, Thakar RG, Wu J, Hsiao BS, Chu B, Lee RJ, Li S. Myotube assembly on nanofibrous and micropatterned polymers. Nano Lett. 2006;6:537–542. doi: 10.1021/nl060060o. [DOI] [PubMed] [Google Scholar]
- 214.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 215.Ono K, Kawamura K. Migration of immature neurons along tangentially oriented fibers in the subpial part of the fetal mouse medulla-oblongata. Exp Brain Res. 1989;78:290–300. doi: 10.1007/BF00228900. [DOI] [PubMed] [Google Scholar]
- 216.Yurchenco PD, Wadsworth WG. Assembly and tissue functions of early embryonic laminins and netrins. Curr Opin Cell Biol. 2004;16:572–579. doi: 10.1016/j.ceb.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 217.Tsiper MV, Yurchenco PD. Laminin assembles into separate basement membrane and fibrillar matrices in Schwann cells. J Cell Sci. 2002;115:1005–1015. doi: 10.1242/jcs.115.5.1005. [DOI] [PubMed] [Google Scholar]
- 218.Hatten ME. Riding the glial monorail — a common mechanism for glial-guided neuronal migration in different regions of the developing mammalian brain. Trends Neurosci. 1990;13:179–184. doi: 10.1016/0166-2236(90)90044-b. [DOI] [PubMed] [Google Scholar]
- 219.Hynes RO, Patel R, Miller RH. Migration of neuroblasts along preexisting axonal tracts during prenatal cerebellar development. J Neurosci. 1986;6:867–876. doi: 10.1523/JNEUROSCI.06-03-00867.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Stepien E, Stanisz J, Korohoda W. Contact guidance of chick embryo neurons on single scratches in glass and on underlying aligned human skin fibroblasts. Cell Biol Int. 1999;23:105–116. doi: 10.1006/cbir.1998.0256. [DOI] [PubMed] [Google Scholar]
- 221.Kofron CM, Liu YT, Lopez-Fagundo CY, Mitchel JA, Hoffman-Kim D. Neurite outgrowth at the biomimetic interface. Ann Biomed Eng. 2010;38:2210–2225. doi: 10.1007/s10439-010-0054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 223.Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. Neurite branching on deformable substrates. Neuroreport. 2002;13:2411–2415. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Leach JB, Brown XQ, Jacot JG, DiMilla PA, Wong JY. Neurite outgrowth and branching of PC12 cells on very soft substrates sharply decreases below a threshold of substrate rigidity. J Neural Eng. 2007;4:26–34. doi: 10.1088/1741-2560/4/2/003. [DOI] [PubMed] [Google Scholar]
- 225.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 226.Ellis-Behnke RG, Liang YX, You SW, Tay DKC, Zhang SG, So KF, Schneider GE. Nano neuro knitting: peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision. Proc Natl Acad Sci. 2006;103:5054–5059. doi: 10.1073/pnas.0600559103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Thonhoff JR, Lou DI, Jordan PM, Zhao X, Wu P. Compatibility of human fetal neural stem cells with hydrogel biomaterials in vitro. Brain Res. 2008;1187:42–51. doi: 10.1016/j.brainres.2007.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Song YL, Li YX, Zheng QX, Wu K, Guo XD, Wu YC, Yin M, Wu Q, Fu XL. Neural progenitor cells survival and neuronal differentiation in peptide-based hydrogels. J Biomater Sci Polym Ed. 2011;22:475–487. doi: 10.1163/092050610X487756. [DOI] [PubMed] [Google Scholar]
- 229.Xie JW, Willerth SM, Li XR, Macewan MR, Rader A, Sakiyama-Elbert SE, Xia YN. The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials. 2009;30:354–362. doi: 10.1016/j.biomaterials.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mahairaki V, Lim SH, Christopherson GT, Xu LY, Nasonkin I, Yu C, Mao HQ, Koliatsos VE. Nanofiber matrices promote the neuronal differentiation of human embryonic stem cell-derived neural precursors in vitro. Tissue Eng Part. 2011;17:855–863. doi: 10.1089/ten.tea.2010.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ahmed I, Liu HY, Mamiya PC, Ponery AS, Babu AN, Weik T, Schindler M, Meiners S. Three-dimensional nanofibrillar surfaces covalently modified with tenascin-C-derived peptides enhance neuronal growth in vitro. J Biomed Mater Res A. 2006;76A:851–860. doi: 10.1002/jbm.a.30587. [DOI] [PubMed] [Google Scholar]
- 232.Patel S, Kurpinski K, Quigley R, Gao HF, Hsiao BS, Poo MM, Li S. Bioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett. 2007;7:2122–2128. doi: 10.1021/nl071182z. [DOI] [PubMed] [Google Scholar]
- 233.Lee JY, Bashur CA, Goldstein AS, Schmidt CE. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials. 2009;30:4325–4335. doi: 10.1016/j.biomaterials.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Delgado-Rivera R, Harris SL, Ahmed I, Babu AN, Patel RP, Ayres V, Flowers D, Meiners S. Increased FGF-2 secretion and ability to support neurite outgrowth by astrocytes cultured on polyamide nanofibrillar matrices. Matrix Biol. 2009;28:137–147. doi: 10.1016/j.matbio.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 235.Wittmer CR, Claudepierre T, Reber M, Wiedemann P, Garlick JA, Kaplan D, Egles C. Multifunctionalized electrospun silk fibers promote axon regeneration in the central nervous system. Adv Funct Mater. 2011;21:4232–4242. doi: 10.1002/adfm.201190103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bakhru S, Nain AS, Highley C, Wang J, Campbell P, Amon C, Zappe S. Direct, cell signaling-based, geometry-induced neuronal differentiation of neural stem cells. Integr Biol. 2011;3:1207–1214. doi: 10.1039/c1ib00098e. [DOI] [PubMed] [Google Scholar]
- 237.Lee YS, Collins G, Arinzeh TL. Neurite extension of primary neurons on electrospun piezoelectric scaffolds. Acta Biomater. 2011;7:3877–3886. doi: 10.1016/j.actbio.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 238.Carlberg B, Axell MZ, Nannmark U, Liu J, Kuhn HG. Electrospun polyurethane scaffolds for proliferation and neuronal differentiation of human embryonic stem cells. Biomed Mater. 2009;4:045004. doi: 10.1088/1748-6041/4/4/045004. [DOI] [PubMed] [Google Scholar]
- 239.Cho YI, Choi JS, Jeong SY, Yoo HS. Nerve growth factor (NGF)-conjugated electrospun nanostructures with topographical cues for neuronal differentiation of mesenchymal stem cells. Acta Biomater. 2010;6:4725–4733. doi: 10.1016/j.actbio.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 240.Yim EKF, Pang SW, Leong KW. Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp Cell Res. 2007;313:1820–1829. doi: 10.1016/j.yexcr.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lee MR, Kwon KW, Jung H, Kim HN, Suh KY, Kim K, Kim KS. Direct differentiation of human embryonic stem cells into selective neurons on nanoscale ridge/groove pattern arrays. Biomaterials. 2010;31:4360–4366. doi: 10.1016/j.biomaterials.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 242.Clark P, Britland S, Connolly P. Growth cone guidance and neuron morphology on micropatterned laminin surfaces. J Cell Sci. 1993;105:203–212. doi: 10.1242/jcs.105.1.203. [DOI] [PubMed] [Google Scholar]
- 243.Dowell-Mesfin NM, Abdul-Karim M-A, Turner AMP, Schanz S, Craighead HG, Roysam B, Turner JN, Shain W. Topographically modified surfaces affect orientation and growth of hippocampal neurons. J Neural Eng. 2004;1:78–90. doi: 10.1088/1741-2560/1/2/003. [DOI] [PubMed] [Google Scholar]
- 244.Jang KJ, Kim MS, Feltrin D, Jeon NL, Suh KY, Pertz O. Two distinct filopodia populations at the growth cone allow to sense nanotopographical extracellular matrix cues to guide neurite outgrowth. PLoS One. 2010;5:e15966. doi: 10.1371/journal.pone.0015966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Staii C, Viesselmann C, Ballweg J, Williams JC, Dent EW, Coppersmith SN, Eriksson MA. Distance dependence of neuronal growth on nanopatterned gold surfaces. Langmuir. 2011;27:233–239. doi: 10.1021/la102331x. [DOI] [PubMed] [Google Scholar]
- 246.Staii C, Viesselmann C, Ballweg J, Shi LF, Liu GY, Williams JC, Dent EW, Coppersmith SN, Eriksson MA. Positioning and guidance of neurons on gold surfaces by directed assembly of proteins using atomic force microscopy. Biomaterials. 2009;30:3397–3404. doi: 10.1016/j.biomaterials.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 247.de Asis ED, Nguyen-Vu TDB, Arumugam PU, Chen H, Cassell AM, Andrews RJ, Yang CY, Li J. High efficient electrical stimulation of hippocampal slices with vertically aligned carbon nanofiber microbrush array. Biomed Microdevices. 2009;11:801–808. doi: 10.1007/s10544-009-9295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Xu XY, Li XT, Peng SW, Xiao JF, Liu C, Fang G, Chen KC, Chen GQ. The behaviour of neural stem cells on polyhydroxyalkanoate nanofiber scaffolds. Biomaterials. 2010;31:3967–3975. doi: 10.1016/j.biomaterials.2010.01.132. [DOI] [PubMed] [Google Scholar]
- 249.Li XR, MacEwan MR, Xie JW, Siewe D, Yuan XY, Xia YN. Fabrication of density gradients of biodegradable polymer microparticles and their use in guiding neurite outgrowth. Adv Funct Mater. 2010;20:1632–1637. doi: 10.1002/adfm.201000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Recknor JB, Sakaguchi DS, Mallapragada SK. Directed growth and selective differentiation of neural progenitor cells on micropatterned polymer substrates. Biomaterials. 2006;27:4098–4108. doi: 10.1016/j.biomaterials.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 251.Oh JS, Recknor JB, Recknor JC, Mallapragada SK, Sakaguchi DS. Soluble factors from neocortical astrocytes enhance neuronal differentiation of neural progenitor cells from adult rat hippocampus on micropatterned polymer substrates. J Biomed Mater Res A. 2009;91A:575–585. doi: 10.1002/jbm.a.32242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Beduer A, Vieu C, Arnauduc F, Sol JC, Loubinoux I, Vaysse L. Engineering of adult human neural stem cells differentiation through surface micropatterning. Biomaterials. 2012;33:504–514. doi: 10.1016/j.biomaterials.2011.09.073. [DOI] [PubMed] [Google Scholar]
- 253.Park KI, Teng YD, Snyder EY. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat Biotechnol. 2002;20:1111–1117. doi: 10.1038/nbt751. [DOI] [PubMed] [Google Scholar]
- 254.Klahr S, Miller SB. Current concepts — acute oliguria. N Engl J Med. 1998;338:671–675. doi: 10.1056/NEJM199803053381007. [DOI] [PubMed] [Google Scholar]
- 255.Humes HD, Fissell WH, Tiranathanagul K. The future of hemodialysis membranes. Kidney Int. 2006;69:1115–1119. doi: 10.1038/sj.ki.5000204. [DOI] [PubMed] [Google Scholar]
- 256.Borenstein JT, Weinberg EJ, Orrick BK, Sundback C, Kaazempur-Mofrad MR, Vacanti JP. Microfabrication of three-dimensional engineered scaffolds. Tissue Eng. 2007;13:1837–1844. doi: 10.1089/ten.2006.0156. [DOI] [PubMed] [Google Scholar]
- 257.Resnick N, Yahav H, Shay-Salit A, Shushy M, Schubert S, Zilberman LC, Wofovitz E. Fluid shear stress and the vascular endothelium: for better and for worse. Prog Biophys Mol Biol. 2003;81:177–199. doi: 10.1016/s0079-6107(02)00052-4. [DOI] [PubMed] [Google Scholar]
- 258.Mulvany MJ. Small artery remodeling in hypertension. Curr Hypertens Rep. 2002;4:49–55. doi: 10.1007/s11906-002-0053-y. [DOI] [PubMed] [Google Scholar]
- 259.Mulvany MJ. Structural abnormalities of the resistance vasculature in hypertension. J Vasc Res. 2003;40:558–560. doi: 10.1159/000075805. [DOI] [PubMed] [Google Scholar]
- 260.Kamiya A, Bukhari R, Togawa T. Adaptive regulation of wall shear stress optimizing vascular tree function. Bull Math Biol. 1984;46:127–137. doi: 10.1007/BF02463726. [DOI] [PubMed] [Google Scholar]
- 261.Kamiya A, Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol. 1980;239:H14–H21. doi: 10.1152/ajpheart.1980.239.1.H14. [DOI] [PubMed] [Google Scholar]
- 262.Clark ER. Studies on the growth of blood-vessels in the tail of the frog larva — by observation and experiment on the living animal. Am J Anat. 1918;23:37–88. [Google Scholar]
- 263.Meeson A, Palmer M, Calfon M, Lang R. A relationship between apoptosis and flow during programmed capillary regression is revealed by vital analysis. Development. 1996;122:3929–3938. doi: 10.1242/dev.122.12.3929. [DOI] [PubMed] [Google Scholar]
- 264.Barakat AI. A model for shear stress-induced deformation of a flow sensor on the surface of vascular endothelial cells. J Theor Biol. 2001;210:221–236. doi: 10.1006/jtbi.2001.2290. [DOI] [PubMed] [Google Scholar]
- 265.Olesen SP, Clapham DE, Davies PF. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- 266.Schwarz G, Callewaert G, Droogmans G, Nilius B. Shear stress-induced calcium transients in endothelial cells from human umbilical cord veins. J Physiol. 1992;458:527–538. doi: 10.1113/jphysiol.1992.sp019432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Traub O, Ishida T, Ishida M, Tupper JC, Berk BC. Shear stress-mediated extracellular signal-regulated kinase activation is regulated by sodium in endothelial cells. Potential role for a voltage-dependent sodium channel. J Biol Chem. 1999;274:20144–20150. doi: 10.1074/jbc.274.29.20144. [DOI] [PubMed] [Google Scholar]
- 268.Park H, Go YM, Darji R, Choi JW, Lisanti MP, Maland MC, Jo H. Caveolin-1 regulates shear stress-dependent activation of extracellular signal-regulated kinase. Am J Physiol Heart Circ Physiol. 2000;278:H1285–H1293. doi: 10.1152/ajpheart.2000.278.4.H1285. [DOI] [PubMed] [Google Scholar]
- 269.Park H, Go YM, St John PL, Maland MC, Lisanti MP, Abrahamson DR, Jo H. Plasma membrane cholesterol is a key molecule in shear stress-dependent activation of extracellular signal-regulated kinase. J Biol Chem. 1998;273:32304–32311. doi: 10.1074/jbc.273.48.32304. [DOI] [PubMed] [Google Scholar]
- 270.Gudi SR, Lee AA, Clark CB, Frangos JA. Equibiaxial strain and strain rate stimulate early activation of G proteins in cardiac fibroblasts. Am J Physiol. 1998;274:C1424–C1428. doi: 10.1152/ajpcell.1998.274.5.C1424. [DOI] [PubMed] [Google Scholar]
- 271.Gloe T, Sohn HY, Meininger GA, Pohl U. Shear stress-induced release of basic fibro-blast growth factor from endothelial cells is mediated by matrix interaction via integrin alpha(V)beta(3) J Biol Chem. 2002;277:23453–23458. doi: 10.1074/jbc.M203889200. [DOI] [PubMed] [Google Scholar]
- 272.Fujiwara K, Masuda M, Osawa M, Kano Y, Katoh K. A PECAM-1 a mechanoresponsive molecule? Cell Struct Funct. 2001;26:11–17. doi: 10.1247/csf.26.11. [DOI] [PubMed] [Google Scholar]
- 273.Shay-Salit A, Shushy M, Wolfovitz E, Yahav H, Breviario F, Dejana E, Resnick N. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc Natl Acad Sci. 2002;99:9462–9467. doi: 10.1073/pnas.142224299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell-surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 275.Hwang SY, Kwon KW, Jang KJ, Park MC, Lee JS, Suh KY. Adhesion assays of endothelial cells on nanopatterned surfaces within a microfluidic channel. Anal Chem. 2010;82:3016–3022. doi: 10.1021/ac100107z. [DOI] [PubMed] [Google Scholar]
- 276.Liliensiek SJ, Wood JA, Yong JA, Auerbach R, Nealey PF, Murphy CJ. Modulation of human vascular endothelial cell behaviors by nanotopographic cues. Biomaterials. 2010;31:5418–5426. doi: 10.1016/j.biomaterials.2010.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Uttayarat P, Chen M, Li M, Allen FD, Composto RJ, Lelkes PI. Microtopography and flow modulate the direction of endothelial cell migration. Am J Physiol Heart Circ Physiol. 2008;294:H1027–H1035. doi: 10.1152/ajpheart.00816.2007. [DOI] [PubMed] [Google Scholar]
- 278.Feinberg AW, Wilkerson WR, Seegert CA, Gibson AL, Hoipkemeier-Wilson L, Brennan AB. Systematic variation of microtopography, surface chemistry and elastic modulus and the state dependent effect on endothelial cell alignment. J Biomed Mater Res A. 2008;86A:522–534. doi: 10.1002/jbm.a.31626. [DOI] [PubMed] [Google Scholar]
- 279.Carpenter J, Khang D, Webster TJ. Nanometer polymer surface features: the influence on surface energy, protein adsorption and endothelial cell adhesion. Nanotechnology. 2008;19:505103. doi: 10.1088/0957-4484/19/50/505103. [DOI] [PubMed] [Google Scholar]
- 280.Zorlutuna P, Rong Z, Vadgama P, Hasirci V. Influence of nanopatterns on endothelial cell adhesion: enhanced cell retention under shear stress. Acta Biomater. 2009;5:2451–2459. doi: 10.1016/j.actbio.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 281.Lu J, Rao MP, MacDonald NC, Khang D, Webster TJ. Improved endothelial cell adhesion, proliferation on patterned titanium surfaces with rationally designed, micrometer to nanometer features. Acta Biomater. 2008;4:192–201. doi: 10.1016/j.actbio.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 282.Miller DC, Thapa A, Haberstroh KM, Webster TJ. Endothelial and vascular smooth muscle cell function on poly(lactic-co-glycolic acid) with nanostructured surface features. Biomaterials. 2004;25:53–61. doi: 10.1016/s0142-9612(03)00471-x. [DOI] [PubMed] [Google Scholar]
- 283.Peng L, Eltgroth ML, LaTempa TJ, Grimes CA, Desai TA. The effect of TiO(2) nanotubes on endothelial function and smooth muscle proliferation. Biomaterials. 2009;30:1268–1272. doi: 10.1016/j.biomaterials.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 284.Zhang XH, Baughman CB, Kaplan DL. In vitro evaluation of electrospun silk fibroin scaffolds for vascular cell growth. Biomaterials. 2008;29:2217–2227. doi: 10.1016/j.biomaterials.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wise SG, Byrom MJ, Waterhouse A, Bannon PG, Ng MKC, Weiss AS. A multilayered synthetic human elastin/polycaprolactone hybrid vascular graft with tailored mechanical properties. Acta Biomater. 2011;7:295–303. doi: 10.1016/j.actbio.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 286.Gasiorowski JZ, Liliensiek SJ, Russell P, Stephan DA, Nealey PF, Murphy CJ. Alterations in gene expression of human vascular endothelial cells associated with nanotopographic cues. Biomaterials. 2010;31:8882–8888. doi: 10.1016/j.biomaterials.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.He W, Yong T, Ma ZW, Inai R, Teo WE, Ramakrishna S. Biodegradable polymer nanofiber mesh to maintain functions of endothelial cells. Tissue Eng. 2006;12:2457–2466. doi: 10.1089/ten.2006.12.2457. [DOI] [PubMed] [Google Scholar]
- 288.He W, Ma ZW, Yong T, Teo WE, Ramakrishna S. Fabrication of collagen-coated biodegradable polymer nanofiber mesh and its potential for endothelial cells growth. Biomaterials. 2005;26:7606–7615. doi: 10.1016/j.biomaterials.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 289.Bettinger CJ, Zhang ZT, Gerecht S, Borenstein JT, Langer R. Enhancement of in vitro capillary tube formation by substrate nanotopography. Adv Mater. 2008;20:99–103. doi: 10.1002/adma.200702487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Santos MI, Fuchs S, Gomes ME, Unger RE, Reis RL, Kirkpatrick CJ. Response of micro- and macrovascular endothelial cells to starch-based fiber meshes for bone tissue engineering. Biomaterials. 2007;28:240–248. doi: 10.1016/j.biomaterials.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 291.Lee SH, Kang DH, Kim HN, Suh KY. Use of directly molded poly(methyl methacrylate) channels for microfluidic applications. Lab Chip. 2010;10:3300–3306. doi: 10.1039/c0lc00127a. [DOI] [PubMed] [Google Scholar]
- 292.Stitzel J, Liu L, Lee SJ, Komura M, Berry J, Soker S, Lim G, Van Dyke M, Czerw R, Yoo JJ, Atala A. Controlled fabrication of a biological vascular substitute. Biomaterials. 2006;27:1088–1094. doi: 10.1016/j.biomaterials.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 293.Ahmad PJ, Osborne LR, Bendeck MP. Bouncing back from elastin deficiency. Circ Res. 2007;101:439–440. doi: 10.1161/CIRCRESAHA.107.160358. [DOI] [PubMed] [Google Scholar]
- 294.Wolinsky H, Glagov S. A lamellar unit of aortic medial structure and function in mammals. Circ Res. 1967;20:99–111. doi: 10.1161/01.res.20.1.99. [DOI] [PubMed] [Google Scholar]
- 295.Wolinsky H, Glagov S. Structural basis for the static mechanical properties of the aortic media. Circ Res. 1964;14:400–413. doi: 10.1161/01.res.14.5.400. [DOI] [PubMed] [Google Scholar]
- 296.Lanir Y. Osmotic swelling and residual stress in cardiovascular tissues. J Biomech. 2012;45:780–789. doi: 10.1016/j.jbiomech.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 297.Isenberg BC, Wong JY. Building structure into engineered tissues. Mater Today. 2006;9:54–60. [Google Scholar]
- 298.Hu W, Yim EKF, Reano RM, Leong KW, Pang SW. Effects of nanoimprinted patterns in tissue-culture polystyrene on cell behavior. J Vac Sci Technol B. 2005;23:2984–2989. doi: 10.1116/1.2121729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Yim EKF, Reano RM, Pang SW, Yee AF, Chen CS, Leong KW. Nanopattern-induced changes in morphology and motility of smooth muscle cells. Biomaterials. 2005;26:5405–5413. doi: 10.1016/j.biomaterials.2005.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Xu CY, Inai R, Kotaki M, Ramakrishna S. Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials. 2004;25:877–886. doi: 10.1016/s0142-9612(03)00593-3. [DOI] [PubMed] [Google Scholar]
- 301.Sarkar S, Dadhania M, Rourke P, Desai TA, Wong JY. Vascular tissue engineering: microtextured scaffold templates to control organization of vascular smooth muscle cells and extracellular matrix. Acta Biomater. 2005;1:93–100. doi: 10.1016/j.actbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 302.Thakar RG, Cheng Q, Patel S, Chu J, Nasir M, Liepmann D, Komvopoulos K, Li S. Cell-shape regulation of smooth muscle cell proliferation. Biophys J. 2009;96:3423–3432. doi: 10.1016/j.bpj.2008.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Cao Y, Poon YF, Feng J, Rayatpisheh S, Chan V, Chan-Park MB. Regulating orientation and phenotype of primary vascular smooth muscle cells by biodegradable films patterned with arrays of microchannels and discontinuous microwalls. Biomaterials. 2010;31:6228–6238. doi: 10.1016/j.biomaterials.2010.04.059. [DOI] [PubMed] [Google Scholar]
- 304.Shen JY, Chan-Park MB, Zhu AP, Zhu X, Beuerman RW, Yang EB, Chen W, Chan V. Three-dimensional microchannels in biodegradable polymeric films for control orientation and phenotype of vascular smooth muscle cells. Tissue Eng. 2006;12:2229–2240. doi: 10.1089/ten.2006.12.2229. [DOI] [PubMed] [Google Scholar]
- 305.Isenberg BC, Tsuda Y, Williams C, Shimizu T, Yamato M, Okano T, Wong JY. A thermoresponsive, microtextured substrate for cell sheet engineering with defined structural organization. Biomaterials. 2008;29:2565–2572. doi: 10.1016/j.biomaterials.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Zorlutuna P, Elsheikh A, Hasirci V. Nanopatterning of collagen scaffolds improve the mechanical properties of tissue engineered vascular grafts. Biomacromolecules. 2009;10:814–821. doi: 10.1021/bm801307y. [DOI] [PubMed] [Google Scholar]
- 307.Isenberg BC, Backman DE, Kinahan ME, Jesudason R, Suki B, Stone PJ, Davis EC, Wong JY. Micropatterned cell sheets with defined cell and extracellular matrix orientation exhibit anisotropic mechanical properties. J Biomech. 2012;45:756–761. doi: 10.1016/j.jbiomech.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 308.Sarkar S, Isenberg BC, Hodis E, Leach JB, Desai TA, Wong JY. Fabrication of a layered microstructured polycaprolactone construct for 3-D tissue engineering. J Biomater Sci Polym Ed. 2008;19:1347–1362. doi: 10.1163/156856208786052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Tan W, Desai TA. Microscale multilayer cocultures for biomimetic blood vessels. J Biomed Mater Res A. 2005;72A:146–160. doi: 10.1002/jbm.a.30182. [DOI] [PubMed] [Google Scholar]
- 310.Feng J, Chan-Park MB, Shen JY, Chan V. Quick layer-by-layer assembly of aligned multilayers of vascular smooth muscle cells in deep microchannels. Tissue Eng. 2007;13:1003–1012. doi: 10.1089/ten.2006.0223. [DOI] [PubMed] [Google Scholar]
- 311.Hu JA, Sun XA, Ma HY, Xie CQ, Chen YE, Ma PX. Porous nanofibrous PLLA scaffolds for vascular tissue engineering. Biomaterials. 2010;31:7971–7977. doi: 10.1016/j.biomaterials.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Godoy P, Hengstler JG, Ilkavets I, Meyer C, Bachmann A, Muller A, Tuschl G, Mueller SO, Dooley S. Extracellular matrix modulates sensitivity of hepatocytes to fibroblastoid dedifferentiation and transforming growth factor beta-induced apoptosis. Hepatology. 2009;49:2031–2043. doi: 10.1002/hep.22880. [DOI] [PubMed] [Google Scholar]
- 313.Mondrinos MJ, Koutzaki S, Jiwanmall E, Li MY, Dechadarevian JP, Lelkes PI, Finck CM. Engineering three-dimensional pulmonary tissue constructs. Tissue Eng. 2006;12:717–728. doi: 10.1089/ten.2006.12.717. [DOI] [PubMed] [Google Scholar]
- 314.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell–matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 315.Cukierman E, Pankov R, Yamada KM. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol. 2002;14:633–639. doi: 10.1016/s0955-0674(02)00364-2. [DOI] [PubMed] [Google Scholar]
- 316.Lammermann T, Sixt M. Mechanical modes of ‘amoeboid’ cell migration. Curr Opin Cell Biol. 2009;21:636–644. doi: 10.1016/j.ceb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 317.Yeh J, Ling YB, Karp JM, Gantz J, Chandawarkar A, Eng G, Blumling J, Langer R, Khademhosseini A. Micromolding of shape-controlled, harvestable cell-laden hydrogels. Biomaterials. 2006;27:5391–5398. doi: 10.1016/j.biomaterials.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 318.Khademhosseini A, Langer R. Microengineered hydrogels for tissue engineering. Biomaterials. 2007;28:5087–5092. doi: 10.1016/j.biomaterials.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 319.Ling Y, Rubin J, Deng Y, Huang C, Demirci U, Karp JM, Khademhosseini A. A cell-laden microfluidic hydrogel. Lab Chip. 2007;7:756–762. doi: 10.1039/b615486g. [DOI] [PubMed] [Google Scholar]
- 320.McMurray RJ, Gadegaard N, Tsimbouri PM, Burgess KV, McNamara LE, Tare R, Murawski K, Kingham E, Oreffo ROC, Dalby MJ. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater. 2011;10:637–644. doi: 10.1038/nmat3058. [DOI] [PubMed] [Google Scholar]
- 321.Dvir T, Timko BP, Kohane DS, Langer R. Nanotechnological strategies for engineering complex tissues. Nat Nanotechnol. 2011;6:13–22. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]