Abstract
Recently, an S haplotype–specific F-box (SFB) gene has been proposed as a candidate for the pollen-S specificity gene of RNase-mediated gametophytic self-incompatibility in Prunus (Rosaceae). We have examined two pollen-part mutant haplotypes of sweet cherry (Prunus avium). Both were found to retain the S-RNase, which determines stylar specificity, but one (S3′ in JI 2434) has a deletion including the haplotype-specific SFB gene, and the other (S4′ in JI 2420) has a frame-shift mutation of the haplotype-specific SFB gene, causing amino acid substitutions and premature termination of the protein. The loss or significant alteration of this highly polymorphic gene and the concomitant loss of pollen self-incompatibility function provides compelling evidence that the SFB gene encodes the pollen specificity component of self-incompatibility in Prunus. These loss-of-function mutations are inconsistent with SFB being the inactivator of non-self S-RNases and indicate the presence of a general inactivation mechanism, with SFB conferring specificity by protecting self S-RNases from inactivation.
INTRODUCTION
Self-incompatibility (SI) prevents inbreeding in a wide range of flowering plants (reviewed in De Nettancourt, 2001). The mechanism can be either gametophytic or sporophytic depending on the genetic control of the pollen SI phenotype. In sweet cherry (Prunus avium), a member of the Rosaceae cultivated for its fruit, SI and cross-incompatibility between various pairs of cultivars was attributed to a multiallelic S locus, expressed gametophytically (Crane and Lawrence, 1929). As in all other SI systems studied, the S locus of cherry is considered to comprise at least two parts, one expressed in the style and a complementary one expressed in the pollen, as originally proposed by Lewis (1949).
Initially the sporophytic SI of Brassica and the gametophytic SI of the Solanaceae were the most intensively studied at the molecular level, but recently several important breakthroughs have been made in the Rosaceae, which appears to have the same SI mechanism as the Solanaceae. The molecular identification of the pistil components of SI has revealed three distinct mechanisms in the sporophytic SI system of Brassica and in the gametophytic SI systems of the Solanaceae/Rosaceae/Scrophulariaceae and of Papaver (De Nettancourt, 2001). In the Solanaceae, the Rosaceae, and the Scrophulariaceae, the stylar part codes for a ribonuclease (S-RNase) (McClure et al., 1989; Broothaerts et al., 1995; Bošković and Tobutt, 1996; Xue et al., 1996). Ribonuclease activity of S-RNases is needed to inhibit the growth of pollen tubes carrying an S allele that matches an S allele present in the style (Huang et al., 1994; Royo et al., 1994), supporting a model for this type of gametophytic SI in which pollen RNA is degraded in an incompatible interaction.
The pollen component (pollen-S) in Brassica has been identified as a ligand for a receptor kinase located in the stigma (reviewed in Brugière et al., 2000). Candidates for the pollen-S gene of RNase-based SI have recently been identified in Antirrhinum of the Scrophulariaceae (Lai et al., 2002), Prunus (Entani et al., 2003; Ushijima et al., 2003; Yamane et al., 2003), and Petunia of the Solanaceae (Qiao et al., 2004; Sijacic et al., 2004). These are all S-linked F-box genes (SLF or SFB) and have implicated ubiquitination in the SI reaction. In this article, we follow the use of the abbreviation SFB (for S haplotype–specific F-box) in cherry (Yamane et al., 2003). Additional F-box genes (SLFL1-3) have been identified in Prunus S haplotypes, although these exhibit relatively low levels of polymorphism (Entani et al., 2003; Ushijima et al., 2003).
Two self-compatible cherry selections raised at the John Innes Institute (Lewis and Crowe, 1954) offer a unique opportunity for studying the molecular basis of loss of pollen-S function at a rosaceous S locus. Gain-of-function or loss-of-function transgenic experiments in Prunus tree species are hindered by the lack of an efficient transformation system and a long juvenile period. The self-compatible selections, JI 2420 and JI 2434, were obtained from the nominally incompatible cross of the cultivars Emperor Francis (S3S4) × Napoleon (S3S4, X-irradiated pollen). Both selections carry pollen-part mutations but retain normal stylar function (Matthews and Lapins, 1967; Matthews, 1970). Recently, the genotypes of JI 2420 and two accessions of JI 2434 were clarified, and the basis of their self-compatibility was studied using stylar ribonuclease analyses and test crosses (Bošković et al., 2000). In JI 2420, self-compatibility is attributed to a mutant S4 allele, denoted S4′, where the prime symbol indicates the loss of pollen-S function (Lewis and Crowe, 1954). Similarly, in JI 2434, the self-compatibility is attributed to a mutant S3 allele, S3′.
Self-compatible pollen-part mutants obtained by selfing or by crossing nominally incompatible plants using x-irradiated pollen have been described not only in P. avium, but also in Oenothera organensis of the Onagraceae (Lewis, 1949, 1951), Petunia inflata (Brewbaker and Shapiro, 1959), and Nicotiana alata (Pandey, 1965). Brewbaker and Natarajan (1960) found that all pollen-part mutants studied in P. inflata carried a centric fragment. Centric fragments, which can result from x-irradiation, are parts of a chromosome including the centromere that segregate randomly at meiosis. The centric fragments in the P. inflata pollen-part mutants carried a duplicated S allele, resulting in some pollen grains being heteroallelic. The self-compatibility of heteroallelic pollen had been advanced by Lewis and Modlibowska (1942) to explain the self-compatibility of a tetraploid pear (Pyrus communis)—pollen grains of genotype S1S2 succeed on S1S1S2S2 styles, whereas S1S1 and S2S2 pollen fails. The cross of the tetraploid as pollen parent on to the original diploid succeeds, whereas the reciprocal cross fails, indicating a breakdown of pollen-part function. This interaction between the two S alleles in heteroallelic pollen has been termed competitive interaction (Lewis, 1943). Brewbaker and Natarajan (1960) also concluded that the pollen-part mutants of Oenothera and Prunus, studied by Lewis, could have resulted from a duplication of an S allele. However, Lewis (1961) concluded that some pollen-part mutations of Oenothera should be the result of a mutation or deletion of the pollen part and stated that most of his arguments were also valid for the pollen-part mutant selections of Prunus.
In recent years, molecular analyses have been performed on pollen-part mutants generated by irradiation mutagenesis in Solanum tuberosum (Thompson et al., 1991) and N. alata (Golz et al., 1999, 2001) of the Solanaceae. In both studies, DNA gel blot analysis with S-RNase probes showed that some of the self-compatible mutants obtained had an extra S allele, either carried on a centric fragment or translocated to another chromosome. In some cases, no additional S-RNase allele was detected, although there was evidence for a chromosomal duplication, presumably including the pollen part but not the S-RNase (Golz et al., 2001). No evidence of a deletion was found in either study.
The cherry pollen-part mutations in JI 2420 and JI 2434 could, we surmised, have resulted from duplication of a pollen-S gene giving heteroallelic pollen or from a mutation in the pollen-S gene. Thus, the S3′ mutant allele of JI 2434 could either be a result of a duplicate S4 pollen-S allele (dS4) giving rise to S3dS4 pollen that would be compatible on an S3S4 style or else a mutation of the S3 pollen-S gene itself. Similarly, explanations of self-compatibility in the S4′ mutant JI 2420 are a duplicate S3 pollen-S allele (dS3) resulting in S4dS3 pollen or a mutation of the S4 pollen-S gene itself. Bošković et al. (2000) found no evidence for a duplicated S-RNase in either mutant selection with ribonuclease activity assays.
A molecular analysis of these cherry mutant S alleles has now been made possible after the cDNA cloning of the S-RNases for the S3- and S4-haplotypes (Sonneveld et al., 2001). We demonstrate that there is no evidence of a duplication in either mutant haplotype, but rather a deletion or mutation affecting the haplotype-specific SFB gene, leading to loss of function. These findings provide further evidence for the pollen-S function of SFB genes, and we discuss how these unique loss-of-function mutations can be reconciled with current models of RNase-based SI.
RESULTS
Genetic Analysis of Pollen-Part Mutations
Pollen-part mutations can theoretically result from duplication of a pollen-S allele or from a mutation of the pollen-S gene itself. Heteroallelic pollen can arise when a duplicated pollen-S gene has been translocated to the chromosome bearing the other S allele, or to a non-S chromosome, or it could be carried on a centric fragment. In the first case, the pollen-part mutation will be linked in coupling to one particular S allele; in the other two cases, the pollen-part mutation will segregate independently from the S locus. A mutation in the pollen part itself would always be associated with one S allele.
To confirm that the pollen-part mutation in JI 2420 is linked in coupling with S4, the progeny of a cross between a pollen parent deriving its self-compatibility from JI 2420 but not having S3 (Lapins, S1S4′) and a female parent with the S1 allele in common (Erika, S1S3) was analyzed. If the mutation was not linked with S4 (i.e., in the case of a duplication of the S3 pollen-S allele elsewhere in the genome), both types of pollen (S1 and S4), when carrying the duplicated S3 pollen-S allele, would be heteroallelic and able to grow on the style of the female parent even if it has the S1 allele in common (Tables 1 and 2).
Table 1.
Erika (S1S3) × Lapins (S1S4′) Expected Gamete Genotypes and Seedling Genotypes (and S-RNase Phenotypes) if S4′ Results from a Duplication of Pollen-S3 (dS3) Not Linked in Coupling with S4
| Female/Male | S1 | S4 | S1dS3 | S4dS3 |
|---|---|---|---|---|
| S1 | X | S1S4 (S1S4) | S1S1dS3 (S1) | S1S4dS3 (S1S4) |
| S3 | X | S3S4 (S3S4) | S1S3dS3 (S1S3) | S3S4dS3 (S3S4) |
X indicates incompatibility of pollen.
Table 2.
Erika (S1S3) × Lapins (S1S4′) Expected Gamete Genotypes and Seedling Genotypes (and S-RNase Phenotypes) if S4′ Results from a Deletion/Mutation of the S4 Pollen-S Allele (for Convenience Indicated by S4′) or from a Duplication of Pollen-S3 (dS3) Linked in Coupling with S4
| Female/Male | S1 | S4′ or S4dS3 |
|---|---|---|
| S1 | X | S1S4′ or S1S4dS3 (S1S4) |
| S3 | X | S3S4′ or S3S4dS3 (S3S4) |
X indicates incompatibility of pollen.
Analysis of stylar ribonucleases showed that 23 seedlings had bands for S1 and S4 (S1S4′ seedlings), and 18 seedlings had bands for S3 and S4 (S3S4′ seedlings) (data not shown). None of the seedlings showed a single band in the S1 position. Consistent rejection of the S1 pollen confirms that the mutation of JI 2420 is linked in coupling with S4 and cannot be attributed to a duplication of the S3 pollen-S allele on a centric fragment or on a non-S chromosome.
Likewise, to confirm that self-compatibility in JI 2434 (S3′S4) is linked in coupling with S3, a progeny from a cross between a pollen parent deriving self-compatibility from JI 2434 but not having the S4 allele (9239-3, S1S3′) and a female parent with the S1 allele in common (Van, S1S3) was raised and analyzed. The presence or absence of S1S1′ genotypes (single S1 band) in the progeny would indicate whether self-compatibility of JI 2434 is linked with S3 (Tables 3 and 4).
Table 3.
Van (S1S3) × 9239-3 (S1S3′) Expected Gamete Genotypes and Seedling Genotypes (and S-RNase Phenotypes) if S3′ Results from a Duplication of Pollen-S4 (dS4) Not Linked in Coupling with S3
| Female/Male | S1 | S3 | S1dS4 | S3dS4 |
|---|---|---|---|---|
| S1 | X | X | S1S1dS4 (S1) | S1S3dS4 (S1S3) |
| S3 | X | X | S1S3dS4 (S1S3) | S3S3dS4 (S3) |
X indicates incompatibility of pollen.
Table 4.
Van (S1S3) × 9239-3 (S1S3′) Expected Gamete Genotypes and Seedling Genotypes (and S-RNase Phenotypes) if S3′ Results from a Deletion/Mutation of the S3 Pollen-S Allele (for Convenience Indicated by S3′) or from a Duplication of Pollen-S4 (dS4) Linked in Coupling with S3
| Female/Male | S1 | S3′ or S3dS4 |
|---|---|---|
| S1 | X | S1S3′ or S1S3dS4 (S1S3) |
| S3 | X | S3S3′ or S3S3dS4 (S3) |
X indicates incompatibility of pollen.
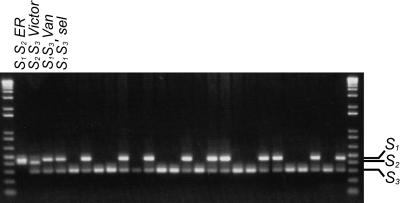
S allele genotyping of 46 seedlings by PCR using consensus primers for the first intron of cherry S-RNases showed that they segregated into two classes (example shown in Figure 1): 17 with bands for S3 and S1 (S1S3′ seedlings) and 29 with a single S3 band (S3S3′ seedlings). The absence of a class with a single S1 band (S1S1′ seedlings) indicates that the pollen-part mutation of JI 2434 (S3′S4) is linked in coupling with S3 and cannot be attributable to a duplication of the S4 pollen component on a centric fragment or on a non-S chromosome.
Figure 1.
Segregation Analysis of S Alleles to Test Cosegregation of the JI 2434 Pollen-Part Mutation with the S3 Allele.
Genomic DNA of seedlings of Van (S1S3) × 9239-3 (S1S3′) was amplified by PCR using consensus primers revealing length polymorphism of the first intron of cherry S-RNases. Samples are as follows: ER, Early Rivers (S1S2); Victor (S2S3) (standards for S1 and S3, respectively); Van (S1S3); S1S3′ selection, 22 seedlings.
DNA Gel Blot Analysis of S-RNase Regions in Pollen-Part Mutants
Because both pollen-part mutant selections appeared to have the mutation linked with a particular S allele, DNA gel blot analysis with an S-RNase probe was performed to look for evidence of a rearrangement in the regions flanking the appropriate S-RNases.
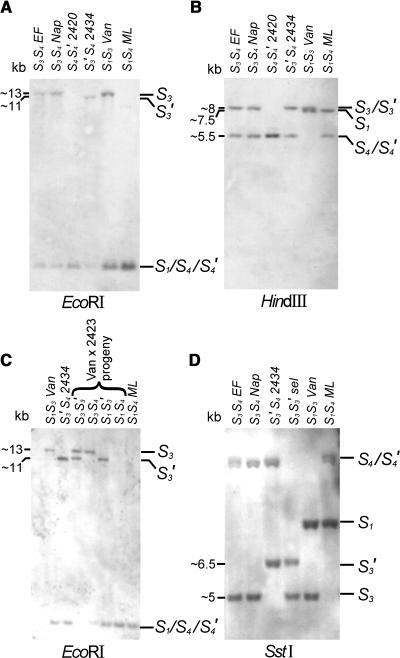
Digestion of genomic DNA with both restriction enzymes EcoRI (Figure 2A) and HindIII (Figure 2B) resulted in a single restriction fragment in the normal S4 position for JI 2420 (S4S4′) (i.e., the S4′ band was indistinguishable from S4). In the HindIII digest, the S3′ restriction fragment of JI 2434 was in the same position as the S3 fragment from the parents and the standard Van (S1S3). In the EcoRI digest, however, the S3′ restriction fragment of JI 2434 appeared to be slightly smaller than the S3 fragment. This indicated an alteration in the region flanking the S3-RNase of JI 2434.
Figure 2.
DNA Gel Blot Analysis of the S3′ and S4′ Mutants Using an S1-RNase cDNA Probe to Test for Rearrangements in the Regions Flanking the S3- and S4-RNase Genes, Respectively.
(A) EcoRI digest of parents and pollen-part mutants. EF, Emperor Francis (S3S4); Nap, Napoleon (S3S4); 2420, JI 2420 (S4S4′); 2434, JI 2434 (S3′S4); Van (S1S3); ML, Merton Late (S1S4).
(B) HindIII digest of parents and pollen-part mutants. EF, Emperor Francis (S3S4); Nap, Napoleon (S3S4); 2420, JI 2420 (S4S4′); 2434, JI 2434 (S3′S4); Van (S1S3); ML, Merton Late (S1S4).
(C) EcoRI digest of the progeny Van (S1S3) × JI 2434 (S3′S4). Van (S1S3); 2434, JI 2434 (S3′S4); S3S3′ selection; S3S4 selection; S1S3′ selection; S1S4 selection; ML, Merton Late (S1S4).
(D) SstI digest of parents and S3′ mutant. EF, Emperor Francis (S3S4); Nap, Napoleon (S3S4); 2434, JI 2434 (S3′S4); S3S3′ selection; Van (S1S3); ML, Merton Late (S1S4).
Low stringency post-hybridization washes allowed the detection of cross-hybridization of the S1-RNase cDNA probe to both the S3 and S4 alleles.
Testing four previously genotyped selections from the cross Van (S1S3) × JI 2434 (S3′S4) confirmed the shift of the S3′ restriction fragment in the EcoRI digest. As shown in Figure 2C, the S3S3′ selection shows two bands, confirming that the S3′ fragment has a lower molecular weight than the normal S3 fragment, indicating a sequence change in the region flanking the S3-RNase of JI 2434. The estimated sizes of the S3 and the S3′ fragments are ∼13 and 11 kb, respectively.
When 11 more restriction enzymes were tested and fragment sizes estimated (Table 5), none of the enzymes showed a fragment size shift for S4′ compared with S4. A band shift for S3′ was, however, found with KpnI, PaeI, PstI, SstI, and XbaI, indicating a major sequence rearrangement flanking the S3-RNase. Whether the rearrangement in the S3′-haplotype is in the 5′ or 3′ flanking region of the S3-RNase was investigated by inclusion of an enzyme cutting at the 5′ end of the S3-RNase sequence (PstI) and an enzyme cutting toward the 3′ end of the S3-RNase sequence (BamHI). Because a band shift was found for PstI, but not for BamHI, it appeared that the rearrangement had occurred downstream of the S3-RNase gene. The SstI digest (Figure 2D) shows that the breakpoint of the rearrangement in the S3′-haplotype must be within ∼3.5 kb of the S3-RNase gene.
Table 5.
Restriction Enzymes Used for DNA Gel Blot Analysis with S-RNase Probe and Estimated Fragment Sizes for Each of the S Alleles Present
| Estimated Size of Restriction Fragments (kb)a
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allele | BamHIb | BclI | EcoRIc | HindIII | KpnIcd | PstIce | NcoI | NdeI | PaeIc | ScaId | SstIc | SspId | XbaIc | EcoRI/HindIII |
| S1 | 14.5 | 7.5 | ∼2 | 7.5 | 21.0 | 23 | 14.5 | 6.5 | 11.5 | 5.5 | 11.5 | ∼2 | 12 | ∼2.0 |
| S3 | 9.5 | 6.0 | 13 | 8.0 | 20.0 | 7 | 8.0 | 9.5 | 7.5 | ∼4.0 | 5.0 | ∼3 + 3.5 | 22 | 7.5 |
| S3′ | 9.5 | 6.0 | 11 | 8.0 | 6.5 | 15 | 8.0 | 9.5 | 16.5 | ∼4.0 | 6.5 | ∼3 + 3.5 | 20 | 7.5 |
| S4 | >30.0 | 7.5 | ∼2 | 5.5 | 10.5 | ∼3 | 4.5 | 17.0 | 13.0 | 6.5 | 12.5 | ∼1 | 8 | ∼2.0 |
| S4′ | >30.0 | 7.5 | ∼2 | 5.5 | 10.5 | ∼3 | 4.5 | 17.0 | 13.0 | 6.5 | 12.5 | ∼1 | 8 | ∼2.0 |
Sizes of the bands were estimated by comparison with the 5-kb DNA ladder (Invitrogen). Please note that the size fragments under 5 kb and over 30 kb could not be estimated accurately.
Restriction site at 3′ end of S3-RNase (just before C5).
Restriction enzyme reveals a fragment size shift for S3′.
Restriction site in S3-RNase between C3 and RC4 (SspI), between C2 and C3 (KpnI), and just after RC4 (ScaI); the probe does not always detect both resulting fragments.
Restriction site at 5′ end of S3-RNase (just after C1).
The DNA gel blot analyses of the S1S3′ and the S3S3′ selection confirmed that JI 2434 does not have a duplicated S4-RNase gene, in accordance with previous reports (Bošković et al., 2000). Similarly, there is no evidence of a duplicated S3-RNase gene in JI 2420.
Cloning of the S4-Haplotype–Specific SFB Sequence
To study the haplotype-specific SFB genes, recently identified as candidates for the pollen-S gene in Prunus (Entani et al., 2003; Ushijima et al., 2003; Yamane et al., 2003), the S4-haplotype–specific SFB (S4-SFB) genomic sequence was cloned by genomic PCR using degenerate primers. The S3-haplotype–specific SFB (S3-SFB) sequence was available from the EMBL database (Yamane et al., 2003).
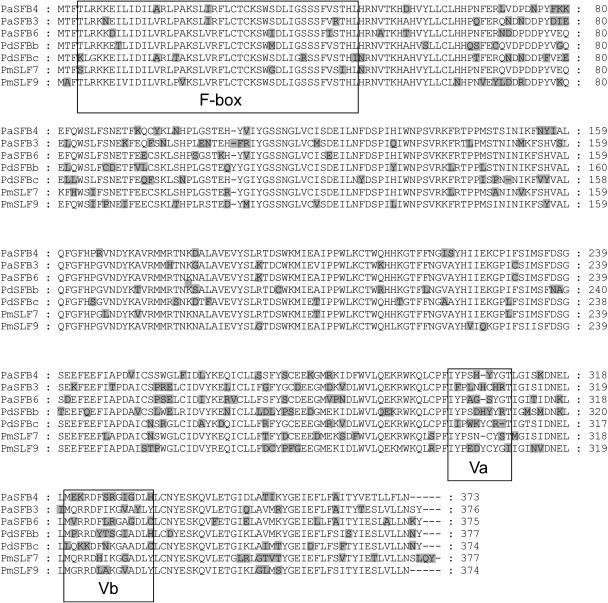
The deduced amino acid sequence of the putative S4-SFB was aligned with other Prunus SFB sequences (Figure 3). It shows high sequence similarity with the other sequences (74.7 to 81.5% amino acid identity) and contains the expected F-box motif and two variable regions toward the 3′ end (Ushijima et al., 2003).
Figure 3.
Alignment of the Predicted Amino Acid Sequence of the S4-Haplotype–Specific SFB with Other Prunus SFB/SLF Sequences.
Seven SFB sequences of Prunus (Pa, P. avium; Pd, P. dulcis; Pm, P. mume) aligned using ClustalW. Residues highlighted in gray denote divergence from the observed consensus, and dashes represent gaps. The F-box region and two variable regions Va and Vb (as indicated in Ushijima et al., 2003) are boxed.
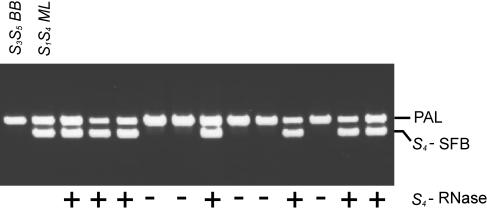
Cosegregation of the cloned putative S4-SFB sequence with the S4 allele was confirmed in a progeny segregating for S4 using allele-specific primers (Figure 4). Seedlings showing amplification had previously been found to carry the S4 allele by stylar ribonuclease activity assays (Bošković et al., 1997) and S-RNase allele-specific PCR (Sonneveld et al., 2001).
Figure 4.
Segregation Analysis of the Putative S4-Haplotype–Specific SFB Sequence to Test Cosegregation with the S4-RNase Allele.
PCR amplification with specific primers for the putative S4-specific SFB sequence is shown for genomic DNA of parents and a representative sample of seedlings of the cross Bradbourne Black (S3S5) × Merton Late (S1S4): BB, Bradbourne Black; ML, Merton Late; 12 seedlings. Presence (+) or absence (−) of the S4-RNase allele is indicated underneath the lanes. The bands amplified by the internal control primers included in each PCR are indicated with PAL.
S-RNase and SFB Allele-Specific PCR
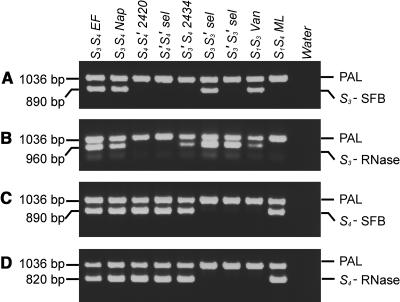
To check for duplications, deletions, or other rearrangements in the pollen-part mutant selections, allele-specific PCR was performed with S3- and S4-specific primers for both S-RNase and SFB sequences. Several conclusions can be drawn from the allele-specific PCR amplifications shown in Figure 5 and summarized in Table 6.
Figure 5.
Genomic PCR Amplification of Parents and Pollen-Part Mutants with Specific Primers for the SFB and S-RNase Genes of the S3- and S4-Haplotypes.
The primers used are specific for S3-haplotype–specific SFB (A), S3-RNase (B), S4-haplotype–specific SFB (C), and S4-RNase (D). Samples are as follows: EF, Emperor Francis (S3S4); Nap, Napoleon (S3S4); 2420, JI 2420 (S4S4′); S4′S4′ selection; 2434, JI 2434 (S3′S4); S3S3′ selection; S3′S3′ selection; Van (S1S3); ML, Merton Late (S1S4); water control. The bands amplified by the internal control primers that were included in each PCR are indicated with PAL.
Table 6.
Presence (+) or Absence (−) of Genomic PCR Amplification in Parents and Pollen-Part Mutants with Specific Primers for the S3- and S4-RNase and -SFB Genes as Shown in Figure 5
| Lane | Cultivar/Selection | S-Genotype | S3-SFB | S3-RNase | S4-SFB | S4-RNase |
|---|---|---|---|---|---|---|
| a | Emperor Francis | S3S4 | + | + | + | + |
| b | Napoleon | S3S4 | + | + | + | + |
| c | JI2420 | S4S4′ | − | − | + | + |
| d | A53 | S4′S4′ | − | − | + | + |
| e | JI 2434 | S3′S4 | − | + | + | + |
| f | 9239-1 | S3S3′ | + | + | − | − |
| g | JI 2434 AH | S3′S3′ | − | + | − | − |
| h | Van | S1S3 | + | + | − | − |
| i | Merton Late | S1S4 | − | − | + | + |
Amplification with the S-RNase primers for parents, standards, and pollen-part mutant selections is consistent with their stylar SI response and with the DNA gel blot analysis described above (Figures 5B and 5D).
Amplification with the SFB primers is as expected for parents and standards. For PCR amplification with the SFB primers for the S4′ mutant, the results of the homozygous S4′S4′ selection are informative (Figures 5A and 5C). The selection clearly shows a band for S4-SFB but not for S3-SFB. This indicates that, if SFB is the pollen-S gene, self-compatibility in the S4′ mutant cannot be attributed to a duplication of the S3 pollen-S allele. This leaves a mutation of the S4 pollen-S allele as the most likely explanation for the pollen-part mutation. If this is the case, the mutation clearly does not affect PCR amplification and does not visibly alter the product size because the S4′ PCR product is indistinguishable on the gel from the product for the S4 allele.
For the S3′ mutant, again the results of the homozygous selection, S3′S3′, are informative (Figures 5A and 5C). This shows no band for the S3- or the S4-SFB, indicating that if SFB is the pollen-S gene, the S3′ mutation is not a duplication of the S4 pollen-S allele but a mutation of the S3 pollen-S allele itself. The nature of the mutation cannot be deduced from these results, but it must affect at least one of the primer binding sites of the S3-SFB sequence or otherwise interfere with PCR amplification.
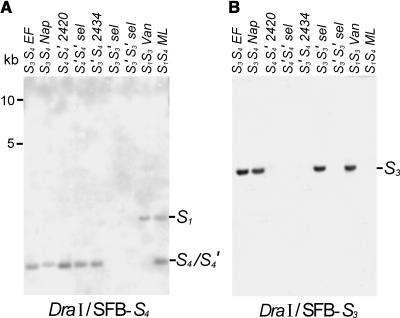
DNA Gel Blot Analysis with SFB Probes
DNA gel blot analyses with S3- and S4-SFB probes were performed to confirm the PCR results described above and to characterize the pollen-part mutant haplotypes further. This approach should indicate whether most of the S3-SFB sequence is missing in the S3′ mutant. In addition, it could also check for rearrangements in the regions flanking the S4-SFB gene in the S4′ mutant.
The results of the DNA gel blot analysis with specific SFB probes are shown in Figure 6. Each of the parents shows a restriction fragment for the S3- and the S4-SFB sequence (Figures 6A and 6B). The S4-SFB probe also weakly hybridizes to the S1 allele of the standards (Figure 6A). With the S4-SFB probe (Figure 6A), the homozygous S4′S4′ selection shows a restriction fragment in the same position as the parents and standards. Therefore, with the restriction enzyme used (DraI), there is no evidence of a rearrangement in the S4′-haplotype. Probing with the S3-SFB probe (Figure 6B) reveals that the S3-SFB sequence, at least that included in the probe, is entirely missing in the S3′ mutant, suggesting that the pollen-part mutation involves a deletion of most of the S3-SFB sequence. Sequencing of the probe confirmed that it represents the S3-SFB sequence because it matched the S3-SFB sequence in the database, which has also been shown to give haplotype-specific hybridization signals in DNA gel blot analysis (Yamane et al., 2003). These DNA gel blot data are consistent with the genomic PCR data in Figure 5.
Figure 6.
DNA Gel Blot Analysis of the S3′ and S4′ Mutants Using SFB Haplotype-Specific Probes.
Genomic DNA was digested with DraI and probed with S4-haplotype–specific SFB probe (A) and S3-haplotype–specific SFB probe (B). The stringent washing conditions resulted in an allele-specific hybridization signal with the S3-SFB probe; the S4-SFB probe also cross-hybridized weakly to the S1-SFB sequence. Samples are as follows: EF, Emperor Francis (S3S4); Nap, Napoleon (S3S4); 2420, JI 2420 (S4S4′); S4′S4′ selection; 2434, JI 2434 (S3′S4); S3S3′ selection; S3′S3′ selection; Van (S1S3); ML, Merton Late (S1S4).
Structure of the S3- and S3′-Haplotypes
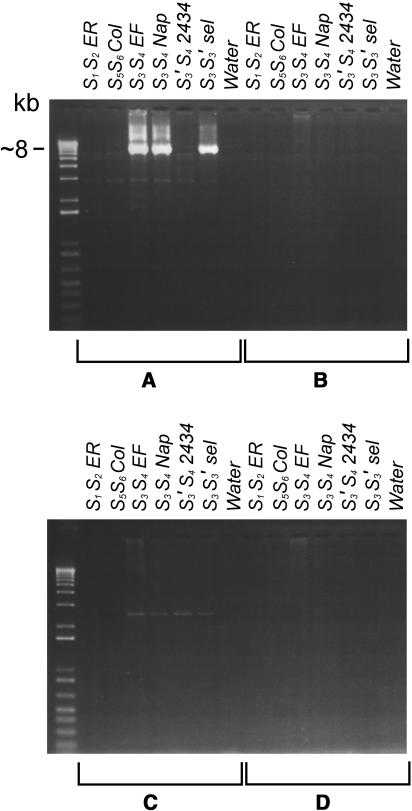
The result of an attempt to amplify the region between the S-RNase and the SFB gene in the S3-haplotype is shown in Figure 7. Allele-specific primers for each of the genes were used in all four possible combinations (Figures 7A to 7D). Only the PCR that included the S3-RNase forward and the S3-SFB forward primers (Figure 7A) gave a product, of ∼8 kb, exclusively in samples containing the S3 allele. The result of the S3 intergenic PCR reveals the relative orientation of the genes, as well as the distance between them: the S3-SFB gene is located ∼6.5 kb downstream of the S3-RNase gene, in opposite transcriptional orientation as shown in Figure 8. This is consistent with the detection of a rearrangement in the S3′-haplotype downstream of the S3-RNase gene (Table 5) that results in loss of the S3-SFB gene. A similar attempt in the S4-haplotype failed, presumably because the distance between the two genes in the S4-haplotype is too great to be amplified under the PCR conditions used.
Figure 7.
Intergenic PCR for the S3-Haplotype Using S3-RNase and S3-SFB Specific Primers in Four Possible Combinations to Determine Distance between and Transcriptional Orientation of the Genes.
The primers used on genomic DNA are S3-RNase forward with S3-haplotype–specific SFB forward (A), S3-RNase forward with S3-haplotype–specific SFB reverse (B), S3-RNase reverse with S3-haplotype–specific SFB forward (C), and S3-RNase reverse with S3-haplotype–specific SFB reverse (D). Samples are as follows: ER, Early Rivers (S1S2); Col, Colney (S5S6); EF, Emperor Francis (S3S4); Nap, Napoleon (S3S4); 2434, JI 2434 (S3′S4); S3S3′ selection; water control. Only combination (A) shows specific amplification for accessions with the S3 allele.
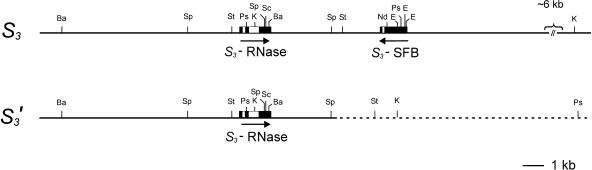
Figure 8.
Preliminary Restriction Maps of the S3- and S3′-Haplotypes of Cherry of Napoleon (S3S4) and JI 2434 (S3′S4).
Restriction enzyme positions and distances were inferred from restriction fragment size estimates on DNA gel blots (Napoleon [S3S4] and JI 2434 [S3′S4]) (Table 5), except for the regions of known sequence. Genes are represented by black boxes, with white boxes for introns. Arrows underneath gene boxes indicate the direction of transcription as deduced from the intergenic PCR (Figure 7). The breakpoint of the rearrangement in the S3′-haplotype was deduced to be ∼3.5 kb downstream of the S3-RNase; sequence downstream of the breakpoint (dotted line) is linked to S3 in the progenitor. Restriction enzyme abbreviations are as follows: Ba, BamHI; E, EcoRI; K, KnpI; Nd, NdeI; Ps, PstI; Sc, ScaI; Sp, SspI; St, SstI.
A preliminary restriction map of the S3- and S3′-haplotypes, of Napoleon (S3S4) and JI 2434 (S3′S4), respectively, is shown in Figure 8. The restriction enzyme sites flanking the S-RNase genes are based on the DNA gel blot analysis (Table 5). A major sequence rearrangement downstream of the S3-RNase in the S3′-haplotype includes a deletion of the S3-SFB sequence. The breakpoint of the rearrangement is ∼3.5 kb from the S3-RNase.
We examined whether other S locus F-box genes (SLFLs) are affected by the rearrangement in the S3′-haplotype. Three SLFL genes (SLFL1-3) have been identified in two haplotypes of P. mume, and the relative gene order (also in relation to S-RNase and SFB) was found to be conserved (Entani et al., 2003). In these two haplotypes, only SLFL2 is located downstream of the S-RNase and upstream of SFB. If the gene order at the Prunus S locus is conserved, SLFL2 is therefore most likely to be affected by the rearrangement in the S3′-haplotype. SLFL2-specific primers amplified a product in Napoleon and in the S3′S3′ homozygous selection, and sequencing of the product showed that they were almost identical. The sequence is ∼96% identical to the two P. mume SLFL2 sequences and ∼67% identical to other Prunus SLFLs, indicating that the SLFL2 gene is not deleted in the S3′-haplotype.
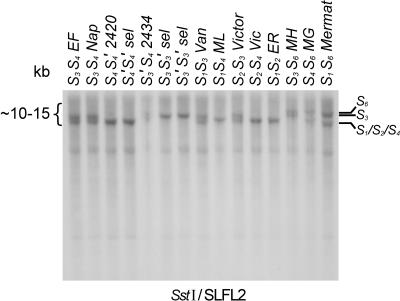
The SLFL2 product was used as a probe in DNA gel blot analysis. Two genomic digests, DraI and SstI, confirmed the presence of an SLFL2 fragment in the S3′-haplotype. The DraI digest gave a monomorphic band of ∼2 kb (data not shown), but the SstI digest revealed limited haplotype-specific polymorphism (Figure 9). The S3′ fragment is approximately the same size as that of S3, indicating that the genomic region around the SLFL2 gene is not rearranged (Figure 9).
Figure 9.
DNA Gel Blot Analysis of the S3′ and S4′ Mutants Using an SLFL2-Specific Probe.
Genomic DNA was digested with SstI and probed with a cherry SLFL2 probe. Samples are as follows: EF, Emperor Francis (S3S4); Nap, Napoleon (S3S4); 2420, JI 2420 (S4S4′); S4′S4′ selection; 2434, JI 2434 (S3′S4); S3S3′ selection; S3′S3′ selection; Van (S1S3); ML, Merton Late (S1S4); Victor (S2S3); Vic (S2S4); ER, Early Rivers (S1S2); MH, Merton Heart (S3S6); MG, Merton Glory (S4S6); Mermat (S1S6).
Cloning of the S4′-Haplotype–Specific SFB
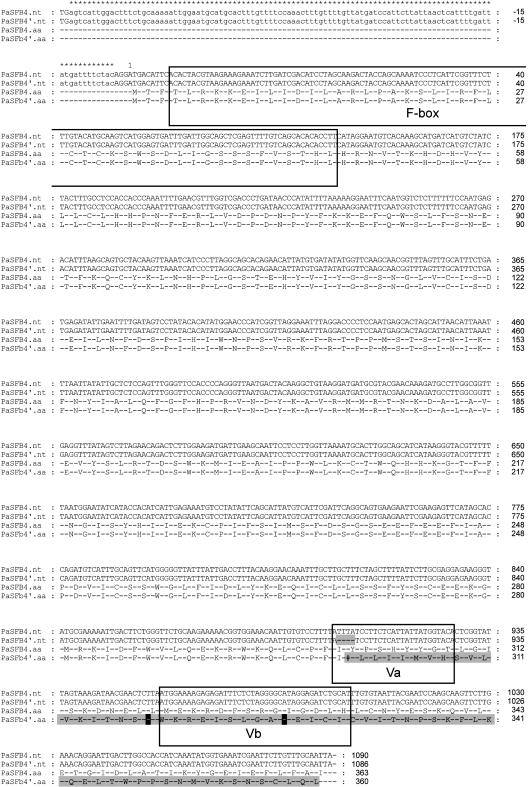
If SFB is the pollen-S gene in cherry, a mutation affecting the S4-SFB is expected because no duplication of the S3-SFB was found in the S4′-haplotype. When the coding region of the S4′-SFB gene was cloned and the sequence aligned with the S4-SFB sequence of the progenitor (Figure 10), a deletion of 4 bp was found toward the 3′ end of the S4′ sequence (position 742 to 745) in the variable region A (as defined in Ushijima et al., 2003), which would result in a frame shift in translation. From that point onwards, the corresponding S4′ protein contains 16 amino acids distinct from the S4-SFB predicted protein sequence and then a premature stop codon would result in a truncated protein. Because the variable regions of the protein are altered significantly and the protein is truncated (317 versus 375 amino acids), the S4′-SFB protein is unlikely to function properly.
Figure 10.
Alignment of Partial Genomic DNA Sequences and Deduced Amino Acid Sequences of the S4- and S4′-Haplotype–Specific SFB Genes.
Nucleotide (nt) and predicted amino acid (aa) sequences representing P. avium SFB sequences for S4 and S4′. Putative intron sequence within the 5′ untranslated region is presented in lower case and indicated by asterisks. Twelve amino acids are missing at the 3′ end of the S4 sequence. Residues highlighted in gray denote divergence from the original S4-SFB sequence. The F-box region and the two variable regions Va and Vb as identified by Ushijima et al. (2003) are boxed. A 4-bp deletion in the S4′-SFB sequence at position 742 to 745 in the first variable region leads to the deletion of a single Tyr residue (#) and a subsequent frame shift in translation. Premature stop codons present in the S4′-SFB sequence are highlighted in black.
DISCUSSION
A genetic analysis of two self-compatible cherry pollen-part mutants has confirmed that both selections have self-compatibility linked with one particular allele (i.e., S3 in JI 2434 [denoted S3′] and S4 in JI 2420 [denoted S4′]), confirming the conclusions of Bošković et al. (2000). A molecular characterization of S locus genes has shown that, in both mutant haplotypes, the S-RNase gene is intact, but each has a mutation affecting the haplotype-specific SFB gene. No evidence of a duplication was found in either mutant selection. In the S3′-haplotype, a rearrangement has been found downstream of the S-RNase gene, with the breakpoint of the rearrangement ∼3.5 kb from the S-RNase gene. In this rearrangement, the S3-haplotype–specific SFB gene, which is located ∼6.5 kb downstream of the S3-RNase gene in the progenitor, is deleted. In the S4′-haplotype, a 4-bp deletion in a variable region of the S4-haplotype–specific SFB gene leads to a shift in the translation reading frame and premature termination of the protein.
Self-Compatibility Is Associated with Loss of Function of SFB
Prunus SFB genes have been reported as good candidates for the pollen component of SI. They are pollen-expressed genes with haplotype-specific polymorphism identified at the S locus in almond (P. dulcis) (Ushijima et al., 2003) and Japanese apricot (P. mume) (Entani et al., 2003). The amino acid identity between different alleles is comparable to S-RNase alleles (Entani et al., 2003; Ushijima et al., 2003), and like S-RNases, they show a trans-specific pattern of evolution (Ikeda et al., 2004). Our finding that two self-compatible pollen-part mutants of cherry are associated with a loss of function of the haplotype-specific SFB gene provides additional support that the SFB gene is the pollen-S gene in Prunus.
Loss of function of the S3-SFB gene in the S3′-haplotype is the result of a deletion of the gene (Figure 8). A variant of the S4-SFB protein is still likely to be produced in the S4′ mutant, but the protein is truncated as the result of a premature stop codon (Figure 10). The C terminus of the S4′-SFB protein will have 16 amino acids of novel sequence and 58 amino acids missing. These alterations are likely to have a significant effect on the protein structure and function. Even though the F-box domain at the N terminus may be unaffected, the variable domains at the C terminus, which are thought to be involved in specific interactions with proteins recruited for degradation (see later), would be changed or lost. The mutant protein is therefore unlikely to perform its normal specific function, and the mutation can be regarded as a loss-of-function mutation. During the preparation of this article, Ushijima et al. (2004) reported identical findings for the S4′-haplotype. They speculate that the truncated S4′ protein, which lacks the two variable regions at the C terminus, may still function partially. Their conclusions are different from ours, as discussed later.
Previously, pollen-part mutations causing self-compatibility in plants with RNase-based SI have been found to be associated with duplications of S alleles (Brewbaker and Natarajan, 1960; Pandey, 1967; Golz et al., 1999, 2001). If SFB is indeed the pollen-S gene, the S3′ mutant characterized in this article would be an unambiguous example of loss of function of the pollen-S gene for an RNase-based SI system.
It is curious that the only two irradiation-induced pollen-part mutants identified in the Rosaceae, studied in this article, appear to be the result of loss-of-function mutations, whereas at least five pollen-part mutants of P. inflata (Brewbaker and Natarajan, 1960) and seven pollen-part mutants of N. alata (Golz et al., 1999, 2001), resulting from similar experiments in the Solanaceae, were attributable to duplications of an S allele, often on a centric fragment. Pandey (1965) also found that 34 pollen-part mutants of N. alata carried a centric fragment. This led Golz et al. (2001) to suggest that deletions of the pollen-S gene are incompatible on any style with S-RNases. If their conclusion is valid, then finding a mutation/deletion of SFB in two pollen-part mutants would indicate that SFB is not the pollen-S gene.
However, it may be that certain S-bearing chromosomes in solanaceous species are particularly prone to breaking as a result of ionizing radiation. In these studies, it was often found that after irradiation of, for example, an S3S6 plant, most pollen-part mutants had a duplication of the S3 allele, indicating that the mutation rate for some alleles is higher than for others. Also, centric fragments carrying the S locus may be frequent in solanaceous species because the S locus is known to be located at the centromere in Petunia (Entani et al., 1999), which may not be true for the S locus in Prunus. Lewis (1961) points out that the absence of the prime-type mutation in the studies of Brewbaker and Natarajan (1960) is not unexpected when taking into account the number of irradiated pollen grains involved and assuming that the mutation rate for the prime-type mutation in Petunia is the same as in his studies of Oenothera. However, it is currently unknown if Oenothera has RNase-based SI.
It should be noted that a loss-of-function approach was not attempted in the transgenic experiments with S locus F-box genes reported in P. inflata (Sijacic et al., 2004) and Antirrhinum/P. hybrida (Qiao et al., 2004b), in which an extra copy of an S locus F-box gene was shown to cause breakdown of SI because of competitive interaction. This may have resulted from the assumption that these F-box proteins inactivate non-self S-RNases, in which case a loss of function should lead to pollen being incompatible on any style with S-RNases. The mode of action of the RNase-mediated SI reaction in the Rosaceae may not be fundamentally different from that in the Solanaceae or Scrophulariaceae because it has recently been suggested they have a common origin based on phylogenetic analyses of S-RNase sequences (Igic and Kohn, 2001; Steinbachs and Holsinger, 2002). It will be interesting to find out whether knockout mutants of S locus F-box genes in the Solanaceae, which is more amenable to transformation than the Rosaceae, also become self-compatible. This would be another way to demonstrate that specific S locus F-box genes encode the pollen component of RNase-mediated SI in this family, in addition to the gain-of-function experiments reported so far.
Organization of the S Locus
The preliminary restriction map of the S3-haplotype (Figure 8) shows that the SFB gene is located downstream of the S-RNase gene in opposite transcriptional direction, as was found in other Prunus S haplotypes, although distances may vary (Entani et al., 2003; Ushijima et al., 2003; Yamane et al., 2003). This organization is also found for the AhSLF-S2 and the S2-RNase gene in Antirrhinum (Lai et al., 2002). By contrast, the two genes determining stigma and pollen specificity at the sporophytic S locus in Brassica are found in various relative orientations (Boyes et al., 1997; Cui et al., 1999; Watanabe et al., 2000). If additional S locus haplotypes continue this pattern, the convergent arrangement of the two genes at the S locus in families with RNase-based SI may be of functional significance.
In previous studies of pollen-part mutants in the Solanaceae, no breakpoint of rearrangements could be identified at a molecular level (Thompson et al., 1991; Golz et al., 1999, 2001). The size of the P. avium genome (2C = 686 Mbp) is only twice that of Arabidopsis (2C = 343 Mbp), whereas genomes of solanaceous species are much larger (e.g., N. alata 2C = 4753 Mbp; P. hybrida 2C = 3283 Mbp) (Bennett and Leitch Angiosperm DNA C-values database, release 4.0, January 2003, http://www.rbgkew.org.uk/cval/homepage.html). The smaller size of the Prunus genome may have increased the likelihood of detecting an irradiation-induced breakpoint. The relatively small genome size makes Prunus a suitable model for studies of the genomic organization of the S locus.
A wide range of mutations can be obtained from x-irradiation, such as deletions, translocations, inversions, and even point mutations. The rearrangement in the S3′-haplotype appears to be relatively major, and preliminary analysis suggests a deletion. Sequencing of the S-RNase/SFB intergenic PCR product for the S3-haplotype and comparing it to sequences downstream of the S3-RNase in the S3′-haplotype, obtained from an ∼4.5-kb SstI inverse PCR fragment, identified the breakpoint of the rearrangement ∼3 kb from the S-RNase (data not shown). Primers based on the new flanking sequence in the S3′-haplotype amplified a PCR product of unique size in samples with S3 and S3′ and cosegregated with the S3 allele in a set of 48 seedlings from the mapping progeny Napoleon (S3S4) × P. nipponica (SaSb) (Bošković and Tobutt, 1997) (data not shown), suggesting that the new flanking sequence originates from the S3 locus. A deletion type rearrangement is therefore more likely than a translocation.
Other S locus F-box genes do not appear to be affected by the rearrangement. In particular, the presence of the SLFL2 gene, which is located downstream of the S-RNase in other Prunus S haplotypes, including S4 of cherry (Ushijima et al., 2004), flanked by the same restriction sites as in the S3-haplotype, suggests that the S3′ rearrangement is not very extensive and may not affect other genes. The simplest interpretation is that the S3′-haplotype has a deletion extending from ∼3 kb downstream of S-RNase to a point in between SFB and SLFL2. However, this cannot be certain without further analysis of the entire S3- and S3′-haplotypes. No evidence of a rearrangement has been found by DNA gel blot analysis in sequences flanking the S-RNase and the SFB genes in the S4′-haplotype and the 4-bp deletion in the coding region of the S4′-SFB gene appears to be the only significant difference in the mutant S4-haplotype.
Loss of Function of SFB and Models of RNase-Mediated SI
Although the mechanism of the RNase-based SI reaction is still largely unknown, the model currently favored is the RNase inhibitor model, variously proposed and developed by McClure et al. (1989), Thompson and Kirch (1992), Kao and McCubbin (1996), and Luu et al. (2000) (2001). Because pollen tubes appear to take up S-RNases indiscriminately (Luu et al., 2000), they must have a mechanism to inactivate the cytotoxic action of non-self S-RNases. In the original inhibitor model, the pollen component is assumed to be an RNase inhibitor inside the pollen tube, which is able to inhibit all S-RNases except the one of corresponding S genotype. In this model, pollen-S encodes a protein that has the two functions of inhibiting non-self S-RNases and conferring specificity. Luu et al. (2001) proposed a modification of the inhibitor model, in which the two functions of S-RNase inhibition and allele-specific recognition are carried by two separate proteins, a general RNase inhibitor, not necessarily encoded by the S locus, and pollen-S. Binding of pollen-S to its corresponding S-RNase would protect it from being inhibited by the general RNase inhibitor. The S-RNase therefore remains active and inhibits pollen tube growth. This version allows for deletions of pollen-S, in contrast with the original inhibitor model as pointed out by Golz et al. (2001), although to date there have been no unambiguous reports of pollen-S deletions in plants with RNase-based SI.
The recent findings of F-box proteins as candidates for the pollen-S component in three families with RNase-mediated SI have suggested that ubiquitin-mediated protein degradation by the 26S proteasome is involved in the mechanism to inactivate non-self S-RNases (Lai et al., 2002; Entani et al., 2003; Ushijima et al., 2003; Sijacic et al., 2004). Biochemical studies in Antirrhinum have supported the role of ubiquitin-mediated protein degradation in compatible pollinations (Qiao et al., 2004a). They showed a nonallelic physical interaction of the S locus F-box protein AhSLF-S2 with S-RNases. The SLF proteins were also found to interact with other proteins involved in the ubiquitin-mediated protein degradation pathway. In addition, proteasomal inhibitors blocked compatible pollinations but had little effect on incompatible pollinations. As pointed out by Zhou et al. (2003), it is difficult to establish whether the Antirrhinum AhSLF gene is the ortholog of SFB in Prunus, so whether studies in Antirrhinum apply to Prunus remains to be seen.
In all three families, it has been proposed that the polymorphic S-locus F-box protein (SLF or SFB) is an inactivator of non-self S-RNases by recruiting non-self S-RNases as a substrate for destruction through the 26S proteasome pathway (Lai et al., 2002; Entani et al., 2003; Ushijima et al., 2003, Sijacic et al., 2004). A different, allele-specific interaction (of unknown nature) of the F-box protein with self S-RNases would prevent these from being degraded. This is in accordance with the original inhibitor model in which the pollen-S component carries the two functions of S-RNase inhibition and allele-specific recognition. Ushijima et al. (2004), who recently reported that the cherry S4′ and P. mume Sf alleles encode truncated SFB proteins, attempt to reconcile their findings with this model by proposing that the truncated proteins lack the specific S-RNase interaction domain but retain a general S-RNase binding domain, leading to inactivation of all S-RNases, including the self S-RNase.
However, in the original inhibitor model, a loss of function of the haplotype-specific F-box gene should lead to universally incompatible pollen because pollen tubes would lack a mechanism to inhibit S-RNases. In this article, we have provided evidence of loss of function of SFB in at least one self-compatible mutant of cherry. Therefore, if SFB is the pollen-S gene in Prunus, it cannot have the role of inactivator of non-self S-RNases.
It seems more likely that SFB proteins provide specificity to the inactivation of S-RNases effected by a general inactivation mechanism present in pollen tubes. This would fit the two-component inhibitor model (Luu et al., 2000). Deletions of the SFB gene would then result in pollen tubes able to inactivate all S-RNases, leading to self-compatibility. In that case, SFB proteins should only prevent self S-RNases from being degraded (not recruit non-self S-RNases for degradation), and so it is difficult to understand the function of their F-box domain. The F-box domain suggests that SFB may act as part of an E3 ubiquitin ligase complex in targeted protein degradation via the 26S proteasome. However, E3 ubiquitin ligase activity has not been demonstrated for SFB, and it may function in an unexpected way. As discussed, Qiao et al. (2004) provide evidence that ubiquitin/26S proteasome activity is essential in compatible but not in incompatible interactions in Antirrhinum. This suggests that a general inhibitor may use the ubiquitin/26S proteasome pathway of protein degradation for the inactivation of non-self S-RNases, but that in incompatible interactions, in which self S-RNases are protected from being degraded, the ubiquitin/26S proteasome pathway is not involved.
Sims and Ordanic (2001) first suggested that ubiquitination and protein degradation may play a role in the SI reaction in P. hybrida. In a yeast two-hybrid assay, they identified a pollen-expressed protein (PhSBP1) with a RING-finger domain that binds to S-RNases in a non-allele–specific manner. Many proteins with a RING-finger domain participate in E3 ubiquitin ligase complexes, like F-box proteins, and they suggest that PhSBP1 is a candidate for the general inhibitor of S-RNases, the existence of which was proposed in the two-component inhibitor model of Luu et al. (2000). It may be that a RING-finger domain protein with E3 ubiquitin ligase activity exists also in Prunus as a general inactivator of S-RNases.
It is clear that other components are involved in the complex interactions of the SI reaction, such as perhaps an S-RNase binding RING-finger domain protein homologous to PhSBP1 as just discussed or other pollen-expressed F-box proteins that are known to be encoded by the S locus (Lai et al., 2002; Entani et al., 2003; Ushijima et al., 2003; Sijacic et al., 2004), although Qiao et al. (2004) report that other F-box proteins identified in Antirrhinum do not interact physically with S-RNases. Further biochemical investigations using the mutants identified in this article and their progenitors are needed to shed light on the mechanism of S-RNase inactivation and the precise role of SFB in protecting self S-RNases from inactivation.
Self-Compatibility and Cherry Breeding
JI 2420, the S4′ mutant, has been used successfully in breeding programs combining self-compatibility with high fruit quality. One of its seedlings, Stella, was the first self-compatible cherry cultivar released, and nearly all other self-compatible cherry cultivars that are now available derive from Stella. JI 2434 has not been used extensively in breeding, but it may be in the parentage of Alex. Although the recorded parentage of Alex indicates a distinct mutant selection, JI 2538, the S3′ allele detected (Sonneveld et al., 2003) displays the same EcoRI shift on DNA gel blots with the S-RNase probe as the S3′ allele of JI 2434 (data not shown).
Self-compatibility is an important agronomic character in sweet cherry, and a PCR-based test can now be developed for the mutant S haplotypes, so seedlings can be selected for self-compatibility soon after germination. Previously, this had to be done by detecting the S-RNases in these haplotypes, which do not differ from the normal S3 and S4 alleles, and self-compatibility had to be deduced from the parentage of the seedlings (Bošković et al., 1997, 2000; Sonneveld et al., 2001, 2003).
METHODS
Plant Material
Two cherry (Prunus avium) pollen-part mutant selections raised at the John Innes Institute from the nominally incompatible cross of the cultivars Emperor Francis (S3S4) × Napoleon (S3S4, x-ray pollen) were used in this investigation, JI 2420 and JI 2434, with mutations affecting the S4 and the S3 alleles, respectively (Lewis, 1949; Matthews and Lapins, 1967; Matthews, 1970). The particular clones used were JI 2420 with genotype S4S4′ and JI 2434 EM with genotype S3′S4 (Bošković et al., 2000). The JI 2434 AH clone, which was subsequently found to be a possible self of JI 2434 EM with the genotype S3′S3′ (data not shown), was used as an example of an S3′S3′ homozygote.
To confirm that the pollen-part mutation in JI 2420 is linked with S4, the progeny of Erika (S1S3) × Lapins (S1S4′) [Van × Stella (JI 2420 × Lambert)] was scored in 1999 using the stylar ribonuclease assay of Bošković and Tobutt (1996). To confirm that self-compatibility in JI 2434 is linked with S3, the backcross Van (S1S3) × 9239-3 (S1S3′) [Van × JI 2434 (S3′S4)] was made in 2000 and the progeny genotyped by PCR using consensus primers for the first intron of cherry S-RNases (Sonneveld et al., 2003).
The S4- and S4′-SFB genes were cloned from the cultivars Inge (S4S9) [and later also Napoleon (S3S4)] and Sonata (S3S4′), respectively. Sonata, Lapins (S3S4′) × [Van × Stella (S3S4′)] is derived from JI 2420 via Lapins and Stella. Cosegregation of the putative S4-SFB sequence with S4 was tested in 44 seedlings of family 9007, Bradbourne Black (S3S5) × Merton Late (S1S4).
The following samples were included in DNA gel blot and S allele-specific PCR analyses: JI 2420 (S4S4′), JI 2434 (S3′S4), Emperor Francis (S3S4), and Napoleon (S3S4), along with standards for S3 and S4, Van (S1S3) and Merton Late (S1S4), respectively. In addition, for some of the analyses, four selections from family 9239 [Van (S1S3) × JI 2434 (S3′S4)] with genotypes S1S3′, S3S3′, S1S4, and S3S4, or only the S3S3′ selection, were used. A selection homozygous for S4′, A53 [Stella (S3S4′) × self] (Bošković et al., 1998), and one homozygous for S3′ (JI 2434 AH) were also included.
All plant material was grown at East Malling Research (UK), except for the progeny of Erika × Lapins, which was supplied by BAZ (Ahrensburg, Germany).
DNA Extraction
Cherry genomic DNA for DNA gel blotting was extracted from winter buds essentially as described by Dellaporta et al. (1983). DNA for genomic PCR and cloning was extracted from two dormant buds using a scaled down cetyl-trimethyl-ammonium bromide extraction method (Doyle and Doyle, 1987) with the addition of 1% (v/v) β-mercaptoethanol and 2% (w/v) polyvinyl pyrollidone (PVP 40) to the extraction buffer.
DNA Gel Blotting
Four micrograms of genomic DNA was digested overnight with the enzymes listed in Table 5 (S-RNase blots), DraI (SFB blot), or DraI and SstI (SLFL blots). The fragments were separated on 0.8% agarose gels for ∼24 h at 25 V and blotted onto a positively charged nylon membrane according to the manufacturer's instructions (Roche Molecular Biochemicals, Indianapolis, IN).
DIG Labeling of Probe
To detect S1-, S3-, and S4-RNases, an S1-RNase cDNA product from the C2 to the C5 region (420 bp) was used as a probe. The region covered by the S1 probe has 82.4% sequence similarity to both S3- and S4-RNase. The probe was labeled with Digoxigenin-11-dUTP (DIG-dUTP; Roche Molecular Biochemicals) by PCR with the consensus primer pair PaConsII-F and PaConsII-R (Sonneveld et al., 2003). The PCR reaction mix contained the following: 1× PCR buffer, 2 mM magnesium chloride, 50 μM dATPs, dCTPs, and dGTPs, 39.6 μM dTTPs, 10.5 μM DIG-dUTPs, 0.2 μM of each of the primers, 1.25 units/50 μL reaction Taq DNA polymerase (Qiagen, Crawley, UK), and ∼20 to 100 pg plasmid DNA containing S1-RNase cDNA (Sonneveld et al., 2001). Cycling conditions were as follows: initial denaturing of 2 min at 95°C, 10 cycles of 10 s at 95°C, 30 s at 57°C, and 2 min at 72°C, 20 cycles of 10 s at 95°C, 30 s at 57°C, and 2 min plus 5 s/cycle at 72°C, followed by 7 min at 72°C.
S3- and S4-SFB genomic PCR products were used as the probes to detect the S3- and the S4-SFB, respectively. The SFB-specific primers listed below under “Genomic PCR with S-RNase and SFB-Specific Primers” were used to amplify the regions from genomic DNA. The labeling reaction was the same as for the S-RNase probe, except for the annealing temperature of the PCR, which was 55°C.
SLFL2-specific primers designed from the P. mume S1 and S7 SLFL2 sequences (AB092625 and AB092626), SLFL2-forward (5′-TGGCAACSTTGAGCAAATTTTCTG-3′) and SLFL2-reverse (5′-CTATCTCATCGTCGTCGTCTTC-3′), were used for amplification of a probe from genomic DNA of Napoleon.
Hybridization and Chemiluminescent Detection of DIG-Labeled Probe
Membranes were hybridized according to the DIG system User's Guide for Filter Hybridizations (Roche Molecular Biochemicals), with 3 μL probe per mL DIG Easy Hyb hybridization buffer. Low stringency post-hybridization washes of membranes that were hybridized with the S-RNase probe allowed the detection of cross-hybridization of the S1-RNase probe to other S-RNase alleles: two washes of 5 min each with 2× SSC, 0.1% SDS at room temperature, followed by two washes of 15 min each with 0.5× SSC, and 0.1% SDS at 68°C. For membranes hybridized with the SFB probes, more stringent washes were used to achieve an allele-specific hybridization signal: two washes of 5 min each with 2× SSC, 0.1% SDS at room temperature, followed by two washes of 15 min each with 0.1× SSC, and 0.1% SDS at 68°C. For the chemiluminescent detection, the substrate CDP-Star (Sigma-Aldrich, Poole, UK) was used.
Cloning S4- and S4′-Haplotype-Specific SFB Genes
Sequence homology analysis was performed using four SFB genes identified in P. dulcis (AB092966, AB092967, AB079776, and AB081648; Ushijima et al., 2003) and three homologous SLF genes identified in P. mume (AB092621, AB092622, and AB092645; Entani et al., 2003) using the DNAstar Megalign (Madison, WI) software. Degenerate primers FBOX5′A (5′-TTKSCHATTRYCAACCKCAAAAG-3′) and FBOX3′A (5′-WATTGAGWAARRSYAAASTTTCTA-3′) were designed to anneal within conserved regions identified upstream of a putative intron within the 5′ untranslated region and towards the 3′ end of the coding sequence, respectively.
Amplification products representing genomic SFB clones were generated using proofreading KOD DNA polymerase (Invitrogen, Paisley, UK) in PCR using 100 ng of genomic DNA as template in a 30 μL reaction mix (1× KOD buffer, 0.2 mM dNTP, 1 mM magnesium sulfate, 0.5 μM forward and reverse primer, and 1 unit of KOD polymerase). PCR cycling conditions were 95°C for 2 min followed by 10 cycles of 94°C for 30 s, 60°C for 60 s with a reduction in temperature of 1°C per cycle, 68°C for 90 s then, 25 cycles of 94°C for 30 s, 50°C for 60 s, 68°C for 90 s, and a final cycle of 68°C for 10 min.
Amplification products were size fractionated by electrophoresis and DNA extracted from agarose using the QIAEX II kit (Qiagen). Amplification products generated from each cultivar were subsequently cloned into the vector pCR4-TOPO (Invitrogen) and transformed into TOP10 chemically competent cells (Invitrogen). Plasmid DNA was then prepared using a mini-spin kit (Qiagen) and inserts sequenced using M13 forward and reverse primers and internal primer, FBOX360 (5′-AGAATTTCAATGGTCTCTTTTTTCC-3′). DNA from four separate colonies containing S4′-SFB was sequenced to substantiate fully the data collected.
Sequences for SFB sequences for S4 and S4′ have been submitted to the EMBL database, and accession numbers are AY649872 and AY649873, respectively. The S4 sequence was initially cloned from the cultivar Inge (S4S9), but we later obtained an identical sequence from Napoleon (data not shown). Our S4 sequences are identical to the S4-SFB sequence now in the database (Ikeda et al., 2004).
The following sequences were used for the alignment of Figure 3: EMBL/GenBank accession numbers PaSFB4 (AY649872), PaSFB3 (AB096857), PaSFB6 (AB096858), PdSFBb (AB092967), PdSFBc (AB079776), PmSLF7 (AB092622), and PmSFB9 (AB092645).
Primers specific for the putative S4-SFB sequence (see next section) were used in genomic PCR to test cosegregation with the S4 allele in the family 9007 Bradbourne Black (S3S5) × Merton Late (S1S4), as described previously for the S4-RNase specific primers (Sonneveld et al., 2001).
Genomic PCR with S-RNase and SFB-Specific Primers
PCR analysis of various selections and cultivars with S3-RNase and S4-RNase specific primers and an internal control was performed as described by Sonneveld et al. (2001) (2003); annealing temperatures of 60°C (S4-RNase) and 63°C (S3-RNase) were used. Specific primers for the S3- and the S4-SFB sequences (S3-SFB, EMBL database accession number AB096857; S4-SFB, AY649872) were designed: PaSFB3-F (S3-SFB forward) 5′-CCACAATTTGAACGTCAGAAC-3′; PaSFB3-R (S3-SFB reverse), 5′-GATTTCGCCATATCTCATGAC-3′; PaSFB4-F (S4-SFB forward), 5′-TTGAACGTTTGGTCGACC-3′; PaSFB4-R (S4-SFB reverse), 5′-TACACAAATGCAGATCTCCTATG-3′. The PAL internal control primers described by Sonneveld et al. (2003) were also included in the PCR with SFB primers. PCR conditions for the SFB primers were as described for the S-RNase allele-specific primers, with an annealing temperature of 54°C.
Intergenic PCR
PCR conditions were based on the Qiagen protocol for long PCR products. Approximately 100 ng of genomic DNA was used in a reaction mix containing the following: 1× PCR buffer (Qiagen), a final concentration of 2 mM magnesium chloride, 0.2 mM dNTPs, 0.2 μM of each of the primers, and 1.25 units/25 μL reaction Taq DNA polymerase (Qiagen). Cycling conditions were as follows: initial denaturing of 2 min at 94°C, 10 cycles of 10 s at 94°C, 1 min 30 s at 55°C, and 6 min at 68°C, 25 cycles of 10 s at 94°C, 1 min 30 s at 55°C, and 6 min plus 10 s/cycle at 68°C.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AY649872 and AY649873.
Acknowledgments
T.S. was supported by a joint studentship from Horticulture Research International–East Malling and the University of Nottingham. Cherry genetics at East Malling is funded by the Department for Environment, Food, and Rural Affairs (London, UK). The authors thank H. Schmidt of BAZ Ahrensburg for plant material, R. Bošković of Imperial College at Wye and F.C.H. Franklin of the University of Birmingham for helpful comments on the manuscript, J. Clarke of East Malling Research for cosegregation analysis of the novel flanking sequence in S3′, and Mike Beard for help with figures.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instruction for Authors (www.plantcell.org) is: Kenneth R. Tobutt (ken.tobutt@emr.ac.uk).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.104.026963.
References
- Bošković, R., and Tobutt, K.R. (1996). Correlation of stylar ribonuclease zymograms with incompatibility alleles in sweet cherry. Euphytica 90, 245–250. [Google Scholar]
- Bošković, R., and Tobutt, K.R. (1997). Inheritance and linkage relationships of isoenzymes in two interspecific cherry progenies. Euphytica 103, 273–286. [Google Scholar]
- Bošković, R., Russell, K., and Tobutt, K.R. (1997). Inheritance of stylar ribonucleases in cherry progenies, and reassignment of incompatibility alleles to two incompatibility groups. Euphytica 95, 221–228. [Google Scholar]
- Bošković, R., Tobutt, K.R., and Russell, K. (1998). Selection of sweet cherry seedlings homozygous for self-compatibility. Acta Hortic. 484, 249–253. [Google Scholar]
- Bošković, R., Tobutt, K.R., Schmidt, H., and Sonneveld, T. (2000). Re-examination of (in)compatibility genotypes of two John Innes self-compatible sweet cherry selections. Theor. Appl. Genet. 101, 234–240. [Google Scholar]
- Boyes, D.C., Nasrallah, M.E., Vrebalov, J., and Nasrallah, J.B. (1997). The self-incompatibility (S) haplotypes of Brassica contain highly divergent and rearranged sequences of ancient origin. Plant Cell 9, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewbaker, J.L., and Natarajan, A.T. (1960). Centric fragments and pollen part mutation of incompatibility alleles in Petunia. Genetics 45, 699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewbaker, J.L., and Shapiro, N. (1959). Homozygosity and S gene mutation. Nature 183, 1209–1210. [DOI] [PubMed] [Google Scholar]
- Broothaerts, W., Janssens, G.A., Proost, P., and Broekaert, W.F. (1995). cDNA cloning and molecular analysis of two self-incompatibility alleles from apple. Plant Mol. Biol. 27, 499–511. [DOI] [PubMed] [Google Scholar]
- Brugière, N., Cui, Y., and Rothstein, S.J. (2000). Molecular mechanisms of self-recognition in Brassica self-incompatibility. Trends Plant Sci. 5, 432–438. [DOI] [PubMed] [Google Scholar]
- Crane, M.B., and Lawrence, W.J.C. (1929). Genetical and cytological aspects of incompatibility and sterility in cultivated fruits. J. Pomol. Hort. Sci. 7, 276–301. [Google Scholar]
- Cui, Y., Brugière, N., Jackman, L., Bi, Y.-M., and Rothstein, S.J. (1999). Structural and transcriptional comparative analysis of the S locus region in two self-incompatible Brassica napus lines. Plant Cell 11, 2217–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellaporta, S.L., Wood, J., and Hicks, J.B. (1983). A plant DNA minipreparation: Version II. Plant Mol. Biol. Rep. 1, 19–21. [Google Scholar]
- De Nettancourt, D. (2001). Incompatibility and Incongruity in Wild and Cultivated Plants. (Berlin: Springer).
- Doyle, J.J., and Doyle, J.L. (1987). A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19, 11–15. [Google Scholar]
- Entani, T., Iwano, M., Shiba, H., Che, F.-S., Isogai, A., and Takayama, S. (2003). Comparative analysis of the self-incompatibility (S-) locus region of Prunus mume: Identification of a pollen-expressed F-box gene with allelic diversity. Genes Cells 8, 203–213. [DOI] [PubMed] [Google Scholar]
- Entani, T., Iwano, M., Shiba, H., Takayama, S., Fukui, K., and Isogai, A. (1999). Centromeric localization of an S-RNase gene in Petunia hybrida Vilm. Theor. Appl. Genet. 99, 391–397. [DOI] [PubMed] [Google Scholar]
- Golz, J.F., Oh, H.-Y., Su, V., Kusaba, M., and Newbigin, E. (2001). Genetic analysis of Nicotiana pollen-part mutants is consistent with the presence of an S-ribonuclease inhibitor at the S locus. Proc. Natl. Acad. Sci. USA 98, 15372–15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golz, J.F., Su, V., Clarke, A.E., and Newbigin, E. (1999). A molecular description of mutations affecting the pollen component of the Nicotiana alata S locus. Genetics 152, 1123–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S., Lee, H.-S., Karunanandaa, B., and Kao, T.-h. (1994). Ribonuclease activity of Petunia inflata S proteins is essential for rejection of self-pollen. Plant Cell 6, 1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igic, B., and Kohn, J.R. (2001). Evolutionary relationships among self-incompatibility RNases. Proc. Natl. Acad. Sci. USA 98, 13167–13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, K., Igic, B., Ushijima, K., Yamane, H., Hauck, N.R., Nakano, R., Sassa, H., Iezzoni, A.F., Kohn, J.R., and Tao, R. (2004). Primary structural features of the S haplotype-specific F-box protein, SFB, in Prunus. Sex. Plant Reprod. 16, 235–243. [Google Scholar]
- Kao, T.-h., and McCubbin, A.G. (1996). How flowering plants discriminate between self and non-self pollen to prevent inbreeding. Proc. Natl. Acad. Sci. USA 93, 12059–12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, Z., Ma, W., Han, B., Liang, L., Zhang, Y., Hong, G., and Xue, Y. (2002). An F-box gene linked to the self-incompatibility (S) locus of Antirrhinum is expressed specifically in pollen and tapetum. Plant Mol. Biol. 50, 29–42. [DOI] [PubMed] [Google Scholar]
- Lewis, D. (1943). Physiology of incompatibility in plants. III. Autotetraploids. J. Genet. 45, 171–185. [Google Scholar]
- Lewis, D. (1949). Structure of the incompatibility gene. II. Induced mutation rate. Heredity 3, 339–355. [DOI] [PubMed] [Google Scholar]
- Lewis, D. (1951). Structure of the incompatibility gene. III. Types of spontaneous and induced mutation. Heredity 5, 399–414. [Google Scholar]
- Lewis, D. (1961). Chromosome fragments and mutation of the incompatibility gene. Nature 190, 990–991. [DOI] [PubMed] [Google Scholar]
- Lewis, D., and Crowe, L.K. (1954). Structure of the incompatibility gene. IV. Types of mutations in Prunus avium L. Heredity 8, 357–363. [Google Scholar]
- Lewis, D., and Modlibowska, I. (1942). Genetical studies in pears. IV. Pollen-tube growth and incompatibility. J. Genet. 43, 211–222. [Google Scholar]
- Luu, D.-T., Qin, X., Laublin, G., Yang, Q., Morse, D., and Cappadocia, M. (2001). Rejection of S-heteroallelic pollen by a dual-specific S-RNase in Solanum chacoense predicts a multimeric SI pollen component. Genetics 159, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu, D.-T., Qin, X., Morse, D., and Cappadocia, M. (2000). S-RNase uptake by compatible pollen tubes in gametophytic self-incompatibility. Nature 407, 649–651. [DOI] [PubMed] [Google Scholar]
- Matthews, P. (1970). Genetics and exploitation of self-fertility in the sweet cherry. In Proceedings of the Eurcarpia Fruit Breeding Symposium, Angers (Versailles, France: INRA), pp. 307–316.
- Matthews, P., and Lapins, K. (1967). Self-fertile sweet cherries. Fruit Var. Hort. Dig. 21, 36–37. [Google Scholar]
- McClure, B.A., Haring, V., Ebert, P.R., Anderson, M.A., Simpson, R.J., Sakiyama, F., and Clarke, A.E. (1989). Style self-incompatibility gene products of Nicotiana alata are ribonucleases. Nature 342, 955–957. [DOI] [PubMed] [Google Scholar]
- Pandey, K.K. (1965). Centric chromosome fragments and pollen-part mutation of the incompatibility gene in Nicotiana alata. Nature 206, 792–795. [Google Scholar]
- Pandey, K.K. (1967). Elements of the S-gene complex. II. Mutation and complementation at the SI locus in Nicotiana alata. Heredity 22, 255–284. [Google Scholar]
- Qiao, H., Wang, H., Zhao, L., Zhou, J., Huang, J., Zhang, Y., and Xue, Y. (2004. a). The F-box protein AhSLF-S2 physically interacts with S-RNases that may be inhibited by the ubiquitin/26S proteasome pathway of protein degradation during compatible pollination in Antirrhinum. Plant Cell 16, 582–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao, H., Wang, F., Zhao, L., Zhou, J., Lai, Z., Zhang, Y., Robbins, T.P., and Xue, Y. (2004. b). The F-box protein AhSLF-S2 controls the pollen function of S-RNase-based self-incompatibility. Plant Cell 16, 2307–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royo, J., Kunz, C., Kowyama, Y., Anderson, M., Clarke, A.E., and Newbigin, E. (1994). Loss of a histidine residue at the active-site of S-locus ribonuclease is associated with self-compatibility in Lycopersicon peruvianum. Proc. Natl. Acad. Sci. USA 91, 6511–6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijacic, P., Wang, X., Skirpan, A.L., Wang, Y., Dowd, P.E., McCubbin, A.G., Huang, S., and Kao, T.-h. (2004). Identification of the pollen determinant of S-RNase-mediated self-incompatibility. Nature 429, 302–305. [DOI] [PubMed] [Google Scholar]
- Sims, T.L., and Ordanic, M. (2001). Identification of a S-ribonuclease-binding protein in Petunia hybrida. Plant Mol. Biol. 47, 771–783. [DOI] [PubMed] [Google Scholar]
- Sonneveld, T., Robbins, T.P., Bošković, R., and Tobutt, K.R. (2001). Cloning of six cherry self-incompatibility alleles and development of allele-specific PCR detection. Theor. Appl. Genet. 102, 1046–1055. [Google Scholar]
- Sonneveld, T., Tobutt, K.R., and Robbins, T.P. (2003). Allele-specific PCR detection of sweet cherry self-incompatibility (S) alleles S1 to S16 using consensus and allele-specific primers. Theor. Appl. Genet. 107, 1059–1070. [DOI] [PubMed] [Google Scholar]
- Steinbachs, J.E., and Holsinger, K.E. (2002). S-RNase-mediated gametophytic self-incompatibility is ancestral in eudicots. Mol. Biol. Evol. 19, 825–829. [DOI] [PubMed] [Google Scholar]
- Thompson, R.D., and Kirch, H.H. (1992). The S locus of flowering plants: When self-rejection is self-interest. Trends Genet. 8, 381–387. [DOI] [PubMed] [Google Scholar]
- Thompson, R.D., Uhrig, H., Hermsen, J.G.Th., Salamini, F., and Kaufmann, H. (1991). Investigation of a self-compatible mutation in Solanum tuberosum clones inhibiting S-allele activity in pollen differentially. Mol. Gen. Genet. 226, 283–288. [DOI] [PubMed] [Google Scholar]
- Ushijima, K., Sassa, H., Dandekar, A.M., Gradziel, T.M., Tao, R., and Hirano, H. (2003). Structural and transcriptional analysis of the self-incompatibility locus of almond: Identification of a pollen-expressed F-box gene with haplotype-specific polymorphism. Plant Cell 15, 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushijima, K., Yamane, H., Watari, A., Kakehi, E., Ikeda, K., Hauck, N.R., Iezzoni, A.F., and Tao, R. (2004). The S haplotype-specific F-box protein gene, SFB, is defective in self-compatible haplotypes of Prunus avium and P. mume. Plant J. 39, 573–586. [DOI] [PubMed] [Google Scholar]
- Watanabe, M., Suzuki, G., Takayama, S., Isogai, A., and Hinata, K. (2000). Genomic organization of the SLG/SRK region of the S locus in Brassica species. Ann. Bot. 85 (suppl.), 155–160. [Google Scholar]
- Xue, Y., Carpenter, R., Dickinson, H.G., and Coen, E.S. (1996). Origin of allelic diversity in Antirrhinum S locus RNases. Plant Cell 8, 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane, H., Ikeda, K., Ushijima, K., Sassa, H., and Tao, R. (2003). A pollen-expressed gene for a novel protein with an F-box motif that is very tightly linked to a gene for S-RNase in two species of cherry, Prunus cerasus and P. avium. Plant Cell Physiol. 44, 764–769. [DOI] [PubMed] [Google Scholar]
- Zhou, J., Wang, F., Ma, W., Zhang, Y., Han, B., and Xue, Y. (2003). Structural and transcriptional analysis of S-locus F-box genes in Antirrhinum. Sex. Plant Reprod. 16, 165–177. [Google Scholar]