Abstract
BACKGROUND
Salvage radiation therapy is often necessary in men who have undergone radical pros-tatectomy and have evidence of prostate-cancer recurrence signaled by a persistently or recurrently elevated prostate-specific antigen (PSA) level. Whether antiandrogen therapy with radiation therapy will further improve cancer control and prolong overall survival is unknown.
METHODS
In a double-blind, placebo-controlled trial conducted from 1998 through 2003, we assigned 760 eligible patients who had undergone prostatectomy with a lymphadenectomy and had disease, as assessed on pathological testing, with a tumor stage of T2 (confined to the prostate but with a positive surgical margin) or T3 (with histologic extension beyond the prostatic capsule), no nodal involvement, and a detectable PSA level of 0.2 to 4.0 ng per milliliter to undergo radiation therapy and receive either antiandrogen therapy (24 months of bicalutamide at a dose of 150 mg daily) or daily placebo tablets during and after radiation therapy. The primary end point was the rate of overall survival.
RESULTS
The median follow-up among the surviving patients was 13 years. The actuarial rate of overall survival at 12 years was 76.3% in the bicalutamide group, as compared with 71.3% in the placebo group (hazard ratio for death, 0.77; 95% confidence interval, 0.59 to 0.99; P=0.04). The 12-year incidence of death from prostate cancer, as assessed by means of central review, was 5.8% in the bicalutamide group, as compared with 13.4% in the placebo group (P<0.001). The cumulative incidence of metastatic prostate cancer at 12 years was 14.5% in the bicalutamide group, as compared with 23.0% in the placebo group (P=0.005). The incidence of late adverse events associated with radiation therapy was similar in the two groups. Gynecomastia was recorded in 69.7% of the patients in the bicalutamide group, as compared with 10.9% of those in the placebo group (P<0.001).
CONCLUSIONS
The addition of 24 months of antiandrogen therapy with daily bicalutamide to salvage radiation therapy resulted in significantly higher rates of long-term overall survival and lower incidences of metastatic prostate cancer and death from prostate cancer than radiation therapy plus placebo. (Funded by the National Cancer Institute and AstraZeneca; RTOG 9601 ClinicalTrials.gov number, NCT00002874.)
Patients with localized prostatic cancer are often treated with radical pros-tatectomy. More than 30% of such patients will subsequently have recurrence. This recurrence manifests first as a rising serum level of prostate-specific antigen (PSA),1–3 termed biochemical recurrence. Large, retrospective studies suggest that salvage radiation therapy after biochemical recurrence may be associated with long-term freedom from cancer recurrence.4,5 However, 50% of the patients who are treated with salvage radiation therapy will have further disease progression, particularly when there are aggressive disease features.4–7
The combination of radiation therapy and either androgen-deprivation therapy or antiandrogen therapy prolongs survival among some men with an intact prostate.8–11 Thus, this combination treatment represents a rational approach to prolong metastasis-free survival and overall survival among men with a postoperative recurrence. In randomized trials, the oral agent bicalutamide, an androgen-receptor blocker, at a dose of 150 mg daily has been shown to be effective against prostate cancer.11,12 Accordingly, the NRG Oncology Radiation Therapy Oncology Group (formed by merging the National Surgical Adjuvant Breast and Bowel Cancer Project, the Radiation Therapy Oncology Group [RTOG], and the Gynecologic Oncology Group) designed a randomized, double-blind, placebo-controlled trial (RTOG 9601) to evaluate whether the addition of antiandrogen therapy for 24 months during and after salvage radiation therapy could prolong overall survival, as compared with radiation therapy plus placebo. Since the initiation of the trial, high-dose bicalutamide has been superseded by injectable gonad-otropin-releasing hormone (GnRH) agonists in therapy, but the hypothesis tested in this trial remains very relevant. A planned interim analysis of the trial in 2010 showed that bicalutamide was associated with significantly lower rates of biochemical recurrence and distant metastases than placebo.13 The primary end point of the protocol of this trial, the overall survival rate, can now be reported.
METHODS
PATIENTS
Eligible patients had all undergone radical pros-tatectomy with lymphadenectomy and had disease that was originally assessed, on the basis of pathological testing, as tumor stage T2 (confined to the prostate but also with a positive surgical margin) or T3 (with histologic extension of tumor beyond the prostatic capsule) without nodal involvement.14 Patients were also required to have a detectable PSA level at least 8 weeks after surgery that was 0.2 to 4.0 ng per milliliter. Eligibility criteria also included a Karnofsky performance-status score of 80 or more (on a 100-point scale, with lower numbers indicating greater disability), no previous chemotherapy or radiation therapy for prostate cancer, and no previous hormone therapy other than preoperative short-term hormonal therapy for 2 to 6 months in some patients (6.4% of those enrolled). In all the patients, abdominal and pelvic computed tomographic (CT) and bone scans showed no metastatic disease. At entry, patients had no evidence of hepatic disease, which was defined as a serum alanine or aspartate aminotransferase level that was at least 2.5 times the normal value and a serum bilirubin level that was more than the institutional upper limit of the normal range. All the patients had a life expectancy of more than 10 years.
TRIAL DESIGN
The trial, sponsored by the National Cancer Institute (NCI), was developed by the first author in collaboration with the RTOG NRG Oncology Genitourinary Committee, RTOG NRG Oncology, and the sponsor. Both the drug and the placebo were provided by AstraZeneca, which had no role in the collection of data, analysis of findings, or preparation of this report. All the data were collected by RTOG NRG Oncology, and analyses were performed by NRG Oncology statisticians. The manuscript was written by the first author with input from all the coauthors, who also reviewed and approved the final version. The first author made the decision to submit the manuscript for publication. All the authors vouch for the completeness and accuracy of the data and analyses and for the adherence of the trial to the protocol and the statistical analysis plan (available with the full text of this article at NEJM.org).
After the protocol was approved by an institutional review board at each center, participants were recruited and treated at NRG Oncology member sites, including community-based sites. All the participants provided written informed consent. Because the trial was placebo-controlled, there were no prophylactic measures to minimize the development of gynecomastia.
Participants were stratified according to the PSA level at trial entry (0.2 to 1.5 ng per milliliter vs. 1.6 to 4.0 ng per milliliter), receipt of short-term androgen-deprivation therapy before surgery (yes vs. no), positive surgical margin (yes vs. no), and the PSA nadir after surgery (<0.5 ng per milliliter vs. =0.5 ng per milliliter). Stratification according to Gleason score was not performed because there was no central review of tumor specimens. Patients underwent randomization to the two groups according to the permuted-block randomization scheme of Zelen.15
TREATMENT
Salvage radiation therapy was initiated within 12 weeks after randomization with the use of photon energies of 6 to 10 MV to the original prostatic site, the tumor resection bed, and the membranous urethra. Two-dimensional and three-dimensional planning systems were used according to institutional choice. A total dose of 64.8 Gy was given in 36 daily fractions of 1.8 Gy at five sessions per week. Regional pelvic lymph-node treatment was omitted because all the patients had negative lymph-node dissections. The trial co-chairs reviewed the simulation and portal treatment films for each treatment field.
Tablets were administered in a double-blind, randomized fashion, with either one 150-mg tablet of bicalutamide or one placebo tablet administered daily, beginning at the initiation of radiation therapy and continuing for 24 months. The medical oncology cochair reviewed all the records of the patients in the two trial groups for treatment completion and assessed the reasons for early terminations and possible adverse events.
ASSESSMENTS
At the beginning and end of radiation therapy, patients were assessed by means of clinical history and physical examination, Karnofsky performance-status score, complete blood count, PSA level, serum alanine aminotransferase level, bilirubin level, and reports of any treatment-related adverse effects. Subsequent follow-up evaluation occurred every 3 months for 2 years, then every 6 months for 3 years, and then yearly. Bone and CT scans were performed at subsequent biochemical recurrence. If metastatic disease was present or if the serum PSA level rose to more than 4.0 ng per milliliter, maximum androgen blockade was recommended. Early and late effects of radiation therapy were assessed with the use of the RTOG Acute and Late Radiation Morbidity Scoring system.16
END POINTS
The primary end point was the rate of overall survival. Prespecified secondary end points included disease-specific death, distant metastases (meta-static prostate cancer), local disease progression, non–disease-specific death, any prostate-cancer progression including a second biochemical recurrence, and adverse events. Disease-specific death included all deaths from prostate cancer or treatment complications as well as death from an unknown process in patients with active prostate cancer, on the basis of centrally reviewed cause of death. Non–disease-specific death was defined as death from any other cause. Scoring of metastatic disease required radiographic confirmation. Local disease progression was defined as the development of a palpable mass in the prostatic fossa, as determined by means of clinical examination.
The definition of biochemical recurrence was complex, because the lowest level of detectable PSA decreased from 0.5 ng per milliliter to 0.2 ng per milliliter during the years of enrollment. Generally, the second biochemical recurrence was defined as an increase of at least 0.3 ng per milliliter above the lowest detectable PSA level after protocol treatment or as the initiation of any subsequent hormone therapy (Table S1 in the Supplementary Appendix, available at NEJM.org). The third PSA biochemical recurrence occurred when the PSA level reached 0.5 ng per milliliter or higher or when there was any disease progression after the start of salvage hormone therapy.
STATISTICAL ANALYSIS
We assumed that the annual death rate among patients who underwent radiation therapy and received placebo would be 0.063 (median overall survival, 11 years). The addition of bicalutamide was hypothesized to result in a death rate that was at least 28.5% lower than the rate with placebo (annual death rate in the bicalutamide group, 0.045; hazard ratio for death, 0.71). We calculated that 230 events would need to be observed in order for the trial to detect this effect with 80% power and at a one-sided significance level of 0.046 (to preserve an overall 0.05 level with three interim analyses) with the use of the log-rank test. Significance levels for the interim and final analyses were determined by means of an alpha-spending function defining the O’Brien–Fleming boundaries.17 The enrollment goal was 725 eligible patients, to be enrolled at a rate of 160 patients per year. After the incorporation of a 10% inflation rate for ineligibility and loss to follow-up, we calculated that the target enrollment was 810 patients.
Outcome times were calculated from the date of randomization to the date of treatment failure or the date of last follow-up. Overall survival was estimated by the Kaplan–Meier method, with treatment groups compared with the use of the log-rank test and the Cox proportional-hazards model used to compute hazard ratios.18–20 The rates of disease-specific death, distant metastasis, non–disease-specific death, disease progression, and second and third biochemical recurrence were estimated by means of cumulative incidence functions.21 We used Gray’s test to compare treatments and the Fine–Gray model to calculate hazard ratios.22,23 Adverse events were graded according to the Cooperative Group Common Toxicity Criteria and the RTOG Acute and Late Radiation Morbidity Scoring system.16 Between-group differences in the frequencies of adverse events were evaluated with the use of the chi-square test.
Subgroup analyses were performed within well-known prognostic classes to better understand treatment effects on overall survival and distant metastases. These post hoc analyses were performed within the following categories: PSA level at trial entry (<0.7 ng per milliliter vs. 0.7 to 1.5 ng per milliliter vs. >1.5 ng per milliliter), Gleason score (2 to 6 vs. 7 vs. 8 to 10, on a scale from 2 to 10, with higher scores indicating a worse prognosis), and the presence of positive surgical margins (yes vs. no). Treatment groups were compared with the use of log-rank tests for overall survival and Gray’s test for distant metastases.19,22 In addition, interaction effects between each factor and treatment were formally tested. To further investigate treatment and other characteristics jointly in relation to outcomes, we conducted stepwise multivariate modeling using the Cox model for overall survival and the Fine–Gray model for end points with competing risks.19,22
RESULTS
CHARACTERISTICS OF THE PATIENTS
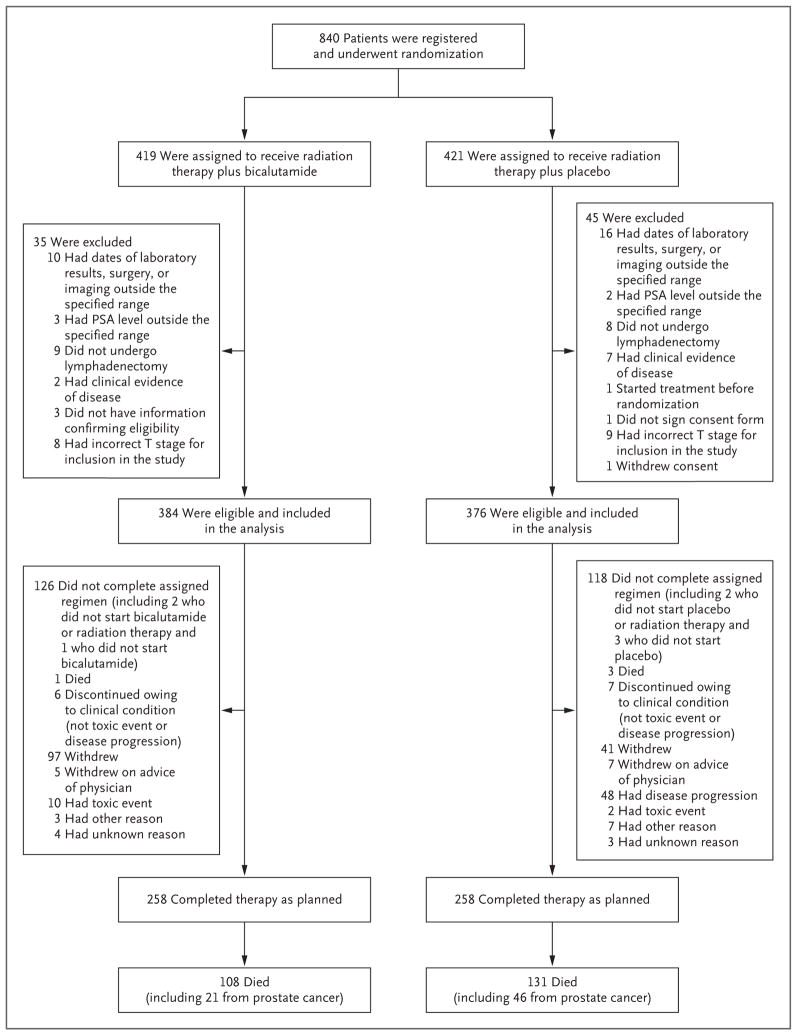
From March 1998 through March 2003, a total of 840 patients underwent randomization (Fig. 1). A total of 79 patients were ineligible (1 did not sign the consent form) and 1 withdrew consent, leaving 760 eligible patients for evaluation (384 patients in the bicalutamide group and 376 in the placebo group). The two groups were well balanced with respect to demographic and tumor-related characteristics (Table 1). The median age of the patients was 65 years, and the median PSA level at trial entry was 0.6 ng per milliliter. The median follow-up among the surviving patients was 13 years. The median interval between surgery and the first detectable PSA level was 1.4 years, and the median interval between surgery and trial entry was 2.1 years.
Figure 1. Enrollment, Randomization, and Follow-up of the Patients.
PSA denotes prostate-specific antigen.
Table 1.
Demographic and Clinical Characteristics of the Patients at Baseline.*
| Characteristic | Bicalutamide Group (N = 384) | Placebo Group (N = 376) | All Patients (N = 760) |
|---|---|---|---|
| Age — no. (%) | |||
| ≤49 yr | 6 (1.6) | 4 (1.1) | 10 (1.3) |
| 50–59 yr | 93 (24.2) | 84 (22.3) | 177 (23.3) |
| 60–69 yr | 192 (50.0) | 194 (51.6) | 386 (50.8) |
| 70–79 yr | 90 (23.4) | 91 (24.2) | 181 (23.8) |
| ≥80 yr | 3 (0.8) | 3 (0.8) | 6 (0.8) |
| Race or ethnic group — no. (%)† | |||
| White | 344 (89.6) | 324 (86.2) | 668 (87.9) |
| Hispanic | 6 (1.6) | 3 (0.8) | 9 (1.2) |
| Black | 28 (7.3) | 40 (10.6) | 68 (8.9) |
| Asian | 5 (1.3) | 4 (1.1) | 9 (1.2) |
| Native American | 0 | 1 (0.3) | 1 (0.1) |
| Other | 1 (0.3) | 4 (1.1) | 5 (0.7) |
| Karnofsky performance-status score — no. (%)‡ | |||
| 80 | 5 (1.3) | 4 (1.1) | 9 (1.2) |
| 90 | 83 (21.6) | 92 (24.5) | 175 (23.0) |
| 100 | 296 (77.1) | 280 (74.5) | 576 (75.8) |
| Gleason score — no./total no. (%)§ | |||
| 2–6 | 111/383 (29.0) | 103/375 (27.5) | 214/758 (28.2) |
| 7 | 205/383 (53.5) | 208/375 (55.5) | 413/758 (54.5) |
| 8–10 | 67/383 (17.5) | 64/375 (17.1) | 131/758 (17.3) |
| T stage — no. (%)¶ | |||
| T2 | 128 (33.3) | 120 (31.9) | 248 (32.6) |
| T3 | 256 (66.7) | 256 (68.1) | 512 (67.4) |
| Neoadjuvant hormone use — no. (%) | |||
| No | 363 (94.5) | 348 (92.6) | 711 (93.6) |
| Yes | 21 (5.5) | 28 (7.4) | 49 (6.4) |
| Positive surgical margin — no. (%) | |||
| No | 96 (25.0) | 95 (25.3) | 191 (25.1) |
| Yes | 288 (75.0) | 281 (74.7) | 569 (74.9) |
| PSA nadir after surgery — no. (%) | |||
| <0.5 ng/ml | 338 (88.0) | 332 (88.3) | 670 (88.2) |
| ≥0.5 ng/ml | 46 (12.0) | 44 (11.7) | 90 (11.8) |
| PSA level at trial entry — no. (%) | |||
| <0.7 ng/ml | 210 (54.7) | 195 (51.9) | 405 (53.3) |
| 0.7–1.5 ng/ml | 119 (31.0) | 118 (31.4) | 237 (31.2) |
| >1.5–4.0 ng/ml | 55 (14.3) | 63 (16.8) | 118 (15.5) |
All the patients underwent radiation therapy in addition to receiving either antiandrogen therapy with bicalutamide or placebo. There were no significant between-group differences in the characteristics listed here. PSA denotes prostate-specific antigen.
Race and ethnic group were self-reported, unless the data were missing, in which case race and ethnic group were determined by the investigator.
The Karnofsky performance-status score is assessed on a 100-point scale, with lower numbers indicating greater disability.
The scale for the Gleason score ranges from 2 to 10, with higher scores indicating a worse prognosis.
A T stage of T2 indicates that the tumor is palpable and confined to the prostate, and a stage of T3 that the tumor is palpable with extension beyond the prostatic capsule.
ADHERENCE
Radiation-therapy review was used to evaluate the total radiation dose, field borders, fractionation, and field administration in a randomly selected subgroup of patients, given that standard techniques were being used. Adherence to the protocol was well balanced between the two trial groups, with 64.8% of the patients treated per protocol, 30.8% treated with an acceptable variation, and 4.4% treated with an unacceptable variation. The percentage of patients who adhered to the assigned regimen, which was defined as continuing to take the tablets for at least 18 months, was 69.8% in the bicalutamide group and 74.7% in the placebo group. At 24 months (the treatment duration designated by the protocol), the adherence rates were 67.2% and 68.6%, respectively.
END POINTS
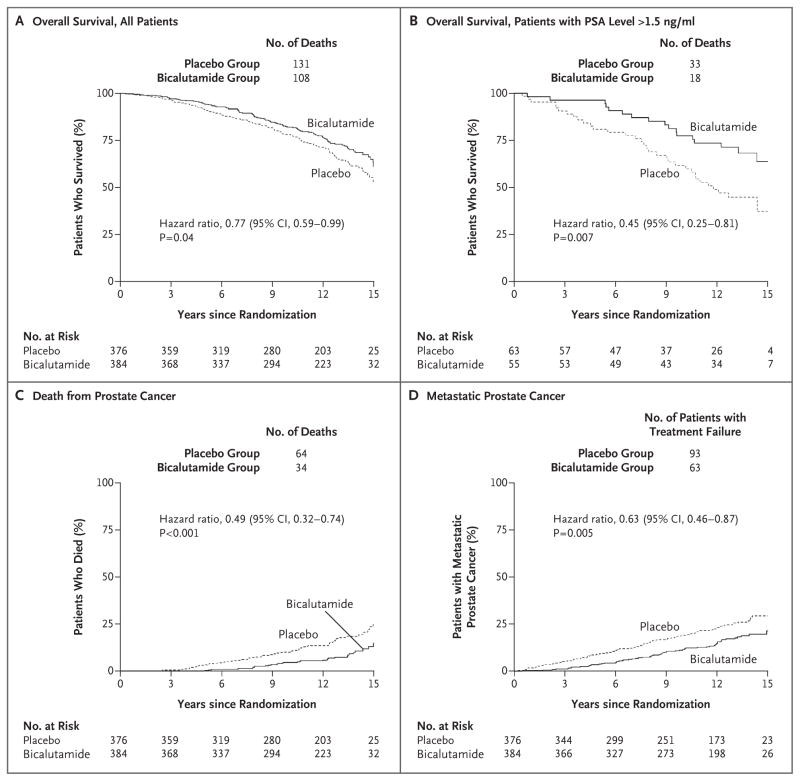
The end-point results at 12 years are shown in Figures 2 and 3 and in Table 2. A total of 21 patients in the bicalutamide group died from prostate cancer, as compared with 46 in the placebo group (Table S2 in the Supplementary Appendix). The actuarial rate of overall survival at 12 years was 76.3% in the bicalutamide group, as compared with 71.3% in the placebo group (hazard ratio for death, 0.77; 95% confidence interval [CI], 0.59 to 0.99; two-sided P = 0.04) (Fig. 2A). The 12-year incidence of death from prostate cancer was 5.8% in the bicalutamide group, as compared with 13.4% in the placebo group (hazard ratio, 0.49; 95% CI, 0.32 to 0.74; P<0.001) (Fig. 2C).
Figure 2. Kaplan–Meier Estimates of Overall Survival and Cumulative Incidence Estimates of Rates of Death from Prostate Cancer and of Metastatic Prostate Cancer.
All patients underwent radiation therapy in addition to receiving either antiandrogen therapy with bicalutamide or placebo. The overall survival analysis among patients with a PSA level of more than 1.5 ng per milliliter at trial entry was a post hoc analysis. Death from prostate cancer included all deaths from prostate cancer or treatment complications as well as death from an unknown process in patients with active prostate cancer on the basis of central review.
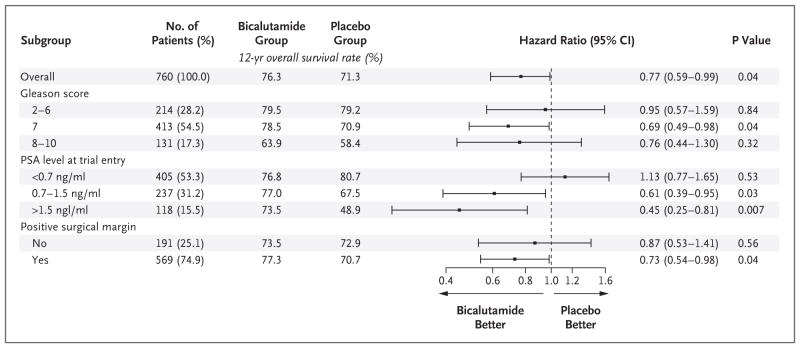
Figure 3. Effect of Antiandrogen Therapy with Bicalutamide on 12-Year Overall Survival.
All patients underwent radiation therapy in addition to receiving either antiandrogen therapy with bicalutamide or placebo. The scale for the Gleason score ranges from 2 to 10, with higher scores indicating a worse prognosis. Data on the Gleason score were missing for one patient in each group. P values were calculated with the use of the log-rank test.
Table 2.
Antitumor Efficacy with Respect to Key Secondary End Points at 12 Years.
| End Point and Subgroup | Bicalutamide Group | Placebo Group | Hazard Ratio (95% CI) | P Value | ||
|---|---|---|---|---|---|---|
| Patients at Risk | Rate of End Point | Patients at Risk | Rate of End Point | |||
| no. | % | no. | % | |||
| Metastatic prostate cancer | ||||||
|
| ||||||
| All patients | 384 | 14.5 | 376 | 23.0 | 0.63 (0.46–0.87) | 0.005 |
|
| ||||||
| Gleason score | ||||||
|
| ||||||
| 2–6 | 111 | 7.8 | 103 | 16.5 | 0.64 (0.30–1.36) | 0.25 |
|
| ||||||
| 7 | 205 | 15.4 | 208 | 19.8 | 0.80 (0.52–1.22) | 0.31 |
|
| ||||||
| 8–10 | 67 | 21.2 | 64 | 44.7 | 0.35 (0.18–0.67) | 0.001 |
|
| ||||||
| PSA level at trial entry | ||||||
|
| ||||||
| <0.7 ng/ml | 210 | 13.4 | 195 | 17.1 | 0.76 (0.47–1.22) | 0.26 |
|
| ||||||
| 0.7–1.5 ng/ml | 119 | 17.4 | 118 | 28.4 | 0.67 (0.40–1.12) | 0.13 |
|
| ||||||
| >1.5 ng/ml | 55 | 13.1 | 63 | 31.1 | 0.36 (0.15–0.84) | 0.01 |
|
| ||||||
| Positive surgical margin | ||||||
|
| ||||||
| No | 96 | 22.9 | 95 | 31.1 | 0.79 (0.47–1.32) | 0.38 |
|
| ||||||
| Yes | 288 | 11.8 | 281 | 20.3 | 0.56 (0.38–0.84) | 0.005 |
|
| ||||||
| Death from prostate cancer* | 384 | 5.8 | 376 | 13.4 | 0.49 (0.32–0.74) | <0.001 |
|
| ||||||
| Death from other causes | 384 | 17.9 | 376 | 15.3 | 1.10 (0.79–1.53) | 0.58 |
Death from prostate cancer included all deaths from prostate cancer or treatment complications as well as death from an unknown process in patients with active prostate cancer on the basis of central review.
The cumulative incidence of distant metastases at 12 years was 14.5% in the bicalutamide group, as compared with 23.0% in the placebo group (hazard ratio, 0.63; 95% CI, 0.46 to 0.87; P = 0.005) (Fig. 2D). The cumulative incidence of a second biochemical recurrence at 12 years was 44.0% in the bicalutamide group, as compared with 67.9% in the placebo group (hazard ratio, 0.48; 95% CI, 0.40 to 0.58; P<0.001) (Fig. S1B in the Supplementary Appendix).
Multivariate analyses of overall survival showed that the significant negative prognostic factors were assignment to the placebo group, a PSA level of more than 1.5 ng per milliliter at trial entry, a Gleason score for prostate cancer of 8 to 10 on the basis of pathological testing, a Karnof-sky performance-status score of 80 or 90, and an age of 65 years or more (Table S3 in the Supplementary Appendix). Other stratification variables (the PSA nadir after surgery, positive surgical margin, and whether short-term androgen-deprivation therapy had been given before surgery) did not meet prespecified significance levels and were not part of the final model. The rates of local disease progression and any form of disease progression including a second biochemical recurrence were all lower in the bicalutamide group than in the placebo group (Table S4 in the Supplementary Appendix).
ADVERSE EVENTS
There was no significant between-group difference in the risk of non–disease-specific death (Table 2). There were no significant between-group differences in the rates of early urinary, bowel, or hematologic reactions. Late genitourinary adverse events of grade 3 occurred in 7.0% of the patients in the bicalutamide group and in 6.0% of those in the placebo group; grade 4 events occurred in 0.3% and 0.8%, respectively. Late grade 2 hepatic toxic effects occurred in 1.6% of the patients in the bicalutamide group and in 0.8% of those in the placebo group; grade 3 effects occurred in 0.8% and 0.3%, respectively. The rate of cardiovascular deaths that were reported as adverse events was not significantly higher in the bicalutamide group than in the placebo group. Details are provided in Tables S5, S6, and S7 in the Supplementary Appendix.
The rates of hot flashes of grade 1, 2, and 3 were similar in the two groups: grade 1 hot flashes were reported in 16.6% of the patients in the bicalutamide group, grade 2 in 4.5%, and grade 3 in 0.8%; the corresponding values in the placebo group were 14.1%, 2.9%, and 0%. In contrast, gynecomastia of grade 1 was reported in 42.4% of the patients in the bicalutamide group, grade 2 in 23.6%, and grade 3 in 3.7% (resulting in 69.7% of the patients in the bicalutamide group having gynecomastia); the corresponding values in the placebo group were 8.8%, 2.1%, and 0% (resulting in 10.9% of the patients in the placebo group having gynecomastia) (P<0.01 for all comparisons). Although the rate of gynecomastia was significantly higher in the bicalutamide group than in the placebo group, this finding was not significantly linked as an explanation in patients taking less than the full course of oral tablets.
SUBGROUP ANALYSES
Post hoc subgroup analyses were performed according to well-known prognostic factors. These factors included the PSA level at trial entry, the Gleason score for prostate cancer, and whether the tumor margins were positive (Table 1). The greatest overall survival benefit was seen in subgroups of patients with more aggressive prostate cancer, such as those with a high PSA level at trial entry (1.5 to 4.0 ng per milliliter) or a Gleason score of 7. Few patients in the trial had a Gleason score of 8, 9, or 10; the difference in overall survival within this subgroup did not reach statistical significance (Figs. 2B and 3). Patients with positive surgical margins also appeared to have a larger benefit than those with negative surgical margins (Fig. S1A in the Supplementary Appendix). With the exception of PSA level, interaction tests did not indicate a significant differential benefit in subgroups that were defined according to these factors. A similar lower rate of distant metastases in the bicalutamide group was also seen among patients with a Gleason score of 8 to 10, those with a PSA level of 1.5 to 4.0 ng per milliliter at trial entry, and those with positive surgical margins (Table 2, and Fig. S2 in the Supplementary Appendix). A significant variation in the extent of benefit across subgroups was not found.
DISCUSSION
This randomized trial showed that the addition of 24 months of bicalutamide to salvage radiation therapy resulted in higher rates of overall survival and other important end points among surgery-treated patients with persistent or recurrent disease that was detected only because of an abnormal PSA level. Because of the relatively slow nature of prostate-cancer progression, a median follow-up of more than a decade was necessary to observe this benefit. The 12-year overall survival benefit with antiandrogen therapy was accompanied by significantly lower rates of disease-specific death, distant metastases, and second biochemical recurrence. Multivariate analysis showed that the factors predicting higher rates of overall survival included assignment to the bicalutamide group, a lower PSA level at trial entry (1.6 to 4.0 ng per milliliter), and younger patient age (<65 years). The between-group differences in other stratification variables (PSA nadir after surgery, positive surgical margin, and whether short-term androgen-deprivation therapy had been given before surgery) were not significant for differences in overall survival. Given the lower rate of death (absolute difference, 5.0 percentage points) and the lack of evidence of higher other-cause mortality in the bicalutamide group than in the placebo group, we calculated that 20 patients would need to be treated with bicalutamide to avoid one death over a 12-year period.24
These gains were achieved without the exacerbation of early or late bladder, bowel, hematologic, or hepatic effects, for which the rates were low in the two groups. Bicalutamide was not associated with a significantly higher risk of cardiac death or serious hepatotoxic effects. Cardiovascular mortality was similar in the two groups, as was seen in three previous RTOG phase 3 trials assessing the use or nonuse of androgen-deprivation therapy.25–27 However, only data on cardiac events that were reported as adverse events were collected, which may have introduced an ascertainment bias.
Wirth and colleagues reported the results of the Early Prostate Cancer Program, a series of randomized, double-blind trials comparing 150 mg of bicalutamide with placebo.28 They found that the rates of cardiovascular death were low and similar in the bicalutamide group and the placebo group (3.5% and 3.1%, respectively). In contrast, they found significant differences between the bicalutamide group and the placebo group in the rates of gynecomastia (68% vs. 8%) and patients’ withdrawal from the trial or stopping of the tablets because of gynecomastia (17% vs. 1%). The double-blind design of the Early Prostate Cancer Program trials, like ours, did not allow for preemptive measures to minimize gynecomastia.
The gains in overall and metastasis-free survival in this trial may be greater among patients whose disease had particular prognostic features — namely, patients with higher Gleason scores (8 to 10), a higher PSA level at trial entry (0.7 to 4.0 ng per milliliter), or positive surgical margins. Although this post hoc risk analysis is hypothesis-generating, it also suggests that patients with a lower Gleason score (=7), a PSA level of less than 0.7 ng per milliliter, or negative surgical margins may have less benefit from the addition of antiandrogen therapy to salvage radiation therapy.
Now, 20 years after this trial was designed, GnRH agonists have superseded bicalutamide as the first-choice hormonal therapy with radiation therapy, and bicalutamide at the 150-mg dose level is not approved for this purpose. Randomized trials involving patients with nonmetastatic disease have shown that high-dose bicalutamide and GnRH agonists have similar systemic anti-cancer efficacy.11,12,28 As such, our trial presents proof of principle that the addition of hormone-based therapy to salvage radiation therapy is associated with significant and clinically important lower rates of prostate-cancer metastases and death. The fully enrolled RADICALS-HD (Radiotherapy and Androgen Deprivation in Combination after Local Surgery) trial in the United Kingdom and the GETUG-16 (Group d’Étude des Tumeurs Urogénitales 16) trial in France are examining the role of contemporary androgen-deprivation therapy in the context of salvage radiation therapy, and these two trials may provide additional insights as these data mature.29,30
In conclusion, the addition of an antiandrogen agent to salvage radiation therapy resulted in higher rates of overall, disease-specific, and metastasis-free survival than radiation therapy plus placebo among patients who were treated for biochemical (PSA) recurrence of prostate cancer after radical prostatectomy. The higher rate of overall survival with antiandrogen therapy than with placebo became evident in the second decade after therapy.
Supplementary Material
Acknowledgments
Supported by grants (U10CA21661, U10CA180868, U10CA180822, and UG1CA189867) from the National Cancer Institute and AstraZeneca. Dr. Shipley reports having held stock in PFS Genomics (he no longer holds the stock); Dr. Lukka, receiving honoraria from AstraZeneca, Janssen Pharmaceuticals, Astellas Pharma, Amgen, AbbVie, Bayer, Actavis Specialty Pharmaceuticals, Sanofi, and Ferring Pharmaceuticals, receiving grant support to his institution from AstraZeneca, AbbVie, Actavis Specialty Pharmaceuticals, and Sanofi, and serving as a meeting coordinator for the GU Radiation Oncologists of Canada, which is sponsored by AstraZeneca, Janssen Pharmaceuticals, Astellas Pharma, Amgen, AbbVie, Bayer, Actavis Specialty Pharmaceuticals, Sanofi, and Ferring Pharmaceuticals; Dr. Feng, receiving fees for serving on advisory boards from Medivation Astellas, GenomeDx, and Celgene, receiving grant support from Varian Medical Systems and Celgene, and being the founder and president (unpaid) of PFS Genomics; Dr. Souhami, receiving lecture fees from Varian Medical Systems; and Dr. Sandler, receiving consulting fees from Sanofi, Medivation, Clovis Oncology, Janssen Pharmaceuticals, Ferring Pharmaceuticals, and Blue Earth Diagnostics and lecture fees from Varian Medical Systems. No other potential conflict of interest relevant to this article was reported.
APPENDIX
The authors’ full names and academic degrees are as follows: William U. Shipley, M.D., Wendy Seiferheld, M.S., Himanshu R. Lukka, M.D., Pierre P. Major, M.D., Niall M. Heney, M.D., David J. Grignon, M.D., Oliver Sartor, M.D., Maltibehn P. Patel, M.D., Jean-Paul Bahary, M.D., Anthony L. Zietman, M.D., Thomas M. Pisansky, M.D., Kenneth L. Zeitzer, M.D., Colleen A.F. Lawton, M.D., Felix Y. Feng, M.D., Richard D. Lovett, M.D., Alexander G. Balogh, M.D., Luis Souhami, M.D., Seth A. Rosenthal, M.D., Kevin J. Kerlin, M.D., James J. Dignam, Ph.D., Stephanie L. Pugh, Ph.D., and Howard M. Sandler, M.D.
The authors’ affiliations are as follows: Massachusetts General Hospital and Harvard Medical School, Boston (W.U.S., N.M.H., A.L.Z.); NRG Oncology Statistics and Data Management Center (W.S., J.J.D., S.L.P.) and Einstein Medical Center (K.L.Z.), Philadelphia; Juravinski Cancer Centre at Hamilton Health Sciences, Hamilton, ON (H.R.L., P.P.M., M.P.P.), Hospital Notre-Dame du Centre Hospitalier de l’Université de Montréal (J.-P.B.) and McGill University Health Centre (L.S.), Montreal, and Tom Baker Cancer Centre, Calgary, AB (A.G.B.) — all in Canada; Indiana University, Indianapolis (D.J.G.); Tulane University, New Orleans (O.S.); Mayo Clinic, Rochester, MN (T.M.P.); Medical College of Wisconsin, Milwaukee (C.A.F.L.); University of Michigan, Ann Arbor (F.Y.F.); University of Vermont Medical Center, Burlington (R.D.L.); Radiation Oncology Center, Sacramento (S.A.R.), and Cedars–Sinai Medical Center, Los Angeles (H.M.S.) — both in California; Wayne Radiation Oncology, Goldsboro, NC (K.J.K.); and the University of Chicago, Chicago (J.J.D.).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990–2013. JAMA. 2015;314:80–2. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 2.Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–13. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mullins JK, Feng Z, Trock BJ, Epstein JI, Walsh PC, Loeb S. The impact of anatomical radical retropubic prostatectomy on cancer control: the 30-year anniversary. J Urol. 2012;188:2219–24. doi: 10.1016/j.juro.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299:2760–9. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–41. doi: 10.1200/JCO.2006.08.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pisansky TM, Kozelsky TF, Myers RP, et al. Radiotherapy for isolated serum prostate specific antigen elevation after prostatectomy for prostate cancer. J Urol. 2000;163:845–50. [PubMed] [Google Scholar]
- 7.Valcenti RK, Thompson I, Albertson P, et al. Adjuvant and salvage radiation therapy after prostatectomy: American Society for Radiation Oncology/American Urological Association guidelines. Int J Radiat Oncol Biol Phys. 2013;86:822–8. doi: 10.1016/j.ijrobp.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Pilepich MV, Caplan R, Byhardt RW, et al. Phase III trial of androgen suppression using goserelin in unfavorable-prognosis carcinoma of the prostate treated with definitive radiotherapy: report of Radiation Therapy Oncology Group Protocol 85–31. J Clin Oncol. 1997;15:1013–21. doi: 10.1200/JCO.1997.15.3.1013. [DOI] [PubMed] [Google Scholar]
- 9.Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337:295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 10.Hanks GE, Pajak TF, Porter A, et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol. 2003;21:3972–8. doi: 10.1200/JCO.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Iversen P, Tyrrell CJ, Kaisary AV, et al. Casodex (bicalutamide) 150-mg mono-therapy compared with castration in patients with previously untreated nonmeta-static prostate cancer: results from two multicenter randomized trials at a median follow-up of 4 years. Urology. 1998;51:389–96. doi: 10.1016/s0090-4295(98)00004-1. [DOI] [PubMed] [Google Scholar]
- 12.Tyrrell CJ, Kaisary AV, Iversen P, et al. A randomised comparison of ‘Casodex’ (bicalutamide) 150 mg monotherapy versus castration in the treatment of meta-static and locally advanced prostate cancer. Eur Urol. 1998;33:447–56. doi: 10.1159/000019634. [DOI] [PubMed] [Google Scholar]
- 13.Shipley WU, Hunt D, Lukka HR, et al. Initial report of RTOG 9601, a phase III trial in prostate cancer: effect of anti-androgen therapy (AAT) with bicalutamide during and after radiation therapy (RT) on freedom from progression and incidence of metastatic disease in patients following radical prostatectomy (RP) with pT2-3,N0 disease and elevated PSA levels. J Clin Oncol. 2011;29(Suppl):7. abstract. [Google Scholar]
- 14.American Joint Commission on Cancer. AJCC cancer staging manual. 5. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 15.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27:365–75. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 16.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–6. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 17.DeMets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med. 1994;13:1341–56. doi: 10.1002/sim.4780131308. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 19.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–70. [PubMed] [Google Scholar]
- 20.Cox DR. Regression models and life-tables. J R Stat Soc [B] 1972;34:187–220. [Google Scholar]
- 21.Gaynor JJ, Feuer EJ, Tan CC, et al. On the use of cause-specific failure and conditional failure probabilities: examples from clinical oncology data. J Am Stat As-soc. 1993;88:400–9. [Google Scholar]
- 22.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. [Google Scholar]
- 23.Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med. 1988;318:1728–33. doi: 10.1056/NEJM198806303182605. [DOI] [PubMed] [Google Scholar]
- 24.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319:1492–5. doi: 10.1136/bmj.319.7223.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Efstathiou JA, Bae K, Shipley WU, et al. Cardiovascular mortality and duration of androgen deprivation for locally advanced prostate cancer: analysis of RTOG 92-02. Eur Urol. 2008;54:816–23. doi: 10.1016/j.eururo.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Efstathiou JA, Bae K, Shipley WU, et al. Cardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG 85-31. J Clin Oncol. 2009;27:92–9. doi: 10.1200/JCO.2007.12.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voog JC, Paulus R, Shipley WU, et al. Cardiovascular mortality following short-term androgen deprivation in clinically localized prostate cancer: an analysis of RTOG 94-08. Eur Urol. 2016;69:204–10. doi: 10.1016/j.eururo.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wirth MP, See WA, McLeod DG, Iversen P, Morris T, Carroll K. Bicalutamide 150 mg in addition to standard care in patients with localized or locally advanced prostate cancer: results from the second analysis of the Early Prostate Cancer Program at median followup of 5. 4 years. J Urol. 2004;172:1865–70. doi: 10.1097/01.ju.0000140159.94703.80. [DOI] [PubMed] [Google Scholar]
- 29.Parker C, Clarke N, Logue J, et al. RADICALS (Radiotherapy and Androgen Deprivation in Combination after Local Surgery) Clin Oncol. 2007;19:167–71. doi: 10.1016/j.clon.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Carrie C, Hasbini A, De Laroche G, et al. Interest of short hormonotherapy associated with radiotherapy as salvage treatment for biological relapse after radical prostatectomy: results of the GETUG-AFU 16 phase III randomized trial. J Clin Oncol. 2015;33(Suppl):5006. abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.