Abstract
Purpose
To evaluate whether patients with Graves’ dysthyroid ophthalmopathy (GDO) have changes in brain anatomy that can be assessed by neuroimaging as compared to age-matched controls.
Methods
We examined 10 female, euthyroid patients with GDO and 14 age and gender matched controls using high-resolution structural MRI scanning. An automatic cortical segmentation algorithm was used to estimate the thickness of each subject’s gray matter across the brain. The resulting cortical thickness measurements were transferred to a standard reference space via a template-based warping procedure. A statistical analysis of between-group cortical thickness differences using a general linear model with a combination of cluster-based and bootstrapped corrections for multiple comparisons was performed.
Results
Patients with GDO have statistically significant thinning of the gray matter sheet in a minimum of eight locations: six in the right hemisphere (insula, paracentral, precuneus, superior frontal cingulate, superioparietal and postcentral) and two in the left hemisphere (lateral occipital sulcus, fusiform gyrus). Left hemisphere thinning occurred in portions of the occipital lobe while right hemisphere thinning occurred in a set of frontal and parietal areas known to be involved in self-evaluation and emotional and cognitive regulation.
Conclusion
Patients with GDO exhibit localized changes in gray matter thickness. These changes may be associated with cognitive changes reported by GDO patients. Further research before and after treatment with larger patient cohorts and experimental protocols including psychiatric evaluation and functional MRI are recommended.
PURPOSE
A pilot study to examine the structural MRI features of patients with Graves’ dysthyroid ophthalmopathy as compared to normal controls. Correlate pathologic MRI changes to clinical signs and symptoms of the disease and compare findings to those of patients with known stress disorders or mild cognitive impairment.
INTRODUCTION
Graves’ dysthyroid ophthalmopathy (GDO) is a debilitating, vision threatening autoimmune disease that largely affects women between the ages of 30 and 50. The physical manifestations of the disease include periorbital edema, double vision, protrusion of the eyes, ocular dryness, light sensitivity, upper and lower eyelid retraction and on occasion loss of vision secondary to optic nerve compression. (Figure 1).
FIGURE 1.

Presentation of a patient with Graves’ dysthyroid ophthalmopathy. Typical is the eyelid retraction, injection and proptosis of a patient with GDO.
In addition to these well-documented ophthalmic symptoms, GDO is associated with functional and psychological abnormalities in the central nervous system. Among these abnormalities are depression, emotional lability, memory deficits and personality irregularities1,2,3,4,5. These psychological disturbances cannot be explained solely by the physical symptoms of the disease, or by the insomnia and fatigue that are commonly associated with it 6, 7. The psychological disturbances may occur prior to the development of the diagnosis of the eye disease, suggesting that they may be a product of subclinical thyroid imbalance8, however the symptoms are evident as well in euthyroid GDO individuals. Our hypothesis is that GDO may be correlated with structural brain abnormalities that are verifiable on MRI. We demonstrate changes in our cohort that are similar to those anatomic changes seen in patients with stress disorders or mild cognitive impairment.
There may be a genetic predisposition to GDO and there is evidence that GDO can be triggered by periods of severe stress due to negative life events 9,10, and 11 Some cases of GDO appear similar to post-traumatic stress disorders resulting in an autoimmune response in susceptible subjects10,11,12,13,14,15,16,17 This hypothesis has anecdotal support from the earliest recorded descriptions of the disease 10,11,12,13,14.
The link between stress and GDO remains controversial. Stress has been implicated in triggering GDO, and physiological paths linking increased glucocorticoid and catecholamine levels to abnormal thyroid regulation have been proposed 15. Other reports suggest that patients who develop GDO have a predisposition to depression, negative affect and an increased tendency to report negative life events 2,4. This could be due to a long-term, undiagnosed thyroid imbalance but could also be related to underlying psychiatric problems that render these patients more likely to experience NLEs; such as marital breakup, loss of a job or a period of severe depression which is, itself, a stressful event 2. Establishing a causal link between severe NLEs and GDO is therefore difficult.
Metabolic abnormalities have been reported in the prefrontal and limbic system of GDO patients16, however there has been no objective neuroanatomic evaluation of GDO. To date, there no clear link between GDO, stress and neurological dysfunction. Although patients with GDO exhibit symptoms similar to those found in mild cognitive impairment (MCI), depression, anxiety and mood disorders, the neural anatomic correlates of the GDO symptoms may be entirely different from those found in these well-recognized neuropsychiatric conditions. Understanding the pattern of cortical deficits associated with GDO is a critical step in understanding the origin of the disease and the mechanism by which it causes psychological illness and its similarity if any to MCI.
The hypothesis we test here is that GDO is associated with structural brain abnormalities compared to controls without GDO. These anatomical changes may be similar to those seen in patients with stress disorders or mild cognitive impairment. Because our approach is purely correlational, we cannot address whether GDO causes these cortical changes but identifying a relationship between GDO and cortical anatomical changes may lead us to reexamine the role that thyroid disorders might play in cognitive dysfunction as well as, potentially, providing a novel way of evaluating or treating this disorder.
We selected a quantitative approach to this problem by examining cortical gray matter thickness in GDO and control groups. Gray matter thickness at particular cortical locations (prefrontal cortex, medial temporal lobe, hippocampus and parietal cortex) is known to correlate with symptom severity in many neurological diseases including dementia and depression17,18 26,27. In most cases, symptom severity is correlated with local decreases in gray matter thickness relative to normal controls. Interestingly in GDO, the associated psychiatric problems are improved substantially or even disappear over time as the disease regresses - a fact that argues against significant preexisting or permanent anatomical changes underlying the cognitive aspects of the disease. Discovering a consistent abnormality in cortical thickness in GDO patients even after the signs and symptoms of the eye disease abate, especially in regions correlated with cognitive dysfunction or depression, would argue in favor of an underlying predisposition to psychiatric illness. Failure to find such a correlation, would support the hypothesis that the psychiatric symptoms of GDO result from relatively transient cortical changes caused by discrete, recent events. The magnitude of the psychiatric disturbance reported in many GDO patients is comparable to that caused by neurological diseases with clear neuroanatomical correlates 16.
Here, we report measurements of cortical gray matter thickness 19,41 in patients suffering from GDO compared to a gender and age-matched control group. The techniques we describe are used routinely by researchers examining the link between gray matter thickness with clinical symptoms 20,21,22,23,24–27. We measured the difference between mean gray matter thickness in our study and control groups at each point across a standardized cortical surface. Our analysis then identified the presence of statistically-significant clustered differences between the groups 28,29.
We identified eight regions in which the cortex of GDO patients is statistically different from those of the control group. These regions can be identified on standardized cortical atlases30. Of the eight areas found to pass our conservative statistical threshold: six were in the right hemisphere and two in the left. Of the six right hemisphere regions, two were relatively small (< 100mm sq.) and although we list them for completeness, we do not consider them in detail. We discuss the significance of the six regions in turn.
METHODS
Two high-resolution, T1-weighted anatomical data sets were created per subject at a resolution of 1×1×1 mm using the standard Siemens MP-RAGE sequence 31. We also collected a single T2-weighted anatomical data set at the same resolution to facilitate the segmentation of brain surfaces. After correcting the 3D data sets for low-spatial-frequency intensity variations, we used the Oxford FSL toolbox (http://www.fmrib.ox.ac.uk/fsl/) to co-align and average the MP-RAGE volumes within each session and to align the T2-weighted volumes to the same reference space.
Subsequent anatomical analysis was performed in Freesurfer. The standard ‘recon-all’ pipeline was used to automatically remove low spatial frequency bias from the images, align them to a standard atlas space, segment the white matter from the left and right hemispheres and then reconstruct the cortical and pial surfaces 19,29. Additional registration steps were included to align each subject’s surfaces to a standard spherical coordinate space and extract gray matter thickness, curvature and area measurements in this space 28.
Segmentations were checked by expert observers’ midway through the pipeline (recon stage ‘1’) to ensure that no gross errors in white matter assignment contaminated the subsequent measurements. Gray matter statistical analysis was performed using the Freesurfer “Query, Design, Estimate, Contrast” (“QDEC”) which fits a general linear model (GLM) to group data at each point on the template-registered cortical surface. Prior to running the GLM, data were smoothed isotropically with a Gaussian kernel (FWHM=10mm) to remove small, non-significant data clusters. The GLM beta values were converted to mm-scale thickness values.
Parametric statistics can be unreliable with small sample sizes because it can be difficult to determine whether data are normally distributed. For this reason, we used a non-parametric resampling technique 29 to generate statistically robust threshold levels. In general, resampling techniques are the preferred option to determine statistical significance in cases where the data cannot be shown to follow a normal distribution. They have the advantage that they are ‘model-free’ and give an unbiased estimate of the true significance level of an effect. The downside, until recently, has been their extremely high computational load: data must be resampled thousands of times per point to obtain an unbiased distribution estimate.
In this study, thresholds were determined by a bootstrapping technique (implemented in QDEC) that resampled then averaged the difference between GDO and control cortical thicknesses 10,000 times at each point on the cortex in order to compute reliable confidence intervals.32 Once the resampling distribution for this difference is available, it is possible to determine the proportion of resampled values that lie above or below the ‘zero’ line (representing the null hypothesis of ‘no difference’). If 95% of the data points lie above zero, the difference in cortical thickness is considered to be statistically significant at the p<.05 level.
By definition, if many tests of this type are performed on uncorrelated noise, positive results will occur by chance in 5% of the tests. Over many voxels, this may lead to dozens, or even hundreds of ‘false positives’. Freesurfer/QDEC corrects for this in two ways. Firstly, the data are spatially smoothed with a Gaussian filter so that random noise levels are reduced and spatial correlation is introduced between neighboring voxels. This reduces the number of independent data points at the cost of reducing the intrinsic spatial resolution of the technique. Secondly the statistical sensitivity of the technique is reduced (or, equivalently, the underlying ‘p’ value of the test is made smaller) so that fewer individual voxels pass the statistical threshold. This combination of smoothing and multiple comparisons correction ensured that the p-values quoted for individual clusters are statistically correct.
Our data were smoothed with a 10 mm Gaussian blurring function (see above) and thresholded at a true, bootstrapped false discovery rate of p<.05. The data presented are from QDEC’s graphical interface which produces plots of statistically-significant gray matter thickness differences rendered onto the inflated, standardized cortex. These are conservative thresholds and larger sample sizes may reveal subtle but statistically significant changes in additional brain regions. Since age and gender were already controlled, the single question the authors asked was whether there was a difference between GDO and non-GDO controls in either the left or right hemispheres. The data we present therefore represents a comparison between two independent datasets illustrated on a single, standardized, cortical surface.
Ten female patients were recruited sequentially from the practice of one of the authors (RZS). These patients were newly referred for the diagnosis and management of Graves’ dysthyroid ophthalmopathy or thyroid eye disease. As part of their normal care, patients underwent hematologic testing evaluating their thyroid function as well as thyroid autoantibody status. Patients included in the study manifested active signs and symptoms of thyroid eye disease with a clinical activity score equal or greater than 3. The clinical activity score is a system designed to evaluate and standardize the signs and symptoms of thyroid eye disease. The clinical activity score, described by Mourits 33 is based on four of the five well known manifestations of inflammation (pain, swelling, redness and impaired function) and consists of ten items. One point is awarded for each item present. The sum of these points is the Clinical Activity Score (CAS: 0 to 10). (Table 1)33
TABLE 1.
THE CLINICAL ACTIVITY SCORE
| Pain | Painful oppressive feeling on or behind the globe during the last 4 weeks Pain on attempted up, side or downgaze during the last 4 weeks |
| Redness | Redness of the eyelid(s) Diffuse redness of the conjunctiva, covering at least one quadrant |
| Swelling | Swelling of the eyelid(s) Chemosis Swollen caruncle |
| Impaired Function | Increase of proptosis ≥2 mm during a period of 1–3 months Decrease of eye movements in any direction ≥5° during a period of 1–3 months Decrease of visual acuity ≥1 line(s) on the Snellen chart (using a pinhole) during a period of 1–3 months. |
For each item, one point is given. The sum of the points is the Clinical Activity Score (CAS).
As there is a known correlation between hyperthyroidism and mental illness9,34 all patients recruited for this study were actively managed to bring them to as close to a hematologically euthyroid state as possible at the time of the MRI study. GDO is more common in females than males 35 and cortical thickness is known to vary between females and males. To eliminate sex differences as a potential confounding variable all patients and controls were female. All patients were right handed. Subject ages ranged from 48 to 63 years. None of the patients had a history of mental illness or were taking antidepressant or other psychoactive medications at the time of study.
Table 2 presents the demographic data of the thyroid patients as well as their thyroid function tests at the time of the MRI scanning. Patient results were compared to age and gender matched normal controls. The control group consisted of female subjects with an age distribution matched to that of the patient group. Control data were extracted from an anonymized database of subjects who underwent identical anatomical scans (same pulse sequence, protocol, scanner and post-processing) as the patient group. Control patients were psychologically normal and had normal- or corrected-to-normal vision.
TABLE 2.
DEMOGRAPHICS, THYROID FUNCTION AND AUTOANTIBODY TESTS OF GRAVES’ DYSTHYROID OPHTHALMOPATHY STUDY PATIENTS
| PT | RACE | DATE OF PRESENTATION/F/U | T3 | T3 TOTAL |
T4, FREE | TSH | TSI | TPO AUTOAb |
ANTITHYROGLOBULIN Ab | TBII | THYROGLOBIN | THYRO TROPIN Ab | HANDEDNESS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| 71–180 ng/dL | 87–167 ng/dL | .5–1.77 ng/dL | .34–3.5 ulU/mL | 0–139 | <35 | <20 | <16 | 2–35 ng/mL | .45–4.5uIU/mL | ||||
| 1 | Cauc | 4/30/12 | 1.7 | 0.01 | 309 | Right | |||||||
| 6/4/12 | 4.6 | 1.5 | 0.01 | ||||||||||
| 7/10/12 | 120 | 1.1 | 0.01 | 430 | <10 | <20 | 42 | ||||||
| 10/15/12 | 90 | 0.83 | 1.560 | 458 | 287 | 457 | 34.46 | Right | |||||
| 2 | Cauc | 5/8/12 | 11 | ||||||||||
| 5/22/12 | 157 | ||||||||||||
| 6/5/12 | 2.71 | 1.0 | 1.09 | ||||||||||
| 3 | Cauc | 3/27/12 | 2.3 | 1.19 | 1.990 | 277 | 10.05 | Right | |||||
| 7/6/12 | 2.3 | 1.01 | 4.380 | ||||||||||
| 11/7/12 | 89 | 1.08 | 3.630 | 378 | |||||||||
| 4 | Cauc | 2/24/12 | 28 | 1.0 | 1.18 | 410 | <10 | <20 | 42 | Right | |||
| 5 | Cauc | 6/8/12 | 93 | 1.3 | 7.58 | 0.40 | >1300 | 213 | Right | ||||
| 6 | Cauc | 2/2/12 | 100 | 0.8 | 1.70 | Right | |||||||
| 3/20/12 | 0.9 | 0.72 | |||||||||||
| 4/13/12 | 0.9 | 1.30 | |||||||||||
| 7/12/12 | 108 | 0.9 | 0.04 | ||||||||||
| 9/5/12 | 1.3 | 0.01 | |||||||||||
| 9/14/12 | 1.3 | <0.01 | |||||||||||
| 9/28/12 | 1.2 | <0.01 | |||||||||||
| 10/26/12 | 0.9 | 0.01 | |||||||||||
| 7 | Hisp | 8/20/12 | 85 | 1.05 | 0.298 | 569 | 90 | <20 | 173 | 5.29 | Right | ||
| 8 | Cauc | 1/27/12 | 528 | <10 | <20 | 42 | 59.6 | Right | |||||
| 5/14/12 | 312 | 3.50 | 0.012 | 455 | 4476.0 | ||||||||
| 6/5/12 | 6.65 | 2.38 | 0.008 | ||||||||||
| 7/5/12 | 3.27 | 1.06 | 0.048 | 523 | |||||||||
| 7/24/12 | 4.74 | 1.60 | <0.008 | 342 | |||||||||
| 8/29/12 | 2.91 | 1.04 | <0.133 | ||||||||||
| 9 | Cauc | 10/15/12 | 90 | 0.83 | 1.560 | 458 | 287 | 457 | 34.46 | Right | |||
| 10 | Asian | 12/10/12 | 82 | 0.94 | 6.040 | 618 | 215 | 210 | 18.32 | Right | |||
| 8/09/11 | 166 | 1.73 | 0.008 | 342 | 12 | <20 | 21.44 | ||||||
T3=Triiodothyronine, T3 Total=Total triiodothyronine levels, T4, Free= Free thyroxine, TSH= Thyroid stimulating hormone, TSI=Thyroid stimulating immunoglobulin, TPO AutoAb= Antithyroid peroxidase antibody, TBII= Thyroid binding inhibiting immunoglobulin
Ethical approval was obtained prospectively from the Institutional Review Boards of both the University of California, San Francisco and the Smith-Kettlewell Eye Research Institute. Informed consent was obtained for all participation in the research. The research was HIPAA compliant and the researchers adhered to the Declaration of Helsinki and all federal or state laws.
RESULTS
Gray matter thickness analysis revealed six regions in the right hemisphere and two in the left hemisphere that passed our statistical threshold. These regions are illustrated in Figures 2 and 3 and the Talairach coordinates 36 are listed in Tables 3 and 4.
FIGURE 2.
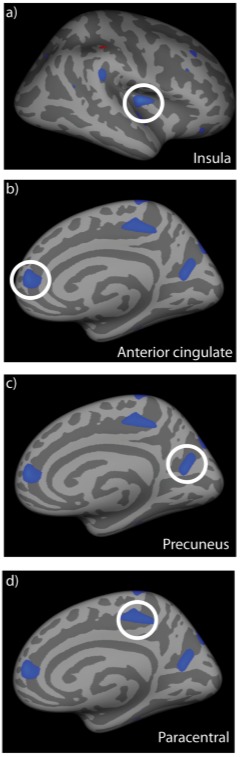
Cortical regions exhibiting significant gray matter thinning in Graves’ dysthyroid ophthalmopathy patients compared to normal controls. Data are rendered on an inflated, standard cortical surface. Significant loss of gray matter was observed in the right insula (a), right anterior cingulate (b), right precuneus (c) and right paracentral gyrus (d). These regions are all implicated in cognitive disorders or emotional regulation. The locations and sizes of all clusters are listed in Table 3.
FIGURE 3.
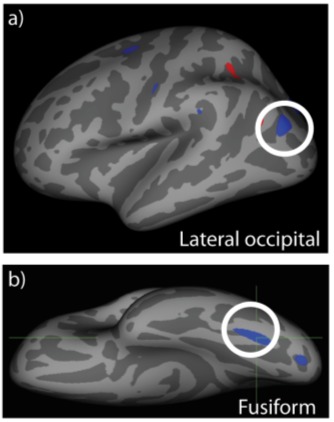
Left hemisphere regions exhibiting significant gray matter thinning in the lateral occipital cortex (a) and fusiform gyrus (b). Both regions lie in occipital cortex in areas associated with mid- or high-level visual processing. The locations and sizes of these clusters are listed in Table 4.
TABLE 3.
RIGHT HEMISPHERE CLUSTER DATA: POSITIONS AND SIZES OF REGIONS SHOWING SIGNIFICANT THINNING IN GRAVES’ DYSTHYROID OPHTHALMOPATHY PATIENTS.
| CLUSTER | MAX | SIZE (mm2) | TALX | TALY | TALZ | ANNOTATION |
|---|---|---|---|---|---|---|
| 1 | −3.0276 | 203.54 | 37.3 | −14.6 | 10.7 | Insula |
| 2 | −2.5315 | 162.74 | 15.6 | −30.7 | 45.2 | Paracentral |
| 3 | −2.4147 | 199.64 | 23.8 | −64.8 | 15.6 | Precuneus |
| 4 | −2.3889 | 138.46 | 19.4 | −84.1 | 39.8 | Superior frontal/cingulate |
| 5 | −2.3742 | 99.15 | 15.7 | 45.0 | 8.6 | Superiorparietal |
| 6 | −2.0515 | 23.31 | 5.4 | −37.4 | 71.6 | Postcentral |
Max: Maximum thickness difference in this cluster between normal and GDO (mm).
Size: Size of cluster in square mm.
TalX, Taly, and TalZ: Location of cluster center in ‘Talairach’ standardized coordinate space
TABLE 4.
LEFT HEMISPHERE CLUSTER DATA: POSITIONS AND SIZES OF REGIONS SHOWING SIGNIFICANT THINNING IN GRAVES’ DYSTHYROID OPHTHALMOPATHY PATIENTS.
| CLUSTER | MAX | SIZE (mm2) | TalX | TalY | TalZ | ANNOTATION |
|---|---|---|---|---|---|---|
| 1 | −2.3947 | 198.78 | −33.6 | 64.7 | −15.2 | Fusiform |
| 2 | −2.2794 | 95.85 | −19.1 | −90.1 | −7.9 | Lateral occipital |
Max: Maximum thickness difference in this cluster between normal and GDO (mm).
Size: Size of cluster in square mm.
TalX, Taly, and TalZ: Location of cluster center in ‘Talairach’ standardized coordinate space
Overall, we found the largest differences in the right insula, right anterior cingulate, right precuneus and right paracentral sulcus. Smaller clusters were also found in the right postcentral gyrus and right superior parietal lobes as well as the left lateral occipital cortex and left fusiform gyrus. In all cases, we found a relative thinning of cortex in GDO patients compared to age-matched controls with average gray matter thickness levels decreasing by between 0.5 to 0.9 mm depending on the precise location.
The significance of the occipital cortex differences remains unclear. Both regions are involved in mid or high level visual processing (for example, face processing or the processing of contours and orientation37,38,39). No significant changes were observed in lower level visual regions (notably primary visual cortex) or in regions that receive highly myelinated projections from lower areas (for example, area hMT+) and this argues against a deficit originating in compromised retinal projections (for example, due to dysthyroid optic neuropathy 40) although gray matter in primary visual cortex is relatively thin and subtle anatomical changes may not be easy to detect in this location. We hypothesize that deficits in these mid-level visual regions may reflect reductions in feedback from higher areas – potentially those involved in directing attention to specific visual features or faces. Additional anatomical and functional studies involving fiber tract tracing and whole-brain voxel-based morphometry41 may resolve these issues in the future.
Cortical thinning in the four frontal regions identified in the right hemisphere is of more interest in the context of the cognitive and emotional disturbances that often accompany GDO. All four regions have been implicated in high level cognition. Three out of the four (the anterior cingulate, the insula and regions around the paracentral sulcus) have been directly linked to emotional regulation while the precuneus is known to be involved in self-reflection, consciousness and linking new information with experience 42,43. We discuss the significance of these deficits in more detail below.
DISCUSSION
Graves’ dysthyroid ophthalmopathy (GDO) or thyroid eye disease is a debilitating, vision threatening condition that largely affects individuals in the most productive years of their life (30 to 50 years of age). This autoimmune, inflammatory disease occurs in women six times more frequently than men, and occurs in association with hyperthyroidism in 94% of cases.
The ocular effects of GDO include proptosis, chemosis, and erythema. Eyelid retraction, extraocular muscle restriction, strabismus, dry eye and potentially optic nerve compression may also occur. However, not all symptoms of GDO are ophthalmologic. Cognitive changes including emotional lability, memory deficits and personality irregularities are frequently reported by patients. Cognitive changes, associated with Graves’ thyroidopathy were described as early as 1933 by Mittleman 13.
The etiology of thyroid eye disease is unknown. Researchers however, have hypothesized that severe emotional shock or stress caused by negative life events may be an instigating feature of thyroid eye disease. The term “crystallized shock” had been used to describe the physiognomy of these patients 12. A case controlled study performed by Radosavljević44 was conducted to assess possible relationships between NLEs and Graves’ disease. The study included 100 newly diagnosed patients with Graves’ disease and 100 controls matched with respect to sex, age (±2 years) and type of residence (rural, urban). Paykel’s Interview for Recent Life Events (a semistructured research interview covering 61 life events) was administered to each subject. In comparison with controls, the Graves’ patients claimed to have had significantly more negative life events in the 12 months preceding the diagnosis (p = 0.0001). These findings indicate that life events may be a risk factor for Graves’ disease. However, despite some statistical correlation between negative life events and the onset of GDO, correlation is not causation. It is possible that cognitive changes caused by the disease may be the cause rather than the effect of some NLEs such as divorce. GDO may interact with a preexisting susceptibility to negative life events and may exacerbate, rather than cause, underlying conditions such as depression or mania. So while it is clear that there is a heightened response to stress and emotional upset in patients with GDO, which came first- the disease or the repeated and/or sustained NLE-remains unclear 9,43,45.
During stress, activation of the hypothalamic-pituitary-adrenal axis and the sympathoadrenal system leads to increased secretion of glucocorticoids and catecholamines in order to maintain homeostasis. Recent evidence suggests that stress hormones, acting on antigen-presenting immune cells, may influence the differentiation of bipotential T helper (Th) cells away from Th1 and toward a Th2 phenotype. This results in suppression of cellular immunity and potentiation of humoral immunity. A predominantly Th2-mediated immune response may induce antigen-specific B lymphocytes to produce anti-TSH receptor (TSHr) antibodies 15. These antibodies bind to receptors on the thyroid gland inducing hyperthyroidism. When similar antibodies bind to surface receptors on the fibro connective tissue in the orbit, they induce T cell recruitment, the release of numerous cytokines including IL-6, IL-17, IGFR-1 and TNF alpha. This cytokine release promotes the deposition of glycosaminoglycans and hyalurons into the orbital muscles. The cytokines also promote adipogenesis and fibrocyte proliferation. This cascade leads to the signs and symptoms of GDO. Whether this cascade promotes a local CNS effect remains unknown.
Studies using quality of life (QoL) questionnaires have shown poor QoL in patients with GDO. A qualitative study of patients with GDO, identified the phenomenon of “altered identity” due to direct consequences of GDO impacting patients’ wellbeing, coping strategies and interactions with healthcare professionals 46. These patients are not only physically ill, they also exhibit emotional distress. Farid, et al 3 analyzed the variance in stress amongst patients with differing severity of GDO. He demonstrated that those patients with moderate/severe thyroid ophthalmopathy showed significantly greater emotional distress than patients with mild/negligible thyroid ophthalmopathy on the Profile of Mood States mean total score (P<.001). Additionally, patients who had disfigurement (proptosis) as the predominant clinical feature had significantly elevated emotional distress compared with the control group (P = .01), whereas no significant difference was detected between the control group and patients with diplopia as the predominant clinical feature (P = .20). Accompanying psychosomatic treatment is indicated among about half of all GDO patients4,47.
Cognitive and emotional changes may outlast the active disease state. Residual complaints after treatment for hyperthyroidism in euthyroid patients were investigated by Fahrenfort 47. On each dimension of psychopathology covered by the Symptoms Check List (SCL-90), including depression and anxiety, approximately one third of the total sample had a score exceeding 80% of adult females. Lack of energy was evident in 53% of all respondents. Over one third of patients with a full-time job were unable to resume the same work after treatment. Many of these patients were in need of ongoing psychological support.
The knowledge that patients with hyperthyroidism show impaired performance on several neuropsychological tests that require complex visual discrimination, conceptualization, mental flexibility or organization and that these findings are consistent with prefrontal lobe dysfunction encouraged a pilot study 16 to characterize the metabolite profile in the right prefrontal cortex in six patients with untreated Graves’ disease using in vivo proton magnetic-resonance spectroscopy (1H-MRS) and healthy controls. The choline/creatine (Cho/Cr) ratios of the hyperthyroid patients were significantly lower than that of controls. Cho/Cr ratios were higher after treatment (euthyroidism) than before treatment. These preliminary data are most consistent with reversible reductions in the concentrations of choline-containing compounds (especially glycerophosphocholine and phosphocholine) in the prefrontal area during hyperthyroidism.
The psychological changes of Graves’ disease have been evaluated using objective psychological measurements 48. There are reports of metabolic abnormalities in the prefrontal and limbic system of GDO patients. However there have been no studies to date of the structural MRI cerebral anatomy of patients with GDO. We questioned whether changes may exist in structural brain MRI as they do in patients with other types of neurological dysfunction and severe stress. To our knowledge, this is the first anatomical neuroimaging study of cortical thickness in patients with GDO.
In this study, the authors adopted a novel quantitative approach to explore the neuroanatomic characteristics of patients with GDO as compared to normal controls. We asked whether our cohort of patients with GDO demonstrated altered neuroanatomy. Once demonstrated, we questioned whether the changes might be implicated in the types of cognitive abnormalities previously reported in this disease 1–13. We used state of the art, high resolution anatomical MRI scans to generate 3D models of both white and gray matter in individual subjects (both GDO patients and age-matched ‘controls’). Gray matter thickness measurements were obtained for each point in the brain and data from different subjects were combined by ‘warping’ each brain to a common template. Statistical analysis based on a General Linear Model (with corrections for multiple comparisons) was then used to identify regions of gray matter that vary in thickness between patient and control groups.
We found clear evidence of significant gray matter thickness differences between the two groups. GDO has been traditionally associated with ophthalmologic symptoms and the presence of clear, demonstrable cortical changes in these patients is novel. All patients with GDO consistently showed thinner gray matter in several cortical regions than controls Most changes occurred in the right hemisphere and interestingly, we found alterations in the right prefrontal region (the anterior cingulate) close to that studied by Bhatara and colleagues using magnetic resonance spectroscopy 16. Bhatara et al identified lower choline/creatine ratios in the frontal lobes of hyperthyroid patients and they hypothesized that these might underlie the psychiatric changes often seen in this patient group. However, they found no evidence of gross anatomical changes in this region and identified a small patient population size (N=6) as a potential shortcoming of their study.
Comparing the psychiatric conditions associated with GDO to well-understood neurological phenomena such as mild cognitive impairment and depression could represent a significant advancement in our understanding of this disease and our ability to monitor and treat its neuropsychological effects. MRI is an informative method for this use, as it elucidates structural changes across the entire brain with sufficient spatial resolution to resolve well-defined anatomical locations. The authors leveraged this effect to produce the first anatomical neuroimaging study of GDO evaluating cortical thickness.
Existing fMRI studies of depression and mid cognitive impairment/attention deficit (MCI/AD) do not always agree on the magnitude or distribution of disease biomarkers but some consensus is emerging in both fields that robust differences between control subjects and patients (or subjects at risk from psychiatric disease) are seen when scanning with certain, well-defined paradigms. For example, in depression, a general hyper activation of cingulate cortex when executing demanding cognitive tasks, such as n-back character recognition or Stroop color naming is seen. In the case of cognitive impairment not associated with depression, researchers typically find changes in the medial temporal lobe and the hippocampus. The type of change depends on the stage of the disease: in early stages of cognitive impairment, studies often report hyper activation in these regions49. Later stages of the disease are characterized by hypo activation in these locations. Evaluation of GDO patients with fMRI with comparison to normal controls and patients with MCI would be very useful.
In this study, we sought to evaluate the neuroanatomy of patients with hematologically quiescent but clinically active thyroid eye disease. The authors compared the GDO population to normal controls. They identified abnormalities in the study population and discussed the similarity between the abnormal responses seen in GDO with those of patients with mood disorders and mild cognitive impairment. There are limitations to this study. Specifically, and most significantly the number of patients studied is limited. The authors would suggest additional studies with a larger n and increased numbers of normal controls. Analyzing control groups for documented absence of psychiatric or thyroid disease would be ideal, although this is already an assumption for the standardized control group data. Study of patients after their disease has abated to determine the return to normalcy, if this exists would be interesting and could serve as an internal control. This initial study is a structural MRI analysis.
Future research should focus on the correlation between cognitive deficits and anatomical changes in a larger patient cohort. This could involve functional MRI (fMRI) as well as structural brain imaging techniques with analysis of both the active and quiescent patient with GDO, hyperthyroid and euthyroid, and their response to treatment. Study of GDO patients placed either preemptively or therapeutically on antidepressants or other appropriate psychoactive medications with follow up MRI scanning could lead to a visual representation of the effect of medication on structural changes, if any, seen in the brain from treatment of the disease process. These future studies could test the use of antidepressants or other psychoactive medications to prevent or modulate the onset of GDO in patients at risk (genetic predisposition, high stress, underlying MCI, etc.) or as a treatment of early Graves’ dysthyroid ophthalmopathy along with thyroid hormone regulation.
CONCLUSION
Patients with Graves’ dysthyroid ophthalmopathy commonly complain of cognitive changes and deficits in emotional regulation during the active disease state. In this research, we asked whether GDO is correlated with structural changes in the brain as seen by MRI that could account for these deficits. We compared these structural alterations with the changes in the size and shape of cortical and subcortical structures demonstrated in many neurological diseases including depression and schizophrenia and mild cognitive impairment. We believe this is the first study to demonstrate that GDO – a disease normally associated with ophthalmologic signs and symptoms – is also associated with changes in cortical thickness in parts of the brain known to regulate emotional state and metacognition. Understanding the cognitive deficits associated with GDO is important to the diagnosis, management and treatment of this disease. The discovery that these cognitive deficits may have a neuroanatomical basis is a significant step toward this goal. Future research should focus on the correlation between cognitive deficits and anatomical changes in a larger patient cohort and may involve functional as well as structural brain imaging techniques to identify networks of areas that are affected by the hyperthyroid or the euthyroid patient with Graves’ ophthalmopathy and their response to treatment. Additionally, consideration may be given to the use of antidepressants or other psychoactive medications to prevent the onset of GDO in patients at risk (high stress or similar environments) or as a treatment of early Graves’ dysthyroid ophthalmopathy along with thyroid hormone regulation.
ACKNOWLEDGEMENTS
Funding/Support: Pacific Vision Foundation
Financial Disclosures: none (RZS, ARW)
-
Contributions of Authors:
Design and conduct of the study; RZS, ARW
Collection, management, analysis, and interpretation of the data: RZS, ARW
Preparation, review, or approval of the manuscript: RZS, ARW
Other Acknowledgments: Adam Gazzaley Ph.D. for use of his laboratory to conduct the study, Morgan Hough Ph.D. for technical support of the study
REFERENCES
- 1.Haggerty JJ, Jr, Garbutt JC, Evans DL, et al. Subclinical hypothyroidism: a review of neuropsychiatric aspects. International Journal of Psychiatry in Medicine. 1990;20(2):193–208. doi: 10.2190/ADLY-1UU0-1A8L-HPXY. [DOI] [PubMed] [Google Scholar]
- 2.Haggerty JJ, Jr, Stern RA, Mason GA, Beckwith J, Morey CE, Prange AJ., Jr Subclinical hypothyroidism: a modifiable risk factor for depression? The American Journal of Psychiatry. 1993 Mar;150(3):508–510. doi: 10.1176/ajp.150.3.508. [DOI] [PubMed] [Google Scholar]
- 3.Farid M, Roch-Levecq AC, Levi L, Brody BL, Granet DB, Kikkawa DO. Psychological disturbance in graves ophthalmopathy. Archives of Ophthalmology. 2005 Apr;123(4):491–496. doi: 10.1001/archopht.123.4.491. [DOI] [PubMed] [Google Scholar]
- 4.Coulter I, Frewin S, Krassas GE, Perros P. Psychological implications of Graves’ orbitopathy. European Journal of Endocrinology/European Federation of Endocrine Societies. 2007 Aug;157(2):127–131. doi: 10.1530/EJE-07-0205. [DOI] [PubMed] [Google Scholar]
- 5.MacCrimmon DJ, Wallace JE, Goldberg WM, Streiner DL. Emotional disturbance and cognitive deficits in hyperthyroidism. Psychosomatic Medicine. 1979 Jun;41(4):331–340. doi: 10.1097/00006842-197906000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Möller MC, Bartfai A, Nygren de Boussard C, Radestad AF, Calissendorff J. High rates of fatigue in newly diagnosed Graves’ disease. Fatigue Biomed Heal Behav. 2014;2(3):153–162. [Google Scholar]
- 7.Rockey PH, Griep RJ. Behavioral dysfunction in hyperthyroidism. Improvement with treatment. Archives of Internal Medicine. 1980 Sep;140(9):1194–1197. [PubMed] [Google Scholar]
- 8.Stern RA, Robinson B, Thorner AR, Arruda JE, Prohaska ML, Prange AJ., Jr A survey study of neuropsychiatric complaints in patients with Graves’ disease. The Journal of Neuropsychiatry and Clinical Neurosciences. 1996 Spring;8(2):181–185. doi: 10.1176/jnp.8.2.181. [DOI] [PubMed] [Google Scholar]
- 9.Matos-Santos A, Nobre EL, Costa JG, et al. Relationship between the number and impact of stressful life events and the onset of Graves’ disease and toxic nodular goitre. Clinical Endocrinology. 2001 Jul;55(1):15–19. doi: 10.1046/j.1365-2265.2001.01332.x. [DOI] [PubMed] [Google Scholar]
- 10.Bram I. Psychic trauma in pathogenesis of exophthalmic goiter. Endocrinology. 1927;11(2):106–116. [Google Scholar]
- 11.Graves RJ. Newly observed affection of the thyroid gland in females. Lond Med Surg, 1835;7(2):516–517. [Google Scholar]
- 12.Lidz T. Emotional factors in the etiology of hyperthyroidism. Psychosomatic Medicine. 1949 Jan-Feb;11(1):2–8. doi: 10.1097/00006842-194901000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Mittleman B. Psychogenic factors and psychoptherapy in hyperthyrosis and rapid heart imbalance. J Nerv Ment Dis. 1933;77(5):457–488. [Google Scholar]
- 14.Parry CH. Collections from the unpublished works of the late Caleb Hillier Parry1825:478. Located at: Underwood. [Google Scholar]
- 15.Tsatsoulis A. The role of stress in the clinical expression of thyroid autoimmunity. Annals of the New York Academy of Sciences. 2006 Nov;1088:382–395. doi: 10.1196/annals.1366.015. [DOI] [PubMed] [Google Scholar]
- 16.Bhatara VS, Tripathi RP, Sankar R, Gupta A, Khushu S. Frontal lobe proton magnetic-resonance spectroscopy in Graves’ disease: a pilot study. Psychoneuroendocrinology. 1998 Aug;23(6):605–612. doi: 10.1016/s0306-4530(98)00028-6. [DOI] [PubMed] [Google Scholar]
- 17.Abe O, Yamasue H, Kasai K, et al. Voxel-based analyses of gray/white matter volume and diffusion tensor data in major depression. Psychiatry Research. 2010 Jan 30;181(1):64–70. doi: 10.1016/j.pscychresns.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Chetelat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron JC. Mapping gray matter loss with voxel-based morphometry in mild cognitive impairment. Neuroreport. 2002 Oct 28;13(15):1939–1943. doi: 10.1097/00001756-200210280-00022. [DOI] [PubMed] [Google Scholar]
- 19.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999 Feb;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 20.Du AT, Schuff N, Kramer JH, et al. Different regional patterns of cortical thinning in Alzheimer’s disease and frontotemporal dementia. Brain : a journal of neurology. 2007 Apr;130(Pt 4):1159–1166. doi: 10.1093/brain/awm016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greve DN, Van der Haegen L, Cai Q, et al. A surface-based analysis of language lateralization and cortical asymmetry. Journal of Cognitive Neuroscience. 2013 Sep;25(9):1477–1492. doi: 10.1162/jocn_a_00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyoo IK, Sung YH, Dager SR, et al. Regional cerebral cortical thinning in bipolar disorder. Bipolar disorders. 2006 Feb;8(1):65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- 23.Salat DH, Buckner RL, Snyder AZ, et al. Thinning of the cerebral cortex in aging. Cerebral cortex. 2004 Jul;14(7):721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 24.Chong CD, Dodick DW, Schlaggar BL, Schwedt TJ. Atypical age-related cortical thinning in episodic migraine. Cephalalgia : an international journal of headache. 2014 Dec;34(14):1115–1124. doi: 10.1177/0333102414531157. [DOI] [PubMed] [Google Scholar]
- 25.Whittle S, Dennison M, Vijayakumar N, et al. Childhood maltreatment and psychopathology affect brain development during adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2013 Sep;52(9):940–952 e941. doi: 10.1016/j.jaac.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Almeida Montes LG, Prado Alcantara H, Martinez Garcia RB, De La Torre LB, Avila Acosta D, Duarte MG. Brain cortical thickness in ADHD: age, sex, and clinical correlations. Journal of Attention Disorders. 2013 Nov;17(8):641–654. doi: 10.1177/1087054711434351. [DOI] [PubMed] [Google Scholar]
- 27.Marques-Iturria I, Garolera M, Pueyo R, et al. The interaction effect between BDNF val66met polymorphism and obesity on executive functions and frontal structure. American Journal of Medical Genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2014 Apr;165B(3):245–253. doi: 10.1002/ajmg.b.32229. [DOI] [PubMed] [Google Scholar]
- 28.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000 Sep 26;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999 Feb;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 30.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006 Jul 1;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Mugler JP, 3rd, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE) Magnetic Resonance in Medicine. 1990 Jul;15(1):152–157. doi: 10.1002/mrm.1910150117. [DOI] [PubMed] [Google Scholar]
- 32.Hagler DJ, Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. NeuroImage. 2006 Dec;33(4):1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in the management of patients with Graves’ ophthalmopathy. Clinical Endocrinology. 1997 Jul;47(1):9–14. doi: 10.1046/j.1365-2265.1997.2331047.x. [DOI] [PubMed] [Google Scholar]
- 34.Kathol RG, Delahunt JW. The relationship of anxiety and depression to symptoms of hyperthyroidism using operational criteria. General Hospital Psychiatry. 1986 Jan;8(1):23–28. doi: 10.1016/0163-8343(86)90060-5. [DOI] [PubMed] [Google Scholar]
- 35.Lazarus JH. Epidemiology of Graves’ orbitopathy (GO) and relationship with thyroid disease. Best practice & research. Clinical endocrinology & metabolism. 2012 Jun;26(3):273–279. doi: 10.1016/j.beem.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Talairach J. Co-Planar Stereotaxic Atlas of the Human Brain: 3-D Proportional System: An Approach to Cerebral Imaging. Thieme; 1988. [Google Scholar]
- 37.Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Research. 2001;41(10–11):1409–1422. doi: 10.1016/s0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- 38.Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. The Journal of Neuroscience : the official journal of the Society for Neuroscience. 1997 Jun 1;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silson EH, McKeefry DJ, Rodgers J, Gouws AD, Hymers M, Morland AB. Specialized and independent processing of orientation and shape in visual field maps LO1 and LO2. Nature Neuroscience. 2013 Mar;16(3):267–269. doi: 10.1038/nn.3327. [DOI] [PubMed] [Google Scholar]
- 40.Trobe JD, Glaser JS, Laflamme P. Dysthyroid optic neuropathy. Clinical profile and rationale for management. Archives of Ophthalmology. 1978 Jul;96(7):1199–1209. doi: 10.1001/archopht.1978.03910060033007. [DOI] [PubMed] [Google Scholar]
- 41.Wright IC, McGuire PK, Poline JB, et al. A voxel-based method for the statistical analysis of gray and white matter density applied to schizophrenia. NeuroImage. 1995 Dec;2(4):244–252. doi: 10.1006/nimg.1995.1032. [DOI] [PubMed] [Google Scholar]
- 42.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain : a journal of neurology. 2006 Mar;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 43.Lou HC, Luber B, Crupain M, et al. Parietal cortex and representation of the mental Self. Proceedings of the National Academy of Sciences of the United States of America. 2004 Apr 27;101(17):6827–6832. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radosavljevic VR, Jankovic SM, Marinkovic JM. Stressful life events in the pathogenesis of Graves’ disease. European journal of endocrinology/European Federation of Endocrine Societies. 1996 Jun;134(6):699–701. doi: 10.1530/eje.0.1340699. [DOI] [PubMed] [Google Scholar]
- 45.Mizokami T, Wu Li A, El-Kaissi S, Wall JR. Stress and thyroid autoimmunity. Thyroid : official journal of the American Thyroid Association. 2004 Dec;14(12):1047–1055. doi: 10.1089/thy.2004.14.1047. [DOI] [PubMed] [Google Scholar]
- 46.Estcourt S, Vaidya B, Quinn A, Shepherd M. The impact of thyroid eye disease upon patients’ wellbeing: a qualitative analysis. Clinical endocrinology. 2008 Apr;68(4):635–639. doi: 10.1111/j.1365-2265.2007.03087.x. [DOI] [PubMed] [Google Scholar]
- 47.Fahrenfort JJ, Wilterdink AM, van der Veen EA. Long-term residual complaints and psychosocial sequelae after remission of hyperthyroidism. Psychoneuroendocrinology. 2000 Feb;25(2):201–211. doi: 10.1016/s0306-4530(99)00050-5. [DOI] [PubMed] [Google Scholar]
- 48.Whybrow PC, Prange AJ, Jr, Treadway CR. Mental changes accompanying thyroid gland dysfunction. A reappraisal using objective psychological measurement. Archives of General Psychiatry. 1969 Jan;20(1):48–63. doi: 10.1001/archpsyc.1969.01740130050004. [DOI] [PubMed] [Google Scholar]
- 49.Pihlajamaki M, DePeau KM, Blacker D, Sperling RA. Impaired medial temporal repetition suppression is related to failure of parietal deactivation in Alzheimer disease. The American Journal of Geriatric Psychiatry : official journal of the American Association for Geriatric Psychiatry. 2008 Apr;16(4):283–292. doi: 10.1097/JGP.0b013e318162a0a9. [DOI] [PMC free article] [PubMed] [Google Scholar]


