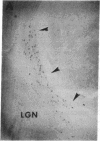
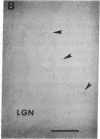
Abstract
Mammalian circadian rhythmicity is endogenously generated by a pacemaker in the suprachiasmatic nuclei and precisely entrained to the 24-hr day/night cycle by periodic environmental light cues. We show that light alters the immunoreactive levels of a transcriptional regulatory protein, Fos, in the suprachiasmatic nuclei of albino rats. Photic regulation of Fos immunoreactivity does not occur in other retino-recipient brain areas except for the intergeniculate leaflet, which appears to be involved in mediating some of the complex effects of light on expressed circadian rhythms. Our results point to a promising new functional marker for the cellular effects of light and suggest that the expression of Fos or a related nuclear protein may be part of the mechanism for photic entrainment of the circadian clock to environmental light/dark cycles.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers H. E., Minamitani N., Stopa E., Ferris C. F. Light selectively alters vasoactive intestinal peptide and peptide histidine isoleucine immunoreactivity within the rat suprachiasmatic nucleus. Brain Res. 1987 Dec 22;437(1):189–192. doi: 10.1016/0006-8993(87)91544-7. [DOI] [PubMed] [Google Scholar]
- Albers H. E., Stopa E. G., Zoeller R. T., Kauer J. S., King J. C., Fink J. S., Mobtaker H., Wolfe H. Day-night variation in prepro vasoactive intestinal peptide/peptide histidine isoleucine mRNA within the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 1990 Jan;7(1):85–89. doi: 10.1016/0169-328x(90)90077-q. [DOI] [PubMed] [Google Scholar]
- Amsterdam E. A., Banas J., Criley J. M., Loeb H. S., Mueller H., Willerson J. T., Mason D. T. Clinical assessment of external pressure circulatory assistance in acute myocardial infarction. Report of a cooperative clinical trial. Am J Cardiol. 1980 Feb;45(2):349–356. doi: 10.1016/0002-9149(80)90658-x. [DOI] [PubMed] [Google Scholar]
- Bravo R., Neuberg M., Burckhardt J., Almendral J., Wallich R., Müller R. Involvement of common and cell type-specific pathways in c-fos gene control: stable induction of cAMP in macrophages. Cell. 1987 Jan 30;48(2):251–260. doi: 10.1016/0092-8674(87)90428-4. [DOI] [PubMed] [Google Scholar]
- Cahill G. M., Menaker M. Effects of excitatory amino acid receptor antagonists and agonists on suprachiasmatic nucleus responses to retinohypothalamic tract volleys. Brain Res. 1989 Feb 6;479(1):76–82. doi: 10.1016/0006-8993(89)91337-1. [DOI] [PubMed] [Google Scholar]
- Card J. P., Brecha N., Karten H. J., Moore R. Y. Immunocytochemical localization of vasoactive intestinal polypeptide-containing cells and processes in the suprachiasmatic nucleus of the rat: light and electron microscopic analysis. J Neurosci. 1981 Nov;1(11):1289–1303. doi: 10.1523/JNEUROSCI.01-11-01289.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card J. P., Fitzpatrick-McElligott S., Gozes I., Baldino F., Jr Localization of vasopressin-, vasoactive intestinal polypeptide-, peptide histidine isoleucine- and somatostatin-mRNA in rat suprachiasmatic nucleus. Cell Tissue Res. 1988 May;252(2):307–315. doi: 10.1007/BF00214373. [DOI] [PubMed] [Google Scholar]
- Chipkin S. R., Stoff J. S., Aronin N. Immunohistochemical evidence for neural mediation of VIP activity in the dogfish rectal gland. Peptides. 1988 Jan-Feb;9(1):119–124. doi: 10.1016/0196-9781(88)90017-4. [DOI] [PubMed] [Google Scholar]
- Cohen D. R., Curran T. fra-1: a serum-inducible, cellular immediate-early gene that encodes a fos-related antigen. Mol Cell Biol. 1988 May;8(5):2063–2069. doi: 10.1128/mcb.8.5.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Curran T., Miller A. D., Zokas L., Verma I. M. Viral and cellular fos proteins: a comparative analysis. Cell. 1984 Feb;36(2):259–268. doi: 10.1016/0092-8674(84)90219-8. [DOI] [PubMed] [Google Scholar]
- Curran T., Morgan J. I. Memories of fos. Bioessays. 1987 Dec;7(6):255–258. doi: 10.1002/bies.950070606. [DOI] [PubMed] [Google Scholar]
- Curran T., Morgan J. I. Superinduction of c-fos by nerve growth factor in the presence of peripherally active benzodiazepines. Science. 1985 Sep 20;229(4719):1265–1268. doi: 10.1126/science.4035354. [DOI] [PubMed] [Google Scholar]
- Curran T., Van Beveren C., Verma I. M. Viral and cellular fos proteins are complexed with a 39,000-dalton cellular protein. Mol Cell Biol. 1985 Jan;5(1):167–172. doi: 10.1128/mcb.5.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M., Robertson H. A. Kindling stimulation induces c-fos protein(s) in granule cells of the rat dentate gyrus. Nature. 1987 Oct 1;329(6138):441–442. doi: 10.1038/329441a0. [DOI] [PubMed] [Google Scholar]
- Dragunow M., Robertson H. A. Localization and induction of c-fos protein-like immunoreactive material in the nuclei of adult mammalian neurons. Brain Res. 1988 Feb 9;440(2):252–260. doi: 10.1016/0006-8993(88)90993-6. [DOI] [PubMed] [Google Scholar]
- Franza B. R., Jr, Sambucetti L. C., Cohen D. R., Curran T. Analysis of Fos protein complexes and Fos-related antigens by high-resolution two-dimensional gel electrophoresis. Oncogene. 1987 May;1(2):213–221. [PubMed] [Google Scholar]
- Gentz R., Rauscher F. J., 3rd, Abate C., Curran T. Parallel association of Fos and Jun leucine zippers juxtaposes DNA binding domains. Science. 1989 Mar 31;243(4899):1695–1699. doi: 10.1126/science.2494702. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Greene L. A., Ziff E. B. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985 Nov 15;260(26):14101–14110. [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B., Greene L. A. Stimulation of neuronal acetylcholine receptors induces rapid gene transcription. Science. 1986 Oct 3;234(4772):80–83. doi: 10.1126/science.3749894. [DOI] [PubMed] [Google Scholar]
- Harrington M. E., Nance D. M., Rusak B. Double-labeling of neuropeptide Y-immunoreactive neurons which project from the geniculate to the suprachiasmatic nuclei. Brain Res. 1987 May 5;410(2):275–282. doi: 10.1016/0006-8993(87)90325-8. [DOI] [PubMed] [Google Scholar]
- Harrington M. E., Rusak B. Lesions of the thalamic intergeniculate leaflet alter hamster circadian rhythms. J Biol Rhythms. 1986 Winter;1(4):309–325. doi: 10.1177/074873048600100405. [DOI] [PubMed] [Google Scholar]
- Harrington M. E., Rusak B. Photic responses of geniculo-hypothalamic tract neurons in the Syrian hamster. Vis Neurosci. 1989;2(4):367–375. doi: 10.1017/s0952523800002170. [DOI] [PubMed] [Google Scholar]
- He X., Treacy M. N., Simmons D. M., Ingraham H. A., Swanson L. W., Rosenfeld M. G. Expression of a large family of POU-domain regulatory genes in mammalian brain development. Nature. 1989 Jul 6;340(6228):35–41. doi: 10.1038/340035a0. [DOI] [PubMed] [Google Scholar]
- Heintz N., Roeder R. G. Transcription of human histone genes in extracts from synchronized HeLa cells. Proc Natl Acad Sci U S A. 1984 May;81(9):2713–2717. doi: 10.1073/pnas.81.9.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S. P., Pini A., Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987 Aug 13;328(6131):632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Johnson R. F., Moore R. Y., Morin L. P. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res. 1988 Sep 20;460(2):297–313. doi: 10.1016/0006-8993(88)90374-5. [DOI] [PubMed] [Google Scholar]
- Johnson R. F., Morin L. P., Moore R. Y. Retinohypothalamic projections in the hamster and rat demonstrated using cholera toxin. Brain Res. 1988 Oct 18;462(2):301–312. doi: 10.1016/0006-8993(88)90558-6. [DOI] [PubMed] [Google Scholar]
- Keefe D. L., Earnest D. J., Nelson D., Takahashi J. S., Turek F. W. A cholinergic antagonist, mecamylamine, blocks the phase-shifting effects of light on the circadian rhythm of locomotor activity in the golden hamster. Brain Res. 1987 Feb 17;403(2):308–312. doi: 10.1016/0006-8993(87)90068-0. [DOI] [PubMed] [Google Scholar]
- Le Gal La Salle G. Long-lasting and sequential increase of c-fos oncoprotein expression in kainic acid-induced status epilepticus. Neurosci Lett. 1988 May 26;88(2):127–130. doi: 10.1016/0304-3940(88)90112-7. [DOI] [PubMed] [Google Scholar]
- Meijer J. H., Groos G. A., Rusak B. Luminance coding in a circadian pacemaker: the suprachiasmatic nucleus of the rat and the hamster. Brain Res. 1986 Sep 10;382(1):109–118. doi: 10.1016/0006-8993(86)90117-4. [DOI] [PubMed] [Google Scholar]
- Meijer J. H., Rietveld W. J. Neurophysiology of the suprachiasmatic circadian pacemaker in rodents. Physiol Rev. 1989 Jul;69(3):671–707. doi: 10.1152/physrev.1989.69.3.671. [DOI] [PubMed] [Google Scholar]
- Meijer J. H., van der Zee E. A., Dietz M. Glutamate phase shifts circadian activity rhythms in hamsters. Neurosci Lett. 1988 Mar 31;86(2):177–183. doi: 10.1016/0304-3940(88)90567-8. [DOI] [PubMed] [Google Scholar]
- Menétrey D., Gannon A., Levine J. D., Basbaum A. I. Expression of c-fos protein in interneurons and projection neurons of the rat spinal cord in response to noxious somatic, articular, and visceral stimulation. J Comp Neurol. 1989 Jul 8;285(2):177–195. doi: 10.1002/cne.902850203. [DOI] [PubMed] [Google Scholar]
- Moore R. Y., Gustafson E. L., Card J. P. Identical immunoreactivity of afferents to the rat suprachiasmatic nucleus with antisera against avian pancreatic polypeptide, molluscan cardioexcitatory peptide and neuropeptide Y. Cell Tissue Res. 1984;236(1):41–46. doi: 10.1007/BF00216511. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Cohen D. R., Hempstead J. L., Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987 Jul 10;237(4811):192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 1989 Nov;12(11):459–462. doi: 10.1016/0166-2236(89)90096-9. [DOI] [PubMed] [Google Scholar]
- O'Shea E. K., Rutkowski R., Stafford W. F., 3rd, Kim P. S. Preferential heterodimer formation by isolated leucine zippers from fos and jun. Science. 1989 Aug 11;245(4918):646–648. doi: 10.1126/science.2503872. [DOI] [PubMed] [Google Scholar]
- Pickard G. E. Bifurcating axons of retinal ganglion cells terminate in the hypothalamic suprachiasmatic nucleus and the intergeniculate leaflet of the thalamus. Neurosci Lett. 1985 Apr 9;55(2):211–217. doi: 10.1016/0304-3940(85)90022-9. [DOI] [PubMed] [Google Scholar]
- Pickard G. E. Morphological characteristics of retinal ganglion cells projecting to the suprachiasmatic nucleus: a horseradish peroxidase study. Brain Res. 1980 Feb 10;183(2):458–465. doi: 10.1016/0006-8993(80)90481-3. [DOI] [PubMed] [Google Scholar]
- Pickard G. E., Ralph M. R., Menaker M. The intergeniculate leaflet partially mediates effects of light on circadian rhythms. J Biol Rhythms. 1987 Spring;2(1):35–56. doi: 10.1177/074873048700200104. [DOI] [PubMed] [Google Scholar]
- Prosser R. A., Gillette M. U. The mammalian circadian clock in the suprachiasmatic nuclei is reset in vitro by cAMP. J Neurosci. 1989 Mar;9(3):1073–1081. doi: 10.1523/JNEUROSCI.09-03-01073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea M. A. Light increases Fos-related protein immunoreactivity in the rat suprachiasmatic nuclei. Brain Res Bull. 1989 Dec;23(6):577–581. doi: 10.1016/0361-9230(89)90204-9. [DOI] [PubMed] [Google Scholar]
- Sagar S. M., Sharp F. R., Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988 Jun 3;240(4857):1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Sagar S. M., Sharp F. R. Light induces a Fos-like nuclear antigen in retinal neurons. Brain Res Mol Brain Res. 1990 Jan;7(1):17–21. doi: 10.1016/0169-328x(90)90068-o. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Ransone L. J., Lamph W. W., Verma I. M. Direct interaction between fos and jun nuclear oncoproteins: role of the 'leucine zipper' domain. Nature. 1988 Dec 15;336(6200):692–695. doi: 10.1038/336692a0. [DOI] [PubMed] [Google Scholar]
- Sharp F. R., Gonzalez M. F., Sharp J. W., Sagar S. M. c-fos expression and (14C) 2-deoxyglucose uptake in the caudal cerebellum of the rat during motor/sensory cortex stimulation. J Comp Neurol. 1989 Jun 22;284(4):621–636. doi: 10.1002/cne.902840409. [DOI] [PubMed] [Google Scholar]
- Sofroniew M. V., Isacson O., O'Brien T. S. Nerve growth factor receptor immunoreactivity in the rat suprachiasmatic nucleus. Brain Res. 1989 Jan 9;476(2):358–362. doi: 10.1016/0006-8993(89)91259-6. [DOI] [PubMed] [Google Scholar]
- Sonnenberg J. L., Macgregor-Leon P. F., Curran T., Morgan J. I. Dynamic alterations occur in the levels and composition of transcription factor AP-1 complexes after seizure. Neuron. 1989 Sep;3(3):359–365. doi: 10.1016/0896-6273(89)90260-2. [DOI] [PubMed] [Google Scholar]
- Sonnenberg J. L., Mitchelmore C., Macgregor-Leon P. F., Hempstead J., Morgan J. I., Curran T. Glutamate receptor agonists increase the expression of Fos, Fra, and AP-1 DNA binding activity in the mammalian brain. J Neurosci Res. 1989 Sep;24(1):72–80. doi: 10.1002/jnr.490240111. [DOI] [PubMed] [Google Scholar]
- Stopa E. G., Minamitani N., Jonassen J. A., King J. C., Wolfe H., Mobtaker H., Albers H. E. Localization of vasoactive intestinal peptide and peptide histidine isoleucine immunoreactivity and mRNA within the rat suprachiasmatic nucleus. Brain Res. 1988 Dec;464(4):319–325. doi: 10.1016/0169-328x(88)90041-1. [DOI] [PubMed] [Google Scholar]
- Szekely A. M., Barbaccia M. L., Costa E. Activation of specific glutamate receptor subtypes increases C-fos proto-oncogene expression in primary cultures of neonatal rat cerebellar granule cells. Neuropharmacology. 1987 Dec;26(12):1779–1782. doi: 10.1016/0028-3908(87)90132-8. [DOI] [PubMed] [Google Scholar]
- Takahashi J. S., DeCoursey P. J., Bauman L., Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984 Mar 8;308(5955):186–188. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- Turner R., Tjian R. Leucine repeats and an adjacent DNA binding domain mediate the formation of functional cFos-cJun heterodimers. Science. 1989 Mar 31;243(4899):1689–1694. doi: 10.1126/science.2494701. [DOI] [PubMed] [Google Scholar]
- Zatz M., Herkenham M. A. Intraventricular carbachol mimics the phase-shifting effect of light on the circadian rhythm of wheel-running activity. Brain Res. 1981 May 11;212(1):234–238. doi: 10.1016/0006-8993(81)90059-7. [DOI] [PubMed] [Google Scholar]