Abstract
Background
Human IgG exists in 4 different subtypes (IgG1, IgG2, IgG3, and IgG4), but it is also now appreciated that there is genetic variation within IgG subtypes (called isoallotypes). 29 different isoallotypes have been described, with 7, 4, 15, and 3 isoallotypes described for IgG1-IgG4, respectively. The reactivity of anti-IgG with different isoallotypes has not been characterized.
Study Design and Methods
A novel monoclonal anti-K antibody (PUMA1) was isolated, sequenced, and a panel of PUMA1 variants was expressed consisting of the 29 known IgG isoallotypes. The resulting panel of antibodies was pre-incubated with K+ RBCs and was then subjected to testing with currently approved anti-IgG, by flow cytometry, solid phase systems, gel card, and tube testing.
Results/Findings
An FDA approved monoclonal anti-IgG (Gamma-clone) failed to recognize 2 out of 15 IgG3 isoallotypes (IgG3-03 and IgG3-13) and 3 out of 3 IgG4 isoallotypes (IgG4-01, 02, 03). In contrast, an FDA approved rabbit polyclonal anti-IgG recognized each of the known human IgG isoallotypes.
Conclusion
These findings demonstrate “blind spots” in isoalloantibody detection by a monoclonal anti-IgG. Should a patient have anti-RBC antibodies predominantly of an IgG3 subtype of the IgG3-03 and/or IgG3-13 variety, it is possible that a clinically significant alloantibody would be missed. IgG-03 and IgG-13 are estimated at a frequency of 1–3% of Caucasian and 20–30% of certain African populations. The non-reactivity with IgG4 is a known characteristic of this monoclonal anti-IgG, but IgG4 isoallotypes have not been previously reported.
Introduction
Alloantibodies to non-ABO red blood cell (RBC) antigens are usually not direct agglutinins, and typically require the addition of anti-IgG to facilitate their detection. The two current methods of manufacturing reagent grade anti-IgG consist of either generating polyclonal anti-IgG from serum of animals (typically rabbits) immunized with polyclonal human IgG or the use of monoclonal antibody based reagents, typically derived from murine sources. While polyclonal anti-IgG contains multiple specificities, and can be a very sensitive reagent, it suffers the potential for variation from animal to animal and from batch to batch. In contrast, monoclonal antibodies are a stable and consistent reagent; however, they may display a more narrow range of reactivity, as typically a single epitope is recognized on the target immunoglobulin.
It has been long appreciated that there are four subclasses of IgG (IgG1, IgG2, IgG3, and IgG4). More recently, genetic variation within IgG subtypes has been discovered (called isoallotypes). 29 different isoallotypes have been described, with 7, 4, 15, and 3 isoallotypes described for IgG1-4, respectively1. While anti-IgG reagents are required to meet specifications and standards for licensure, the reactivity of anti-IgG with different isoallotypes has not been characterized.
The generation of test reagents to assess specificity of anti-IgG has historically been challenging and difficult to standardize2,3. Typically, solid media is coated with purified IgG of different types to determine anti-IgG reactivity (e.g. ELISAs). While meaningful, such approaches are outside of the context in which IAT and DAT tests are run (e.g. testing IgG bound to the surface of RBCs), and thus may not reflect anti-IgG performance for clinical testing. To create the ability to characterize anti-IgG sensitivities and specificities in the context in which anti-IgG is used in immunohematology, we generated a new monoclonal antibody against the K antigen and isolated the cDNA sequence for the heavy and light chain variable regions. The heavy chain variable region was then ligated into expression vectors using a strategy that fused it in frame with the constant region of human IgG. Separate expression vectors for each of the 29 known IgG isoallotypes were created, allowing expression and purification of isoallotypic variants. This approach isolates isoallotypic variation as an independent variable, as the antigen binding domain is the same for each anti-K variant.
The panel of 29 isoallotypes was utilized to characterize the performance of different, commercially available anti-IgG reagents. Herein we report that an FDA approved monoclonal anti-IgG fails to recognize two particular isoallotypes of IgG3 (IgG3-03 and IgG3-13). In addition, we confirmed the known property that this monoclonal anti-IgG does not react with canonical IgG4, and also observed that this non-reactivity extends to the 3 known isoallotypes of IgG4. IgG4 is not known to typically result in acute hemolytic transfusion reactions, and thus non-reactivity with IgG4 is not typically considered as a weakness of monoclonal anti-IgG. However, IgG3 is often considered the most hemolytic IgG subclass. The identified IgG3 isoallotypes have a significant frequency in certain populations, including Africans.
Materials and Methods
Mice
K transgenic mice (published as KEL1 mice) were generated and characterized as previously described and were bred in the BloodworksNW vivarium4. CBy.RBF-Rb(8.12)5Bnr/J mice were purchased from Jackson Labs, Bar Harbor ME (Cat # 001802). All mice were maintained on standard rodent chow and water in a temperature- and light-controlled environment. All experiments were performed according to approved Institutional Animal Care and Use Committee (IACUC) procedures.
Immunizations and Isolation of Monoclonal Antibody
A new anti-K monoclonal antibody was isolated and sequenced by routine methods; details provided in supplemental section.
Recombinant Antibody Production
Recombinant antibodies were produced via transient co-transfection of the plasmids encoding the PUMA1 light chain and appropriate PUMA1 heavy chain into suspension CHO cells as part of the FreeStyle MAX CHO Expression System (ThermoFisher). Briefly, 24hr prior to transfection, suspension CHO cells were seeded at a density of 0.5×106 cells/ml. Transfections were performed using a heavy chain to light chain plasmid ratio of 2:3, and cultures were grown at 37°C for 7 days. To harvest, cultures were centrifuged at 4000 RPM for 30min at 4°C, followed by filtration of the supernatant through a .45um filter apparatus to remove any remaining cell debris. In some cases, antibodies were purified from supernatants using rProteinA/G columns (GE Healthcare, Pittsburgh, PA), dialyzed, aliquoted and stored at −20°C. Samples from each purification were assessed by SDS-PAGE for both purity and concentration by comparison to a standard curve of purified PUMA1 of known concentration.
Flow Cytometry
Flow cytometry consisted of incubating test RBCs with PUMA1 variants, followed by the anti-IgG being evaluated, followed by a detection reagent. Test RBCs included RBCs from K mice, wild-type mice, and reagent RBCs from humans with the phenotype of (K+k+) or (K-k+). Test RBCs were resuspended in 50 microliters of supernatants of PUMA1 isoallotypes; in some cases a 1/10 dilution in phosphate buffered saline (PBS) was used. For purified IgG1-IgG4 PUMA1, the antibodies were diluted in PBS at the indicated concentrations. The tested anti-IgG reagents were used undiluted. Detection reagents consisted of donkey anti-rabbit conjugated to phycoerythrin at a 1/200 dilution (Affymetrix cat# 12-4739-81, Santa Clara, CA), goat anti-mouse IgM conjugated to allophycocyanin at a 1/100 dilution (BD Biosciences, cat# 550826, San Jose, CA), and goat anti-mouse Igs conjugated to allophycocyanin at a 1/100 dilution (SouthernBiotech cat# 1020-11s,Birmingham, AL). All anti-IgG subclass antibodies were conjugated to phycoerythrin, were purchased from SouthernBiotech (catalog numbers 9056-09, 9070-09, 9210-09, 9200-09), and were used at a dilution of 1/200. All incubations were performed for 30 min at room temperature, followed by three washes with PBS. Antibodies were titrated to define working concentrations. All flow cytometry was performed on an Accuri 4 color cytometer (BD biosciences, San Jose CA) and all data was analyzed with Flo-Jo version 10.
Solid Phase Testing
Daily instrument maintenance and quality control were performed as described in the Galileo Echo (Immucor, Norcross, GA) Operator Manual prior to testing. The PUMA1 subtypes were tested using combinations of in-date lots of Capture-R Ready Screen 3 strips (CRRS3), Capture LISS and Capture-R Indicator Cells (CRIND) on 2 Galileo Echo instruments (Immucor). The instrument reads and interprets the test results of the individual test wells, which in the case of CRRS3 meant that one of the three test wells contained a K+k+ red cell monolayer and 2 K-k+ red cell monolayers. The antibody samples were evaluated on 2 instruments using 2 lots of CRRS3 strips and 2 lots of CR-IND. Two lots of Capture-P indicator cells were also used.
Tube Antiglobulin Test (AGT)
PUMA1 was tested with Panoscreen I, II & III reagent RBCs (Immucor) by a standard saline tube AGT as described in the direction insert. Briefly, equal volumes of antibody and 2–4% reagent RBCs were incubated for 45 minutes at 37°C. After incubation the tubes were washed 3 times with an excess of PBS. Anti-IgG reagent was added and the tubes centrifuged and then examined for the presence of agglutination. Gammaclone Murine Monoclonal Anti-IgG (Immucor) was used as Anti-IgG reagents.
Gel Testing
Gel testing was carried out using antibody-screening reagent RBCs (0.8% Surgiscreen RBCs I, II, and III) MTS Anti-IgG Cards, as per manufacturer’s instructions (ID-Micro Typing System Gel Test; Ortho Clinical Diagnostics, Inc., Raritan, NJ).
Results
Generation of a novel monoclonal anti-K antibody
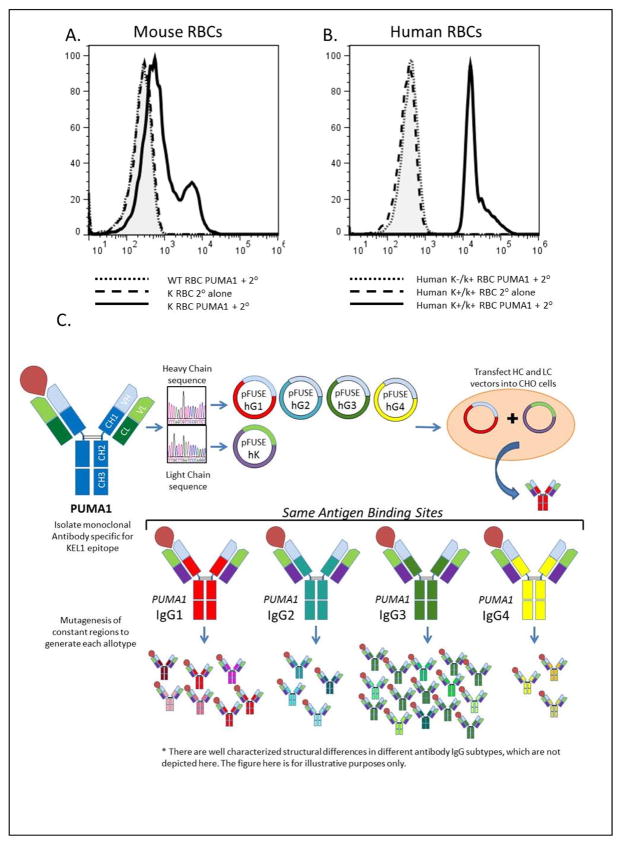
We utilized RBCs from previously reported K transgenic mice as an immunogen4. Mice with high-titer antisera specific for K transgenic RBCs were identified, spleens were harvested, and fusions were performed with an immortal myeloma line. Through traditional cell cloning methods, a new monoclonal line (PUMA1) was isolated, that secreted an antibody with specificity for the K antigen. PUMA1 was reactive with RBCs from the K transgenic donor mouse used for immunization; no signal was observed on wild-type RBCs compared to secondary antibody alone (Figure 1A). Similarly, PUMA1 was reactive with human RBCs of the K+k+ phenotype but not with K-k+ RBCs by flow cytometry (Figure 1B) and as confirmed by other methods (see below). Characterization of PUMA1 indicates that it was of the murine IgG2a subclass and expressed a kappa light chain (data not shown).
Figure 1.
Specificity of PUMA1 for the K antigen and strategy to generate PUMA1 variants. (A) Mouse RBCs were stained with PUMA1 antibody followed by secondary antibody (wild-type RBCs dotted line, K transgenic RBCs solid line). K transgenic RBCs were also stained with secondary antibody alone (dashed line). (B) Human RBCs with a K+k+ phenotype were stained with PUMA 1 followed by secondary antibody (solid line) or with secondary antibody alone (dashed line). RBCs with a K-k+ phenotype were stained with PUMA1 followed by secondary antibody (dotted line). (C) Diagram showing the general cloning and expression strategy.
Detection of IgG subtypes and isoallotypes by different anti-IgG reagents
In order to allow engineering of the PUMA1 antibody, the cDNA for the heavy and light chains were isolated from the PUMA1 hybridoma, and the coding region for the antibody complementary determining region (CDR) was determined. The heavy chain CDR was cloned, including a Kozak sequence, ATG, and leader sequence, in frame, into expression vectors for human IgG1, IgG2, IgG3, and IgG4 (Figure 1C). Each expression vector was used as a starting material to generate additional expression vectors for the known isoallotypic variants of IgG1-4 1. Likewise, the light chain CDR was cloned in frame, into an expression vector for human kappa light chain. The plasmid encoding the light chain was then co-transfected with expression vectors for each of the canonical IgG subclasses and isoallotypes, and cell culture supernatants which expressed PUMA1 IgG variants were collected. Each PUMA1 variant was incubated with K+k+ RBCs, followed by incubation with either monoclonal or polyclonal anti-IgG, followed by the appropriate detection reagents (see methods). Binding of PUMA1 variants was assessed by flow cytometry. Background staining was determined using K-k+ RBCs that don’t express the K antigen.
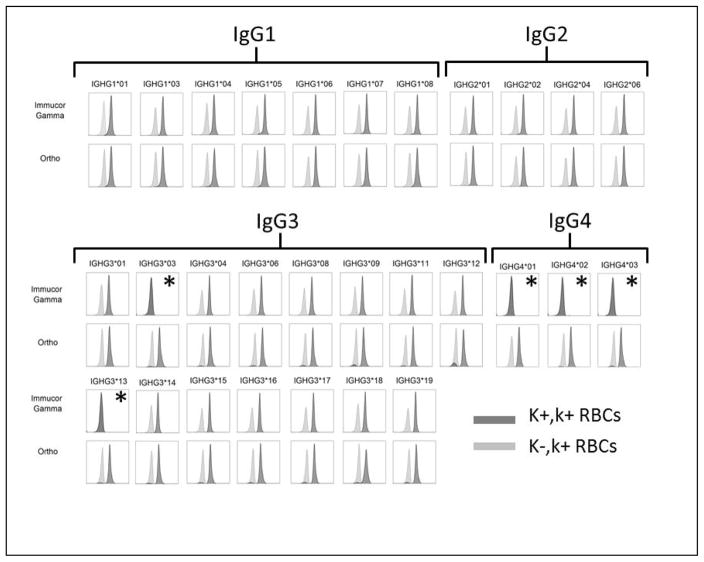
All 29 isoallotypes of human IgG were detected by polyclonal anti-IgG. In contrast, the tested monoclonal anti-IgG bound to 24 out of 29 isoallotypes, failing to recognize IgG3-03, IgG3-13, IgG4-01, IgG4-02, and IgG4-03 (see figure 2). The lack of detection of these isoallotypic variants was not due to their lack of expression or inability to recognize the K antigen, as equivalent signal was detected on K+k+ but not K-k+ RBCs for all 29 isoallotypes by polyclonal anti-IgG. These studies were carried out with saturating quantities of anti-K (PUMA1), and resulted in no detectable signal for the isoallotypes that are not detected by the tested monoclonal anti-IgG. Detailed titrations were not carried out to determine limits of detection of fine differences in sensitivity at low concentrations.
Figure 2.
K+k+ RBCs were incubated with each of the indicated PUMA1 IgG isoallotype, followed by the test anti-IgG and relevant detection reagent (see methods) [dark gray histograms]. K-k+ RBCs were also incubated with each IgG isoallotype as a background control [light gray histograms]. Isoallotypes that were not recognized are indicated by (*). A separate stain was performed with each IgG isoallotype against K+,k+ and K-,k+ RBCs; the corresponding histograms for each isoallotype are overlaid in each panel.
Characterization of anti-IgG regents for sensitivity to human IgG subclasses
To allow standards of known quantity, so as to allow precise determination of the sensitivity of anti-IgG, the canonical forms of IgG1-IgG4 were expressed and purified by affinity chromatography (All canonical forms have an isoallotype designation of *01). Protein electrophoresis was performed on each purified IgG subclass of PUMA1, both to assess expression and purity (Supplemental Figure 1A). The same single band was observed at 25 kD for each preparation, consistent with the same kappa light chain of PUMA1. In addition, bands corresponding to each of the heavy chains were also observed, at the predicted molecular weights consistent with the known size of each of the IgG subclasses. As predicted, only the IgG3 heavy chain displayed a higher molecular weight in accordance with its longer hinge region. To test if the purified PUMA1 IgG1-IgG4 maintained antigen binding properties and as a further confirmation as to the correct expression, of IgG subtypes, K+k+ human RBCs were stained with each of the PUMA1 preparations, followed by secondary antibodies specific for the human IgG subclasses. Each of the PUMA1 IgG subclasses bound to (K+k+) but not (K-k+) RBCs, was reactive with the secondary antibody specific for the appropriate IgG subclass, and was nonreactive with secondary antibodies of other specificities by flow cytometry (Supplemental Figure 1B). Together, these findings demonstrate the successful purification to homogeneity of the expressed panel of antibodies. Accordingly, determination of protein concentration in these preparations reflects the quantity of anti-K IgG, allowing quantitative standard reagents.
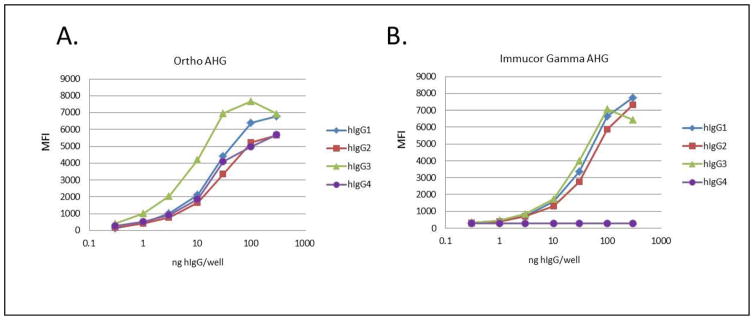
To assess the sensitivity of commercially available anti-IgG preparations for different human IgG subclasses bound to RBCs, K+k+ RBCs were first incubated with PUMA1 of the different human IgG subclasses, followed by incubation with anti-IgG. Treated RBCs were then stained with a fluorescently labeled antibody specific for the species in which the anti-IgG was generated (and non-reactive with human IgG). For each anti-IgG, the same secondary antibody was used to detect binding of the anti-IgG to each human PUMA1 IgG subclass. To assess relative sensitivity, titrations of each PUMA1 IgG subclass were carried out and samples were analyzed by flow cytometry using mean fluorescent intensity (MFI) to quantify staining (Figure 3). The polyclonal rabbit anti-IgG reagent produced by Ortho Diagnostics (Ortho anti-IgG) had an increased sensitivity for IgG3 compared to other IgG subtypes (Figure 3A). In contrast, the mouse monoclonal anti-IgG reagent produced by Immucor-Gamma (Immucor Gamma anti-IgG) had equivalent sensitivity for IgG1, IgG2, and IgG3; however, as observed in the qualitative screening above (see figure 2), the Immucor Gamma anti-IgG had no detectable reactivity with IgG4 (Figure 3B).
Figure 3.
Human K+k+ RBCs sere incubated with a titration of each human IgG subclass of PUMA1 in the indicated amounts, followed by staining with the appropriate secondary antibodies (see methods). This was carried out for both of the indicated anti-IgG reagents. All data are derived from mean fluorescent intensity determined by flow cytometry.
Specificity of anti-IgG reagents in common platforms used in immunohematology labs
Flow cytometry remains a technique predominantly utilized for research purposes. Accordingly, the select forms of PUMA1 IgG1-IgG4 were evaluated in assay systems and on platforms currently in use in clinical immunohematology labs. The canonical PUMA1 IgG1-IgG4 subtypes were analyzed by the BloodworksNW Immunohematology Reference Laboratory and also by the research and development labs at Immucor Inc. PBS was used as a negative control. The five samples were evaluated using both solid phase assay systems (Galileo Echo platform, Immucor), by tube testing, or using gel – those performing the analysis were blinded as to the identity of the specimens tested. Overall results are shown, which were in agreement with flow cytometry results (Table 1). In all cases, systems that utilized the Immucor monoclonal 16H8 based reagent, either by solid phase (Capture-R indicator cells) or tube testing with Gammaclone anti-IgG reagent, detected. PUMA1 IgG1, IgG2, and IgG3 (but not IgG4) Capture-P indicator cells are prepared using a polyclonal rabbit anti-IgG, and detect all 4 PUMA1 IgG subclasses. In other systems using polyclonal rabbit anti-IgG from Ortho, all four IgG subclasses of PUMA1 were detected.
Table 1.
PUMA1 of the indicated human IgG subclasses was analyzed using test RBCs of a K+k+ phenotype and using the indicated platforms (see methods for details).
| Sample ID | Echo (M00211) Test Results | Tube AGT | Gel Testing | ||
|---|---|---|---|---|---|
| Capture-R Indicator Cells | Capture-P Indicator Cells | Gammaclone AHG Reagent | Ortho AHG | Ortho | |
| (PUMA1-IgG1) | Positive | Positive | 4+ | 4+ | 3+ |
| (PUMA1-IgG2) | Positive | Positive | 4+ | 3+ | 3+ |
| (PUMA1-IgG3) | Positive | Positive | 4+ | 4+ | 3+ |
| (PUMA1-IgG4) | Negative | Positive | 0 | 3+ | 3+ |
| (PUMA1-IgG3-03) | untested | untested | 0 | 3+ | 3+ |
| (PUMA1-IgG3-6) | untested | untested | 3+ | 3+ | 3+ |
| (PUMA1-IgG3-13) | untested | untested | 0 | 3+ | 3+ |
| (PBS) | 0 | 0 | 0 | 0 | 0 |
| Source of anti-IgG | Clone 16H8 | Polyclonal Rabbit | Clone 16H8 | Polyclonal Rabbit | Polyclonal Rabbit |
Additional gel testing and tube testing was carried out on the IgG3 isoallotypes that were not detected by the monoclonal anti-IgG using flow cytometry. Consistent with the flow cytometry results, the canonical IgG3-01 was detected by Gamma clone reagent in tube testing and in gel; however, neither IgG3-03 nor IgG3-13 was detected in either platform. Also consistent with flow cytometry, the polyclonal anti-IgG detected IgG3-03 and IgG3-13 both in tube testing and in gel. In no case was a positive signal observed in the PBS control sample. Positive signals were also not detected whenever K-k+ RBCs were used as screening RBCs (data not shown). Thus, in all cases, standardly utilized immunohematology methods generated data that was in agreement with flow cytometry. Sensitivity titrations were not carried out in each of the clinically used immunohematology platforms.
Discussion
The sensitivity and specificity of the anti-IgG component of AHG reagents are essential for immunohematology labs to detect RBC alloantibodies and autoantibodies, most of which are not direct agglutinins. However, human IgG is not a monomorphic entity; rather, it consists of 4 distinct IgG subclasses (IgG1-IgG4), each of which has natural genetic variation in their constant regions, giving rise to at least 29 isoallotypes1. To the best of our knowledge, this report is the first assessment of anti-IgG reactivity to the different human IgG isoallotypes. The difficulty in generating test systems of this type has been acknowledged in past efforts2,3; however, the current use of recombinant antibodies circumvents the previous barriers. By expressing a panel of antibodies with the same antigen-binding domain, but different IgG constant regions, isoallotype has been isolated as an independent variable.
Monoclonal anti-IgGs have a number of distinct advantages, including the relative ease of production and consistency of the reagent over time. Moreover, they do not require the ongoing immunization and housing of animals to maintain a polyclonal antisera, which can vary from batch-to-batch. However, the downside to monoclonal reagents can be a more myopic focus on a smaller number of epitopes (or a single epitope) on the target molecule, potentially decreasing the range of recognized entities. In the current manuscript, we report that a commonly used monoclonal anti-IgG does not recognize 5 of the 29 known isoallotypes of human IgG.
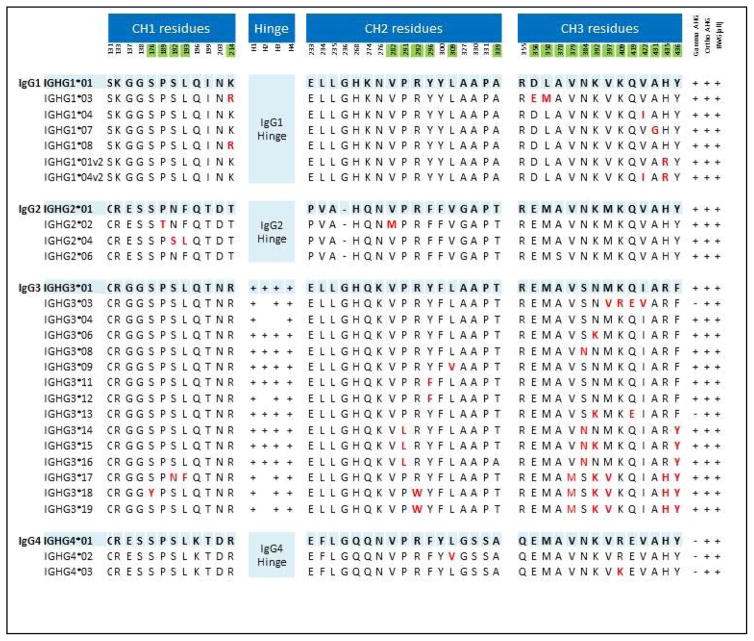
IgG3-03 and IgG3-13 are found at their highest frequencies in a number of ethnic groups of African origin or with origins in Yemen and parts of the Middle East5. IgG3 is typically considered a clinically significant IgG subtype, which is often associated with hemolytic pathology6; however, patients with IgG3 and no hemolytic anemia have also been described 7,8. To the best of our knowledge, the hemolytic potential of different IgG isoallotypes has not been assessed; thus, it is unclear where IgG3-03 and/or IgG3-13 fall on the hemolytic spectrum. Juxtaposition of the amino acid sequences indicates that the presence of a glutamic acid (instead of glutamine) at position 419 is a common characteristic of the IgG3-03, IgG3-13, and each of the IgG4 isoallotypes. Thus, the Q to E changes may be responsible for an alteration in epitope recognized by the characterized monoclonal anti-IgG. As IgG4 is generally considered to not cause acute hemolytic pathology, it is possible that Q to E change in IgG3-03 and IgG3-13 disrupts IgG3 effector function. However, given the potential for hemolysis by IgG3 in general, prudence would dictate an assumption of hemolytic potential by IgG-03 and/or IgG-13, until proven otherwise.
The non-reactivity of Immucor Gamma anti-IgG with IgG4 is a previously known property of this particular monoclonal antibody (clone 16H8)9, which is listed as a limitation of the antibody in its package insert. The observation that this non-reactivity extends to different IgG4 isoallotypes is a novel observation contained herein. Although there are no data on the hemolytic potential of different IgG4 isoallotypes, it is precisely because IgG4 is typically considered benign that the inability of the monoclonal Immucor Gamma anti-IgG (clone 16H8) to bind IgG4 has been acceptable. Indeed, it has been argued that since IgG4 are typically benign, then non-reactivity to IgG4 is of benefit, since it avoids costly and time-consuming serological workups, which may ultimately have a negative impact on patient care through delay in blood product delivery.
While IgG4 is not associated with acute hemolytic events, it is unclear that IgG4 is entirely benign. Studies by Baldwin et al. convincingly demonstrated that an anti-JMH, which was an isolated IgG4, did indeed fail to cause an acute hemolytic transfusion reaction after transfusion of a whole unit of JMH+ RBCs10. However, whereas short term 51Cr studies in this patient showed a greater than 70% 1 hr recovery, the long term T1/2 51Cr survival was only 12 days. Thus, while not acutely hemolytic, it does appear that an anti-JMH IgG4 can substantially decrease the circulatory life-span of JMH+ RBCs. This may affect not only long-term efficacy for chronically transfused patients (e.g. increase chances of iron overload due to need of more units over time), but it is also unclear if ongoing clearance of RBCs by antibody is a benign process. Such sequelae would not cause signs or symptoms that physicians or patients would experience or report, as one would need to perform RBC survival studies to detect the problem. Accordingly, it is of little value to exclude this as a potential problem by the argument that this reagent has been used for decades without reports of any problems. In addition, an isolated IgG4 (anti-Ch) has been reported to be responsible for a severe anaphylactic reaction following transfusion 11. Finally, it is possible that an IgG4 alloantibody may predict an immune response of an IgG1-IgG3 type upon subsequent transfusion, as it has been shown that repeat exposure can alter IgG subtype12. Failure of a screen to pick up an alloantibody (due to it being an isolated IgG4) would increase the likelihood of inadvertent re-exposure to the antigen, which may cause such a class switch. This would not be due to IgG4 secreting cells further switching, but rather due to a new B cell response or IgM+ memory B cells13. Thus, on balance, it is unclear if an inability to recognize IgG4 is a desirable or undesirable property in an anti-IgG regent.
The monoclonal anti-IgG had the same sensitivity for each IgG subtype that it recognized, which may be an attribute of a monoclonal reagent that recognizes an epitope that is common to the IgG types that it recognizes. In contrast, the rabbit polyclonal reagent tested was more sensitive to IgG3. It is unclear how such differential sensitivity of anti-IgG for different IgG subtypes may affect serological testing; however, it raises the possibility of differential detection of alloantibodies in a given patient sample by different anti-IgG as a property of the relative levels of IgG subtypes, which can change over time as a patient’s alloimmune response evolves.
It could be argued that there is little likelihood of an adverse patient outcome due to the lack of reactivity of some anti-IgG (as demonstrated herein). The patient populations to consider include alloimmunized transfusion patients, pregnant women with a possibility of HDN, and patients with autoimmune hemolytic anemia (AIHA). Detailed studies in AIHA patients have demonstrated that a mixture of IgG subclasses is the most common presentation, and has a stronger association with hemolysis than isolated IgG subclasses14,15. In such patients, the monoclonal anti-IgG would still pick up the anti-RBC antibodies, as a mixture of IgG subclasses is present. However, a significant number of patients have also been observed who have only a single detectable IgG subclass on their RBCs. In a combined study of both healthy blood donors who had a positive DAT and patients with AIHA, isolated IgG1, IgG2, or IgG3 were all observed in the context of clinically significant hemolysis; no hemolysis was observed with isolated IgG416.
Although infrequent in the Caucasian population (overall about 1%), IgG3-03 and IgG3-13 have up to a 30% frequency in African populations of certain distributions 5. Thus, it is likely that there are some patients who are homozygous for either IgG3-03 or IgG3-13. In addition, some patients are likely to be compound heterozygotes for IgG3-03/IgG3-13. Alternatively, even if patients are heterozygous for IgG3-03 or IgG3-13, B cells that make alloantibodies may develop predominantly from clones that express the IgG3-03 or IgG3-13 isoallotype. In such patients, in the event that an anti-RBC antibody response is predominantly of the IgG3 subtype (and thus of the IgG3-03 and/or IgG3-13 isoallotype), then they may not be detected by platforms using the monoclonal anti-IgG characterized herein. This ethnic distribution of IgG-03 and IgG-13 raises the possibility of a higher representation in patients with Sickle Cell Disease, who have the highest rates of alloimmunization to RBC transfusion.
It is important to note, that while isolated IgG3 has been reported both in patients with AIHA and also in some alloimmunized patients 16,17, the frequency with which an isolated IgG3 would be found is predicted to be low (about 2% - although this estimate comes from a small samples size of 42 patients)17. Moreover, the frequency with which any given individual would be homozygous or compound heterozygous for IgG3-03 or IgG3-13 is also unclear. Thus, the clinically significant cases that would likely be missed by the 16H8 reagent are likely to be rare events, potentially very rare. We are not presenting herein, nor are we aware of, any clinical data to indicate that clinically significant hemolytic events have occurred because of alloantibodies that were not detected by the 16H8 reagent. However, this issue has also never been studied in detail with a focus on the blind spot described herein, thus it remains unknown. In any event, because approved and licensed alternatives exist that have no such blind spot, prudence may require the cessation of utilizing monoclonals with known inabilities to detect isoallotypes.
The overall approach used herein can be utilized to further assess sensitivity and specificity of other anti-IgG reagents, for any diagnostic platform that has an anti-IgG component. In our view, the findings in this report suggest that an evaluation of additional anti-IgG reagents for isoallotype reactivity may be warranted. As knowledge of the human genome evolves, and new isoallotypes of IgG are identified, this approach can be expanded to continue to refine our understanding of the diagnostic specifics of anti-IgG against human immunoglobulins.
Supplementary Material
Figure 4.
Amino acid basis for variation amongst IgG isoallotypes. For each IgG subtype, the *01 designation is the canonical sequence
Acknowledgments
We would like to express our extreme gratitude to the expert and generous contributions made by Dr. Tom Frame at Immucor Inc.
Footnotes
Disclosure of Conflicts of Interest: BloodworksNW has filed intellectual properties around the PUMA1 reagents in this report-HLH, XW, LK, YW, JPA, JCZ and MD are each employees of BloodworksNW. JCZ has a sponsored research agreement with Immucor (unrelated to the current work) and is on the scientific advisory board for Rubius Therapeutics.
References
- 1.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stratton F, Rawlinson VI. Preparation of test cells for the antiglobulin test. J Clin Pathol. 1974;27:359–67. doi: 10.1136/jcp.27.5.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawlinson VI, Stratton F, Merry AH. Test cells for use with anti-IgG sub-typing antisera in the antiglobulin test. Transfusion. 1985;25:27–9. doi: 10.1046/j.1537-2995.1985.25185116496.x. [DOI] [PubMed] [Google Scholar]
- 4.Smith NH, Henry KL, Cadwell CM, Bennett A, Hendrickson JE, Frame T, Zimring JC. Generation of transgenic mice with antithetical KEL1 and KEL2 human blood group antigens on red blood cells. Transfusion. 2012;52:2620–30. doi: 10.1111/j.1537-2995.2012.03641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dugoujon JM, Hazout S, Loirat F, Mourrieras B, Crouau-Roy B, Sanchez-Mazas A. GM haplotype diversity of 82 populations over the world suggests a centrifugal model of human migrations. Am J Phys Anthropol. 2004;125:175–92. doi: 10.1002/ajpa.10405. [DOI] [PubMed] [Google Scholar]
- 6.Engelfriet CP, Borne AE, Beckers D, Van Loghem JJ. Autoimmune haemolytic anaemia: serological and immunochemical characteristics of the autoantibodies; mechanisms of cell destruction. Ser Haematol. 1974;7:328–47. [PubMed] [Google Scholar]
- 7.Dubarry M, Charron C, Habibi B, Bretagne Y, Lambin P. Quantitation of immunoglobulin classes and subclasses of autoantibodies bound to red cells in patients with and without hemolysis. Transfusion. 1993;33:466–71. doi: 10.1046/j.1537-2995.1993.33693296807.x. [DOI] [PubMed] [Google Scholar]
- 8.Sokol RJ, Hewitt S, Booker DJ, Bailey A. Erythrocyte autoantibodies, subclasses of IgG and autoimmune haemolysis. Autoimmunity. 1990;6:99–104. doi: 10.3109/08916939008993374. [DOI] [PubMed] [Google Scholar]
- 9.Moulds MK, Spruell P, Lomas C. Experience in the use of a monoclonal polyspecific anti-human globulin reagent for antibody investigations. Vox Sang. 1994;67:121. [Google Scholar]
- 10.Baldwin ML, Ness PM, Barrasso C, Kickler TS, Drew H, Tsan MF, Shirey RS. In vivo studies of the long-term 51Cr red cell survival of serologically incompatible red cell units. Transfusion. 1985;25:34–8. doi: 10.1046/j.1537-2995.1985.25185116499.x. [DOI] [PubMed] [Google Scholar]
- 11.Westhoff CM, Sipherd BD, Wylie DE, Toalson LD. Severe anaphylactic reactions following transfusions of platelets to a patient with anti-Ch. Transfusion. 1992;32:576–9. doi: 10.1046/j.1537-2995.1992.32692367205.x. [DOI] [PubMed] [Google Scholar]
- 12.AuBuchon JP, Brightman A, Anderson HJ, Kim B. An example of anti-Yta demonstrating a change in its clinical significance. Vox Sang. 1988;55:171–5. doi: 10.1111/j.1423-0410.1988.tb05087.x. [DOI] [PubMed] [Google Scholar]
- 13.Della Valle L, Dohmen SE, Verhagen OJ, Berkowska MA, Vidarsson G, van der Schoot E. The majority of human memory B cells recognizing RhD and tetanus resides in IgM+ B cells. J Immunol. 2014;193:1071–9. doi: 10.4049/jimmunol.1400706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabijanska-Mitek J, Lopienska H, Zupanska B. Gel test application for IgG subclass detection in auto-immune haemolytic anaemia. Vox Sang. 1997;72:233–7. [PubMed] [Google Scholar]
- 15.Sokol RJ, Hewitt S, Booker DJ, Bailey A. Red cell autoantibodies, multiple immunoglobulin classes, and autoimmune hemolysis. Transfusion. 1990;30:714–7. doi: 10.1046/j.1537-2995.1990.30891020331.x. [DOI] [PubMed] [Google Scholar]
- 16.Petz LD, Garratty G. Immune Hemolytic Anemias. 2. Churchill Livingstone; 2004. [Google Scholar]
- 17.Zupanska B, Brojer E, McIntosh J, Seyfried H, Howell P. Correlation of monocyte-monolayer assay results, number of erythrocyte-bound IgG molecules, and IgG subclass composition in the study of red cell alloantibodies other than D. Vox Sang. 1990;58:276–80. doi: 10.1111/j.1423-0410.1990.tb04999.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.