Abstract
Background
Induction therapy leads to significant improvement in survival for selected patients with Stage IIIA non-small cell lung cancer (NSCLC). The ideal time interval between induction therapy and surgery remains unknown.
Methods
Clinical Stage IIIA NSCLC patients receiving induction therapy and surgery were identified in the National Cancer Database (NCDB). Delayed surgery was defined as ≥ 3 months after starting induction therapy. A logistic regression model identified variables associated with delayed surgery. Cox proportional hazards modeling and Kaplan Meier analysis were performed to evaluate variables independently associated with overall survival.
Results
From 2006 to 2010, 1,529/2380 (64.2%) received delayed surgery. Delayed surgery patients were older (61.2 ± 10.0 years versus 60.3 ± 9.2, p=0.03), more likely to be non-Caucasian (12.4% versus 9.7%, p=0.046), and less likely to have private insurance (50% versus 58.2%, p=0.002). Delayed surgery patients were also more likely to have a sublobar resection (6.3% versus 2.9%). On multivariate analysis, age > 68 years (OR 1.37, 95% CI 1.1–1.7) was associated with delayed surgery, while Caucasian race (OR 0.75, 95% CI 0.57–0.99) and private insurance status (OR 0.82, 95% CI 0.68–0.99) were associated with early surgery. Delayed surgery was associated with higher risk of long-term mortality (HR 1.25, 95% CI 1.07–1.47).
Conclusions
Delayed surgery after induction therapy for stage IIIA lung cancer is associated with shorter survival, and is influenced by both social and physiologic factors. Prospective work is needed to further characterize the relationship between patient comorbidities and functional status with receipt of timely surgery.
Keywords: Locally advanced lung cancer, Induction Therapy, Lobectomy, Survival Analysis
Locally advanced lung cancer is estimated to represent almost 25% of non-small cell lung cancer (NSCLC) diagnoses in the United States, with a five-year survival of 27.4%. [1] Stage IIIA NSCLC (T1a-T2bN2, T2N1-N2, or T4N0-N1) specifically is amenable to trimodality therapy (neoadjuvant chemotherapy and radiation therapy, followed by surgery) for patients that are operable candidates and do not show evidence of disease progression. A recent review of the National Cancer Database (NCDB), examining over 60,000 clinical Stage IIIA patients found that while a minority were receiving trimodality therapy (15%), these patients experienced superior median overall survival when compared to definitive chemoradiation therapy patients (32.4 months versus 15.7 months, respectively, p<0.001). [2] While not all Stage IIIA patients may be operable candidates and may have poorer overall survival due to additional comorbidities, this study did have novel and significant survival findings compared to randomized trials that were not able to detect a survival difference between trimodality and definitive therapy treatment in Stage IIIA NSCLC. [3,4] Current National Comprehensive Cancer Network (NCCN) guidelines also suggest induction chemotherapy with or without radiation therapy, followed by surgery if there is no apparent progression of disease. [5]
While consideration and inclusion of appropriate clinical State IIIA candidates for surgery is crucial, much is currently unknown regarding the timing of these sequential therapies, and if there is a point at which surgery should be considered ‘delayed’, with patients experiencing poorer long term outcomes. To study this question, we used the NCDB to investigate possible time points to define delayed surgery, and evaluate which factors may be associated with receiving delayed surgery.
Patients and Methods
The NCDB Participant User File (PUF) for NSCLC was reviewed to identify all clinical Stage IIIA NSCLC patients receiving induction chemotherapy with or without radiation therapy, followed by surgical resection. The NCDB, established in 1989, is a joint program of the American College of Surgeons and the American Cancer Society. It is a comprehensive clinical oncology database that captures approximately 70% of all diagnosed malignancies nationally at Commission on Cancer accredited centers. As patients and facilities are both deidentified in the PUF, this database is exempt from our Institutional Review Board.
Patient characteristic variables that were abstracted and dichotomized for analysis included race (Caucasian or non-Caucasian), population type (patient’s home is in a metropolitan, urban, or rural county type as determined by the United States Department of Agriculture Economic Research Service, income (average income in patient’s zip code is < or ≥ $38,000 per year), education level (percent of population in patients zip code without a high school education is ≥21% or <21%), insurance status (uninsured, private, Medicare, Medicaid, or other government insurance), and center type (academic or community cancer center). The Charlson/Deyo comorbidity score is recorded in the NCDB as 0, 1, or ≥2, and does not include the patient’s known primary lung malignancy. Surgical margin status was dichotomized as either R0 (negative margins) or ≥R1 (microscopic or macroscopic residual margins). This was due to the fact that of 188 patients with positive surgical margins, 43% were recorded as having residual tumor at the margins, but not categorized into microscopic disease versus gross tumor present.
We limited the years of analysis in this study to begin in 2006, as this is when the NCDB regularly recorded the timing of systemic therapy relative to surgery. To calculate the time interval from the start of induction therapy to surgical resection, we subtracted the ‘time from diagnosis to systemic therapy’ value from the ‘time from diagnosis to surgery’ value. This value was cross-checked with the NCDB’s ‘systemic surgery sequence’ variable. Patients with ≤12 days (for likely non-completion of induction therapy regimen) or >200 days (for likely salvage surgery) were excluded from analysis. The NCDB does not record number of cycles of therapy, completion of chemotherapy date, or types of agents used, so these factors could not be used to further classify induction regimens. To identify a potential time point that would constitute delayed care, we used X-tile software, an assisted marker cutpoint analysis program (Version 3.6.1, Yale University, New Haven, CN 2005). This program uses the marker of interest (here, interval from beginning of induction therapy to date of surgery) and survival data (with length of follow-up and vital status) to create possible division thresholds in a training set and then evaluating their survival outcome differences in a validation set, using a different patient cohort from the database. [6] This program has been previously used to examine breast cancer survival patterns with various patient characteristic markers from the national Surveillance, Epidemiology, and End Results (SEER) Program database. [6]
Once a time-point was identified that resulted in significantly different median overall survival, clinical stage IIIA NSCLC patients receiving induction therapy followed by surgery were then divided into ‘early’ and ‘delayed’ groups. Descriptive statistics of continuous variables were reported as mean ± standard deviation. Independent sample t tests were used to analyze normally distributed continuous data and χ2 tests were used to compare categorical data. Backwards stepwise logistic regression was performed to identify variables independently associated with receiving delayed surgery after induction therapy. Variables with a significant difference <0.05 on univariate analysis were selected for entry into the logistic regression model. A Cox proportional hazards model was also created to identify variables independently associated with increased overall mortality for Stage IIIA patients who underwent trimodality therapy. All statistical analyses were performed in SPSS for Windows (Version 23.0. Armonk, NY: IBM Corporation, 2015)
Results
From 2006 to 2012, 2,380 clinical Stage IIIA patients receiving induction therapy followed by surgical resection were identified. Specifically, 2,032/2,380 (85.4%) of patients received induction chemoradiation therapy, while 348/2,380 (14.6%) of patients received induction chemotherapy only. 2,053/2,380 (86.3%) were classified with clinical N2 disease. On cutpoint analysis, receiving surgical resection ≥3 months from the time induction therapy was started resulted in significantly lower median overall survival than patients that proceeded to resection <3 months after starting induction therapy. Based on this division, 851/2,380 (35.8%) were classified as receiving early surgery, and 1,529/2,380 (64.2%) were classified as receiving delayed surgery. A histogram displaying the range of times from the start of induction therapy to surgery are shown in Figure 1. For early surgery patients, the mean time from the start of induction therapy to surgical resection was 69.8 days ± 10.2 and for delayed patients, the mean time was 113.0 days ± 25.3.
Figure 1.
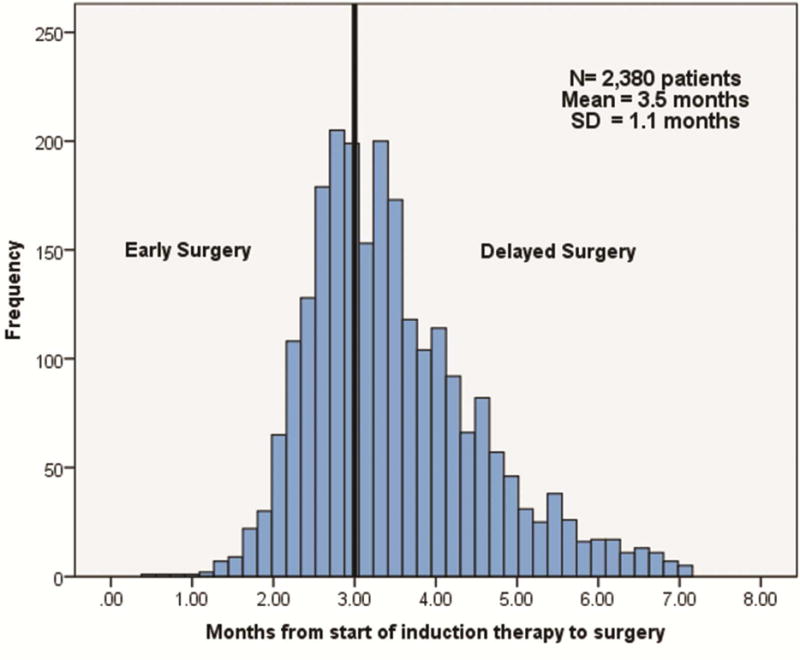
Histogram of clinical Stage IIIA NSCLC patients in the NCDB from 2006–2012, by length of time between start of induction therapy and date of surgical resection.
Characteristics of patients receiving delayed resection are shown in Table 1. Being in an age category ≥68 years old was independently associated with an increased likelihood of receiving delayed surgery (OR 1.37, 95% CI 1.10–1.72, p=0.006), while variables independently associated with a decreased likelihood of receiving delayed surgery included Caucasian race (OR 0.75, 95% CI 0.57–0.99, p=0.04) and private insurance status (reference: uninsured, OR 0.82, 95% CI 0.68–0.99, p=0.04).
Table 1.
Univariate comparison of patients receiving early (<3 months) verus delayed (≥3 months) surgical resection after the initiation of induction therapy.
| Variable | Early Induction to Surgery Interval (<3 months) (n=851, 35.8%) |
Late Induction to Surgery Interval (≥ 3 months) (n=1529, 64.2%) |
P value |
|---|---|---|---|
|
| |||
| Age | 60.31 ± 9.18 | 61.23 ±9.98 | 0.027 |
|
| |||
| Gender | |||
| Male | 438 (51.5%) | 784 (51.3%) | 0.928 |
| Female | 413 (48.5%) | 745 (48.7%) | |
|
| |||
| Race | |||
| Caucasian | 763 (90.3%) | 1332 (87.6%) | 0.046 |
| Non-Caucasian | 82 (9.7%) | 189 (12.4%) | |
|
| |||
| Income | |||
| <$35,000 | 212 (26.4%) | 411 (28.8%) | 0.219 |
| ≥$35,000 | 592 (73.6%) | 1016 (71.2%) | |
|
| |||
| Population type | |||
| <250,000 | 221 (27.8%) | 393 (27.8%) | 0.968 |
| ≥250,000 | 573 (72.2%) | 1023 (72.2%) | |
|
| |||
| Greatest circle distance | 96.82 ± 808.81 | 129.93 ± 982.01 | 0.414 |
|
| |||
| Insurance status | |||
| Uninsured | 14 (1.7%) | 35 (2.3%) | 0.002 |
| Private Insurance | 493 (58.2%) | 755 (50.0%) | |
| Medicaid | 35 (4.1%) | 98 (6.5%) | |
| Medicare | 293 (34.6%) | 603 (39.9%) | |
| Other government | 12 (1.4%) | 20 (1.3%) | |
|
| |||
| Facility type | |||
| Academic | 325 (38.2%) | 612 (40.0%) | 0.380 |
| Community | 526 (61.8%) | 917 (60.0%) | |
|
| |||
| Charlson/Deyo Score | |||
| 0 | 565 (66.4%) | 1053 (68.9%) | 0.258 |
| 1 | 239 (28.1%) | 383 (25.0%) | |
| ≥2 | 47 (5.5%) | 93 (6.1%) | |
|
| |||
| Clinical T stage | |||
| 1 | 200 (23.5%) | 366 (23.9%) | 0.828 |
| 2 | 390 (45.8%) | 713 (46.6%) | |
| 3 | 209 (24.6%) | 374 (24.5%) | |
| 4 | 25 (2.9%) | 38 (2.5%) | |
| X | 27 (3.2%) | 38 (2.5%) | |
|
| |||
| Clinical N2 stage | 720 (84.6%) | 1333 (87.2%) | 0.080 |
|
| |||
| Tumor size (mm) | 44.51 ± 33.44 | 43.75 ± 27.25 | 0.557 |
|
| |||
| Surgery type | |||
| Sublobar Resection | 25 (2.9%) | 96 (6.3%) | <0.001 |
| Lobar Resection | 666 (78.3%) | 1236 (80.8%) | |
| Pneumonectomy | 151 (17.7%) | 186 (12.2%) | |
| Surgery, NOS | 9 (1.1%) | 11 (0.7%) | |
|
| |||
| Positive Surgical Margins | 94 (11.4%) | 94 (6.4%) | <0.001 |
|
| |||
| Number of Positive Lymph Nodes | 2.13 ± 3.34 | 1.86 ± 2.78 | 0.052 |
|
| |||
| Inpatient Length of Stay (days) | 6.81 ± 5.61 | 6.79 ± 5.44 | 0.937 |
|
| |||
| Thirty Day Readmission | 57 (7.0%) | 86 (5.9%) | 0.295 |
|
| |||
| Thirty Day Mortality | 28 (3.3%) | 54 (3.5%) | 0.757 |
|
| |||
| Received adjuvant therapy (chemotherapy and/or radiation therapy) | 277 (32.5%) | 434 (28.4%) | 0.03 |
There was no difference in the use of radiation therapy with induction chemotherapy among early versus delayed patients (86.1% versus 85.0%, respectively, p=0.44). In terms of perioperative outcomes, there was no significant difference in inpatient length of stay (days), thirty day readmission, or thirty day mortality (Table 1). Delayed patients were significantly more likely to receive a sublobar resection (6.3% versus 2.9%) and early patients had a higher pneumonectomy rate (17.7% versus 12.2%), p<0.001. Of note, early surgery patients were more likely to have positive surgical margins (≥R1) than delayed patients (11.4% versus 6.4% respectively, p<0.001). When analyzed by resection type, early patients still had higher rates of ≥R1 margins compared to delayed patients: for lobectomy 8.6% (56/651) versus 5.7% (69/1201), p=0.02, for pneumonectomy 21.5% (31/144) versus 8.0% (14/176), p=0.001. For sublobar resection, positive margins rates approached but did not reach significance for early surgery patients, but this was likely due to small sample sizes 25.0% (6/18) versus 11.1% (10/80), p=0.08. Of patients with ≥R1 resection, 103/188 (54.8%) did not receive any adjuvant therapy, 30/188 (16.0%) received additional adjuvant chemotherapy, 35/188 (18.6%) received adjuvant radiotherapy, and 20/188 (10.6%) received both adjuvant chemotherapy and radiotherapy. Early surgery patients were significantly more likely to receive adjuvant therapy (chemotherapy ± radiation therapy. 32.5% versus 28.4%, p=0.03)
On Kaplan-Meier analysis, early surgery patients demonstrated significantly longer overall median survival compared to delayed surgery patients (39.8 months ± 3.5 versus 33.2 months ± 1.8, p=0.03, Figure 2). Even among patients with positive surgical margins, early surgery patients continued to have improved median overall survival compared to delayed surgery patients (24.7 months ± 3.2 versus 19.5 months ±1.9, p=0.009), Figure 3. For patients with negative surgical margins, an increase in median overall survival for early surgery patients approached, but did not reach, significance (43.6 months ± 4.5 versus 36.3 months ± 1.9, p=0.06). Results of the Cox proportional hazards model are shown in Table 2. Of note, both positive surgical margins (HR 1.84, 95% CI 1.44–2.34, p<0.001) and delayed surgical resection (HR 1.19, 95% CI 1.02–1.40, p=0.03) were independently associated with increased likelihood of long-term mortality.
Figure 2.
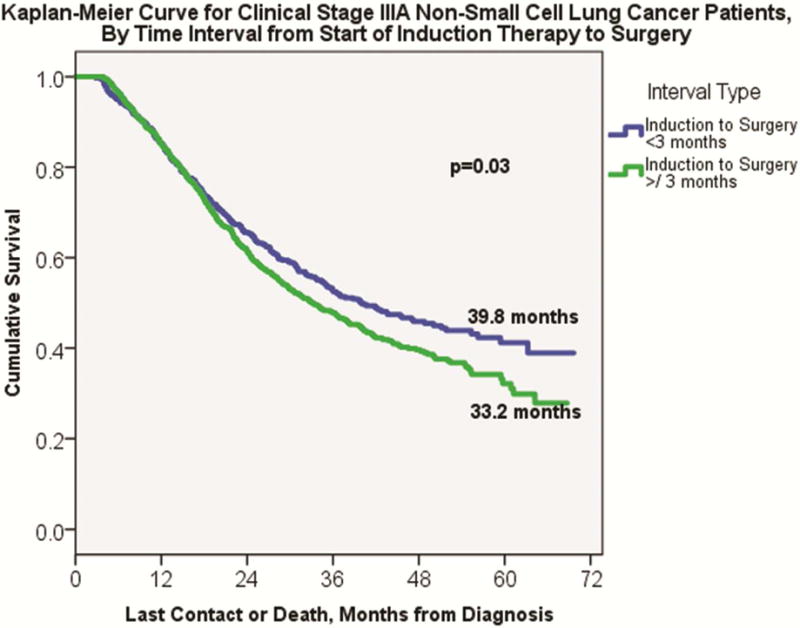
Kaplan-Meier analysis for clinical Stage IIIA NSCLC patients, by early versus delayed interval from the start of induction therapy to date of surgery.
Figure 3.
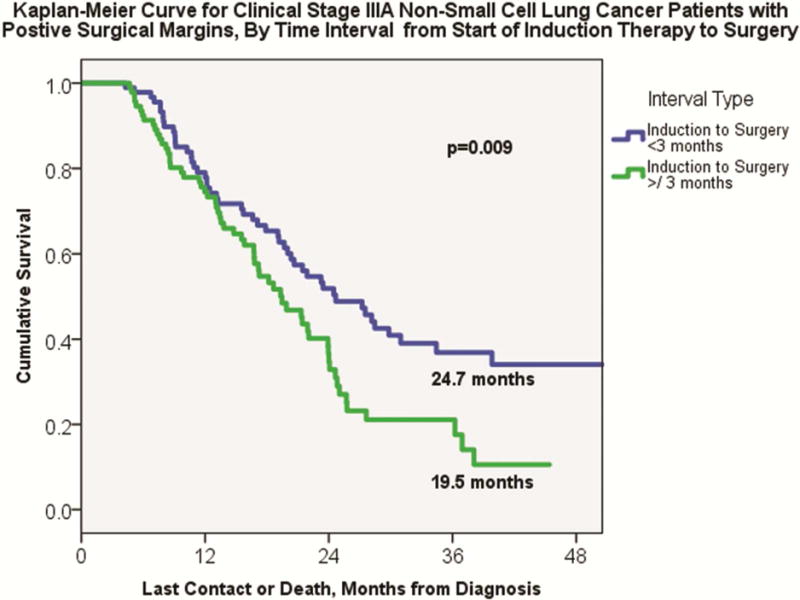
Kaplan-Meier analysis for clinical Stage IIIA NSCLC patients with positive surgical margins (≥R1), by early versus delayed interval from the start of induction therapy to date of surgery.
Table 2.
Cox proportional hazards model for variables independently associated with overall survival. Inputs into the model included: age, gender, race, population status of zip code, income level, insurance status, Charlson/Deyo comorbidity score, induction radiation therapy status, early versus delayed surgery interval, extent of surgical resection, number of pathologically positive lymph nodes, surgical margins, thirty-day readmission status, and adjuvant therapy status.
| Variable | Hazard Ratio (95% CI) | P value |
|---|---|---|
|
| ||
| Age (per year increase) | 1.02 (1.01–1.03) | <0.001 |
|
| ||
| Caucasian race | 0.74 (0.57–0.95) | 0.02 |
|
| ||
| Charlson-Deyo Score (ref: 0) | ||
| 1 | 1.21 (1.03–1.43) | 0.03 |
| ≥2 | 1.14 (0.83–1.56) | 0.42 |
|
| ||
| Positive Lymph Nodes (per node) | 1.04 (1.01–1.06) | 0.004 |
|
| ||
| Delay ≥ 3 months in interval from induction therapy initiation to surgery | 1.21 (1.04–1.43) | 0.02 |
|
| ||
| Positive surgical margins (≥R1) | 1.73 (1.35–2.20) | <0.001 |
Comment
This analysis sought to characterize the time interval between starting induction therapy and receiving surgery for clinical Stage IIIA NSCLC in the NCDB and examine what variables were associated with receiving delayed surgery and its association with overall survival. Using cut-point analysis in separate training and validation cohorts, an overall survival detriment was seen among patients receiving surgery ≥3 months from the start of induction therapy. This remained true on Cox modeling, which adjusted for other covariates including surgical margin status.
A particularly interesting finding of this study was that although early surgery patients were significantly more likely to have positive surgical margins (with both lobectomies and pneumonectomies), early surgery patients with ≥R1 resection had significantly longer median overall survival than delayed patients with ≥R1 resection. We hypothesize this finding may be caused by two factors. The first is that patients in the delayed surgery cohort may be relatively more physiologically compromised or frail after induction therapy, and therefore may be more susceptible to earlier mortality from any cause, than patients that are able to receive induction therapy and proceed to surgery in less than 3 months. While our univariate comparison did not show a significant difference in the Charlson/Deyo comorbidity score between early and delayed surgical patients, it is possible that this is not an inclusive enough parameter to capture a patient’s true spectrum or severity of comorbidities, which may be exacerbated during induction chemoradiation therapy. In this analysis, we are not able to account for specific comorbidities or frailty indices that could help nuance our description of early and delayed surgical patients. The finding that a small, but significantly higher proportion of early surgery patients are able to receive additional adjuvant therapy may support this ‘healthier population’ hypothesis.
A second possibility that could be contributing to a higher positive surgical margin rate in the early surgery population is that these patients are receiving more expeditious surgery due to little to no response on induction therapy, and surgical intervention is undertaken while the tumor is still deemed operable. This may partially explain the particularly high margin rate for early versus delayed pneumonectomy patients. If this is true, and explains the higher surgical margin rate in early surgery patients, then this likely makes our true difference in median overall survival smaller (and more conservative) than expected. Unfortunately, we have no data on pre- and post-induction imaging or staging, and cannot account for what proportion of early surgery patients had no or marginal response to their induction therapy treatment. Information on pre- and post-induction CT and FDG-PET imaging would be particularly helpful, as this would not only allow us to categorize patients by response type, but also evaluate for possible improvement in long term recurrence and survival outcomes. [7,8] This would also explain why, in our Cox proportional hazards model delayed surgery is independently associated with increased mortality when adjusting for surgical margin status, and vice versa.
In order to provide context of the practice patterns and time to surgery characteristics of clinical Stage IIIA NSCLC patients in the NCDB, it is useful to compare our findings to those described in clinical trials of induction chemoradiation therapy regimens. While many trials report treatment associated toxicities, the proportion of patients who receive reduced chemotherapy and/or radiotherapy doses, or do not complete treatment, the duration of treatment is not typically reported. [9–12] We do know from trials such as Southwest Oncology Group Study 8805 and Intergroup Trial 0139, which evaluated one of the most commonly used induction regimens still in practice today (two cycles of cisplatin and etoposide, with concurrent radiotherapy of 45 Gy, with 1.8Gy fractions beginning on day one) took approximately 6 weeks for completion of therapy, followed by a 3–5 week recovery period prior to surgery and to minimize the degree of radiation associated fibrosis. [11,12] Therefore, even with an ideal schedule and no treatment delays due to leukopenia, thrombocytopenia, renal sufficiency or other toxicities, a patient would complete induction therapy and be ready for surgery at a minimum of 9 weeks, and a maximum of 12 weeks. [11,12] This further seems to support our hypothesis that delay of surgery ≥3 months from the start time of induction therapy may be a marker for patient comorbidities and physiologic compromise that ultimately lead to decreased median overall survival.
There are limitations to this study and identifying these helps pose new questions for more granular retrospective and prospective studies. As mentioned earlier, we are not able to analyze reasons for why the majority of clinical Stage IIIA patients undergoing induction therapy followed by surgery experience an interval ≥3 months. Therefore we do not know if these delays are due to patient factors such as compromised physiologic function (requiring modified chemotherapy regimen scheduling), oncologic factors (difference in evaluation practices or referral patterns based on burden of N2 disease), or institutional factors (scheduling of surgery after completion of chemotherapy). Since the date of completion of induction therapy is unavailable in the NCDB, we are unable to discern which patients had a prolonged treatment course, and received surgery in a relatively timely manner, versus those patients that received induction therapy without interruption, but experienced a delay between completion of therapy and surgery due to deconditioning or increased frailty. Although both of those groups of patients would be considered delayed by the ≥3 month cut point used in this analysis, it is possible that these are two distinct groups of patients with different survival outcomes.
Additionally, we do not know the specific induction therapy regimens delivered to these cohorts, which could certainly affect the associated toxicities and/or timing intervals. A better comorbidity and frailty profile of these cohorts would also give a more nuanced comparison between early and delayed surgery patients. Also, a better capture of microscopic R1 margins versus grossly positive R2 margins may assist in characterizing the disease status of both groups (i.e. if some early stage surgery patients are receiving salvage surgery, they may be more likely to have R2 disease, while delayed patients may be more likely to have R1 disease). Studying disease-free survival (unavailable from the NCDB), rather than overall survival, would also help us understand the role of cancer in the mortality of these patients who often have multiple comorbidities.
In conclusion, this analysis found that there was a decrease in median overall survival for clinical Stage IIIA NSCLC patients receiving surgery ≥3 months after the start of induction therapy. Future clinical questions to enhance our understanding of this delay (and its possible implications in survival) include comparing early versus delayed surgery in radiologic responders versus non-responders that receive surgery, comparing specific comorbidities and functional assessments, and disease-free survival patterns. Trimodality therapy is an intensive effort for the patients that receive this approach, and attempts to maximize the potential survival benefits are needed.
Acknowledgments
Pamela Samson, MD, MPHS has grant support through NIH Cardiothoracic Surgery T32 HL07776. Varun Puri, MD, MSCI has grant funding through NIH K07CA178120 and K12CA167540-02. The NCDB has not verified and is not responsible for the analytic methodology used in this study, and the conclusions drawn are solely those of the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Poster Session of the Fifty-second Annual Meeting of the Society of Thoracic Surgeons, Phoenix, AZ, Jan 23–27, 2016.
References
- 1.Surveillance, Epidemiology, and End Results Program. SEER Statistical Fact Sheets: Lung and Bronchus Cancer. http://seer.cancer.gov/statfacts/html/lungb.html. Accessed 1/23/2016.
- 2.Patel AP, Crabtree TD, Bell JM, et al. National Patterns of Care and Outcomes After Combined Modality Therapy for Stage IIIA Non-Small Cell Lung Cancer. J Thorac Oncol. 2014;9:612–621. doi: 10.1097/JTO.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomized controlled trial. Lancet. 2009;374:379–386. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Meerbeeck JP, Kramer GW, Van Schil PE, et al. European Organization for Research and Treatments of Cancer-Lung Cancer Group Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small cell lung cancer. J Natl Cancer Inst. 2007;99:442–450. doi: 10.1093/jnci/djk093. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer, Version 2.2016. doi: 10.6004/jnccn.2016.0051. http://www.NCCN.org. Accessed 1/23/2016. [DOI] [PubMed]
- 6.Camp RL, Dolled-Filhart M, Rimm DL. X-Tile: A New Bioinformatics Tool for Biomarker Assessment and Outcome-Based Cut-Point Optimization. Clin Cancer Res. 2004;10:7252–59. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 7.Lee HY, Lee HJ, Kim YT, Kang CH, et al. Value of Combined Interpretation of Computed Tomography Response and Positron Emission Tomography Response for Prediction of Prognosis after Neoadjuvant Chemotherapy in Non-small Cell Lung Cancer. J Thorac Oncol. 2010;5:497–503. doi: 10.1097/JTO.0b013e3181d2efe7. [DOI] [PubMed] [Google Scholar]
- 8.Tanvetyanon T, Eikman EA, Sommers E, Robinson L, et al. Computed tomography response, but not positron emission tomography scan response, predicts survival after neoadjuvant chemotherapy for resectable non-small cell lung cancer. J Clin Oncol. 2008;26(28):4610–6. doi: 10.1200/JCO.2008.16.9383. [DOI] [PubMed] [Google Scholar]
- 9.Katakami N, Tada H, Mitsudomi T, Kudoh S, et al. A Phase 3 Study of Induction Treatement with Concurrent Chemoradiothreapy Versus Chemotherapy Before Surgery in Patients with Pathologically Confirmed N2 Stage IIIA Nonsmall Cell Lung Cancer (WJTOG9903) Cancer. 2012;118:6126–35. doi: 10.1002/cncr.26689. [DOI] [PubMed] [Google Scholar]
- 10.Thomas M, Rube C, Hoffknecht P, Macha HN, et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomized trial in stage III non-small cell lung cancer. Lancet Oncol. 2008;9:636–648. doi: 10.1016/S1470-2045(08)70156-6. [DOI] [PubMed] [Google Scholar]
- 11.Albain KS, Rusch VW, Crowley JJ, Rice TW, et al. Concurrent Cisplatin/Etoposide Plus Chest Radiotherapy Followed by Surgery for Stages IIIA(N2) and IIIB Non-Small Cell Lung Cancer: Mature Results of Southwest Oncology Group Phase II Study 8805. J Clin Oncol. 1995;13:1880–1892. doi: 10.1200/JCO.1995.13.8.1880. [DOI] [PubMed] [Google Scholar]
- 12.Albain KS, Swann RS, Rusch VW, Turrisi AT, 3rd, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small cell lung cancer: a phase III randomized controlled trial. Lancet. 2009;374(9687):379–86. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


