Abstract
We have analyzed the development of leaf shape and vascular pattern in leaves mutant for ASYMMETRIC LEAVES1 (AS1) or AS2 and compared the timing of developmental landmarks to cellular response to auxin, as measured by expression of the DR5:β-glucuronidase (GUS) transgene and to cell division, as measured by expression of the cycB1:GUS transgene. We found that the earliest visible defect in both as1 and as2 first leaves is the asymmetric placement of auxin response at the distal leaf tip. This precedes visible changes in leaf morphology, asymmetric placement of the distal margin gap, formation of margin gaps along the leaf border, asymmetric distribution of marginal auxin, and asymmetry in cell division patterns. Moreover, treatment of developing leaves with either exogenous auxin or an auxin transport inhibitor eliminates asymmetric auxin response and subsequent asymmetric leaf development. We propose that the initial asymmetric placement of auxin at the leaf tip gives rise to later asymmetries in the internal auxin sources, which subsequently result in asymmetrical cell differentiation and division patterns.
INTRODUCTION
Growth and development of the plant shoot requires that a group of indeterminate stem cells, the shoot apical meristem (SAM), continuously replenishes itself and produces determinate lateral organs from its flanks. The SAM is radially symmetrical, and lateral organs are produced from it in a regular pattern. The maintenance of SAM symmetry and formation of organs require integration and coordination of processes controlling cell growth and cell division in diverse tissue types. Asymmetric growth of the shoot does occur in response to either light or gravity. Both gravitropism and phototropism are regulated through asymmetric distribution of the hormone auxin, with both environmental stimuli proposed to cause an altered distribution of auxin carrier proteins in the plasma membrane (Friml, 2003).
Lateral organs produced from the SAM, such as leaves, usually attain both adaxial–abaxial and proximodistal asymmetry. A large set of genes, isolated from a range of angiosperm species, has been implicated in the specification of leaf adaxial–abaxial asymmetry. In Arabidopsis thaliana, mutations in the genes PHABULOSA (PHB), FILAMENTOUS FLOWER (FIL), PINHEAD/ZWILLE, ARGONAUTE1 (AGO1), GYMNOS, CRABS CLAW, ETTIN, YABBY2, KANADI (KAN), ASYMMETRIC LEAVES2 (AS2), and AS1 result in aberrant adaxial–abaxial specification of foliar organs (Bohmert et al., 1998; McConnell and Barton, 1998; Eshed et al., 1999, 2004; Lynn et al., 1999; Sawa et al., 1999; Siegfried et al., 1999; Douglas et al., 2002; Emery et al., 2003; Lin et al., 2003; Xu et al., 2003). In Antirrhinum majus, phantastica (phan) results in abaxialization of leafy organs (Waites and Hudson, 1995; Waites et al., 1998). Whereas phan results in abaxialized and phb in adaxialized lateral organs, in both mutants organs are radially symmetrical with only a single vascular trace and no blade expansion. In phan mutants, the leaf margin, a region of distinct epidermal cells at the leaf edge, does not form. Plants multiply mutant for members of the KAN genes show progressive leaf adaxialization and loss of lamina expansion, with adaxial leaf outgrowths (Eshed et al., 2004). Single mutants of FIL and YAB3 show no obvious vegetative phenotype, but fil yab3 double mutants produce linear leaves, somewhat adaxialized abaxial leaf surfaces, and a simple leaf vascular pattern that shows reduced meeting at the leaf margin (Siegfried et al., 1999). Ectopic expression of FIL results in linear leaves with reduced or no vascular tissue and incomplete or abnormal margin formation (Sawa et al., 1999), and leaf outgrowths characteristic of KAN loss of function are dependent upon YABBY activity (Eshed et al., 2004). Ectopic expression of AS2 results in adaxialization, a lack of blade expansion, and altered vein patterning (Lin et al., 2003). Finally, mutations in AGO1 result in elongated leaves with a simplified vascular pattern (Bohmert et al., 1998). These phenotypes suggest a complex interplay amongst a set of leaf characters: margin formation, blade expansion, and vascular pattern.
Specification of proximodistal asymmetry is less well characterized, although AS1 and AS2 in Arabidopsis and orthologs ROUGH SHEATH2 (RS2) in maize (Zea mays) and PHAN in Antirrhinum seem to play a direct or indirect role. Mutations in rs2 show defects in proximodistal specification, with blade cells adopting a sheath-like identity (Schneeberger et al., 1998). The phan phenotype may also be interpreted as a proximodistal defect, with radially symmetrical leaves resulting from a conversion of blade (distal) to petiole (proximal) (Tsiantis et al., 1999b). The interpretation that multiple midveins form within the blade of AS1 mutant leaves could also indicate leaf proximalization (Byrne et al., 2000). AS1 and its orthologs act to downregulate a set of genes required to maintain meristem indeterminacy, including WUSCHEL and the class 1 KNOX homeobox-containing genes (KNAT1 [BREVIPEDICELLUS], KNAT2, and SHOOT MERISTEMLESS [STM]; Schneeberger et al., 1998; Timmermans et al., 1999; Tsiantis et al., 1999b; Byrne et al., 2000; Ori et al., 2000; Barton, 2001; Semiarti et al., 2001; Venglat et al., 2002), an action proposed to establish determinate cell fate. In wild-type leaves, cell division ceases in first distal and then proximal cells, suggesting that the KNAT genes may be negatively regulated in a spatial manner. A possible explanation for the proximalization seen in AS1 mutant leaves is that lack of KNAT downregulation causes cells to adopt a proximal fate; however, loss-of-function mutations in KNAT genes do not suppress the as1 phenotype, suggesting that either significant redundancy exists amongst the KNOX genes or that other factors must play a role (Byrne et al., 2002; Micol and Hake, 2003).
Plant growth regulators also seem to play a role in the establishment of the indeterminant versus determinant pathways and hence may act to establish proximal/distal asymmetries. Cytokinin induces expression of STM and KNAT1 (Rupp et al., 1999; Frank et al., 2000), whereas polar auxin transport is necessary for their downregulation (Scanlon, 2003). In turn, KNAT genes downregulate the gibberellin pathway within the meristem (Hay et al., 2002). It is now postulated that the lobes in leaves mutant for AS1 or AS2 result when faulty downregulation of KNAT1 and KNAT2 causes a reinitiation of the meristematic program, including a reduction in gibberellin biosynthesis within leaf primordia and subsequent aberrant cell divisions (Hay et al., 2002).
Although each leaf displays proximodistal and adaxial–abaxial polarity, the expansion of the leaf blade, position of leaf serrations, and loops of vascular tissue create an essentially bilaterally symmetrical organ across the midvein. Moreover, changes in leaf shape are coupled with changes to vascular pattern, suggesting a link between the controls on cell growth, division, and differentiation (Dengler and Kang, 2001). A probable explanation is that, in conjunction with mechanisms controlling polarity, a coordinating mechanism controls these cell processes. A possible candidate is the plant hormone auxin, whose symmetric distribution in roots and shoots maintains symmetric growth and whose asymmetric localization upon stimulation with light or gravity results in asymmetric cell elongation and growth (Friml, 2003). As well as its role in cell elongation, auxin in leaves is known to control progression into the cell cycle (Riou-Khamlichi et al., 1999; den Boer and Murray, 2000; Meijer and Murrary, 2001; Stals and Inzé, 2001; Himanen et al., 2002). The auxin resistant mutants axr2 and axr1 have smaller leaves (Lincoln et al., 1990; Wilson et al., 1990; Steynen and Schultz, 2003), which in axr1 have been shown to result from a reduction in cell number (Lincoln et al., 1990). More recently, expression of the auxin inducible gene ARGOS has been shown to influence rates of cell division in leaves (Hu et al., 2003). Directed transport of auxin is thought to regulate leaf position and establish the developing leaf primordial tip as an auxin sink (Reinhardt et al., 2003), thus generating a proximodistal auxin gradient with its maximum at the tip of the developing primordium (Benkova et al., 2003). Later in leaf development, auxin, proposed to be derived from the margin, is symmetrically distributed on either side of the midvein (Mattsson et al., 1999, 2003; Sieburth, 1999; Aloni et al., 2003), a pattern postulated to drive the ordered and symmetrical differentiation of the leaf vascular pattern (Aloni et al., 2003; Steynen and Schultz, 2003). The coordinate control of cell division and vascular differentiation by auxin could provide a link between vascular pattern and leaf shape and provide a molecular explanation for the importance of the leaf margin in both processes. As a first step toward testing this hypothesis, we screened mutants with altered leaf shape for margin defects. The margin is characterized by readily identified files of cells more elongated than those on either the adabaxial or adaxial lamina. All mutants identified in this screen were found to have margin gaps (small, unelongated cells interrupting the files of margin cells) and to be homozygous for recessive alleles of AS1 or AS2. To further test our idea, we compared changes in auxin distribution to changes in cell division and leaf morphology in as1 and as2 mutant leaves. We found that the asymmetric shape of the mature leaves is predicted firstly by asymmetric auxin distribution, secondly by asymmetric placement of margin gaps, and finally by asymmetric cell division patterns. Furthermore, we found that treatment of developing as1 and as2 leaves with either exogenous auxin or auxin transport inhibitor eliminated asymmetric leaf morphology.
RESULTS
Complementation Tests
Mutants having leaf shape abnormalities were assessed for defective margins. Eight mutants having asymmetric or lobed leaves were found to have margin gaps and were tested for allelism to as1-1 and as2-1 by intercrossing (Table 1). Five new alleles of AS1 were identified (CS444, CS3240, CS3250, GW4-0-0, and AW179, designated as1-15, as1-16, as1-17, as1-18, and as1-19, respectively) and two new alleles of AS2 (CS230 and CS3381, designated as2-6 and as2-7, respectively). Preliminary examination of plants homozygous for the new AS1 alleles indicated that their phenotypes were not distinct from previously described alleles. Therefore, we choose alleles as1-1 and as1-16 from Landsberg erecta (Ler) and Columbia (Col) ecotypes, respectively, for further analysis. Plants homozygous for as2-7 have a phenotype somewhat different from other as2 alleles; therefore, we choose plants homozygous for as2-7 (Col background) as well as for as2-1 (Ler background) for further analysis.
Table 1.
Complementation Analysis of Asymmetric Leaf Mutants
| Female Parent | Male Parent | F1 Progeny Phenotype |
|---|---|---|
| as1-1 | cs444 | as1 (19) |
| cs444 | as1-1 | as1 (12) |
| as1-1 | AW179 | as1 (12) |
| cs3240 | as1-1 | as1 (28) |
| cs3250 | as1-1 | as1 (28) |
| cs3240 | GH 4-0-0 | as1 (28) |
| GH 4-0-0 | cs3240 | as1 (17) |
| as2-1 | cs230 | as2 (21) |
| as2-1 | cs3381 | as2 (32) |
Number of plants scored is given in parentheses.
as1 and as2 Leaf Shape
First and fifth leaves of both as1 and as2 plants are obviously distinct from the wild type in shape and size, generally having smaller, more curled, and heart-shaped blades, shorter petioles from which asymmetric lobes emerge, and protrusions from the leaf blade that are larger and asymmetrically placed compared with wild-type serrations (Table 2). When seedlings are dissected to expose the SAM and young leaf primordia, no differences are evident in the size, shape, or spacing of the first two primordia in mutant plants compared with the wild type up to 5 d after germination (DAG; where transfer to growth chamber is 0 DAG) or for the fifth primordium up to 10 DAG (data not shown). By 6 DAG for the mutant first leaf and by 11 DAG for the fifth, both shape and cell differentiation are noticeably different than the wild type (Figures 1D, 1E, 1G, 1H, 2E, 2F, 2I, and 2J). Leaf primordia of both as1 and as2 plants curl inward, are smaller than wild-type leaves, wider at the distal than the proximal end of the blade, and show little petiole development. Moreover, some asymmetric protrusions are evident at the base of the leaf. At this stage, differentiation of margin cell files begins in the wild type, first visible adjacent to the distal leaf tip as two to three files of slightly larger and more elongate cells than the cells on either the adaxial or abaxial blade surface (Figures 1A, 1B, 2A, and 2B) and progressing proximally. Cells at the distal primordial tip remain small and unelongated and often include a large number of stomata and/or trichomes and associated basal cells (Figures 1A and 1B). We term this region the distal margin gap. In as1 and as2 leaves at this stage, margin cells are smaller and less elongated than in the wild type (Figures 1D, 1E, 1G, 1H, 2E, 2F, 2I, and 2J), and the distal margin gap is sometimes asymmetrically positioned at the leaf tip (Figures 1D, 2F, and 2I).
Table 2.
Morphology of First and Fifth Leaves
| Blade Length (mm) | Blade Width (mm) | Petiole Width (mm) | Petiole Length (mm) | Distance Widest to Base (mm) | Length/Width | Margin Protrusions | Petiole Lobes | |
|---|---|---|---|---|---|---|---|---|
| First Leaf | ||||||||
| Ler (30) | 6.25 ± 0.51 | 5.97 ± 0.38 | 0.93 ± 0.13 | 4.00 ± 0.83 | 2.90 ± 0.38 | 1.05 ± 0.07 | 0.00 ± 0.0 (25) | 0% (25) |
| +s (30) | 6.25 ± 0.79 | 5.96 ± 0.73 | 0.66 ± 0.15 | 5.03 ± 0.76 | 2.59 ± 0.52 | 1.05 ± 0.07 | 0.00 ± 0.0 (35) | 0% (35) |
| as1-1 (30) | 6.08 ± 1.02 | 8.06 ± 1.43a | 1.82 ± 0.47a | 2.13 ± 0.83a | 1.92 ± 0.50a | 0.76 ± 0.05a | 0.29 ± 0.5 (48)a | 4% (48) |
| as1-16 (30) | 3.42 ± 1.06b | 2.98 ± 0.86b | 0.63 ± 0.20b | 1.57 ± 0.56b | 1.19 ± 0.45b | 1.16 ± 0.18b | 0.19 ± 0.4 (32)b | 0% (32) |
| as2-1 (30) | 4.90 ± 0.71a | 4.28 ± 0.75a | 0.51 ± 0.18a | 3.69 ± 0.97 | 1.52 ± 0.42a | 1.15 ± 0.10a | 0.31 ± 0.5 (45)a | 1% (45) |
| as2-14 (30) | 3.83 ± 0.84b | 2.41 ± 0.52b | 0.37 ± 0.08b | 2.76 ± 0.94b | 1.55 ± 0.57b | 1.60 ± 0.18b | 0.24 ± 0.4 (41)b | 3% (41) |
| Fifth Leaf | ||||||||
| Ler (30) | 17.64 ± 3.36 | 11.05 ± 1.10 | 1.36 ± 0.29 | 6.89 ± 1.52 | 9.94 ± 2.61 | 1.60 ± 0.28 | 0.00 ± 0.0 (41) | 0% (41) |
| +s (30) | 18.95 ± 2.75 | 8.65 ± 1.25 | 1.30 ± 0.26 | 11.98 ± 3.12 | 10.31 ± 2.23 | 2.20 ± 0.21 | 6.03 ± 1.94 (30) | 0% (30) |
| as1-1 (30) | 8.21 ± 1.40a | 10.43 ± 1.65 | 1.62 ± 0.52a | 3.07 ± 1.09a | 2.33 ± 0.94a | 0.79 ± 0.10a | 0.69 ± 0.67 (45)a | 17% (45) |
| as1-16 (30) | 8.90 ± 1.63b | 8.62 ± 1.77 | 1.24 ± 0.44 | 3.69 ± 1.42b | 2.31 ± 1.38b | 1.05 ± 0.14b | 2.38 ± 1.00 (37)b | 15% (37) |
| as2-1 (30) | 11.12 ± 1.72a | 7.75 ± 1.26a | 1.06 ± 0.33a | 10.60 ± 2.05a | 3.74 ± 1.06a | 1.45 ± 0.20 | 3.15 ± 1.5 (39)a | 32% (39) |
| as2-14 (30) | 17.07 ± 3.07b | 8.25 ± 1.48 | 0.84 ± 0.19b | 13.91 ± 3.25 | 7.86 ± 2.07b | 2.08 ± 0.27b | 3.08 ± 1.31 (35)b | 10% (35) |
Values represent mean ± se, except in the case of lobes, where value is percentage of leaves with lobes. Number in parentheses represents number of organs scored.
Significantly different from Ler, P > 0.05.
Significantly different from Col, P > 0.05.
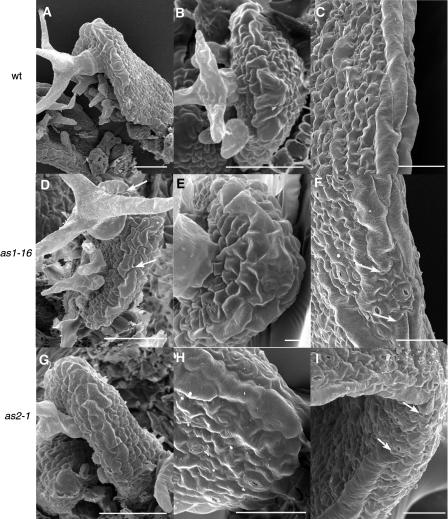
Figure 1.
Development of Wild-Type, as1-16, and as2-1 First Leaves.
(A) to (C) The wild type.
(D) to (F) as1-16.
(G) to (I) as2-1.
At 5 DAG, elongated margin cells differentiate basipetally, beginning adjacent to a group of nonelongated cells that remains at the distal leaf tip (the distal margin gap) in all genotypes ([A] and [B], [D] and [E], and [G] and [H]). In both as1 and as2 leaves, the distal margin gap may be asymmetrically placed (arrows in [D]). At 10 DAG, files of margin cells are continuous in the wild type (C) but show gaps in as1-16 and as2-1 (indicated by arrows in [F] and [I]). Scale bars = 50 μm.
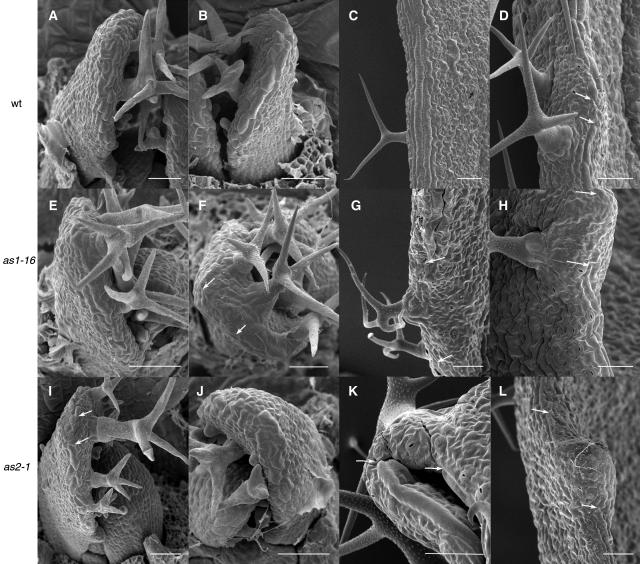
Figure 2.
Development of Wild-Type, as1-16, and as2-1 Fifth Leaves.
(A) to (D) The wild type.
(E) to (H) as1-16.
(I) to (L) as2-1.
At 11 DAG, elongated margin cells differentiate in all genotypes ([A], [B], [D], [E], [I], and [J]), beginning adjacent to the distal margin gap. In as1 and as2 leaves, the distal margin gap may be asymmetrically placed (arrows in [F] and [I]). At maturity, margin cells usually form continuous files in the wild type (C), although rarely a margin gap is seen (arrows in [D]). Margin gaps are common in as1-16 and as2-1 leaves (arrows in [G], [H], [K], and [L]). Scale bars = 50 μm.
Because of the proposed relationship amongst the leaf margin, leaf expansion, and abaxial/adaxial specification (Waites and Hudson, 1995; Ori et al., 2000), we assessed the integrity of the margin by examining mature leaf segments of as1 and as2 first and fifth leaves by SEM and in all found disruptions (Figures 1F, 1I, 2G, 2H, 2K, and 2L). In wild-type leaves (Col and Ler ecotypes), the margin consists of two to six files of linear and elongated cells that span the leaf edge at the abaxial/adaxial boundary (Figures 1C, 2C, and 2D). In first leaves, the margin is continuous except for the distal margin gap. In fifth leaves, the distal margin gap is also present (Figures 2A and 2B), and a margin gap involving a similar number of cells also occurs infrequently at the tips of leaf serrations (one gap observed in four leaves, Figure 2D). In all of as1 and as2 first leaves, margin gaps are seen in variable positions within the proximal half of the leaf and contain cell types similar to those seen on the abaxial leaf surface, often interspersed with stomata (Figures 1F and 1I). as1 and as2 fifth leaves show as many as four margin gaps per leaf (Figures 2G, 2H, 2K, and 2L). In as1 mutants, margin gaps are most common in the proximal portion of the leaf (as1-1, 86%, n = 14; as1-16, 85%, n = 13), whereas in as2 mutants, they are observed exclusively in the proximal portion of the leaf. Because such gaps have been found associated with lobe sinuses in other alleles (Ori et al., 2000), we assessed the position of margin gaps relative to lobes (Table 3). In all alleles except as1-1, gaps are most common at the lobe tip. Other differences in as1 and as2 margins compared with the wild type include margin cells that are not positioned at the leaf edge but rather are found adjacent to the margin in place of laminar epidermal cells and variable numbers of margin cell files, such that, in places, the margin consists of as many as 15 cell files (data not shown).
Table 3.
Position of Margin Gaps Relative to Lobes in as1 and as2 Fifth Leaves
| Number Scored
|
Position of Gap within Lobe
|
||||
|---|---|---|---|---|---|
| Genotype | n (Leaves) | n (Gaps) | Base | Middle | Tip |
| as-1 | 5 | 12 | 50% | 33% | 17% |
| as1-16 | 6 | 11 | 9% | 45% | 45% |
| as2-1 | 5 | 10 | 20% | 0% | 80% |
| as2-14 | 6 | 14 | 0% | 0% | 100% |
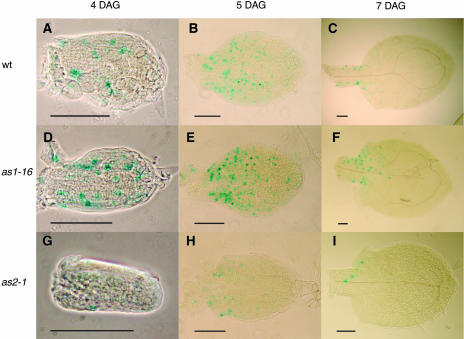
We looked at the patterns of cell division in the wild type, as1-16, and as2-1 first leaves using the cycB1:β-glucuronidase (GUS) reporter construct (Doerner et al., 1996) and found differences in the patterns of cell division in mutant leaves (Table 4, Figure 3). At 4 DAG, cycB1:GUS expression is similar in distal and proximal leaf halves and shows no significant difference between the wild type and as1-16 (Table 4, Figures 3A and 3D), but the number of cells expressing the reporter gene is reduced in both halves of as2-1 leaves (Table 4, Figure 3G). Consistent with basipetal decrease in cell division (Donnelly et al., 1999), at 5 DAG, cycB1:GUS expression is diminishing in the distal half of both wild-type and mutant leaves, and the number of cells expressing is again lower in as2-1 (Table 4, Figures 3B, 3E, and 3H). At 7 DAG, cycB1:GUS expression is virtually absent in the distal half of all leaves (Table 4, Figures 3C, 3F, and 3I). We next assessed asymmetrical expression of cycB1:GUS by determining cell divisions in four sections of the leaf: left and right sides of the midvein within both distal and proximal halves. Based on the number of cell divisions in each side, left and right sides of distal and proximal halves were categorized as high or low, and the mean high versus mean low number of cell divisions was compared by Student's t test. At some stages, the comparison between high and low showed significant differences in the wild type, presumably because these stages had a low number of cell divisions and relatively high standard error. In these cases, we discounted similar differences in the mutants because they presumably would not contribute to asymmetry. Based on this analysis, mutants showed significant difference between high and low sides in the proximal half at 7 DAG (Figures 3F and 3I). Whereas in the wild type, the number of cells expressing GUS in the wild type low proximal side and the high proximal sides was not significantly different (27.8 ± 14.7 versus 33.6 ± 16.7), in both as1-16 and as2-1, this difference was significant (27.1 ± 14.2 versus 50.2 ± 17.8 and 12.0 ± 8.0 versus 17.2 ± 9.2, respectively). Thus, at 7 DAG, the proximal half of mutant leaves shows distinct asymmetry in the number of dividing cells, consistent with the asymmetrical leaf lobes within the proximal leaf blade observed at leaf maturity.
Table 4.
Landmarks of Vascular Development and Cell Division Patterns in First Leaves
| Wild Type | as1-16 | as2-1 | |
|---|---|---|---|
| Day 3 | |||
| DR5:GUS in distal tip, asymmetric expression | 100%, 2% (22) | 100%, 48% (22) | 100%, 51% (27) |
| DR5:GUS in 2° veins | 68% (22) | 14% (22) | 11% (27) |
| DR5:GUS in 3° veins | 14% (22) | 0% (22) | 0% (27) |
| Day 4 | |||
| DR5:GUS in 2° veins | 91% (46) | 71% (28) | 87% (38) |
| DR5:GUS in 3° veins | 91% (46) | 21% (28) | 63% (38) |
| DR5:GUS in hydathodes (1) | 8% (35) | 0% (28) | 0% (38) |
| DR5:GUS in hydathodes (2) | 51% (35) | 0% (28) | 0% (38) |
| Midvein maturation | 28% (46) | 0% (28) | 0% (38) |
| cycB1:GUS in proximal half | 10.9 ± 6.2 | 9.0 ± 3.4 | 1.6 ± 1.2a |
| cycB1:GUS in distal half | 10.0 ± 4.8 | 10.7 ± 6.5 | 1.4 ± 1.5a |
| Day 5 | |||
| DR5:GUS in 3° veins | 100% (31) | 75% (38) | 81% (21) |
| DR5:GUS in hydathodes (1) | 13% (31) | 2% (40) | 18% (17) |
| DR5:GUS in hydathodes (2) | 81% (31) | 1% (40) | 59% (17) |
| DR5:GUS in hydathodes (3) | 0% (31) | 0% (19) | 6% (17) |
| Midvein maturation | 86% (22) | 10% (40) | 54% (22) |
| cycB1:GUS in proximal half | 57.9 ± 19.9 | 54.1 ± 25.6 | 10.4 ± 7.6a |
| cycB1:GUS in distal half | 24.7 ± 14.2 | 30.1 ± 20.2 | 2.8 ± 2.8a |
| Day 6 | |||
| DR5:GUS in hydathodes (1) | 2% (42) | 26% (27) | 18% (39) |
| DR5:GUS in hydathodes (2) | 86% (42) | 11% (27) | 44% (39) |
| DR5:GUS in hydathodes (3) | 7% (42) | 0% (27) | 33% (39) |
| DR5:GUS in hydathodes (4) | 12% (34) | 0% (27) | 3% (39) |
| Midvein maturation | 100% (41) | 78% (27) | 87% (39) |
| Day 7 | |||
| cycB1:GUS in proximal half | 61.4 ± 30.9 | 77.3 ± 27.2a | 29.6 ± 16.6a |
| cycB1:GUS in distal half | 2.2 ± 1.8 | 3.4 ± 2.9 | 1.1 ± 1.4a |
| Day 10 | |||
| DR5:GUS in hydathodes (1) | 16% (31) | 10% (41) | 33% (42) |
| DR5:GUS in hydathodes (2) | 74% (31) | 58% (41) | 5% (42) |
| DR5:GUS in hydathodes (3) | 6% (31) | 20% (41) | 0% (42) |
| DR5:GUS in hydathodes (4) | 3% (31) | 12% (41) | 0% (42) |
| DR5:GUS in veins | 3% (31) | 41% (41) | 43% (42) |
Values represent percentage of leaves showing the character, except for cycB1:GUS, where values represent mean number of cells expressing the reporter gene ± se. Number in parentheses represents number of organs scored.
Significantly different from the wild type.
Figure 3.
CycB1:GUS Expression in Developing First Leaves of the Wild Type and as1-16 and as2-1 Viewed with Phase Contrast or Bright-Field Optics.
(A) to (C) The wild type.
(D) to (F) as1-16.
(G) to (I) as2-1.
(A), (D), and (G) Phase contrast images.
(B), (C), (E), (F), (H), and (I) Bright-field images.
CycB1:GUS expression is similar in the wild type and as1-16 at 4 DAG ([A] and [D]) but is expressed in fewer cells in as2-1 (G). At 5 DAG, cycB1:GUS expression is lower in the distal half compared with the proximal half of all leaves ([B], [E], and [H]), with fewer cells expressing cycB1:GUS in as2-1 leaves. At 7 DAG, very few cells express cycB1:GUS in leaf distal halves, and in the proximal half, asymmetric expression is seen on either side of the midvein in as1-16 and as2-1 ([F] and [I]). Scale bars = 0.1 mm.
as1 and as2 Venation Pattern
As described by other studies (Semiarti et al., 2001), venation pattern is simpler through the as1 and as2 leaf blades but shows increased numbers of veins within the petiole (Table 5). It is possible that the simplicity of the vascular pattern indicates delayed vascular development in as1 and as2 leaves, such that full maturity is never reached. In fact, although landmarks of vascular differentiation are reached somewhat later than in the wild type (Table 4), all events seem to occur, indicating that the as1 and as2 leaves are fully mature.
Table 5.
Vein Patterning in First and Fifth Leaves
| Number of Veins in Petiole | Number Secondary from Midvein | Vein Count across Width | Veins/mm Length | Veins/mm Width | |
|---|---|---|---|---|---|
| First Leaf | |||||
| Ler (30) | 1.03 ± 0.18 | 7.24 ± 0.83 | 9.93 ± 1.17 | 0.87 ± 0.16 | 1.66 ± 0.20 |
| +s (30) | 1.00 ± 0.00 | 6.70 ± 0.99 | 9.20 ± 1.49 | 0.95 ± 0.16 | 1.55 ± 0.20 |
| as1-1 (30) | 3.03 ± 1.07a | 4.40 ± 0.67a | 12.13 ± 2.27a | 1.41 ± 0.31a | 1.52 ± 0.20a |
| as1-16 (30) | 2.50 ± 0.73b | 4.20 ± 1.27b | 8.40 ± 2.58 | 0.84 ± 0.21b | 2.91 ± 0.76b |
| as2-1 (30) | 2.27 ± 0.74a | 4.27 ± 0.64a | 7.87 ± 1.41a | 1.17 ± 0.23a | 1.88 ± 0.40a |
| as2-14 (30) | 1.10 ± 0.40 | 4.33 ± 0.92b | 5.77 ± 1.81b | 0.91 ± 0.23 | 2.42 ± 0.64b |
| Fifth Leaf | |||||
| Ler (30) | 4.23 ± 0.94 | 12.30 ± 2.55 | 19.70 ± 2.94 | 1.47 ± 0.33 | 1.80 ± 0.30 |
| +s (30) | 4.87 ± 1.01 | 11.60 ± 1.38 | 21.20 ± 2.83 | 1.66 ± 0.32 | 2.47 ± 0.28 |
| as1-1 (30) | 4.33 ± 1.27 | 6.03 ± 1.83a | 20.37 ± 3.75 | 1.45 ± 0.39a | 1.97 ± 0.33a |
| as1-16 (30) | 6.23 ± 1.41b | 4.97 ± 0.93b | 17.07 ± 3.60b | 1.84 ± 0.40 | 2.01 ± 0.38b |
| as2-1 (30) | 6.03 ± 1.07a | 6.47 ± 1.07a | 16.17 ± 2.68a | 1.78 ± 0.50a | 2.11 ± 0.34a |
| as2-14 (30) | 3.90 ± 0.96b | 8.53 ± 1.25b | 15.00 ± 3.17b | 2.04 ± 0.47b | 1.83 ± 0.32b |
Values represent mean ± se. Number in parentheses represents number of organs scored.
Significantly different from Ler, P > 0.05.
Significantly different from Col, P > 0.05.
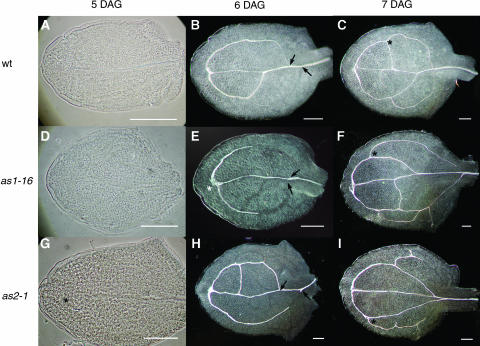
To determine if the sequence and direction of vascular pattern formation was similar to the wild type, we examined development of vascular pattern in as1 and as2 first leaves (Table 4, Figure 4). The differentiating midvein in mutant first leaves was visible in essentially the normal position and normal acropetal direction, although it occurred slightly later (6 DAG) than in the wild type (5 DAG; Figure 4A). Moreover, midvein differentiation frequently terminated before the leaf apex or veered away from the leaf tip (Figures 4E and 4G). As in the wild type, distal secondary veins were initiated at the apical end of the midvein (Figures 4A and 4D), although more frequently than in the wild type, one secondary vein was initiated later than the other or was initiated below the distal tip of the midvein (Figures 4E and 4G). The aberrant differentiation of the midvein and asymmetric initiation of secondary veins often correlated with an asymmetrically placed margin gap. The distal secondary veins rejoined the midvein at a more proximal point of the midvein than in the wild type, resulting in larger distal secondary loops (Figures 4E, 4F, 4H, and 4I). These large loops were often divided by subsequent intercalary formation of secondary veins (Figures 4E, 4F, 4H, and 4I). Differentiation of secondary veins, as indicated by xylem thickenings, occurred basipetally in both the wild type and mutants (Figures 4B, 4E, and 4H). Initiation of proximal secondary veins, which occurs from the distal secondaries at about the midpoint of the leaf blade in the wild type (Figures 4B and 4C), occurs at a more distal point in the mutant leaves (Figures 4E, 4F, 4H, and 4I).
Figure 4.
Vascular Pattern Development in the Wild Type, as1-16, and as2-1 Viewed with Phase Contrast or Dark-Field Optics.
(A), (F), and (G) The wild type
(B), (E), and (H) as1-16.
(C), (F), and (I) as2-1.
(A), (D), and (G) Phase contrast images.
(B), (C), (E), (F), (H), and (I) Dark-field images.
At 5 DAG, the xylem of the midvein is differentiating in the wild type (A) but not in the mutants ([D] and [G]). Distal secondary veins are initiated from the wild type 5 DAG ([A], [D], and [G]) but may be asymmetrically placed in the mutant (asterisks in [G] and [E]). Distal secondary maturation is evident by 6 DAG ([B], [E], and [H]), with the proximal point of secondary joining to the midvein lower in mutants (arrows in [E] and [H]) than in the wild type (arrows in [B]). Proximal secondaries are differentiating by day 7 and are often initiated at a more distal position in mutant leaves (asterisks in [F] and [I]) than in the wild type (asterisk in [C]).
Auxin Response in as1 and as2 Leaves
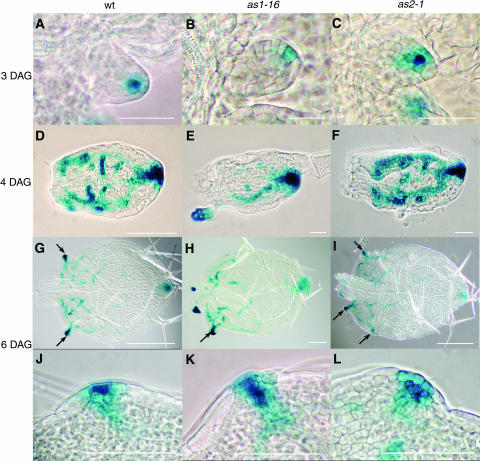
Reasoning that the altered venation pattern in as1 and as2 might be predicted by an altered auxin response pattern, we crossed DR5:GUS into the as1-16 and as2-1 background. The DR5:GUS construct links the reporter gene to a synthetic auxin inducible enhancer, so that reporter gene expression is an indicator of high auxin response (Ulmasov et al., 1997). In wild-type first leaves, DR5:GUS appears 3 DAG in a single cell at the leaf distal tip (Table 4, Figure 5A). In mutant first leaves, DR5:GUS is evident with similar timing. However, the position is often asymmetric relative to the leaf, shifted to one side of the distal tip (Table 4, Figures 5B and 5C). Subsequent DR5:GUS expression in mutant leaf blades is slightly later than in the wild type (Table 4), and no differences in pattern are evident, although expression within the blade at these stages is very transient, so that pattern differences would be difficult to detect (Figures 5D to 5F). At this stage, distal tip expression coincides with the distal margin gap, and in mutant leaves, both sometimes appear asymmetrically placed (Figure 5E). Asymmetric DR5:GUS expression is especially evident when expression begins in hydathodes (Table 4). In wild-type first leaves, hydathode expression usually appears in two points at equivalent positions along the leaf margin (Figure 5G). By contrast, expression in both as1-16 and as2-1 may begin at just one point and be followed by expression in another one or two, often unequally positioned points in as2-1 leaves (Figures 5H and 5I). Expression of DR5:GUS at this stage coincides with margin gaps (Figures 5J to 5L), and the margin gaps seem to contain more cells in the mutants than the wild type (cf. Figures 5K and 5L to 5J).
Figure 5.
DR5:GUS Expression in Developing First Leaves of the Wild Type, as1-16, and as2-1 Viewed with Phase Contrast Optics.
(A), (D), (G), and (J) The wild type.
(B), (E), (H), and (K) as1-16.
(C), (F), (I), and (L) as2-1.
At 3 DAG, DR5:GUS expression is visible in the distal tip of wild-type leaves (A), but this expression is often asymmetrically placed in as1-16 (B) and as2-1 (C) leaves. By 4 DAG, expression of DR5:GUS within the mutant leaf blades ([E] and [F]) is usually indistinguishable from the wild type (D), although asymmetries may exist within the blade or more frequently at the distal tip (E). At 6 DAG, DR5:GUS is expressed strongly in hydathodes (arrows), usually at opposite points along the leaf blade in the wild type (G), but often asymmetrically placed in as1-16 (H) and as2-1 (I). At high magnification, hydathodes show a larger number of small cells expressing GUS in as1-16 (K) and as2-1 (L) than in the wild type (J). Scale bars = 0.1 mm in (A) to (C) and (J) to (L), 0.2 mm in (D) to (F), and 0.25 mm in (G) to (I).
Response of as1 and as2 Phenotypes to Exogenous Auxin and Auxin Transport Inhibitor
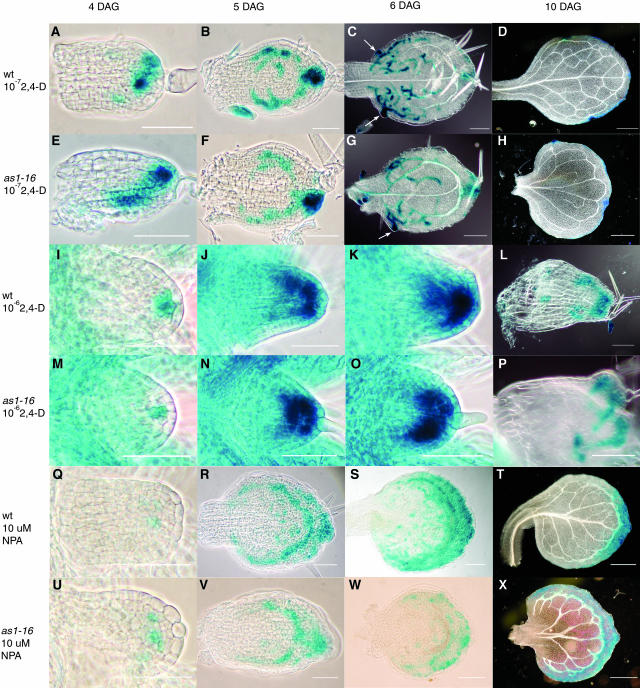
The correlation of early asymmetric auxin response with later asymmetric leaf morphology suggests that altered leaf morphology may result from altered auxin response. If this notion is correct, one might expect that altering auxin response in mutant leaves could alter the mutant phenotype. We therefore treated developing wild-type and mutant leaves with either the synthetic auxin 2,4-D or the auxin transport inhibitor naphthylphthalamic acid (NPA; Sigma-Aldrich). We then compared the pattern of auxin response (DR5:GUS expression), leaf shape, and leaf venation in developing mutant and wild-type leaves (Figure 6, data not shown for as2-1). Treatment of developing mutant and wild-type leaves with 10−7 M 2,4-D results in an auxin response that appears somewhat more intense and more widespread than in untreated leaves (cf. Figures 6A to 6H with 5A to 5L). Nevertheless, the mature leaf shape and vascular pattern of treated leaves (Figures 6D and 6H) appears unchanged compared with untreated leaves. By contrast, treatment with 10−6 M 2,4-D, 10 μM NPA, or 30 μM NPA results in drastically altered auxin response and leaf development in both wild-type and mutant leaves such that mutant leaves are indistinguishable from the wild type. In both mutant and wild-type leaves treated with 10−6 M 2,4-D, the distal tip auxin maximum is visible slightly later and initially occupies several cells (Figures 6I and 6M) rather than the single cell typical of untreated leaves (Figures 5A and 5B). By 5 DAG, expression increases to occupy approximately half the developing primordium (Figures 6J and 6N). The increased size of the distal maximum makes it difficult to compare the symmetry to that in untreated leaves; however, we are unable to detect any difference in position of the larger distal maximum in the treated wild type compared with treated mutants. Moreover, in contrast with untreated leaves, no vascular differentiation is visible by 6 DAG in treated leaves (Figures 6K and 6O), and by 10 DAG treated leaves usually remain small with little midvein differentiation (Figures 6L and 6P). Similarly, mutant and wild-type leaves treated with 10 or 30 μM NPA were indistinguishable (data not shown for 30 μM). Initial expression of DR5:GUS in 4 DAG treated wild-type and mutant primordia show no clear distal maximum, but rather a less intense expression in several cells across the apex (Figures 6Q and 6U). Because of the variable number and position of cells showing DR5:GUS expression, we were unable to compare the symmetry to that in untreated leaves. However, there is no apparent difference in symmetric position of expressing cells in the treated wild type compared with treated mutant primordia. As development proceeds in both treated wild-type and mutant leaves, DR5:GUS is expressed in two loops of cells (Figures 6R, 6S, 6V, and 6W), the inner loop coinciding later with the formation of vascular tissue (Figures 6S and 6W). In contrast with untreated leaves, in NPA-treated wild-type and mutant leaves, DR5:GUS expression never resolves to the hydathodes, but rather is seen in a broad band of cells between the leaf margin and the vascular tissue (Figures 6S, 6T, 6W, and 6X). No differences in symmetry of leaf shape or vascular pattern are evident between wild-type and mutant leaves at 10 DAG; the only difference is a simpler venation pattern in mutant leaves (Figures 6T and 6X).
Figure 6.
DR5:GUS Expression in Developing Leaves of the Wild Type and as1-16 Treated with 10−7 M 2,4-D, 10−6 2,4-D, and 10 μM NPA Viewed with Phase Contrast Optics or Differential Interference Contrast Optics.
(A) to (D), (I) to (L), and (Q) to (T) The wild type.
(E) to (H), (M) to (P), (U) to (X) as1-16.
(A) to (H) Treated with 10−7 M 2,4-D.
(I) to (P) Treated with 10−6 2,4-D.
(Q) to (X) Treated with 10 μM NPA.
(A), (B), (E), (F), (I), (K), (M), (O), (Q), (S), (U), and (W) Phase contrast images.
(C), (D), (G), (H), (L), (P), (T), and (X) Differential interference contrast images.
At 3 DAG, leaves treated with 10−7 M 2,4-D show DR5:GUS expression in the distal tip, which is somewhat more intense than in untreated leaves, and symmetrically placed in wild-type leaves (A), but is often asymmetrically placed in as1-16 (E) leaves. By 5 DAG, expression of DR5:GUS within the treated mutant leaf blades (F) is usually indistinguishable from the wild type (B). At 6 DAG, DR5:GUS is expressed strongly in hydathodes (arrows), usually at opposite points along the leaf blade in the wild type (C) but often asymmetrically placed in as1-16 (G). At 10 DAG, 10−7 M 2,4-D–treated wild-type leaves show symmetrical shape and vascular pattern (D), whereas in as1-16, shape and vascular pattern are often asymmetric (H). At 4 DAG, leaves treated with 10−6 M 2,4-D of both the wild type (I) and as1-16 (M) express DR5:GUS in a distal maximum containing more cells than that in untreated leaves. At 5 and 6 DAG in the wild type ([J] and [K]) and as1-16 ([O] and [P]), distal maximum expression increased both in intensity and number of cells. At 10 DAG, both wild-type (L) and as1-16 (P) leaves are small with little vascular development. At 4 DAG, leaves treated with 10 μM NPA of both the wild type (Q) and as1-16 (U) express DR5:GUS at a low level in a group of often disconnected cells across the distal region of the primordium. At 5 and 6 DAG in the wild type ([R] and [S]) and as1-16 ([V] and [W]), DR5:GUS is expressed in two loops of cells; the inner loop at 6 DAG is coincident with developing vascular tissue. At 10 DAG, both wild-type (T) and as1-16 (X) leaves have a loop of vascular tissue adjacent to the leaf margin with veins extending from the margin into the leaf interior. Scale bars = 0.05 mm in (A), (B), (E), (F), (I), (K), (M), (O), (Q), (R), (U), and (V), 0.1 mm in (C), (G), (P), (S), and (W), and 0.5 mm in (D), (H), (T), and (X).
DISCUSSION
We have analyzed several defects in developing as1 and as2 leaves, including vascular pattern, leaf shape, leaf margin defects, cell division patterns, and auxin response. Early in development of wild-type leaves, an auxin response maximum is located symmetrically within the distal leaf tip. We found that the earliest visible defect in as1 and as2 leaves is the asymmetric placement of this auxin response maximum. Subsequent auxin response pattern is also asymmetric. Furthermore, cell division patterns are shown to be asymmetric in the mutants compared with the wild type. Finally, when a more symmetric auxin response is induced in mutant leaves through treatment with either exogenous auxin or auxin transport inhibitor, a more symmetric leaf morphology and venation pattern results. We suggest that the early, distal tip asymmetry may lead to subsequent asymmetries in the auxin response pattern and hence generate an asymmetric vein pattern. We further propose that the asymmetric auxin distribution in as1 and as2 leaves affects cell division patterns and hence generates asymmetric leaf shape. This suggests a mechanism whereby cell differentiation patterns, including vascular pattern, and cell divisions may be coordinated.
Asymmetric Auxin Response Predicts Asymmetric Vascular Pattern
The proposal that auxin directs the formation of vascular pattern within leaves is supported by many studies (Mattsson et al., 1999, 2003; Sieburth, 1999; Aloni et al., 2003; Steynen and Schultz, 2003). Moreover, it has been suggested that three sequential sources of auxin are responsible for sequential elements of the vein pattern: (1) auxin from an external source, likely the cotyledons, directs the midvein, (2) auxin from the leaf margin directs secondary veins, and (3) auxin from sources within the leaf lamina directs tertiary and quaternary veins (Aloni et al., 2003; Steynen and Schultz, 2003). PIN1 has recently been proposed to transport external auxin through the epidermal cells to the tip of the incipient organ primordium, generating an auxin response maximum. Auxin is then transported basipetally through the center of the primordium anticipating the position of the midvein (Benkova et al., 2003; Reinhardt et al., 2003). Our analysis of DR5:GUS expression in as1-16 and as2-1 leaves indicates that all three sources exist and direct the appropriate veins in the normal sequence and direction. However, differences in the auxin response pattern are evident and may account for the differences in vascular pattern. In wild-type leaves, the auxin response maximum is visible at the distal tip at 3 DAG. In as1-16 and as2-1 leaves, this point of auxin response is often displaced to one side of the distal leaf tip. Subsequent development of the midvein may be displaced in the distal region, supporting the idea that the auxin maximum defines the distal position of the midvein. Initiation of secondary veins is often asymmetric and seems to correspond to the placement of the distal point of auxin response. Finally, wild-type and as leaves treated with 2,4-D or NPA show indistinguishable position of the distal tip maximum and subsequent vein pattern.
Altered placement of secondary veins in as1 and as2 leaves suggests that the second, marginal auxin source may be altered. However, changes in symmetry of auxin distribution from the marginal source to the leaf lamina are difficult to assess using DR5:GUS expression because auxin response is quite transient in cells that will become vascular tissue. Later in leaf development a less transient response to marginal auxin becomes concentrated to distinct points, the hydathodes, along the leaf margin. In wild-type leaves, there are usually two points symmetrically positioned along the leaf margin, whereas in as1-16 and as2-1 leaves, the number of points varies, and their position is often asymmetric. When wild-type or as leaves are treated with 2,4-D or NPA, both marginal and later hydathode DR5:GUS expression are altered to produce phenotypes indistinguishable from one another. Subsequently, symmetry of secondary and higher order vein patterns are also indistinguishable. Thus, our results suggest strong correlations firstly between asymmetries of different auxin responses and secondly between asymmetries of auxin response and vascular pattern. When the auxin response maxima resulting from both the primary, external auxin source is asymmetrically positioned, subsequent secondary, marginal sources are also asymmetrically positioned. Although it is possible that the two are independently controlled, we suggest that the first asymmetry results in the second. Such coordination would allow the generation of an integrated vascular pattern even if the distribution of auxin driving the pattern is altered.
Ectopic Expression of KNAT Genes Results in Aberrant Regulation of Growth Regulators
Within the lateral primordia, AS1 and AS2 are known to downregulate the KNAT genes. It is not clear if the absence of this downregulation is responsible for the as1 and as2 phenotypes; losing KNAT gene expression does not eliminate the mutant phenotype, suggesting either significant redundancy in KNAT gene action or another role for AS1 and AS2 (Byrne et al., 2002). Moreover, there is significant interplay between the KNAT genes and plant growth regulators. Polar auxin transport, which is necessary for appropriate positioning and outgrowth of leaves (Reinhardt et al., 2000, 2003; Benkova et al., 2003), is also necessary for downregulation of the KNAT genes (Scanlon, 2003). Conversely, polar auxin transport is defective if KNOX genes are not properly downregulated (Schneeberger et al., 1998; Tsiantis et al., 1999a). This suggests a possible regulatory loop whereby polar auxin transport defines leaf position, in part by downregulating KNOX genes. In turn, this establishes appropriate polar auxin transport, perhaps reinforcing primordial position and/or influencing the auxin gradient.
In this study, we have shown that leaves mutant for AS1 or AS2 also show altered positions of auxin response, suggesting either that the auxin source is altered or that auxin transport from source to sink is altered. Changes to the auxin source could result from as1 and as2 defects in the previously formed primordia (in the case of the first leaf, the cotyledons). Alternatively, changes in auxin transport could be the result of as1 and as2 induced changes within the leaf primordia, such as KNAT gene misexpression. Although we do not know how misexpression of KNAT genes might lead to altered auxin transport, one possibility is that downregulation of KNAT genes predisposes cells to transport auxin, such that ectopic KNAT expression disrupts routes of auxin transport. Consistent with this idea, downregulation of KN1 is a characteristic of vein initiation (Smith et al., 1992; Schneeberger et al., 1998), and the simplification of vein pattern in rs2 mutants is proposed to be from prolonged expression of KNOTTED-like genes that disrupts auxin levels (Schneeberger et al., 1998). A second possibility is that as1 and as2 induced KNAT misexpression changes cell division patterns, and this results in a more random decision as to which cells will transport auxin in the leaf.
Asymmetric Auxin Response Precedes Asymmetric Cell Division Patterns
We propose that the symmetrical distribution of auxin, firstly from the distal leaf tip and subsequently from the leaf margin, is a requirement for symmetrical leaf expansion by inducing symmetrical cell division patterns. Auxin gradients in young leaves (10 to 20% expanded) result in high auxin at the base and low auxin at the tip (Chen et al., 2001; Benkova et al., 2003); the gradient has been proposed to regulate cell division and elongation patterns (Chen et al., 2001), primordial outgrowth (Reinhardt et al., 2000, 2003; Stieger et al., 2002), and cell differentiation patterns (Tsiantis et al., 1999a). Our data support the notion that the gradient is critical to both cell division and cell differentiation because leaf primordia that are exposed to high levels of 2,4-D show higher and more widespread DR5:GUS expression and also lack blade expansion and vascular cell differentiation. There are clear links between auxin and the control of cell division (den Boer and Murray, 2000; Stals and Inzé, 2001). The stimulation of G1 to S transition that is the first step in forming lateral roots is known to be regulated by auxin (Reed et al., 1998; Casimiro et al., 2001; Bhalerao et al., 2002; Himanen et al., 2002). In tobacco (Nicotiana tabacum) leaves, high auxin levels are correlated with high levels of cell division, whereas low auxin levels are correlated with cell elongation (Chen et al., 2001). Plants mutant for AXR1 have smaller leaves as a result of reduced numbers of cell divisions (Lincoln et al., 1990). Recently, the gene ARGOS has been proposed to act downstream of AXR1 to control cell division in lateral organs in response to auxin (Hu et al., 2003). Asymmetric induction of cell division can cause asymmetric growth because localized induction of cell cycle regulators on the flanks of young tobacco leaf primordia results in increased cell division and ultimately asymmetry of the mature leaf (Wyrzykowska et al., 2002). We propose that the asymmetric auxin response seen early in as1 and as2 leaf development is coupled to the later asymmetry in cell division seen within the proximal leaf half.
Our data support the idea that symmetric response to auxin along the leaf margin is critical to symmetric cell division patterns and leaf expansion. Studies on leaf vascular pattern indicate that the leaf margin is a source of auxin (Mattsson et al., 1999, 2003; Sieburth, 1999; Aloni et al., 2003). Consistent with the requirement of the leaf margin for leaf blade expansion (Waites and Hudson, 1995; Waites et al., 1998; Sawa et al., 1999; Lin et al., 2003; Eshed et al., 2004), we suggest that the auxin produced by the leaf margin also controls cell division and blade expansion. In wild-type first-leaf margins, cells at the distal leaf tip and at two symmetrically placed points along the leaf edge do not differentiate as elongated margin cells and also show prolonged auxin response. In as mutant leaves, these groups of cells at the distal leaf tip are often asymmetrically placed, whereas those along the leaf edge are much larger than in the wild type and are usually asymmetrically placed. Those along the leaf edge are most often at the tips of leaf lobes. We propose that the asymmetric placement of these cells along the leaf edge and/or their larger size results in an asymmetric auxin gradient within as mutant leaves. This asymmetry in auxin gradient results in asymmetry in cell division and, hence, asymmetric leaf outgrowth.
The pattern of response to auxin, which is initially transient throughout the margin and subsequently prolonged at defined points along the margin, suggests a possible mechanism whereby the marginal auxin and its symmetrical and basipetal response may be regulated. Margin cells differentiate first near the leaf apex, and subsequent differentiation is basipetal along the leaf circumference. If, once fully differentiated, margin cells no longer serve as a source of auxin, auxin response would diminish basipetally. This is consistent with both the basipetal reduction in cell division and the basipetal development of leaf vascular pattern, two processes proposed to occur in response to auxin.
Symmetrical Auxin Distribution Coordinates Symmetrical Cell Differentiation and Division Patterns
Our proposal whereby symmetrical auxin distribution within the developing leaf controls both vascular pattern and cell division pattern suggests a compelling model whereby processes of differentiation and division might be coordinated. First, an auxin gradient with response maximum at the leaf tip provides positional information for midvein development. Second, auxin produced basipetally along the margin and then resolving into response maxima symmetrically positioned along the margin provides positional information for development of higher order veins and ensures that cell division occurs in a symmetrical fashion. The symmetrical auxin response in wild-type leaves allows a coordinated and symmetrical differentiation pattern and blade expansion. By contrast, if, as in as1 and as2 mutants, the auxin response maxima are not symmetrically positioned, cell differentiation and cell division patterns are disrupted, resulting in abnormal venation patterns and leaf outgrowth, which are nevertheless coordinated to generate an integrated pattern.
METHODS
Plant Material and Growth Conditions
Mutant lines cs146 (as1-1), cs444 (as1-14), cs3240 (as1-16), cs3250 (as1-17), cs3117 (as2-1), cs3118 (as2-1), cs230 (as2-13), and cs3381 (as2-14) were obtained from the Arabidopsis Biological Resource Centre (Columbus, OH); line GH4-0-0 is from an ethyl methanesulfonate–mutagenized Col-1 population obtained from G. Haughn (University of British Columbia); line AW179 is from a γ-irradiated Ler population from A. Wilson (John Innes Centre). The cycB1:GUS line was kindly provided by P. Doerner (University of Edinburgh) and the DR5:GUS line by J. Murfett (University of Missouri, Columbia, MO). To generate AS1 and AS2 mutants expressing the cycB1:GUS or DR5:GUS fusion, as1-16 and as2-1 were crossed to the respective expression line. Plants showing the as mutant phenotype in the F2 populations were screened for cycB1:GUS expression in root tips or DR5:GUS expression in leaves. F3 seed was collected from those showing expression, and lines expressing GUS in all F3 plants were used for subsequent analysis. Plants for analysis of mature leaves were grown on soil, and seedlings for analysis of developing leaves were grown on media unsupplemented or supplemented with 10−6 M 2,4-D, 10−7 M 2,4-D, 10 μM NPA, or 30 μM NPA (Sigma-Aldrich, St. Louis, MO) as described by Steynen and Schultz (2003).
Microscopy and Analysis
Primordial development and differentiation of epidermal cells of first and fifth leaves of as1-1, as1-16, as2-1, and as2-14 mutants and Col and Ler wild-type plants were examined by scanning electron microscopy, with specimens prepared as described by Steynen et al. (2001). Seedlings used to examine first leaf development were collected at 12-h intervals between 2.5 and 4 DAG and at 24 h intervals between 4 and 11 DAG. Plants used to examine fifth leaf development were collected at 24-h intervals between 10 and 13 DAG. Approximately 10 to 15 plants were examined for each genotype at each developmental stage. For each genotype, the entire circumferences of five to six fully expanded first and fifth leaves were also examined for margin defects by scanning electron microscopy; first leaves were collected when the inflorescence bolt reached a height of 1 cm and fifth leaves when the first silique began to elongate. Development of leaf vascular pattern was examined in 20 seedlings of each genotype at 24-h intervals from 2 to 11 DAG and cleared as described by Steynen and Schultz (2003). For analysis of mature leaf shape and vascular pattern, first leaves were gathered from the plants when the inflorescence bolt was at least 1 cm and fifth leaves when the first silique was elongated. Leaves were fixed and images taken and analyzed as described by Steynen and Schultz (2003). For analysis of DR5:GUS and cycB1:GUS expression, 20 seedlings were taken from day 3 to day 7, and GUS staining and leaf clearing was done as described by Steynen and Schultz (2003).
Acknowledgments
Seed was kindly provided by Peter Doerner, George Haughn, Jane Murfet, Alison Wilson, and the Arabidopsis Biological Resource Centre. We thank John Bain, George Haughn, Shelley Hepworth, Ljerka Kunst, and members of the Schultz lab for insightful comments on the manuscript. This work was supported through Natural Sciences and Engineering Research Council of Canada Summer Undergraduate Research Awards to J.M.Z. and D.A.B. and a Natural Sciences and Engineering Research Council of Canada Operating Grant to E.A.S.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Elizabeth A. Schultz (schultz@uleth.ca).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.104.026898.
References
- Aloni, R., Schwalm, K., Langhans, M., and Ullrich, C.I. (2003). Gradual shifts in sites of free-auxin production during leaf-primordium development and their role in vascular differentiation and leaf morphogenesis in Arabidopsis. Planta 216, 841–853. [DOI] [PubMed] [Google Scholar]
- Barton, M.K. (2001). Leaving the meristem behind: Regulation of KNOX genes. Genome Biology 2, 1002.1–1002.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkova, E., Michniewicz, M., Sauer, M., Teichmann, T., Seifertova, D., Jurgens, G., and Friml, J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602. [DOI] [PubMed] [Google Scholar]
- Bhalerao, R.P., Eklof, J., Ljung, K., Marchant, A., Bennett, M., and Sandberg, G. (2002). Shoot derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J. 29, 325–332. [DOI] [PubMed] [Google Scholar]
- Bohmert, K., Camus, I., Bellini, C., Bouchez, D., Caboche, M., and Benning, C. (1998). AGO1 defines a novel locus of Arabidopsis controlling leaf development. EMBO J. 17, 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne, M.E., Barley, R., Curtis, M., Arroyo, J.M., Dunham, M., Hudson, A., and Martienssen, R.A. (2000). ASYMMETRIC LEAVES1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 408, 967–971. [DOI] [PubMed] [Google Scholar]
- Byrne, M.E., Simorowski, J., and Martienssen, R.A. (2002). ASYMMETRIC LEAVES1 reveals knox gene redundancy in Arabidopsis. Development 129, 1957–1965. [DOI] [PubMed] [Google Scholar]
- Casimiro, I., Marchant, A., Bhalerao, R.P., Beeckman, T., Dhooge, S., Swarup, R., Graham, N., Inze, D., Sandberg, G., Casero, P.J., and Bennett, M. (2001). Auxin transport promotes lateral root initiation. Plant Cell 13, 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J.-G., Shimomura, S., Sitbon, F., Sandberg, G., and Jones, A.M. (2001). The role of auxin-binding protein 1 in the expansion of tobacco leaf cells. Plant J. 28, 607–617. [DOI] [PubMed] [Google Scholar]
- den Boer, B.G.W., and Murray, J.A.H. (2000). Triggering the cell cycle in plants. Trends Cell Biol. 10, 245–250. [DOI] [PubMed] [Google Scholar]
- Dengler, N., and Kang, J. (2001). Vascular patterning and leaf shape. Curr. Opin. Plant Biol. 4, 50–56. [DOI] [PubMed] [Google Scholar]
- Doerner, P., Jorgensen, J.E., You, R., Steppuhn, J., and Lamb, C. (1996). Control of root growth and development by cyclin expression. Nature 380, 520–523. [DOI] [PubMed] [Google Scholar]
- Donnelly, P.M., Bonetta, D., Tsukaya, H., Dengler, R.E., and Dengler, D.G. (1999). Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev. Biol. 215, 407–419. [DOI] [PubMed] [Google Scholar]
- Douglas, S.J., Chuck, G., Dengler, R.E., Pelecanda, L., and Riggs, C.D. (2002). KNAT1 and ERECTA regulate inflorescence architecture in Arabidopsis. Plant Cell 14, 547–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery, J.F., Floyd, S.K., Alvarez, J., Eshed, Y., Hawker, N.P., Izhaki, A., Baum, S.F., and Bowman, J.L. (2003). Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr. Biol. 13, 1768–1774. [DOI] [PubMed] [Google Scholar]
- Eshed, Y., Baum, S.F., and Bowman, J.L. (1999). Distinct mechanisms promote polarity establishment in carpels of Arabidopsis. Cell 99, 199–209. [DOI] [PubMed] [Google Scholar]
- Eshed, Y., Izhaki, A., Baum, S.F., Floyd, S.K., and Bowman, J.L. (2004). Asymmetric leaf development and blade expansion in Arabidopsis are mediated by KANADI and YABBY activities. Development 131, 2997–3006. [DOI] [PubMed] [Google Scholar]
- Frank, M., Rupp, H.-M., Prinsen, E., Motyka, V., Van Onckelen, H., and Schümulling, T. (2000). Hormone autotrophic growth and differentiation identifies mutant lines of Arabidopsis with altered cytokinin and auxin content or signaling. Plant Physiol. 122, 721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml, J. (2003). Auxin transport—Shaping the plant. Curr. Opin. Plant Biol. 6, 7–12. [DOI] [PubMed] [Google Scholar]
- Hay, A., Kaur, H., Phillips, A., Hedden, P., Hake, S., and Tsiantis, M. (2002). The gibberellin pathway mediates KNOTTED1-type homeobox function in plants with different body plans. Curr. Biol. 12, 1557–1586. [DOI] [PubMed] [Google Scholar]
- Himanen, K., Boucheron, E., Vanneste, S., de Almeida Engler, J., Inze, D., and Beeckman, T. (2002). Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14, 2339–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Y., Xie, Q., and Chua, N.-H. (2003). The Arabidopsis auxin-inducible gene ARGOS controls lateral organ size. Plant Cell 15, 1951–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, W.-C., Shuai, B., and Springer, P.S. (2003). The Arabidopsis LATERAL ORGAN BOUNDARIES-domain gene AWYMMETRIC LEAVES2 functions in the repression of KNOX gene expression and in adaxial-abaxial patterning. Plant Cell 15, 2241–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln, C., Britton, J.H., and Estelle, M. (1990). Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 2, 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn, K., Fernandez, A., Aida, M., Sedbrook, J., Tasaka, M., Masson, P., and Barton, M.K. (1999). The PINHEAD/ZWILLE gene acts pleiotropically in Arabidopsis development and has overlapping functions with the ARGONAUTE1 gene. Development 126, 1–13. [DOI] [PubMed] [Google Scholar]
- Mattsson, J., Ckurshumova, W., and Berleth, T. (2003). Auxin signaling in Arabidopsis leaf vascular development. Plant Physiol. 131, 1327–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson, J., Sung, Z.R., and Berleth, T. (1999). Responses of plant vascular systems to auxin transport inhibition. Development 126, 2979–2991. [DOI] [PubMed] [Google Scholar]
- McConnell, J.R., and Barton, M.K. (1998). Leaf polarity and meristem formation in Arabidopsis. Development 125, 2935–2942. [DOI] [PubMed] [Google Scholar]
- Meijer, M., and Murrary, J.A.H. (2001). Cell cycle controls and the development of plant form. Curr. Opin. Plant Biol. 4, 44–49. [DOI] [PubMed] [Google Scholar]
- Micol, J.L., and Hake, S. (2003). The development of plant leaves. Plant Physiol. 131, 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori, N., Eshed, Y., Chuck, G., Bowman, J., and Hake, S. (2000). Mechanisms that control knox gene expression in the Arabidopsis shoot. Development 127, 5523–5532. [DOI] [PubMed] [Google Scholar]
- Reed, R.C., Brady, S.R., and Muday, G.K. (1998). Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol. 118, 1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt, D., Mandel, T., and Kuhlemeier, C. (2000). Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12, 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt, D., Pesce, E.-R., Stieger, P., Mandel, T., Baltensperger, K., Bennett, M., Traas, J., Friml, J., and Kuhlemeier, C. (2003). Regulation of phyllotaxis by polar auxin transport. Nature 426, 255–260. [DOI] [PubMed] [Google Scholar]
- Riou-Khamlichi, C., Huntley, R., Jacqmard, A., and Murray, J.A.H. (1999). Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 283, 1541–1544. [DOI] [PubMed] [Google Scholar]
- Rupp, H.-M., Frank, M., Werner, T., Strnad, M., and Schmülling, T. (1999). Increased steady state mRNA levels of the STM and KNAT1 homeobox genes in cytokinin overproducing Arabidopsis thaliana indicate a role for cytokinins in the shoot apical meristem. Plant J. 18, 557–563. [DOI] [PubMed] [Google Scholar]
- Sawa, S., Watanabe, K., Goto, K., Kanaya, E., Morita, E.H., and Okada, K. (1999). FILAMENTOUS FLOWER, a meristem and organ identity gene of Arabidopsis, encodes a protein with zinc finger and HMG-related domains. Genes Dev. 13, 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon, M. (2003). The polar auxin transport inhibitor N-1-napthylphthalamic acid disrupts leaf initiation, KNOX protein regulation ad formation of leaf margins in maize. Plant Physiol. 133, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger, R., Tsiantis, M., Freeling, M., and Landale, J.A. (1998). The rough sheath2 gene negatively regulates homeobox gene expression during maize leaf development. Development 125, 2857–2865. [DOI] [PubMed] [Google Scholar]
- Semiarti, E., Ueno, Y., Tsukaya, H., Iwakawa, H., Machida, C., and Machida, Y. (2001). The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana regulates formation of a symmetric lamina, establishment of venation and repression of mersitem-related homeobox genes in leaves. Development 128, 1771–1783. [DOI] [PubMed] [Google Scholar]
- Sieburth, L.E. (1999). Auxin transport is required for leaf vein pattern in Arabidopsis. Plant Physiol. 121, 1179–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried, K.R., Eshed, Y., Baum, S.F., Otsuga, D., Drews, G.N., and Bowman, J.L. (1999). Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development 126, 4117–4128. [DOI] [PubMed] [Google Scholar]
- Smith, L.G., Greene, B., Veit, B., and Hake, S. (1992). A dominant mutation in the maize homeobox gene Knotted-1 causes its ectopic expression in leaf cells with altered fates. Development 116, 21–30. [DOI] [PubMed] [Google Scholar]
- Stals, H., and Inzé, D. (2001). When plant cells decide to divide. Trends Plant Sci. 6, 359–364. [DOI] [PubMed] [Google Scholar]
- Steynen, Q.J., Bolokoski, D.A., and Schultz, E.A. (2001). Alteration in flowering time causes accelerated or decelerated progression through Arabidopsis vegetative phases. Can. J. Bot. 79, 657–665. [Google Scholar]
- Steynen, Q.J., and Schultz, E.A. (2003). The FORKED genes are essential for distal vein meeting in Arabidopsis. Development 130, 4695–4708. [DOI] [PubMed] [Google Scholar]
- Stieger, P.A., Reihnardt, D., and Kuhlemeier, C. (2002). The auxin influx carrier is essential for correct leaf positioning. Plant J. 32, 509–517. [DOI] [PubMed] [Google Scholar]
- Timmermans, M.C.P., Hudson, A., Becraft, P.W., and Nelson, T. (1999). ROUGH SHEATH2: A Myb protein that represses knox homeobox genes in maize lateral organ primordia. Science 284, 151–153. [DOI] [PubMed] [Google Scholar]
- Tsiantis, M., Brown, M.I.N., Skibinski, G., and Langdale, J.A. (1999. a). Disruption of axuin transport is associated with aberrant leaf development in maize. Plant Physiol. 121, 1163–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiantis, M., Schneeberger, R., Golz, J.F., Freeling, M., and Langdale, J.A. (1999. b). The maize rough sheath2 gene and leaf development in monocot and dicot plants. Science 284, 154–156. [DOI] [PubMed] [Google Scholar]
- Ulmasov, T., Murfett, J., Hagen, G., and Guilfoyle, T.J. (1997). Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9, 1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venglat, S.P., Dumonceaux, T., Rozwadowki, K., Parnell, L., Babic, V., Keller, W., Martienssen, R., Selvaraj, G., and Datla, R. (2002). The homeobox gene BREVIPEDICELLUS is a key regulator of inflorescence architecture in Arabidopsis. Proc. Natl. Acad. Sci. USA 99, 4730–4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites, R., and Hudson, A. (1995). phantastica: A gene required for dorsoventrality of leaves in Antirrhinum majus. Development 121, 2143–2154. [Google Scholar]
- Waites, R., Selvadurai, H.R.N., Oliver, I.R., and Hudson, A. (1998). The phantastica gene encodes a MYB transcription factor involved in growth and dorsoventrality of lateral organs in Antirrhinum. Cell 93, 779–789. [DOI] [PubMed] [Google Scholar]
- Wilson, A.K., Pickett, F.B., Turner, J.C., and Estelle, M. (1990). A dominant mutation in Arabidopsis confers resistance to auxin, ethylene and abscisic acid. Mol. Gen. Genet. 222, 377–383. [DOI] [PubMed] [Google Scholar]
- Wyrzykowska, J., Pien, S., Shen, W.H., and Fleming, A.J. (2002). Manipulation of leaf shape by modulation of cell division. Development 129, 957–964. [DOI] [PubMed] [Google Scholar]
- Xu, L., Xu, Y., Dong, A., Sun, Y., Pi, L., Xu, Y., and Huang, H. (2003). Novel as1 and as2 defects in leaf adaxial-abaxial polarity reveal the requirement for ASYMMETRIC LEAVES1 and 2 and ERECTA functions in specifying leaf adaxial identity. Development 130, 4097–4107. [DOI] [PubMed] [Google Scholar]