Abstract
Objective
: Diffuse Large B Cell Lymphoma (DLBCL) is a heterogeneous group of tumors with different biological and clinical characteristics that have diverse clinical outcomes and response to therapy. Stromal-1 signature of tumor microenvironment of DLBCL represents extracellular matrix deposition and histiocytic infiltrate, whereas stromal-2 represents angiogenesis that could affect tumor progression.
Methods
: The aim of the present study is to assess the significance of stromal-1 signature using SPARC-1 and stromal-2 signature using CD31 expression and then finally to construct biologic prognostic model (BPM) in 60 cases of DLBCL via immunohistochemistry.
Results
: Microvessel density (P<0.05) and SPARC percentage of expression (P<0.001) were higher in DLBCL, including germinal and nongerminal cases, compared with reactive follicular hyperplasia. High microvessel density was significantly associated with splenic involvement (P=0.008), high mitotic count (P=0.045), and presence of capsular invasion (P=0.035). Percentage of SPARC expression was significantly associated with splenic involvement (P=0.03). Constructing BPM showed that 42 cases (70%) were of low biologic score (0–1) and 18 cases (30%) were of high biologic score (2–3). Low BPM cases showed less probability for splenic involvement (P=0.04) and a higher rate of complete response to therapy compared with high score cases (P=0.08).
Conclusions
: The DLBCL microenvironment could modulate tumor progression behavior since angiogenesis and SPARC positive stromal cells promote dissemination by association with spleen involvement and capsular invasion. Biologic prognostic models, including modified BPM, which considered cell origin of DLBCL and stromal signature pathways, could determine DLBCL progression and response to therapy.
Keywords: DLBCL, stromal-1, stromal-2, SPARC, angiogenesis, BPM
Introduction
Diffuse large B-cell lymphoma (DLBCL) accounts for 30% of non-Hodgkin’s lymphoma (NHL) diagnosed in adults1. In Egypt, DLBCL is the most common subtype of NHL and represents roughly 54.4% of NHL cases at the National Cancer Institute, Cairo University2. Based on the pathology-based registry of Ain Shams, Faculty of Medicine (2001–2010), NHL cases constituted 73.4% of all lymphoma cases and DLBCL represented 44.8% of them3. DLBCL is a heterogeneous group of tumors with different biological and clinical characteristics, emphasized by the diverse clinical outcome of the patients4.
A survival-predictor score based on a multivariate model derived from the germinal center B-cell, stromal-1, and stromal-2 gene expression signatures has the capability of predicting survival among patients with DLBCL treated with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone). Although the “stromal-1-signature” that is related to extracellular matrix deposition and histiocytic infiltration is prognostically favorable, the angiogenesis-related signature (stromal-2 signature) is associated with an unfavorable outcome5.
A subset of macrophages expresses one of the markers that are present in the stromal-1 panel called SPARC (secreted protein, acidic and rich in cysteine)6. SPARC-induced changes in the tumor microenvironment can suppress or promote progression of different cancers depending on the tissue and cell type7.
Biological factors that predict the survival of patients with DLBCL, such as cell of origin and stromal signatures, have been discovered by gene expression profiling. Biologic prognostic model (BPM) is an attempt to simulate this profiling via immunohistochemistry8. In this model, low scores are related to germinal cases, those with high SPARC (stromal signature-1), and low microvessel density (stromal signature-2); by contrast, high scores represented the opposite features.
In the present study, we aimed to assess the significance of stromal-1 signature via SPARC-1, stromal-2 signature via CD31 expression, and finally BPM in 60 cases of DLBCL; these were classified into germinal and non-germinal subtypes by using Han’s algorithm.
Patients and methods
This retrospective selective study was performed on 60 cases of DLBCL and 11 cases of reactive follicular hyperplasia as a control group. The cases were retrieved from the archive of the Pathology Department, Faculty of Medicine, Menoufia University. The cases received treatment in Menoufia Cancer Institute during the period between January 2009 and March 2015. These cases were selected based on the availability of paraffin-embedded blocks and clinical data of patients. The study was approved according to the institutional ethics of Faculty of Medicine, Menuofia University.
Clinical data of the patients were retrieved from hospital records that included age, gender, maximal tumor size of affected lymph node, splenic involvement, bone marrow involvement, B symptoms, performance status, revised IPI, age adjusted IPI, staging, LDH level, recurrence, and response to therapy.
The patients received cyclophosphamide, hydroxydaunorubicin, oncovin, prednisolone, and rituximab. Response to therapy was evaluated following the criteria described by Cheson et al.9, who divided the response into complete and incomplete responses in which the latter included partial response, stable, and progressive diseases. Patients were evaluated for response to therapy at the end of 6 months of treatment; such data were available for 48 patients.
Overall survival
Follow-up data were available for 44 patients where overall survival time was calculated from the date of diagnosis until death or the last contact with patient.
Histopathological evaluation of cases and immunohistochemical staining
Several 4-micron thick sections were cut from each block. Hematoxylin and eosin staining was performed to confirm the diagnosis and evaluate effacement, capsular and perinodal fat invasion, necrosis, and mitotic figures count. The method used for immunostaining was a streptavidin-biotin-amplified system. The primary antibodies used were mouse monoclonal antihuman CD10 (ready to use antibody, Cat. #No.081287, Labvision, USA), BCL6 (ready to use antibody, Cat. #MS-1114-R7, Labvision, USA), MUM-1 (concentrated antibody, Cat. # MA1-25525, Labvision, USA, Diluted as 1:100), CD31 (ready to use, clone QBE, DAKO), and SPARC (H-90, SANTA CRUZ, Cat. #.25574, diluted as 1:100). The slides were subjected to subsequent steps of deparaffinization and rehydration. Antigen retrieval was performed by boiling in citrate buffer saline (pH 6) followed by cooling at room temperature. The primary antibodies were incubated overnight at room temperature, and then the secondary antibody (Ultravision detection system anti-polyvalent HRP/DAB, ready-to-use, Neomarker) was applied with DAB as a chromogenic substrate and Mayer’s hematoxylin as a counter stain. Kidney tissue and hemangioma were used as a positive control for SPARC and CD31, respectively. Replacement of the primary antibody step by a blocking buffer was included in the staining procedure as a negative control. Classification of cases into germinal center and non-germinal center cases was applied using Han’s algorithm, which depended on the immunoreactivity for CD10, BCl6, and MUM110.
Evaluation of mean vascular density (MVD) using CD31 immunostaining
All specimens were analyzed semiquantitatively via image analyzer software program after thorough examination of all immunostained slides. Slides were scanned in a light microscope at 40× magnifications, and three areas of maximal MVD, the so-called “hot spots, ” were identified. In each hot spot, microvessels (capillaries and small venules) were counted at 400× magnification (each field representing an area of 0.375 mm2). For each slide, the mean number of microvessels from these three areas was calculated. The calculated figures were expressed as mean, median, and range11. The median value of MVD was used as the cutoff point, and then cases were classified into cases with low MVD “≤ median” and cases with high MVD “> median.” SPARC positivity was assigned when any number of non-neoplastic stromal cells showed cytoplasmic staining, evaluated as percentage of expression, and then expressed as median, mean, and range. Cases were also categorized based on extent of expression into cases with low SPARC (<5%) and cases with high SPARC (≥5%) to be used for construction of biologic prognostic model8.
BPM
We used SPARC, MVD, and molecular subtyping (germinal versus non-germinal) based on Han’s algorithm to construct BPM. One point was awarded for each adverse prognostic marker: non-GCB subtype, SPARC <5%, and high MVD. After lumping of cases, two groups were delineated; one with a low biologic score (0–1) and the other with a high score (2–3) 8.
Modified BPM (mBPM)
According to the result of the present study, modification on the previously described biologic prognostic model was performed. One point was awarded for the following: non-GCB subtype, SPARC ≥5%, and high MVD. After lumping of cases, two groups were delineated: one with a low biologic score (0–1–2) and the other with a high score3.
Statistical analysis
Data were collected, tabulated, and statistically analyzed by using a personal computer with SPSS version 20. The χ2 and Fisher exact tests were used for comparisons between qualitative variables. The Mann-Whitney (U) and Kruskal-Wallis (K) tests were used for comparisons between quantitative variables. The Kaplan-Meier survival curves were used to represent the overall survival (OS) distributions. Variables significantly related to OS were then included in the multivariate Cox proportional hazard regression model. P≤0.05 was considered significant.
Results
The clinical and histopathological data of DLBCL cases are presented in Tables 1 and 2, respectively.
Mean vascular density (MVD) in reactive follicular hyperplasia and DLBCL
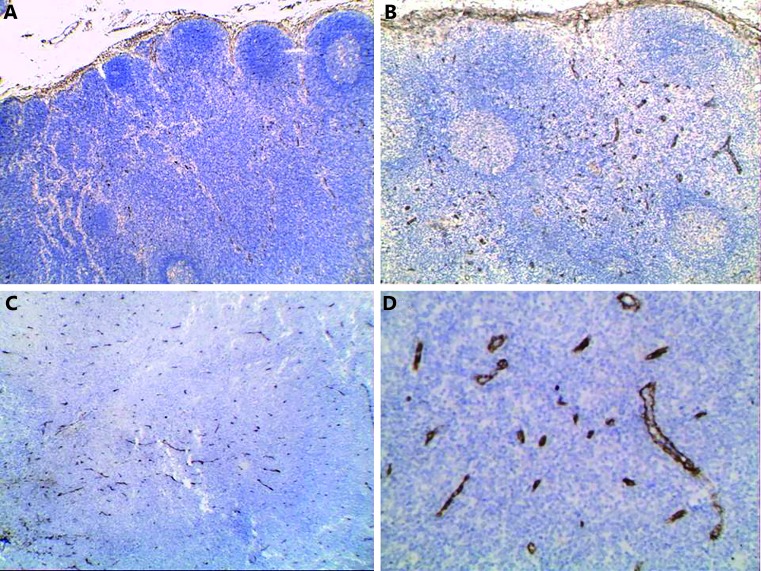
In reactive follicular hyperplasia, MVD ranged between 15 and 159 with a mean of 75.6 and a median of 78.6. Ten (90.9%) cases showed low MVD ( Figure 1A ), and only one case (9.1%) showed high MVD ( Figure 1B ).
1.
Reactive follicular hyperplasia showing low MVD (A) and high MVD (B). DLBCL showed high MVD (C and D) (IHC staining, 100× for A and C, 200× for B, and 400× for D).
In DLBCL cases, MVD ranged between 15 and 579 with a mean of 170.6 and a median of 128.5. A total of 30 (50%) cases showed high MVD, and another 30 (50%) cases showed low MVD ( Figures 1C and D ).
In germinal center DLBCL, MVD ranged between 15 and 579 with a mean of 177 and median of 120. Fifteen (50%) cases showed high MVD and another 15 (50%) cases showed low MVD. In non-germinal DLBCL, MVD ranged between 28 and 424 with a mean of 163.9 and a median of 106.7. Fifteen (50%) cases showed high MVD, and another 15 (50%) cases showed low MVD.
Comparison between reactive follicular hyperplasia and DLBCL including both GCB and non-GCB DLBCL subgroups regarding MVD
DLBCL cases showed higher mean and median values of MVD compared to follicular hyperplasia (P=0.027). Furthermore, germinal (P=0.028) and non-germinal DLBCL (P=0.028) showed higher MVD compared to follicular hyperplasia. However, MVD did not differ between germinal and non-germinal DLBCL ( Table 3).
The association between MVD and the studied parameters in DLBCL
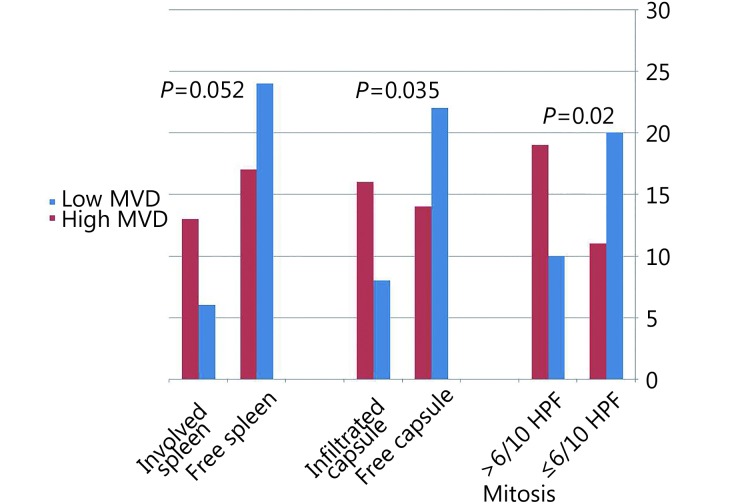
In DLBCL cases, MVD measured as quantitative values was significantly associated with splenic involvement (P=0.029) and high mitoses (P=0.045). When MVD was evaluated as low versus high using the median value as cut-off point, high MVD was found to be significantly associated with splenic involvement (P=0.052), high mitotic count (P=0.02), and presence of capsular invasion (P=0.035) ( Figure 2 ).
2.
Relationship of MVD with splenic involvement, mitotic count and infiltration of lymph node capsule in DLBCL.
SPARC immunostaining
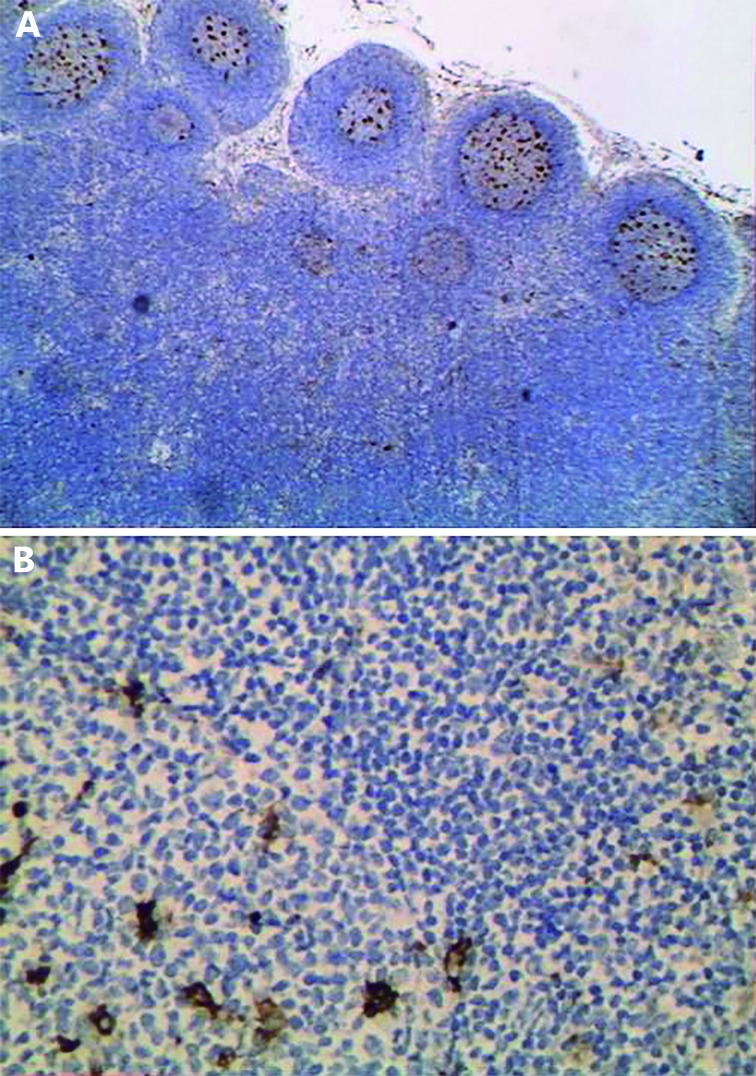
In reactive follicular hyperplasia cases, the expression was localized to germinal centers and the extent of positivity ranged from 1% to 5% with a mean±SD of 1.54±1.43 and a median of 1 ( Figures 3A and B ). In DLBCL, the extent of positivity ranged between 1% and 60% with a mean±SD of 28.63%±17.83 and a median of 30 ( Figure 4 ).
3.
SPARC positive expression in follicular hyperplasia localized to reactive germinal centers (IHC staining, 100× for A and 200× for B).
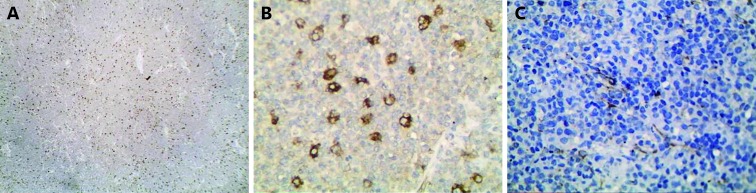
4.
DLBCL showed high SPARC in A and B and low SPARC in C (IHC staining, 100× for A, 200× for B, and 400× for C).
In germinal DLBCL, SPARC positivity ranged between 1 and 60% with a mean±SD of 31.06±18.08 and a median of 32.5. In non-germinal center DLBCL, SPARC positivity ranged between 1 and 60% with a mean±SD of 26.2±17.5 and a median of 30.
Differences between reactive follicular hyperplasia and DLBCL including germinal and non germinal subgroups as regards SPARC expression
DLBCL cases showed a higher expression of SPARC (median=30) in comparison to reactive follicular hyperplasia cases (median=1) with a statistically significant difference (P<001). By contrast, no significant differences were observed between GCB and non-GCB cases regarding SPARC expression. However, GCB cases tended to show higher SPARC (median=32.5) compared with non-germinal cases (median=30) ( Table 4).
Correlation between SPARC immunostaining and different clinico-pathological data of DLBCL
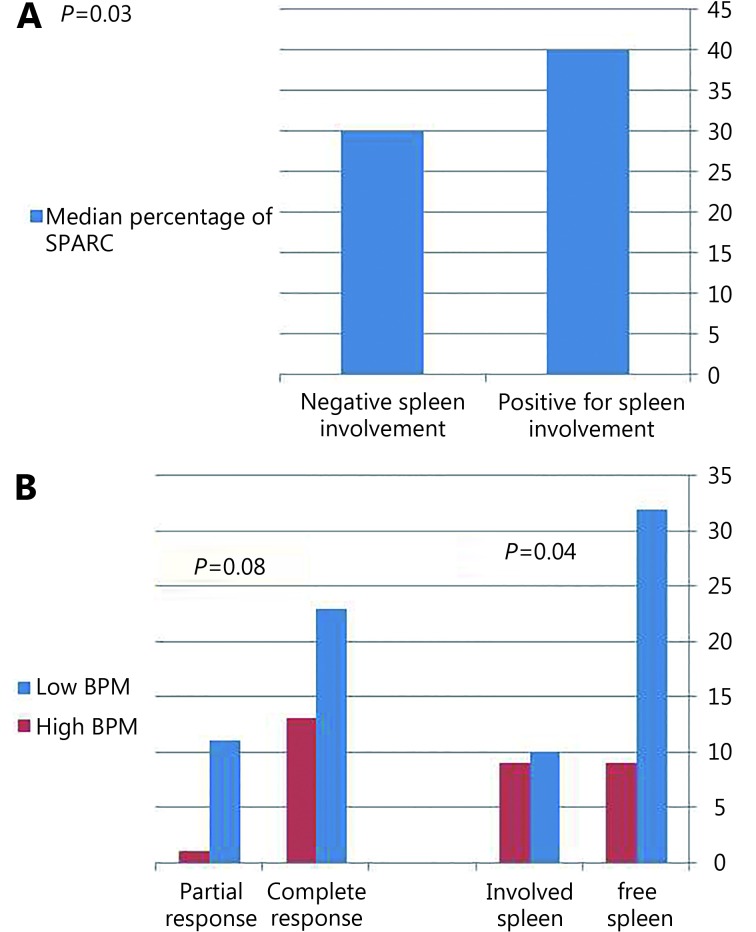
The extent of SPARC positivity was significantly correlated with splenic involvement, where cases with involved spleen showed higher percentage of SPARC positivity compared to cases that lacked splenic involvement (P=0.03) ( Figure 5A ).
5.
Relationship between percentage of SPARC and spleen involvement (A). Effect of BPM on splenic involvement and response to therapy (B).
BPM
Constructing the biologic prognostic model showed that 42 cases (70%) were of low biologic score (0–1), and 18 cases (30%) were of high biologic score (2–3). As regards germinal DLBCL, 29 cases (96.7%) were of low score (0–1), and only 1 case (3.3%) was of high score (2–3). Regarding non-germinal DLBCL, 13 cases (43.3%) were of low score, and 17 cases (56.7%) were of high score (2–3).
Relationship between BPM score and different clinicopathologic parameters in all cases of DLBCL
A statistically significant association was found between splenic involvement and BPM score, where cases with high score (2–3) showed a higher rate of splenic involvement (P=0.04) ( Figure 5B ). Furthermore, cases with low BPM score showed a higher rate of complete response to therapy [23 cases (63.9%)] in comparison to cases with high BPM score (13 cases, 36.1%) with a near statistical significance (P=0.08) ( Figure 5B ).
mBPM
Constructing the modified BPM revealed that 46 cases (76.7%) were of low modified score (0–1–2) and 14 cases (23.3%) were of high modified score (score 3). As regards germinal DLBCL, all 30 cases (100%) were of low score (0–1–2). Regarding non-germinal DLBCL, 16 cases (53.3%) were of low score, and 14 cases (46.7%) were of high score (score 3).
Relationship between mBPM and different clinicopathologic parameters in all DLBCL cases
A statistically significant association was found between response to therapy and mBPM score, where cases with low score (0–1–2) showed a higher rate of complete response to therapy (P=0.04) compared with cases with high scores.
OS
Survival data were available for 44 out of the 60 studied DLBCL cases (72.1%) (25 GCB and 19 non-GCB). Survival time ranged between 1 and 105 months with a mean±SD of 16.25±17.87 months and a median of 9.5 months. Seven out of 44 DLBCL cases (15.9%) died from their offending disease.
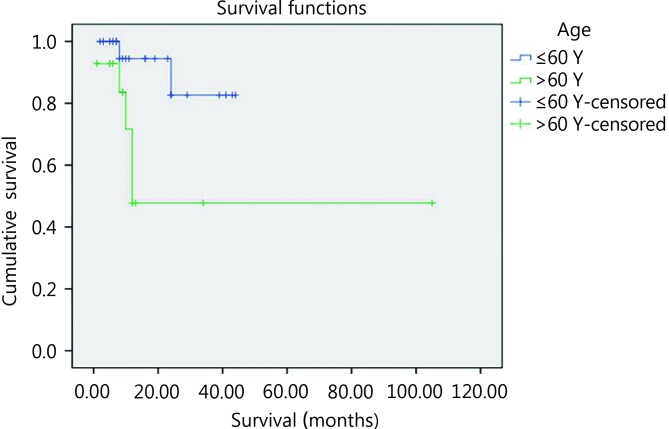
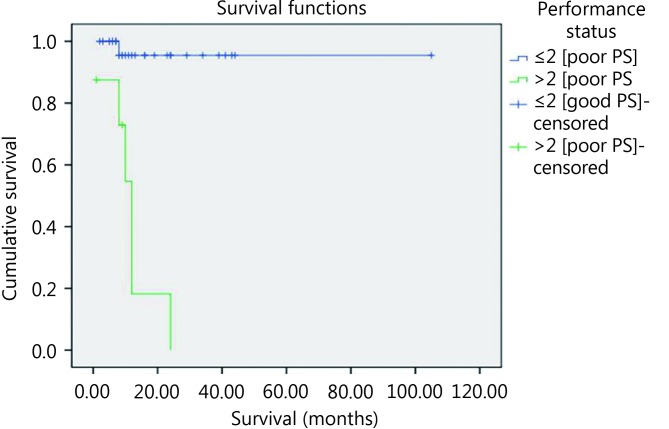
By univariate survival analysis, younger patients experienced shorter survival compared with older patients (P=0.02) ( Figure 6 ). Furthermore, patients with poor performance status (PS>2) showed poorer survival compared with cases associated with good performance status (PS≤2) (P<0.001) ( Figure 7 ). Using Cox regression analysis revealed the independence of performance status in affecting OS.
6.
The impact of age on patients’ OS using log rank equation.
7.
The impact of performance status on patients’ OS using log rank equation.
Discussion
Assessment of angiogenesis (mean vascular density) was performed using CD31, where MVD values were significantly lower in reactive follicular hyperplasia cases compared with all DLBCL (P=0.022), GCB cases (P=0.028), and non-GCB cases (P=0.028). This was similar to Ribatti et al.12, who reported that MVD is higher in lymphomas than in reactive nodes and is higher in aggressive than in indolent lymphomas13.
The microvessel number was usually low in lymphadenopathies and increased significantly in low-grade B-NHL. Intermediate-grade tumors displayed a further significant increase that mainly occurred because of their diffuse subtypes rather than to the follicular subtype; high-grade B-NHL showed the highest counts14. A possible reason is that angiogenesis is a multistep process that is crucial in progression and metastasis of various tumors, including those of visceral organs and hemato-lymphoid malignancies.
Angiogenesis is required by the tumor for ensuring adequate supply of oxygen and nutrients to the proliferating tumor cells15. However, such a result is in contrast with Aggarwal et al.16, who found that reactive lymphoid hyperplasia showed a significantly higher MVD than did individual NHL subgroups, except for peripheral T cell lymphoma “PTCL.” These results are also contrary to Passalidou et al.13, who found that MVD in the paracortex of reactive nodes is higher than that in the paracortex of follicular lymphomas and is also higher than in DLBCL. They suggested that physiologic angiogenesis in the reactive nodes is more effective than the tumor-induced angiogenesis.
In the present study, high MVD in DLBCL cases was significantly associated with splenic involvement, high mitoses, and presence of capsular invasion (P<0.05). Such a result was in agreement with Ganjoo et al.17, who found that angiogenesis and lymphangiogenesis have important roles in the development and progression of lymphoma.
The association of high MVD with poor prognostic factors in DLBCL seen in the present study and in others could be explained by the association of angiogenesis-related signatures (stromal signature-2) with unfavorable outcome5. Stromal-2 signature may be an “angiogenic switch” in which the progression of a hyperplastic lesion to a fully malignant tumor is accompanied by new blood-vessel formation18.
Previous reports suggested that non-GCB-type DLBCLs showed a higher MVD than did tumors with a GCB profile4; this finding was not proved in the present study. Constitutive activation of the NF-κB signaling pathway in non-germinal DLBCL could promote cell survival and proliferation and inhibit apoptosis19.
As regards SPARC immunostaining in the present study, DLBCL cases showed significant higher expression of SPARC (median=30) in comparison to reactive follicular hyperplasia cases (median=1) ( P<0.001). This can be explained by the role of SPARC in modulating ECM assembly, integrin activity, and growth factor signaling, thereby controlling a range of cellular functions including adhesion, proliferation, survival, and migration20.
According to Sangaletti et al.21, SPARC was expressed by germinal center-associated macrophages and FDCs in reactive follicles, findings that are also seen in the present study. By contrast, its expression was redistributed to scattered mesenchymal elements and lymphoid cells in lymphomatous follicles21. Thus, the altered stromal features and SPARC expression may accompany common events that occur early in B-cell transformation, including the imbalance between proliferation and apoptosis in expanding B-cell clones. SPARC expression has been found to vary among the different B-NHLs, with the highest expression in DLBCL and MZL and the lowest expression in SLL/CLL21.
In the present study, the extent of SPARC positivity was significantly associated with splenic involvement, in which cases that involved spleen showed higher percentages of SPARC positivity compared with cases that lacked splenic involvement (P=0.03). This may be explained by the crucial role of SPARC in the process of tumor invasion and metastasis in certain malignancies22. SPARC also influenced the microenvironment and signaling pathways involved in disease progression20.
SPARC was reported to be an unfavorable prognostic factor in nasopharyngeal carcinoma22, associated with nodal and distant metastasis in gastric carcinoma23, involved in cell migration in endometrial carcinoma24, and related to transformation into aggressive forms of renal cell carcinoma25.
Furthermore, SPARC showed oncogenic properties in many tumor types including gliomas, astrocytomas26, melanomas27, and colorectal carcinoma28. Furthermore, in invasive ductal carcinoma, the expression of SPARC is enhanced in tumor tissue compared with normal controls, and an increased level of SPARC is associated with higher histological grade and advanced pathological stage29.
SPARC has tumor suppressor and promoter roles in certain neoplasms. In hematologic neoplasms, for example, in leukemias that do not express SPARC, such as acute myelogenous leukemia with MLL gene abnormalities, exogenous SPARC from the microenvironment promotes tumor suppression30. This suppression may occur because of decreased production of necessary growth factors, alteration of the ECM preventing tumor cell interaction, or decreased integrin production by the tumor cells, thereby resulting in altered ECM interactions31. Furthermore, in pancreatic carcinoma, tumor-derived fibroblasts strongly expressed SPARC mRNA and secreted SPARC protein. Treatment of pancreatic cancer cells, with exogenous SPARC resulted in growth suppression32. The same was reported in ovarian carcinoma, in which addition of exogenous SPARC, as well as ectopic expression by an adenoviral vector, resulted in decreased proliferation of ovarian cancer cell lines 33.
By contrast, lymphomas or leukemias in which the tumor cells expressed high levels of SPARC, such as mantle cell lymphoma and acute myelogenous leukemia associated with the inv16 chromosomal abnormality, high levels of SPARC are associated with increased tumor growth34.
As regards the biologic prognostic model originally described by Perry et al.8, the present study showed that cases with high biologic prognostic score (2–3) were statistically associated with a higher rate of splenic involvement (P=0.04). This agreed with Perry et al.8, who found that a higher proportion of patients with advanced-stage disease are in group “2–3” score because this group included cases with previously evidenced poorer prognostic factors such as high MVD and non-germinal molecular profiling. Furthermore, we found that cases with low BPM score showed higher rate of complete response to therapy in comparison to cases with high BPM score with a nearly statistical significance. This again agreed with Perry et al.8, who found that patients with a low BPM score (0–1) had a significantly better survival rate than those with a high BPM score (2–3).
Cases that have a low BPM score may have germinal center profiling, and it is a prognostically favorable profile because cell lines derived from germinal-center DLBCL have decreased activity of the NF-κB signaling pathway19. The transcriptional activation of genes that are associated with cell proliferation, angiogenesis, metastasis, and suppression of apoptosis appears to lie at the heart of the ability of NF-κB to promote oncogenesis and cancer therapy resistance35. In addition, the group with low BPM score has a higher SPARC; certain studies have demonstrated the role for SPARC in sensitizing therapy-resistant cancers36.
Given that the present study showed the association of high percentage of SPARC with adverse parameters such as splenic involvement, the idea of modification of BPM arises, in which high SPARC was considered an adverse feature. The modified BPM demonstrated the statistically significant effect of high score on response to therapy (P=0.04), which is a relationship that was not clearly statistically proved in the original BPM. This can be explained by the role of SPARC in ECM remodeling, and growth factor signaling that may enhance cellular proliferation when combined with higher angiogenesis “high MVD.”
Patients with a low score (0–1) were associated with good survival, whereas those with a high score (2–3) were associated with poor survival8. Unfortunately, neither biologic model scores nor stromal signatures showed any effect on patient overall survival based on the present results because of small number of cases that were followed. In addition, most cases lied in the censored group.
In brief, the DLBCL microenvironment could modulate tumor progression behavior since angiogenesis and SPARC positive stromal cells promote dissemination by association with spleen involvement and capsular invasion. Biologic prognostic models, including modified BPM, which considered cell origin of DLBCL and stromal signature pathways, could determine DLBCL progression and response to therapy. Further studies are recommended to examine the prognostic role of stromal signatures and biologic prognostic models by using a large number of cases and longer follow-up.
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Stein H, Warnke RA, Chan WC. Diffuse large B-cell lymphoma, not otherwise specified. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC; 2008. p. 233-7.
- 2.Mokhtar N, Gouda I, Adel I. Cancer pathology registry 2003-2004 and time trend analysis. Egypt: El Sherss for Advertising; 2007.
- 3.El A Helal T, Salma MI, Ezz-Elarab SS. Malignant tumors of lymph node. Pathology-based cancer registry 2001-2010, Ain-Shams faculty of medicine, Cairo, Egypt, 2015.
- 4.Cardesa-Salzmann TM, Colomo L, Gutierrez G, Chan WC, Weisenburger D, Climent F, et al. High microvessel density determines a poor outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus chemotherapy. Haematologica. 2011;96:996–1001. doi: 10.3324/haematol.2010.037408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359:2313–23. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reed MJ, Puolakkainen P, Lane TF, Dickerson D, Bornstein P, Sage EH. Differential expression of sparc and thrombospondin 1 in wound repair: Immunolocalization and in situ hybridization. J Histochem Cytochem. 1993;41:1467–77. doi: 10.1177/41.10.8245406. [DOI] [PubMed] [Google Scholar]
- 7.Chlenski A, Cohn SL. Modulation of matrix remodeling by SPARC in neoplastic progression. Semin Cell Dev Biol. 2010;21:55–65. doi: 10.1016/j.semcdb.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Perry AM, Cardesa-Salzmann TM, Meyer PN, Colomo L, Smith LM, Fu K, et al. A new biologic prognostic model based on immunohistochemistry predicts survival in patients with diffuse large B-cell lymphoma. Blood. 2012;120:2290–6. doi: 10.1182/blood-2012-05-430389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 10.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large b-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–82. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 11.Mazur G, Wróbel T, Dzięgiel P, Jeleń M, Kuliczkowski K, Zabel M. Angiogenesis measured by expression of CD34 antigen in lymph nodes of patients with non-hodgkin’s lymphoma. Folia Histochem Cytobiol. 2004;42:241–3. [PubMed] [Google Scholar]
- 12.Ribatti D, Vacca A, Marzullo A, Nico B, Ria R, Roncali L, et al. Angiogenesis and mast cell density with tryptase activity increase simultaneously with pathological progression in B-cell non-hodgkin’s lymphomas. Int J Cancer. 2000;85:171–5. [PubMed] [Google Scholar]
- 13.Passalidou E, Stewart M, Trivella M, Steers G, Pillai G, Dogan A, et al. Vascular patterns in reactive lymphoid tissue and in non-hodgkin’s lymphoma. Br J Cancer. 2003;88:553–9. doi: 10.1038/sj.bjc.6600742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribatti D, Vacca A, Nico B, Fanelli M, Roncali L, Dammacco F. Angiogenesis spectrum in the stroma of B-cell non-Hodgkin’s lymphomas. An immunohistochemical and ultrastructural study. Eur J Haematol. 1996;56:45–53. doi: 10.1111/j.1600-0609.1996.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 15.Bellamy WT, Richter L, Frutiger Y, Grogan TM. Expression of vascular endothelial growth factor and its receptors in hematopoietic malignancies. Cancer Res. 1999;59:728–33. [PubMed] [Google Scholar]
- 16.Aggarwal D, Srivastava G, Gupta R, Pant L, Krishan G, Singh S. Angiogenesis in non-Hodgkin’s lymphoma: An intercategory comparison of microvessel density. ISRN Hematol. 2012;2012: Article ID 943089.
- 17.Ganjoo KN, Moore AM, Orazi A, Sen JA, Johnson CS, An CS. The importance of angiogenesis markers in the outcome of patients with diffuse large B cell lymphoma: A retrospective study of 97 patients. J Cancer Res Clin Oncol. 2008;134:381–7. doi: 10.1007/s00432-007-0294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 19.Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor κB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001;194:1861–74. doi: 10.1084/jem.194.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnold SA, Brekken RA. Sparc: A matricellular regulator of tumorigenesis. J Cell Commun Signal. 2009;3:255–73. doi: 10.1007/s12079-009-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sangaletti S, Tripodo C, Vitali C, Portararo P, Guarnotta C, Casalini P, et al. Defective stromal remodeling and neutrophil extracellular traps in lymphoid tissues favor the transition from autoimmunity to lymphoma. Cancer Discov. 2014;4:110–29. doi: 10.1158/2159-8290.CD-13-0276. [DOI] [PubMed] [Google Scholar]
- 22.Wang HY, Li YY, Shao Q, Hou JH, Wang F, Cai MB, et al. Secreted protein acidic and rich in cysteine (SPARC) is associated with nasopharyngeal carcinoma metastasis and poor prognosis. J Transl Med. 2012;10:27. doi: 10.1186/1479-5876-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao ZS, Wang YY, Chu YQ, Ye ZY, Tao HQ. Sparc is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2010;16:260–8. doi: 10.1158/1078-0432.CCR-09-1247. [DOI] [PubMed] [Google Scholar]
- 24.Yusuf N, Inagaki T, Kusunoki S, Okabe H, Yamada I, Matsumoto A, et al. Sparc was overexpressed in human endometrial cancer stem-like cells and promoted migration activity. Gynecol Oncol. 2014;134:356–63. doi: 10.1016/j.ygyno.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Sakai N, Baba M, Nagasima Y, Kato Y, Hirai K, Kondo K, et al. Sparc expression in primary human renal cell carcinoma: Upregulation of sparc in sarcomatoid renal carcinoma. Hum Pathol. 2001;32:1064–70. doi: 10.1053/hupa.2001.28244. [DOI] [PubMed] [Google Scholar]
- 26.Huang HT, Colella S, Kurrer M, Yonekawa Y, Kleihues P, Ohgaki H. Gene expression profiling of low-grade diffuse astrocytomas by cDNA arrays. Cancer Res. 2000;60:6868–74. [PubMed] [Google Scholar]
- 27.Alonso SR, Tracey L, Ortiz P, Pérez-Gómez B, Palacios J, Pollán M, et al. A high-throughput study in melanoma identifies epithelial-mesenchymal transition as a major determinant of metastasis. Cancer Res. 2007;67:3450–60. doi: 10.1158/0008-5472.CAN-06-3481. [DOI] [PubMed] [Google Scholar]
- 28.Porter PL, Sage EH, Lane TF, Funk SE, Gown AM. Distribution of SPARC in normal and neoplastic human tissue. J Histochem Cytochem. 1995;43:791–800. doi: 10.1177/43.8.7622842. [DOI] [PubMed] [Google Scholar]
- 29.Barth PJ, Moll R, Ramaswamy A. Stromal remodeling and sparc (secreted protein acid rich in cysteine) expression in invasive ductal carcinomas of the breast. Virchows Arch. 2005;446:532–6. doi: 10.1007/s00428-005-1256-9. [DOI] [PubMed] [Google Scholar]
- 30.DiMartino JF, Lacayo NJ, Varadi M, Li L, Saraiya C, Ravindranath Y, et al. Low or absent SPARC expression in acute myeloid leukemia with MLL rearrangements is associated with sensitivity to growth inhibition by exogenous SPARC protein. Leukemia. 2006;20:426–32. doi: 10.1038/sj.leu.2404102. [DOI] [PubMed] [Google Scholar]
- 31.Meyer PN, Fu K, Greiner T, Smith L, Delabie J, Gascoyne R, et al. The stromal cell marker sparc predicts for survival in patients with diffuse large B-cell lymphoma treated with rituximab. Am J Clin Pathol. 2011;135:54–61. doi: 10.1309/AJCPJX4BJV9NLQHY. [DOI] [PubMed] [Google Scholar]
- 32.Sato N, Fukushima N, Maehara N, Matsubayashi H, Koopmann J, Su GH, et al. Sparc/osteonectin is a frequent target for aberrant methylation in pancreatic adenocarcinoma and a mediator of tumor-stromal interactions. Oncogene. 2003;22:5021–30. doi: 10.1038/sj.onc.1206807. [DOI] [PubMed] [Google Scholar]
- 33.Socha MJ, Said N, Dai Y, Kwong J, Ramalingam P, Trieu V, et al. Aberrant promoter methylation of sparc in ovarian cancer. Neoplasia. 2009;11:126–35. doi: 10.1593/neo.81146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martínez N, Camacho FI, Algara P, Rodríguez A, Dopazo A, Ruíz-Ballesteros E, et al. The molecular signature of mantle cell lymphoma reveals multiple signals favoring cell survival. Cancer Res. 2003;63:8226–32. [PubMed] [Google Scholar]
- 35.Orlowski RZ, Baldwin AS Jr. NF-κB as a therapeutic target in cancer. Trends Mol Med. 2002;8:385–9. doi: 10.1016/s1471-4914(02)02375-4. [DOI] [PubMed] [Google Scholar]
- 36.Tai IT, Tang MJ. Sparc in cancer biology: Its role in cancer progression and potential for therapy. Drug Resist Updat. 2008;11:231–46. doi: 10.1016/j.drup.2008.08.005. [DOI] [PubMed] [Google Scholar]