Abstract
Kappa opioid (KOP) receptor antagonists and delta opioid (DOP) receptor agonists have antidepressant-like effects in animal tests and may be useful for treatment-resistant depression in humans. In this study, we examined whether the combination of a KOP receptor antagonist and a DOP receptor agonist would produce a better than additive effect (i.e. synergy). LY2444296 is a short-acting selective nonpeptide KOP receptor antagonist. ADL5859 is a selective nonpeptide DOP receptor agonist which does not produce seizures and EEG disturbances. Each compound and combinations of the two were examined in the forced swim test (FST) one h post injection, a screening test for antidepressant-like effect, in male adult C57BL/6J mice (Jackson Lab). LY2444296 [subcutaneous (s.c.) injection] at 10 and 30 mg/kg, but not 3 mg/kg, significantly decreased immobility time in a dose-dependent manner. Intraperitoneal (i.p.) injections of ADL5859 also reduced immobility time dose-dependently at doses of 3 and 10 mg/kg, but not at 1 mg/kg. An analysis was conducted using the method of Tallarida and Raffa (2010), which employed dose equivalence. The relative potency of the drugs was determined to be LY2444296: ADL5859=1:0.28, which was the dose ratio for combination studies. Six combinations of the two compounds were tested in mice at a fixed dose ratio. We found that LY2444296 and ADL5859 yielded significant synergistic effects for the antidepressant-like effect at the combined dose ranging from 3.84 mg/kg to 9.0 mg/kg. ADL5859 (10 mg/kg), LY2444296 (30 mg/kg) and their combined dose (3.84 mg/kg) had no effects on locomotor activities. Since the two drugs have distinct pharmacological profiles, such a synergism will allow use of lower doses of both drugs to achieve desired antidepressant effects with fewer side effects.
Keywords: kappa opioid antagonist, delta opioid agonist, antidepressant, synergy, forced swim test
1. Introduction
The dynorphin / κ opioid (KOP) receptor system has been demonstrated to mediate negative emotional states. Activation of the KOP receptor by selective agonists produces depression and dysphoria in humans (Barber and Gottschlich, 1997; Pfeiffer et al., 1986) and conditioned place aversion in animals (Shippenberg et al., 2007). In laboratory animals, many stress-induced behavioral responses are mediated by the KOP receptor (McLaughlin et al., 2006; McLaughlin et al., 2003) and prolonged stress produces depression-like behaviors [see (Bruchas et al., 2010) for a review]. Several groups have reported that in animal tests KOP receptor antagonists, including norbinaltorphimine (norBNI), JDTic and DIPPA, have antidepressant-like effects (Beardsley et al., 2005; Carr et al., 2010; Mague et al., 2003; Reindl et al., 2008; Shirayama et al., 2004; Zhang et al., 2007). Both nor-BNI and JDTic have slow onsets of maximal KOP receptor antagonist actions (24-48 h) and show very long durations of action, lasting more than two weeks after a single injection (Carroll et al., 2004; Endoh et al., 1992; Horan et al., 1992). In addition, the short-acting KOP receptor antagonists PF-04455242 (Grimwood et al., 2011) and LY2456302 (Rorick-Kehn et al., 2014) were shown to have antidepressant-like effects. The selective KOP receptor antagonist CERC-501 (also known as LY2456302) has passed phase I clinical trial (Lowe et al., 2014) and is currently in phase II trial for adjunctive treatment of major depressive disorder and for substance use disorders (e.g., nicotine, alcohol, and/or cocaine) (http://www.cerecor.com/pipeline/cerc-501.php).
The enkephalin / delta opioid (DOP) receptor system has been shown to be involved in emotional responses. Mutant mice devoid of the DOP receptor or preproenkephalin gene displayed enhanced depression- and anxiety-like behaviors (Filliol et al., 2000; Konig et al., 1996; Ragnauth et al., 2001). Several selective DOP receptor agonists reduced depressive-like behaviors in animal tests, including SNC80, NIH11082, UFP512, KNT-127, and AZD2327 [for a review, see (Chung and Kieffer, 2013)]. The antidepressant-like effects were comparable to those produced by selective serotonin reuptake inhibitors and tricyclic antidepressants (Naidu et al., 2007; Saitoh et al., 2004). DOP receptor agonists decreased anxiety- and depression-like behaviors in mice withdrawn from alcohol and cocaine (Ambrose-Lanci et al., 2010; Perrine et al., 2008; van Rijn et al., 2010)
The DOP receptor and KOPR receptor have distinct distributions in the brain (Mansour et al., 1988). KOPR antagonists and DOPR agonists produce antidepressant-like effects in rodents by acting on some similar and some distinct brain regions. Sites of action of KOPR antagonists include the ventral tegmental area, nucleus accumbens, hippocampus, prefrontal cortex and dorsal raphe nucleus [reviewed in (Van't Veer and Carlezon, 2013)], whereas those of DOPR agonists are cingulate, frontal and insular cortices, hippocampus, nucleus accumbens, and amygdala [reviewed in (Chung and Kieffer, 2013)].” We thus hypothesized that a DOP agonist and a KOPR antagonist may have synergistic antidepressant-like effects. In this study, we tested the hypothesis by examining if combinations of a KOP receptor antagonist (LY2444296) and a DOP receptor agonist (ADL5859) exhibited synergistic antidepressant-like effects in the mouse forced swim test. LY2444296, an analogue of LY2456302, is a selective short-acting KOP receptor antagonist with a Ki value of ∼1 nM for the KOP receptor and κ / μ and κ / δ selectivity of ∼60 and ∼350, respectively [compound 25 in (Mitch et al., 2011)]. ADL5859 is a highly selective nonpeptide DOP receptor agonist with a Ki value of 0.8 nM for the DOP receptor and δ / κ and δ / μ selectivity >1000 (Le Bourdonnec et al., 2008). Unlike the prototypic selective nonpeptide DOP receptor agonists BW373U86 and SNC80, ADL5859 does not induce seizures and electroencephalogram (EEG) disturbances in rodents (Chung et al., 2015). In addition, ADL5859 has passed phase I clinical trials demonstrating its safety in humans, but it was shown to be ineffective in reducing osteoarthritis pain in phase II trials (https://clinicaltrials.gov/ct2/show/NCT00979953). Its safety in humans makes it attractive for further investigation as an anti-depressant.
2. Materials and Methods
2.1. Drugs
ADL5859 hydrochloride, purchased from MedChem Express (Monmouth Junction, NJ), was prepared in deionized water. LY2444296, a generous gift from Eli Lilly and Co. (Indianapolis, IN), was dissolved in 85% DL- lactic acid (20 μl per mg compound), then diluted with saline by vortex, and lastly added with 1N NaOH (150 μl per mg compound) by vortex for final pH∼5. Both drugs were prepared freshly on the test days and mixed thoroughly before syringe aspiration. ADL5859 [intraperitoneal (i.p.) injection], LY2444296 [subcutaneous (s.c.) injection] or their combination was administered in a volume of 10 mL/kg to animals 60 min prior to testing, while control animals received injections of water i.p., saline s.c. or water i.p. plus saline s.c.
2.2. Animals
Male adult C57BL/6J mice (∼23 g) were purchased from The Jackson Laboratory (Bar Harbor, ME). The total number of mice used was 230 (see figure legends for details). After arrival, mice were habituated to the animal facility for about 7 days before any experiments. Mice were housed under a 12-hr light/dark cycle with food and water available ad libitum. Other housing conditions were as follows: cage dimension of 32×18×15 cm3, 5 mice per cage without enrichment and housing room temperature of 21 ± 1°C. Experimental procedures were approved by the Temple University Institutional Animal Care and Use Committee.
2.3. Forced swim test (FST)
FST in mice or rats has been commonly used for screening of antidepressant activities of drugs. It should be noted that it is a screening test, but not a model of depression. FST in mice was performed as described by Lucki et al. (2001). Each mouse was used only once. On the day of experiment, mice were allowed to acclimate to the test room for one h. All experimental sessions were conducted between 1:00 and 6:00 pm. Mice were injected with vehicle or a dose of LY2444296 (s.c.) or ADL5859 (i.p.) or a combination of both drugs. Sixty min later, mice were placed for 6 min in a cylindrical tank (46 cm tall × 20 cm diameter) of 23-25°C water filled to a depth of 15 cm. The swim sessions were videotaped for later analysis by the experimenter. Some of the FST videos were also scored by other experienced researchers in the lab who were blind to the treatments. The scores obtained were consistent with the data shown. Duration of immobility (minimum movements necessary to stay afloat) in the last 4-min of the swim was measured. A reduction of immobility in the swim session (relative to vehicle controls) is used as a measure of antidepressant-like effects.
2.4. Determination of drug combination effects
From the dose-effect data of the individual compounds, we selected doses for joint application (in a fixed ratio based on the individual potency values). Subsequent analysis of the combination dose-effect data gave combination dose-effect values for comparison with the expected (additive) effect. Getting the additive effect employs the concept of dose equivalence (the basis of the isobole method) (Tallarida and Raffa, 2010). That methodology calculates the expected (additive) effect of each dose combination and thus allows comparison of these effects (statistically) with the observed combination effect. The calculation proceeds from using each dose of ADL5859 in the combination and finding its equivalent dose of LY2444296 (the higher efficacy drug). That equivalent plus the actual quantity of LY2444296 allows calculation of the expected effect by use of LY's dose-effect equation. If the observed effect is greater than the calculated expected effect then that interaction is synergistic. Combinations that give the expected effect are termed additive, while those combinations that give effects less than expected are termed sub-additive. Synergy means that the observed effect of the combination is greater than the expected.
2.5. Locomotor activities
Motor activities were measured using a Digiscan D Micro System (Accuscan, Columbus, OH, USA) and eight individual activity monitors. A single activity monitor consists of an aluminum frame equipped with 16 horizontal infrared light beams and detectors where the activity chamber (a standard clear plastic animal cage, 42cm×20cm×20 cm) is placed. As the mouse moves within the chamber, light beams are broken and recorded by a computer connected to the Digiscan system. Activity was recorded as total activity, ambulatory activity and stereotypy. Total activity represents all beam breaks by a single mouse, and is the sum of the ambulatory and stereotypy counts. Ambulatory activity represents successive beam breaks. Stereotypic counts identify repeated breaks of the same beam indicative of a stationary animal engaged in a repetitive behavior as opposed to ambulation, but they do not identify a specific stereotypic behavior. Activity was measured over 1.5 h post injections with 5-min collection intervals under normal laboratory lighting conditions.
2.6. Data analysis
Data were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni post hoc tests (Prism v.5, GraphPad, San Diego, CA) for results of behavioral tests and by paired Student's t test for synergy analysis. Each value is expressed as the mean ± S.E.M.
3. Results
3.1. ADL5859 and LY2444296 show dose-dependent antidepressant-like effects
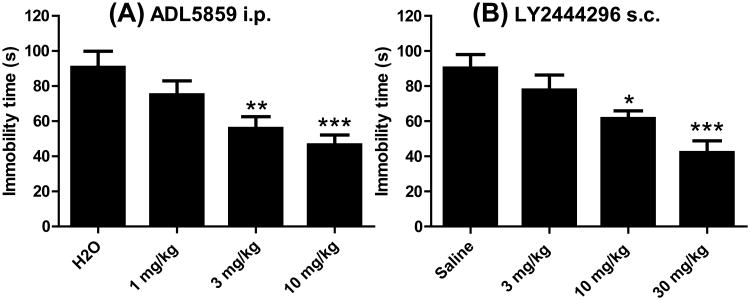
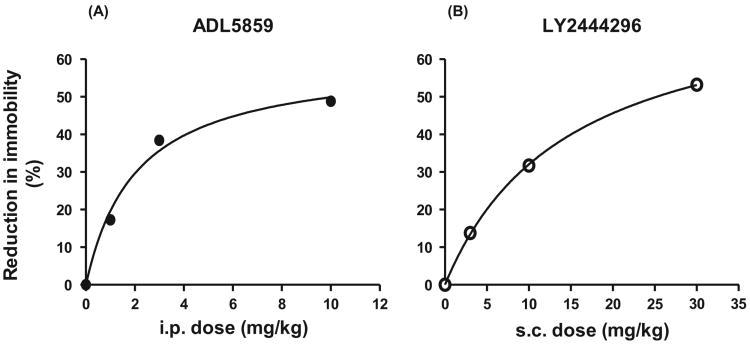
Sixty min following i.p. injection, ADL5859 at 3 and 10 mg/kg, but not at 1 mg/kg, reduced immobility time of mice in the FST in a dose-dependent manner (Fig. 1A). One h after subcutaneous (s.c.) injection of LY2444296, mice displayed significantly decreased immobility time in the FST at 10 and 30 mg/kg in a dose-dependent fashion, but not 3 mg/kg (Fig. 1B). The data were analyzed with nonlinear regression and produced excellent fits to the dose-effect curves which are E = 60.7 D / (D + 2.2) for ADL5859 and E = 81.5 D / (D +16.0) for LY2444296, where D is dose and E is effect (Fig. 2). The equations so derived provide the potencies.
Figure 1. The DOR agonist ADL5859 and the KOR antagonist LY2444296 showed antidepressant-like effects in the FST in C57BL/6J mice.
One h after drug administration, (A) ADL5859 (i.p.) and (B) LY2444296 (s.c.) dose-dependently reduced immobility time (in sec), indicating antidepressant-like effects. ***P<0.001, **P<0.01 and *P<0.05, compared with the respective vehicle groups (n=10-15 per group), by one-way ANOVA followed by Bonferroni's multiple comparison tests (Prism 5). The animal numbers for each treatment were as follows: in (A), H2O (n=14), 1 mg/kg (n=13), 3 mg/kg (n=12) and 10 mg/kg (n=12); in (B), H2O (n=15), 3 mg/kg (n=12), 10 mg/kg (n=14) and 30 mg/kg (n=10).
Figure 2. Dose-effect curves of ADL5859 (left) and LY2444296 (right) in the FST where the effect is the magnitude (%) of the decrease in immobility time.
The data in Fig. 1 were transformed into % reduction in immobility and were fitted to the dose-effect curves by nonlinear regression.
3.2. Synergistic antidepressant-like effects of combinations of ADL5859 and LY2444296
The potencies of the individual compounds serve as a guide in the selection of the dose ratio to be tested and thus the individual dose – effect curves suggested the dose ratio LY2444296: ADL5859 = 1: 0.28.
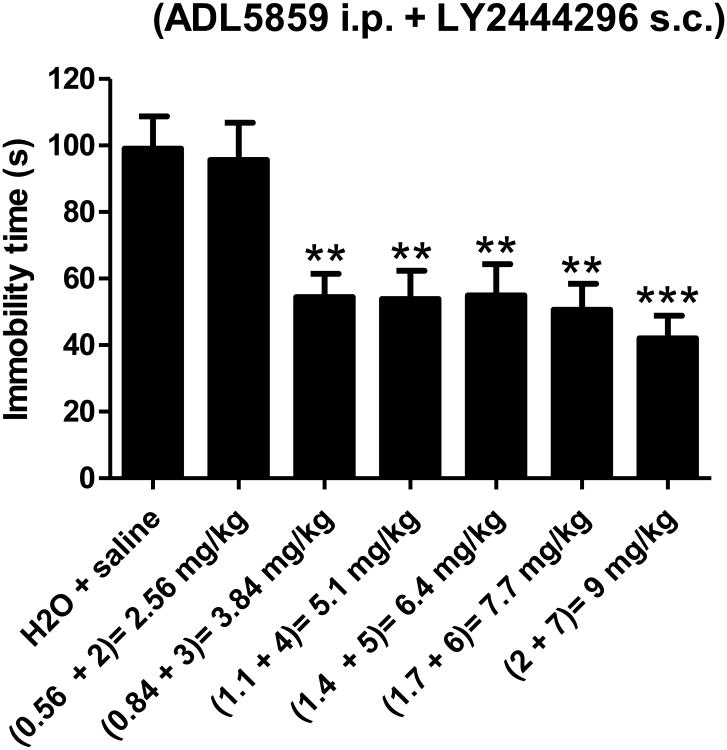
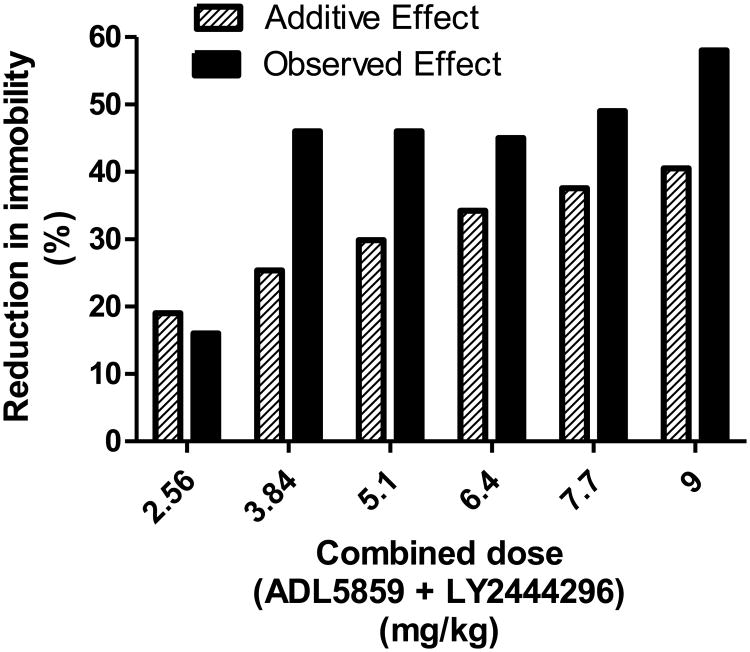
Six combinations of the two compounds were tested in mice at this fixed dose ratio (LY2444296: ADL5859=1:0.28) (Fig. 3). Sub-active or active doses of ADL5859 i.p. and LY2444296 s.c. led to significant reduction of mouse immobility for 5 out of 6 drug combinations (Fig.3). The combined active doses ranged from 3.84 mg/kg to 9.0 mg/kg. For each of these dose combinations there is an expected (additive) effect that was calculated from the dose equivalence procedure of (Tallarida and Raffa, 2010) (Fig. 4). Comparison of the observed effects and the expected effects indicates that these two drugs in combination produce significant synergistic antidepressant-like effects at these low doses (Fig. 4). Notably, the combination of 0.84 mg/kg ADL5859 and 3 mg/kg LY2444296 showed as great an effect as higher dose combinations although either one alone barely had any effect (See Fig.1).
Figure 3. Effects of co-administration of a fixed dose ratio of LY2444296 and ADL5859 (1:0.28) on immobility time in the FST in mice.
LY2444296 and ADL5859 were administered at indicated dose combinations and 60 min later, the FST was performed on mice (n=10-15/group). ***P<0.001, **P<0.01, compared with the vehicle group, by one-way ANOVA followed by Bonferroni's multiple comparison tests. The animal numbers for each treatment were as follows: H2O + saline (n=15), 2.56 mg/kg (n=11), 3.84 mg/kg (n=15), 5.1 mg/kg (n=10), 6.4 mg/kg (n=15), 7.7 mg/kg (n=13) and 9 mg/kg (n=13).
Fig. 4. Observed (red) and expected / additive (black) effects of combinations of ADL5859 and LY2444296 shown on horizontal scale as the dose sum.
For each of these dose combinations, expected (additive) effects were calculated from the dose equivalence procedure as described in Methods. Expected and observed effects were compared by the application of paired Student's t test and the results shows that the paired differences are significantly different (P = 0.015, comparing all six combined doses; P = 0.0012, comparing five combined doses except for 2.56 mg/kg), thereby indicating synergy.
3.3. Effects of ADL5859, LY2444296 or combined administration on locomotor activities
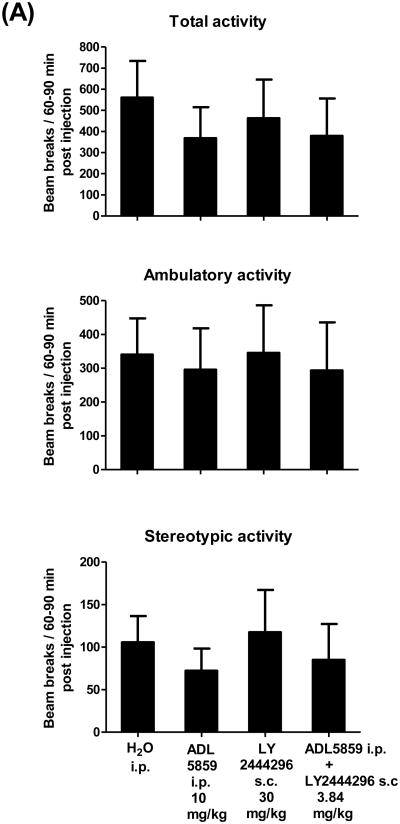
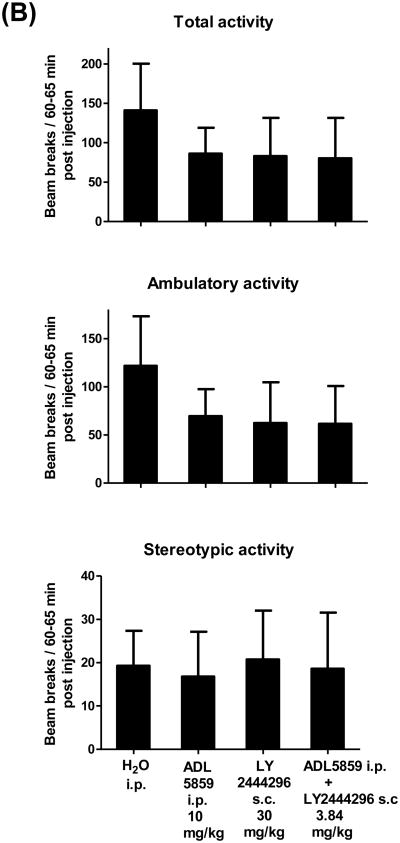
We then examined if the reduction in immobility in the FST of ADL5859, LY2444296 or combined administration was due to enhanced locomotor activity. Mice were habituated in the locomotor chambers for 2 h, and then injected with water (i.p.), ADL5859 (i.p., 10 mg/kg), LY2444296 (s.c., 30 mg/kg) or ADL5859 (i.p., 0.84 mg/kg) plus LY2444296 (s.c., 3 mg/kg). After drug administration, mouse activities were recorded continuously for 90 min. Thirty-min cumulative data from 60-90 min post-injections and five-min data 60-65 min post-injections were presented and analyzed, and either drug or the combination did not have significant effects on mouse locomotor activities (Fig. 5A, 5B). Thus, the effect of either drug or combination is not due to enhanced locomotor activity.
Fig. 5. Effects of ADL5859, LY2444296 or their combined administration vs water on locomotor activity in mice.
Mice were habituated in the locomotor chambers for 2 h first and then injected with water or drug(s). Total, ambulatory and stereotypic activities were continuously monitored after injections for 90 min using automated locomotor chambers. Data are expressed as mean ± S.E.M. beam breaks per 30-min period (60-90 min post injections) in (A) or per 5-min period (60-65 min post injections) in (B) and analyzed by one-way ANOVA. n = 8-10/group. The animal numbers for each treatment are as follows: H2O (n=10), ADL5859 10 mg/kg (n=10), LY2444296 30 mg/kg (n=8) and the combined dose 3.84 mg/kg (n=8).
4. Discussion
We have shown that LY2444296 and ADL5859 at a fixed dose ratio of 1:0.28 have highly synergistic antidepressant-like effects in the FST at combined doses as low as 3.84 mg/kg. To the best of our knowledge, this is the first to show that a KOP receptor antagonist and a DOP receptor agonist have synergistic antidepressant-like effects. The findings have implications for development of these classes of drugs for clinical applications.
Drug combinations have often been employed clinically; however, rigorous studies on the doses used in combinations that would produce synergy are not performed regularly. Classifying an interaction as we did here is a quantitative pursuit. It does not require a description of mechanism. All that is required are the dose-response curve of each drug acting alone and the dose-response curve of the combination. In most cases these will be drugs that give overtly similar effects. When a combination of such two drugs or compounds show synergism, both drugs can be used at lower doses, which likely lead to reduced side effects. For example, the combination of tramadol and acetaminophen at a fixed dose ratio have synergistic analgesic effects, and the combination was patented and further developed as the combination drug marketed as ULTRACET®. There are many drug combination studies in animals, in which both drugs are active or only one is active for the pharmacological end point. For example, Fairbanks and Wilcox (1999) demonstrated that either morphine or clonidine administered intrathecally had antinociceptive effect and when given at a dose ratio of 1:20 or 1:2 the combination displayed synergistic antinociceptive effects. Rawls et al. (2004) reported that the NO synthetase inhibitor L-NAME had no effect on body temperature, but it greatly potentiated the hypothermic effect of the cannabinoid agonist WIN 55212-2.
This is the first demonstration that ADL5859 has antidepressant-like effects. This finding is consistent with many previous studies showing that selective DOP receptor agonists reduced depressive-like behaviors in the FST, tail suspension test and learned helplessness. The nonpeptide DOP receptor agonists examined include BW373U86 (Broom et al., 2002b), SNC80 (Broom et al., 2002b; Saitoh et al., 2004), KNT-127 (Nozaki et al., 2014; Saitoh et al., 2011) and AZD2327 (Hudzik et al., 2011). The peptide agonists DPDPE, JOM-13, deltorphin II, and H-Dmt-Tic-NH-CH2-Bid (Torregrossa et al., 2006), NIH11082 (Naidu et al., 2007) and UFP-512 (Vergura et al., 2008) also displayed antidepressant-like effects. Like KNT-127, but unlike BW363U86 and SNC-80, ADL5859 does not cause convulsions (Broom et al., 2002a; Chung et al., 2015; Comer et al., 1993; Saitoh et al., 2011).
Our finding is the first one to show that LY2444496 exhibits antidepressant-like activity. This observation is consistent with several previous reports that KOP receptor antagonists have antidepressant-like activity in animal tests, including norBNI, JDTic, DIPPA, PF-04455242 and LY2456302 (Beardsley et al., 2005; Carr et al., 2010; Grimwood et al., 2011; Mague et al., 2003; Reindl et al., 2008; Rorick-Kehn et al., 2014; Shirayama et al., 2004; Zhang et al., 2007). LY2444496 has a short duration of action, similar to PF-04455242 (Grimwood et al., 2011) and LY2456302 (Rorick-Kehn et al., 2014), but different from long acting KOP receptor antagonists, such as norBNI and JDTic (Carroll et al., 2004; Endoh et al., 1992; Horan et al., 1992).
For the FST, we used C57BL/6J mice from Jackson Labs. This strain has been used previously to demonstrate the anti-depressant-like effects of KOP receptor antagonists in the mouse FST (Falcon et al., 2015; McLaughlin et al., 2003).
The mechanisms underlying this synergistic interaction remain to be determined. The KOP receptor in several mesolimbic areas are involved in KOP receptor-mediated depressive-like behaviors, including the ventral tegmental area, dorsal raphe nucleus, nucleus accumbens, hippocampus and prefrontal cortex [reviewed in (Bruchas et al., 2010; Van't Veer and Carlezon, 2013)]. Carr et al. (2010) reported that the piriform cortex and nucleus accumbens shell in WKY rats play important roles in antidepressant-like effect of the KOP receptor antagonist norBNI. DOP receptor agonists act on the frontal, cingulate and insular cortices, hippocampus, amygdala and nucleus accumbens to regulate emotional responses [reviewed in (Chung and Kieffer, 2013)]. It is likely that the two drugs may act on different neuronal circuitries to produce synergistic antidepressant-like effects. In addition, there may be interactions of the effects of the two drugs at the cellular levels in some of the regions. The synergistic antidepressant-like effect of ADL5859 and LY2444296 may also be due to pharmacokinetic interactions. Whether this synergistic effect is generally applicable to combinations of other DOP receptor agonists and KOP receptor antagonists remains to be investigated.
In conclusion, ADL5859, a DOP receptor agonist, and LY2444296, a KOP receptor antagonist, produced synergistic antidepressant-like effects in the mouse FST at a fixed dose ratio of 0.28 to 1. Such a synergism will allow use of lower doses of both drugs to achieve desired effects. Studies to examine if these two drugs display synergistic anxiolytic-like effects will be conducted.
Acknowledgments
This work was supported by NIH grants (R03 DA036802, P30 DA13429 and AT006899). We thank Eli Lilly and Co. for providing LY2444296 and Dr. Linda Rorick-Kehn of Eli Lilly and Co. for discussions.
Footnotes
Disclosure: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambrose-Lanci LM, Sterling RC, Van Bockstaele EJ. Cocaine withdrawal-induced anxiety in females: impact of circulating estrogen and potential use of delta-opioid receptor agonists for treatment. J Neurosci Res. 2010;88:816–824. doi: 10.1002/jnr.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A, Gottschlich R. Novel developments with selective, non-peptidic kappa-opioid receptor agonists. Expert Opin Investig Drugs. 1997;6:1351–1368. doi: 10.1517/13543784.6.10.1351. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Convulsant activity of a non-peptidic delta-opioid receptor agonist is not required for its antidepressant-like effects in Sprague-Dawley rats. Psychopharmacology (Berl) 2002a;164:42–48. doi: 10.1007/s00213-002-1179-y. [DOI] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology. 2002b;26:744–755. doi: 10.1016/S0893-133X(01)00413-4. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I. Antidepressant-like effects of kappa-opioid receptor antagonists in Wistar Kyoto rats. Neuropsychopharmacology. 2010;35:752–763. doi: 10.1038/npp.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll I, Thomas JB, Dykstra LA, Granger AL, Allen RM, Howard JL, Pollard GT, Aceto MD, Harris LS. Pharmacological properties of JDTic: a novel kappa-opioid receptor antagonist. Eur J Pharmacol. 2004;501:111–119. doi: 10.1016/j.ejphar.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Chung PC, Boehrer A, Stephan A, Matifas A, Scherrer G, Darcq E, Befort K, Kieffer BL. Delta opioid receptors expressed in forebrain GABAergic neurons are responsible for SNC80-induced seizures. Behav Brain Res. 2015;278:429–434. doi: 10.1016/j.bbr.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung PCS, Kieffer BL. Delta opioid receptors in brain function and diseases. Pharmacol Ther. 2013;140:112–120. doi: 10.1016/j.pharmthera.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Hoenicke EM, Sable AI, McNutt RW, Chang KJ, De Costa BR, Mosberg HI, Woods JH. Convulsive effects of systemic administration of the delta opioid agonist BW373U86 in mice. J Pharmacol Exp Ther. 1993;267:888–895. [PubMed] [Google Scholar]
- Endoh T, Matsuura H, Tanaka C, Nagase H. Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch Int Pharmacodyn Ther. 1992;316:30–42. [PubMed] [Google Scholar]
- Fairbanks CA, Wilcox GL. Spinal antinociceptive synergism between morphine and clonidine persists in mice made acutely or chronically tolerant to morphine. J Pharmacol Exp Ther. 1999;288:1107–1116. [PubMed] [Google Scholar]
- Falcon E, Maier K, Robinson SA, Hill-Smith TE, Lucki I. Effects of buprenorphine on behavioral tests for antidepressant and anxiolytic drugs in mice. Psychopharmacology (Berl) 2015;232:907–915. doi: 10.1007/s00213-014-3723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Grimwood S, Lu Y, Schmidt AW, Vanase-Frawley MA, Sawant-Basak A, Miller E, McLean S, Freeman J, Wong S, McLaughlin JP, Verhoest PR. Pharmacological characterization of 2-methyl-N-((2′-(pyrrolidin-1-ylsulfonyl)biphenyl-4-yl)methyl)propan-1-ami ne (PF-04455242), a high-affinity antagonist selective for kappa-opioid receptors. J Pharmacol Exp Ther. 2011;339:555–566. doi: 10.1124/jpet.111.185108. [DOI] [PubMed] [Google Scholar]
- Horan P, Taylor J, Yamamura HI, Porreca F. Extremely long-lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J Pharmacol Exp Ther. 1992;260:1237–1243. [PubMed] [Google Scholar]
- Hudzik TJ, Maciag C, Smith MA, Caccese R, Pietras MR, Bui KH, Coupal M, Adam L, Payza K, Griffin A, Smagin G, Song D, Swedberg MD, Brown W. Preclinical pharmacology of AZD2327: a highly selective agonist of the delta-opioid receptor. J Pharmacol Exp Ther. 2011;338:195–204. doi: 10.1124/jpet.111.179432. [DOI] [PubMed] [Google Scholar]
- Konig M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ, Zimmer A. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature. 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- Le Bourdonnec B, Windh RT, Ajello CW, Leister LK, Gu M, Chu GH, Tuthill PA, Barker WM, Koblish M, Wiant DD, Graczyk TM, Belanger S, Cassel JA, Feschenko MS, Brogdon BL, Smith SA, Christ DD, Derelanko MJ, Kutz S, Little PJ, DeHaven RN, DeHaven-Hudkins DL, Dolle RE. Potent, orally bioavailable delta opioid receptor agonists for the treatment of pain: discovery of N,N-diethyl-4-(5-hydroxyspiro[chromene-2,4′-piperidine]-4-yl)benzamide (ADL5859) J Med Chem. 2008;51:5893–5896. doi: 10.1021/jm8008986. [DOI] [PubMed] [Google Scholar]
- Lowe SL, Wong CJ, Witcher J, Gonzales CR, Dickinson GL, Bell RL, Rorick-Kehn L, Weller M, Stoltz RR, Royalty J, Tauscher-Wisniewski S. Safety, tolerability, and pharmacokinetic evaluation of single- and multiple-ascending doses of a novel kappa opioid receptor antagonist LY2456302 and drug interaction with ethanol in healthy subjects. J Clin Pharmacol. 2014;54:968–978. doi: 10.1002/jcph.286. [DOI] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitch CH, Quimby SJ, Diaz N, Pedregal C, de la Torre MG, Jimenez A, Shi Q, Canada EJ, Kahl SD, Statnick MA, McKinzie DL, Benesh DR, Rash KS, Barth VN. Discovery of aminobenzyloxyarylamides as kappa opioid receptor selective antagonists: application to preclinical development of a kappa opioid receptor antagonist receptor occupancy tracer. J Med Chem. 2011;54:8000–8012. doi: 10.1021/jm200789r. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Lichtman AH, Archer CC, May EL, Harris LS, Aceto MD. NIH 11082 produces anti-depressant-like activity in the mouse tail-suspension test through a delta-opioid receptor mechanism of action. Eur J Pharmacol. 2007;566:132–136. doi: 10.1016/j.ejphar.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki C, Nagase H, Nemoto T, Matifas A, Kieffer BL, Gaveriaux-Ruff C. In vivo properties of KNT-127, a novel delta opioid agonist: receptor internalisation, antihyperalgesia and antidepressant effects in mice. Br J Pharmacol. 2014 doi: 10.1111/bph.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine SA, Sheikh IS, Nwaneshiudu CA, Schroeder JA, Unterwald EM. Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology. 2008;54:355–364. doi: 10.1016/j.neuropharm.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Ragnauth A, Schuller A, Morgan M, Chan J, Ogawa S, Pintar J, Bodnar RJ, Pfaff DW. Female preproenkephalin-knockout mice display altered emotional responses. Proc Natl Acad Sci U S A. 2001;98:1958–1963. doi: 10.1073/pnas.041598498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Tallarida RJ, Gray AM, Geller EB, Adler MW. L-NAME (N omega-nitro-L-arginine methyl ester), a nitric-oxide synthase inhibitor, and WIN 55212-2 [4,5-dihydro-2-methyl-4(4-morpholinylmethyl)-1-(1-naphthalenyl-carbonyl)-6 H-pyrrolo[3,2,1ij]quinolin-6-one], a cannabinoid agonist, interact to evoke synergistic hypothermia. J Pharmacol Exp Ther. 2004;308:780–786. doi: 10.1124/jpet.103.054668. [DOI] [PubMed] [Google Scholar]
- Reindl JD, Rowan K, Carey AN, Peng X, Neumeyer JL, McLaughlin JP. Antidepressant-like effects of the novel kappa opioid antagonist MCL-144B in the forced-swim test. Pharmacology. 2008;81:229–235. doi: 10.1159/000112867. [DOI] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Witkin JM, Statnick MA, Eberle EL, McKinzie JH, Kahl SD, Forster BM, Wong CJ, Li X, Crile RS, Shaw DB, Sahr AE, Adams BL, Quimby SJ, Diaz N, Jimenez A, Pedregal C, Mitch CH, Knopp KL, Anderson WH, Cramer JW, McKinzie DL. LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology. 2014;77:131–144. doi: 10.1016/j.neuropharm.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Kimura Y, Suzuki T, Kawai K, Nagase H, Kamei J. Potential anxiolytic and antidepressant-like activities of SNC80, a selective delta-opioid agonist, in behavioral models in rodents. J Pharmacol Sci. 2004;95:374–380. doi: 10.1254/jphs.fpj04014x. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Sugiyama A, Nemoto T, Fujii H, Wada K, Oka J, Nagase H, Yamada M. The novel delta opioid receptor agonist KNT-127 produces antidepressant-like and antinociceptive effects in mice without producing convulsions. Behav Brain Res. 2011;223:271–279. doi: 10.1016/j.bbr.2011.04.041. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Raffa RB. The application of drug dose equivalence in the quantitative analysis of receptor occupation and drug combinations. Pharmacol Ther. 2010;127:165–174. doi: 10.1016/j.pharmthera.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Jutkiewicz EM, Mosberg HI, Balboni G, Watson SJ, Woods JH. Peptidic delta opioid receptor agonists produce antidepressant-like effects in the forced swim test and regulate BDNF mRNA expression in rats. Brain Res. 2006;1069:172–181. doi: 10.1016/j.brainres.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Brissett DI, Whistler JL. Dual efficacy of delta opioid receptor-selective ligands for ethanol drinking and anxiety. J Pharmacol Exp Ther. 2010;335:133–139. doi: 10.1124/jpet.110.170969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van't Veer A, Carlezon WA., Jr Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology (Berl) 2013;229:435–452. doi: 10.1007/s00213-013-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergura R, Balboni G, Spagnolo B, Gavioli E, Lambert DG, McDonald J, Trapella C, Lazarus LH, Regoli D, Guerrini R, Salvadori S, Calo G. Anxiolytic- and antidepressant-like activities of H-Dmt-Tic-NH-CH(CH2-COOH)-Bid (UFP-512), a novel selective delta opioid receptor agonist. Peptides. 2008;29:93–103. doi: 10.1016/j.peptides.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Zhang H, Shi YG, Woods JH, Watson SJ, Ko MC. Central kappa-opioid receptor-mediated antidepressant-like effects of nor-Binaltorphimine: behavioral and BDNF mRNA expression studies. Eur J Pharmacol. 2007;570:89–96. doi: 10.1016/j.ejphar.2007.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]