Abstract
Phosphorylation sites of KOPR following treatment with the selective agonist U50,488H were identified after affinity purification, SDS-PAGE, in-gel digestion with Glu-C and LC-MS/MS. Single- and double-phosphorylated peptides were identified containing phosphorylated S356, T357, T363 and S369 in the C-terminal domain. Antibodies were generated against three phosphopeptides containing pS356/pT357, pT363, and pS369, respectively, and affinity-purified antibodies were found to be highly specific for phospho-KOPR. U50,488H markedly enhanced staining of the KOPR by pT363, pS369 and pS356/pT357 antibodies in immunoblotting, which was blocked by the selective KOPR antagonist norbinaltorphimine. S369 phosphorylation affected T363 phosphorylation and vice versa and T363 or S369 phosphorylation was important for S356/T357 phosphorylation, revealing phosphorylation hierarchy. U50,488H, but not etorphine, promoted robust KOPR internalization, although both were full agonists. U50,488H induced higher degrees of phosphorylation than etorphine at S356/T357, T363 and S369 by immunoblotting. Using SILAC (stable isotope labeling by amino acids in cell culture) and LC-MS/MS, we found that compared with control (C), U50,488H (U) and etorphine (E) KOPR promoted single phosphorylation primarily at T363 and S369 with U/E ratio of 2.5 and 2, respectively. Both induced double phosphorylation at T363+S369 and T357+S369 with ratios of U/E=3.3 and 3.4, respectively. Only U50,488H induced triple phosphorylation at S356+T357+S369. An unphosphorylated KOPR(354–372) fragment containing all the phosphorylation sites was detected with a ratio of C/E/U =1/0.7/0.4, indicating that ~60% and ~30% of the mKOPR are phosphorylated following U50,488H and etorphine, respectively. Thus, KOPR internalization requires receptor phosphorylation above a certain threshold and higher-order KOPR phosphorylation may be disproportionally important.
Keywords: kappa opioid receptor, phosphorylation sites, endocytosis threshold, phospho-kappa opioid receptor specific antibody, SILAC
(a) Introduction
Kappa opioid receptor (KOPR) is one of the three opioid receptors (μ, δ and κ), which mediate the effects of opioid drugs and endogenous peptides. KOPR belongs to the rhodopsin sub-family of the seven transmembrane receptor (7TMR) family. Activation of the KOPR in vivo produces many effects including analgesia (1), dysphoria/aversion (2,3), water diuresis (4), and antipruritic effects (5). U50,488H is the prototypic selective nonpeptide KOPR full agonist (1).
It has been demonstrated that signal transduction of 7TMRs involves both G proteins- and β-arrestin-mediated pathways [reviewed in (6)]. The KOPR acts through Gi/o proteins to inhibit adenylyl cyclase and N-type and L-type Ca++ channels and activate inwardly rectifying K+ channels and ERK1/2 [reviewed in (7)]. In addition, activated KOPR undergoes GRK-mediated phosphorylation and β-arrestin-dependent desensitization, internalization and down-regulation [reviewed in (8)]. Moreover, activation of the KOPR enhances ERK1/2 and p38 MAP kinase phosphorylation in β-arrestin-dependent manner (9,10). Therefore, agonist-promoted phosphorylation of KOPR is a key event in signaling, trafficking and regulation of the receptor. Chavkin and colleagues have suggested that KOPR-induced analgesia is mediated by the G protein pathway (11), whereas aversion is produced by the β-arrestin-mediated p38 MAP kinase phosphorylation (9). However, recently White et al. (12) reported that the G protein-biased KOPR agonist RB64 produced analgesia and conditioned place aversion (CPA). Because of the pivotal role of KOPR phosphorylation in signaling, it is important to understand molecular details of this event.
Previously MacLaughlin et al. (13) showed that S369A mutation in the rat KOPR, which has the same C-terminal domain sequence as the mouse KOPR, inhibited agonist-promoted internalization and desensitization. However, S369 may not be the only phosphorylation site. In this study, we identified the sites of U50,488H-induced phosphorylation in the mouse KOPR by high performance liquid chromatography - tandem mass spectrometry (LC-MS/MS) analysis of Glu-C treated purified KOPR. We then generated antiserum against phosphopeptides and purified antibodies were proven to be specific for phospho-KOPR in immunoblotting.
We found that U50,488H promoted robust KOPR internalization, whereas etorphine caused low or no internalization, even though both were full agonists in enhancing [35S]GTPγS binding. We then examined whether the two agonists differentially phosphorylated KOPR. Two approaches were employed: immunoblotting with phospho-specific KOPR antibodies and stable isotope-labeled amino acids in cells (SILAC) followed by LC-MS/MS. SILAC uses stable isotope-labeled amino acids to differentially label proteins in cultured cells and utilizes the high resolution of mass spectrometry to quantify the differences in the abundance of the same peptide, which is differentially labeled with stable isotopes, following different treatments (14,15).
(b) Experimental Procedures
Materials
Mouse neuro2A (N2A) cell was purchased from ATCC. U50,488H, etorphine and nor-binaltorphimine (nor-BNI) were provided by the National Institute on Drug Abuse (Bethesda, MD). Rabbit anti-FLAG antibody (F7425) and mouse monoclonal anti-FLAG M2 affinity agarose were purchased from Sigma-Aldrich (St. Louis, MO). The rabbit anti-mKOPR (PA847) and anti-phophopeptides antisera were custom-developed by Covance Co. (Denver, PA) and purified in our own laboratory. All peptides were custom-synthesized by EZBiolab Inc. (Carmel, IN). The horseradish peroxidase (HRP) conjugated goat anti-rabbit or anti-mouse IgG antibodies were purchased from Jackson ImmunoResearch (West Grove, PA). The minimal essential medium (MEM), blasticidin, Lipofectamine 2000, pcDNA3.1, pcDNA6/Myc-6His plasmids and the precast gel (4–12% Bis-Tris plus) were purchased from Invitrogen Thermo Fisher Scientific (Carlsbad, CA). The fetal bovine serum (FBS) was purchased from Atlanta Biochemicals (Flowery Branch, GA). The SulfoLink coupling resin column, chromatography columns, protease inhibitor tablet, 13C6 L-Arginine, 13C615N4 L-Arginine, MEM medium minus L-arginine and dialyzed fetal bovine serum and enhanced chemiluminescence (ECL) reagent Supersignal West Pico were purchased from Pierce Thermo Fisher Scientific (Rockford, IL). The lambda phosphatase was purchased from New England BioLabs (Ipswich, MA). The Ni-NTA Agarose was purchased from Qiagen GmbH (Hilden, Germany). Dodecyl-β-D-maltoside (DDM) was purchased from EMD Biosciences, Inc. (La Jolla, CA). All other chemicals were purchased from Sigma-Aldrich.
cDNA constructs of mouse KOPRs
The mouse KOPR (mKOPR) was epitope-tagged with FLAG including a signal peptide at the N-terminus (16) to facilitate receptor purification. The construct was generated by PCR and cloned into KpnI/AgeI sites of pcDNA6/6His vector with blasticidin resistance gene. The mKOPR thus generated is epitope-tagged with FLAG at the N-terminus and 6XHis at the C-terminus (FmK6H).
Cell Culture
Neuro2A mouse neuroblastoma (N2A) cells were used in the studies because of its neuron-like nature. Cells were transfected with the FmK6H construct and grown in the presence of 5 μg/ml blasticidin and clonal cells stably expressing the receptor were established. One cell line that expressed FmK6Hat ~ 2 pmole/mg membrane protein was used for the study. Cells were maintained in 10% fetal bovine serum, 1 μg/ml blasticidin and minimum essential medium at 37°C in a humidified 5% CO2 atmosphere.
For SILAC experiments, cells were grown in the presence of regular Arg, Arg containing six 13C (Arg6) or Arg containing six 13C and four 15N (Arg10) for at least 10 passages to label proteins to ~99% with Arg, Arg6 or Arg10.
Receptor purification
The following buffers were used for receptor purification: Buffer A, 20mM Tris, pH 7.4, 1% n-Dodecyl-β-D-maltoside (DDM), 0.1 M NaCl, phosphatase inhibitors including 20 mM Na4 pyrophosphate, 20 mM Na2 glycerophosphate, 20 mM NaF, 1mM orthovanadate, 10 mM imidazole and protease inhibitors tablet, pH adjusted to 8.0; Buffer B, 20mM Tris, pH 7.4, 0.1% DDM, 0.5M NaCl, phosphatase inhibitors mix in buffer A at 1/10 of the original concentrations, 10 mM imidazole; Buffer C, Buffer B + 0.25M imidazole; Buffer D, 20mM Tris, pH 7.4, 0.05% DDM; Buffer E, Buffer D + 0.5M NaCl; Buffer F, Buffer D + 0.15M NaCl + 100μg/ml FLAG peptide.
Twenty 10-cm plates of cells at ~70% confluence (1.4 × 108 cells) were washed, incubated with serum-free medium for 2 h and then treated with or without 10 μM U50,488H for 30 min at 37°C and medium aspirated. Cells were immediately solubilized for 5 min at room temperature in 1 ml/plate buffer A. The lysate was collected, centrifuged at 51,500 × g for 30 min at 4°C to remove unsolubilized materials, and the supernatant was passed through a 0.22μm syringe filter.
For purification of the KOPR, the supernatant was loaded onto a 1ml Ni-NTA agarose column (7.5X 77 mm, Pierce column with extender reservoir), passed once by gravity at 4°C, washed with buffer B for 20 volumes by gravity. The Ni-NTA absorbed proteins were eluted with 5 ml of buffer C. The eluate from Ni-NTA column was loaded onto a mini-column (Pierce, 3X37mm, modified in-lab) packed with 0.1 ml of anti-FLAG M2 antibody-conjugated agarose, passed twice by gravity at 4°C, washed alternately with buffer D and buffer E for a total of 3ml. The receptor was eluted with 0.5ml buffer F. The final eluate was concentrated to 20μl using an Amicon centrifugal filter with a 100KDa cut-off, immediately mixed with SDS–PAGE loading buffer to final concentrations of 2.5% sodium dodecyl sulfate (SDS) and 50mM dithiothreitol (DTT) and incubated at room temperature for 1h.
The samples were resolved with SDS-PAGE using a 4–12% bis-tris pre-casted gel with MES [2-(N-morpholino)ethanesulfonic acid] running buffer. Gel was stained with GelCode (Pierce) and destained with MilliQ water, sealed in a plastic bag and stored at 4°C until LC-MS/MS analysis. The GelCode-stained FmK6H protein appeared as a diffused band with median size of ~52kDa (see Fig. 7A), which was consistent with the band verified by immunoblotting with FLAG antibodies (F7425, Sigma-Aldrich). The two–step purification had ~50% yield of total receptors and afforded ~1 μg KOPR protein. The KOPR protein band was marked on the gel image and sent along with the gel for mass spectrometry analysis.
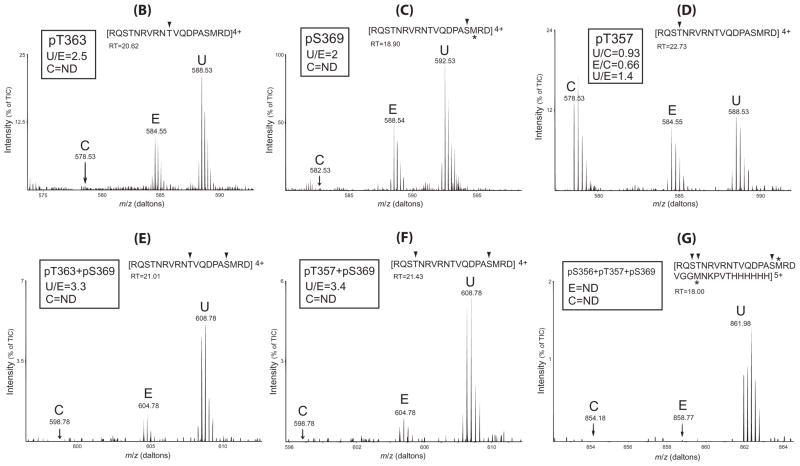
Figure 7. Differential effects of U50,488H and etorphine on KOPR phosphorylation: Determination with SILAC-LC/MS/MS.
(A) Schematic drawing of the experimental procedures. N2A-FmK6H cells were cultured in medium containing regular Arg (Control) (C), Arg6 (etorphine) (E) or Arg10 (U50,488H) (U) for at least 10 passages to label all the proteins. Cells were treated with vehicle, etorphine (1 μM), U50,488H (10 μM) for 30 minutes. Similar numbers of cells from each group were combined and solubilized and mKOPR was purified with Ni-NTA agarose column followed by anti-FLAG affinity column. Eluates were resolved with SDS-PAGE and a broad band of ~52 kDa was excised. In-gel digestion was performed with Glu-C and digested materials were subject to LC-MS/MS for identification and quantitation.
(B–G) phospho-peptide fragments identified and quantitation and relative abundance of the phosphopeptides in control (C), etorphine (E) and U50,488H (U) groups. The peptide fragment detected is shown at the top of each figure with the phosphorylated residue(s) indicated with arrow head. The retention time (RT) of each phosphopeptide in the HPLC run is shown. For each phosphopeptide, the ratio of the amount in the U50,488H sample vs. that in the etorphine sample (U/E) is shown. For the phosphopeptide containing pT357, the ratios of 50,488H/control (U/C) and etorphine/control (E/C) and U/C are shown. Results shown are from one of the two experiments performed with similar results. m/z: mass/charge ratio; ND: not detected; TIC: total ion currents. *: methionine sulfoxide.
LC-MS/MS analysis
Mass spectrometry analysis was performed by Proteomics Facility of Wistar Institute in Philadelphia, PA, which is under the direction of Hsin-Yao Tang, Ph.D. The KOPR protein band was excised from the gel and in-gel micro-digestion was carried out with the endopeptidase Glu-C in phosphate buffer (pH 7.8). Our sequence analysis revealed that Glu-C digestion, which cut at the C-terminal side of Glu and Asp in phosphate buffer, would yield a 19-aa fragment (354 to 372) in the C-terminal domain containing all the possible S and T phosphorylation sites. The digested materials were then subject to LC-MS/MS analysis using Thermo Fisher Hybrid LTQ-OrbitrapXL™ Mass Spectrometers with online Eksigent® Nano-capillary HPLC instrument. Data were analyzed with Peaks Studio 6.0 software (Bioinformatics Solutions Inc) against the Uniprot/SwissProt database in which the sequence of mFK6H is added. Scores for confidence were set at −10logP>15 for peptides, −10logP>20 for proteins, FDR<5%. We did not use trypsin for digestion because it would render the fragment containing S356TN358 too small for analysis.
Generation of antiserum
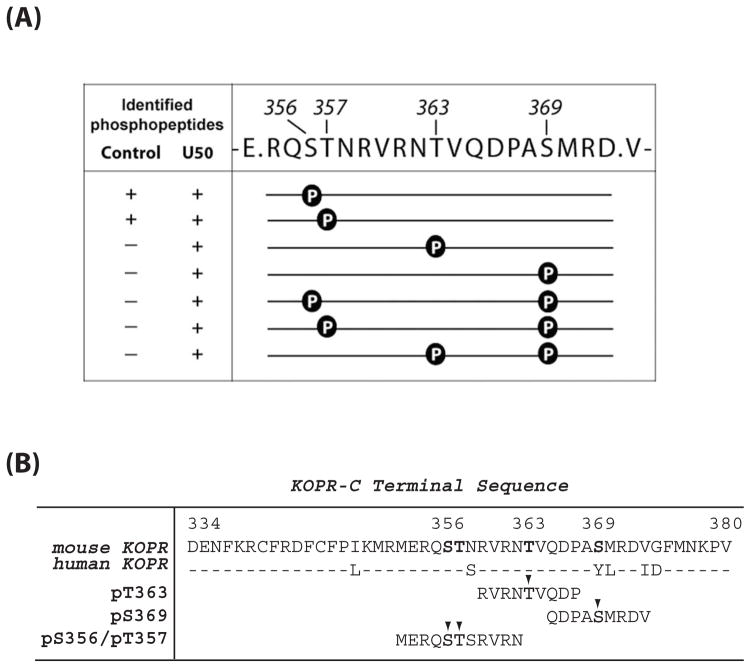
We custom-synthesized three phosphopeptides: RVRNpTVQDP-NH2 (pT363, mKOPR 359–367), QDPApSMRDV (pS369, mKOPR 365–373) and MERQpSpTSRVRN-NH2 (pS356/p357, KOPR 352–360) (see Fig. 1B). Cys was added to the N-terminus to facilitate conjugation to keyhole limpet hemocyanin (KLH). We chose to use short phosphopeptides with the pS or pT in the middle as the antigens based on the approaches of Schulz et al. (17,18) and Busillo et al. (19). Antisera against each phosphopeptide-KLH were generated in two rabbits and tested in ELISA for immunoreactivity with the phosphopeptide antigen by Covance Co. (Denver, PA). Three corresponding unphosphorylated peptides (T363, S369 and S356/T357, respectively) were also synthesized.
Figure 1. (A) Sites of U50,488H-induced phosphorylation in the mouse KOPR.
N2A-FmK6H cells were treated with vehicle or 10 μM U50,488H at 37°C for 30 min. The receptor was purified using a Ni-NTA agarose column followed by an anti-FLAG M2 antibody-agarose affinity column. Eluates were resolved with SDS-PAGE and the KOPR bands were excised and in-gel digested with the endopeptidase Glu-C and subject to LC-MS/MS. Glu-C cuts at the C-terminal to Glu and Asp. The fragment shown here contains all the possible Ser and Thr phosphorylation sites in the C-terminal domain. Data were analyzed with Peaks Studio 6.0. The presence and absence of the phosphopeptide in the samples are indicated by “+” and “−“, respectively. The results were pooled from two experiments.
(B) Sequence of C-terminal domain of the mouse and human KOPRs and sequences of the phosphopeptides used as the antigens for antiserum generation. The phosphorylated residues are indicated with arrow heads.
Purification of antibodies
Antiserum with high titer for the phosphopeptide antigen by ELISA testing was purified by affinity chromatography using the phosphopeptide conjugated to SulfoLink Resin (Pierce). Antiserum was diluted with an equal volume of 25 mM Tris-HCl buffer (pH 7.4) and loaded onto a column containing 1 ml of resin by gravity. The resin was washed with 20x volumes of 25 mM Tris-HCl buffer (pH 7.4) followed by 0.5 M NaCl in 25 mM Tris-HCl buffer (pH 7.4). Elution was carried out with 2 ml/fraction of 0.1 M glycine-HCl buffer (pH 2.5) for 5 fractions and eluates were neutralized immediately with 1 M Tris-HCl buffer (pH 8.0). The fractions were further purified by passing through a column of unphosphorylated peptide-conjugated to SulfoLink Resin to enhance specificity against the phosphorylated peptide. Purification was carried out at 4°C.
Dot blot analysis
Purified antibodies were examined for their phospho-site specificity by dot bot with phosphopeptides vs. unphosphorylated peptides. Each phosphopeptide and its corresponding unphosphorylated peptide were serially diluted and 1.0 μL of each concentration was applied onto a nitrocellulose membrane as a dot in duplicate. The membrane was then dried at 50°C for 30 min, soaked in water and blocked with 2% BSA in 25 mM Tris-buffered saline containing 0.1% Tween 20 (TBS-T, pH7.4) for 30 min at room temperature. Membrane was incubated overnight at 4°C with purified antibody (1μg/mL), followed by goat anti-rabbit IgG conjugated with horseradish peroxidase (HRP) and reacted with enhanced chemiluminescence (ECL) reagent. The chemiluminescence signal was acquired with LAS 1000 plus Image Analyzer (Fuji Photo Film Co., Ltd, Tokyo, Japan) and the intensity of signal of each dot was quantified using the ImageGauge software (Version 4.1, Fuji Photo Film Co. Ltd.).
Immunoblotting (IB)
IB was performed according to our published protocols (20,21). N2A-FmK6H cells were treated with vehicle or 10 μM U50,488H for 30 min. Cells were solubilized and the receptors were partially purified using Ni-NTA agarose. Eluate was resolved with SDS-PAGE and transferred to PVDF membranes (Millipore). IB was performed with affinity purified rabbit pS356/pT357, pS363 or pS369 antibodies (1–2 μg/ml), followed by HRP-conjugated goat anti-rabbit IgG and ECL reagents. Images were captured with a LAS1000 plus system and staining intensities of bands were quantitated using the ImageGauge software. The blots were stripped and re-blotted with rabbit FLAG antibodies (F7425, Sigma-Aldrich) to reveal total KOPRs. Images were again captured and band staining intensity was quantitated. Staining intensity of phosphorylated KOPR was normalized against that of the total KOPR of the same lane.
Treatment with lambda protein phosphatase
Duplicate FmK6H samples were resolved with SDS-PAGE and transferred to PVDF membranes. One of the membranes was incubated with lambda phosphatase at 2000 unit/ml buffer in sealed plastic bag for 3 hours at 30°C and then subject to IB along with the untreated membrane with antibodies indicated.
Generation of phosphorylation site mutants of FmK6H
Each of the phosphorylation sites (serine or threonine) was mutated to alanine using the overlap the PCR method (22). The following mutants were generated: S356A, T357A, S356A/T357A, T363A and S369A in the mKOPR. Each of the mutation (±) primer pairs was designed by replacing the codon of serine or threonine with that of alanine and then flanking the mutation with 9 original neighboring nucleotides added at both 5′ and 3′. The 5′ primer was mK.EcoRI510(+) (TTG ATG AAT TCT TGG CCT TTT). The 3′ primer was BGHR (ACT AGA AGG CAC AGT CGA GG), which is a vector sequence located downstream from the multiple cloning sites. The PCR fragment (~0.8kb) was cut by EcoRI and AgeI (New England Biolab) and cloned into pcDNA6-FmK6H vector cut by the same two enzymes (~5.7kb). DNA sequences were determined with the BGHR primer to confirm desired mutations and to ensure no unwanted mutations (Genewiz, South Plainfield, NJ).
Immunofluorescence staining of receptor internalizaton
N2A-FmK6H cells grown on cover slips for 48h were washed and incubated with serum free MEM medium for 1 h. Cells were incubated with M1 mouse anti-FLAG antibodies for 30 min and then treated with 1 and 10 μM U50,488H or 0.1 and 1 μM etorphine for 30 min. Cells were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH7.0), permeabilized, stained with goat anti-mouse IgG conjugated with Alex-Fluor 488. Cells were examined under a fluorescence microscope and images captured.
Effects of S356A, T357A, S356A/T357A, T363A and S369A mutations on U50,488H-promoted KOPR phosphorylation
Each of the wildtype and S356A, T357A, S356A/T357A, T363A and S369A mutants of FmK6H was transiently transfected into N2A cells with Lipofectamine. Cells were grown in the presence of 5 μg/ml blasticidin for at least 7 days and mixed clonal cells stably transfected with the receptor were established. Cells were harvested and lysed, receptors were partially purified and IB was carried out as described above.
(c) Results
U50,488H promoted mKOPR phosphorylation at S356, T357, T363 and S369
Cells were incubated with vehicle or U50,488H for 30 min. This incubation time was shown previously to detect KOPR phosphorylation. Two samples each of vehicle- and U50,488H-treated mouse KOPR were analyzed with LC-MS/MS. All the phosphopeptides in the C-terminal domain were found in the fragment [KOPR(354–372)] shown in Fig. 1A. Glu-C cleaves E-P and D-P bonds poorly (23), which resulted in generation of KOPR(354–372) as the predominant peptide product.
Peptides containing 1 or 2 phosphates were identified. In control samples, peptides containing pS356 or pT357 were identified. In U50,488H (10 μM, 30 min)-treated samples, peptides containing pS356, pT357, pT363, pS369, pS356+pS369, pT357+pS369 and pT363+pS369 were found.
Specificity of antibodies for phospho-peptide antigens by dot blot analysis of antibodies against phosphopeptides
Antiserum was generated in rabbits against three phosphopeptides, pS356/pT357, pT363 and pS369 (Fig. 1B) conjugated to KLH and each of the three antibodies was purified with affinity chromatography with the phosphopeptide antigen followed by adsorption of antibodies against its corresponding unphosphorylated peptide.
Antibodies thus purified were tested for their reactivity with phosphopeptides vs. unphosphorylated peptides using dot blot and staining intensity was quantified. As shown in Supplemental Fig. 1A, each of anti-p363 and anti-p369 reacted with its phosphopeptide antigen in a dose-dependent manner, but it did not react with the corresponding unphosphorylated peptide. Interestingly, anti-pS356/pT357 reacted with the pT357 peptide better than the pS356/pT357 peptide, but it barely recognized the pS356 peptide and it did not react with the unphosphorylated peptides. These results indicate that each antibody has high specificity for the phosphopeptide used as the antigen, compared with the corresponding unphosphorylated peptide.
We also tested cross-reactivity of antibodies with phosphopeptides other than the antigens (Supplemental Fig. 1B). None of the three antibodies showed cross-reactivity with non-antigen phospho-peptides.
Antibody specificity: Immunoblotting of KOPRs with purified antibodies against phosphopeptides
N2A-FmK6H cells were treated with vehicle or 10 μM U50,488H for 30 min. Cells were solubilized and FmK6H was partially purified with a Ni-NTA agarose column. The eluate was resolved with SDS-PAGE and IB with each of anti-pS356/pT357, anti-pS363 and anti-pS369 revealed a high-intensity, broad and diffuse band of Mr~52 kDa in U50,488H-treated samples (Fig. 2A, phosphorylated mKOPR). In the control samples, there was no staining with anti-pS363 and anti-pS369; however, there was a faint staining with anti-pS356/pT357. Without Ni-NTA agarose purification, none of the antibodies worked.
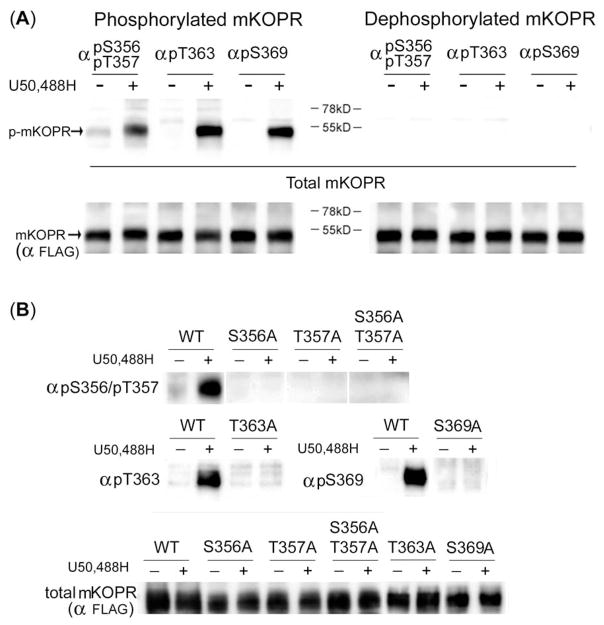
Figure 2. (A) phospho-peptide antibodies have high specificity for phosphorylated KOPR.
left: U50,488H greatly enhanced anti-pS356/pT357, anti-pT363 and anti-pS369 immunoblotting intensity of the mKOPR. N2A-FmK6H cells were treated with vehicle or 10 μM U50,488H for 30 min. Cells were solubilized and the receptors were partially purified with a Ni-NTA agarose column. Eluates were subject to SDS-PAGE and immunoblotting with indicated antibodies. Membranes were stripped and re-blotted with anti-FLAG antibodies for total KOPR. The experiments were performed three times with similar results.
right: Immunoblotting intensity was greatly reduced by dephosphorylation. Experiments were performed as described above, except that PVDF membranes with transferred proteins were incubated with lambda phosphatase and then subject to immunoblotting with indicated antibodies. Membranes were stripped and re-blotted with anti-FLAG antibodies for total KOPR. Phosphatase treatment reduced staining of U50,488H-treated samples, indicating phospho-specificity of the antibody. In addition, phosphatase reduced pS356/pT357 staining in the control, demonstrating constitutive phosphorylation of the sites. These experiments were performed four times with similar results.
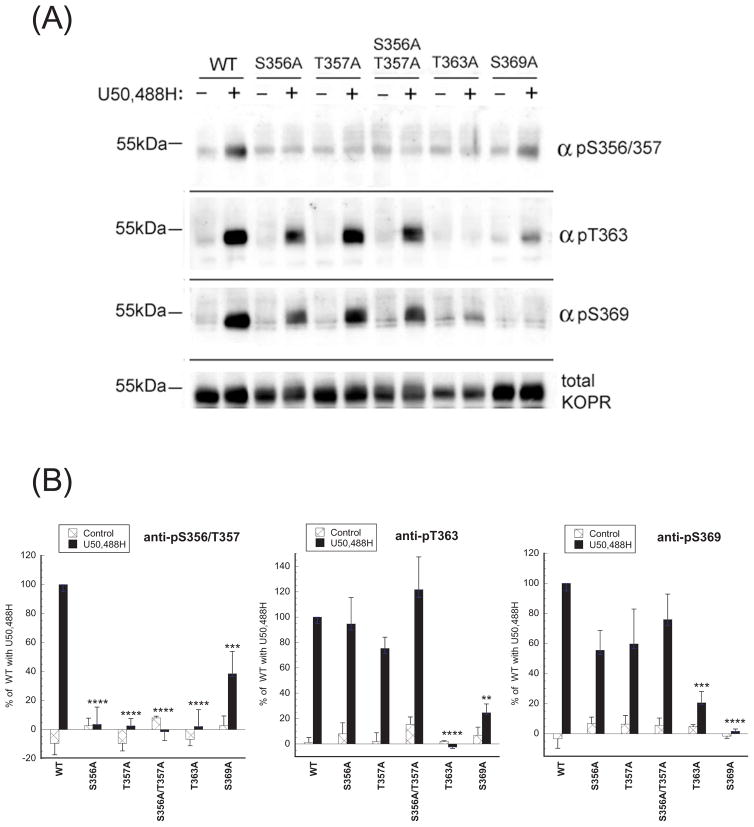
(B) Effects of mutations in the mKOPR on U50,488H-promoted receptor phosphorylation detected with phospho-KOPR antibodies. The cDNA construct of the wildtype, S356A, S357A, S356A/S357A, T363A or S369A mutant of the mKOPR was transfected into N2A cells and stable mixed clonal cells were established. Cells were treated with vehicle or U50,488H (10 μM) for 30 min, harvested and receptor proteins were purified and resolved with SDS-PAGE. Immunoblotting was performed with the indicated antibodies. The amount of total mKOPR was determined with another gel loaded with the same aliquots. S356A, S357A or S356A/S357A substitutions abolished basal and U50,488H-promoted mKOPR phosphorylation detected by anti-pS356/pT357. T363A and S369A mutations eliminated mKOPR phosphorylation detected by anti-pT363 and anti-pS369 staining, respectively. The experiments were performed two times with similar results.
Blots were then stripped and re-blotted with rabbit anti-FLAG antibodies to stain total KOPR. The mKOPR was revealed as a diffuse band of 52 kDa (Fig. 2A, total mKOPR) and a sharp band of 45 kDa (not shown) and the amounts of total KOPR were not different between the vehicle- and U50,488H-treated groups.
Untransfected N2A cells were subject to similar treatment and purification procedures and none of anti-pS356/pT357, anti-pS363 and anti-S369 detected a 52-Ka band (data not shown).
When mKOPR samples were treated with lambda protein phosphatase, which dephosphorylates phospho-S, -T and -Y, U50,488H-promoted staining in the 52-kDa protein band by anti-pS356/pT357, anti-pS363 and anti-pS369 was eliminated (Fig. 2A, dephosphorylated mKOPR). These results indicate that the immunoreactivity at 52-kDa band is due to phosphorylated KOPR. Similarly, lambda phosphatase reduced the levels of anti-pS356/pT357 staining of the control and U50,488H-treated mKOPR samples to undetectable levels, demonstrating that S356/T357 in the mKOPR are constitutively phosphorylated, consistent with the results shown in Fig. 1.
Antibody specificity: Effects of mutations in KOPRs on antibody reactivity
Alanine substitution of serine or threonine residue abolishes phosphorylation at either amino acid. S356A, T357A or S356A/T357A substitution abrogated anti-pS356/pT357 staining in control and U50,488H-treated mKOPR (Fig. 2B). T363A and S369A mutations of the FmK6H eliminated U50,488H-induced staining by anti-pT363 and anti-pS369, respectively (Fig. 2B).
Thus, in the mKOPR, anti-pS356/pT357, anti-pS363 and anti-pS369 react with the mKOPR phosphorylated at S356/T357, S363 and S369, respectively. These results further validate the phospho-specificity of antibodies.
U50,488H-induced KOPR phosphorylation was blocked by nor-binaltorphimine (nor-BNI)
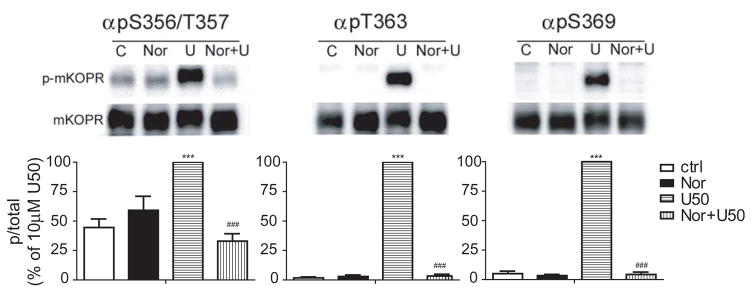
N2A-FmK6H cells were treated with the selective KOPR antagonist norBNI (1 μM) for 1 h followed by 30-minute treatment with 10 μM U50,488H. Cells were lysed and the receptor partially purified. While norBNI itself had no effect on staining of the control mKOPR samples by any of the antibodies (Fig. 3), norBNI pretreatment abolished U50,488H-promoted phosphorylation of the mKOPR as detected with anti-pS356/pT357, pT363 and pS369 antibodies (Fig. 3), indicating that phosphorylation is mediated by activation of the KOPR.
Figure 3. U50,488H-induced phosphorylation of the mouse KOPR was blocked by norBNI pretreatment.
N2A-FmK6H cells were treated with vehicle or 1 μM norBNI for 1 h followed by 30-minute treatment with vehicle or 10 μM U50,488H. Cells were lysed and receptors were partially purified. The eluted samples were used for immunoblotting with indicated phospho-specific antibodies. Staining intensity of each phosphorylated KOPR was normalized against that of the total KOPR in the same lane. Data were then normalized against those of 10 μM U50,488H-treated group and each value represents the mean ± S.E.M of 4–6 independent experiments. Data were analyzed by one-way ANOVA followed by Newman-Keuls post hoc test. (***:p<0.001, compared to untreated group; ###:p<0.001, compared to U50,488H-treated group).
Effects of mutations in KOPRs on U50,488H-promoted KOPR phosphorylation
We then examined if mutations of S356, T357, T363 or S369 affected phosphorylation other residues following U50,488H treatment (Fig. 4).
Figure 4. Effects of S356A, S357A, S356A/S357A, T363A or S369A mutation on U50,488H-promoted phosphorylation of the mouse KOPR.
(A) Stable mixed clonal N2A cells expressing the wildtype or one of the mutants were treated with vehicle or U50,488H (10 μM) for 30 min, harvested and receptor proteins were purified and resolved with SDS-PAGE. Immunoblotting was performed with the indicated antibodies. Membranes were stripped and re-blotted with anti-FLAG antibodies for total KOPR. (B) Staining intensity of each phosphorylated KOPR was normalized against that of the total KOPR in the same lane. Data were then normalized to those of 10 μM U50,488H-treated wildtype group and each value represents the mean ± S.E.M of 3 independent experiments. Data were analyzed by one-way ANOVA followed by Newman-Keuls post-hoc test. (**:p<0.01, ***:p<0.001, ****:p<0.0001, compared to U50,488H-treated group).
Anti-pS356/pT357 staining was eliminated by S356A, T357A, S356A/T357A and T363A substitution, and reduced by S369A mutation. These results indicate that both T363 and S369 are important for phosphorylation of S356 and T357.
Anti-pT363 staining was abolished by T363A mutation as expected. Unexpectedly, S369A substitution greatly reduced anti-pT363 staining. In contrast, S356A, T357A or S356A/T357A mutations did not affect anti-pT363 staining. Thus, S369 is important for phosphorylation of T363.
S369A substitution eliminated and T363A mutation greatly reduced anti-pS369 staining, indicating that T363 is important for phosphorylation of S369. In contrast, S356A, T357A, and S356A/T357A did not have any effect.
Taken together, these results demonstrate that phosphorylation of either S369 or T363 impacts on phosphorylation of the other and affects phosphorylation of S356/T357.
At a saturation concentration, U50,488H promoted higher levels of KOPR phosphorylation than etorphine
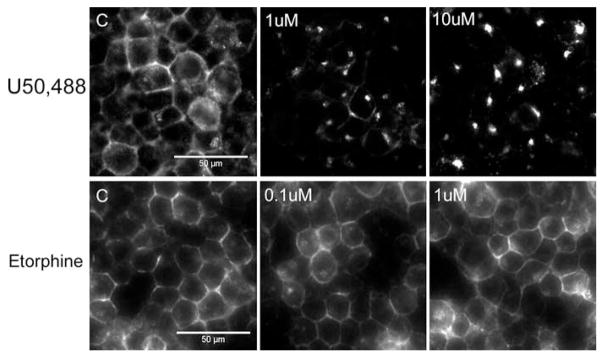
We previously reported that both U50,488H and etorphine were full agonists in promoting KOPR-mediated [35S]GTPγS binding with EC50 values of 4.1 nM and 0.87 nM, respectively (24). In addition, after 60-min treatment while U50,488H caused internalization of ~41% cell surface mKOPR (EC50 1.4 nM), etorphine did not at the highest concentration of 1 μM, when determined with on-cell western (24). To focus on agonist efficacy and avoid influence of affinity, we treated cells with a saturation concentration of each agonist (U50,488H 1 or 10 μM; etorphine 0.1 or 1 μM) to maximize receptor occupancy. Fig. 5 shows that treatment with 1 or 10 μM U50,488H for 30 min caused robust internalization of the mKOPR detected by immunocytochemical staining, whereas treatment with 0.1 or 1 μM etorphine did not. We have shown previously that net internalization of KOPR could be observed at 30 min (25), so 30 min was used.
Figure 5. U50,488H induced mKOPR internalization, but etorphine did not.
N2A-FmK6H cells grown on cover slips were incubated with M1 anti-FLAG antibodies for 30 min and then treated with 1 and 10 μM U50,488H or 0.1 and 1 μM etorphine for 30 min. Cells were fixed with paraformaldehyde, permeated, stained with goat anti-mouse IgG conjugated with Alex-Fluor 488. Images were acquired using 40X objective. The experiment was performed three times with similar results.
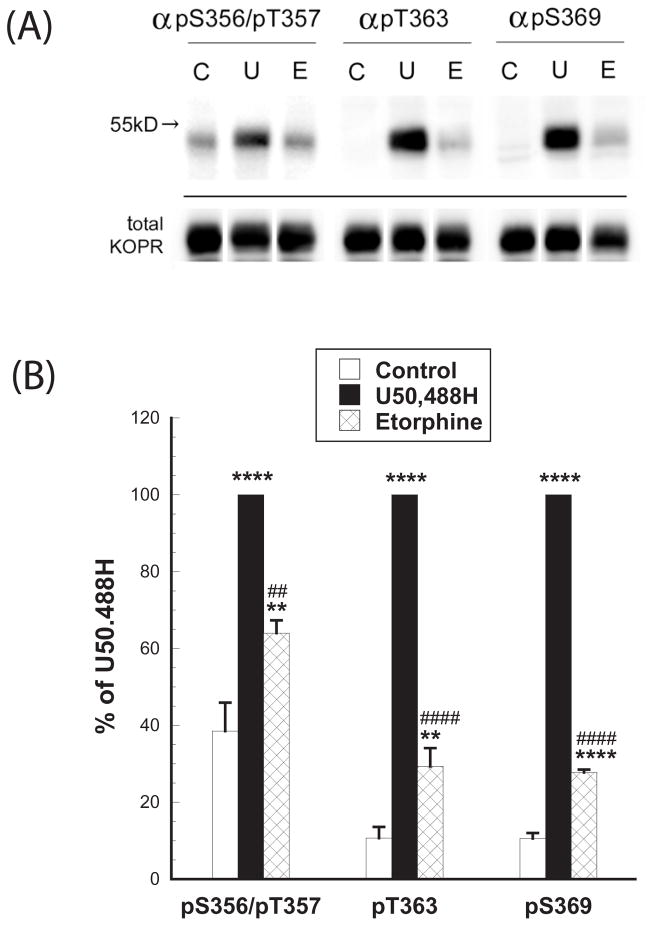
We examined if the two agonists caused differential mKOPR phosphorylation. Both agonists promoted significant KOPR phosphorylation, detected with immunoblotting using anti-pS356/pT357, anti-pT363 and anti-pS369 (Figs. 6A and 6B). In addition, U50,488H promoted much higher levels of KOPR phosphorylation than etorphine at all the sites (Figs. 6A and 6B). Staining intensity with anti-pS356/pT357 was in the order of U50,488H > etorphine > control. Anti-pT363 and anti-pS369 staining was in the order of U50,488H ≫ etorphine > control. (Fig. 6B).
Figure 6. At a saturation concentration, U50,488H promoted higher levels of KOPR phosphorylation than etorphine: Determination with immunoblotting.
N2A-FmK6H cells were treated with vehicle, 10 μM U50,488H or 1 μM etorphine for 30 min. Cells were lysed and mKOPR was partially purified. The eluted samples were subject to SDS-PAGE and then immunoblotting with indicated phospho-specific antibodies. The blots were stripped and stained with rabbit FLAG antibodies (F7425) to reveal total KOPRs (bottom blots). Intensity of phospho-KOPR was normalized against that of the total KOPR of the same lane. Data were then normalized against that of U50,488H in the same group. The experiment was performed three times with similar results. Data were analyzed by one-way ANOVA and Newman-Keuls post-hoc test. (**:p<0.01, ****:p<0.0001, compared to the control group; ##:p<0.01, ####:p<0.0001, compared to the U50,488H-treated group).
U50,488H induced much higher levels of single-, double- and triple-site KOPR phosphorylation than etorphine
We then used a combined SILAC and LC-MS/MS approach to quantitatively compare U50,488H- and etorphine-induced KOPR phosphorylation. Fig. 7A shows the experimental procedures. N2A-FmK6H cells were grown in the presence of Arg, Arg6 or Arg10 for at least 10 passages to label proteins to 99% containing Arg6 or Arg10, which were determined by LC-MS/MS (not shown). Cells were treated with vehicle (C, Arg), etorphine (E, Arg6) or U50,488H (U, Arg10) for 30 min and similar amounts of FmK6H were combined from the three groups and purified with Ni-NTA agarose column followed by anti-FLAG affinity chromatography. The FmK6H was resolved as a broad band of Mr 52kDa by SDS-PAGE, in-gel digested with endoproteinase Glu-C and analyzed with LC-MS/MS. This approach ensures co-elution in the LC step of the same peptides labeled with different Arg isotopes prior to MS ionization (14,15).
NFKRCFRDFCFPIKMRME, a peptide fragment in the proximal region of the C-terminal domain, was detected. The amount of this phosphorylation-independent KOPR fragment was used to quantify relative amounts of the KOPR loaded, which were in the ratio of 1.0 (Control): 0.9 (U50,488H): 1.1 (etorphine) (Supplemental Fig. 2). These results indicate that there were similar amounts of the KOPR loaded from the three groups. This ratio was used for calibration in the following quantitation of the phosphopeptides.
The same peptide fragment KOPR(354–372) containing one or two phosphate groups were identified (Figs. 7B–7F). In addition, a longer peptide KOPR(354–380) plus 6XHis epitope containing three phosphate groups was found (Fig. 7G). These phosphopeptides were quantified, revealing distinct imprints of the two different ligands on KOPR phosphorylation.
In the control samples, basal phosphorylation was detected at T357 (Fig. 7D), but not for any other sites. The two ligands promoted single-site phosphorylation primarily at T363 (Fig. 7B) and S369 (Fig. 7C) with U/E=2.5 and 2, respectively. In contrast, U50,488H or etorphine did not increase T357 phosphorylation (Fig. 7D), indicating constitutive phosphorylation. Both agonists also induced phosphorylation at two sites at T363+S369 (Fig. 7E), T357+S369 (Fig. 7F) with the ratios of U/E=3.3 and 3.4, respectively, while only the U50,488H yield peptides containing three phosphorylated resides at S356+T357+S369 (Fig. 7G). Therefore, U50,488H produces higher levels of single-, double- and triple-phosphorylated forms of the KOPR and U50,488H differs more markedly from etorphine in higher-order KOPR phosphorylation. Since the phosphopeptide fragments contain one or two phosphates have the same mass and charges (Fig. 7B–7F), in U50,488-treated samples the relative abundance of phosphopeptides, which can be determined from total ion currents (%TIC), was pS369 > pT363 ~pT357 > pT363+pS369 ~ pT357 +pS369. The triple phosphorylated pS356+pT357+pS369 fragment showed the lowest abundance (Fig. 7G).
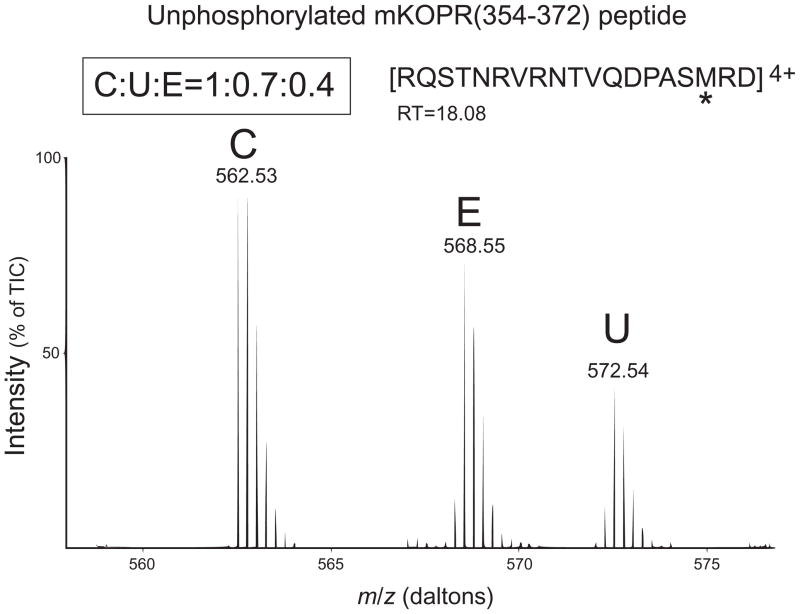
In addition, because all the phosphorylation sites are within the fragment R354QSTNRVRNTVQDPASMRD372, the unphosphorylated fragment was used to calculate the % of total KOPR being phosphorylated. Fig. 8 shows that the ratio of the amount of the unphosphorylated fragment is control: etorphine: U50,488H = 1: 0.7: 0.4. Thus, about 60% and 30% of the receptors are phosphorylated following U50,488H and etorphine treatment, respectively.
Figure 8. Relative abundance of the unphosphorylated peptide KOPR(354–372), in control (C), etorphine (E) and U50,488H (U) groups, determined with SILAC-LC/MS/MS.
Experiments were performed as described in Fig. 7 legend. The corresponding unphosphorylated peptide KOPR(354–372) was identified in LC-MS/MS analysis. This peptide contains all the four phosphorylation sites and the relative abundance was determined to be C:U:E=1:0.7:0.4. Results shown are from one of the two experiments performed with similar results.
(d) Discussion
We have shown that U50,488H promotes phosphorylation of the mKOPR at S356, T357, T363 and S369 in the C-terminal domain, which is the first to directly identify phosphorylation sites of the KOPR. We have generated and purified antibodies that recognize with high specificity mKOPR phosphorylated at S356/T357, T363 and S369 in immunoblotting. These antibodies are the first to recognize all the phosphorylation sites in the C-terminal domain of the mKOPR; therefore, they will be very useful for studies of KOPR biochemical pharmacology.
In addition, we compared U50,488H and etorphine, two full agonists in activating G proteins, but differing in their abilities to promote KOPR internalization. U50,488H induced higher degrees of phosphorylation and the differences were more pronounced for double and triple phosphorylation. Etorphine did not induce KOPR internalization even though it promoted phosphorylation, albeit at lower levels, demonstrating that an above-threshold KOPR phosphorylation is required for KOPR internalization and higher-order phosphorylation may be more important.
Etorphine caused lower KOPR phosphorylation than U50,488H as determined by immunoblotting and SILAC-LC/MS/MS
Etorphine, a non-selective opioid agonist, activated KOPR-mediated [35S]GTPγS binding as a full agonist, similar to U50,488H. However, while U50,488H promoted robust mKOPR internalization, etorphine caused no mKOPR internalization. We found by immunoblotting that etorphine caused lower levels of KOPR phosphorylation at S356/T367, T363 and S369.
SILAC and LC-MS/MS results showed that U50,488H caused 2–2.5x, 3.3–3.4x of KOPR single and double phosphorylation as etorphine, respectively. U50,488H, but not etorphine, caused triple phosphorylation. Therefore, there are quantitative and qualitative differences in KOPR phosphorylation between the two agonists. Although etorphine induced phosphorylation of ~30% of KOPR phosphorylation, it did not cause KOPR internalization, indicating that there is a threshold level of KOPR phosphorylation needed for internalization and etorphine did not reach the threshold. This finding is consistent with that of Lau et al. (26) that morphine did not cause MOPR internalization even though it induced MOPR phosphorylation, albeit at lower levels than DAMGO.
In addition, because the differences between U50,488H and etorphine are more pronounced in double and triple phosphorylation, higher-order phosphorylation may be more important for agonist-promoted KOPR phosphorylation. This observation is in accord with that on the MOPR. Lau et al. (26) showed that DAMGO promoted higher levels of multi-phosphorylated states of S375TANT379 in the MOPR than morphine, which were critical for agonist-induced internalization and β-arrestin recruitment.
T363 and S369 are two main and initial phosphorylation sites
Using the phospho-KOPR specific antibodies and KOPR phosphorylation site mutants, we demonstrated that T363 and S369 were the primary phosphorylation sites and phosphorylation of either residue appeared to be important for phosphorylation of the other and phosphorylation of either T363 or S369 was critical for phosphorylation of S356/T357. Our SILAC-LC/MS/MS results that U50,488H or etorphine did not increase single phosphorylation at T357 (Fig. 7D), but increased double phosphorylation at T357+S369 (Fig. 7F) and triple phosphorylation at S356+T357+S369 (Fig. 7G) are consistent with the notion that S369 phosphorylation permits phosphorylation at S356 and T357. These results are also consistent with our immunoblotting data that U50,488H enhanced pS356/pT357 staining. The finding that S369A mutation alone inhibited rat KOPR internalization (13) can be reconciled with our observations that S369 phosphorylation is important for phosphorylation of T363 and S356/T357 and that higher-order of KOPR phosphorylation may contribute more to KOPR internalization.
Combined SILAC and LC-MS/MS approach
The combination of SILAC and LC-MS/MS is an extremely powerful approach and has been used to quantify differences in phosphorylation at certain residues of the MOPR and the β2-adrenergic receptor (β2-AR) induced by different agonists (26,27). The ratios of the fragments with phosphorylation at multiple sites revealed greater differences between morphine- and DAMGO-treated MOPR (26) and among isoproterenol-, epinephrine- and dopamine-treated β2-AR (27) than those with single phosphorylation.
Comparison of combined SILAC and LC-MS/MS approach with immunoblotting using phospho-site specific antibodies
The SILAC-LC-MS/MS approach and immunoblotting with phospho-site specific antibodies complement each other. Each approach has its strengths and weaknesses. Combined SILAC- and LC-MS/MS reveals KOPR phosphorylated at one, two or three sites and relative abundance of each phosphorylated form among different treatments, whereas immunoblotting detects KOPR phosphorylated at a particular residue regardless of phosphorylation states of other residues. For example, anti-pS369 antibodies likely detect pS369-, pT363/pS369-, pT357/pS369- and pS356/pT357/pS369-containing KOPR. SILAC-LC-MS/MS provides more precise quantitation, whereas density in immunoblotting is semi-quantitative. SILAC-LC-MS/MS requires access to labs specializing in doing LC-MS/MS, whereas immunoblotting can be done by labs experienced in such a biochemistry technique, provided that specific antibodies are available. However, phospho-specific antibodies have been generated for only a few 7TMRs (see below).
Specificity of the antibodies for phospho-KOPR
Specific antibodies against 7TMRs have been difficult to obtain [(28) and references therein] and those against phosphorylated 7TMRs are even more scarce. There are a few specific antibodies against phosphorylated 7TMRs, including, for example, the MOPR (17,18), CXCR4 (19), M3 muscarinic receptor (29,30) and β2-adrenergic receptor (31). Chavkin and colleagues have previously reported antibodies that recognize rat KOPR phosphorylated at S369 (11).
Specificity of 7TMR antibodies has always been an issue, which was why we went to great lengths to characterize specificities of these antibodies. We purified the antibodies with affinity chromatography using the phospho-peptide antigen followed by removal of antibodies recognizing its unphosphorylated peptide counterpart.
The specificity of each purified antibody was tested using several approaches. Using dot blot analysis, we found that each purified antibody had high specificity for phosphopeptide vs. the corresponding unphosphorylated peptide.
When the purified antibodies were used in immunoblotting, U50,488H treatment greatly enhanced anti-pT363 and anti-pS369 staining in the mKOPR. Anti-pS356/pT357 showed high basal staining and U50,488H slightly increased the staining. Each of the antibodies recognized a diffuse band of ~52kDa. This band was absent in similarly-treated untransfected N2A cells. The diffuse nature and relative molecular weights of the KOPR are consistent with our previous findings [for example, (20,21)]. We have shown that the 52-kDa band is fully glycosylated form residing in trans-Golgi and plasma membranes, whereas the 45-kDa band is glycosylated intermediates, residing in the endoplasmic reticulum and cis-Golgi (21). The immunoblotting results that U50,488H enhanced phosphorylation of the 52-kDa band, but not the 45-kDa band are consistent with the notion that agonist increases phosphorylation of fully glycosylated KOPR, most likely in plasma membranes.
Phosphatase treatment profoundly reduced or eliminated the staining in both control and U50,488H-treated samples. S356A/T357A, T363A and S369A substitutions in the mKOPR eliminated anti-pS356/pT357, anti-pS363 and anti-pS369 staining, respectively.
Taken together the results indicate that these antibodies have high specificity for phosphorylated KOPR.
Antibodies specific for phosphorylated KOPR will be very useful for biochemical pharmacology of KOPR in general and for trafficking, signaling and regulation of the KOPR in particular. Using these antibodies, we are carrying out studies to determine the time course and dose-response relationship of phosphorylation at each site, differential phosphorylation of KOPR by agonists at each site, involvement of various G protein-coupled receptor kinases and other kinases in phosphorylation of each site and cell- and tissue-specific KOPR phosphorylation. Of particular interest is whether KOPR agonists biased for the G protein- or the arrestin-mediated pathways cause differential phosphorylation at these sites. Several G protein- and β-arrestin-biased KOPR agonists have been reported (12,24,32–34). These phospho-specific KOPR antibodies may be great tools for differentiating biased agonists.
Supplementary Material
Summary statement.
U50,488H-promoted KOPR phosphorylation sites were identified and phospho-KOPR specific antibodies obtained. U50,488H, but not etorphine, induced KOPR internalization. U50,488H promoted higher phosphorylation than etorphine and higher-order phosphorylation may be important for KOPR internalization, which requires KOPR phosphorylation above a threshold.
Acknowledgments
(f) Funding
This work was supported by National Institutes of Health National Institute on Drug Abuse grants R01 DA017302, R03 DA036802 and P30 DA013429.
Abbreviations
- 7TMR
seven transmembrane receptor
- DDM
n-Dodecyl-β-D-maltoside
- ELISA
enzyme-linked immunosorbant assay
- ECL
enhanced chemiluminescence
- FLAG epitope tag
DYKDDDDK
- FBS
Fetal bovine serum
- FmK6H
mouse KOPR epitope-tagged with FLAG at the N-terminus and 6xHis at the C-terminus
- GRKs
G protein-coupled receptor kinases
- GTPγS
guanosine-5′-O-(3-thio) triphosphate
- HRP
horseradish peroxidase
- IB
immunoblotting or western blot
- IP
immunoprecipitation
- ICC
immunocytochemistry
- KOPR
kappa opioid receptor
- KLH
keyhole limpet hemocyanin
- LC-MS/MS
liquid chromatography tandem mass spectroscopy
- MEM
Minimum Essential Media
- Ni-NTA agarose
nickel-nitrilotriacetic acid agarose
- nor-BNI
nor-binaltorphimine
- N2A
Neuro2A mouse neuroblastoma cells
- PVDF membrane
polyvinylidene fluoride membrane
- PCR
polymerase chain reaction
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- U50
488H, (−)(trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidiny) cyclohexyl] benzeneacetamide
Footnotes
(e) Declarations of interest
There is no conflict of interests.
(g) Author contribution statement
Design experiments: Chongguang Chen*, Yi-Ting Chiu*, Lee-Yuan Liu-Chen*
Perform experiments: Chongguang Chen*, Yi-Ting Chiu*, Wenman Wu*, Peng Huang*, Anika Mann†
Data analysis: Chongguang Chen*, Yi-Ting Chiu*, Wenman Wu*, Lee-Yuan Liu-Chen*
Provide reagents: Anika Mann†, Stefan Schulz†,
Write manuscript: Lee-Yuan Liu-Chen, Chongguang Chen*, Yi-Ting Chiu*.
(h) References
- 1.von Voigtlander PF, Lahti RA, Ludens JH. U-50,488: a selective and structurally novel non-Mu (kappa) opioid agonist. J Pharmacol Exp Ther. 1983;224:7–12. [PubMed] [Google Scholar]
- 2.Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- 3.Barber A, Gottschlich R. Novel developments with selective, non-peptidic kappa-opioid receptor agonists. Expert Opin Investig Drugs. 1997;6:1351–1368. doi: 10.1517/13543784.6.10.1351. [DOI] [PubMed] [Google Scholar]
- 4.Slizgi GR, Ludens JH. Studies on the nature and mechanism of the diuretic activity of the opioid analgesic ethylketocyclazocine. J Pharmacol Exp Ther. 1982;220:585–591. [PubMed] [Google Scholar]
- 5.Togashi Y, Umeuchi H, Okano K, Ando N, Yoshizawa Y, Honda T, Kawamura K, Endoh T, Utsumi J, Kamei J, Tanaka T, Nagase H. Antipruritic activity of the kappa-opioid receptor agonist, TRK-820. Eur J Pharmacol. 2002;435:259–264. doi: 10.1016/s0014-2999(01)01588-6. [DOI] [PubMed] [Google Scholar]
- 6.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 7.Law P-Y. Opioid receptor signal transduction mechanisms. In: Pasternak GW, editor. The Opiate Receptors. The Humana Press; New York, NY: 2011. [Google Scholar]
- 8.Liu-Chen LY. Agonist-induced regulation and trafficking of kappa opioid receptors. Life Sci. 2004;75:511–536. doi: 10.1016/j.lfs.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 9.Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci. 2007;27:11614–11623. doi: 10.1523/JNEUROSCI.3769-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLennan GP, Kiss A, Miyatake M, Belcheva MM, Chambers KT, Pozek JJ, Mohabbat Y, Moyer RA, Bohn LM, Coscia CJ. Kappa opioids promote the proliferation of astrocytes via Gbetagamma and beta-arrestin 2-dependent MAPK-mediated pathways. J Neurochem. 2008;107:1753–1765. doi: 10.1111/j.1471-4159.2008.05745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLaughlin JP, Myers LC, Zarek PE, Caron MG, Lefkowitz RJ, Czyzyk TA, Pintar JE, Chavkin C. Prolonged kappa opioid receptor phosphorylation mediated by G-protein receptor kinase underlies sustained analgesic tolerance. J Biol Chem. 2004;279:1810–1818. doi: 10.1074/jbc.M305796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White KL, Robinson JE, Zhu H, DiBerto JF, Polepally PR, Zjawiony JK, Nichols DE, Malanga CJ, Roth BL. The G Protein-Biased kappa-Opioid Receptor Agonist RB-64 Is Analgesic with a Unique Spectrum of Activities In Vivo. J Pharmacol Exp Ther. 2015;352:98–109. doi: 10.1124/jpet.114.216820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLaughlin JP, Xu M, Mackie K, Chavkin C. Phosphorylation of a carboxy-terminal serine within the kappa opioid receptor produces desensitization and internalization. J Biol Chem. 2003;278:34631–34640. doi: 10.1074/jbc.M304022200. [DOI] [PubMed] [Google Scholar]
- 14.Mann M. Functional and quantitative proteomics using SILAC. Nat Rev Mol Cell Biol. 2006;7:952–958. doi: 10.1038/nrm2067. [DOI] [PubMed] [Google Scholar]
- 15.Ong SE, Mann M. A practical recipe for stable isotope labeling by amino acids in cell culture (SILAC) Nat Protoc. 2006;1:2650–2660. doi: 10.1038/nprot.2006.427. [DOI] [PubMed] [Google Scholar]
- 16.Guan XM, Kobilka TS, Kobilka BK. Enhancement of membrane insertion and function in a type IIIb membrane protein following introduction of a cleavable signal peptide. J Biol Chem. 1992;267:21995–21998. [PubMed] [Google Scholar]
- 17.Schulz S, Mayer D, Pfeiffer M, Stumm R, Koch T, Hollt V. Morphine induces terminal micro-opioid receptor desensitization by sustained phosphorylation of serine-375. EMBO J. 2004;23:3282–3289. doi: 10.1038/sj.emboj.7600334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doll C, Konietzko J, Poll F, Koch T, Hollt V, Schulz S. Agonist-selective patterns of micro-opioid receptor phosphorylation revealed by phosphosite-specific antibodies. Br J Pharmacol. 2011;164:298–307. doi: 10.1111/j.1476-5381.2011.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Busillo JM, Armando S, Sengupta R, Meucci O, Bouvier M, Benovic JL. Site-specific phosphorylation of CXCR4 is dynamically regulated by multiple kinases and results in differential modulation of CXCR4 signaling. J Biol Chem. 2010;285:7805–7817. doi: 10.1074/jbc.M109.091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C, Li JG, Chen Y, Huang P, Wang Y, Liu-Chen LY. GEC1 interacts with the kappa opioid receptor and enhances expression of the receptor. J Biol Chem. 2006;281:7983–7993. doi: 10.1074/jbc.M509805200. [DOI] [PubMed] [Google Scholar]
- 21.Li JG, Chen C, Liu-Chen LY. N-Glycosylation of the human kappa opioid receptor enhances its stability but slows its trafficking along the biosynthesis pathway. Biochem. 2007;46:10960–10970. doi: 10.1021/bi700443j. [DOI] [PubMed] [Google Scholar]
- 22.Higuchi R, Krummel B, Saiki RK. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breddam K, Meldal M. Substrate preferences of glutamic-acid-specific endopeptidases assessed by synthetic peptide substrates based on intramolecular fluorescence quenching. Eur J Biochem. 1992;206:103–107. doi: 10.1111/j.1432-1033.1992.tb16906.x. [DOI] [PubMed] [Google Scholar]
- 24.DiMattio KM, Ehlert FJ, Liu-Chen LY. Intrinsic relative activities of kappa opioid agonists in activating Galpha proteins and internalizing receptor: Differences between human and mouse receptors. Eur J Pharmacol. 2015;761:235–244. doi: 10.1016/j.ejphar.2015.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li JG, Luo LY, Krupnick JG, Benovic JL, Liu-Chen LY. U50,488H-induced internalization of the human kappa opioid receptor involves a beta-arrestin- and dynamin-dependent mechanism. Kappa receptor internalization is not required for mitogen-activated protein kinase activation. J Biol Chem. 1999;274:12087–12094. doi: 10.1074/jbc.274.17.12087. [DOI] [PubMed] [Google Scholar]
- 26.Lau EK, Trester-Zedlitz M, Trinidad JC, Kotowski SJ, Krutchinsky AN, Burlingame AL, von Zastrow M. Quantitative encoding of the effect of a partial agonist on individual opioid receptors by multisite phosphorylation and threshold detection. Sci Signal. 2011;4:ra52. doi: 10.1126/scisignal.2001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trester-Zedlitz M, Burlingame A, Kobilka B, von Zastrow M. Mass spectrometric analysis of agonist effects on posttranslational modifications of the beta-2 adrenoceptor in mammalian cells. Biochem. 2005;44:6133–6143. doi: 10.1021/bi0475469. [DOI] [PubMed] [Google Scholar]
- 28.Michel MC, Wieland T, Tsujimoto G. How reliable are G-protein-coupled receptor antibodies? Naunyn Schmiedebergs Arch Pharmacol. 2009;379:385–388. doi: 10.1007/s00210-009-0395-y. [DOI] [PubMed] [Google Scholar]
- 29.Butcher AJ, Prihandoko R, Kong KC, McWilliams P, Edwards JM, Bottrill A, Mistry S, Tobin AB. Differential G-protein-coupled receptor phosphorylation provides evidence for a signaling bar code. J Biol Chem. 2011;286:11506–11518. doi: 10.1074/jbc.M110.154526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torrecilla I, Spragg EJ, Poulin B, McWilliams PJ, Mistry SC, Blaukat A, Tobin AB. Phosphorylation and regulation of a G protein-coupled receptor by protein kinase CK2. J Cell Biol. 2007;177:127–137. doi: 10.1083/jcb.200610018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nobles KN, Xiao K, Ahn S, Shukla AK, Lam CM, Rajagopal S, Strachan RT, Huang TY, Bressler EA, Hara MR, Shenoy SK, Gygi SP, Lefkowitz RJ. Distinct phosphorylation sites on the beta(2)-adrenergic receptor establish a barcode that encodes differential functions of beta-arrestin. Sci Signal. 2011;4:ra51. doi: 10.1126/scisignal.2001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rives ML, Rossillo M, Liu-Chen LY, Javitch JA. 6′-Guanidinonaltrindole (6′-GNTI) is a G protein-biased kappa-opioid receptor agonist that inhibits arrestin recruitment. J Biol Chem. 2012;287:27050–27054. doi: 10.1074/jbc.C112.387332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmid CL, Streicher JM, Groer CE, Munro TA, Zhou L, Bohn LM. Functional Selectivity of 6′-Guanidinonaltrindole (6′-GNTI) at kappa-Opioid Receptors in Striatal Neurons. J Biol Chem. 2013;288:22387–22398. doi: 10.1074/jbc.M113.476234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou L, Lovell KM, Frankowski KJ, Slauson SR, Phillips AM, Streicher JM, Stahl E, Schmid CL, Hodder P, Madoux F, Cameron MD, Prisinzano TE, Aube J, Bohn LM. Development of functionally selective, small molecule agonists at kappa opioid receptors. J Biol Chem. 2013;288:36703–36716. doi: 10.1074/jbc.M113.504381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.