SUMMARY
Few studies have evaluated the impact of complications, sociodemographic and clinical factors on early mortality (death ≤ 60 days from diagnosis) in acute myeloid leukaemia (AML) patients. Using data from the California Cancer Registry linked to hospital discharge records from 1999–2012, we identified patients aged ≥ 15 years with AML who received inpatient treatment (N=6359). Multivariate logistic regression analyses were used to assess the association of complications with early mortality, adjusting for sociodemographic factors, comorbidities and hospital type.
Early mortality decreased over time (25.3%, 1999–2000; 16.8%, 2011–2012) across all age groups, but was higher in older patients (6.9%, 15–39, 11.4%, 40–54, 18.6% 55–65, and 35.8%, > 65 years). Major bleeding (Odds ratio [OR] 1.5, 95% confidence interval [CI] 1.3–1.9), liver failure (OR 1.9, 95% CI 1.1–3.1), renal failure (OR 2.4, 95% CI 2.0–2.9), respiratory failure (OR 7.6, 95% CI 6.2–9.3) and cardiac arrest (OR 15.8, 95% CI 8.7–28.6) were associated with early mortality. Higher early mortality was also associated with single marital status, low neighbourhood socioeconomic status, lack of health insurance and comorbidities. Treatment at National Cancer Institute-designated cancer centres was associated with lower early mortality (OR 0.5, 95% CI 0.4–0.6).
In conclusion, organ dysfunction, hospital type and sociodemographic factors impact early mortality. Further studies should investigate how differences in healthcare delivery affect early mortality.
Keywords: AML, epidemiology, outcomes research, acute leukaemia, early mortality
INTRODUCTION
Acute myeloid leukaemia (AML) is associated with a poor overall prognosis, especially in older adults. Survival decreases with age, with 5-year relative survival of 65.9% in patients aged less than 15 years old, 49.9% in patients aged 15–39 years old and 16.7% in older adults.(Keegan et al, 2016; Siegel et al., 2015) Initial standard treatment of AML consists of induction chemotherapy, which usually requires an inpatient hospitalization of at least one month, a period that is associated with a high early mortality both due to the underlying disease and complications of treatment.(Walter et al., 2011) Early mortality has been reported to occur primarily from infection, bleeding or hyperleucostasis.(Ferrara & Schiffer, 2013)
Recent studies have shown an improvement in early mortality, defined as death within the first 30- or 60-days of diagnosis, over the past few decades.(Appelbaum et al., 2006; Othus et al., 2014; Percival et al., 2015) This improvement may be related to advances in supportive care in patients undergoing intensive chemotherapy, including rigorous transfusions and advancements in the prevention and treatment of infections, specifically with newer antifungal therapy.(Higby et al., 1974; Walsh et al., 1999; Cornely et al., 2007) However, improvements in early mortality may not be occurring among all patients, as recent studies have highlighted the disparities in long-term survival between different patient populations, with public or lack of insurance coverage, African-African race/ethnicity and lower income all associated with inferior long-term survival.(Kristinsson et al., 2009; Bierenbaum et al., 2012; Patel et al., 2015)
Few studies have examined the types of complications that have impacted AML patients in the last 15 years and how these complications and sociodemographic and clinical factors contribute to early mortality. Prior studies have evaluated the rates of febrile neutropenia, bleeding and invasive fungal infections in AML patients, but these were primarily in the clinical trial setting or were single centre studies and did not present differences by age. (Camera et al., 2003; Lowenberg et al., 2011; De Rosa et al., 2013; Ferrara & Schiffer, 2013; Garcia et al., 2013; Buckley et al., 2014) To our knowledge, no population-based studies have considered the effect of complications on early mortality in AML patients.(Juliusson et al., 2012) Because early mortality continues to be a barrier to improving long-term survival and age is a major prognostic factor, (Juliusson et al., 2009) evaluating the factors that impact early mortality on a population-based level will identify targeted areas for improvement.
Therefore, we examined trends in 60-day mortality, complications requiring any hospitalization within 60 days of diagnosis, and sociodemographic and clinical factors associated with 60-day mortality among adult AML patients by age. We hypothesized that early mortality and medical complications would be higher in older patients and sociodemographic factors would be more strongly associated with early mortality among younger patients.
METHODS
Study Population
Adolescent and adult patients (>15 years of age) diagnosed with a first primary AML and treated at a hospital in California from 1999–2012 were eligible for the study. To identify cases of AML, we used the following morphology codes from the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) (World Health Organization [WHO], 2000): 9840, 9861, 9867, 9869–9874, 9891, 9895–9898, 9910, 9911, 9920 and 9931. We excluded patients with a diagnosis of acute promyelocytic leukaemia because the treatment and management differs from AML. We also excluded children because the treatment and biology may be different in this group. In addition, we excluded patients without a record linkage number to hospital data; with an AML diagnosis at autopsy or death certificate only; who did not receive chemotherapy within 30 days of diagnosis as indicated in either databases; and without an inpatient hospitalization (figure 1). Our final cohort included 6359 AML patients.
Figure 1.
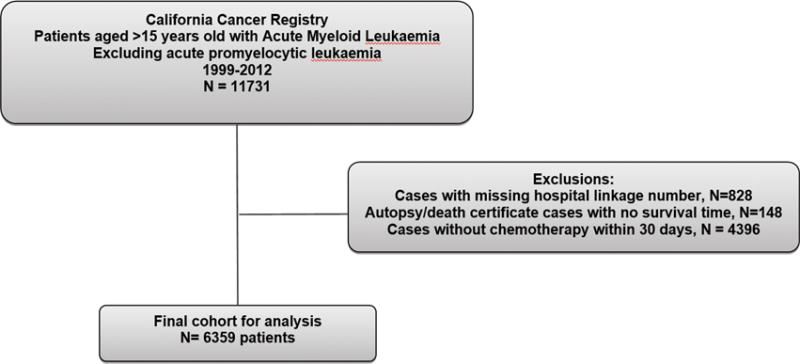
Analysis cohort of patients with acute myeloid leukaemia in California.
Databases
This study used a linked database between the California Cancer Registry (CCR) and the California Office of Statewide Health Planning and Development Patient Discharge Database (PDD) (http://www.ccrcal.org/Data_and_Statistics/Cancer_Data_for_Research.shtml). The CCR contains sociodemographic and clinical information on all patients diagnosed with cancer in California. Reporting is mandatory and completeness of cases is at least 98%. From the CCR, we obtained information on age at diagnosis, race/ethnicity, year of diagnosis, gender, marital status, neighbourhood socioeconomic status (SES), health insurance at diagnosis or initial treatment and date of initial chemotherapy.
The PDD contains information about all patients hospitalized in the state, except patients admitted to one of 14 Federal hospitals (12 Veterans Affairs hospitals and two military hospitals). Serial records from a single person are linked using an encrypted form of the social-security number, called the record linkage number. (Grannis et al., 2002; Hser & Evans, 2008) PDD records include a principal medical diagnosis, up to 24 additional ‘secondary’ diagnoses, and a principal and up to 20 secondary procedures coded using International Classification of Diseases, 9th Revision, Clinical Modification codes (ICD-9-CM). From the PPD, we obtained information on complications, chemotherapy administration, leukapheresis (a procedure used as a surrogate for hyperleucocytosis; ICD-9, 99.72), and comorbidities 2 years prior to or at AML diagnosis using the Elixhauser index.(Schoenman et al., 2007) The database also includes a hospital identifier. From this list of hospitals, we were able to classify hospitals into those associated with a National Cancer Institute (NCI)-designated cancer centre, Kaiser (a large vertically integrated health organization in California), teaching (academic centres without NCI designation) and private non-academic hospitals.
Outcomes
The primary outcome was death ≤60 days from AML diagnosis. Secondary outcomes were the following complications: major bleeding, sepsis, venous thrombosis, renal failure, liver dysfunction, respiratory failure, or cardiac arrest (ICD-9 codes in Supplemental Table 1). These complications were chosen as they have been previously identified in prior studies as being primary complications during AML induction treatment.(Ferrara & Schiffer, 2013) Complications were included if they occurred within any hospitalization from the time of diagnosis to 60 days or death.
Statistical Analysis
Chi-square tests were used to assess differences in complications and mortality by age group. Multivariate logistic regression was used to obtain odds ratios (ORs) and 95% confidence intervals (CIS) to assess the associations between sociodemographic and clinical factors, including complications, with early mortality. Interactions by age were examined for each variable in the model. Analyses were performed using SAS® (9.4) software (SAS Institute, Cary, NC) and a two-sided p-value <0.05 was considered statistically significant, including interactions.
RESULTS
From 11731 patients with AML, we identified 6359 patients that fulfilled our inclusion criteria (Figure 1). While most younger patients (84.5% aged 15–39 years, 80.8% aged 40–54 years, 75.5% aged 55–65 years) were hospitalized for chemotherapy, only 40.4% of AML patients aged >65 years were hospitalized and received chemotherapy and included in this study. The proportion of AML patients aged >65 years that were hospitalized and received chemotherapy did not substantially change from 1999 to 2012 (Supplementary Table 2). The majority of the 6359 patients in our analytic cohort were white (61.4%), married (59.4%), had private insurance (50.8%) and were treated at a private hospital (45.9%) (Table I). Seventy-nine per cent of patients had at least one comorbidity prior to or at diagnosis. Only 3.5% of the patients underwent leukapheresis. When evaluating by age, a greater percentage of younger patients, aged 15–39 years, lived in lower SES neighborhoods, had public insurance and were treated at an NCI-designated cancer centre when compared to those >65 years old.
Table I.
Sociodemographic and clinical characteristics of hospitalized acute myeloid leukaemia patients receiving chemotherapy by age at diagnosis, California, 1999–2012.
| Variables | Total N = 6359 |
15–39 N = 1059 |
40–54 N = 1479 |
55–65 N = 1556 |
>65 N = 2265 |
P-value | |
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Male | 55.4% | 51.3% | 52.1% | 56.8% | 58.6% | ||
| Female | 44.6% | 48.7% | 47.9% | 43.2% | 41.4% | <0.0001 | |
| Race/Ethnicity | |||||||
| Non-Hispanic White | 61.4% | 42.2% | 55.1% | 65.8% | 71.5% | ||
| African American | 5.3% | 6.2% | 7.4% | 5.3% | 3.7% | ||
| Hispanic | 19.7% | 34.7% | 22.3% | 16.7% | 13.0% | ||
| Asian | 13.0% | 15.8% | 14.9% | 11.9% | 11.3% | <0.0001 | |
| Year of diagnosis | |||||||
| 1999–2002 | 29.3% | 28.8% | 29.9% | 26.5% | 31.2% | ||
| 2003–2006 | 26.9% | 27.7% | 28.1% | 28.0% | 25.0% | ||
| 2007–2009 | 22.5% | 22.9% | 22.9% | 22.0% | 22.3% | ||
| 2010–2012 | 21.3% | 20.7% | 19.1% | 23.6% | 21.5% | 0.020 | |
| Comorbidities | |||||||
| 0 comorbidities | 21.1% | 33.0% | 27.2% | 17.0% | 14.5% | ||
| 1–2 comorbidities | 43.2% | 45.9% | 46.0% | 44.7% | 39.1% | ||
| 3+ comorbidities | 35.7% | 21.2% | 26.8% | 38.4% | 46.4% | <0.0001 | |
| Marital status at diagnosis | |||||||
| Married | 59.4% | 37.9% | 63.7% | 67.1% | 61.4% | ||
| Single marital status | 39.0% | 60.5% | 35.1% | 30.9% | 37.0% | <0.0001 | |
| Neighbourhood Socioeconomic status | |||||||
| Low | 52.7% | 61.8% | 53.6% | 52.2% | 48.2% | ||
| High | 45.3% | 37.1% | 44.5% | 46.1% | 49.2% | <0.0001 | |
| Health insurance status | |||||||
| Public insurance | 12.8% | 26.4% | 18.8% | 12.4% | 2.7% | ||
| Private insurance | 50.8% | 58.6% | 66.5% | 62.9% | 28.7% | ||
| Medicare | 27.6% | 2.9% | 3.0% | 13.4% | 64.9% | ||
| Uninsured | 1.8% | 2.6% | 3.0% | 2.0% | 0.4% | <0.0001 | |
| Hospital type | |||||||
| Private | 45.9% | 37.3% | 37.7% | 42.4% | 57.6% | ||
| NCI-designated | 24.3% | 29.7% | 28.8% | 27.1% | 17.0% | ||
| Teaching | 10.6% | 15.3% | 12.2% | 8.7% | 8.5% | ||
| Kaiser | 16.8% | 15.8% | 19.5% | 19.3% | 13.8% | <0.0001 | |
| Leukapheresis | |||||||
| Yes | 3.5% | 5.3% | 4.7% | 2.5% | 2.6% | ||
| No | 96.5% | 94.7% | 95.3% | 97.5% | 97.4% | <0.0001 | |
NCI-designated=National Cancer Institute designated cancer centre
For patients of all ages, there was an overall decrease in 60-day mortality from 1999 to 2012 (Figure 2). While those aged >65 years had the highest early mortality rate, this cohort also had the greatest absolute decrease in early mortality over time from 43% in 1999–2002 to 28% in 2010–2012. In the younger cohort, aged 15–39 years, the relative 60-day mortality decreased by more than 50% from 1999 to 2012. There were differences in the rate of early mortality by WHO/French-American-British (FAB) classifications, with a range of 11% in AML with t(8;21)(q22;q22) up to 26% in those with AML-M7 subtype (Supplemental Table 3).
Figure 2.
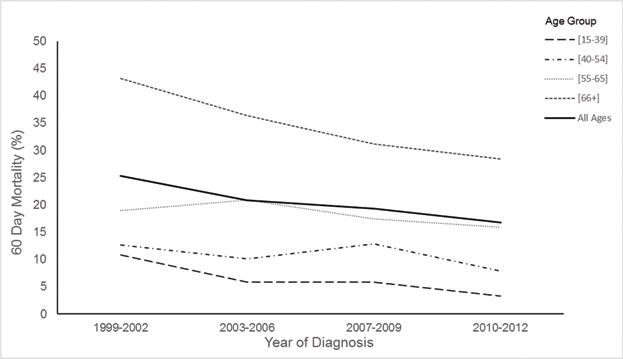
60-day mortality in hospitalized acute myeloid leukaemia patients receiving chemotherapy by age (in years), in California, 1999–2012.
The majority of complications across all ages were sepsis (35%), major bleeding (12%), renal failure (18%) and respiratory failure (12%) (Table II). Complications were similar across all age groups, except that those aged 55–65 years had higher rates of most complications than patients of other ages (sepsis 40.6%, p<0.001; venous thrombosis 2.4%, p=0.003; renal failure 21.7%, p<0.001; and respiratory failure 17%, p<0.001). Complications were also similar across racial/ethnic groups, except that African Americans had higher rates of renal failure, Hispanics had higher rates of sepsis and Asians had higher rates of respiratory failure (Supplemental Table 4).
Table II.
Complications in hospitalized acute myeloid leukaemia patients receiving chemotherapy by age at diagnosis, California, 1999–2012.
| Complication | Total N = 6359 |
15–39 years N = 1059 |
40–54 years N = 1479 |
55–65 years N = 1556 |
>65 years N = 2265 |
P-value |
|---|---|---|---|---|---|---|
| Sepsis | 34.6% | 32.2% | 35.8% | 40.6% | 33.2% | <0.0001 |
| Bleeding | 11.9% | 11.0% | 11.5% | 13.4% | 13.9% | 0.044 |
| Thrombosis | 1.7% | 0.6% | 2.4% | 2.4% | 1.8% | 0.003 |
| Renal failure | 17.9% | 12.8% | 17.4% | 21.7% | 22.1% | <0.0001 |
| Liver failure | 1.4% | 1.6% | 1.8% | 1.7% | 1.1% | 0.290 |
| Respiratory failure | 12.0% | 9.3% | 10.6% | 16.7% | 14.2% | <0.0001 |
| Cardiac arrest | 1.7% | 1.0% | 1.9% | 2.2% | 2.0% | 0.140 |
In multivariate analyses, age was associated with 60-day mortality, with those of younger age having lower odds of dying within 60-days compared to those >65 years of age (Table III). African American (OR=0.66; 95% CI: 0.46, 0.94) patients also had a lower odds of dying within 60-days compared to non-Hispanic white patients. Improvements in early mortality occurred after 2002. Higher 60-day mortality was also associated with single marital status, low neighbourhood SES, and lack of health insurance. Increasing number of comorbidities (vs none) was also associated with increased early mortality, with higher mortality in those with ≥3 comorbidities (OR=1.97, 95% CI: 1.56, 2.49) than 1–2 comorbidities (OR=1.46, 95% CI: 1.16–1.84).
Table III.
Multivariate model of the relationship of sociodemographic and clinical factors to 60-day mortality in hospitalized acute myeloid leukaemia patients receiving chemotherapy, California 1999–2012.
| Variable | Odds Ratio | (95% confidence interval) | P-value |
|---|---|---|---|
| Gender | |||
| Female vs Male | 0.91 | (0.78, 1.06) | 0.213 |
| Age (years) | |||
| 15–39 vs >66 | 0.11 | (0.08, 0.16) | <0.0001 |
| 40–54 vs >66 | 0.20 | (0.16, 0.26) | <0.0001 |
| 55–65 vs >66 | 0.32 | (0.26, 0.40) | <0.0001 |
| Race/Ethnicity | |||
| Asian vs Non-Hispanic White | 0.81 | (0.64, 1.04) | 0.094 |
| Hispanic vs Non-Hispanic White | 0.82 | (0.66, 1.01) | 0.064 |
| African American vs Non-Hispanic White | 0.66 | (0.46, 0.94) | 0.022 |
| Year of diagnosis | |||
| 2003–2006 vs 1999–2002 | 0.66 | (0.54, 0.80) | <0.0001 |
| 2007–2009 vs 1999–2002 | 0.50 | (0.40, 0.61) | <0.0001 |
| 2010–2012 vs 1999–2002 | 0.38 | (0.30, 0.47) | <0.0001 |
| Marital status at diagnosis | |||
| Single marital status vs Married | 1.25 | (1.07, 1.46) | 0.006 |
| Neighbourhood Socioeconomic status | |||
| Low vs High | 1.21 | (1.03, 1.41) | 0.017 |
| Health insurance status | |||
| Medicare vs Private insurance | 1.07 | (0.88, 1.30) | 0.519 |
| Public insurance vs Private insurance | 1.08 | (0.81, 1.44) | 0.585 |
| Uninsured vs Private insurance | 2.44 | (1.45, 4.13) | <0.001 |
| Comorbidities | |||
| 1–2 comorbidities vs 0 comorbidities | 1.46 | (1.16, 1.84) | 0.001 |
| 3+ comorbidities vs 0 comorbidities | 1.97 | (1.56, 2.49) | <0.0001 |
| Bleeding vs none | 1.54 | (1.56, 1.88) | <0.0001 |
| Sepsis vs none | 1.05 | (0.89, 1.23) | 0.568 |
| Thrombosis vs none | 0.85 | (0.50, 1.46) | 0.563 |
| Liver failure vs none | 1.87 | (1.12, 3.12) | 0.017 |
| Renal failure vs none | 2.39 | (2.00, 2.86) | <0.0001 |
| Respiratory failure vs none | 7.59 | (6.18, 9.33) | <0.0001 |
| Cardiac arrest vs none | 15.79 | (8.73, 28.56) | <.00001 |
| Hospital type | |||
| Kaiser vs Private | 0.91 | (0.73, 1.14) | 0.410 |
| NCI-designated vs Private | 0.45 | (0.36, 0.56) | <0.0001 |
| Teaching vs Private | 0.92 | (0.72, 1.19) | 0.536 |
| Leukapheresis vs none | 1.26 | (0.85, 1.86) | 0.256 |
NCI-designated=National Cancer Institute designated cancer centre
Significant interactions by age were only found for marital status (p-value <0.001). Single marital status was only significantly associated with increased odds of early mortality in those aged >65 years (OR 1.37, 95% CI: 1.09–1.71) (data not shown in Tables). In those aged 40–54 years, lack of health insurance (OR 5.81, 95% CI: 2.26–14.30) was associated with increased odds of early mortality compared to private health insurance.
Across all age groups, the complications of bleeding, liver, renal, respiratory failure and cardiac arrest were associated with a higher OR of dying within 60 days (Table III). There were no significant differences by age group, except that respiratory failure (OR=13.00, 95% CI: 6.14–27.53) and bleeding (OR=2.69, 95% CI: 1.38–5.25) had higher odds of 60-day mortality in patients 15–39 years of age (data not shown in tables). Treatment at an NCI-designated cancer centre (vs private hospital) was associated with lower odds of dying within 60-days (OR=0.45, 95% CI: 0.36, 0.56). There were no differences in mortality between the other hospital types.
Table IV shows the number of complications by hospital type. Private hospitals had higher rates of sepsis (p<0.001), bleeding (p=0.001) and cardiac arrest (p<0.001). Renal failure accounted for more than a fifth of all complications in those treated at teaching and NCI-designated cancer centres.
Table IV.
Complications in hospitalized acute myeloid leukaemia patients receiving chemotherapy by hospital type, California, 1999–2012
| Complication | Total N = 6204 |
Kaiser N = 1069 |
Teaching N = 671 |
Private N = 2917 |
NCI-designated N = 1547 |
P-value |
|---|---|---|---|---|---|---|
| Sepsis | 35.6% | 32.1% | 34.7% | 38.2% | 33.3% | <0.001 |
| Bleeding | 12.7% | 9.4% | 13.7% | 14.1% | 11.8% | 0.001 |
| Thrombosis | 1.9% | 1.6% | 1.6% | 1.8% | 2.3% | 0.586 |
| Renal failure | 19.2% | 17.9% | 22.5% | 17.5% | 21.9% | <0.001 |
| Liver failure | 1.5% | 0.9% | 1.8% | 1.8% | 1.2% | 0.277 |
| Respiratory failure | 13.2% | 11.0% | 15.6% | 14.6% | 11.0% | <0.001 |
| Cardiac arrest | 1.8% | 0.9% | 1.9% | 2.3% | 1.2% | <.0001 |
NCI-designated=National Cancer Institute designated cancer centre
DISCUSSION
In our analysis using a large, sociodemographically and geographically diverse cohort of hospitalized AML patients receiving chemotherapy, we found significant sociodemographic and age disparities in early mortality. Specifically, marital status, location of care, neighbourhood SES and health insurance type were all significantly associated with early mortality. In terms of complications, major organ dysfunction continues to be a main driver of early mortality across adult patients of all ages, although the presence of sepsis, thrombosis and leukapheresis, a poor prognostic marker, did not impact mortality when adjusted for other factors. Our study also confirms that early mortality has continued to decline across all adult AML age groups in more recent times.
Receiving care at an NCI-designated cancer centre was significantly associated with lower early mortality across all age groups compared to all other types of hospitals. This finding adds to the growing body of evidence that the setting where patients receive their cancer care affects patient outcomes.(Onega et al., 2008; Onega et al., 2010) Prior studies in solid tumours have shown lower mortality and better surgical outcomes in patients treated at NCI-designated cancer centres compared to non-NCI designated facilities.(Birkmeyer et al., 2005; Paulson et al., 2008; Luchtenborg et al., 2013) Population-based studies conducted in Los Angeles County also showed improved overall survival among adult patients with adult-onset cancers (breast, colorectal, pancreatic, gastric and lung) and adolescent and young adult patients with haematological malignancies treated at NCI-designated cancer centres.(Wolfson et al, 2014; Wolfson et al., 2015)
Outcomes are thought to be better at NCI-designated cancer centres due to clinical trial availability, organizational affiliation and evidence-based cancer care.(Birkmeyer et al., 2005; Bilimoria et al., 2007; Bilimoria et al., 2008; Huang et al., 2014) However, few studies have evaluated how treatment at a NCI-designated cancer centre differs from other hospitals. It has been reported that NCI-designated cancer centres may have better expertise at performing a high volume of specialized care than low volume non-NCI designated facilities.(Hillner et al., 2000; Bilimoria et al., 2008) Similarly, a recent study showed reduced inpatient mortality rates in AML patients treated at high versus low volume chemotherapy centres.(Giri et al., 2015) The present study did not find substantial differences in the number of complications by hospital type. However, for induction therapy in AML, staff at high volume centres may be more capable of recognizing and managing complications earlier, which could improve early mortality rates. Future research should evaluate how the differences in health care delivery and access to care at a NCI-designated cancer centre leads to improved outcomes.
In this study, we also found that the type of health insurance was associated with early mortality. While our study did not observe differences in 60-day mortality between public and private health insurance, our findings that lack of health insurance was associated with 60-day mortality is consistent with a prior population-based cancer registry analysis of 19–64 year-old AML patients (Borate et al., 2015). Future research should continue to monitor the impact of health insurance on outcomes after the implementation of the Affordable Care Act (ACA) in 2014, as the ACA has resulted in a decrease in the population of uninsured,(Wherry & Miller, 2016) but it is not yet clear if this results in better health outcomes.
Racial/ethnic differences have been thought to play a major role in the presentation and survival after AML. In a single-centre study, black patients more frequently presented with high-risk cytogenetics and had lower rates of clinical trial participation than whites, but the overall survival did not differ between these groups.(Bierenbaum et al., 2012) In contrast, a population-based, California Cancer Registry study revealed that black AML patients had a lower odds of chemotherapy or transplant and worse long-term survival compared to non-Hispanic whites.(Patel et al., 2015) Our study, however, observed a lower 60-day mortality in patients of African American race/ethnicity than non-Hispanic whites. We observed some differences in complications by race/ethnicity, but these did not follow any pattern. African Americans had higher rates of renal failure, Hispanics had higher rates of sepsis and Asians had higher rates of respiratory failure (Supplemental Table 4). The lack of any specific trend suggests that disparities in survival may not be related to initial treatment and treatment-related complications but may reflect disparities in long-term health care delivery.
In the present study, we also found that marital status and neighbourhood SES impacted early mortality in AML patients. Similar to studies that found better cancer survival among married patients (Goodwin et al., 1987; Aizer et al., 2013), we found that married patients had lower 60-day mortality, particularly among patients >65 years. There are several proposed theories underlying the beneficial effects of being married, including stronger support networks, having medical insurance, economic security and better lifestyle practices.(Ayanian et al., 1993; Seeman, 2000; Waite & Lehrer, 2003; Manzoli et al., 2007; Bernstein et al., 2008) Our finding of higher 60-day mortality among patients residing in low SES neighbourhoods is consistent with a prior study (Hilner et al 2000) and may be related to disparities in access to healthcare and more advanced disease at presentation.
We observed that major organ dysfunction and bleeding continue to be the main drivers of early mortality. We did not see significant differences across age groups, except that young patients with respiratory failure and bleeding had higher relative odds of early mortality than older patients with these complications. Further, we did not observe sepsis to be associated with early mortality, which may be a reflection of improvements in supportive care, rigorous transfusions and introduction of newer antibiotics and antifungals.(Higby et al., 1974; Pagano et al., 2012; Cowan et al., 2015) Clinical pathways that result in increased awareness, early detection and aggressive management of respiratory failure, renal failure and bleeding may lead to improved outcomes.
Our findings are consistent with prior studies that found improvements in early mortality among AML patients over time(Othus et al., 2014; Percival et al., 2015), across all age groups, with the greatest improvement in older patients. Retrospective data showed that the 5–10% of AML patients treated on clinical trials had significant improvements in the early-mortality rate at 28 days, from 18–19% to 3–4% in one study and 20% to 12% in another study.(Appelbaum et al., 2006; Othus et al., 2014) In population-based, cancer registry analyses a decrease from 18% 30-day mortality in patients treated from 1973 to 1977 to 5.8% in patients treated in 2008–2010 was seen.(Percival et al., 2015) Our study extends these prior findings to include more recent data, showing continued improvements in early mortality, potentially due to advancements in management of infections and sepsis.
There are several limitations to this study. While we were able to identify patients that received chemotherapy while hospitalized, we did not have detailed information on the type of chemotherapy given or whether there were differences in curative versus palliative intent of treatment. We did not include patients who received therapy in the outpatient setting. As a result, our findings are only applicable to adult patients who receive chemotherapy requiring hospitalization. While this comprises the majority of patients aged ≤65 years, only 40.4% of elderly patients with AML in California were hospitalized for treatment and this percentage of elderly patients hospitalized for treatment did not change substantially over the study period. Therefore, the large decline in early mortality in older patients may be reflective of healthier elderly patients who received in-patient chemotherapy for their treatment, given that elderly AML patients in general may be less likely to be offered intensive in-patient therapy due to adverse leukaemia prognostic factors and patient-related factors, including comorbidities and performance status. (Othus et al., 2014; Medeiros et al., 2015; Ossenkoppele & Lowenberg, 2015) The large decline in early mortality in older patients may also be reflective of recent advances in treatment, including the use of low intensity hypomethylating agents, which have been associated with lower adverse events. (Dombret et al., 2015) Our study also lacked individual-level measures of SES to consider separately or with our neighbourhood measure. While neighbourhood and individual SES are associated, neighbourhood SES has been found to underestimate associations observed with individual-level SES (Krieger, 1992). Our study may also be subject to potential misclassification of race/ethnicity, although we previously have detected excellent overall agreement with self-reported race/ethnicity for Whites and Blacks, and good agreement for Hispanics and Asians (Gomez & Glaser, 2006; Clegg et al., 2007). Despite these limitations, our study includes a diverse, population-based patient population treated across all types of hospitals with findings that are more generalizable of treatment and health care delivery in the modern era than clinical trials or single institution studies.
This population-based study in adult patients with AML found a significant association between sociodemographic factors and early mortality. Being unmarried, living in a low socioeconomic neighbourhood and lacking insurance led to worse outcomes. Cancer care setting also greatly influenced outcomes, with lower early mortality observed in those treated at a NCI-designated cancer centre. We show that early mortality continues to decline across all age groups, but major organ dysfunction and bleeding continue to impact early deaths. Newer disease-directed therapies may minimally impact early mortality without more robust initiatives to prevent or mitigate these complications. Other strategies to improve outcomes include wider insurance coverage and treatment at specialty centres, where lower early mortality may result from better supportive care.
Supplementary Material
Acknowledgments
Funding sources
TW is supported by TR000002 from NCATS, NIH. BAJ is supported by K12 CA138464 from NIH.
Footnotes
Conflict of Interest
The authors report no relevant conflicts of interest.
Contributions of the Authors:
Concept and design: Ho, Jonas, Li, Brunson, Wun, Keegan
Acquisition and Analysis of Data: Brunson, Li, Ho, Keegan
Drafting of the manuscript: Ho, Keegan
Final approval: Brunson, Ho, Jonas, Wun, Keegan
References
- Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, et al. Marital status and survival in patients with cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(31):3869–3876. doi: 10.1200/JCO.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayanian JZ, Kohler BA, Abe T, Epstein AM. The relation between health insurance coverage and clinical outcomes among women with breast cancer. The New England journal of medicine. 1993;329(5):326–331. doi: 10.1056/NEJM199307293290507. [DOI] [PubMed] [Google Scholar]
- Bernstein AB, Cohen RA, Brett KM, Bush MA. Marital status is associated with health insurance coverage for working-age women at all income levels, 2007. NCHS data brief. 2008;(11):1–8. [PubMed] [Google Scholar]
- Bierenbaum J, Davidoff AJ, Ning Y, Tidwell ML, Gojo I, Baer MR. Racial differences in presentation, referral and treatment patterns and survival in adult patients with acute myeloid leukemia: a single-institution experience. Leukemia research. 2012;36(2):140–145. doi: 10.1016/j.leukres.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilimoria KY, Bentrem DJ, Ko CY, Tomlinson JS, Stewart AK, Winchester DP, et al. Multimodality therapy for pancreatic cancer in the U.S.: utilization, outcomes, and the effect of hospital volume. Cancer. 2007;110(6):1227–1234. doi: 10.1002/cncr.22916. [DOI] [PubMed] [Google Scholar]
- Bilimoria KY, Talamonti MS, Wayne JD, Tomlinson JS, Stewart AK, Winchester DP, et al. Effect of hospital type and volume on lymph node evaluation for gastric and pancreatic cancer. Archives of surgery. 2008;143(7):671–678. doi: 10.1001/archsurg.143.7.671. discussion 678. [DOI] [PubMed] [Google Scholar]
- Birkmeyer NJ, Goodney PP, Stukel TA, Hillner BE, Birkmeyer JD. Do cancer centers designated by the National Cancer Institute have better surgical outcomes? Cancer. 2005;103(3):435–441. doi: 10.1002/cncr.20785. [DOI] [PubMed] [Google Scholar]
- Borate UM, Mineishi S, Costa LJ. Nonbiological factors affecting survival in younger patients with acute myeloid leukemia. Cancer. 2015;121(21):3877–3884. doi: 10.1002/cncr.29436. [DOI] [PubMed] [Google Scholar]
- Buckley SA, Othus M, Vainstein V, Abkowitz JL, Estey EH, Walter RB. Prediction of adverse events during intensive induction chemotherapy for acute myeloid leukemia or high-grade myelodysplastic syndromes. American journal of hematology. 2014;89(4):423–428. doi: 10.1002/ajh.23661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camera A, Andretta C, Villa MR, Volpicelli M, Picardi M, Rossi M, et al. Intestinal toxicity during induction chemotherapy with cytarabine-based regimens in adult acute myeloid leukemia. The hematology journal: the official journal of the European Haematology Association / EHA. 2003;4(5):346–350. doi: 10.1038/sj.thj.6200304. [DOI] [PubMed] [Google Scholar]
- Clegg LX, Reichman ME, Hankey BF, Miller BA, Lin YD, Johnson NJ, et al. Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies. Cancer Causes Control. 2007;18(2):177–187. doi: 10.1007/s10552-006-0089-4. [DOI] [PubMed] [Google Scholar]
- Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. The New England journal of medicine. 2007;356(4):348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- Cowan AJ, Altemeier WA, Johnston C, Gernsheimer T, Becker PS. Management of Acute Myeloid Leukemia in the Intensive Care Setting. Journal of intensive care medicine. 2015;30(7):375–384. doi: 10.1177/0885066614530959. [DOI] [PubMed] [Google Scholar]
- De Rosa FG, Motta I, Audisio E, Frairia C, Busca A, Di Perri G, et al. Epidemiology of bloodstream infections in patients with acute myeloid leukemia undergoing levofloxacin prophylaxis. BMC infectious diseases. 2013;13:563. doi: 10.1186/1471-2334-13-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126:3. doi: 10.1182/blood-2015-01-621664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara F, Schiffer CA. Acute myeloid leukaemia in adults. Lancet. 2013;381(9865):484–495. doi: 10.1016/S0140-6736(12)61727-9. [DOI] [PubMed] [Google Scholar]
- Garcia JB, Lei X, Wierda W, Cortes JE, Dickey BF, Evans SE, et al. Pneumonia during remission induction chemotherapy in patients with acute leukemia. Annals of the American Thoracic Society. 2013;10(5):432–440. doi: 10.1513/AnnalsATS.201304-097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri S, Pathak R, Aryal MR, Karmacharya P, Bhatt VR, Martin MG. Impact of hospital volume on outcomes of patients undergoing chemotherapy for acute myeloid leukemia: a matched cohort study. Blood. 2015;125(21):3359–3360. doi: 10.1182/blood-2015-01-625764. [DOI] [PubMed] [Google Scholar]
- Gomez SL, Glaser SL. Misclassification of race/ethnicity in a population-based cancer registry (United States) Cancer Causes Control. 2006;17(6):771–781. doi: 10.1007/s10552-006-0013-y. [DOI] [PubMed] [Google Scholar]
- Goodwin JS, Hunt WC, Key CR, Samet JM. The effect of marital status on stage, treatment, and survival of cancer patients. Jama. 1987;258(21):3125–3130. [PubMed] [Google Scholar]
- Grannis SJ, JM O, McDonald CJ. Analysis of identifier performance using a deterministic linkage algorithm. Proc Amia Symp. 2002:305–309. [PMC free article] [PubMed] [Google Scholar]
- Higby DJ, Cohen E, Holland JF, Sinks L. The prophylactic treatment of thrombocytopenic leukemic patients with platelets: a double blind study. Transfusion. 1974;14(5):440–446. doi: 10.1111/j.1537-2995.1974.tb04558.x. [DOI] [PubMed] [Google Scholar]
- Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: importance in quality of cancer care. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2000;18(11):2327–2340. doi: 10.1200/JCO.2000.18.11.2327. [DOI] [PubMed] [Google Scholar]
- Hser Y, Evans E. Cross-system data linkage for treatment outcome evaluation: lessons learned from the California Treatment Outcome Project. Eval Program Plann. 2008;(31):2. doi: 10.1016/j.evalprogplan.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LC, Ma Y, Ngo JV, Rhoads KF. What factors influence minority use of National Cancer Institute-designated cancer centers? Cancer. 2014;120(3):399–407. doi: 10.1002/cncr.28413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliusson G, Antunovic P, Derolf A, Lehmann S, Mollgard L, Stockelberg D, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113(18):4179–4187. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- Juliusson G, Lazarevic V, Horstedt AS, Hagberg O, Hoglund M, Swedish Acute Leukemia Registry G Acute myeloid leukemia in the real world: why population-based registries are needed. Blood. 2012;119(17):3890–3899. doi: 10.1182/blood-2011-12-379008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan T, Ries LA, Barr RD, Geiger AM, Vollmer D, Pollock BH, Bleyer A. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122:1009–1016. doi: 10.1002/cncr.29869. [DOI] [PubMed] [Google Scholar]
- Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82(5):703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristinsson SY, Derolf AR, Edgren G, Dickman PW, Bjorkholm M. Socioeconomic differences in patient survival are increasing for acute myeloid leukemia and multiple myeloma in sweden. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(12):2073–2080. doi: 10.1200/JCO.2008.18.2006. [DOI] [PubMed] [Google Scholar]
- Lowenberg B, Pabst T, Vellenga E, van Putten W, Schouten HC, Graux C, et al. Cytarabine dose for acute myeloid leukemia. The New England journal of medicine. 2011;364(11):1027–1036. doi: 10.1056/NEJMoa1010222. [DOI] [PubMed] [Google Scholar]
- Luchtenborg M, Riaz SP, Coupland VH, Lim E, Jakobsen E, Krasnik M, et al. High procedure volume is strongly associated with improved survival after lung cancer surgery. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(25):3141–3146. doi: 10.1200/JCO.2013.49.0219. [DOI] [PubMed] [Google Scholar]
- Manzoli L, Villari P, G MP, Boccia A. Marital status and mortality in the elderly: a systematic review and meta-analysis. Social science & medicine. 2007;64(1):77–94. doi: 10.1016/j.socscimed.2006.08.031. [DOI] [PubMed] [Google Scholar]
- Medeiros BC, Satram-Hoang S, Hurst D, Hoang KQ, Momin F, Reyes C. Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Annals of hematology. 2015;94(7):1127–1138. doi: 10.1007/s00277-015-2351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onega T, Duell EJ, Shi X, Wang D, Demidenko E, Goodman D. Geographic access to cancer care in the U.S. Cancer. 2008;112(4):909–918. doi: 10.1002/cncr.23229. [DOI] [PubMed] [Google Scholar]
- Onega T, Duell EJ, Shi X, Demidenko E, Goodman DC. Race versus place of service in mortality among medicare beneficiaries with cancer. Cancer. 2010;116(11):2698–2706. doi: 10.1002/cncr.25097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele G, Lowenberg B. How I treat the older patient with acute myeloid leukemia. Blood. 2015;125(5):767–774. doi: 10.1182/blood-2014-08-551499. [DOI] [PubMed] [Google Scholar]
- Othus M, Kantarjian H, Petersdorf S, Ravandi F, Godwin J, Cortes J, et al. Declining rates of treatment-related mortality in patients with newly diagnosed AML given ‘intense’ induction regimens: a report from SWOG and MD Anderson. Leukemia. 2014;28(2):289–292. doi: 10.1038/leu.2013.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano L, Caira M, Candoni A, Aversa F, Castagnola C, Caramatti C, et al. Evaluation of the practice of antifungal prophylaxis use in patients with newly diagnosed acute myeloid leukemia: results from the SEIFEM 2010-B registry. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;55(11):1515–1521. doi: 10.1093/cid/cis773. [DOI] [PubMed] [Google Scholar]
- Patel MI, Ma Y, Mitchell B, Rhoads KF. How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24(2):344–349. doi: 10.1158/1055-9965.EPI-14-0963. [DOI] [PubMed] [Google Scholar]
- Paulson EC, Mitra N, Sonnad S, Armstrong K, Wirtalla C, Kelz RR, et al. National Cancer Institute designation predicts improved outcomes in colorectal cancer surgery. Annals of surgery. 2008;248(4):675–686. doi: 10.1097/SLA.0b013e318187a757. [DOI] [PubMed] [Google Scholar]
- Percival ME, Tao L, Medeiros BC, Clarke CA. Improvements in the early death rate among 9380 patients with acute myeloid leukemia after initial therapy: A SEER database analysis. Cancer. 2015;121(12):2004–2012. doi: 10.1002/cncr.29319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenman JA, Sutton JP, Elixhauser A, Love D. Understanding and enhancing the value of hospital discharge data. Medical care research and review: MCRR. 2007;64(4):449–468. doi: 10.1177/1077558707301963. [DOI] [PubMed] [Google Scholar]
- Seeman TE. Health promoting effects of friends and family on health outcomes in older adults. American journal of health promotion: AJHP. 2000;14(6):362–370. doi: 10.4278/0890-1171-14.6.362. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- Waite LJ, Lehrer EL. The Benefits from Marriage and Religion in the United States: A Comparative Analysis. Population and development review. 2003;29(2):255–276. doi: 10.1111/j.1728-4457.2003.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh TJ, Finberg RW, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. The New England journal of medicine. 1999;340(10):764–771. doi: 10.1056/NEJM199903113401004. [DOI] [PubMed] [Google Scholar]
- Walter RB, Othus M, Borthakur G, Ravandi F, Cortes JE, Pierce SA, et al. Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: a novel paradigm for treatment assignment. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29(33):4417–4423. doi: 10.1200/JCO.2011.35.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry LR, Miller S. Early Coverage, Access, Utilization, and Health Effects Associated With the Affordable Care Act Medicaid Expansions: A Quasi-experimental Study. Annals of internal medicine. 2016;164(12):795–803. doi: 10.7326/M15-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J, Sun CL, Wyatt L, Stock W, Bhatia S. Impact of Care at NCI Comprehensive Cancer Centers (NCICCC) on Outcomes in Children, Adolescents and Young Adults (AYA) with Hematologic Malignancies. Blood. 2014;124(21):556. [Google Scholar]
- Wolfson JA, Sun CL, Wyatt LP, Hurria A, Bhatia S. Impact of care at comprehensive cancer centers on outcome: Results from a population-based study. Cancer. 2015;121(21):3885–3893. doi: 10.1002/cncr.29576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. International Classification of Diseases for Oncology. third. Geneva: World Health Organization; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


