Abstract
Background
Soy foods possess both anti-estrogenic and estrogen-like properties. It remains controversial whether women diagnosed with breast cancer should be advised to eat more or less soy foods, especially for those who receive hormonal therapies as part of cancer treatments.
Methods
We examined the association of dietary intake of isoflavone, the major phytoestrogen in soy, with all-cause mortality in 6,235 women with breast cancer enrolled in the Breast Cancer Family Registry. Dietary intake was assessed using a Food Frequency Questionnaire developed for the Hawaii-Los Angeles Multiethnic Cohort, with 5,178 women who reported pre-diagnosis diet and 1,664 women who reported post-diagnosis diet. Cox proportional hazard models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI).
Results
During a median follow-up of 113 months (approximately 9.4 years), 1,224 deaths were documented. We observed a 21% decrease in all-cause mortality for women with the highest vs. lowest quartile of dietary isoflavone intake (≥1.5 vs. <0.3 mg/d: HR=0.79, 95% CI: 0.64–0.97, p trend=0.01). Lower mortality associated with higher intake was limited to women with negative tumor hormone receptors (HR=0.49, 95% CI: 0.29–0.83, p trend=0.005) and women who were not treated with hormonal therapy for their breast cancer (HR=0.68, 95% CI: 0.51–0.91, p trend=0.02). Interactions, however, did not reach statistical significance.
Conclusions
In this large ethnically-diverse cohort of women with breast cancer living in North America, a higher dietary intake of isoflavone was associated with reduced all-cause mortality.
Keywords: Soy, isoflavone, mortality, survival, breast cancer, breast cancer survivors
INTRODUCTION
Isoflavone has been suggested to inhibit breast cancer development by decreasing estrogen production, inhibiting cell proliferation, and reducing reactive oxygen species production.1 However, isoflavone is also known for its estrogenic activity by binding and activating estrogen receptors in breast tumors,1 which may interfere with tamoxifen therapy by reducing its treatment effect. It remains controversial whether women should be advised to avoid or to increase intake of food products or supplements that contain isoflavone to reduce breast cancer risk or progression.2,3
Only a few epidemiologic studies have evaluated the association between intake of soy foods or dietary isoflavone, either before or after cancer diagnosis, and survival in women with breast cancer.4 Although several lines of evidence indicate a reduced risk of mortality or recurrence associated with increasing soy consumption in Chinese women,5–7 the evidence is still very limited for women living in Western countries where soy product consumption is much lower than that in Asian countries. In Western countries, the intake of soy products varies by race/ethnicity.8 It remains unclear whether dietary isoflavone intake is associated with different mortality rates among Caucasian, Hispanic, African American, and Asian American women living in the United States (U.S.). Importantly, studies are needed to further quantify whether associations between dietary isoflavone intake and survival vary by tumor hormone receptor status, and by use of hormonal therapy for treatment of breast cancer. These analyses will contribute to the evidence-base for developing targeted dietary recommendations for breast cancer survivors.
The present study examined the association between dietary intake of isoflavone and all-cause mortality in a multiethnic cohort of women diagnosed with breast cancer living in the U.S. and Canada, and assessed whether the associations differ by race/ethnicity, tumor hormone receptor status, and receipt of hormone therapy.
METHODS
Study Population
The Breast Cancer Family Registry (BCFR) is an international research infrastructure established in 1995, with six participating sites from the U.S., Canada, and Australia that recruited breast cancer families either through population-based cancer registries (population-based families) or cancer clinics and community outreach (clinic-based families).9 Population-based families were recruited through incident breast cancer cases identified by the regional cancer registries in the Greater San Francisco Bay area, the province of Ontario, Canada, and the metropolitan areas of Melbourne and Sydney, Australia. Clinic-based families were recruited from the local populations in New York City, Philadelphia, Utah, Ontario, and Melbourne/Sydney. The first family member recruited into the BCFR is referred to as the proband, regardless of breast cancer status. Population-based probands were sampled according to site-specific criteria based on sex, race/ethnicity, family history, and age at diagnosis. Permission was sought from the probands to contact eligible family members. Between 1996 and 2011, more than 13,000 families were recruited and followed prospectively. Because a different food frequency questionnaire (FFQ) was used to assess dietary intake in Australia, the current analysis only includes women from the five North American BCFR sites who completed the same FFQ at baseline and is limited to women diagnosed with a first primary invasive breast cancer (n=7,471). We further excluded 588 women who died within the first year of the baseline questionnaire to minimize the impact of reverse causation. The remaining 6,883 women included 5,279 (77%) population-based cases with breast cancer (5,105 women enrolled in the BCFR as affected probands, and 174 enrolled as unaffected relatives who were diagnosed with breast cancer during follow-up) and 1,604 (23%) women from the clinic-based BCFR sites (1,471 women enrolled with breast cancer and 133 enrolled as unaffected relatives who developed breast cancer during follow-up).
At enrollment into the BCFR, probands completed a detailed questionnaire on family history of cancer in first- and higher-degree relatives. All participants completed a structured questionnaire on menstrual and reproductive histories, hormone use, physical activity, alcohol drinking, cigarette smoking, height and weight, as well as a food frequency questionnaire (see below). For women diagnosed with breast cancer, self-reported information on treatment was collected by questionnaire, and information on tumor characteristics (i.e., tumor size, number of affected lymph nodes, grade, histology, estrogen receptor (ER) and progesterone receptor (PR) status) was abstracted from pathology reports or obtained from cancer registry records. The population-based probands reported on their exposures and dietary intake up to or during the year before diagnosis. All other women reported on their exposures and dietary intake up to or during the year before enrollment.
Self-reported weight and height were used to calculate body mass index (BMI) (kg/m2) and classified as normal weight (BMI<25 kg/m2), overweight (BMI=25–29.9 kg/m2) and obese (BMI ≥30 kg/m2). Minutes per week of recent recreational physical activity (i.e., during the three years prior to diagnosis for population-based probands or prior to enrollment for all others) were calculated by summarizing moderate and vigorous physical activities (MVPA) reported as hours per week and months per year, and by weighting vigorous physical activity at 1.67 minutes for each minute of activity. A binary variable (active vs. inactive) was created to reflect whether a women’s MVPA meets the Center for Disease Control and Prevention (CDC) Physical Activity Guidelines for Americans (≥150 minutes per week).10 Usual alcohol consumption was self-reported drinks per week consumed prior to diagnosis (population-based probands) or enrollment (all others) and categorized as nondrinkers, <7 drinks/week, or ≥7 drinks/week. Smokers were defined as women who reported smoking at least 1 cigarette a day for 3 months or longer. Cigarette smoking status was defined as never, former or current. Pack-years of smoking were calculated using smoking intensity (packs/day) multiplied by duration (years smoked).
Dietary Assessment
Dietary intake data were collected at enrollment using a self-administered FFQ which was developed for the Hawaii-Los Angeles Multiethnic Cohort to assess dietary intake in a racially/ethnically diverse population. It has been previously validated with repeated 24-hour diet recalls and demonstrated high correlations for most food groups and nutrients.11 The FFQ asked study participants about their usual dietary intake of 108 food items and assessed frequency of consumption (never or hardly ever, once a month, 2–3 times a month, once a week, 2–3 times a week, 4–6 times a week, once a day, and ≥2 times a day) and portion size (three categories).
There were 6,883 women who completed the FFQ at baseline and were alive within the first year of completing the FFQ. From these we excluded 55 women with potentially unreliable reporting of dietary intake, defined as total caloric intake exceeding three standard deviations above or below the mean value of the natural log-transformed caloric intake in the study population. We also excluded 459 population-based probands who completed the FFQ more than 5 years after diagnosis in order to reduce error associated with recalling more distant dietary intake. For all other women we excluded 134 who reported their dietary intake more than 5 years before diagnosis. Of the remaining 6,235 women, 4,769 women reported on their dietary intake within 5 years prior to breast cancer diagnosis (i.e., pre-diagnosis diet) and 1,466 women reported on their dietary intake within 5 years after diagnosis (i.e., post-diagnosis diet).
Survival Outcomes
Vital status of cases was ascertained through several follow-up activities to ensure completeness, including annual telephone contacts or mailed questionnaires with probands or family members, linkage to cancer registry and death registry records, and review of medical records or contact with physicians’ offices. Causes of death were not available for these analyses.
Statistical Analyses
We performed multivariable Cox proportional hazard models to evaluate the association between dietary isoflavone intake and all-cause mortality. Days since diagnosis was used as the time scale, with follow-up time left-truncated at the date of interview to minimize potential survival bias. Cases were censored at the date of death or date of last contact. Rate ratio of all-cause mortality was estimated as hazard ratio (HR) with 95% confidence interval (CIs). All models were adjusted for age, study site, and total caloric intake. We then examined a pre-defined list of additional confounders and adjusted for variables that altered the parameter estimates by more than 10%, including race/ethnicity, education, total fiber intake, Healthy Eating Index (HEI)-2010, treatment type, recent recreational physical activity, BMI, usual alcohol consumption, and cigarette smoking status. A binary variable (yes vs. no) was created for each treatment type (surgery, radiation therapy, chemotherapy, and hormonal therapy) based on self-reported treatment. The HEI-2010 was calculated using the methods provided by the USDA, and measures the overall diet quality by assessing adherence to the 2010 Dietary Guidelines for Americans (DGA).12,13 The total HEI-2010 score ranges from 0 (non-adherence) to 100 (perfect adherence). A higher score indicates a better adherence to the dietary guidelines. Wald tests for trend were used to evaluate associations with increasing dietary intake of isoflavone categorized as quartiles based on the intake of all women.
We first performed the analysis in all 6,235 women for total isoflavone intake and specific types of isoflavone (genistein, daidzein, and glycitein). Separate analyses were conducted for 4,769 women who reported pre-diagnosis diet and 1,466 women who reported post-diagnosis diet (three women who did not report age at diagnosis were excluded in this analysis). For all women, we evaluated potential effect modification by race/ethnicity (non-Hispanic White, Black, Hispanic, and Asian/Pacific Islander/other), menopausal status (pre- vs. post-menopausal), receipt of hormonal therapy (yes vs. no), BMI (<25, 25–29.9, ≥30 kg/m2), and levels of recreational physical activity (inactive vs. active) by comparing the log-likelihood statistics of models that included interaction terms and models without interaction terms. P values <0.05 were considered statistically significant effect modification at the multiplicative scale. We further evaluated whether associations differed for tumor hormone receptors defined by ER and PR status (any hormone receptor positive vs. hormone receptor negative) by multinominal Cox-proportional hazard regression. All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Table 1 describes the characteristics of the 6,235 women diagnosed with a first primary invasive breast cancer enrolled in the BCFR who reported reliable dietary intake. The mean (SD) dietary intake of isoflavone was 1.8 (3.9) mg/day and the median (interquartile range (IQR)) was 0.7 (1.2) mg/day. Genistein was the major source of isoflavone, followed by daidzein and glycitein. Women who consumed high levels of dietary isoflavone were more likely to be Asian Americans, young, pre-menopausal, physically active, more educated, not overweight or obese, never smokers, and drink no alcohol or <7 drinks/week (Supplemental Table 1). Women with the lowest or highest quartiles of isoflavone intake had a higher diet quality index compared to those in the middle quartiles.
Table 1.
Characteristics of Women with Breast Cancer: the Breast Cancer Family Registry
| N (%) or Mean (SD) (n=6,235) |
|
|---|---|
| Age at enrollment, y, mean (SD) | 51.8 (10.6) |
| Race/ethnicity, N (%) | |
| Non-Hispanic Whites | 3,647 (58.5) |
| Hispanics | 1,033 (16.6) |
| Blacks | 751 (12.0) |
| Asians | 690 (11.1) |
| Other | 114 (1.8) |
| Education, N (%) | |
| High school or less | 2,299 (37.1) |
| Some college or bachelor’s degree | 2,957 (47.7) |
| Graduate degree | 945 (15.2) |
| Menopausal status at enrollment, N (%) | |
| Pre-menopausal | 3,056 (49.0) |
| Post-menopausal | 3,176 (51.0) |
| Body Mass Index (BMI) at enrollment, kg/m2, mean (SD) | 26.4 (5.9) |
| <18.5 | 143 (2.3) |
| 18.5–24.9 | 2,848 (45.7) |
| 25–29.9 | 1,723 (28.5) |
| ≥30 | 1,336 (22.1) |
| Recreational Physical Activity1, N (%) | |
| Active | 2,725 (45.5) |
| Inactive | 3,262 (54.5) |
| Cigarette Smoking, N (%) | |
| Never | 3,626 (58.3) |
| Ever | 2,589 (41.7) |
| Pack-years (among smokers), mean (SD) | 17.2 (18.0) |
| Usual Alcohol Consumption, N (%) | |
| Nondrinkers | 3,702 (60.7) |
| <7 drinks/week | 1,614 (26.5) |
| ≥7 drinks/week | 787 (12.9) |
| Cancer Treatment Received, N (%) | |
| Surgery | 5,378 (86.3) |
| Radiation therapy | 3,634 (58.3) |
| Chemotherapy | 3,271 (52.5) |
| Hormonal therapy | 2,862 (45.9) |
| Tumor Estrogen receptor (ER) Status | |
| Positive | 3,260 (52.3) |
| Negative | 1,394 (22.4) |
| Undetermined | 120 (1.9) |
| Missing/unknown | 1,461 (23.4) |
| Tumor Progesterone receptor (PR) Status | |
| Positive | 2,937 (47.1) |
| Negative | 1,679 (26.9) |
| Undetermined | 110 (1.8) |
| Missing/unknown | 1,509 (24.2) |
Physic al activity was defined as active if their current levels (during the three years prior to diagnosis or questionnaire completion) of moderate-to-vigorous recreational physical activities (MVPA) was ≥150 minutes/week, and inactive otherwise.
After a median follow-up of 113 months (approximately 9.4 years), 1,224 deaths were documented. Women in the highest quartile of dietary isoflavone intake (≥1.5 mg/d) had a 21% decrease in all-cause mortality compared to women in the lowest quartile (<0.3 mg/d) (Q4 vs. Q1: HR = 0.79, 95% CI: 0.64 – 0.97, p trend=0.01) (Table 2). The inverse association was statistically significant for women who reported post-diagnosis intake of total isoflavone (Q4 vs. Q1: HR = 0.65, 95% CI: 0.41 – 1.00, p trend=0.02). The association with pre-diagnosis intake was weaker and not statistically significant (Q4 vs. Q1: HR = 0.84, 95% CI: 0.66 – 1.06, p trend=0.13). The three common types of isoflavone (genistein, daidzein, and glycitein) were associated with similar reductions in all-cause mortality (Figure 1).
Table 2.
Dietary Intake of Isoflavone and All-Cause Mortality in Women with Breast Cancer: the Breast Cancer Family Registry
| Number of Deaths | Person Years | HR (95% CI)1 | HR (95% CI)2 | |
|---|---|---|---|---|
| All women (n=6,235) | ||||
| Total Isoflavone, mg/d | ||||
| Q1 (< 0.342) | 359 | 13,938 | 1.0 (ref.) | 1.0 (ref.) |
| Q2 (0.343 – 0.674) | 343 | 14,072 | 0.96 (0.83, 1.12) | 1.01 (0.85, 1.19) |
| Q3 (0.675 – 1.493) | 291 | 14,227 | 0.81 (0.68, 0.95) | 0.89 (0.74, 1.07) |
| Q4 (≥1.494) | 231 | 13,560 | 0.67 (0.56, 0.80) | 0.79 (0.64, 0.97) |
| Ptrend <0.0001 | Ptrend = 0.01 | |||
| Women who reported pre-diagnosis diet (n=4,769)3 | ||||
| Total Isoflavone, mg/d | ||||
| Q1 (< 0.342) | 270 | 10,178 | 1.0 (ref.) | 1.0 (ref.) |
| Q2 (0.343 – 0.674) | 265 | 11,440 | 0.90 (0.76, 1.07) | 0.96 (0.80, 1.15) |
| Q3 (0.675 – 1.493) | 235 | 11,124 | 0.80 (0.67, 0.97) | 0.90 (0.74, 1.10) |
| Q4 (≥1.494) | 193 | 10,531 | 0.68 (0.55, 0.83) | 0.84 (0.66, 1.06) |
| Ptrend <0.0001 | Ptrend = 0.13 | |||
| Women who reported post-diagnosis diet (n=1,466)3 | ||||
| Total Isoflavone, mg/d | ||||
| Q1 (< 0.342) | 89 | 3,761 | 1.0 (ref.) | 1.0 (ref.) |
| Q2 (0.343 – 0.674) | 78 | 2,632 | 1.28 (0.92, 1.77) | 1.26 (0.90, 1.77) |
| Q3 (0.675 – 1.493) | 56 | 3,102 | 0.84 (0.59, 1.20) | 0.80 (0.55, 1.16) |
| Q4 (≥1.494) | 38 | 3,029 | 0.62 (0.41, 0.93) | 0.65 (0.41, 1.00) |
| Ptrend =0.008 | Ptrend = 0.02 | |||
Adjusted for age (continuous), study site, and total caloric intake (quartiles).
Additionally adjusted for race/ethnicity (non-Hispanic White, Black, Hispanic, and Asian/Pacific Islander/other), education (high school or less, some college or bachelor’s degree, or graduate degree), total fiber intake (quartiles), Health Eating Index (HEI)-2010 (quartiles), treatment type (surgery, radiation, chemotherapy, and hormone therapy), recreational physical activity (active, inactive), BMI (<25, 25–29.9, ≥30 kg/m2), alcohol use (never, <7 drinks/wk, ≥7 drinks/wk), smoking status (never, ever), and pack-years (continuous).
Three women did not report age at diagnosis and were excluded in the stratified analyses by pre- vs. post-diagnosis diet.
Figure 1. Types of Isoflavone Intake and All-Cause Mortality in Women With Breast Cancer: The Breast Cancer Family Registry.
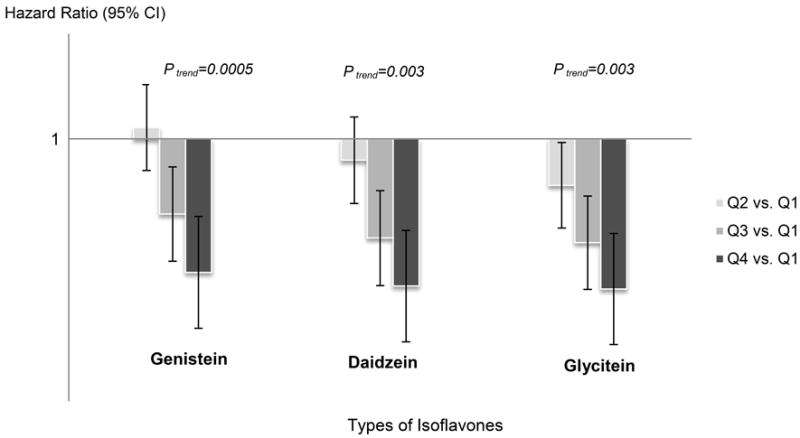
The three bars with different gray colors correspond to the harzard ratios (HRs) compring the highest quartile (Q4), the third quartile (Q3) and the second quartile (Q2) to the lowest quartile (Q1) for different types of isoflavone intake. The three lines correspond to the 95% confidence intervals (CIs).
In stratified analyses (Table 3), reduced risk of all-cause mortality associated with high (highest vs. lowest quartile) dietary isoflavone intake was statistically significant for women with ER−PR− tumors (HR=0.49, 95% CI: 0.29–0.83, p trend=0.006) and women who did not receive hormonal therapy as a component of their treatment for breast cancer (Q4 vs. Q1: HR=0.68, 95% CI: 0.51–0.91, p trend=0.02). No associations were found for women with hormone receptor positive tumors and for women who received hormonal therapy. The interactions, however, were not statistically significant. Analyses stratified by race/ethnicity, menopausal status, BMI, and physical activity showed borderline significant trends across quartiles of isoflavone intake for Hispanics (P trend=0.05) and normal weight women (BMI < 25 kg/m2; P trend=0.05), and a significant trend for physically active women (P trend=0.04), but none of the HR estimates or interactions was statistically significant.
Table 3.
Associations between Dietary Intake of Isoflavone and All-Cause Mortality in Women with Breast Cancer by Patient and Treatment Characteristics: the Breast Cancer Family Registry
| Isoflavone, mg/d | Number of death | Person-years | HR (95% CI)1 | HR (95% CI)2 |
|---|---|---|---|---|
| By Tumor Hormone Receptor Status | ||||
| Tumor hormone receptor positive (ER+PR+, ER+PR−, ER−PR+) (n=3,348) | ||||
| Q1 (< 0.342) | 192 | 7,665 | 1.0 (ref.) | 1.0 (ref.) |
| Q2 (0.343 – 0.674) | 190 | 7,966 | 1.00 (0.81, 1.24) | 1.06 (0.85, 1.31) |
| Q3 (0.675 – 1.493) | 168 | 8,209 | 0.85 (0.68, 1.06) | 0.96 (0.76, 1.22) |
| Q4 (≥1.494) | 146 | 8,067 | 0.72 (0.57, 0.91) | 0.90 (0.69, 1.19) |
| Ptrend = 0.002 | Ptrend = 0.41 | |||
| Tumor hormone receptor negative (ER−PR−) (n=1,167) | ||||
| Q1 (< 0.342) | 65 | 2,554 | 1.0 (ref.) | 1.0 (ref.) |
| Q2 (0.343 – 0.674) | 61 | 2,463 | 0.92 (0.63, 1.34) | 0.95 (0.64, 1.41) |
| Q3 (0.675 – 1.493) | 44 | 2,177 | 0.69 (0.45, 1.04) | 0.69 (0.44, 1.08) |
| Q4 (≥1.494) | 37 | 2,349 | 0.49 (0.31, 0.76) | 0.49 (0.29, 0.83) |
| Ptrend = 0.001 | Ptrend = 0.005 | |||
| P for Interaction = 0.55 | P for Interaction = 0.53 | |||
| By Breast Cancer Treatment with Hormonal Therapy | ||||
| Treated with Hormonal Therapy (n=2,862) | ||||
| Q1 (< 0.342) | 153 | 6,266 | 1.0 (ref.) | 1.0 (ref.) |
| Q2 (0.343 – 0.674) | 158 | 6,487 | 0.99 (0.78, 1.24) | 0.99 (0.78, 1.26) |
| Q3 (0.675 – 1.493) | 123 | 6,904 | 0.72 (0.56, 0.93) | 0.78 (0.60, 1.02) |
| Q4 (≥1.494) | 123 | 6,476 | 0.75 (0.58, 0.97) | 0.90 (0.66, 1.22) |
| Ptrend = 0.005 | Ptrend = 0.19 | |||
| Not Treated with Hormonal Therapy (n=3,373) | ||||
| Q1 (< 0.342) | 206 | 7,672 | 1.0 (ref.) | 1.0 (ref.) |
| Q2 (0.343 – 0.674) | 185 | 7,585 | 0.93 (0.76, 1.15) | 1.03 (0.83, 1.28) |
| Q3 (0.675 – 1.493) | 168 | 7,323 | 0.88 (0.71, 1.10) | 0.95 (0.75, 1.20) |
| Q4 (≥1.494) | 108 | 7,083 | 0.58 (0.45, 0.75) | 0.68 (0.51, 0.91) |
| Ptrend <0.0001 | Ptrend = 0.02 | |||
| P for Interaction = 0.08 | P for Interaction = 0.20 | |||
| By Race/Ethnicity | ||||
| Non-Hispanic Whites (n=3,647) | ||||
| Q1 (< 0.342) | 232 | 9,844 | 1.0 (ref.) | 1.0 (ref.) |
| Q2 (0.343 – 0.674) | 260 | 10,885 | 1.03 (0.85, 1.24) | 1.07 (0.88, 1.29) |
| Q3 (0.675 – 1.493) | 206 | 10,268 | 0.88 (0.72, 1.08) | 0.97 (0.78, 1.19) |
| Q4 (≥1.494) | 104 | 6,617 | 0.73 (0.57, 0.94) | 0.86 (0.66, 1.12) |
| Ptrend =0.008 | Ptrend = 0.25 | |||
| Blacks (n=751) | ||||
| Q1 (< 0.342) | 92 | 2,342 | 1.0 (ref.) | 1.0 (ref.) |
| Q2 (0.343 – 0.674) | 31 | 869 | 0.85 (0.55, 1.29) | 0.82 (0.52, 1.27) |
| Q3 (0.675 – 1.493) | 26 | 1,040 | 0.53 (0.33, 0.87) | 0.52 (0.31, 0.87) |
| Q4 (≥1.494) | 23 | 642 | 0.82 (0.49, 1.37) | 0.76 (0.42, 1.39) |
| Ptrend = 0.10 | Ptrend = 0.08 | |||
| Hispanics (n=1,033) | ||||
| Q1 (< 0.342) | 28 | 1,380 | 1.0 (ref.) | 1.0 (ref.) |
| Q2 (0.343 – 0.674) | 40 | 1,710 | 1.23 (0.73, 2.07) | 1.24 (0.71, 2.16) |
| Q3 (0.675 – 1.493) | 39 | 2,092 | 0.92 (0.54, 1.57) | 0.82 (0.45, 1.47) |
| Q4 (≥1.494) | 27 | 1,854 | 0.71 (0.39, 1.28) | 0.62 (0.32, 1.21) |
| Ptrend = 0.11 | Ptrend = 0.05 | |||
| Asians3 (n=690) | ||||
| Q1 (< 1.677) | 29 | 1,380 | 1.0 (ref.) | 1.0 (ref.) |
| Q2 (1.678 – 3.699) | 22 | 1,373 | 0.74 (0.42, 1.30) | 0.83 (0.46, 1.49) |
| Q3 (3.700 – 7.999) | 19 | 1,384 | 0.61 (0.34, 1.11) | 0.56 (0.30, 1.03) |
| Q4 (≥8.000) | 27 | 1,424 | 0.90 (0.52, 1.58) | 0.81 (0.44, 1.46) |
| Ptrend = 0.59 | Ptrend = 0.26 | |||
| P for Interaction = 0.52 | P for Interaction = 0.52 | |||
| By Menopausal Status | ||||
| Pre-menopausal women (n=3,056) | ||||
| Q1 (< 0.342) | 126 | 5,838 | 1.0 (ref.) | 1.0 (ref.) |
| Q2 (0.343 – 0.674) | 156 | 6,650 | 1.06 (0.83, 1.35) | 1.17 (0.91, 1.51) |
| Q3 (0.675 – 1.493) | 146 | 7,700 | 0.86 (0.67, 1.11) | 0.99 (0.75, 1.29) |
| Q4 (≥1.494) | 123 | 7,295 | 0.77 (0.59, 1.01) | 0.93 (0.68, 1.27) |
| Ptrend = 0.02 | Ptrend = 0.46 | |||
| Post-menopausal women (n=3,176) | ||||
| Q1 (< 0.342) | 231 | 8,080 | 1.0 (ref.) | 1.0 (ref.) |
| Q2 (0.343 – 0.674) | 187 | 7,423 | 0.94 (0.77, 1.15) | 0.96 (0.78, 1.18) |
| Q3 (0.675 – 1.493) | 145 | 6,527 | 0.83 (0.66, 1.04) | 0.88 (0.69, 1.11) |
| Q4 (≥1.494) | 108 | 6,265 | 0.65 (0.50, 0.83) | 0.78 (0.59, 1.05) |
| Ptrend =0.001 | Ptrend =0.09 | |||
| P for interaction=0.49 | P for interaction= 0.45 | |||
| By BMI Status | ||||
| Normal weight (BMI <25 kg/m2) (n=2,991) | ||||
| Q1 (< 0.342) | 150 | 6,520 | 1.0 (ref.) | 1.0 (ref.) |
| Q2 (0.343 – 0.674) | 146 | 6,858 | 0.94 (0.74, 1.19) | 0.97 (0.76, 1.24) |
| Q3 (0.675 – 1.493) | 120 | 6,828 | 0.78 (0.61, 1.01) | 0.86 (0.66, 1.13) |
| Q4 (≥1.494) | 122 | 8,147 | 0.67 (0.52, 0.87) | 0.74 (0.54, 1.01) |
| Ptrend =0.001 | Ptrend = 0.05 | |||
| Overweight (BMI 25–29.9 kg/m2) (n=1,723) | ||||
| Q1 (< 0.342) | 104 | 4,006 | 1.0 (ref.) | 1.0 (ref.) |
| Q2 (0.343 – 0.674) | 81 | 3,636 | 0.93 (0.68, 1.26) | 1.01 (0.74, 1.39) |
| Q3 (0.675 – 1.493) | 93 | 4,247 | 0.93 (0.68, 1.26) | 0.95 (0.69, 1.30) |
| Q4 (≥1.494) | 68 | 3,342 | 0.89 (0.64, 1.24) | 0.97 (0.66, 1.41) |
| Ptrend = 0.51 | Ptrend = 0.75 | |||
| Obese (BMI ≥30 kg/m2) (n=1,336) | ||||
| Q1 (< 0.342) | 91 | 2,992 | 1.0 (ref.) | 1.0 (ref.) |
| Q2 (0.343 – 0.674) | 103 | 3,014 | 1.06 (0.79, 1.44) | 1.17 (0.86, 1.60) |
| Q3 (0.675 – 1.493) | 70 | 2,741 | 0.74 (0.53, 1.05) | 0.83 (0.58, 1.20) |
| Q4 (≥1.494) | 38 | 1,767 | 0.59 (0.39, 0.90) | 0.76 (0.48, 1.19) |
| Ptrend = 0.005 | Ptrend = 0.13 | |||
| P for Interaction=0.76 | P for Interaction = 0.77 | |||
| By Levels of Recreational Physical Activity | ||||
| Physically Inactive (n=3,262) | ||||
| Q1 (< 0.342) | 170 | 6,457 | 1.0 (ref.) | 1.0 (ref.) |
| Q2 (0.343 – 0.674) | 155 | 6,357 | 0.88 (0.71, 1.12) | 0.94 (0.74, 1.18) |
| Q3 (0.675 – 1.493) | 127 | 5,809 | 0.78 (0.61, 1.00) | 0.84 (0.64, 1.08) |
| Q4 (≥1.494) | 107 | 5,321 | 0.70 (0.54, 0.91) | 0.85 (0.62, 1.15) |
| Ptrend =0.005 | Ptrend = 0.19 | |||
| Physically Active (n=2,725) | ||||
| Q1 (< 0.342) | 171 | 6,850 | 1.0 (ref.) | 1.0 (ref.) |
| Q2 (0.343 – 0.674) | 167 | 7,110 | 1.01 (0.81, 1.27) | 1.09 (0.86, 1.38) |
| Q3 (0.675 – 1.493) | 153 | 7,839 | 0.86 (0.68, 1.08) | 0.93 (0.73, 1.20) |
| Q4 (≥1.494) | 118 | 7,815 | 0.66 (0.51, 0.85) | 0.75 (0.56, 1.01) |
| Ptrend = 0.001 | Ptrend = 0.04 | |||
| P for Interaction = 0.62 | P for Interaction = 0.64 | |||
Adjusted for age (continuous), study site, and total caloric intake (quartiles).
Additionally adjusted for race/ethnicity (non-Hispanic White, Black, Hispanic, and Asian Americans), education (high school or less, some college or bachelor’s degree, or graduate degree), total fiber intake (quartiles), Health Eating Index (HEI)-2010 (quartiles), treatment type (surgery, radiation, chemotherapy, and hormone therapy), physical activity (active, inactive), BMI (categorical: <25, 25–29.9, ≥30), alcohol use (never, <7 drinks/wk, ≥7 drinks/wk), smoking status (never, ever), and pack-years (continuous).
Quartiles were based on the distribution of dietary intake of isoflavone in Asian Americans.
DISCUSSION
The present study examined the association between dietary intake of isoflavone and all-cause mortality in 6,235 women diagnosed with a first primary breast cancer who had been followed for a median of over nine years. Overall, we observed a 21% lower all-cause mortality associated with high isoflavone intake. The reduced mortality was largely confined to women with ER−PR− tumors and women who were not treated with hormonal therapy.
Although a number of epidemiologic studies reported that higher soy consumption was associated with a lower risk of breast cancer recurrence and/or mortality in Chinese women,7,14,15 studies in the U.S. have reported inconsistent findings.16,17 For pre-diagnosis intake of dietary isoflavone, one study of 3,842 women enrolled in the Multiethnic Cohort did not find an association with all-cause mortality,18 but an earlier report in 1,210 women found a 48% reduction in all-cause mortality for those who consumed the highest quintile of isoflavone (>7.5 mg/day).19 For post-diagnosis intake, a pooled analysis of two U.S. cohorts did not find significant reduction in all-cause mortality associated with high isoflavone intake (>10 mg/day).16,17,20 However, the two U.S. cohorts were predominantly non-Hispanic Whites (82–85%), with a small proportion of racial/ethnic minorities (approximately 5% Hispanics, 4% Blacks, and 3% Asian Americans). In our more diverse cohort of women living in North America (approximately 17% Hispanics, 12% Blacks, and 11% Asian Americans), we found a significant trend of lower all-cause mortality associated with a higher dietary intake of isoflavone. This association was similarly observed across all racial/ethnic groups, although the trend was slightly stronger in Hispanic women. Although Asian American women enrolled in BCFR had a higher mean intake of dietary isoflavone than women from other racial/ethnic groups (6.1 vs. 1.3 mg/day, p<0.0001), these intake levels are substantially lower than those of women living in Asian countries (e.g., mean intake of 45.9 mg/day in women living in China). Our findings suggest that women living in North American, despite an overall low consumption of isoflavone from diet, may still benefit from increasing their isoflavone intake to a higher level.
Consistent with the pooled analysis in the two U.S. cohorts,16,17,20 we found high isoflavone intake was significantly associated with a reduction in all-cause mortality only in women with ER−PR− tumors. Cell line studies suggest soy isoflavone may interact with tamoxifen therapy and potentially reduce the effect of cancer treatment.1 However, our study did not find a negative impact of isoflavone on all-cause mortality among women treated with hormonal therapy. Among those who did not receive hormonal therapy as part of their cancer treatment, high isoflavone intake was associated with reduced all-cause mortality. These results, taken together, may indicate that dietary isoflavone is unlikely to negatively impact survival for women treated with hormonal therapy, but the potential benefit may be limited to women with negative tumor hormone receptors (ER−PR−) or those not receiving hormonal therapy.
For women with breast cancer in the BCFR, information is available on both pre-diagnosis and post-diagnosis dietary intake. This provides a unique opportunity to assess the potential timing effect of women’s diet on survival. A statistically significant inverse trend was found for post-diagnosis isoflavone intake only, with an approximately 35% reduction in all-cause mortality associated with high intake. For pre-diagnosis intake, the inverse association was weaker and not statistically significant. It is possible that dietary assessment for pre-diagnostic intake is associated with more measurement errors that post-diagnostic intake, which can bias the findings towards the null. Alternatively, this finding may suggest women’s recent diet plays a more important role in survival than a more remote diet, thus highlighting the opportunity for women to improve their survival by increasing dietary intake of isoflavone after cancer diagnosis.
Some limitations of this study should be considered when interpreting our results. First, the use of an FFQ to assess habitual dietary intake is subject to measurement error in estimating absolute intake. We noticed that the mean intake levels of isoflavone in our study population were lower than those reported in other US women, which may be due to the differences of the FFQs used in capturing dietary isoflavone intake. Nevertheless, comparing higher versus lower intake such as comparing the highest quartile to the lowest quartile is still valid in evaluating diet assessed from FFQs with health outcomes.21 The FFQ used in the BCFR has also been shown to have reasonable validity when compared against repeated 24-hour diet recalls (validity coefficient r = 0.5 for dietary isoflavone intake).11 To further improve validity, we excluded women with unreliable dietary reporting and those who completed the FFQ more than 5 years after diagnosis to reduce errors associated with recalling more distant diet. We also adjusted for total energy intake in all analyses to reduce confounding and improve validity by removing correlated errors. Although we cannot rule out misclassification of isoflavone intake, the misclassification error is likely to be non-differential, thus attenuating the results towards the null. Second, a higher dietary intake of isoflavone was associated with socioeconomic and lifestyle factors such as education, body mass index, recreational physical activity, cigarette smoking and alcohol consumption. To minimize the chance of residual confounding, we carefully adjusted for all these factors in the multivariable models. Third, information on treatment was based on women’s self-report, and we lacked information on type and length of hormonal therapy, preventing a more in-depth analysis of potential different effects in women treated with different types and lengths of hormonal therapy. However, previous validation studies in the BCFR have shown high agreement between self-reported treatment information and medical records.22,23 Fourth, tumor hormone receptor status was not available for approximately 28 percent of the women; the mean intake of dietary isoflavone, however, was similar for women with and without information on tumor hormone receptor status (1.9 vs. 1.8 mg/day, P=0.49). Lastly, our study outcome was limited to all-cause mortality, preventing us from evaluating breast cancer-specific mortality, recurrence, and other prognostic endpoints. Prior studies of women with breast cancer have found that 48–70% of all deaths were due to breast cancer. We also lacked information on co-morbidities, which could influence all-cause mortality. While prior studies suggest that lifestyle risk factors may impact all-cause mortality through effects on deaths unrelated to breast cancer, the pooled analysis of two U.S. cohorts reported that women who consumed a high level of dietary isoflavone had a significantly reduced breast cancer recurrence, a surrogate for breast cancer-specific survival.
The strengths of this study include a large number of women from racial/ethnic minority populations that allowed us to evaluate the potential heterogeneous effect by race/ethnicity groups, and the availability of clinical and interview data that allowed us to consider different subtypes of breast cancer and subgroups of patients, and to adjust for confounders to minimize confounding. Bias due to differential follow-up was minimized by the use of linkages to population-based cancer registries and death registry record outcomes of all cases. We adjusted for any survival bias by left-truncating all cases at the time of recruitment.
In conclusion, in this large ethnically-diverse cohort of women with breast cancer, a higher dietary intake of isoflavone was associated with a reduced total mortality. High isoflavone intake may be associated with lower mortality only for women with ER−PR− tumors or those who do not receive hormonal therapy as part of their cancer treatment.
Supplementary Material
Acknowledgments
Sources of Support: This work was supported by grant UM1 CA164920 from the USA National Cancer Institute. The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the Breast Cancer Family Registry (BCFR), nor does mention of trade names, commercial products, or organizations imply endorsement by the USA Government or the BCFR.
Footnotes
Conflict of Interests Statement: The authors have no conflicts of interests to disclose.
References
- 1.Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999 Mar;129(3):758S–767S. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- 2.Messina M, McCaskill-Stevens W, Lampe JW. Addressing the soy and breast cancer relationship: review, commentary, and workshop proceedings. Journal of the National Cancer Institute. 2006 Sep 20;98(18):1275–1284. doi: 10.1093/jnci/djj356. [DOI] [PubMed] [Google Scholar]
- 3.Velentzis LS, Woodside JV, Cantwell MM, Leathem AJ, Keshtgar MR. Do phytoestrogens reduce the risk of breast cancer and breast cancer recurrence? What clinicians need to know. European journal of cancer (Oxford, England : 1990) 2008 Sep;44(13):1799–1806. doi: 10.1016/j.ejca.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Research WCRFAIfC. Diet, nutrition, physical activity, and breast cancer survivors. 2014 http://www.wcrf.org/sites/default/files/Breast-Cancer-Survivors-2014-Report.pdf.
- 5.Chi F, Wu R, Zeng YC, Xing R, Liu Y, Xu ZG. Post-diagnosis soy food intake and breast cancer survival: a meta-analysis of cohort studies. Asian Pacific journal of cancer prevention : APJCP. 2013;14(4):2407–2412. doi: 10.7314/apjcp.2013.14.4.2407. [DOI] [PubMed] [Google Scholar]
- 6.Dong JY, Qin LQ. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast cancer research and treatment. 2011 Jan;125(2):315–323. doi: 10.1007/s10549-010-1270-8. [DOI] [PubMed] [Google Scholar]
- 7.Shu XO, Zheng Y, Cai H, et al. Soy food intake and breast cancer survival. JAMA : the journal of the American Medical Association. 2009 Dec 9;302(22):2437–2443. doi: 10.1001/jama.2009.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morimoto Y, Maskarinec G, Park SY, et al. Dietary isoflavone intake is not statistically significantly associated with breast cancer risk in the Multiethnic Cohort. The British journal of nutrition. 2014 Sep 28;112(6):976–983. doi: 10.1017/S0007114514001780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.John EM, Hopper JL, Beck JC, et al. The Breast Cancer Family Registry: an infrastructure for cooperative multinational, interdisciplinary and translational studies of the genetic epidemiology of breast cancer. Breast Cancer Res. 2004;6(4):R375–389. doi: 10.1186/bcr801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Center for Disease Control and Prevention (CDC) Physical Activity Guidelines for Americans. 2008 http://www.health.gov/paguidelines/. Accessed October 1, 2014.
- 11.Stram DO, Hankin JH, Wilkens LR, et al. Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. Am J Epidemiol. 2000 Feb 15;151(4):358–370. doi: 10.1093/oxfordjournals.aje.a010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Developing the Healthy Eating Index–2010. 2014 http://appliedresearch.cancer.gov/hei/developing.html?&url=/tools/hei/developing.html.
- 13.Guenther PM, Kirkpatrick SI, Reedy J, et al. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J Nutr. 2014 Mar;144(3):399–407. doi: 10.3945/jn.113.183079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang X, Zhang Q, Wang S, Huang X, Jin S. Effect of soy isoflavones on breast cancer recurrence and death for patients receiving adjuvant endocrine therapy. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2010 Nov 23;182(17):1857–1862. doi: 10.1503/cmaj.091298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang YF, Kang HB, Li BL, Zhang RM. Positive effects of soy isoflavone food on survival of breast cancer patients in China. Asian Pacific journal of cancer prevention : APJCP. 2012;13(2):479–482. doi: 10.7314/apjcp.2012.13.2.479. [DOI] [PubMed] [Google Scholar]
- 16.Guha N, Kwan ML, Quesenberry CP, Jr, Weltzien EK, Castillo AL, Caan BJ. Soy isoflavones and risk of cancer recurrence in a cohort of breast cancer survivors: the Life After Cancer Epidemiology study. Breast cancer research and treatment. 2009 Nov;118(2):395–405. doi: 10.1007/s10549-009-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caan BJ, Natarajan L, Parker B, et al. Soy food consumption and breast cancer prognosis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011 May;20(5):854–858. doi: 10.1158/1055-9965.EPI-10-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conroy SM, Maskarinec G, Park SY, Wilkens LR, Henderson BE, Kolonel LN. The effects of soy consumption before diagnosis on breast cancer survival: the Multiethnic Cohort Study. Nutrition and cancer. 2013;65(4):527–537. doi: 10.1080/01635581.2013.776694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fink BN, Steck SE, Wolff MS, et al. Dietary flavonoid intake and breast cancer survival among women on Long Island. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007 Nov;16(11):2285–2292. doi: 10.1158/1055-9965.EPI-07-0245. [DOI] [PubMed] [Google Scholar]
- 20.Nechuta SJ, Caan BJ, Chen WY, et al. Soy food intake after diagnosis of breast cancer and survival: an in-depth analysis of combined evidence from cohort studies of US and Chinese women. The American journal of clinical nutrition. 2012 Jul;96(1):123–132. doi: 10.3945/ajcn.112.035972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willett WC. Nutritional Epidemiology. New York: Oxford University Press; 1998. [Google Scholar]
- 22.Barisic A, Glendon G, Weerasooriya N, Andrulis IL, Knight JA. Accuracy of Self-Reported Breast Cancer Information among Women from the Ontario Site of the Breast Cancer Family Registry. Journal of cancer epidemiology. 2012;2012:310804. doi: 10.1155/2012/310804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips KA, Milne RL, Buys S, et al. Agreement between self-reported breast cancer treatment and medical records in a population-based Breast Cancer Family Registry. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005 Jul 20;23(21):4679–4686. doi: 10.1200/JCO.2005.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


