Abstract
The ability of the autonomic nervous system to flexibly adapt to environmental changes is thought to indicate efficient use of self-regulatory resources. Deficits in autonomic reactivity appear to characterize current depression; however, whether autonomic reactivity confers vulnerability to future depression when individuals encounter environmental stressors is unknown. Fluctuations in respiratory sinus arrhythmia (RSA) and heart rate (HR) were evaluated in response to emotion-eliciting films among 134 undergraduates. Negative events and depressive symptoms were assessed five times across twelve weeks. Multilevel modeling demonstrated that smaller decreases in RSA in response to sadness, greater increases in HR following sadness, and smaller increases in HR to amusement were prospectively associated with greater depressive symptoms when individuals encountered high levels of idiographically-assessed negative events. These results demonstrate that the lack of contextually-appropriate autonomic reactivity may confer vulnerability to depression under conditions of environmental stress, perhaps due to attenuated capacity for effective self-regulation.
Keywords: depression, respiratory sinus arrhythmia, vulnerability, autonomic nervous system, stress
Major Depressive Disorder (MDD) is the most common mental disorder, with an estimated lifetime prevalence of 16.6% (Kessler et al., 2005). It is associated with tremendous impairment and considerable comorbidity with other psychiatric conditions, resulting in major personal, economic, and societal costs (Kessler et al., 2006; Kessler & Wang, 2009). The onset of depression often occurs following exposure to stressful life events, but there are considerable individual differences in susceptibility to depression following stressors (Kendler et al., 1999). Thus, recent work has sought to identify behavioral and biological factors that serve as indicators of vulnerability to or protection against depression following negative life events (e.g., Abramson et al., 2002). In the present study, we used a multi-wave design to evaluate whether patterns of reactivity of the autonomic nervous system in response to different emotional contexts would confer vulnerability to depression in the face of negative life events.
The autonomic nervous system, which contains sympathetic and parasympathetic branches, can facilitate adaptive behavioral and emotional responses to meet contextual demands (Beauchaine, 2001; Kashdan & Rottenberg, 2010; Kreibig, 2010; Thayer & Lane, 2009). Autonomic nervous system reactivity can be measured as the extent to which individuals show patterns of change in activity across different environmental or emotional contexts. One index of parasympathetic activity is respiratory sinus arrhythmia (RSA), a measure of variability in heart rate that occurs over the respiration cycle. During periods of rest, the medial prefrontal cortex exerts inhibitory control over the amygdala, indirectly enhancing cardiac control via the vagus nerve, and resulting in elevated resting RSA (Thayer et al., 2009, 2012). However, during periods of emotional or environmental challenge (e.g., stressors, attention to salient stimuli, or during emotion regulation), the parasympathetic nervous system typically withdraws its inhibitory control over heart rate, which results in reductions in RSA, allowing the body to mobilize resources needed to flexibly respond to the challenge (Beauchaine, 2001). When the challenge remits, inhibitory vagal control typically is augmented, resulting in a return of RSA to resting levels (Rottenberg, 2007). Given the presence of shared neural pathways, RSA has been proposed as a biological index of capacity for effective emotion regulation (Beauchaine & Thayer, 2015; Thayer et al., 2012).
Although RSA reactivity is a common method of assessing vagal control, fluctuations in heart rate (HR) in response to emotional stimuli also can be used as an indicator of both vagal and sympathetic activity (Cacioppo, Uchino, & Berntson, 1994; Kreibig, 2010). Increases in HR are thought to represent emotional arousal or regulatory effort, whereas decreases in HR may represent decreased arousal, shifting attention toward salient stimuli, or regulatory success (Allen et al., 2012; Somers et al., 2015). Alterations of typical patterns of responding to emotional stimuli may represent the inability of the autonomic nervous system to respond flexibly to changes in the environmental context (Kashdan & Rottenberg, 2010). These patterns may be attenuated in MDD, consistent with the theory that depression is characterized by insensitivity to emotional context (Rottenberg, 2005; Rottenberg & Hindash, 2015).
Evaluating fluctuations in the autonomic nervous system (e.g., using RSA and HR) in response to emotional stimuli such as sadness, one of the core features of depression, may provide useful information about the capacity for flexible adaptation (e.g., Rottenberg, 2007; Gentzler et al., 2009; Porges, 2007). One common method of studying physiological responses to varying emotional contexts is the presentation of films that are designed to elicit specific emotions, such as sadness and amusement (e.g., Gross & Levenson, 1997; Rottenberg et al., 2007b). Based on the existing literature, it can be difficult to articulate “normative” patterns of autonomic responses to specific stimuli (Kreibig, 2010). In part, this is because studies have used a variety of types of stimuli (e.g., various films, pictures, autobiographical recall tasks) and a variety of indices of autonomic functioning, which seem to be imperfect measures of psychological processes. For example, RSA and HR each are influenced to some degree by both parasympathetic and sympathetic systems, vary based on interactions among autonomic branches and respiration, and each may be related to emotional experience as well as effortful emotion regulation (Berntson et al., 1997; Kreibig, 2010; Overbeek et al., 2012). Thus, the field has not come to a consensus on exactly what patterns of response to emotional stimuli are likely to be adaptive, and results across studies have been quite mixed (Kreibig, 2010). Nevertheless, several studies that have used films to elicit sadness have demonstrated reductions in RSA to sad films relative to neutral films, likely representing vagal withdrawal, which allows the body to respond to the emotional challenge (Rottenberg et al., 2005; El-Sheikh et al., 2015; Overbeek et al., 2012; Porges, 2007). That attenuated vagal withdrawal to sadness is associated with depression concurrently and prospectively is consistent with the hypothesis that RSA withdrawal during sad films is normative and may be adaptive for the regulation of sadness (Rottenberg, 2007).
With respect to HR, the majority of studies using sad films have reported a deactivating response to sadness in which sympathetic withdrawal occurs (Kreibig, 2010; Overbeek et al., 2012), which may represent concerned attention or sympathy (Eisenberg et al., 1989). It is important to note, however, that sadness, including crying, sometimes is characterized by an increase in HR (Kreibig, 2010; Rottenberg, Bylsma, & Vingerhoets, 2008; Vingerhoets & Bylsma, in press). Others have suggested that sympathetic activation is characteristic of impending loss, whereas deactivating responses (e.g., decreases in HR) are more common after loss has occurred (such as in response to commonly-used film clips such as “The Champ” in which a boy’s father has died; Gross & Levenson, 1997; Kreibig, 2010; Overbeek et al., 2012; Rottenberg, 2007). Thus, although decreases in HR are expected overall, there is likely to be variability in affective and sympathetic responses to sadness films, and it may not be clear what constitutes a “psychologically healthy” HR response to sad stimuli as the associated psychological processes may be complex (Kreibig et al., 2007; Kreibig, 2010). Although initially it might seem contradictory to anticipate decreases in both RSA and HR to sad films given that in general RSA and HR are inversely related, these measures actually do not index completely overlapping processes (Berntson et al., 1997). For example, decreases in HR to sad films could represent concerned attention or an appropriate deactivating response following loss (Kreibig, 2010; Eisenberg et al., 1989), whereas simultaneous decreases in RSA could represent the body attempting to adapt to the emotional demands of the situation or preparation for a regulatory response. In line with these hypotheses, previous work has documented decreases in both RSA and HR to sad films (Overbeek et al., 2012).
Few studies in the literature have evaluated normative autonomic responses during recovery periods following sad films, when a variety of regulatory processes may occur. On the one hand, following a sad film, a return to baseline (an increase in HR) might be expected given that concerned attention no longer is directed toward the film (Eisenberg et al., 1989). At the same time, regulatory efforts to reduce sadness would be expected during recovery, which also should increase HR (Frazier et al., 2004; Obrist, 1981; Overbeek et al., 2012) and maintain RSA withdrawal (LeMoult et al., 2016). However, once regulation is complete, conservation of autonomic resources and energy should take place by increasing parasympathetic activation (augmentation of RSA) and decreasing sympathetic activation (decrease in HR from regulatory levels toward resting levels) (Rottenberg, 2007). Although it is difficult to interpret these dynamic and opponent processes by averaging autonomic responses across a recovery period (Rottenberg et al., 2007a), it might be expected that across recovery, excessive increases in HR and attenuated RSA augmentation could map onto psychological processes such as a relatively greater time spent regulating, excessive perceived need for continued regulation (Frazier et al., 2004; Obrist, 1981; Overbeek et al., 2012), unsuccessful regulation, or greater use of maladaptive regulatory strategies such as expressive suppression or rumination that inhibit autonomic recovery (Gross & Levenson, 1997; LeMoult et al., 2016). Relatively few studies have examined normative patterns of autonomic response to positive affective stimuli such as amusement, but the available literature suggests that RSA may decrease (Overbeek et al., 2012) or stay the same (Kreibig, 2010), whereas HR may increase or show no change (Kreibig, 2010).
Given that RSA and HR likely index a variety of physiological and psychological processes (Berntson et al., 1997, 2007; El-Sheikh et al., 2015; Kreibig et al., 2007; Kreibig, 2010), it is difficult to completely disentangle emotional experience from emotion regulation by measuring autonomic responses to films. Nevertheless, it is possible that evaluating individual differences in patterns of response to emotional stimuli would be useful in identifying individuals who may be vulnerable to depression. However, the depression literature on autonomic reactivity and recovery in response to emotional stimuli is relatively sparse. A few studies have found that RSA withdrawal to sadness and RSA recovery from sadness or other stressors is attenuated among individuals with current MDD (Rottenberg et al., 2003, 2005, 2007a; Bylsma et al., 2014). Similarly, depressed individuals show blunted HR responses to stressful stimuli (Bylsma et al., 2008; Salomon et al., 2013; Taylor, 2010). However, although case-controlled studies suggest that impairments in autonomic reactivity are associated with depression, they provide limited information about whether atypical autonomic reactivity could serve as a vulnerability factor for future depression. These studies use a logically backward design, selecting participants based on the outcome (depression or no depression) and then comparing on the potential vulnerability factor; they ignore the possible activating role of stressful events (or shifts in environmental or emotional context) that are particularly relevant to depression; and the use of postmorbid participants to test causal hypotheses precludes the ability to test whether atypical autonomic activity is a vulnerability to future depression, versus a scar of past depression or simply a correlate of current depressive symptoms (e.g., Just, Abramson, & Alloy, 2001).
A modest number of prospective studies have improved upon these methodological limitations. These studies found evidence that attenuated RSA reactivity to sadness (but not to amusement) predicted a poorer course of current depressive episodes among adults (Fraguas et al., 2007; Panaite et al., 2016; Rottenberg et al., 2005) and that combinations of RSA reactivity were associated with depressive symptom trajectories among children and adolescents (Yaroslavsky, Rottenberg, & Kovacs, 2014). With respect to HR reactivity, smaller decreases in HR to sadness were associated with prospective increases in depressive symptoms among children (Somers et al., 2015), and a lack of HR increase to amusement predicted a lower likelihood of recovery from a major depressive episode among adults (Rottenberg et al., 2002).
Vulnerability factors may be particularly likely to manifest in psychopathology following context shifts (such as negative life events) that require flexible adaptation. This consideration is particularly important given that autonomic reactivity may be helpful for adapting to difficult experiences brought about by recent stressors. Knowing whether atypical autonomic reactivity is a vulnerability factor, as opposed to a correlate of depression, is a critical gap in the literature that could inform efforts for early intervention to attenuate vulnerability. Indeed, researchers have called for prospective studies that evaluate autonomic reactivity in the context of naturally-occurring stressors (e.g., Panaite et al., 2016).
To date, three prospective studies have suggested that atypical autonomic reactivity could serve as a vulnerability to depression that is apparent only in the presence of contextual factors such as marital conflict (El Sheikh & Whitson, 2006), lack of social support (Hopp et al., 2013), and social withdrawal (Morgan et al., 2013). In addition, one study did not find evidence that resting RSA protected against the impact of stressors on depressive symptoms (Bosch et al., 2009). However, no prospective studies have evaluated whether exposure to negative life events is particularly likely to lead to depression among individuals with atypical reactivity or recovery of RSA and HR. Thus, prior work has provided an incomplete test of whether atypical autonomic reactivity confers vulnerability to future depression.
In the present study, we completed the first multi-wave study of atypical autonomic reactivity as a vulnerability to depression. Importantly, we sought to address many of the limitations of past studies by (a) evaluating fluctuations in both RSA and HR in response to emotional contexts (sadness and amusement, emotions relevant to the core features of depression) relative to neutral baselines; (b) examining the effects of controlling for respiration, to rule out this important potential confound (Overbeek et al., 2012); (c) evaluating interactions between naturally-occurring negative life events and indices of autonomic reactivity when predicting prospective fluctuations in depressive symptoms; and (d) using a multi-wave design, which allowed us to evaluate fluctuations in negative life events using an idiographic (person-centered) approach, providing a stronger test of vulnerability than is possible with the nomothetic (sample mean-centered) approach that is commonly-used with two time-point study designs (Abela & Hankin, 2008). Thus, the present study represents the first to conduct a complete test of whether atypical autonomic reactivity confers vulnerability to future symptoms of depression, with a number of methodological improvements on the existing literature.
Given that vagal withdrawal is expected in response to emotionally-salient stimuli, and that vagal augmentation is expected during recovery from negative affective states (Frazier et al., 2004; Rottenberg, 2007; Overbeek et al., 2012), we hypothesized that individuals with smaller reductions in RSA to sadness and amusement and smaller augmentations in RSA recovery from sadness would be more vulnerable to symptoms of depression, particularly when exposed to stressful events. Because HR often decreases in response to sad films (Gross & Levenson, 1997; Overbeek et al., 2012), we hypothesized that individuals with smaller reductions in HR to sadness would be vulnerable to symptoms of depression (e.g., Somers et al., 2015) following stressful events. During recovery from sadness, HR is expected to increase to resting levels, representing attentional disengagement from the sad film and/or active coping processes (Frazier et al., 2004; Obrist, 1981; Overbeek et al., 2012); however, excessive HR increases during recovery also could represent ineffective regulation or excessive perceived need for coping with sadness, and no studies to our knowledge have evaluated HR recovery from sadness in relation to depression. Thus, our analyses with respect to HR recovery were exploratory. Given that the existing literature is mixed on the degree to which RSA withdrawal to amusement is normative (Kreibig, 2010; Overbeek et al., 2012) and is associated with depression (Fraguas et al., 2007; Panaite et al., 2016; Rottenberg et al., 2005), we hypothesized that RSA reactivity to amusement would not predict symptoms of depression following stressors. Finally, we expected relative increases in HR during amusement, as amusement is a relatively high-arousal emotion (Kreibig, 2010; Rottenberg et al., 2007b). Given that depression often is characterized by anhedonia and a lack of responsivity to positive stimuli (Pizzagalli, 2013; Rottenberg, 2005), and that lack of HR reactivity to amusement was associated with a longer course of current MDD (Rottenberg et al., 2002), we hypothesized that individuals exhibiting smaller increases in HR during an amusing film would be vulnerable to depression, particularly in the context of negative life events.
Method
Participants and Procedure
Participants were undergraduate students at Temple University and could range from 18 to 65. For inclusion, participants were fluent in English and had normal or corrected-to-normal vision. Participants were recruited from undergraduate psychology classes using the department’s online listing of studies, and from the diverse student body via flyers posted around campus. Students either received psychology course credit or were compensated in cash for participation. All participants completed informed consent approved by the University’s Institutional Review Board.
The sample included 178 participants (Mage = 21.94, SD = 5.71). The sample was 57.9% female, 9.0% Hispanic or Latino, 20.2% African American/Black, 64.0% Caucasian/White, 12.4% Asian, 0.6% Pacific Islander, 1.1% Native American, and 6.2% “other” race.
At Time 1, participants completed a laboratory assessment that involved completing questionnaires in an online survey and watching videos while heart rate and respiration were assessed. Every three weeks after Time 1, participants completed follow-up assessments remotely that included measures of current symptoms of depression and life events experienced in the prior three weeks (Times 2–5). At the Time 5 assessment, participants also completed an interview to confirm that life events reported at Times 1–5 fell in the relevant three-week intervals and that events reported met a priori definitional criteria for each event. Follow-up responses at Times 2–5 were required to be completed within a five-day window surrounding each participant’s follow-up target date (i.e., two days on either side of the target date). This approach allowed some flexibility in case participants were unable to complete the survey on a given day, but not so much time that it would bias the time period over which life events and symptoms were assessed.
To qualify for inclusion in the present multi-wave analyses (see Data Analysis), participants were required to have completed at least two of the four possible follow-up assessments, as using at least three observations is recommended when for the idiographic assessment of life events that are centered on each participant’s mean (Abela & Hankin, 2008). This yielded a final sample size of 134. Participants included in the present analyses did not differ from participants excluded from analyses (n = 44) on any study variables or demographic characteristics (ps > .08).
Measures
Autonomic Reactivity and Recovery
At the Time 1 assessment, participants were seated comfortably in a small assessment room and the experimenter attached cardiovascular sensors. After a five-minute rest period to become acclimated to the room and sensors, participants watched a series of video clips, which were presented on a desktop computer approximately 24 inches in front of them. Film selection was based on criteria recommended by Rottenberg et al. (2007b). To establish physiological parameters during a neutral baseline film, participants first viewed a two-minute nature film clip (a documentary on Denali National Park), followed by a film depicting a boy who is distraught at the death of his father (the movie The Champ), which has been demonstrated to elicit sadness (Rottenberg et al., 2007b). Previous work indicates that vagal recovery is most likely to occur during an unchallenged period immediately following emotional challenges (e.g., 60–120 seconds following the challenge; Rottenberg et al., 2003, 2007a; Mezzacappa et al., 2001). Thus, participants then completed a two-minute recovery period of rest in which they were asked to sit quietly. For a second resting baseline, participants then viewed another two-minute nature film clip (another clip from Denali), followed by an improvisational comedy film clip (Whose Line is it Anyway?) that has been demonstrated to elicit amusement (Rottenberg et al., 2007b). Participants completed a brief self-report measure of affect between film and recovery periods.
Electrocardiogram (ECG) and respiration signals were assessed with a three-lead electrocardiogram and BioPac BioHarness, and were continuously recorded on a PC with AcqKnowledge 4.3 software (equipment and software from Biopac Instruments Inc., Goleta, CA), sampled at 1000Hz. Cloth base disposable Ag/AgCl electrodes were placed in a modified Lead-II configuration on the chest. ECG signals were amplified with a Biopac MP150 with an ECG100 amplifier. We measured respiration rate (RR) with an RSP100C amplifier with a TSD100C respiratory transducer, which was placed around the chest, crossing under the armpits and on top of the breastbone. Respiration data were high-pass filtered and visually inspected for artifacts and corrected when needed, following well-established procedures outlined elsewhere (Grossman et al., 1990; Rottenberg et al., 2007). HR data were manually visually inspected for artifacts with the aid of a channel that computed momentary inter-beat interval; artifacts were manually adjusted as necessary (< 1% of heartbeats required adjustment). RSA was calculated using the well-validated peak-valley method (Grossman et al., 1990). The maximum heart rate during the expiration window of respiration was subtracted from the minimum heart rate during the inspiration window of respiration. RSA was computed in milliseconds such that higher values reflected greater vagal tone (or parasympathetic activity).
Average RSA, HR, and RR were computed separately for each experimental period (neutral film #1, sad film, recovery period, neutral film #2, and amusement film). Given that RR typically is correlated with RSA and HR (e.g., Overbeek et al., 2012), to examine the possible influence of respiration as a confound, analyses were conducted with and without inclusion of RR during the relevant periods as a covariate.
Life Events Scale (LES) and Interview (LEI)
The LES (Alloy & Clements, 1992; Safford et al., 2007) includes 134 major and minor life events in a variety of domains relevant to college students (e.g., school, finances, family, social and romantic relationships). Individuals indicate which events have occurred for them over a given time period. The validity of these reports is then evaluated objectively by an interviewer with the LEI to reduce any problems related to subjective report biases. Explicit criteria for event definition and a priori probes are provided to the interviewer to help him/her determine whether reported events on the LES meet the criteria of these provided definitions. Any events that do not meet the stringent event definition criteria or that did not occur within the relevant interval(s) are disqualified. For example, one LES item is “did poorly on or failed an exam or major project in an important class (i.e., grade less than C).” The LEI a priori probes require that the exam or project be worth at least 20% of the final grade to be classified as major. In the event that the exam or project was not worth at least 20% of the final grade, or the grade was not less than a C, the event would be disqualified, thus reducing the subjectivity of the event (as “poorly” and “major” might be interpreted differently across participants). Interviewers verify the timing of events using a variety of strategies to anchor events to days of the week, time of year, time during the semester or school break, and in relation to the timing of other major events. Interviews were completed by phone or in person by an advanced graduate student, who was blind to individuals’ autonomic reactivity scores, following extensive training and supervision on interview procedures. The LES and LEI have shown excellent reliability and predictive validity (Alloy & Clements, 1992; Boland et al., 2016; Francis-Raniere et al., 2006; Safford et al., 2007; Stange et al., 2014; Weiss et al., 2015).
Beck Depression Inventory (BDI-II; Beck et al., 1996)
The BDI-II assesses the severity of cognitive, affective, and somatic symptoms of depression during the previous two weeks. It is the most commonly-used self-report measure of depressive symptoms and has demonstrated excellent internal consistency and validity in undergraduate samples (Storch et al., 2004; Dozois et al., 1998). In the present study, αs = .87–.90 at Times 1–5.
Positive and Negative Affect Scale (PANAS), Brief Version (Mackinnon et al., 1999)
The PANAS – Brief version is a brief, 10-item version of the original self-report measure (Watson, Clark, & Tellegen, 1988) that assesses current emotions and affective experiences. Participants indicate the extent to which a number of different affective words describe their current state. It contains two subscales, each with five items: positive affect (inspired, alert, excited, enthusiastic, determined) and negative affect (afraid, upset, nervous, scared, distressed). The PANAS is a commonly-used measure of affect in experimental studies, and it has excellent validity and reliability (Crawford & Henry, 2004; Watson et al., 1988). It was administered as a manipulation check before and after each of the film and rest periods in the study to evaluate shifts in affect. In the present study, the PANAS had excellent internal consistency across the experimental periods (positive affect α = .84–.91, negative affect α = .90–.97).
Data Analysis
Given the nested structure of the data (multiple observations of negative life events and depressive symptoms within each person), multilevel modeling (MLM) was used (Raudenbush & Bryk, 2002). This design allowed for an idiographic (person-centered) approach to the measurement of stress, which provides a more accurate test of vulnerability-stress models of depression than is possible with a nomothetic (sample mean-centered) approach (Abela & Hankin, 2008). Additionally, MLM is advantageous in terms of maximizing data usage because it can flexibly handle cases with missing data, so participants with missing data (e.g., participants who miss a follow-up visit) are not eliminated from the data analyses. However, using at least three observations typically is recommended for idiographic assessment of life events that are centered on each participant’s mean (Abela & Hankin, 2008). Thus, as noted earlier, participants were included only if they had completed at least two follow-up assessments (a total of three assessments of life events and depressive symptoms when including the baseline assessment). Analyses were conducted with the Mplus 6.12 statistical software package (Muthen & Muthen, 2011), which allowed for use of full information maximum likelihood (FIML) estimation of data (Enders & Bandalos, 2001), using maximum likelihood estimation with robust standard errors.
To test the study hypotheses, fluctuations in person-centered life events at each wave were evaluated as the focal predictor of depressive symptoms (BDI) at each wave, with level-2 observations of the six indices of autonomic reactivity at Time 1 serving as moderators (in separate analyses) of this relationship, accounting for lagged BDI at the previous wave (e.g., controlling for Time 1 BDI when predicting Time 2 BDI). To compute the measures of autonomic reactivity and recovery that were of primary interest in the present study, rather than computing difference scores representing change in RSA and HR across periods, we entered RSA or HR as predictors during the current period as well as the previous period. For example, to assess RSA reactivity to the sad film, RSA during the first neutral film and RSA during the sad film were entered simultaneously as predictors; thus, the value of RSA during the sad film represents residual change in RSA after accounting for RSA during the neutral film. To explore the effects of accounting for respiration rate, we conducted each analysis with and without RR during the relevant periods as covariates. An example MLM equation for the analysis of RSA reactivity to sadness is provided in the Appendix. Significant interactions between indices of autonomic reactivity and negative life events were probed by testing the simple slopes of life events on depressive symptoms at ± 1 standard deviation from the mean of the given index of autonomic reactivity (Aiken & West, 1991). Final models included a random intercept and a random slope for the relationship between negative life events and symptoms of depression. All measures of autonomic reactivity were standardized.
Power analyses were conducted a priori following Raudenbush and Xiao-Feng (2001) using Optimal Design software (Spybrook et al., 2006) for multi-wave, repeated-measure longitudinal research designs. We assumed a medium effect size given the lack of prior literature evaluating interactions between autonomic reactivity and life events predicting symptoms of depression. Power analyses suggested that to detect significant interactions of moderate effect size with Power = .80 and α = .05 using a 5-time point study, a sample size of 139 would be required.
Results
Preliminary Analyses
First, we conducted manipulation checks to evaluate the extent to which the emotionally-salient films induced the intended emotions. As expected, compared to the first neutral baseline film, there was an increase in negative affect following the sad film (t = −9.87, p < .001, d = .59), as well as a decrease in positive affect (t = 10.36, p < .001, d = .53). Compared to the sad film, following the recovery period there was a significant decrease in negative affect (t = 8.57, p < .001, d = .38) and an increase in positive affect (t = −4.55, p < .001, d = .18). Following the amusement film relative to the second neutral baseline film, there was an increase in positive affect (t = −7.74, p < .001, d = .35) and no change in negative affect (t = 0.42, p = .67, d = .002).
As expected, participants showed decreases in RSA (t = 4.52, p < .001, d = .19) and HR (t = 6.06, p < .001, d = .12) from the first neutral film to the sad film. From the sad film to the recovery period, there was a marginally-significant increase in RSA (t = −1.87, p = .06, d = .08) and a significant increase in HR (t = −16.76, p < .001, d = .37). From the recovery period to the second neutral film, there was no significant change in RSA (t = 0.27, p = .79, d = .01), but there was a significant decrease in HR (t = 6.21, p < .001, d = .17). From the second neutral film to the amusement film, there was no significant change in RSA (t = −0.23, p = .82, d = .01), but there was a small but significant decrease in HR (t = 1.98, p = .05, d = .06). Finally, a comparison of the first neutral film to the second neutral film revealed that RSA remained significantly lower (t = 3.10, p < .005, d = .13) and HR remained significantly higher (t = −2.86, p = .005, d = .09) during the second neutral film, suggesting that autonomic recovery from the sad film may not have been complete following the recovery period (a limitation to which we return in the Discussion). As we were more interested in how individual differences in autonomic reactivity predicted vulnerability to depression than we were in absolute changes across individuals, we proceeded to test our main hypotheses.
Overall, participants had relatively low symptoms of depression (BDI Mean = 6.90, SD = 7.28, range = 0–46)1, but displayed moderate levels of negative life events (Mean = 8.64, SD = 6.94, range = 0–37). Descriptive statistics for RSA and HR across each period are displayed in Table 1. Correlations between autonomic variables, BDI, and negative life events at Time 1 are displayed in Table 2. In general, increases in RSA were associated with decreases in HR. BDI and negative life events were positively correlated, but were not associated with RSA and HR reactivity at Time 1.
Table 1.
Descriptive Statistics for Respiratory Sinus Arrhythmia (RSA), Heart Rate (HR), Positive Affect, and Negative Affect Across Each Period.
| RSA | HR | PA | NA | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Neutral Film #1 | 102.68 | 58.69 | 69.16 | 10.79 | 13.48 | 5.24 | 6.52 | 3.70 |
| Sad Film | 92.28 | 48.24 | 67.90 | 10.82 | 10.76 | 4.92 | 8.87 | 4.32 |
| Recovery Period | 96.44 | 49.16 | 71.92 | 10.72 | 11.67 | 5.41 | 7.25 | 4.21 |
| Neutral Film #2 | 95.94 | 49.95 | 70.11 | 10.62 | 12.08 | 5.51 | 6.40 | 4.00 |
| Amusement Film | 95.92 | 46.20 | 69.46 | 10.71 | 13.93 | 4.90 | 6.39 | 4.19 |
Note. RSA = Respiratory Sinus Arrhythmia (RSA); HR = Heart Rate; PA = Positive Affect; NA = Negative Affect; SD = Standard Deviation.
Table 2.
Correlations between Depression Symptoms and Negative Life Events at Time 1, and Residual Change Scores for Respiratory Sinus Arrhythmia (ΔRSA) and Heart Rate (ΔHR) Across Each Period.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. BDI | --- | |||||||
| 2. Negative Life Events | .43** | --- | ||||||
| 3. ΔRSA (Sad Film) | .11 | .10 | --- | |||||
| 4. ΔRSA (Recovery) | .01 | −.10 | −.37** | --- | ||||
| 5. ΔRSA (Amusement Film) | −.04 | .04 | .21* | .004 | --- | |||
| 6. ΔHR (Sad Film) | .16 | .05 | −.19* | .02 | −.10 | --- | ||
| 7. ΔHR (Recovery) | .08 | −.02 | −.02 | −.10 | −.04 | −.41** | --- | |
| 8. ΔHR (Amusement Film) | −.11 | −.02 | .02 | −.06 | −.14 | .21* | −.32** | --- |
p < .05,
p < .01.
Note. BDI = Beck Depression Inventory. Residual change scores represent RSA or HR for the stated period, controlling for RSA or HR during the prior period.
Prospective Analyses
Including the baseline assessment, a total of 619 observations were completed by the 134 participants who completed at least two of the four follow-up assessments (M = 3.62, SD = 0.71). Number of assessments completed was not related to any study variables. The intra-class correlation for an empty model predicting BDI was .651, indicating that 65.1% of the variance in depressive symptoms occurred at the between-subjects level (Level 2), whereas 34.9% of the variance occurred at the within-subjects level (Level 1).
Results of MLM analyses are presented in Table 22. In all models, negative life events significantly predicted more symptoms of depression. In a model containing only negative life events and lagged BDI as predictors, negative life events predicted significantly greater levels of depressive symptoms (B = 0.56, SE = 0.05, t = 10.61, p < .001, R2 = .92, 95% CI for R2 = .89–.94).
As hypothesized, when controlling for RR, there was a significant interaction between RSA reactivity and negative life events (Figure 1a), such that negative life events predicted symptoms of depression more strongly among individuals with less RSA withdrawal (B = 1.02, SE = 0.28, t = 3.63, p < .001, R2 = .40, 95% CI for R2 = .27–.52) than among individuals with greater (more contextually-appropriate) RSA withdrawal (B = −0.13, SE = 0.30, t = −0.42, p = .67, R2 = .02, 95% CI for R2 = −.02–.04) to the sad film.3 However, this interaction was attenuated to non-significance when not accounting for RR. In contrast, HR reactivity did not predict symptoms of depression as a main effect or in interaction with negative life events, regardless of whether RR served as a covariate.
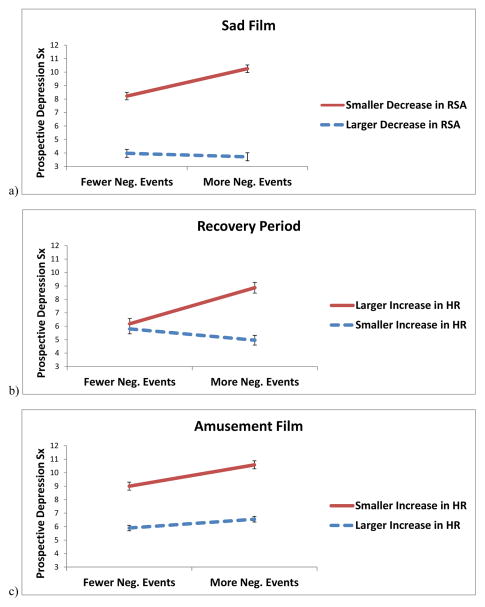
Figure 1.
Interactions between negative life events and (a) respiratory sinus arrhythmia (RSA) reactivity to a sad film, (b) heart rate (HR) recovery from a sad film, and (c) HR reactivity to an amusing film, predicting symptoms of depression across five waves of observation. Error bars represent standard errors of the simple slopes of the relationship between negative life events and prospective symptoms of depression, at high and low levels of the moderator.
RSA recovery did not predict symptoms of depression as a main effect or in interaction with negative life events, regardless of whether RR served as a covariate. In contrast, HR recovery from the sad film did interact with life events (Figure 1b), such that life events predicted symptoms of depression among individuals who showed larger increases in HR (B = 1.34, SE = 0.40, t = 3.36, p = .001, R2 = .41, 95% CI for R2 = .29–.54), but not among individuals who showed smaller increases in HR (B = −0.42, SE = 0.36, t = −1.16, p = .25, R2 = .08, 95% CI for R2 = −.01–.16) during recovery from the sad film. When controlling for RR, this interaction was reduced to nonsignificance.
RSA reactivity to the amusement film did not predict symptoms of depression as a main effect or in interaction with negative life events. In contrast, consistent with hypotheses, HR reactivity to the amusement film interacted with negative life events (Figure 1c), such that life events predicted symptoms of depression more strongly among individuals who showed smaller increases in HR (B = 0.86, SE = 0.30, t = 2.88, p < .01, R2 = .34, 95% CI for R2 = .21–.47), than among individuals who showed larger (more contextually-appropriate) increases in HR (B = 0.23, SE = 0.14, t = 1.12, p = .26, R2 = .07, 95% CI for R2 = −.01–.16) during the amusement film. This interaction maintained significance when controlling for RR.
Discussion
The present study represented the first complete test of components of atypical autonomic reactivity to loss and amusement as vulnerability factors for depression in the context of negative life events, using a multi-wave prospective study design. Consistent with hypotheses, we found that less contextually-appropriate vagal withdrawal to sadness was associated with greater levels of depressive symptoms when individuals were exposed to recent life stressors, but only when accounting for effects of respiration. Inconsistent with hypotheses, neither smaller reductions in HR to the sad film nor attenuated RSA augmentation during recovery from the sad film predicted future symptoms of depression. Our exploratory analyses with HR recovery from the sad film showed that individuals with greater increases in HR during recovery from sadness had greater vulnerability to depression in the context of stressful events. Consistent with hypotheses, RSA withdrawal to the amusing film was not associated with future symptoms of depression. Finally, consistent with hypotheses, attenuated heart rate reactivity to amusement was associated with vulnerability to depression in the context of stressful events. These results provide initial evidence that differential patterns of autonomic reactivity may not only characterize current depression (Rottenberg, 2007), but may actually constitute vulnerability to future symptoms of depression, above and beyond current depressive symptoms.
Results with RSA reactivity to the sad film suggest that the lack of ability to engage with sad stimuli may be an important indicator of vulnerability. The rapid withdrawal of the vagal brake is thought to help individuals to meet changing environmental demands, such as responding to physical activity, increasing attention and information processing (Suess, Porges, & Plude, 1994), and coping with negative emotion (Beauchaine, 2001). Indeed, prior empirical work suggests that RSA reactivity to sadness is positively associated with the ability to engage with sad stimuli and adaptively regulate emotions (Gentzler et al., 2009; LeMoult et al., 2016; Yaroslavsky, Bylsma, Rottenberg, & Kovacs, 2013). It is possible that RSA reactivity to sadness reflects emotional and behavioral adaptability which facilitates goal-directed behavior (e.g., attending to sad stimuli when relevant, or preparing the body for a regulatory response), a capacity that likely is useful when encountering negative life events throughout life (Kashdan & Rottenberg, 2010; Thayer & Lane, 2009). Thus, RSA reactivity may be an important substrate for flexible behavior in response to environmental and emotional changes. Given that in some prior work (e.g., Bylsma et al., 2014; Salomon et al., 2013) attenuated RSA reactivity to sadness was associated more with current MDD than with remitted MDD, it is possible that attenuated RSA reactivity represents a short-term, state-like vulnerability similar to a depressive prodrome, which may be particularly likely to lead to the onset of depression following exposure to stressful environmental contexts. Future studies are needed to establish the temporal stability of RSA reactivity to sadness, and to track how it may change within individuals across different mood states. Interestingly, attenuated RSA withdrawal to the sad film only appeared to confer vulnerability to depression in analyses that controlled for respiration. Prior researchers have argued that it is important to account for respiration when evaluating effects of autonomic constructs, as respiration rate impacts RSA (Overbeek et al., 2012) and could represent regulatory effort that is independent of true vagal withdrawal. The present results suggest that respiration rate could suppress the relationship between RSA and depression vulnerability, as the relationship between attenuated RSA withdrawal and vulnerability only was apparent after removing variance associated with respiration.
RSA recovery from the sad film was not associated with vulnerability to depression across follow-up. This contrasts with prior cross-sectional work that found that individuals with MDD showed attenuated recovery of RSA during crying (Rottenberg et al., 2003) and during a stressor task (Rottenberg et al., 2007), relative to controls. However, to our knowledge, no other prospective studies to date have evaluated RSA recovery from sadness as a predictor of depression course. It is possible that attenuated RSA recovery is a marker of a current depressive state rather than a vulnerability to later depression. In addition, RSA reactivity to the amusement film also did not predict symptoms of depression across follow-up, suggesting specificity of RSA reactivity as a vulnerability factor in the context of sadness. Although vagal withdrawal is expected in amusement contexts, sadness typically elicits greater withdrawal (Overbeek et al., 2012); thus, atypical RSA fluctuations in response to sad films may be more detectable and more relevant to depression vulnerability. Indeed, prior prospective studies in current MDD have demonstrated that RSA reactivity to amusement was not associated with illness course (Fraguas et al., 2007; Panaite et al., 2016; Rottenberg et al., 2005). The present study represents the first to evaluate individual differences in RSA reactivity to amusement as a vulnerability factor for future depressive symptoms in the context of negative life events, finding no evidence of such a relationship.
With respect to HR reactivity to the sad film, we did not find evidence that attenuated withdrawal conferred vulnerability to depression. This contrasts with a recent study of children (Somers et al., 2015), but is consistent with a null finding in a study of adults receiving treatment for current MDD (Rottenberg et al., 2002). Given that HR decreases to sad films likely are influenced by many sources, including parasympathetic and sympathetic activity, sadness following loss, and concerned attention (Berntson et al., 1997; Eisenberg et al., 1989; Gross & Levenson, 1997; Kreibig, 2010; Overbeek et al., 2012; Rottenberg, 2007), it may be difficult to detect relationships between HR reactivity and vulnerability to depression in this context. It is possible that using a more “pure” measure of sympathetic activity (Berntson et al., 1997; Kreibig, 2010; Mezzacappa et al., 2001) would help to elucidate patterns of sympathetic reactivity that confer vulnerability to depression. In addition, future work could evaluate autonomic responses to anticipated losses (when “activating” responses are expected; Kreibig, 2010) rather than after loss has occurred as a predictor of vulnerability.
In contrast, individuals with greater increases in HR during recovery from the sad film were more likely to experience prospective symptoms of depression under conditions of stress than were individuals with smaller increases in HR. To our knowledge, our study is the first to evaluate this question. Although some increase in HR during the recovery period was expected to be adaptive as attention no longer was drawn to the film, exaggerated HR increases could represent increased regulatory effort (Frazier et al., 2004; Obrist, 1981; Overbeek et al., 2012) or greater use of maladaptive regulatory strategies such as expressive suppression or rumination which inhibit autonomic recovery (Gross & Levenson, 1997; LeMoult et al., 2016). However, it is important to reiterate that HR likely is influenced by multiple autonomic and psychological processes, so these interpretations of the effects of HR recovery in the present study are admittedly speculative. Nevertheless, these results could suggest that individuals who lack the ability to efficiently and effectively regulate physiological responses to sadness may be more prone to experiencing prolonged periods of distress such as depression, particularly following difficult experiences (e.g., negative events) that elicit sadness.
Consistent with hypotheses, blunted HR reactivity to amusement conferred vulnerability to depression in the context of negative life events. This result extends prior work in individuals with current MDD that found that attenuated HR reactivity to amusement predicted a lower likelihood of recovery from depression (Rottenberg et al., 2002). These findings are consistent with perspectives suggesting that a lack of HR reactivity reflects blunted activity in the behavioral approach system (Fowles, Fisher, & Tranel, 1982; Kasch et al., 2002). In contexts during which amusement is expected, the approach system may be sensitive to signals of impending reward and to actual reward (Fowles et al., 1982). Given that blunted hedonic capacity is common in MDD (Pizzagalli, 2013), the present results could suggest that similar processes are reflected in attenuated HR reactivity to amusement, which also may characterize vulnerability to future depression. Thus, autonomic reactivity is relevant to evaluating components of the positive valence system (e.g., approach motivation, responsiveness to reward), in addition to the negative valence system (e.g., loss) and the arousal and modulatory systems that are proposed by NIMH’s Research Domain Criteria (RDoC) initiative (Sanislow et al., 2010; Beauchaine & Thayer, 2015).
The results of the present study suggest that evaluating autonomic reactivity may be useful for identifying individuals who are vulnerable to experiencing symptoms of depression following stressors, and who may benefit from interventions to attenuate risk. Biofeedback is one intervention that has promise for helping individuals to directly improve their autonomic activity in response to emotional stimuli (Karavidas et al., 2007; Nolan et al., 2007; Siepmann et al., 2008). Future work also should explore the extent to which existing psychosocial treatments can improve autonomic reactivity indirectly, perhaps by improving mindfulness (e.g., present-moment awareness of emotional states) or the ability to engage temporarily with sad stimuli. It is possible that the use of strategies to reduce extended engagement following sadness (e.g., training reappraisal or distraction rather than rumination) would help to attenuate maladaptive HR recovery following sadness, thereby reducing risk. Neurocognitive approaches also hold promise for improving autonomic activity. For example, repetitive transcranial magnetic stimulation may be able to directly improve autonomic reactivity to stress (e.g., Remue et al., 2016), and attentional control training (e.g., Siegle et al., 2014; Wells, 2011) could help people to learn to disengage from repetitive negative thinking.
The current study has a number of limitations. Video clip order was not counterbalanced, given that we wanted participants to end the study on a positive note (the amusement film) for ethical reasons; nevertheless, there appeared to be autonomic carry-over effects from the sad film that were not resolved before the amusement film, so the autonomic responses to the amusement film must be interpreted with this important caveat in mind. In addition, as we were more interested in recovery from sadness given its theoretical relevance to depression, and in part due to time constraints of the study session, we did not assess autonomic and affective responses during a similar “recovery” period following the amusement film. This limits the ability to determine whether autonomic responses during recovery from the sad film are specific to sadness versus applicable to other emotions. It also is important to note that video clips that are experimentally intended to be “neutral” in valence may in fact elicit other emotions (e.g., awe or interest; Rottenberg et al., 2007b; Shiota et al., 2011); thus, the ecological validity of these indices of autonomic reactivity and recovery to real-world responses to loss and amusement is not entirely clear. We also did not evaluate behavioral responses to the films, such as crying or laughing; this would have provided useful contextual information, given that crying is associated with increases in sympathetic activation during sadness (Kreibig, 2010; Rottenberg, Bylsma, & Vingerhoets, 2008; Vingerhoets & Bylsma, in press) as well as improved vagal augmentation during periods of recovery from sadness in healthy individuals, but not in depressed individuals (Rottenberg et al., 2003).
In addition, the study sample was composed of university students; although there are high rates of depression in college students and substantial variability in stressors between individuals as well as within individuals across the course of semesters, the results can not necessarily be extended to the general public. We also examined fluctuations in symptoms of depression rather than clinically-significant depressive episodes; although evaluating depression on a continuum resulted in greater potential power to detect nuanced effects and is consistent with evidence supporting the dimensional structure of depression (e.g., Liu, 2016), the immediate clinical significance of the present results is not clear (although findings in clinical samples have paralleled those in the present study; e.g., Fraguas et al., 2007; Panaite et al., 2016; Rottenberg et al., 2002, 2005). In addition, although our primary findings remained after accounting for potential confounds of autonomic activity such as respiration, antidepressant use, BMI, and age, we cannot rule out other possible confounds such as physical activity and smoking. Finally, although the present study evaluated vulnerability for depression, atypical autonomic reactivity also holds promise as a potential transdiagnostic factor that may be implicated in other types of psychopathology, including anxiety disorders (Beauchaine & Thayer, 2015).
Despite these limitations, the present study had numerous strengths. We evaluated fluctuations in both RSA and HR in response to different types of emotional contexts relevant to the core features of depression relative to neutral baseline periods, and controlled for respiration, self-reported affect shifts, and other potential confounds. We also used a multi-wave design to evaluate interactions between naturally-occurring negative life events and autonomic reactivity when predicting prospective fluctuations in depression, using a person-centered approach to assessing fluctuations in negative events, which allowed for strong tests of the vulnerability-stress model that have not been tested in the literature to date.
The present study provides the most complete test to date of atypical autonomic reactivity as a vulnerability to future depression, with a number of methodological improvements on the existing literature. Our results suggest that components of autonomic reactivity may serve as protective factors against depression when individuals encounter stressful life events. Alternatively, individuals with deficits in autonomic reactivity may be vulnerable to experiencing symptoms of depression following recent stressors. However, the precise psychological mechanisms of these autonomic constructs require further study. These results also lend support to theories of self-regulation that suggest the importance of context in determining when regulatory strategies are likely to lead to adaptive versus maladaptive outcomes (e.g., Aldao, 2013; Aldao, Sheppes, & Gross, 2015; Bonanno & Burton, 2013). In conclusion, the present study suggests that evaluating psychophysiological components of flexibility in response to shifts in environmental and emotional context holds promise for improving our understanding of the pathophysiology of depression and for targeting these mechanisms to reduce the personal and societal impact of this illness.
Supplementary Material
Table 3.
Results of Hierarchical Linear Regression Models Assessing Main Effects and Interactions with Negative Life Events for Fluctuations in Respiratory Sinus Arrhythmia (RSA) and Heart Rate (HR) to Sadness, Recovery from Sadness, and Amusement, Predicting Symptoms of Depression Across Five Waves of Observation over Twelve Weeks.
| Main Effect | Interaction with Negative Life Events | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Model | Predictor | B | SE | p | R2 | 95% CI for R2 | B | SE | p | R2 | 95% CI for R2 |
| Reactivity to Sad Film | |||||||||||
| 1 | RSA | 1.14 | 1.28 | .43 | .07 | −.01–.16 | 0.21 | 0.26 | .43 | .06 | −.02–.14 |
| 2 | RSA (controlling RR) | 2.69 | 1.54 | .08 | .18 | .06–.30 | 0.57 | 0.27 | .04 | .24 | .11–.36 |
| 3 | HR | 4.68 | 2.70 | .08 | .16 | .05–.27 | −0.35 | 0.19 | .07 | .17 | .06–.29 |
| 4 | HR (controlling RR) | 4.71 | 2.67 | .08 | .13 | .03–.24 | −0.29 | 0.17 | .08 | .13 | .03–.23 |
| Recovery from Sad Film | |||||||||||
| 5 | RSA | 1.72 | 1.09 | .12 | .13 | .03–.24 | 0.05 | 0.13 | .99 | 0 | 0–0 |
| 6 | RSA (controlling RR) | 1.06 | 0.61 | .77 | .004 | −.02–.03 | 0.04 | 0.15 | .78 | .06 | −.02–.14 |
| 7 | HR | 1.07 | 1.85 | .56 | .02 | −.03–.07 | 0.88 | 0.37 | .02 | .26 | .14–.39 |
| 8 | HR (controlling RR) | 1.12 | 1.84 | .54 | .02 | −.03–.06 | 0.59 | 0.38 | .12 | .11 | .01–.20 |
| Reactivity to Amusement Film | |||||||||||
| 9 | RSA | 0.79 | 0.82 | .34 | .03 | −.02–.09 | 0.01 | 0.08 | .95 | 0 | 0–0 |
| 10 | RSA (controlling RR) | 0.41 | 1.21 | .74 | .01 | −.02–.03 | −0.01 | 0.11 | .95 | <.001 | −.01–.01 |
| 11 | HR | −1.79 | 1.01 | .08 | .16 | .05–.27 | −0.23 | 0.09 | .01 | .28 | .15–.40 |
| 12 | HR (controlling RR) | −1.82 | 1.08 | .09 | .12 | .02–.23 | −0.24 | 0.10 | .01 | .24 | .11–.36 |
Note. R2 = partial R2 of focal predictor (proportion of variance in depression symptoms predicted) after accounting for covariates. In all models, RSA or HR represents the value during the specified period, while controlling for the relevant value during the prior period (e.g., in Model 1, controlling RSA during neutral film when RSA to sad film is focal predictor).
Acknowledgments
This work was supported by grants to Jonathan P. Stange from the National Institute of Mental Health (F31MH099761), the Association for Psychological Science, the American Psychological Foundation, and the American Psychological Association. Jessica L. Hamilton was supported by National Research Service Award MH106184 from the National Institute of Mental Health. Lauren B. Alloy was supported by NIMH Grant MH101168.
Footnotes
BDI was positively skewed, as is common in studies of non-clinical samples. However, values for skewness were within the recommended range (George & Mallery, 2010) for BDI (1.54) and the residual BDI change score (1.17). Sensitivity analyses indicated that removing the most extreme outliers (cases in which BDI observations were > 3 SDs from the mean) did not alter any of the results meaningfully. For interpretability and consistency with the prior literature, we report results using non-transformed BDI scores.
Although not the primary focus of the manuscript, we also evaluated the incremental utility of autonomic variables in predicting fluctuations in depressive symptoms beyond self-reported shifts in affect. None of the PANAS change variables predicted symptoms of depression as main effects or in interaction with negative life events. Furthermore, controlling for the corresponding PANAS change variables in analyses of autonomic flexibility did not substantively change the results (all statistically significant results remained significant). In addition, results were consistent when controlling for other factors that may influence cardiovascular activity, including body mass index, antidepressant use, any psychiatric medication use, and age (Kemp et al., 2010). Sex did not moderate any of the relationships between autonomic activity and fluctuations in depressive symptoms.
At the suggestion of a reviewer, we also tested for interactions between baseline RSA (first neutral film) and RSA to the sad film predicting symptoms of depression. This two-way interaction was not significant, nor was the corresponding three-way interaction with life events, with or without controlling for RR.
Contributor Information
Jonathan P. Stange, Temple University and the University of Illinois at Chicago
Jessica L. Hamilton, Temple University
Thomas M. Olino, Temple University
David M. Fresco, Kent State University
Lauren B. Alloy, Temple University
References
- Abela JRZ, Hankin BL. Cognitive vulnerability to depression in children and adolescents: A developmental psychopathology perspective. In: Abela JRZ, Hankin BL, editors. Handbook of Depression in Children and Adolescents. New York: Guilford; 2008. pp. 35–78. [Google Scholar]
- Abramson LY, Alloy LB, Hankin BL, Haeffel GJ, MacCoon DG, Gibb BE. Cognitive vulnerability-stress models of depression in a self-regulatory and psychobiological context. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. New York: Guilford; 2002. pp. 268–294. [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Aldao A. The future of emotion regulation research: Capturing context. Perspectives on Psychological Science. 2013;8(2):155–172. doi: 10.1177/1745691612459518. [DOI] [PubMed] [Google Scholar]
- Aldao A, Sheppes G, Gross JJ. Emotion regulation flexibility. Cognitive Therapy and Research. 2015;39(3):263–278. [Google Scholar]
- Allen NB, Kuppens P, Sheeber LB. Heart rate responses to parental behavior in depressed adolescents. Biological Psychology. 2012;90:80–87. doi: 10.1016/j.biopsycho.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Clements CM. Illusion of control: invulnerability to negative affect and depressive symptoms after laboratory and natural stressors. Journal of Abnormal Psychology. 1992;101(2):234–245. doi: 10.1037//0021-843x.101.2.234. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13(2):183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Thayer JF. Heart rate variability as a transdiagnostic biomarker of psychopathology. International Journal of Psychophysiology. 2015;98(2):338–350. doi: 10.1016/j.ijpsycho.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Boland EM, Stange JP, LaBelle DR, Shapero BG, Weiss RB, Abramson LY, Alloy LB. Affective disruption from social rhythm and behavioral approach system (BAS) sensitivities: A test of the integration of the social zeitgeber and BAS theories of bipolar disorder. Clinical Psychological Science. 2016;4(3):418–432. doi: 10.1177/2167702615603368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno GA, Burton CL. Regulatory flexibility: An individual differences perspective on coping and emotion regulation. Perspectives on Psychological Science. 2013;8(6):591–612. doi: 10.1177/1745691613504116. [DOI] [PubMed] [Google Scholar]
- Bosch NM, Riese H, Ormel J, Verhulst F, Oldehinkel AJ. Stressful life events and depressive symptoms in young adolescents: Modulation by respiratory sinus arrhythmia? The TRAILS study. Biological Psychology. 2009;81(1):40–47. doi: 10.1016/j.biopsycho.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review. 2008;28(4):676–691. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Salomon K, Taylor-Clift A, Morris BH, Rottenberg J. Respiratory sinus arrhythmia reactivity in current and remitted major depressive disorder. Psychosomatic Medicine. 2014;76(1):66–73. doi: 10.1097/PSY.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Uchino BN, Berntson GG. Individual differences in the autonomic origins of heart rate reactivity: The psychometrics of respiratory sinus arrhythmia and preejection period. Psychophysiology. 1994;31(4):412–419. doi: 10.1111/j.1469-8986.1994.tb02449.x. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Henry JD. The Positive and Negative Affect Schedule (PANAS): Construct validity, measurement properties and normative data in a large non-clinical sample. British Journal of Clinical Psychology. 2004;43(3):245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Demaree HA, Robinson JL, Everhart DE, Schmeichel BJ. Resting RSA is associated with natural and self-regulated responses to negative emotional stimuli. Brain and Cognition. 2004;56(1):14–23. doi: 10.1016/j.bandc.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Dozois DJ, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory-II. Psychological Assessment. 1998;10(2):83–89. [Google Scholar]
- Eisenberg N, Fabes RA, Miller PA, Fultz J, Shell R, Mathy RM, Reno RR. Relation of sympathy and personal distress to prosocial behavior: a multimethod study. Journal of Personality and Social Psychology. 1989;57(1):55–66. doi: 10.1037//0022-3514.57.1.55. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Hinnant JB, Erath SA. Marital conflict, vagal regulation, and children’s sleep: A longitudinal investigation. Monographs of the Society for Research in Child Development. 2015;80(1):89–106. doi: 10.1111/mono.12146. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Whitson SA. Longitudinal relations between marital conflict and child adjustment: vagal regulation as a protective factor. Journal of Family Psychology. 2006;20(1):30–39. doi: 10.1037/0893-3200.20.1.30. [DOI] [PubMed] [Google Scholar]
- Fowles DC, Fisher AE, Tranel DT. The heart beats to reward: The effect of monetary incentive on heart rate. Psychophysiology. 1982;19:506–513. doi: 10.1111/j.1469-8986.1982.tb02577.x. [DOI] [PubMed] [Google Scholar]
- Fraguas R, Jr, Marci C, Fava M, Iosifescu DV, Bankier B, Loh R, Dougherty DD. Autonomic reactivity to induced emotion as potential predictor of response to antidepressant treatment. Psychiatry Research. 2007;151(1):169–172. doi: 10.1016/j.psychres.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Francis-Raniere EL, Alloy LB, Abramson LY. Depressive personality styles and bipolar spectrum disorders: Prospective tests of the event congruency hypothesis. Bipolar Disorders. 2006;8(4):382–399. doi: 10.1111/j.1399-5618.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Strauss ME, Steinhauer SR. Respiratory sinus arrhythmia as an index of emotional response in young adults. Psychophysiology. 2004;41:75–83. doi: 10.1046/j.1469-8986.2003.00131.x. [DOI] [PubMed] [Google Scholar]
- Gentzler AL, Santucci AK, Kovacs M, Fox NA. Respiratory sinus arrhythmia reactivity predicts emotion regulation and depressive symptoms in at-risk and control children. Biological Psychology. 2009;82(2):156–163. doi: 10.1016/j.biopsycho.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George D, Mallery M. SPSS for Windows Step by Step: A Simple Guide and Reference, 17.0 update. 10. Boston: Pearson; 2010. [Google Scholar]
- Goldston DB, O’Hara MW, Schartz HA. Reliability, validity, and preliminary normative data for the Inventory to Diagnose Depression in a college population. Psychological Assessment. 1990;2(2):212–215. [Google Scholar]
- Gross JJ, Levenson RW. Hiding feelings: the acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology. 1997;106(1):95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- Grossman P, Beek JV, Wientjes C. A comparison of three quantification methods for estimation of respiratory sinus arrhythmia. Psychophysiology. 1990;27(6):702–714. doi: 10.1111/j.1469-8986.1990.tb03198.x. [DOI] [PubMed] [Google Scholar]
- Hopp H, Shallcross AJ, Ford BQ, Troy AS, Wilhelm FH, Mauss IB. High cardiac vagal control protects against future depressive symptoms under conditions of high social support. Biological Psychology. 2013;93(1):143–149. doi: 10.1016/j.biopsycho.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just N, Abramson LY, Alloy LB. Remitted depression studies as tests of the cognitive vulnerability hypotheses of depression onset: A critique and conceptual analysis. Clinical Psychology Review. 2001;21(1):63–83. doi: 10.1016/s0272-7358(99)00035-5. [DOI] [PubMed] [Google Scholar]
- Karavidas MK, Lehrer PM, Vaschillo E, Vaschillo B, Marin H, Buyske S, … Hassett A. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Applied Psychophysiology and Biofeedback. 2007;32(1):19–30. doi: 10.1007/s10484-006-9029-z. [DOI] [PubMed] [Google Scholar]
- Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. Journal of Abnormal Psychology. 2002;111(4):589–597. doi: 10.1037//0021-843x.111.4.589. [DOI] [PubMed] [Google Scholar]
- Kashdan TB, Rottenberg J. Psychological flexibility as a fundamental aspect of health. Clinical Psychology Review. 2010;30(7):865–878. doi: 10.1016/j.cpr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biological Psychiatry. 2010;67(11):1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. American Journal of Psychiatry. 1999;136:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Akiskal HS, Ames M, Birnbaum H, Greenberg P, Hirschfeld RM, … Wang PS. Prevalence and effects of mood disorders on work performance in a nationally representative sample of U.S. workers. American Journal of Psychiatry. 2006;163(9):1561–1568. doi: 10.1176/appi.ajp.163.9.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Wang PS. Epidemiology of depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2. New York: Guilford; 2009. pp. 5–22. [Google Scholar]
- Kreibig SD. Autonomic nervous system activity in emotion: A review. Biological Psychology. 2010;84(3):394–421. doi: 10.1016/j.biopsycho.2010.03.010. [DOI] [PubMed] [Google Scholar]
- LeMoult J, Yoon KL, Joorman J. Rumination and cognitive distraction in major Depressive Disorder: an examination of respiratory sinus arrhythmia. Journal of Psychopathology and Behavioral Assessment. 2015 doi: 10.1007/s10862-015-9510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RT. Taxometric evidence of a dimensional latent structure for depression in an epidemiological sample of children and adolescence. Psychological Medicine. 2016 doi: 10.1017/S0033291715002792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR. Can exaggerated stress reactivity and prolonged recovery predict negative health outcomes? The case of cardiovascular disease. Psychosomatic Medicine. 2015;77(3):212–214. doi: 10.1097/PSY.0000000000000173. [DOI] [PubMed] [Google Scholar]
- Mackinnon A, Jorm AF, Christensen H, Korten AE, Jacomb PA, Rodgers B. A short form of the Positive and Negative Affect Schedule: Evaluation of factorial validity and invariance across demographic variables in a community sample. Personality and Individual Differences. 1999;27(3):405–416. [Google Scholar]
- Mezzacappa ES, Kelsey RM, Katkin ES, Sloan RP. Vagal rebound and recovery from psychological stress. Psychosomatic Medicine. 2001;63:650–657. doi: 10.1097/00006842-200107000-00018. [DOI] [PubMed] [Google Scholar]
- Morgan JK, Shaw DS, Forbes EE. Physiological and behavioral engagement in social contexts as predictors of adolescent depressive symptoms. Journal of Youth and Adolescence. 2013;42(8):1117–1127. doi: 10.1007/s10964-012-9815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 6. Los Angeles, CA: Muthén & Muthén; 1998–2011. [Google Scholar]
- Nolan RP, Kamath MV, Floras JS, Stanley J, Pang C, Picton P, Young QR. Heart rate variability biofeedback as a behavioral neurocardiac intervention to enhance vagal heart rate control. American Heart Journal. 2005;149(6):1137e1–1137.e7. doi: 10.1016/j.ahj.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Obrist P. Cardiovascular Psychophysiology. Plenum Press; New York: 1981. [Google Scholar]
- Overbeek TJ, van Boxtel A, Westerink JH. Respiratory sinus arrhythmia responses to induced emotional states: effects of RSA indices, emotion induction method, age, and sex. Biological Psychology. 2012;91(1):128–141. doi: 10.1016/j.biopsycho.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Panaite V, Hindash AC, Bylsma LM, Small BJ, Salomon K, Rottenberg J. Respiratory sinus arrhythmia reactivity to a sad film predicts depression symptom improvement and symptomatic trajectory. International Journal of Psychophysiology. 2016 doi: 10.1016/j.ijpsycho.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annual Review of Clinical Psychology. 2013;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- Raudenbush SW, Xiao-Feng L. Effects of study duration, frequency of observation, and sample size on power in studies of group differences in polynomial change. Psychological Methods. 2001;6(4):387–401. [PubMed] [Google Scholar]
- Remue J, Vanderhasselt MA, Baeken C, Rossi V, Tullo J, De Raedt R. The effect of a single HF-rTMS session over the left DLPFC on the physiological stress response as measured by heart rate variability. Neuropsychology. 2016 doi: 10.1037/neu0000255. [DOI] [PubMed] [Google Scholar]
- Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biological Psychology. 2007;74(2):200–211. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Bylsma LM, Vingerhoets AJ. Is crying beneficial? Current Directions in Psychological Science. 2008;17(6):400–404. [Google Scholar]
- Rottenberg J, Clift A, Bolden S, Salomon K. RSA fluctuation in major depressive disorder. Psychophysiology. 2007a;44(3):450–458. doi: 10.1111/j.1469-8986.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Hindash AC. Emerging evidence for emotion context insensitivity in depression. Current Opinion in Psychology. 2015;4:1–5. [Google Scholar]
- Rottenberg J, Kasch KL, Gross JJ, Gotlib IH. Sadness and amusement reactivity differentially predict concurrent and prospective functioning in major depressive disorder. Emotion. 2002;2(2):135–146. doi: 10.1037/1528-3542.2.2.135. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Ray RD, Gross JJ. Emotion elicitation using films. In: Coan JA, BAllen JJ, editors. Handbook of emotion elicitation and assessment. New York: Oxford University Press; 2007b. [Google Scholar]
- Rottenberg J, Salomon K, Gross JJ, Gotlib IH. Vagal withdrawal to a sad film predicts subsequent recovery from depression. Psychophysiology. 2005;42(3):277–281. doi: 10.1111/j.1469-8986.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Wilhelm FH, Gross JJ, Gotlib IH. Vagal rebound during resolution of tearful crying among depressed and nondepressed individuals. Psychophysiology. 2003;40(1):1–6. doi: 10.1111/1469-8986.00001. [DOI] [PubMed] [Google Scholar]
- Safford SM, Alloy LB, Abramson LY, Crossfield AG. Negative cognitive style as a predictor of negative life events in depression-prone individuals: a test of the stress generation hypothesis. Journal of Affective Disorders. 2007;99(1–3):147–154. doi: 10.1016/j.jad.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon K, Bylsma LM, White KE, Panaite V, Rottenberg J. Is blunted cardiovascular reactivity in depression mood-state dependent? A comparison of major depressive disorder remitted depression and healthy controls. International Journal of Psychophysiology. 2013;90(1):50–57. doi: 10.1016/j.ijpsycho.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, … Cuthbert BN. Developing constructs for psychopathology research: research domain criteria. Journal of Abnormal Psychology. 2010;119(4):631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- Shiota MN, Neufeld SL, Yeung WH, Moser SE, Perea EF. Feeling good: autonomic nervous system responding in five positive emotions. Emotion. 2011;11(6):1368–1378. doi: 10.1037/a0024278. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Price RB, Jones NP, Ghinassi F, Painter T, Thase ME. You gotta work at it pupillary indices of task focus are prognostic for response to a neurocognitive intervention for rumination in depression. Clinical Psychological Science. 2014;2(4):455–471. [Google Scholar]
- Siepmann M, Aykac V, Unterdörfer J, Petrowski K, Mueck-Weymann M. A pilot study on the effects of heart rate variability biofeedback in patients with depression and in healthy subjects. Applied Psychophysiology and Biofeedback. 2008;33(4):195–201. doi: 10.1007/s10484-008-9064-z. [DOI] [PubMed] [Google Scholar]
- Somers JA, Borelli JL, Smiley PA, West JL, Hilt LM. Concurrent and prospective associations between emotion reactivity and depressive symptoms in middle childhood. Journal of Psychopathology and Behavioral Assessment. 2015;37(4):692–704. [Google Scholar]
- Spybrook J, Raudenbush SW, Liu XF, Congdon R, Martínez A. Optimal design for longitudinal and multilevel research: Documentation for the “Optimal Design” software. Survey Research Center of the Institute of Social Research at University of Michigan; 2006. [Google Scholar]
- Stange JP, Hamilton JL, Abramson LY, Alloy LB. A vulnerability-stress examination of response styles theory in adolescence: Stressors, sex differences, and symptom specificity. Journal of Clinical Child & Adolescent Psychology. 2014;43(5):813–827. doi: 10.1080/15374416.2013.812037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Roberti JW, Roth DA. Factor structure, concurrent validity, and internal consistency of the Beck Depression Inventory-Second Edition in a sample of college students. Depression and Anxiety. 2004;19(3):187–189. doi: 10.1002/da.20002. [DOI] [PubMed] [Google Scholar]
- Taylor CB. Depression, heart rate related variables and cardiovascular disease. International Journal of Psychophysiology. 2010;78:80–88. doi: 10.1016/j.ijpsycho.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews. 2012;36(2):747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine. 2009;37(2):141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Review. 2009;33(2):81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Vingerhoets AJ, Bylsma LM. The riddle of human emotional crying: A challenge for emotion researchers. Emotion Review. doi: 10.1177/1754073915586226. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weiss RB, Stange JP, Boland EM, Black SK, LaBelle DR, Abramson LY, Alloy LB. Kindling of life stress in bipolar disorder: comparison of sensitization and autonomy models. Journal of Abnormal Psychology. 2015;124(1):4–16. doi: 10.1037/abn0000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A. Metacognitive therapy for anxiety and depression. New York: Guilford; 2011. [Google Scholar]
- Yaroslavsky I, Rottenberg J, Kovacs M. Atypical patterns of respiratory sinus arrhythmia index an endophenotype for depression. Development and Psychopathology. 2014;26(4pt2):1337–1352. doi: 10.1017/S0954579414001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky I, Bylsma LM, Rottenberg J, Kovacs M. Combinations of resting RSA and RSA reactivity impact maladaptive mood repair and depression symptoms. Biological Psychology. 2013;94(2):272–281. doi: 10.1016/j.biopsycho.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.