Abstract
Objectives
Recent evidence for abnormal thalamic connectivity in autism spectrum disorders (ASD) and sensory processing disorders (SPD) suggests the thalamus may play a role in sensory over-responsivity (SOR), an extreme negative response to sensory stimuli, which is common in ASD. However, there is yet little understanding of changes in thalamic connectivity during exposure to aversive sensory inputs in individuals with ASD. In particular, the pulvinar nucleus of the thalamus is implicated in atypical sensory processing given its role in selective attention, regulation, and sensory integration. This study aimed to examine the role of pulvinar connectivity in ASD during mildly aversive sensory input.
Method
Functional magnetic resonance imaging was used to examine connectivity with the pulvinar during exposure to mildly aversive auditory and tactile stimuli in 38 youth (age 9-17; 19 ASD, 19 IQ-matched typically developing (TD)). Parents rated children’s SOR severity on two standard scales.
Results
Compared to TD, ASD participants displayed aberrant modulation of connectivity between pulvinar and cortex (including sensory-motor and prefrontal regions) during sensory stimulation. In ASD participants, pulvinar-amygdala connectivity was correlated with severity of SOR symptoms.
Conclusions
Deficits in modulation of thalamocortical connectivity in youth with ASD may reflect reduced thalamo-cortical inhibition in response to sensory stimulation, which could lead to difficulty filtering out and/or integrating sensory information. An increase in amygdala connectivity with the pulvinar might be partially responsible for deficits in selective attention as the amygdala signals the brain to attend to distracting sensory stimuli.
Keywords: sensory over responsivity, thalamus, amygdala, functional connectivity, fMRI
The thalamus plays a central role in relaying and integrating sensory information in the brain (e.g., Sherman, 2005). Connectivity between the thalamus and other brain areas is extremely complex, including connections with most cortical and many subcortical regions (Barron, Eickhoff, Clos, & Fox, 2015; Behrens et al., 2003). These connections are not only involved in projecting incoming sensory information to appropriate cortical regions, but also in feedback loops between cortex and “higher-level” thalamic nuclei that serve to modulate attention and coordinate sensory processing (John, Zikopoulos, Bullock, & Barbas, 2016; Shipp, 2003).
Given the involvement of the thalamus in processing sensory information, the atypical sensory reactivity seen in autism spectrum disorders (ASD) could be related to altered thalamic connectivity. Over half of children with ASD meet criteria for sensory over-responsivity (SOR; Baranek, David, Poe, Stone, & Watson, 2006; Ben-Sasson et al., 2007), which is characterized by an extreme, negative response to, or avoidance of, sensory stimuli (Liss, 2006). SOR is related to increased impairment, including higher anxiety, more severe autism symptoms, and worse social and adaptive skills (Ben-Sasson et al., 2008; Ben-Sasson, Carter, & Briggs-Gowan, 2009; Horder, Wilson, Mendez, & Murphy, 2013). The stimulus types most commonly reported as aversive are auditory and tactile stimuli such as noisy environments, sudden loud noises, scratchy clothing, or being touched unexpectedly (Haigh, Minshew, Heeger, Dinstein, & Behrmann, 2016; Rogers, Hepburn, & Wehner, 2003; Tomchek & Dunn, 2007). Although to date there are no studies of thalamic functional connectivity during sensory processing in ASD, sensory over-responsivity (SOR) has been found to be related to over-active brain responses in the thalamus, as well as in brain areas responsible for salience detection and attention (e.g., amygdala, insula) and primary sensory processing (e.g., visual, auditory, and somatosensory cortices; Green et al., 2013, 2015).
Additionally, there is evidence for abnormal intrinsic thalamic connectivity in individuals with ASD (Cerliani et al., 2015; Nair, Treiber, Shukla, Shih, & Müller, 2013). The direction of these findings is mixed; for example, Cerliani et al. (2015) found increased functional connectivity between thalamus and primary sensory regions during resting-state, whereas Nair et al. (2013) found underconnectivity between thalamus and frontal and primary sensory cortical regions. A more recent study suggests that ASD in children and adolescents is related to thalamic overconnectivity at rest with early-developing sensory-motor areas but underconnectivity with later-developing prefrontal areas (Nair et al., 2015). Severity of ASD symptoms has also been found to correlate with extent of alterations in thalamocortical connectivity (Cerliani et al., 2015; Nair et al., 2013). Further research implicating the thalamus in SOR includes findings of reduced structural thalamocortical connectivity in children with Sensory Processing Disorder (Owen et al., 2013).
The pulvinar nucleus of the thalamus was of particular interest for this study because this area was found to be overactive in response to mildly aversive sensory stimuli in youth with ASD, as compared to typically developing controls (Green et al., 2013, 2015). The pulvinar is a unique thalamic nucleus in that it mainly receives input from and outputs to cortical regions, and thus is thought to aid in regulation and integration of sensory information (Sherman & Guillery, 1996; Shipp, 2003). The pulvinar is also involved in selective attention as inhibitory inputs from the cortex to the pulvinar via the thalamic reticular nucleus can help decrease attention to a stimulus (Zhou, Schafer, & Desimone, 2016). Thus, a hyperactive pulvinar in response to sensory stimuli could indicate lack of cortical inhibition. Additionally, pulvinar connectivity with the amygdala is thought to play a role in directing attention to emotionally salient information (Barron et al., 2015; Troiani & Schultz, 2013). Given evidence that SOR is related to an over-attribution of salience to irrelevant sensory information (Green et al., in press), we hypothesized that individuals with ASD and SOR would show a decrease in pulvinar-cortical connectivity and an increase in pulvinar-amygdala connectivity during exposure to mildly aversive sensory stimuli.
Methods
Participants
Participants were 19 youth with ASD and 19 TD matched controls aged 9-17.6 years (M=13.66; SD=2.11) recruited through flyers posted around the University of California Los Angeles (UCLA) campus as well as through referrals from the UCLA autism clinic. All participants had a full-scale IQ within the normal range based on an assessment with the Weschler Abbreviated Scales of Intelligence (Wechsler, 1999) (WASI), or the Weschler Intelligence Scale for Children – 4th Edition (Wechsler, 2003) (WISC-IV). ASD and TD groups did not differ significantly in age, FSIQ, performance IQ, verbal IQ, and mean or maximum head motion during fMRI (see Table 1). However, the TD group had marginally greater variability in age (TD stdev=2.6, ASD stdev=1.6, F(36)=3.4, p=.07). Therefore, to be conservative, we included age as a covariate in all imaging analyses. ASD participants had a prior diagnosis of autism spectrum disorder, confirmed using the Autism Diagnostic Interview – Revised (Lord, Rutter, & Le Couteur, 1994) (ADI-R) and the Autism Diagnostic Observation Schedule – Generic (Lord et al., 2000) (ADOS-G). All study procedures were approved by the UCLA Institutional Review Board.
Table 1.
Descriptive statistics.
| ASD | TD | t or ς2 | |
|---|---|---|---|
| Age | 13.71 (1.60) | 13.61 (2.57) | 0.13 |
| Gender (% male) | 84% (n=16) | 84% (n=16) | 0.00 |
| Handedness (% right-handed) | 95% (n=18) | 100% (n=19) | 0.31 |
| FSIQ | 104.63 (13.22) | 107.37 (15.06) | −0.59 |
| VIQ | 103.74 (13.49) | 107.63 (13.17) | −0.90 |
| PIQ | 103.70 (14.47) | 105.76 (16.00) | −0.42 |
| Mean Absolute Motion | 0.33 (.17) | 0.31 (.23) | 0.21 |
| Max Absolute Motion | 0.94 (.64) | 0.87 (.97) | 0.29 |
| Mean Relative motion | 0.09 (.04) | 0.13 (.20) | −0.90 |
| Max Relative Motion | 0.80 (.63) | 0.61 (1.15) | 0.62 |
| SensOR tactile count | 4.79 (5.57) | 2.76 (4.12) | 1.22 |
| SensOR auditory count | 6.89 (7.06) | 1.56 (3.90) | 2.87** |
| SSP auditory/visual | 19.32 (5.10) | 24.28 (2.11) | −3.90** |
| SSP auditory filtering | 17.42 (6.00) | 26.11 (4.01) | −5.20*** |
| SSP tactile sensitivity | 27.32 (6.19) | 32.89 (3.64) | −3.31** |
| SOR composite | 0.45 (.93) | −0.45 (.51) | 3.71** |
| SCARED anxiety total | 13.84 (9.44) | 5.47 (5.88) | 3.28** |
p<.01;
p<.001.
Note: Lower SSP scores indicate higher symptom severity. N=19 ASD, 19 TD except for SSP analyses where N=19 ASD, 18 TD, and SensOR analyses where N=19 ASD, 17 TD.
MRI data acquisition
Scans were acquired on a Siemens Trio 3 Tesla magnetic resonance imaging scanner. A high-resolution structural T2-weighted echo-planar imaging volume (spin-echo, TR=5000 ms, TE=33 ms, 128×128 matrix, 20cm FOV, 36 slices, 1.56mm in-plane resolution, 3mm thick) was acquired coplanar to the functional scans in order to ensure identical distortion characteristics to the fMRI scan. Each functional run involved the acquisition of 137 EPI volumes (gradient-echo, TR=2500ms, TE=30ms, flip angle=90, 64×64 matrix, 20cm FOV, 33 slices, 3.125mm in-plane resolution, 3 mm thick). Auditory stimuli were presented to the participant using magnet-compatible headphones under computer control (Resonance Technologies, Inc.). The stimuli were presented using E-Prime. Participants wore earplugs and headphones to reduce interference of the auditory stimuli from the scanner noise.
Measures
The ADI-R, ADOS, WISC, and WASI were administered at a clinical assessment visit prior to the MRI scan. Parents completed the additional questionnaires listed below while their child was in the scanner.
Short Sensory Profile
(SSP; Dunn, 1999) The SSP is a widely used, 38-item parent report measure of youth sensory dysregulation across a number of sensory modalities. Parents rate the frequency with which their child responds in an atypical way to sensory stimuli on a five-point Likert scale from “never” responds this way to “always” responds this way. This measure yields both a total score of sensory dysregulation as well as subscale scores for Tactile, Taste/Smell, Movement, and Auditory/Visual Sensitivity, Underresponsive/Seeks Sensation, Auditory Filtering, and Low Energy/Weak. For the purposes of this study, we used only the subscales relevant to the auditory and tactile stimuli administered during the scan, namely the Auditory/Visual Sensitivity scores, the Auditory Filtering score, and the Tactile Sensitivity score. Higher scores on the SSP indicate lower impairment.
Sensory Over-Responsivity (SensOR) Inventory
(Schoen, Miller, & Green, 2008) The SensOR Inventory is a parent checklist of sensory sensations that bother their child. For the purposes of this study, only the tactile and auditory subscales were used. The number of items parents rate as bothering their child has been shown to discriminate between TD children and children with SOR (Schoen et al., 2008).
SOR composite
An SOR composite score was created by standardizing and averaging relevant subscales of the SOR measures (SensOR auditory and tactile scores and reverse-scored SSP auditory/visual sensitivity, tactile sensitivity, and auditory filtering scales and) across all participants.
fMRI sensory experiment and analysis
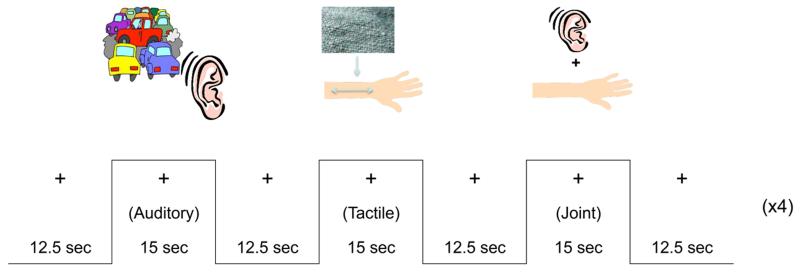
As reported in Green et al. (2015) on this same sample, participants were passively exposed to three mildly aversive stimulus conditions in an event-related paradigm (see Figure 1): an auditory condition, a tactile condition, and a “joint” condition where the auditory and tactile stimuli were presented simultaneously. The Joint condition was the focus of the current study because this condition was shown to result in the greatest hyperactivity in youth with SOR (Green et al., 2013, 2015), and the only condition in which the ASD group showed greater pulvinar activation than the TD group. We were interested in further probing the mechanisms underlying this greater activation, particularly given the pulvinar’s role in integrating multiple sensory inputs. However, for purposes of comparison, pulvinar activation results from the tactile condition are presented in supplemental online materials. There was no significant pulvinar activation in either group for the auditory condition. The auditory stimuli consisted of different traffic noises. The tactile stimulus was a scratchy wool fabric rubbed on participants’ inner arms at the rate of one stroke/sec. Stimuli were chosen that best differentiated ASD versus TD groups based on pilot testing with the Sensory Over-Responsivity Checklist. Participants were instructed to focus on a central fixation cross throughout the task. Each condition was presented 4 times, lasting 15 sec (each defined as one block), with 12.5 sec of fixation between trials. Total scan length was 5 min, 42.5 sec including 12.5-sec initial and final fixations.
Figure 1.
Experimental design.
FSL’s fMRI Expert Analysis Tool (FEAT), Version 5.98 was used for statistical analyses. Preprocessing included motion correction to the mean image, spatial smoothing (Gaussian Kernel FWHM = 5mm), and normalization to Montreal Neurological Institute (MNI) standard space. Within- and between-group analyses were run as described in Green et al., (2015). For these analyses, fixed-effects models were run separately for each subject, then combined in a higher-level mixed-effects model to investigate within and between-group differences. Single-subject models included six motion parameters as covariates. Each experimental condition (Auditory, Tactile, or Joint condition) was modeled with respect to the fixation condition during rest. Higher-level group analyses were carried out using FSL’s FLAME (FMRIB’s Local Analysis of Mixed Effects State) stage 1 (Beckmann, Jenkinson, & Smith, 2003; M. Woolrich, 2008; Woolrich, Behrens, Beckmann, Jenkinson, & Smith, 2004). Peak coordinates from pulvinar activation in the Joint condition were used for subsequent functional connectivity analyses, as described below.
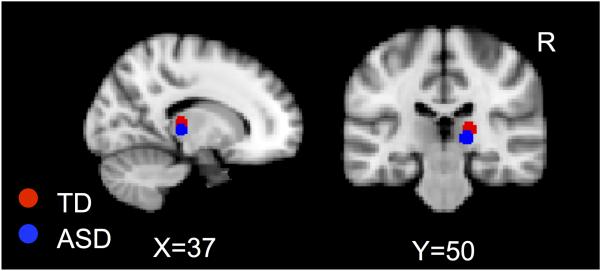
A psychophysiological interaction (PPI) analysis was used to examine functional connectivity during the Joint condition compared to fixation. This analysis examines the interaction between task (psychological context) and the time series of a seed region (physiological context) to identify brain regions where activity is more correlated with the seed region during the task than during baseline. The right pulvinar nucleus of the thalamus was used as a seed region because this was the area of significant activation as well as significantly greater activation in the ASD>TD contrast during the Joint sensory stimulation. The pulvinar seed was defined by masking a 5mm sphere around the peak coordinate of activation in each group during the Joint condition (at Z>1.7, corrected for multiple comparisons at p<.05) and adding the two masks (TD peak coordinates: x=18, y=−26, z= 8; ASD peak coordinates: x=16, y=−26, z=2; see Figure 2). For each subject, this mask was transformed into subject space, and then we extracted the region-of-interest (ROI) time-series from each subject’s filtered functional data. At the individual subject level, we used a general linear model (GLM) to model the subject’s ROI time series, the “task” (i.e., timing of the Joint stimulus), and the interaction between the two. This analysis generates single-subject correlation maps showing brain regions that are more correlated with the pulvinar ROI during the Joint condition compared to baseline. Individual correlation maps were then converted into z-statistic maps using Fischer’s r-to-z transformation. At the group level, we modeled a paired-sample mixed-effects design (Z>1.7, corrected for multiple comparisons at the cluster level p<.05) using Gaussian random field (GRF) method (FWHMx=4.67, FWHMy=4.56, FWHMz=5.26) and examined both within-group results as well as direct comparisons between groups. Participants’ ages were entered as covariates. Because of a priori interest in the amygdala (defined by the Harvard-Oxford probabilistic atlas, thresholded at 75%; 493 voxels), small volume correction was used to correct for multiple comparisons within this region of interest (ROI) using 3dClustSim (https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html). Parameter estimates for significant clusters were extracted from each participant and plotted in a graph to rule out the presence of outliers. Clusters where correlation with pulvinar activity were not significant after removal of an extreme outlier are not reported.
Figure 2.
Pulvinar masks.
Results
Task-based functional connectivity
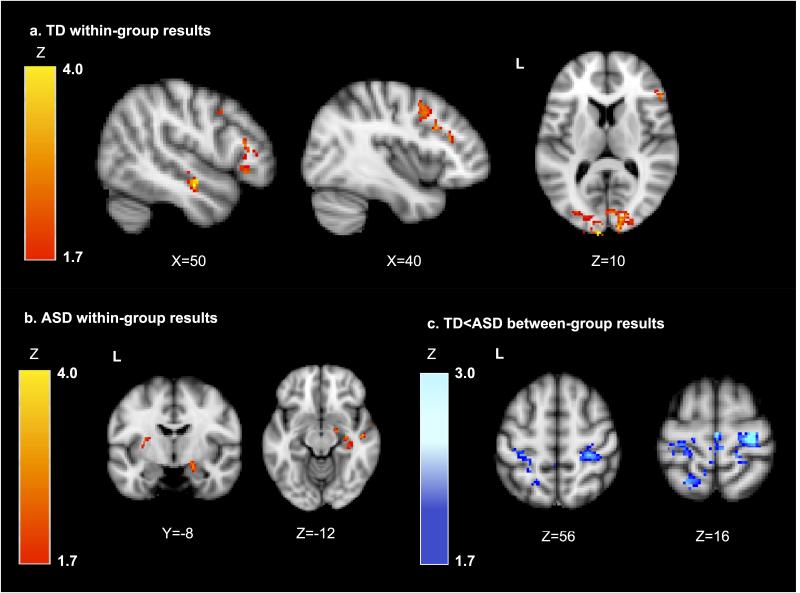
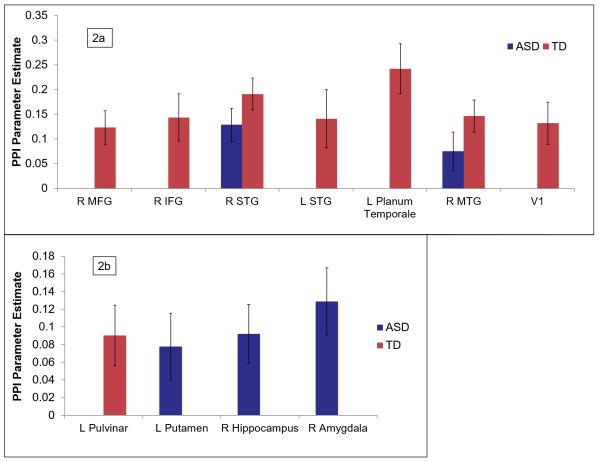
First, we used a whole-brain PPI analysis to examine regions showing significant functional connectivity with the pulvinar seed as a function of the joint auditory and tactile sensory stimulation (see Figures 3-5 and Table 2). Within the TD group, the right pulvinar showed significant increases in connectivity with sensory cortical regions (bilateral temporal lobes, primary visual cortex (V1)), dorsolateral prefrontal cortex, and left pulvinar. The ASD group showed significant increases in cortical connectivity only with right temporal lobe. The ASD group also had increased pulvinar connectivity with putamen and hippocampus.
Figure 3.
Brain maps showing PPI results for areas of significant changes in connectivity with the right pulvinar seed a) in the TD group only; b) in the ASD group only, and c) TD<ASD (indicating areas where the TD group has significantly greater decreases in connectivity or increases in negative connectivity compared to the ASD group). Results are thresholded at Z>1.7 and corrected for multiple comparisons at p<.05, except for amygdala which was corrected using a small volume correction (Z>1.7, p<.01 within the amygdala).
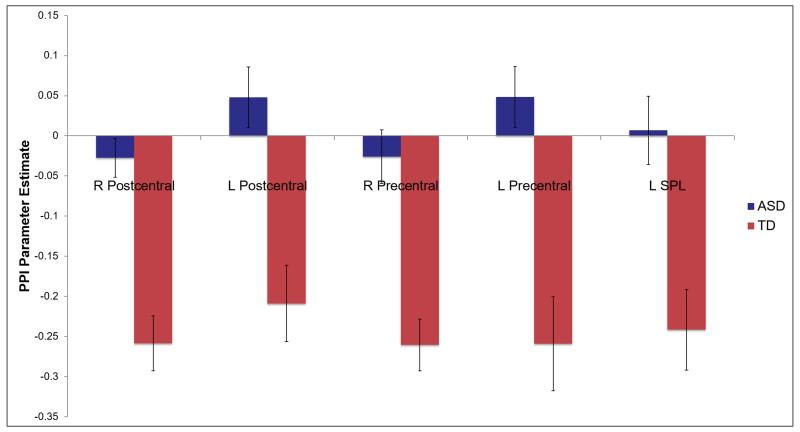
Figure 5.
PPI results: Areas of significant between-group differences in changes in connectivity with right pulvinar seed region.
Note. All clusters shown have significantly more negative connectivity (i.e. connectivity with this region decreases or becomes more negative as a function of the task) in the TD group (red bars) compared to the ASD group (blue bars) during sensory stimulation. The ASD clusters are not significantly different from zero. Results are corrected for multiple comparisons across the whole brain (Z>1.7, p<.05). Abbreviations: R: right; L: left; SPL: superior parietal lobe.
Table 2.
MNI coordinates for regions showing significant changes in functional connectivity with the pulvinar as a function of joint (auditory and tactile) sensory stimulation.
| TD+ | ASD+ | ASD>TD+ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| MNI Peak (mm) | Max | MNI Peak (mm) | Max | MNI Peak (mm) |
Max | ||||||||||
| x | y | z | Z | voxels | x | y | z | Z | voxels | x | y | z | Z | voxels | |
| Right Postcentral Gyrus | 16 | −36 | 66 | 2.50 | 659 | ||||||||||
| Right Precentral Gyrus | 28 | − 22 | 66 | 3.66 | − | ||||||||||
| Left Postcentral Gyrus | −32 | −32 | 56 | 2.64 | 870 | ||||||||||
| Left Precentral Gyrus | −22 | −24 | 70 | 3.44 | − | ||||||||||
| Left Superior Parietal Lobule | − 16 | − 54 | 68 | 3.08 | − | ||||||||||
| Right Middle Frontal Gyrus | 38 | 8 | 46 | 3.11 | 950 | ||||||||||
| Right Inferior Frontal Gyrus | 54 | 28 | 10 | 3.19 | − | ||||||||||
| Right Superior Temporal Gyrus | 52 | −8 | −12 | 3.92 | 947 | 48 | −14 | −2 | 4.41 | 1516 | |||||
| Right Middle Temporal Gyrus | 62 | − 46 | 4 | 3.21 | − | 58 | − 46 | 0 | 3.37 | − | |||||
| Left Superior Temporal Gyrus | −54 | −2 | −6 | 3.97 | 993 | ||||||||||
| Left Planum Temporale | − 32 | − 30 | 16 | 4.44 | − | ||||||||||
| Left Thalamus (Pulvinar) | − 18 | − 32 | − 2 | 3.14 | − | ||||||||||
| Occipital Pole/V1 | −8 | −104 | 12 | 4.50 | 292 | ||||||||||
| Right Hippocampus/Parahippocampus | 32 | − 32 | − 16 | 3.99 | − | ||||||||||
| Left Putamen | −28 | −10 | 14 | 2.60 | 839 | ||||||||||
| Cerebellum | −16 | −82 | −34 | 3.84 | 632 | −22 | −64 | −52 | 3.92 | 1494 | 10 | −64 | − 52 |
2.96 | 328 |
Note: x, y, and z refer to the left–right, anterior–posterior, and inferior–superior dimensions, respectively; Z refers to the Z-score at those coordinates (local maxima or submaxima). Voxels indicates cluster size; coordinates in itallics are local maxima within the same cluster as the coordinates above them. Within- and between-group analyses are cluster corrected for multiple comparisons, Z>1.7, p<.05. Within-group coordinates indicate positive functional connectivity; between-group ASD>TD coordinates indicate clusters where the TD group has significant negative connectivity and the ASD group does not have significant connectivity.
Direct comparisons between groups showed significant group differences in connectivity in bilateral sensory-motor cortex and left superior parietal lobule. Parameter estimates were extracted to determine the direction of effect; these indicated that the TD group had significant, negative changes in connectivity within each of these regions, whereas the ASD group did not have significant changes in connectivity. Overall, the TD group showed more extensive positive and negative changes in thalamocortical connectivity during sensory stimulation compared to the ASD group.
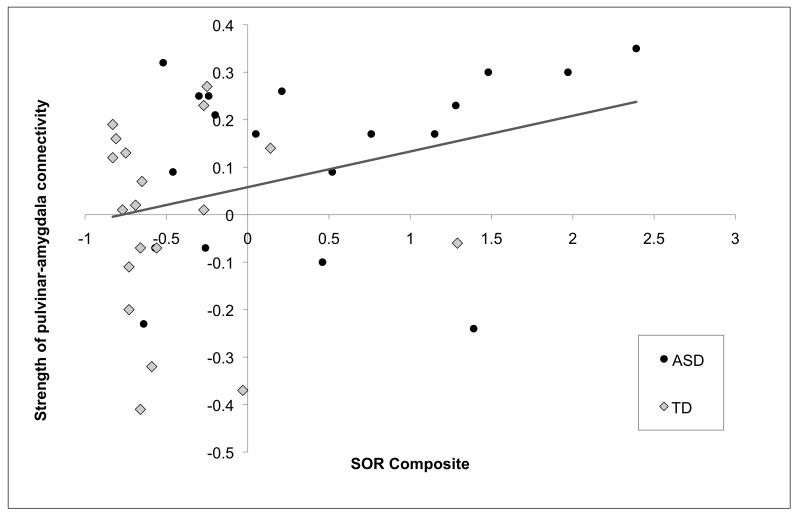
We next examined whether there were significant changes in pulvinar connectivity with the amygdala using a small volume correction. The ASD group had significant increases in connectivity between the pulvinar and right amygdala (peak coordinate=20,−8,−16, val=2.92, p<.01, voxels=32). In order to test whether this increase in connectivity was associated with SOR, parameter estimates were extracted for both groups from the area of significant connectivity with the pulvinar, and correlated with SOR composite scores using a one-tailed test; there was a significant positive correlation (r(36)=.33, p=.02). This correlation was clearly driven by the ASD group (see Figure 4), though the sample size was too low to reach significance within the ASD group alone (r(17)=.32, p=.09); in the TD group: r(17)=−.04, p=.45. No significant correlations were found between SOR and any of the other clusters showing significant changes in connectivity with the pulvinar in either group.
Figure 4.
PPI results: Areas of within-group changes in connectivity of right pulvinar seed with a) cortical regions and b) subcortical regions during Joint (auditory+tactile) sensory stimulation.
Note. Blue bars indicate significant clusters in ASD group, red bars indicate significant clusters in TD group. All results are corrected for multiple comparisons across the whole brain (Z>1.7, p<.05) except for the amygdala, which is corrected using a small volume correction (Z>1.7, p<.01). Abbreviations: R: right; L: left; MFG: middle frontal gyrus; IFG: inferior frontal gyrus, STG: superior temporal gyrus; MTG: middle temporal gyrus; V1: primary visual cortex.
Discussion
The aim of this study was to compare functional connectivity with the thalamus during auditory and tactile stimulation in youth with and without ASD. We focused on the pulvinar nucleus of the thalamus based on its role in regulation and integration of sensory information (Sherman & Guillery, 1996; Shipp, 2003), as well as its demonstrated over-responsivity to mildly aversive sensory stimuli in youth with ASD (Green et al., 2013, 2015). Overall, we found evidence for greater stimulus-elicited changes in pulvinar-cortical connectivity within the TD group compared to the ASD group in response to combined tactile and auditory stimulation. The TD group showed positive changes in connectivity with prefrontal regions and with auditory and visual sensory cortex and negative changes in pulvinar connectivity with somatosensory cortex and sensory association areas. Given that the pulvinar increased in activation in response to the sensory stimulation, the most cogent explanation for these negative PPI findings in the TD group is that sensory cortex activation decreased while pulvinar activation increased as a function of the task. In comparison, the ASD group showed significant changes in cortical connectivity only with right temporal cortex. Furthermore, the ASD group showed additional changes in pulvinar connectivity with subcortical areas including putamen, hippocampus, and amygdala. These results are specific to the Joint condition as the other conditions did not show the same pattern of results (see supplementary materials).
Most previous studies of thalamic connectivity in ASD have examined structural or resting-state connectivity (Cerliani et al., 2015; Nair et al., 2015, 2013); the current study is one of the first to examine changes in thalamic connectivity during information processing. Our findings are consistent with recent research demonstrating decreased structural thalamocortical connectivity in children with ASD (Nair et al., 2013) and those with sensory processing disorder (Owen et al., 2013). Our findings should also be interpreted in the context of resting-state studies showing that youth with ASD have increased functional connectivity with the thalamus, including both whole-thalamus over-connectivity with sensory-motor regions (Nair et al., 2015) and pulvinar over-connectivity with temporal cortex, prefrontal cortex, and sensory-motor regions (Woodward, Giraldo-Chica, Rogers, & Cascio, in press). Further research is needed to differentiate atypical pulvinar connectivity during information processing compared to resting-state, though it is possible that resting-state over-connectivity may reflect “over-use” of sensory relay pathways, whereas increases in pulvinar-cortical connectivity in response to sensory information seen in our TD participants may reflect modulation during mildly aversive sensory input. Thus, our results may indicate that brain activity in ASD is less responsive to changing environmental inputs rather than reflecting overall pulvinar-cortical under-connectivity. Additionally, two recent papers have examined age-related effects in resting-state thalamocortical connectivity in ASD, suggesting that resting-state atypicalities may increase from childhood to adolescence (Chen, Uddin, Zhang, Duan, & Chen, 2016; Woodward et al., in press); future studies should thus also examine age-related changes in task-based connectivity.
The current findings contribute to the existing understanding of the neurobiological basis of SOR. Previously, we found that youth with ASD, and particularly those with SOR, are over-responsive to mildly aversive sensory stimuli and show slower neural habituation in amygdala and sensory cortex to these stimuli (Green et al., 2013, 2015). Lack of pulvinar-cortical connectivity modulation in response to sensory stimuli in the ASD group could partially explain difficulties with filtering and/or integrating sensory information, especially given that the ASD group showed greater modulation of connectivity in response to tactile stimulation only compared to tactile combined with auditory stimulation. The prefrontal cortex in particular can influence attentional selection through connections with the thalamic reticular nucleus (Zikopoulos & Barbas, 2006), which inhibits the pulvinar from further inputs to sensory cortical regions (Phillips, Kambi, & Saalmann, 2016; Zhou et al., 2016). In this study, the combination of increases and decreases in connectivity with the pulvinar in the TD group could reflect such coordination through the pulvinar’s involvement in up-regulating activity in prefrontal and association regions and downregulating activity in sensory regions. Specific areas of the pulvinar may play discrete roles; for example, the medial pulvinar nucleus projects to the prefrontal cortex, perhaps initiating an inhibitory feedback loop (Barbas, Henion, & Dermon, 1991). The ventrolateral pulvinar receives inputs from the prefrontal cortex, a process which helps modulate visual processing and attention (Zhou et al., 2016). The methods in the current study are not sensitive enough to examine particular pulvinar nuclei but future studies should examine this in more detail.
While the ASD group displayed overall fewer changes in connectivity between pulvinar and cortex, this group did have significant stimulus-related increases in connectivity between the pulvinar and right amygdala, which was greater in individuals with higher SOR symptoms. Structural connectivity between the pulvinar and amygdala has been demonstrated in primate studies (Aggleton, Burton, & Passingham, 1980; Jones & Burton, 1976; Price & Amaral, 1981). Positive functional connectivity between pulvinar and amygdala is thought to relate to disruption in inhibitory cortical feedback (Williams et al., 2006), consistent with our previous findings that SOR is related to increased amygdala activity and decreased functional connectivity between prefrontal cortex and amygdala (Green et al., 2015). Other research supports this hypothesis; for example, findings from functional connectivity studies suggest that projections from the amygdala to the pulvinar, as well as to the thalamic reticular nucleus (e.g., Zikopoulos & Barbas, 2012), play a role in affective salience and selective attention (John et al., 2016; Troiani & Schultz, 2013). Thus, the association between SOR and increased amygdala-pulvinar connectivity may lead to increased attention to the sensory stimuli and increased negative arousal, reflecting the aversive perception of the sensory stimulation. These results are consistent with our previous findings that SOR is related to heterogeneity in brain function within youth with ASD (Green et al., 2013, 2015)
This is the first fMRI study examining pulvinar connectivity in response to sensory stimulation in ASD, and as such it provides important insight into difficulties with sensory over-responsivity and sensory integration seen in this population. However, the study does have some limitations. The relatively small sample size may have limited our ability to find significant within-group correlations with SOR; SOR was found to be correlated only with changes in pulvinar-amygdala across the entire sample and did not have significant correlations with extent of connectivity changes in any other regions. Similarly, we had relatively low power to find between-group differences; while the TD group had many more regions of positive connectivity with the pulvinar, these group differences did not survive correction for multiple comparisons; the ASD group had essentially zero connectivity in these same regions so it is likely that with additional subjects the group difference would be significant.
Together, these results extend our understanding of the neurobiological basis of SOR in autism by demonstrating that, during sensory stimulation, children and adolescents with ASD show reduced modulation of pulvinar connectivity with the cortex that could serve to down-regulate the brain’s response to extraneous sensory information to direct attention to more socially-relevant stimuli. Instead, youth with ASD showed increases in pulvinar connectivity with subcortical areas, including the amygdala, which may play a role in maintaining attention and affective responses to the sensory stimuli.
Supplementary Material
Figure 6.
Scatterplot showing correlation between change in amygdala-pulvinar connectivity and sensory over-responsivity (SOR) composite score.
Note. Right amygdala with right pulvinar change in functional connectivity during sensory stimulation was positively correlated with more severe symptoms of sensory over-responsivity (SOR) (r(38)=.33, p=.046). This correlation was driven by the ASD group (r(19)=.32, p=.19); in the TD group: r(19)=−.04, p=.89.
Many individuals with autism spectrum disorders (ASD) have sensory over-responsivity (SOR), an extreme negative response to sensory stimuli. The thalamus plays an important role in brain processing of sensory information, and there is recent evidence for abnormal thalamic connectivity in ASD and in sensory processing disorders such as SOR. This study used functional magnetic resonance imaging (fMRI) during sensory stimulation to examine functional connectivity with the pulvinar, a thalamic nucleus involved in selective attention, regulation, and sensory integration. Thirty-eight children and adolescents (half with ASD, half typically developing) participated in an fMRI scan while experiencing mildly aversive auditory and tactile sensory stimulation. We examined functional connectivity with the pulvinar by identifying brain regions whose activity was synchronized with activation observed in the pulvinar during the sensory stimulation. Results indicated that children with ASD had fewer changes in connectivity between pulvinar and cortex (including regions involved in sensory processing and inhibition) during sensory stimulation, which could lead to difficulty filtering out and/or integrating sensory information. The ASD participants, particularly those with higher sensory over-responsivity, had greater increases in pulvinar connectivity with the amygdala. An increase in amygdala connectivity with the pulvinar might be partially responsible for deficits in selective attention as the amygdala signals the brain to attend to distracting sensory stimuli.
Acknowledgments
This work was supported by grants from the National Institute of Child Health and Human Development (grant numbers P50 HD055784 and 1R01 HD065280) as well as the National Institute of Mental Health (grant number T32 MH073526 to L.H. and grant number F32 MH105167 to S.G.).
For generous support the authors also wish to thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family and Northstar Fund. The project described was supported by Grant Numbers RR12169, RR13642 and RR00865 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCR or NIH.
The funding sources and organizations listed above had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Funding: Grant sponsor: NICHD; grant numbers: P50 HD055784 and 1R01 HD073983; Grant sponsor: NIMH T32 MH073526 and F32 MH105167
Footnotes
The authors (S.G., L.H., S.B., and M.D.) have no conflicts of interests to declare.
References
- Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Research. 1980;190(2):347–368. doi: 10.1016/0006-8993(80)90279-6. http://doi.org/10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- Baranek GT, David FJ, Poe MD, Stone WL, Watson LR. Sensory experience questionnaire: Discriminating sensory features in young children with autism, developmental delays, and typical development. Journal of Child Psychology and Psychiatry. 2006;47(6):591–601. doi: 10.1111/j.1469-7610.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Barbas H, Henion THH, Dermon CR. Diverse thalamic projections to the prefrontal cortex in the rhesus monkey. The Journal of Comparative Neurology. 1991;313(1):65–94. doi: 10.1002/cne.903130106. http://doi.org/10.1002/cne.903130106. [DOI] [PubMed] [Google Scholar]
- Barron DS, Eickhoff SB, Clos M, Fox PT. Human pulvinar functional organization and connectivity. Human Brain Mapping. 2015;36(7):2417–2431. doi: 10.1002/hbm.22781. http://doi.org/10.1002/hbm.22781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. http://doi.org/10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott C. a. M., Boulby PA, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience. 2003;6(7):750–757. doi: 10.1038/nn1075. http://doi.org/10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A, Carter AS, Briggs-Gowan MJ. Sensory Over-Responsivity in Elementary School: Prevalence and Social-Emotional Correlates. Journal of Abnormal Child Psychology. 2009;37(5):705–716. doi: 10.1007/s10802-008-9295-8. http://doi.org/10.1007/s10802-008-9295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson A, Cermak SA, Orsmond GI, Carter AS, Kadlec MB, Dunn W. Extreme sensory modulation behaviors in toddlers with autism. American Journal of Occupational Therapy. 2007;61(5):584–592. doi: 10.5014/ajot.61.5.584. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A, Cermak SA, Orsmond GI, Tager-Flusberg H, Kadlec MB, Carter AS. Sensory clusters of toddlers with autism spectrum disorders: Differences in affective symptoms. Journal of Child Psychology and Psychiatry. 2008;49(8):817–825. doi: 10.1111/j.1469-7610.2008.01899.x. [DOI] [PubMed] [Google Scholar]
- Cerliani L, Mennes M, Thomas RM, Di Martino A, Thioux M, Keysers C. INcreased functional connectivity between subcortical and cortical resting-state networks in autism spectrum disorder. JAMA Psychiatry. 2015;72(8):767–777. doi: 10.1001/jamapsychiatry.2015.0101. http://doi.org/10.1001/jamapsychiatry.2015.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Uddin LQ, Zhang Y, Duan X, Chen H. Atypical effective connectivity of thalamo-cortical circuits in autism spectrum disorder. Autism Research. 2016 doi: 10.1002/aur.1614. n/a-n/a. http://doi.org/10.1002/aur.1614. [DOI] [PubMed] [Google Scholar]
- Dunn W. The Sensory Profile: User’s Manual. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Green SA, Hernandez L, Tottenham N, Krasileva K, Bookheimer SY, Dapretto M. Neurobiology of sensory overresponsivity in youth with autism spectrum disorders. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.0737. http://doi.org/10.1001/jamapsychiatry.2015.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Rudie JD, Colich NL, Wood JJ, Shirinyan D, Hernandez L, Bookheimer SY. Overreactive Brain Responses to Sensory Stimuli in Youth With Autism Spectrum Disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52(11):1158–1172. doi: 10.1016/j.jaac.2013.08.004. http://doi.org/10.1016/j.jaac.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh SM, Minshew N, Heeger DJ, Dinstein I, Behrmann M. Over-Responsiveness and Greater Variability in Roughness Perception in Autism. Autism Research. 2016;9(3):393–402. doi: 10.1002/aur.1505. http://doi.org/10.1002/aur.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horder J, Wilson CE, Mendez MA, Murphy DG. Autistic Traits and Abnormal Sensory Experiences in Adults. Journal of Autism and Developmental Disorders. 2013;44(6):1461–1469. doi: 10.1007/s10803-013-2012-7. http://doi.org/10.1007/s10803-013-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John YJ, Zikopoulos B, Bullock D, Barbas H. The Emotional Gatekeeper: A Computational Model of Attentional Selection and Suppression through the Pathway from the Amygdala to the Inhibitory Thalamic Reticular Nucleus. PLoS Computational Biology. 2016;12(2) doi: 10.1371/journal.pcbi.1004722. http://doi.org/10.1371/journal.pcbi.1004722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Burton H. A projection from the medial pulvinar to the amygdala in primates. Brain Research. 1976;104(1):142–147. doi: 10.1016/0006-8993(76)90654-5. http://doi.org/10.1016/0006-8993(76)90654-5. [DOI] [PubMed] [Google Scholar]
- Liss M. Sensory and attention abnormalities in autistic spectrum disorders. Autism. 2006;10(2):155–172. doi: 10.1177/1362361306062021. http://doi.org/10.1177/1362361306062021. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Nair A, Carper RA, Abbott AE, Chen CP, Solders S, Nakutin S, Müller R-A. Regional specificity of aberrant thalamocortical connectivity in autism. Human Brain Mapping. 2015 doi: 10.1002/hbm.22938. n/a-n/a. http://doi.org/10.1002/hbm.22938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Treiber JM, Shukla DK, Shih P, Müller R-A. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain: A Journal of Neurology. 2013;136(Pt 6):1942–1955. doi: 10.1093/brain/awt079. http://doi.org/10.1093/brain/awt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen JP, Marco EJ, Desai S, Fourie E, Harris J, Hill SS, Mukherjee P. Abnormal white matter microstructure in children with sensory processing disorders. NeuroImage: Clinical. 2013;2:844–853. doi: 10.1016/j.nicl.2013.06.009. http://doi.org/10.1016/j.nicl.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JM, Kambi NA, Saalmann YB. A Subcortical Pathway for Rapid, Goal-Driven, Attentional Filtering. Trends in Neurosciences. 2016;39(2):49–51. doi: 10.1016/j.tins.2015.12.003. http://doi.org/10.1016/j.tins.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. The Journal of Neuroscience. 1981;1(11):1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn S, Wehner E. Parent Reports of Sensory Symptoms in Toddlers with Autism and Those with Other Developmental Disorders. Journal of Autism and Developmental Disorders. 2003;33(6):631–642. doi: 10.1023/b:jadd.0000006000.38991.a7. http://doi.org/10.1023/B:JADD.0000006000.38991.a7. [DOI] [PubMed] [Google Scholar]
- Schoen SA, Miller LJ, Green KE. Pilot study of the sensory over-responsivity scales: Assessment and inventory. American Journal of Occupational Therapy. 2008;62:393–406. doi: 10.5014/ajot.62.4.393. [DOI] [PubMed] [Google Scholar]
- Sherman SM. In: Thalamic relays and cortical functioning. B.-P. in B. Research, editor. Vol. 149. Elsevier; 2005. pp. 107–126. Retrieved from http://www.sciencedirect.com/science/article/pii/S0079612305490093. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Functional organization of thalamocortical relays. Journal of Neurophysiology. 1996;76(3):1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- Shipp S. The functional logic of cortico–pulvinar connections. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2003;358(1438):1605–1624. doi: 10.1098/rstb.2002.1213. http://doi.org/10.1098/rstb.2002.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchek SD, Dunn W. Sensory processing in children with and without autism: a comparative study using the short sensory profile. The American Journal of Occupational Therapy. 2007;61(2):190–200. doi: 10.5014/ajot.61.2.190. [DOI] [PubMed] [Google Scholar]
- Troiani V, Schultz RT. Amygdala, pulvinar, and inferior parietal cortex contribute to early processing of faces without awareness. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00241. http://doi.org/10.3389/fnhum.2013.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation: Harbourt Brace & Company; New York, NY: 1999. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 4th ed Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- Williams LM, Das P, Liddell BJ, Kemp AH, Rennie CJ, Gordon E. Mode of Functional Connectivity in Amygdala Pathways Dissociates Level of Awareness for Signals of Fear. The Journal of Neuroscience. 2006;26(36):9264–9271. doi: 10.1523/JNEUROSCI.1016-06.2006. http://doi.org/10.1523/JNEUROSCI.1016-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Giraldo-Chica M, Rogers B, Cascio CJ. Thalamocortical Dysconnectivity in Autism Spectrum Disorder: An Analysis of the Autism Brain Imaging Data Exchange. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. doi: 10.1016/j.bpsc.2016.09.002. (in press) http://doi.org/10.1016/j.bpsc.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M. Robust group analysis using outlier inference. NeuroImage. 2008;41(2):286–301. doi: 10.1016/j.neuroimage.2008.02.042. http://doi.org/10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 2004;21(4):1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. http://doi.org/10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Zhou H, Schafer RJ, Desimone R. Pulvinar-Cortex Interactions in Vision and Attention. Neuron. 2016;89(1):209–220. doi: 10.1016/j.neuron.2015.11.034. http://doi.org/10.1016/j.neuron.2015.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Prefrontal Projections to the Thalamic Reticular Nucleus form a Unique Circuit for Attentional Mechanisms. The Journal of Neuroscience. 2006;26(28):7348–7361. doi: 10.1523/JNEUROSCI.5511-05.2006. http://doi.org/10.1523/JNEUROSCI.5511-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Pathways for Emotions and Attention Converge on the Thalamic Reticular Nucleus in Primates. The Journal of Neuroscience. 2012;32(15):5338–5350. doi: 10.1523/JNEUROSCI.4793-11.2012. http://doi.org/10.1523/JNEUROSCI.4793-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.