Abstract
Objective
To validate a hypothesis that prostate cancer (PCa) can be detected noninvasively by a simple and reliable assay by targeting genomic VPAC receptors expressed on malignant PCa cells shed in voided urine.
Materials and Methods
VPAC receptors were targeted with a specific biomolecule, TP4303, developed in our laboratory. With an IRB “exempt” approval of use of de-identified discarded samples, an aliquot of urine collected as a standard of care, from patients presenting to the urology clinic, (N=207, M= 176, F= 31, 21 years or older) was cytospun. The cells were fixed and treated with TP4303 and 4, 6 Dimidino-2-phenylindole, Dihydrochloride (DAPI). The cells were then observed under a microscope and cells with TP4303 orange fluorescence around the blue (DAPI) nucleus were considered malignant and those only with blue nucleus were regarded as normal. VPAC presence was validated using receptor blocking assay and cell malignancy was confirmed by PCa gene profile examination.
Results
The urine specimens were labeled only with gender and presenting diagnosis, with no personal health identifiers or other clinical data. The assay detected VPAC positive cells in 98.6% of the patients having a PCa diagnosis, (N=141), and none (0%) of the males with benign prostatic hyperplasia (BPH) (N=10). Of the 56 “normal” patients, 62.5% (N=35, M=10, F=25) were negative for VPAC cells; 19.6% (N=11, M=11, F=0) had VPAC positive cells; and 17.8% (N=10, M=4, F=6) were uninterpretable due to excessive crystals in the urine. Although data are limited, the sensitivity of the assay was 99.3% with confidence interval of 96.1%–100% and the specificity was 100% with confidence interval of 69.2%–100%. Receptor blocking assay and FACS analyses demonstrated the presence of VPAC receptors and gene profiling examinations confirmed that the cells expressing VPAC receptors were malignant PCa cells.
Conclusion
These preliminary data are highly encouraging and warrant further evaluation of the assay to serve as a simple and reliable tool to detect PCa noninvasively.
Keywords: Imaging Prostate cancer, Targeting VPAC receptors, Urinary Assay, Optical imaging
Introduction
Increased understanding of human diseases at cellular and molecular levels has paved the way for development of, several novel, life-sciences technologies. Detecting circulating tumor cells (CTC) in human blood is one. CTC associated liquid biopsy approach has drawn considerable attention and is advancing into clinical applications [1, 2]. Broadly, such approaches are based upon collection of body fluids, cell isolation, followed by multiplex genomic profiling and identification of disease specific fingerprints. Although innovative and state of the art, such approaches can be technically complex, expensive, subject to errors leading to inconsistent results [1, 2].
Among men worldwide, prostate cancer (PCa) is the most common malignancy. With increasing lifespan, the incidence of PCa is on the rise. In the USA alone, every hour of each day, in 2016, more than 25 new cases of PCa were identified and greater than three men died of PCa [3–5]. PCa is a heterogeneous disease in its presentation, biochemistry and even in its histology. Despite the recent advances in understanding of the molecular basis of the origin of PCa, its diagnosis by non-invasive or minimally invasive methods has continued to be challenging. With prostate specific antigen (PSA) serum test, there is a considerable controversy, with no consistent recommendations from major medical organizations with the best approach to screening [5, 6]. Digital rectal examination (DRE) remains a commonly performed physical examination but is unreliable and accurate diagnosis of PCa requires histologic identification of cancer cells on invasive prostate biopsy tissue. More than 66% of prostate biopsy procedures reveal benign pathology without evidence of malignancy [7–9].
The era of molecular profiling has led investigators to new discoveries in the field of PCa. One minimally invasive diagnostic test approved recently by FDA is the urinary PCA3 multiplex gene test. It targets a PCa molecular signature, and requires that urine must be collected following prostate massage performed in a specific manner during DRE [10]. The PCA3 sensitivity ranged from 62% to 94%, specificity from 37% to 99%, positive predicative value (PPV) from 42% to 98%, and negative predicative value (NPV) was 36% to 96%. As a result, the predictive accuracy of PCA3 test has been questioned and its clinical utility when examined in the context of its high cost is considered controversial [10].
Another relatively recent test, now performed by the commercial OPKO Laboratory, is the 4K score test. Based on measurements of specific Kallikrein markers in blood serum of patients, the test determines the serum concentration of i) total PSA, ii) free PSA, iii) intact PSA and iv) human Kallikrein (hK2). Although early results of the 4K score test appear promising, its general clinical acceptance as a PCa screening test remains undetermined [11]. Therefore, there is a clear need for a noninvasive examination that shall improve accurate detection of PCa.
Over the past few years, we have targeted VPAC1 genomic receptors that are overexpressed on the surface of malignant cells at the onset of cancers as those of the breast, prostate and lung. The human VPAC1 receptor, named for the combined vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating peptide (PACAP) family of cell surface receptors, encodes a G protein coupled receptor that recognizes with high affinity both VIP and PACAP related peptides. It has been demonstrated that VPAC1 receptors are expressed in men with prostate cancer irrespective of their hormonal status (68/68 men with PCa, 100%) including 15 men with metastatic lesions [12]. High expression of VPAC1 receptors (104/cell) on prostate cancer has been confirmed by others [13–15]. Both VIP and PACAP have high affinity for VPAC (VPAC1 and VPAC2) receptors. VIP is a 28-amino acid peptide initially isolated from porcine intestine [16]. VIP, whose structure is conserved in humans, pigs and rats, is a hydrophobic, basic peptide containing three lysines (no. 15, 20 and 21) and two arginines (no. 12 and 14). From the essential histidine residue at the N-terminus to the amidated C-terminus, all 28 amino acids of VIP are required for high affinity binding and biological activity [17].
Using a Cu-64 labeled VPAC1 specific peptide designed in our laboratories, we imaged breast and prostate cancers in humans with >97% sensitivity. [18, 19]. Encouraged by these results, we hypothesized that cells shed in voided urine of PCa patients can be imaged optically, by targeting the VPAC1 receptors with the same peptide labeled with a fluorophore. We report on a pilot feasibility study examining VPAC on shed urinary cells.
Methods
a) Peptide synthesis
In order to validate the hypothesis, the peptide was synthesized on solid state TG Sieber resin as described previously with added cysterin at the N terminus [20–23]. The peptide was cleaved off the resin, purified by HPLC, and purity was examined by Matrix Assisted Laser Desorption Ionization (MALDI) spectroscopy. To the peptide dissolved in 1:1 water:acetonitrile was added as a maleimide moiety, near infrared fluorophore PSVue 794, (MTTI, Westchester, PA), in 1:1 molar ratio. The pH was adjusted to 8 and stirred at 22°C for two hours. Following HPLC purification, fractions were collected, lyophilized and characterized using MALDI spectroscopy. The product, with >98% purity was named TP4303 after its molecular weight 4303, (observed 4304). The Kd value for such a sequence of peptide was determined to be 3.3x10−9 M (22, 23). TP4303 was dissolved in deionized water and 0.5 mg/ml fractions were stored at −80°C till use.
b) Patient population
From patients (M/F), 21 years of age or older (N=207, M=176, F=31) presenting to the Urology clinic at our institution, 10 to 20 ml of discarded, de-identified urine was obtained for this pilot investigation on an IRB exemption wavier. If the protocol processing did not begin within four hours of urine collection the samples were stored at −10°C for up to 72 hours. For up to four hours the samples were kept at 22°C and processed at 22°C.
c) Processing urine sample
No patient health information was provided to the laboratory personnel. The samples were identified only by the patient gender, date of collection and presenting diagnosis.
Samples were centrifuged at 2000xg for 10 minutes and all but approximately 250 µl of supernatant was discarded. The cells were then suspended, and cytocentrifuged (Shandon Cytospin 4, Thermo Fisher Scientific, PA), and fixed in 97% ethyl alcohol. TP4303 solution (0.5 µg) was added to the cells to cover the entire cell area, approximately one cm in diameter. The slide was then kept in dark, at 22°C for approximately 20 minutes and then thoroughly rinsed with deionized water and air dried. On the cells was then added, 20 µl of 4,6 Dimidino-2-phenylindole, Dihydrochloride (DAPI, Fisher Scientific, PA) which strongly binds to A-T rich region of DNA in the cell nucleus. A coverslip was then placed and slide was observed using an inverted confocal microscope (Ex: 630 nm, Em: 730 nm, LSM 510 Carl Zeiss, Germany). Cells with TP4303 interaction presented themselves with dark orange fluorescence around the nucleus and thereby indicated the presence of VPAC receptor molecules around the cell surface. In the absence of VPAC receptors, only the DAPI bound cell nucleus was seen in dark blue. Normal epithelial cells that may only have minimal or no expression of VPAC therefore do not interact with TP4303 and show only cell nucleus.
d) Statistical Analysis
Since the assay determines only “yes” (orange fluorescence positive) or “no” (orange fluorescence negative) malignant cells, no AUC (area under the curve) analysis was performed. Instead for these relatively small sample size, Clopper-Pearson exact 95% confidence intervals were computed for sensitivity and specificity.
e) Validating the presence of VPAC receptors and cell malignancy
i) Validation that TP4303 truly targeted VPAC receptors
Human PCa cell line PC3 was obtained from ATCC (American Type Culture Collection) and grown in tisue culture (10% FBS containing RPMI 1640). The confluent cells were detached, washed with phosophate buffered 0.9% NaCl (PBS), 10x105 cells were suspended in one ml PBS and cytospun on two glass slides A and B. The cells on slides were then fixed with 97% ethanol and to the cells on slide A, VPAC receptor specific peptide (10 µg) that was not conjugated with the fluorophore was added and incubated for 10 minutes. The slide was washed, air dried and 0.5 µg of TP4303 was added to the cells on both slides, A and B, incubated for 20 minutes and prepared similarly for microscopic examination.
ii) Fluorescence Activated Cell Sorting (FACS)
In order to further demonstrate that VPAC receptors are truly targeted by TP4303 and that they are cell surface receptors, PC3 cells were subjected to FACS analysis before and after receptor blocking. PC3 cells were grown as stated above, washed with PBS and fixed with 97% ethanol. In three separate test tubes (A, B, C), approximately 50x103 cells were dispensed. Cells in A, served as controls, cells in B were incubated with 2 µg TP4303 for 10 minutes, washed with PBS and subjected to (APC-CY7) FACS using Ex 750 nm and Em 810 nm filters. Cells in test tube C were incubated first for 10 minutes, with 10 µg of VPAC receptor specific peptide that was not conjugated with the fluorophore washed, and then incubated for 10 minutes with 2 µg TP4303. Cells were then washed with PBS and subjected to FACS as cells in B. Cells in A were also analyzed by FACS similarly.
iii) Validation that the VPAC1 expressing cells were malignant
The purpose was to ascertain that the cells that do express VPAC1 receptors and to which binds TP4303 selectively, are true malignant PCa cells. PCa is heterogeneous disease and the molecular basis of it’s clinical, pathological and genetic heterogeneity remains poorly understood. However, to determine the cell malignancy, we chose to examine mutation in gene expression on these cells, as compared to the genes in urine cells, collected from young normal males. Non-DRE urine was collected from four patients diagnosed with PCa, and from three normal male volunteers. Urine was centrifuged for 10 minutes at 2000 xg, cells were fixed, washed with PBS and suspended in 0.5 ml PBS. To the suspension was added one µg TP4303, incubated at 25°C for 10 minutes, cells were washed and resuspended. The cells were then subjected to orange fluorescence activated cell sorter (FACS, Becton, Dickinson).
A pure cell population with TP4303 orange fluorescence was selectively collected, centrifuged and the supernatant was discarded. To the cells was then added 250 µl of cell lysis buffer provided with the Norgen Biotek RNA purification kit. Following the kit procedure step by step, RNA was extracted from the cells and quantified using a nanodrop ND-100 spectrophotometer followed by RNA quality assessment analysis using Agilent 2200 Tapetation (Agilent Technologies, Palo Alto, CA). Using pure RNA, and GeneChip WT Pico Kit (Affimax, Santa Clara, CA) fragmented biotin-labeled cDNA was synthesized. Affymetrix gene chips, Human Transcriptome Array 2.0 (Affymetrix) were hybridized with five µg fragmented and biotin-labeled cDNA in 200 µg of hybridization cocktail. Target denaturation was performed at 99°C for five minutes and then at 45°C for 15 minutes, followed by hybridization with rotation at 60 rpm for 16 hour at 45°C. Arrays were then washed and stained using Gene Chip Fluidic Station 450, using Affymetrix GeneChip hybridization wash and the stain kit. Chips were scanned on an Affymetrix Gene Chip Scanner 3000, using Comman Console Software. Quality Control of the experiment was performed by Expression Console Software. Quality Control of the experiment was performed by Expression Console Software V 1.4.1.
Analysis
Chip file was generated by sst-rma normalization from Affymetrix cell file by using Expression Console Software. Experimental group was compared with control group by using transcriptome array console software. Differentially expressed gene list was used for pathway analysis using IPA software.
Results
TP4303 was >98% pure and had the molecular weight of 4304 (data not shown). The calculated weight was 4303. The 0.5 mg/ml TP4303 solution, kept frozen, has been stable for the past 20 months. As for the biofluid analysis, we have examined 207 samples (as of September 2016). Out of these, 141 had a diagnosis of PCa, 10 patients with a diagnosis of BPH, and 56 (M=25, F=31) presented for non-oncology or prostate diagnoses and were considered “controls”. The data presented in Table 1, depicts that out of 141 patients with PCa 139 (98.6%) were positive for VPAC positive shed urinary cells. Two patient slides were technically unclear due to sediment which compromised image quality. All 10 BPH patients (100%) had negative (no cell orange fluorescence) results. While computing Clopper-Pearson exact 95% confidence intervals, we focused only on the PCa and BPH patient data. Furthermore, one “Technically unclear” patient data was eliminated (N=140). Calculations showed that the sensitivity of the assay was 99.3% with confidence interval of 96.1%–100%% and the specificity was 100% with confidence interval of 69.2%–100%.
Table 1.
Urine Analysis (N=207)
| Presenting Diagnosis | Total No. | VPAC Positive | VPAC Negative | Technically unclear |
|---|---|---|---|---|
| BPH | 10 | 0 | 10 | 0 |
| Prostate Cancer (M=141) |
141 | 139 | 1 | 1 |
| Non-Oncology Controls (M=25, F=31) |
56 | 11 | 35 | 10 |
Out of 56 ”controls”, 35 (62.5%) were negative for VPAC cells, ten had technical failure (17.8%) and 11 (19.6%) were positive for VPAC cells. The final diagnosis of those eleven subjects with VPAC positive cells remains unknown and could have latent prostate cancer (latent true positives). The majority of the technical failures were due to hypercalciuria or sediment. The deposit of calcium crystals on the slides made the slide difficult to read and were placed in the technical failure group. In addition to VPAC positive malignant cells, generally large in size but variable in number, of epithelial cells that had no orange fluorescence around them due to the absence of VPAC receptors. Examples of images are given in Fig. 1 and normal images are given in Fig. 2. Fig. 3 depicts the results of receptor blocking studies in which it was observed that all PC3 cells prior to blocking VPAC receptors, had intense orange fluorescence around them (Fig. 3.A and B) which was abscent on all cells after the receptors were blocked (Fig. 3.C and D). The data strongly indicate that the cells with orange fluorescence represent the presence of VPAC receptors.
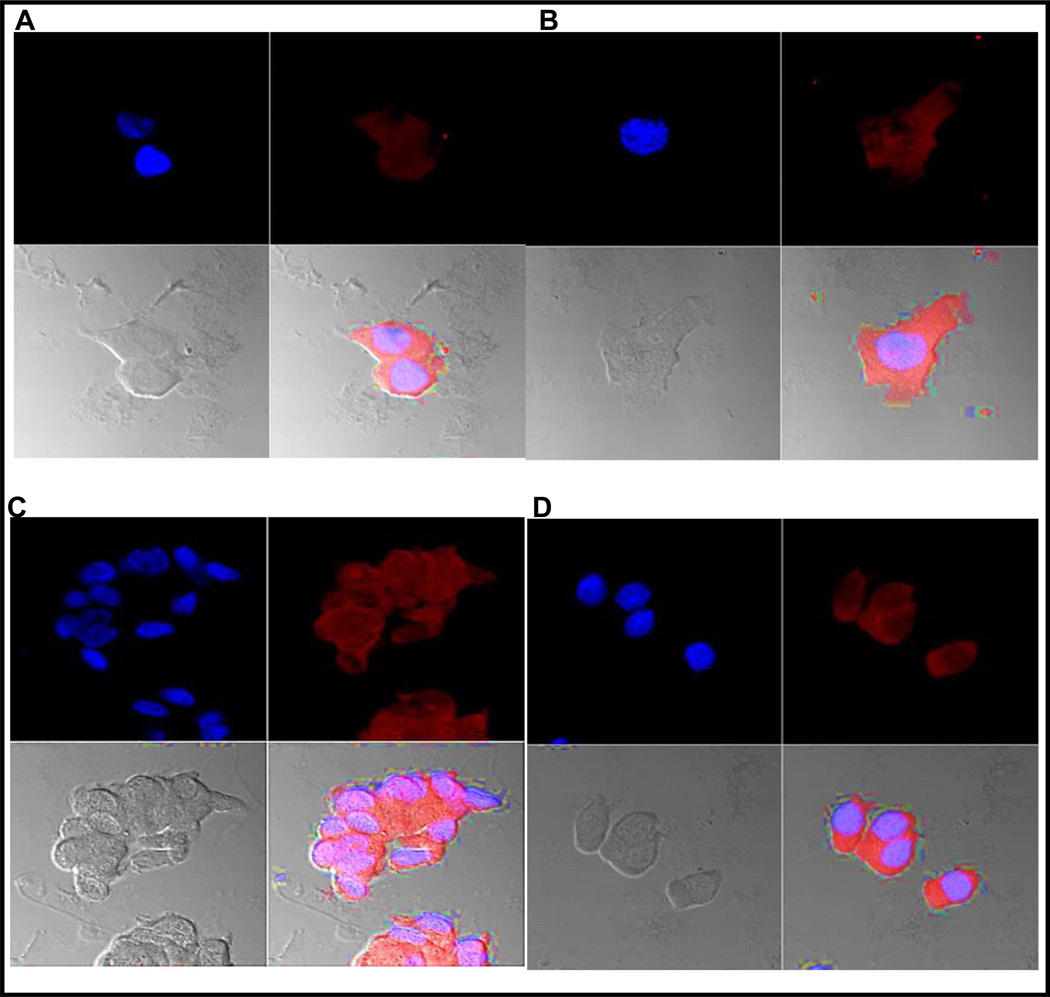
Fig. 1.
Optical imaging of cells prepared from voided urine of patients A, B, C and D. Each image is presented in four subsections. Top left of the four subsections is the cell nucleus in blue, top right is the orange fluorescence of TP4303 bound to VPAC receptors expressed the cell membrane, the lower right is fusion of the two showing the orange fluorescence around the cell, and lower left is cell morphology. Patients A and B had known PCa with Gleason score 3+3 and patients C and D known with PCa had a Gleason score 9.
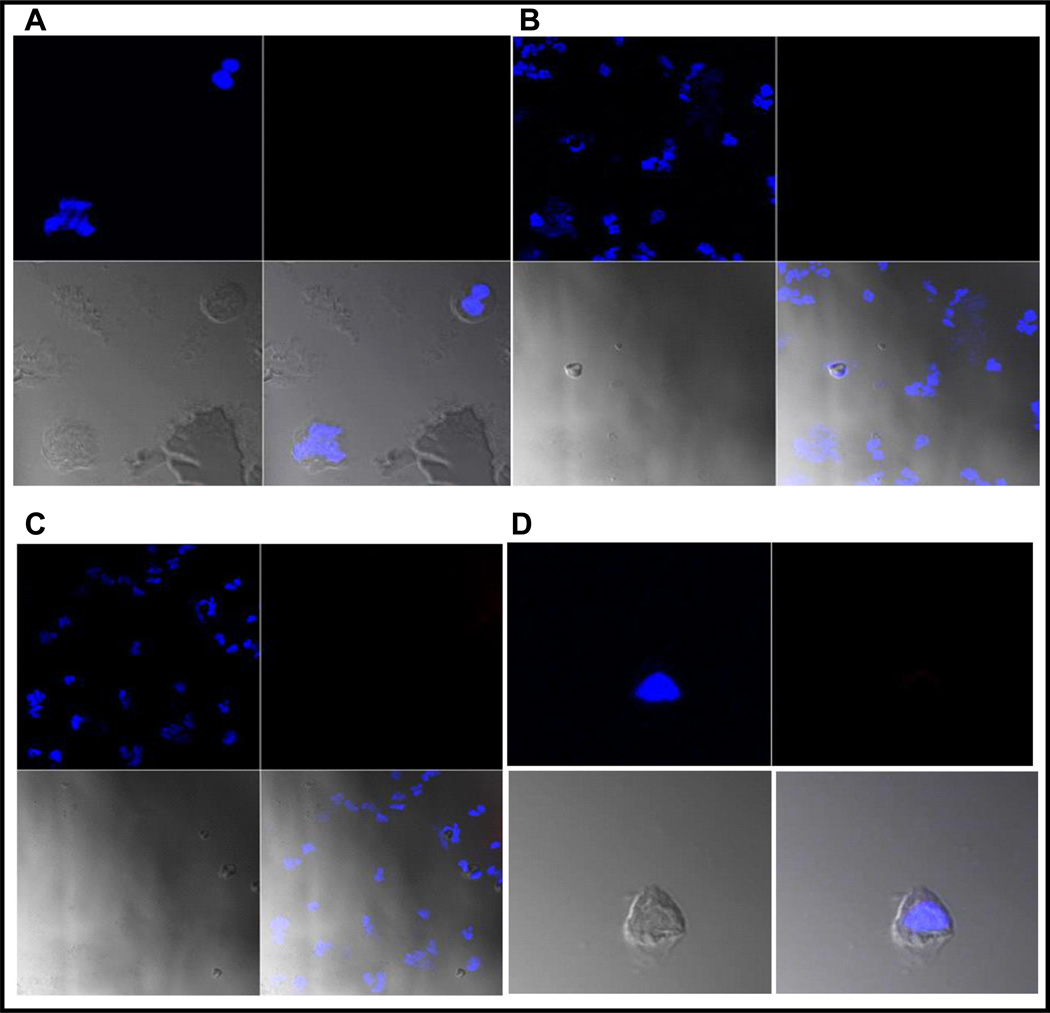
Fig. 2.
Optical imaging of cells prepared from voided urine of subjects A, B, C and D. Again each image is presented in four subsections as described in Fig. 2. Subjects A and B had negative biopsy and they were PCa free. Patient C had BPH and subject D was a normal volunteer. Please note the absence of the orange fluorescence as these subjects had no PCa and normal (epithelial) cells shed in urine had no VPAC receptors leading to the absence of TP4303 orange fluorescence.
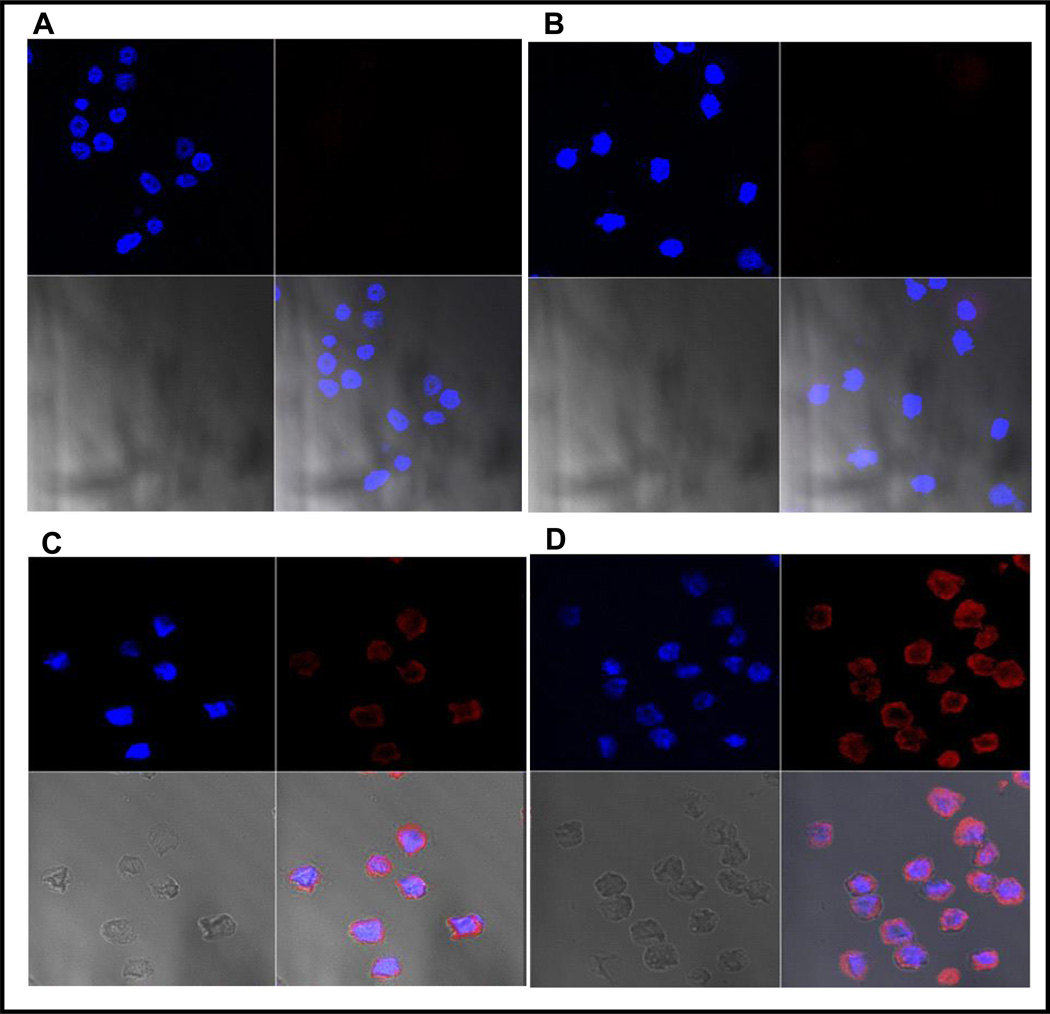
Fig. 3.
This figure demonstrates VPAC1 receptor specificity of TP4303. As described in the text, the two separate images shown in A and B, VPAC1 receptors of the human PCa, PC3 cells were blocked (pre-separated) using TP4303 without the fluorophore attached the TP4303 molecules. This resulted in seeing only the cell nucleus (blue) but no orange fluorescence around them. In image C and D, were the PC3 cells in which the receptors were not blocked, and incubated with fluorescence labeled TP4303 show the customary, orange fluorescence indicating the presence of VPAC receptors.
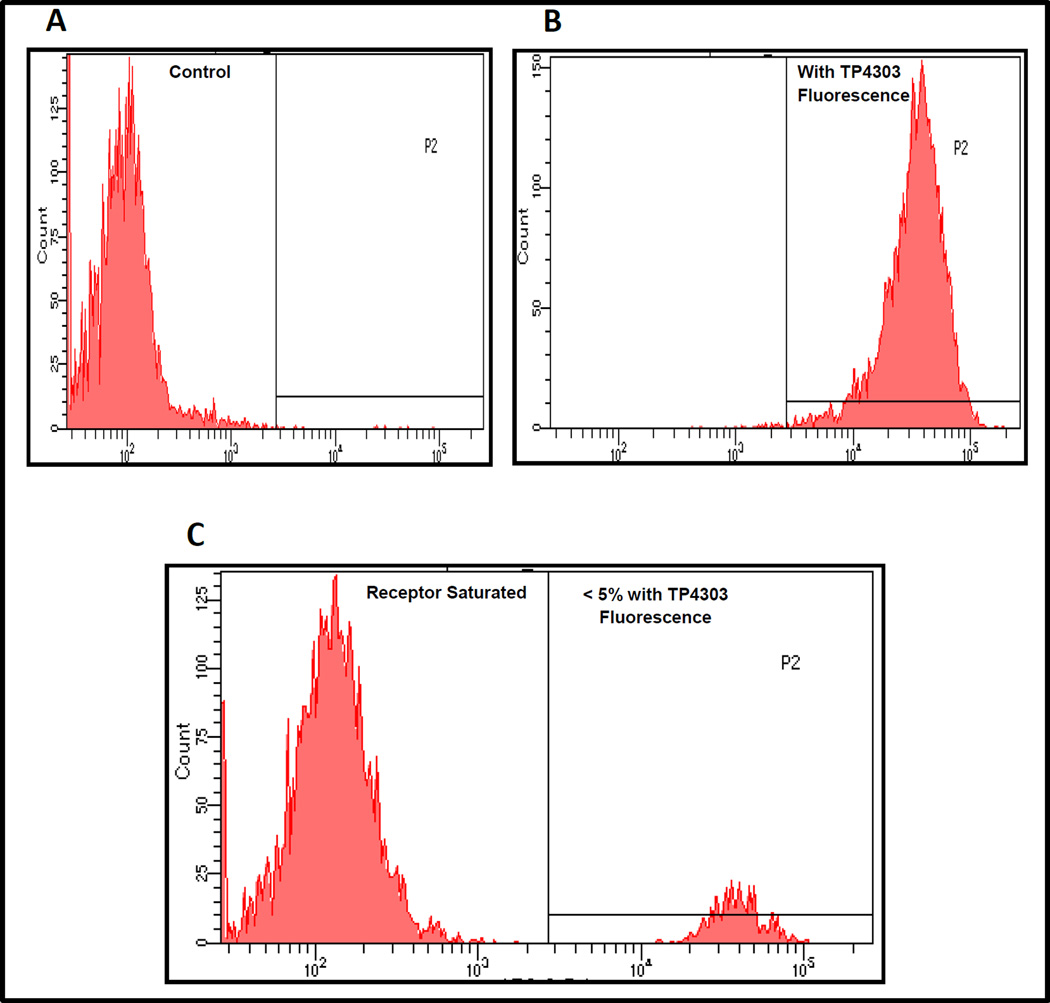
The FACS analysis data presented in Fig. 4 illustrated that VPAC genomic receptors can be selectively blocked on >95% of the PC3 cells with the receptor specific peptide and that 100% of the PC3 cells express VPAC receptors and are expressed on the cell surface (Fig. 4 B).
Fig. 4.
FACS analysis of human PCa, PC3 cells (A control) depicts that 100% of the PC3 cells express VPAC cell surface receptors (B), and that these receptors can be selectively blocked by the receptors specific peptide TP4303 (C).
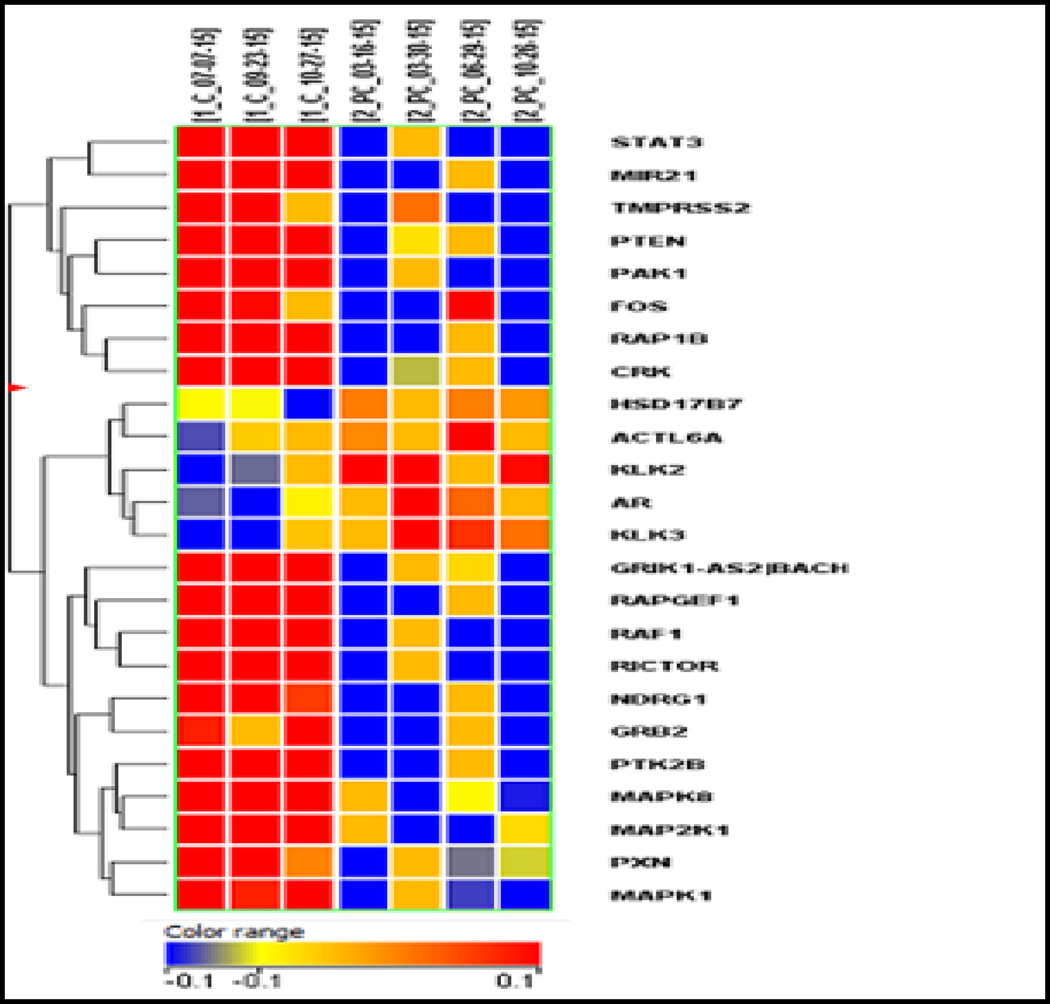
Fig. 5 is a heat chart of the genomic profiling analysis. The results showed that five PCa coding genes (AR, HSD17B, KLK3, KLK2 and ACTL6A) were upregulated and 19 coding PCa genes (e.g. PTEN, MR01, BACH1, etc.) were down regulated (>1.5 fold, P=<0.05) [24, 25]. Data thereby confirm that the urine shed cells targeted for VPAC1 using TP4303 are malignant and validate our hypothesis.
Fig. 5.
Differential gene expression of four patients with known PCa (at the right) verses three normal volunteers at the left. Five PCa genes were upregulated (>1.5 fold with p<0.05) and 19 genes were down regulated (>1.5 fold with P < 0.05) in a paired sample test.
Discussion
In an attempt to develop a simple, completely non-invasive, inexpensive and reliable test to screen or detect PCa, we have chosen to target VPAC receptors known to express in high density on PCa cells at the onset of oncogenesis. In this preliminary evaluation our approach was to validate our hypothesis by optically detecting, shed PCa cells that are eliminated in voided urine without prostate stimulation.
It was 1869 when Thomas Ashworth reported that cells similar to those in tumors were seen in blood of a patient [26]. Subsequently it has been observed that one gram of a growing tumor, sheds nearly 0.4% (3.4x106 cells) every 24 hours [27]. Parts of these cells, in the case of PCa, through prostatic ducts, pass into the urine, 15 to 20 ml of which we used upon natural voiding without performing DRE.
At the time of this writing, urine samples studied from more than 141 PCa patients have correctly identified PCa cells with 98.5% sensitivity. In urine from BPH (n=10) patients, no PCa cells were detectable. We have further validated our data by i) receptor blocking studies using VPAC expressing human PCa cells PC3, and ii) demonstrated that these genomic receptors are expressed on the cell surface. Furthermore, we performed PCa gene expression profiling studies using VPAC expressing malignant cells, separated from voided urine of patients with known PCa. These data strongly demonstrated that not only did TP4303 specifically targeted, cell surface VPAC receptors but also confirmed that the cells we identified as PCa cells, were truly malignant.
The VPAC genomic biomarker belongs to the superfamily of G-protein coupled surface receptors, which are expressed in high density (104–105/ cell) at the onset of oncogenesis, and prior to the alterations in cell morphology [12–14], VPAC receptors are also expressed on breast and lung cancer [12]. On stroma, normal cells and benign masses, VPAC1 receptors are minimally present [15, 28].
The VPAC1 receptor specific peptide was designed and thoroughly evaluated in our laboratory [18–23]. When labeled with a positron emitting radionuclide 64Cu (β+, 19%, t ½-12.7 hours), it permits us to image spontaneously grown breast cancer in MMTVnue transgenic mice, and spontaneously grown prostate cancer in TRAMP (Transgenic adenocarcinoma of mouse prostate) mice [20–23].These findings have now been transformed into successful imaging of breast and prostate cancers in humans [18–19]. This agent is named Cu-64-TP3805. These investigations demonstrated, the ability of the peptide to image with high sensitivity only malignant lesions, but not benign masses and prompted us to hypothesize that the VPAC1 receptor specific peptide, covalently bound to a fluorophore (TP4303), will allow us to optically image PCa cells, but not the normal epithelial cells shed in voided urine.
This feasibility study was performed under IRB exempt waiver using discarded urine specimens from de-identified subjects with limited clinical information. The study therefore restricts us to rigorously evaluate the data statistically. However the study with its sensitivity for PCa (N=140) detection was 99.3% with confidence interval of 96.1%–100%,and specificity for BPH was 100% (N=10) with confidence interval of 69.2% –100%validates our hypothesis that PCa malignant cells shed in voided urine without prostate stimulation, can be correctly identified for a reliable detection of PCa. Although the number of observations in this pilot study are limited, the results warrant a further evaluation to scrutinize if it could serve as a much needed simple and reliable assay to detect PCa noninvasively. Our long term goal therefore is to i) fully and statistically validate our hypothesis in patients known to have elevated PSA and PCa as proven by histology, ii) substantiate our initial observations in normal volunteers (21 years or older) and those with benign prostatic hyperplasia (BPH). Furthermore, the goal iii) is to determine a quantitative threshold above which a physician can distinguish with >95% confidence that a patient with elevated PSA has either PCa or BPH. Our long term goal is to minimize a number of unnecessary invasive biopsies, sparing the patient from anxiety and trauma and to reduce the expenditure of healthcare dollars.
As lifespan continues to increase, the number of PCa cases will increase dramatically in the aging population worldwide. Today, for diagnosis of PCa however, histology remains a gold standard which requires invasive biopsy, that is morbid, and 66% of the time finds benign pathology.
Although great strides have been made in detecting CTCs and urinary proteins, to diagnose PCa, the tests are either cost prohibitive, controversial or have not yet become widely acceptable for routine clinical practice. Due to the rarity of CTC (10–100 malignant-cells/ml blood) the detection, separation, and characterization of CTC remains a major challenge. Furthermore, the most commonly used method for identifying CTC’s is based upon enumeration of epithelial cells using antiepithelial cell adhesion molecule (EpCAM) antibody and subsequent staining for cell visualization. However, approximately 40% of the cancers have low EpCAM expression and 20% of the cancers are EpCAM negative. These parameters lead to low sensitivity of CTC detection (29). There remain therefore an unmet need for a simple and reliable test that can i) screen a patient, ii) serve as a diagnostic test, iii) reduce a number of unnecessary biopsies that can minimize patient morbidity and decrease the healthcare cost, and iv) play a vital role in the determination of the effectiveness of therapeutic intervention.
We believe that the present optical imaging assay promises to be simple, innovative, and specific for detecting VPAC1 expressing malignant PCa cells and demonstrates a potential to address the three, above stated important parameters needed for the management of genitourinary conditions of the aging population in North America and beyond.
Since this optical urinary assay requires only voided urine and does not need any patient physical contact, the assay has a fair probability to be of a clinical use provided that the results of the current evaluation continues to demonstrate >95% sensitivity and ability to distinguish between malignant and benign conditions. Once the urine optical imaging is positive, further VPAC based imaging tests can be performed to localize the disease.
VPAC receptors are known to express in many solid tumors, such as those of the breast, lung and colorectal. Success of such an optical screening liquid biopsy test may pave the way to detect shed malignant cells in nipple discharge to detect breast cancer, lung lavage to determine lung cancer, saliva to screen cancers of the head and neck and stool sample to detect colorectal cancer to name a few.
Conclusion
In patients with prostate cancer, malignant cells are shed in voided urine. These cells express VPAC receptors. Targeting VPAC receptors with high sensitivity, for optical imaging of these cells, may represent a novel diagnostic assay for an early, accurate and simple non-invasive detection of these malignancies. Such an assay once fully validated may play a vital role in the management of prostate cancer.
Acknowledgments
The research in part, was supported by NIH/NCI RO1CA157372 (M. L. Thakur, PI). The PI, M. L. Thakur thanks his colleagues for their enthusiastic support and continued collaboration. Ms. Anne Calvaresi, CRNP of the Department of Urology collected urine samples and Ms. Alice Johnny of the Department of Pathology and Molecular Cytology cytospun them. As a part of the SKCC core facility Drs. S. Addya and P. Fortina performed gene expression assays. Dr. Pardeep Kumar provided technical assistance as and when needed. Ms. Kim Lee assisted in the preparation of the manuscript. Their contributions are gratefully acknowledged. The PI also thanks NuView Life Sciences, Inc. for their interest in further development of this technology.
Footnotes
Author Contribution:
MLT conceived, demonstrated and established that VPAC genomic receptors can be targeted for scintigraphic and optical imaging of oncologic diseases. MLT and LG conceptualized that PCa Cells shed in urine can be imaged by targeting VPAC receptors. MLT gathered, coordinated the team of investigators and designed the protocol. SKT performed all the laboratory work including RNA extraction. MLT and SKT blinded to patient condition analyzed the data and presented the data for validation, to LG and EJT, who made the urine samples available. CS facilitated cytospun urine slides and participated in frequent discussion of results. EW supervised synthesis of TP4303. MLT and EJT wrote the manuscript.
Financial Disclosure:
Mathew Thakur and Leonard Gomella hold a pending patent through their employer, Thomas Jefferson University. At the time of this submission, Mathew Thakur is a consultant to NuView Life Sciences Inc. The other authors disclose no current conflict of interest.
References
- 1.Hessels D, Schalken A. Urinary biomarkers for prostate cancer: a review. Asian Journal of Andrology. 2013;15:333–339. doi: 10.1038/aja.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cristofanilli M, Hayes DF, Budd GT, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23:1420–1430. doi: 10.1200/JCO.2005.08.140. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer Facts & Figures. Atlanta: American Cancer Society; http://www.cancer.org/research/canceractsstatistics/cancerfactsfigures2015index. Updated 2015. [Google Scholar]
- 4.Cancer Research, UK, www.cancerresearch.uk.org. 2015
- 5.Bell N, Gorber SC, Shane A, et al. Recommendations on screening for prostate cancer with the prostate-specific antigen test. Canadian Medical Association Journal. 2014;186:1225–1234. doi: 10.1503/cmaj.140703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loeb S. Guideline of guidelines: prostate cancer screening. BJU Int. 2014;114:323–325. doi: 10.1111/bju.12854. [DOI] [PubMed] [Google Scholar]
- 7.Bryant RJ, Lilja H. Emerging PSA-based tests to improve screening. Urol Clin North Am. 2014;41:267–276. doi: 10.1016/j.ucl.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjurlin MA, Carter HB, Schellhammer P, et al. Optimization of initial prostate biopsy in clinical practice: sampling, labeling and specimen processing. J Urol. 2013;189:2039–2046. doi: 10.1016/j.juro.2013.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loeb S, Roehl K, Antenor JA, Catalona WJ, Suarez BK, Nadler RB. Baseline prostate-specific antigen compared with median prostate-specific antigen for age group as predictor of prostate cancer risk in men younger than 60 years old. Urology. 2006;67:316–320. doi: 10.1016/j.urology.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 10.Dijkstra S, Mulders PFA, Schalken JA. Clinical use of novel urine and blood based prostate cancer biomarkers: a review. Clinical Biochemistry. 2014;47:888–896. doi: 10.1016/j.clinbiochem.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Bryant RJ, Sjoberg DD, Vickers AJ, et al. Predicting high-grade cancer at ten-core prostate biopsy using four Kallikrein markers measured in blood in the ProtecT study. National Cancer Institute. 2015;107:1–6. doi: 10.1093/jnci/djv095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reubi JC, Laderach U, Waser B, Gebbers JO, Robberecht P, Laissue JA. Vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide receptor subtypes in human tumors and their tissues of origin. Cancer Res. 2000;60:3105–3112. [PubMed] [Google Scholar]
- 13.Lelievre V, Pineau N, Waschek J. Vaudry H, Arimura A. Pituitary Adenylate Cyclase-Activating Polypeptide. Endocrine Updates. Vol. 20. New York: Springer-Verlag; 2003. The biological significance of PACAP and PACAP receptors in human tumors from cell lines to cancer; pp. 361–399. [Google Scholar]
- 14.Zia H, Hida T, Jakowlew S, et al. Breast cancer growth is inhibited by vasoactive intestinal peptide, (VIP) hybrid, a synthetic VIP receptor antagonist. Cancer Res. 1996;56:3486–3489. [PubMed] [Google Scholar]
- 15.Leyton J, Gozes Y, Pisegna J, et al. PACAP (6–38) is a PACAP receptor antagonist for breast cancer cells. Breast Cancer Res Treat. 1999;56:177–186. doi: 10.1023/a:1006262611290. [DOI] [PubMed] [Google Scholar]
- 16.Said SI, Mutt V. Polypeptide with broad biological activity: isolation from the small intestine. Science. 1970;69:1217–1218. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- 17.Chakder S, Rattan S. The entire vasoactive intestinal polypeptide molecule is required for the activation of the vasoactive intestinal polypeptide receptor: functional and binding studies. J Phar and Expt Therapeutics. 1993;266:392–399. [PubMed] [Google Scholar]
- 18.Thakur ML, Zhang K, Berger A, et al. VPAC1 receptors for imaging breast cancer: a feasibility study. J Nucl Med. 2013;54:1019–1025. doi: 10.2967/jnumed.112.114876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tripathi SK, Trabulsi EJ, Gomella L, et al. VPAC1 targeted Cu-64-TP-3805 PET imaging of prostate cancer: preliminary evaluation in man. Urology. 2016;31:29–36. doi: 10.1016/j.urology.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thakur ML, Aruva M, Gariepy J, et al. PET imaging oncogene overexpression using 64Cu-VIP analog: comparison with 99mTc-VIP analog. J Nucl Med. 2004;45:1381–1389. [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang K, Aruva M, Shanthly N, et al. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating peptide (PACAP) receptor specific peptide analogues for PET imaging of breast cancer: in vitro/in vivo evaluation. Regul Pept. 2007;144:91–100. doi: 10.1016/j.regpep.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thakur ML, Devadhas D, Zhang K, et al. Imaging spontaneous MMTVneu transgenic murine mammary tumors: targeting metabolic activity versus genetic products. J Nucl Med. 2010;51:106–111. doi: 10.2967/jnumed.109.069542. [DOI] [PubMed] [Google Scholar]
- 23.Zhang K, Aruva MR, Shanthly N, et al. PET imaging of VPAC1 expression in experimental and spontaneous prostate cancer. J Nucl Med. 2008;48:112–121. doi: 10.2967/jnumed.107.043703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandran UR, Ma C, Dhir R, et al. Gene expression profiles of prostate cancer reveal involvement of multiple molecular pathways in the metastatic process. BMC Cancer. 2007;7:64. doi: 10.1186/1471-2407-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gregg JL, Brown KE, Mintz EM, Piontkivska H, Frazier GC. Analysis of gene expression in prostate cancer epithelial and interstitial stromal cells using laser capture microdissection. BMC Cancer. 2010;10:165. doi: 10.1186/1471-2407-10-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Australian Medical Journal. 1869;14:146–147. [Google Scholar]
- 27.Butler TP, Gullino PM. Quantitation of Cell Shedding into Efferent Blood of Mammary Adenocarcinoma. Cancer Research. 1975;35:512–526. [PubMed] [Google Scholar]
- 28.Valdehita A, Bajo AM, Fernandez-Martinez AB, et al. Nuclear localization of vasoactive intestinal peptide (VIP) receptors in human breast cancer. Peptides. 2010;31:2035–2045. doi: 10.1016/j.peptides.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 29.Castro CM, Im H, Le C, Lee H, Weissleder R, Birrer MJ. Exploring alternative ovarian cancer biomarkers using innovative nanotechnology strategies. Cancer Metastasis Rev. 2015;34:75–82. doi: 10.1007/s10555-014-9546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]