Summary
Blood transfusion plays a prominent role in the management of patients with sickle cell disease (SCD), but causes significant iron overload. As transfusions are used to treat the severe complications of SCD, it remains difficult to distinguish whether organ damage is a consequence of iron overload or is due to the complications treated by transfusion. Better management has resulted in increased survival, but prolonged exposure to iron puts SCD patients at greater risk for iron-related complications that should be treated. The success of chelation therapy is dominated by patient adherence to prescribed treatment; thus, adjustment of drug regimens to increase adherence to treatment is critical.
This review will discuss the current biology of iron homeostasis in patients with SCD and how this informs our clinical approach to treatment. We will present the clinical approach to treatment of iron overload at our centre using serial assessment of organ iron by magnetic resonance imaging.
Keywords: sickle cell disease, anaemia, chelator, iron overload, management
Introduction
Sickle cell disease (SCD) is an inherited chronic haemolytic anaemia that results from a single amino acid substitution in the β-globin chain, producing the abnormal haemoglobin-S (HbS). Unlike normal haemoglobin, HbS polymerizes at low oxygen tension, triggering the conversion of the normally flexible red blood cell (RBC) into a rigid, crescent or “sickled” shape RBC that obstructs blood flow in the microcirculation. This sickling process is continual; however, episodic exacerbations occur that result in severe vaso-occlusion and pain, pulmonary failure and stroke. The median survival for SCD patients in the United States is about 42 years, with significant pre-morbid complications (Powars, et al 2005), although recent data suggest survival may be better in some western countries (Gardner, et al 2016). Currently, about 95% of children in the US and Europe survive until 18 years of age (Quinn, et al 2010), but have significant vascular compilations by age 20 years as well as chronic organ failure leading to premature death in their fourth and fifth decade (Bernaudin, et al 2015, Powars, et al 2005) A high proportion of adults suffer from severe, chronic pain that significantly diminishes their quality of life (Smith, et al 2005, Smith, et al 2008). About 240,000 children born annually in Africa have SCD, and only 20% survive to their second birthday (Makani, et al 2011). All of the morbidity seen in SCD is due to vascular disease and tissue necrosis that occur as a consequence of the chronic haemolytic anaemia (Detterich, et al 2015, Novelli and Gladwin 2016).
Blood transfusion plays a prominent role in the management of patients with SCD, but causes significant iron overload (Ballas 2001, Fung, et al 2007, Puliyel, et al 2014, Vitrano, et al 2016, Wood, et al 2005). Chronic transfusions are used to treat patients with severe complications of SCD. Despite the significant morbidity associated with iron overload (Ballas 2001) it remains difficult to distinguish whether organ damage in SCD is a consequence of iron from transfusions used to treat SCD complications or due to the complications themselves. We know from experience with thalassaemia, where the causal relation of iron overload to mortality is clear (Berdoukas and Modell 2008, Modell, et al 2008, Modell, et al 1982), that iron overload is toxic, and can be lethal. Nevertheless, we also know that the same degree of iron loading is less toxic in SCD than in thalassaemia (Vichinsky, et al 2005, Walter, et al 2006). Some investigators have even questioned whether treatment of iron overload in SCD is advisable (Lucania, et al 2011), although it is strongly recommended by the United States National Institutes of Health Guidelines based on evidence of moderate quality (Yawn, et al 2014) and iron may be responsible for up to 11% of deaths in SCD subjects (Darbari, et al 2006, Perronne, et al 2002). While rare, iron cardiomyopathy is detectable in about 2.5% of chronically transfused SCD patients (Meloni, et al 2014), and it is one iron toxicity that is separable from SCD damage, indicating that iron can cause serious health issues in some SCD patients. We suspect that the incidence of iron-related complications is actually higher than reported because the diagnosis is often not considered and the magnetic resonance imaging (MRI) methodology for tissue iron detection is not readily available. Iron toxicity is related to the duration and severity of iron overload and, over decades, can result in multiple problems including malignant transformation. As survival is improving in adults with SCD, we feel that iron overload should ideally be treated with the goal of bringing iron levels to a normal range. This opinion is based largely on the general effect of iron on survival and the strong association with malignant transformation [reviewed in (Coates, et al 2016)].
This review will discuss the current biology of iron homeostasis in humans and how this new knowledge has informed our thinking and has modified our approach to clinical management in this population with transfusional iron overload. We will present the clinical practice at our centre, where we follow approximately 120 patients on chronic transfusion each year, from early childhood to 40 years of age. Our approach relies heavily on ready access to serial assessment of organ iron by MRI, established at our centre since 2003.
Transfusion in SCD
In general, transfusion is used to treat symptoms of anaemia or to stop or prevent complications of SCD-related vaso-occlusion. There is general agreement for using transfusion to prevent strokes in children, to treat severe acute chest syndrome, and as a preoperative precaution (de Montalembert, et al 2011, Habibi, et al 2015, Yawn, et al 2014). About 20% of SCD children in the US are on regular transfusion programmes; however, the use of transfusions in SCD is variable around the globe, as is the practice of chelation (Vichinsky, et al 2011a, Vichinsky, et al 2011b).
SCD subjects do not hyper-absorb iron, as is the case in thalassaemia. In fact, intermittently transfused SCD patients can lose up to 5.5 mg of iron per day in their urine, presumably because of haemoglobinuria, accounting for about 10 units of RBC per year (Inati, et al 2009). Each millilitre of packed red cells contains about 0.8 mg of iron as haemoglobin, directly linking the rate of iron loading with transfusion to the rate of transfusion. Iron loading from transfusion occurs under two circumstances: 1) regular transfusions, about every three weeks, with the intent of lowering HbS to < 30% and thereby preventing SCD complications; and 2) episodic transfusions for anaemia, usually during hospitalisation. Iron overload is anticipated in patients on chronic transfusion and treatment is more likely to be prescribed. With episodic transfusion, iron overload is usually not recognized and often not treated (Ballas 2001). Because of the uncertainty of transfusion history in SCD patients, ferritin levels should be obtained annually in any SCD patient who may have been transfused to screen for possible iron overload.
Iron homeostasis and toxicity in humans
The field of transfusional iron overload has significantly progressed in the past two decades, thanks to advances in our understanding of iron transport in mammals as well as by the ability to non-invasively monitor iron burden in humans by MRI (Wood 2015). It is clear from serial MRI testing that iron loading and unloading occurs at significantly different rates in different tissues (Anderson, et al 2004, Noetzli, et al 2011, Noetzli, et al 2012). Liver iron concentration (LIC) is highly correlated (r2 = 0.98; p<0.001) with total body iron (Angelucci, et al 2000); however, there is a very poor correlation between LIC and pancreatic, pituitary or cardiac iron concentrations, indicating that besides total iron, other factors control iron trafficking in these organs. In response to intensive iron chelation, the LIC can be reduced by 50% in 1.5 months, whereas it takes about 13 months to remove half of the iron from the heart (Anderson, et al 2004). Pancreatic loading and unloading rates are intermediate. These significant differences in loading and unloading between various tissues (see Figure 1), derived from serial MRI measurements in iron-loaded humans, are consistent with very elegant biochemistry studies of iron regulation over the past two decades [reviewed in (Coates 2014)], and have a direct impact on the diagnosis, monitoring and treatment of SCD patients with iron overload, as summarized below.
Figure 1.
Temporal partitioning of total body iron (liver iron concentration, LIC) into pancreas and heart in a chronically transfused sickle cell patient who was minimally adherent to chelation. Serial MRI measurements of the organ iron are plotted (left axis) as fold change relative to normal. The pancreas iron (normal = 27 Hz) reaches the level associated with glucose intolerance (100 Hz) at 18.8 years of age, well before the cardiac T2* drops below 20 ms at about 21 years. The left ventricular ejection fraction (LVEF) by magnetic resonance imaging (MRI) drops at age 23 years.
Biological organisms have evolved to conserve iron, an essential metal for numerous biochemical processes in humans, and thus, humans have no mechanisms to excrete iron. Approximately 1 to 2 mg iron per day (0.05% of total body iron) is lost through desquamation of the gastro-intestinal tract lining and skin, and in smaller amounts, through blood loss (Green, et al 1968). Iron balance is maintained entirely through the regulation of dietary iron absorption, primarily in the duodenum, and the recycling of iron from RBC. About 25 mg of iron are reclaimed daily through the phagocytosis and degradation of senescent autologous or transfused RBC by macrophages. The reclaimed iron in the macrophage is in the ferrous state (Fe+2), and is called labile cellular iron (LCI). LCI increases the levels of ferritin and is either utilised by the cell, taken up and buffered by ferritin, or exported to the plasma via ferroportin (FPN), the cellular iron exporter. Ferritin, while primarily intracellular, can leak into the plasma through damaged cell membranes. Thus, serum/plasma ferritin levels are a rough measure of iron loading. Ferritin also serves as a major intracellular buffer of toxic Fe+2 by converting it to the ferric state (Fe+3). Haemosiderin, which is made up of large aggregates of ferritin, is the primary species that is detected by MRI [reviewed in (Coates 2014, Frazer and Anderson 2014, Ganz 2013)].
Normally, Fe+3 is bound to transferrin in the circulation, and it enters cells by receptor-mediated endocytosis via the transferrin receptor, TfR1, also termed TFRC. TfR1 transcription decreases when Fe+2/LCI increases, thereby preventing cellular iron overload. However, when total body iron increases dramatically, as it is the case in multiply transfused patients, the transferrin-binding ability is rapidly exceeded, and circulating non-transferrin bound iron (NTBI) appears in the plasma. NTBI rises considerably when the transferrin saturation reaches around 60%, and a highly reactive Fe+2 subspecies of NTBI called labile plasma iron (LPI) increases concomitantly. LPI can enter cells through ion transporters that are normally designed to carry divalent cations like zinc and calcium. For the most part, these ion transporters are not regulated by intracellular iron concentration; thus, iron loading proceeds, even when cytosolic iron levels are very high. The iron transport through these channels is organ-specific and may explain why the rate of loading observed by serial MRI is different (liver>pancreas>heart) (Coates 2014, Khamseekaew, et al 2016, Murphy and Oudit 2010). While most information regarding the physiology of cardiac iron loading is based on studies in animals, the recent demonstration that the calcium channel blocker amlodipine decreases cardiac iron loading in patients with thalassaemia suggests that this mechanism is also operative in humans (Fernandes, et al 2016).
The flow of iron from enterocytes and macrophages into the plasma is regulated by hepcidin, a 25 amino acid, defensin-like peptide that is made in the liver and binds to FPN, thereby causing FPN internalization and degradation (Nemeth 2010). Hepcidin is elevated in iron overload and inflammatory states (Ganz 2013, Nemeth, et al 2003). While inflammation increases hepcidin and blocks iron release into the plasma (Ganz and Nemeth 2012), hypoxia, anaemia and erythropoiesis reduce hepcidin production, increasing the release of Fe+2 into the plasma and iron absorption. Thus, the state of bone marrow activity has a significant impact on hepcidin production and on the levels of reactive LPI.
The toxicity from iron is due to NTBI/LPI and LCI, the Fe+2 reactive forms of iron, and is mediated through production of reactive oxygen species (ROS), either through direct effects or through ROS signalling. Fe2+ reacts with ROS, such as hydrogen peroxide (H2O2) to produce hydroxyl radical (HO•; Fenton reaction). HO• is a potent oxidant that can react rapidly with most molecules, including DNA, thereby permanently altering genetic material. In conditions of iron overload when NTBI and LPI/LCI levels are high, and during inflammation when high levels of ROS are produced, severe oxidant damage to tissues can occur [reviewed in (Koskenkorva-Frank, et al 2013)].
Clinical evidence also supports the idea that NTBI/LPI is the toxic form of iron. NTBI/LPI represents the pool of iron that is immediately accessible to chelators, and NTBI/LPI levels in the blood can be reduced to near zero within minutes to hours of starting chelation with deferoxamine (DFO) (Porter, et al 1996) (see below). When patients with severe myocardial iron loading and decreased left ventricular function were treated with continuous iron chelation therapy, normalization of ejection fraction occurred within three months of starting chelation, even though the cardiac iron levels detected by MRI remained remarkably elevated. Thus, chelating the NTBI/LPI was sufficient to improve cardiac function (Anderson, et al 2004), and having a chelator in the circulation can be protective, even before tissue levels of iron have been reduced.
Iron loading and toxicity in SCD
Generally, the toxicity of iron overload is substantially less in patients with SCD than in patients with thalassaemia. When populations of SCD and thalassaemia patients who had equal exposure to blood transfusion and equal LIC were compared, thalassaemia patients had a 3.5-fold greater risk of developing heart failure and hypogonadism after controlling for differences in transfusion duration (Vichinsky, et al 2005), consistent with the known lower levels of NTBI/LPI in SCD (Koren, et al 2010, Porter, et al 2016) and the role played by LPI in toxicity [reviewed in (Coates 2014, Porter and Garbowski 2014)]. Nonetheless, we have followed several SCD patients who had clear-cut, clinically symptomatic iron cardiomyopathy (Meloni, et al 2014). Interestingly, SCD patients with significant cardiac iron loading (T2* < 20 ms) have surprisingly low HbS concentration and reticulocyte counts in the 3 to 10% range (Meloni, et al 2014). Iron is toxic to the bone marrow, probably because iron and oxidants play a role in causing ineffective erythropoiesis (Breda and Rivella 2014, Camaschella and Nai 2016, Dussiot, et al 2014, Gardenghi, et al 2007, Rivella 2009). Thus, we suspect that the surprisingly low HbS levels in these extremely overloaded SCD patients are due to iron-mediated marrow toxicity, resulting in an ineffective erythropoiesis phenotype that favours development of iron cardiomyopathy.
The occurrence of iron cardiomyopathy clearly shows that iron toxicity arises in SCD. One of our SCD patients, a 24-year-old woman who was in clear clinical congestive heart failure (CHF) with a cardiac T2* < 10 ms and an ejection fraction of 45% by MRI, was asymptomatic 3 weeks after starting aggressive chelation with deferiprone, essentially proving her CHF was related to her high levels of cardiac iron. While we are aware of several other cases of cardiac iron in SCD and are convinced that iron overload is a serious contributor to morbidity in SCD in general, we have yet to find a published case series that clearly separates iron-related toxicity from SCD-related complications. The toxicity of iron depends on the tissue iron concentration, the duration of the exposure, and the adequacy of the individual anti-oxidant systems. In general, measurable changes in organ function due to iron exposure require years to develop, though the exact timing is not known.
Regardless, as survival in SCD is increasing (Gardner, et al 2016), the lifetime exposure to iron will increase and we can expect iron-related complications to become more frequent in these patients, as is the case in thalassaemia (reviewed in (Coates, et al 2016)).
Principles of iron overload management in SCD
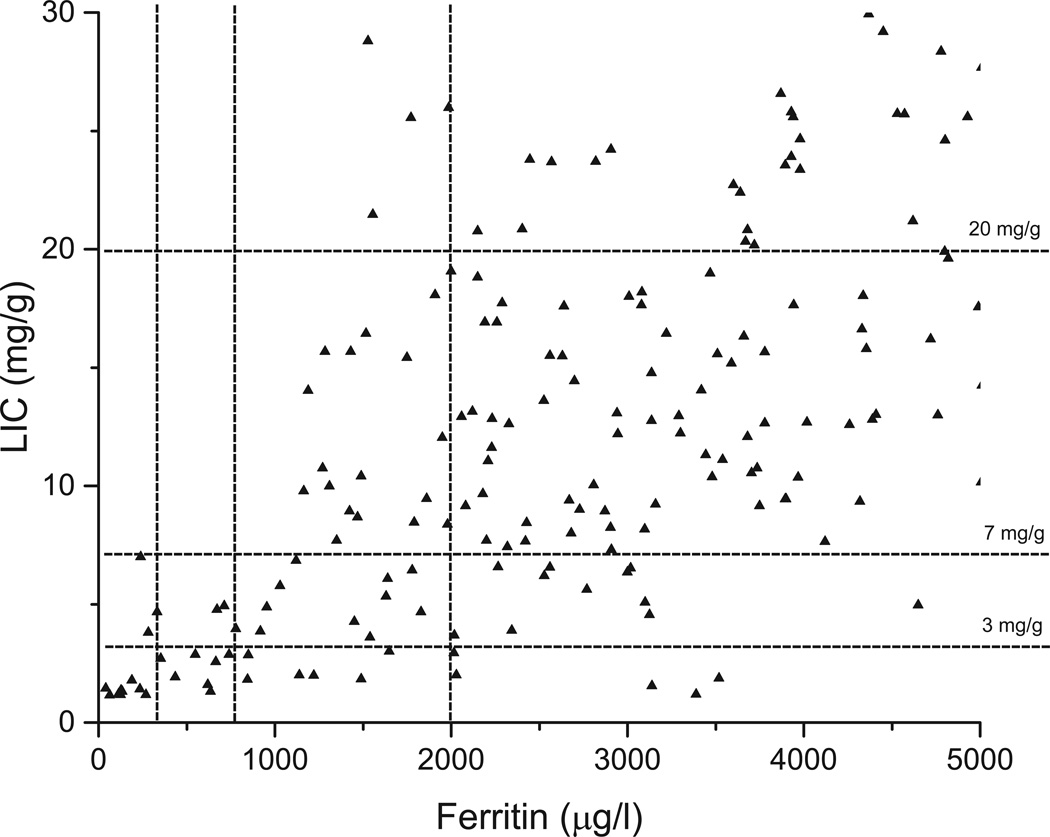
While there are some differences in iron homeostasis between SCD and other transfusion-dependent anaemias, the approach to chelation therapy and monitoring of the overload is not different. The treatment goals for iron overload are to reduce plasma and cytosolic levels of reactive labile iron (Fe+2;NTBI/LPI) as quickly as possible, and to remove all excess iron from the body. In our opinion, regardless of the underlying disease process, the major goal of treatment should be to maintain these reactive forms of iron in the normal range throughout life. If this can be achieved, most of the complications of iron overload can be reduced or even eliminated (Coates, et al 2016, Farmaki, et al 2010, Kolnagou, et al 2010, Kolnagou and Kontoghiorghes 2010a, Kolnagou and Kontoghiorghes 2010b, Kontoghiorghes 2010). However, achieving this goal without risking unacceptable treatment toxicity may not be possible, and some experts do not agree with trying to keep iron at near normal levels. Maintaining iron levels in the low range (LIC < 3 mg/g) should only be attempted at centres with the ability to monitor organ iron by MRI and expertise in chelation. These levels cannot be safely achieved using ferritin measures alone as a surrogate for organ iron loading.
Diagnosis and monitoring of patients with iron overload
The methods for clinical diagnosis and monitoring of iron overload are summarized in Table I. Ferritin levels and transferrin saturation are simple tests that have been used for many decades to diagnose and monitor iron overload. Intracellular ferritin levels increase in response to LCI, but are also significantly increased by interleukin 6 in response to inflammation. Thus, the blood levels of ferritin reflect the degree of cellular membrane leakage, the LCI and the degree of inflammation. A good correlation has been found between ferritin levels and total iron, but only in patient populations. The relation of ferritin to total iron in individual patients is poor and the trends in ferritin are opposite to the trends in total iron 23% of the time (Aubart, et al 2016, Puliyel, et al 2014). Figure 2 shows the scatter in ferritin at low LIC and demonstrates that attempting to achieve tight iron control (LIC < 3 mg /g) with ferritin alone would increase the risk of over chelation. Ferritin cut-off levels have been published that reduce the risk of over-chelation when ferritin alone is used to estimate LIC (Taher, et al 2014). If MRI LIC measures are available, we use these values to adjust chelation, regardless of the ferritin. Because of its great variability, ferritin should be measured frequently and only the trends over many measurements should be used for any therapeutic decision-making. We routinely measure ferritin after each transfusion, usually every three weeks. Generally, we recommend acquiring LIC measurements annually, and sooner if ferritin trends are not consistent with the clinical circumstance (Table I). Critical treatment decisions based on ferritin alone should be made with great caution, especially at low LIC levels.
TABLE I.
Monitoring Iron Overload
| Ferritin µg/l |
LIC (MRI) mg/g dw |
Cardiac T2* | R2* ms/s |
Pancreatic R2* Hz (Noetzli et al 2011) |
Comments | |
|---|---|---|---|---|---|
| Key values | Normal: 25–300 Low: 300–800 Moderate: 800–1700 High: 1700–2500 Very high: >2500 |
Normal :0.8–1.5 Low: 1.5–3.5 Mild: 3.5–5.0 Moderate: 5.0–0 High: 10–20 Very high: >20 |
Normal: >30 | <33 Low: 20–30 | 50–33 Moderate: 10–20 | 100–50 Moderate-severe: 8–10 | 100–125 Severe: 6–8 | 125–167 Very severe: <6 | >167 |
Normal: <40 Moderate: 40–100 Severe: 100–300 Very severe: >300 |
R2 = 1000 × 1/T2 R2* = 1000 × 1/T2* Heart Fe mg/g tissue = 45 x (T2*)1.22 Liver R2 and R2* are highly correlated with each other, but can diverge so use one or the other or the average of both. |
| First study | >10 transfusions Suspect iron loading |
Ferritin >500 Suspect iron loading |
Pancreatic R2* >100 or LIC >20 especially if reticulocytes and %HbS persistently <15% (Meloni, et al 2014) (b) |
(a) When LIC is done |
(a) Can be measured in same image plane as LIC (b) Chronically low reticulocytes and %HbS suggests Fe toxicity to marrow and switch to ineffective erythropoiesis. |
|
Start chelation |
>1000 | > 3.5 | (<20) | (>100) | (Not usually part of the decision to initiate therapy) |
|
Monitoring Frequency |
With each transfusion, at least monthly |
(a) 12–18 months. If ferritin trends don’t fit with clinical picture Low – Nl every 6 to 8 months. |
(b) Annual or when “first study” criteria persist. Depends on last value. Much more often in mod or worse iron cardiac iron |
With each LIC measurement |
(a) Depends on initial LIC, less often if LIC is moderate-high, every 6 months when first entering the mild to low LIC range. LIC is the main marker for over-chelation risk. (b) Once cardiac loading starts, it can progress quickly. Every 4–6 months is suggested for moderate- severe. |
|
Consider Chelation Combination (see Table IV) |
(a) >1700 or no apparent response after 6 to 12 months on adequate monotherapy |
(b) High or greater LIC with apparent inadequate response. |
Add DFP if T2* < 10 Arrhythmia or failure is an emergency. Follow AHA guidelines (Pennell, et al 2013). |
Not a major factor in starting combination therapy. |
(a) We would confirm by LIC and prefer not to use ferritin alone. (b) LIC is main determinant of all treatment dose decisions. |
|
Therapy changes (see Table IV) |
<2000 (a) single agent < 1000 reduce dose < 800 reduce dose, hold depending on transfusion burden |
Moderate-mild ⇒ monotherapy. Normal (a) -low ⇒ decrease dose |
(b) Low or higher cardiac iron levels ⇒ adjust dose to even out plasma chelator levels (e.g. BID DFX). |
(b) Moderate or higher ⇒ adjust dose to even out plasma levels (e.g. BID DFX). |
(a) If only ferritin is available (Taher, et al 2015). Otherwise, base decision on LIC regardless of ferritin level. (b) If slower rate of iron reduction, adjust to eliminate valleys in plasma chelator levels even if LIC is low. |
Superscripted letters in parentheses (x) refer to comments in the far-right column of the table on the same line.
AHA, American Heart Association; BID, twice daily; DFP, Deferiprone; DFX, Deferasirox; LIC, liver iron concentration; MRI, magnetic resonance imaging. .
Figure 2.
Ferritin levels in patients with sickle cell disease show very broad scatter. The liver iron concentration (LIC) levels at 3, 7 and 20 mg/g dry weight liver correspond to levels that have been related to ferritin in Table IV (Taher et al, 2015).
Transferrin saturation is the only common test that reflects the toxic NTBI/LPI pool. If the transferrin saturation is greater than 50%, NTBI/LPI levels are likely to be high, and if it is >70%, NTBI/LPI levels are significantly elevated (Porter, et al 2016, Sahlstedt, et al 2001). Transferrin saturation has been used to screen for iron overload in epidemiology studies and can predict long-term complications of iron overload in large populations [reviewed in (Coates, et al 2016, Puliyel, et al 2015)]. However, transferrin saturation can change literally within minutes to hours, and should be assessed while the patient is fasting. It can go from 5% to 60% and back down to 5 % within 60 min of a single oral iron dose, and it drops as quickly after a dose of iron chelator. As the half-life of NTBI/LPI inferred by transferrin saturation is very short, transferrin saturation and direct LPI measurements are of limited help for monitoring iron overload therapy (de Swart, et al 2016, Porter, et al 2016). The parameter we would like to have is the area under the curve of exposure to NTBI/LPI over time.
LIC accurately reflects total body iron loading (Angelucci, et al 2000), and is best measured by MRI imaging (Wood 2015). LIC measurements can be accurately made on commercially available 1.5 Tesla MRI machines that are equipped with the proper software packages. However, significant errors in quantitation can occur if the radiology centre has not been properly trained to make these measurements. The MRI scanner measures relaxation times that are described as T2 and T2* (“t two star”) and expressed in milliseconds. T2/T2* decreases in a non-linear fashion as iron increases. R2 and R2* are the reciprocals of T2 and T2*, respectively, and increase as iron increases. While R2 and R2* are highly correlated, they cannot be used interchangeably (Wood, et al 2015). Table I shows the relationships between these parameters as well as the conversion factors to iron concentration. A normal LIC is between 0.8 and 1.5 mg/g dry weight of liver. Quantitation by MRI becomes very unreliable at LIC > 35 mg/g. Details of MRI techniques have recently been reviewed (Wood 2015). Many centres are now routinely using 3 Tesla (3T) MRI machines because of better image resolution. Iron measurements on 3T MRI machines require special calibration that is not routinely available, but is under development (Wood 2015).
MRI has allowed us to study partitioning of iron into multiple organs, most importantly, the heart. Cardiac iron must be measured directly by MRI, as it cannot be predicted accurately based on LIC values. In thalassaemia, cardiac T2* of 8 ms or less predicts arrhythmia and heart failure (Kirk, et al 2009, Wood 2011). While significant cardiac iron overload occurs only in about 2.5% of SCD patients, it is life threatening and requires a more intensive approach to chelation, using guidelines established in thalassaemia patients (Pennell, et al 2013). Certainly, cardiac T2* should be measured in any SCD patient who has had significant liver iron loading (LIC > 20 mg/g) over many years or a pancreatic R2* > 100 Hz (Table I).
Pituitary iron, pituitary volume, pancreatic iron and renal iron can also be measured by MRI, but they are not standard or routinely available. Our own studies have demonstrated the usefulness of these measures (Wood 2011, Wood 2014). In particular, pancreatic iron deserves more attention. Pancreas iron measures can be conveniently obtained with the MRI images taken for LIC quantitation. The detection of iron in the pancreas gives us some sense of the “area under the curve” exposure to toxic LPI, as the pancreas only loads when there has been persistent exposure to LPI. Pancreatic R2* reflects the “area under the curve” exposure to LPI, in the same way that HbA1c reflects glucose exposure in diabetes. Mild levels of pancreatic iron were seen in 38% of SCD patients in the TWiTCH (Transcranial doppler With Transfusions Changing to Hydroxyurea) trial, but no patient had pancreas R2* exceeding 100 Hz, the value associated with pancreatic damage and cardiac iron deposition(Wood, et al 2016). This observation paralleled our single centre results (Noetzli, et al 2011) and is consistent with the higher levels of hepcidin and lower levels of LPI in SCD patients (Koren, et al 2010, Porter, et al 2016). Interestingly, all SCD patients with cardiac iron had pancreatic R2* >100 Hz, with some as high as 450 Hz (Meloni, et al 2014). In fact, we have never observed cardiac iron loading in the absence of pancreatic iron loading, though the converse certainly is not true (Noetzli, et al 2009). Thus, if pancreatic iron is not detectable, we believe it is not necessary to assess cardiac iron.
While tissue-specific measures of iron correlate with organ function, it is still important to serially monitor organ function. It is very difficult to make specific recommendations for patients with SCD, as there is no clear association between iron overload and end organ failure. The liver is a primary target for iron toxicity in the third decade and beyond, and liver function needs to be monitored. We suggest an annual panel consisting of monitoring of thyroid function, glucose metabolism, morning cortisol, adrenocorticotropic hormone and sex hormones in severely iron-loaded SCD patients (LIC > 20 mg/g), and certainly if pancreatic, pituitary or cardiac iron has been detected. We do not recommend routine monitoring of echocardiograms or electrocardiograms to detect iron-related cardiac pathology.
Severely iron-loaded SCD patients had low levels of thiamine (38.5% of patients), ascorbate (56.7%), vitamin A (73.7%), selenium (67.5%) and pyridoxine (34.2 %) (Claster, et al 2009), although we do not understand the mechanism behind these findings. We monitor these micronutrients annually in all severely iron-loaded patients and prescribe replacement therapy if the levels are low.
Treatment of iron overload
Obviously, reducing the amount of transfused blood can help decrease iron loading. Phlebotomy has been the mainstay for treatment of iron overload in settings where marrow function is normal, and can be considered in SCD patients who have adequate elevation of their haemoglobin on hydroxycarbamide/hydroxyurea (Aygun, et al 2015, Ware, et al 2016). In chronically transfused patients, red cell exchange (RCE) transfusion significantly reduces iron loading and maintains HbS levels in a therapeutic range (Danielson 2002, Kim 2014, Yawn, et al 2014). If the patients have adequate venous access, this approach can take less time than simple transfusion, may permit keeping HbS at a low level when used at four to five week intervals, and can leave a patient in neutral iron balance without the need for chelators. However, if the patient is already significantly iron overloaded, chelation will have to be added. Programmatic administrative costs, amount of blood required and venous access can be major barriers (Kim 2014). Nonetheless, this approach should be considered in all SCD patients who require long-term chronic transfusion. Partial exchange transfusion approaches where a moderate amount of blood is drawn and replaced with saline at the time of simple periodic transfusion is favoured by some providers, but its benefits are not well documented (Fasano, et al 2016, Savage, et al 2013).
Iron chelation is the primary treatment for transfusional iron overload in patients with SCD. The agents and their toxicities are listed in Table II and III). Treatment should start when patients have received 10 transfusions, have a serum ferritin > 1000 µg/l, or LIC > 3 mg/g dry weight liver (Table I and IV). We do not start chelation in children before two years of age and increase the dose over a period of one year to avoid toxicity. Currently, there are three licensed iron chelators available in the US and Europe. These agents along with their properties are listed in Table II. There are decades of experience with these agents, showing that they are effective at reducing iron in both SCD and thalassaemia patients (Aydinok, et al 2015, Calvaruso, et al 2014, Hoffbrand, et al 2012, Maggio, et al 2011, Piga, et al 2013, Tsouana, et al 2015, Vichinsky, et al 2011c). There is substantial evidence that these agents improve the clinical outcome in thalassaemia(Modell, et al 2008). As it is extremely difficult, if not impossible, to separate the complications of iron overload from those of SCD, there are minimal and conflicting data suggesting chelation improves SCD outcomes (Jordan, et al 2015, Vekeman, et al 2016, Vitrano, et al 2016).
TABLE II.
Chelators
| Deferoxamine (DFO) | Deferiprone (DFP) | Deferasirox (DFX) | |
|---|---|---|---|
| Molecular weight | 560 | 139 | 373 |
| First clinically available | 1968 | 1999 | 2005 |
| Route of administration | Parenteral | Oral (tablet or solution) | Oral Dispersible tablet (ExJade) Film-coated tablet (Jadenu) |
| Plasma half-life | 30 min | 3 h | 8–16 h |
| Usual dose | 40–50 mg/kg/day | 75–100 mg/kg/day | 20–40 mg/kg/day (ExJade) 14–28 mg/kg/day (Jadenu) |
| Administration | Over 10 to 24 h Should be continuous infusion over 24 hours in heart failure. |
Every 8 h; TID | Once daily; used BID to improve tolerance and perhaps efficacy, but no clear data on BID dosing yet. |
| Route of excretion | Urine, faecal especially at higher dose |
Urine | Faecal |
|
Use in decreased renal function |
Can be used, dialysable | Can be used, dialysable | Nephrotoxic. Should not be used unless totally dialysis dependent. |
| Removal of cardiac iron | Effective especially by continuous infusion |
Most effective at removal of cardiac iron |
Effective over long periods of time |
| Cardiac function | Effective by continuous infusion |
Most effective for improving cardiac function, even at high levels of cardiac iron. |
Functional improvement has not been demonstrated. |
| Reduction in total Fe (LIC) | All three agents are effective as single agents at the high end of the dose ranges above. Higher doses required with higher transfusion burden. |
||
| Advantage | Long experience, can be given intravenously |
Most effective for cardiomyopathy |
Longest half life, can be given once a day |
| Toxicity | Local reactions, severe allergic reactions, retinal damage, hearing loss, osteoporosis, growth failure |
Gastrointestinal, arthralgias, transient transaminitis, rare idiosyncratic agranulocytosis |
Increase GFR in 30%, proteinuria, renal failure is rare, moderate gastrointestinal toxicity, rare gastrointestinal bleeding. |
BID, twice daily; GFR, glomerular filtration rate; LIC, liver iron concentration; TID, three times daily.
TABLE III.
Chelator Toxicity
| Toxicity | Incidence | Management |
|---|---|---|
| Desferal (DFO) | ||
|
Injection site reaction |
Common | Make sure a small subcutaneous needle is used that is perpendicular to the skin and goes all the way through the dermis. Intra-dermal injection is a major cause of local reaction. Rotate the injection sites. Lower the DFO concentration. A small amount of hydrocortisone can be put into the DFO, but should be avoided. |
| Anaphylaxis | Rare | This is a rare serious reaction. If the patient has systemic allergic symptoms from DFO, we would discontinue the drug. Desensitization can be done. However, given the adherence issues with DFO, patients stop the DFO and then restart several days later and can have a serious reaction. We do not recommend desensitization. |
| Infection | Rare | Increased risk of infection with ferophilic organisms like Yersina Enterocolitca, V. Vulcanificus and Mucorales |
| Retinal / Auditory | Rare | Change in colour perception or visual impairment should prompt immediate stopping of DFO. May restart when symptoms resolve. DFP and DFX essentially do not have these complications (isolated cases) and are alternatives. |
| Deferiprone (DFP) | ||
| Gastrointestinal | 33 % at first |
Symptoms are usually mild and transient. Starting at a lower dose (40 mg/mg/day) and increasing over a period of weeks to months can help. Liquid formulation may be better tolerated. DFP is well tolerated and gastrointestinal symptoms usually resolve after month or two. |
| Transaminitis | 7% | Transaminases can be 2 to 4 times normal levels from severe iron overload. There may be transient increase in the first few months of DFP treatment. If there is significant increase, hold the drug and restart at lower dose after return to baseline levels to see if elevations are truly due to DFP. Evidence of cholestasis suggests other pathology. |
|
Neutropenia (ANC <1.5 × 109/l) |
8% | Hold medication until recovery and re-challenge. Make sure patients know to stop DFP and seek medical attention for any fever/mouth sores. |
|
Agranulocytosis (ANC <0.5 × 109/l) |
1.5% | Most cases occur during first year on DFP. Make sure patients know to stop DFP if any significant fever or mouth sores and seek immediate medical attention and notify the emergency personal they are on a drug that causes agranulocytosis. They should be treated with parenteral antibiotics if febrile. GCSF may be helpful. |
| Arthropathy | 3.9–40% | More likely at high LIC. Stop DFP and restart at lower dose. Treat with antiinflammatory agents. |
| Deferasirox (DFX) | ||
| Gastrointestinal | 15% | Major toxicity of DFX. Starting at lower dose increasing slowing can help with nausea. LactAid can help some with lactose intolerance. Splitting the dose twice a day can also help. Some patients find taking the dose at night is helpful. The new formulation Jadenu, is much better tolerated and does not contain lactose. Significant gastrointestinal bleeding is rare but has occurred and the risk may be higher at low LIC levels. Patients should be warned to stop the drug and seek attention for several abdominal pain. |
| Renal | 36% | 33% will have a 30% increase in creatinine. Increase above the upper limit of normal is very rare, though we have seen renal failure and severe hypertension when the renal complication was unrecognised. The urine protein/creatine ratio must be followed carefully although this can be difficult in SCD because of SCD-related renal disease. Hold DFX and re-challenge at a 10 mg/kg lower dose per the specific recommendations in the DFX package insert. We monitor renal function and urine protein/creatinine at each transfusion visit. |
| Transaminitis | < 5% | Monitor liver function every three months. Liver toxicity is rare with DFX. Comments for DFP apply. |
ANC, absolute neutrophil count; GCSF, granulocyte colony-stimulating factor; LIC, liver iron concentration, SCD, sickle cell disease.
The above toxicities are not exhaustive, see references
Table IV.
Chelator Dosing
| Iron Load Ferritin (µg/l) LIC (mg/g dw) |
DFO mg/mg/day over 10 h |
DFP mg/kg/day Q 8 h/TID |
DFX(a) mg/mg/day QD or BID |
Comments |
|---|---|---|---|---|
|
Ferritin < 300 (e)3: 14%⇓ 60%⇑ LIC ≤ 3 |
30–40 [hold] |
75–100 [hold] |
20–30 [hold] |
Keep at low end of dose range if no LIC measure available (d) [hold] => if low iron input; on occasional transfusion or on red cell exchange. |
|
Ferritin < 800 (e)5: 8% ⇓ 46% ⇑ 3 < LIC < 7 |
30–40 (b) [hold] |
75–100 (b) [hold] |
20–30 (b) [hold] |
If LIC near 3, stay in mid dose range. If near 7, consider increase in dose. If LIC is dropping, consider lowering dose as LIC approaches 3 mg/g. |
|
Ferritin > 1700 (e)7: 0% ⇓ 39% ⇑ 7 < LIC < 15 |
40–50 | 75–100 | 20–40 |
(c) Increase based on tolerance. Most patients in negative balance at upper end of dose scale. If LIC dropping, may change to single agent or reduce dose as LIC approaches 7. |
|
Ferritin > 2000 LIC > 15 |
40–50 | 75–100 | 20–40 |
(c)Increased based on tolerance. Consider splitting DFX dose BID or combination therapy. |
|
Ferritin > 2500 LIC > 20 |
40–50 | 75–100 | 20–40 | Get cardiac T2* especially if reticulocyte count <15% and %HbS <15%. (c)Increase based on tolerance. Consider splitting DFX dose BID or combination therapy. |
|
Ferritin > 2500 LIC > 20 T2* < 10 ms |
40–50 | 75–100 | 20–40 | Combination therapy containing DFP moved to 100 mg/kg as fast as tolerated. Cardiac T2* every 4 to 6 months. Critical to have circulating chelator 24 hr. a day 7 days a week. Follow AHA consensus statement (Pennell, et al 2013). Consult physician with experience managing severe iron cardiomyopathy. |
|
LIC < 5 T2* < 10 |
40–50 | 75–100 | 20–40 | LIC clears first during intense chelation leaving heart loaded. (d) Toxicity is related to LIC. Monitor toxicity carefully. Need to continue chelation to clear heart. DFP at 100 as a single agent is probably safest in this circumstance. |
Superscripted letters in parentheses (x) refer to comments in the far-right column of the table on the same line. (a) Exjade dosing; for Jadenu multiply by 0.75; (b) If LIC measurement not available consider holding if ferritin decreasing; (c) Increase dose incrementally to high end of dose range every 2–3 months based on toxicity/tolerance. (d) Risk of over chelation is highest at low ferritin/LIC. However, if regular transfusions are occurring, chelation should not be stopped. If ferritin/LIC is dropping in this low iron load range, reduce chelator dose. If ferritin is climbing but low, check the LIC before increasing the dose. (e) cut-off-LIC: x% ⇓ y% ⇑ means based on ferritin, there is x% chance the LIC is lower than the cut-off, and y% chance is it higher than the cut-off (Taher, et al 2015). Delay starting chelation in children until 2 years of age and increase the dose over a year to maximum tolerated.
AHA, American Heart Association; BID, twice daily; DFO, deferoxamine; DFP, Deferiprone; DFX, Deferasirox; LIC, liver iron concentration.
The most important differences between these agents relate to the route of administration, the half-life and the toxicities. DFO (Desferal) was the first effective chelator. Because it is not orally absorbed and it has a very short half-life (30 min), it has to be administered by continuous subcutaneous or intravenous infusion (Table II). While DFO is effective, the parenteral route of administration limits its acceptability to patients. Deferiprone (Ferriprox, DFP) is the first oral iron chelator and has a half-life of six to eight hours. Deferasirox (ExJade, JadeNu; DFX) is an oral agent with a 14-h half-life, allowing once-daily dosing and prolonged circulating chelator levels. While all three chelators can lower cardiac iron, DFP appears to be the most effective in protecting and restoring cardiac function (Berdoukas, et al 2015, Pennell, et al 2013).
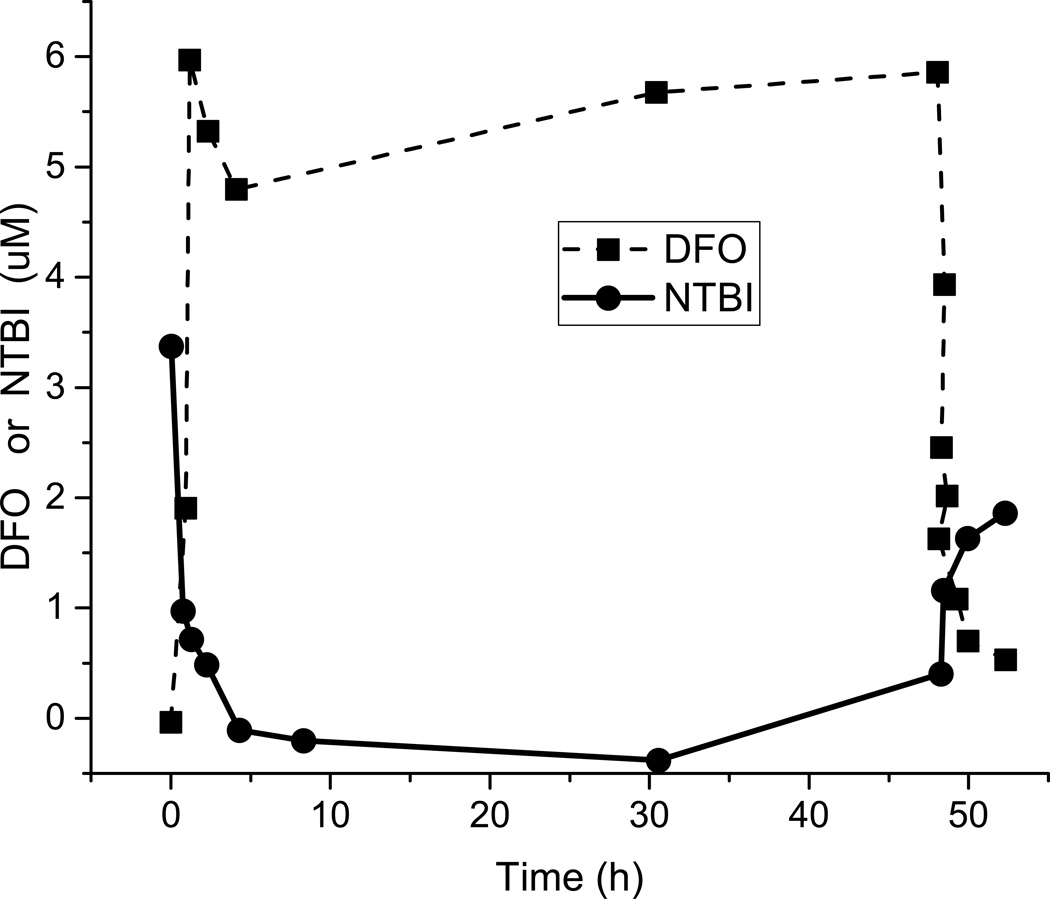
The primary goal of chelation is to clear the circulating reactive forms of iron and to protect tissue from iron toxicity. Clinically, this translates into keeping the NTBI/LPI levels in the normal range, essentially zero, at all times. NTBI/LPI constitutes the so-called “chelatable pool” and, as can be seen in Figure 3, NTBI/LPI levels drop to zero coincident with the onset of DFO infusion and increase again very quickly when the DFO infusion is stopped. All three chelators have the ability to rapidly drop plasma levels of NTBI/LPI. This pharmacology drives our clinical approach to chelation. As LPI is the reactive sub-species of NTBI that enters the heart and the endocrine and other tissues in an unregulated fashion, and can be quickly reduced by chelation, the ideal chelator would be the one with significant levels in the circulation at all times in order to bind reactive iron and block entry into tissue.
Figure 3.
non-transferrin bound iron (NTBI) levels drop quickly after starting infusion of deferoxamine (DFO) and return quickly when DFO is stopped (Porter, et al 1996). NTBI/labile plasma iron, which are free to enter organs through non-regulated ion transporters, are rapidly reduced when chelators are circulating and, until total iron excess is reduced, return when there is no chelator present.
The other goal of chelation is to eliminate excess stored iron. This is driven primarily by the degree of total body iron loading reflected by LIC and ferritin. As the toxicity of iron is related to the total amount of iron and the duration of exposure, the objective of treatment is to lower tissue iron levels as quickly as possible and in a practical and tolerable manner for the patient. The presence of organ failure is a primary determinant of treatment intensity and urgency. Organ failure due to iron is less common in SCD than it is in thalassaemia; nonetheless, it definitely occurs in SCD (Meloni, et al 2014), supporting our recommendation of reducing iron as quickly and as safely as possible. Organ failure generally occurs over a period of several years. However, it is very unpredictable and it is impossible to know when end organ damage is no longer reversible. In general, the physician has sufficient time to work with the patient to arrive at an acceptable and effective chelation regimen. Furthermore, there is some protection from iron toxicity as soon as the chelator begins circulating (Anderson, et al 2004, Coates, et al 2016). For this reason, we try to adjust chelator regimens to maximize circulating chelator levels, recommending chelation seven days a week as the ideal, though not always achievable, goal.
Though rare, iron cardiomyopathy is a life-threatening issue in SCD. Based on data from thalassaemia, if the cardiac T2* is 8 ms or less, there is a high risk of arrhythmia and heart failure and aggressive chelation is indicated. Excellent guidelines have been published for the management of iron cardiomyopathy (Pennell, et al 2013). It is important to stress that even severe cardiomyopathy due to iron is reversible, usually with full recovery if the patient can survive the first several days of severe symptoms, and if exposure to the chelator is maintained without interruption.
Iron overload treatment only works if patients adhere to therapy
All three chelators are very effective at controlling iron individually or in combination. Overwhelmingly, the dominant reason for failure of chelation therapy is failure of the patient to take the prescribed medicine (Origa, et al 2013). While there are data showing different absorption rates for some chelators (Chirnomas, et al 2009), the effect of poor adherence to recommended therapy is the dominant cause of treatment failure. SCD and transfusional iron overload are chronic, lifelong disorders, and thus patients are subject to all of the psychosocial and quality-of-life issues related to chronic disease. Patient adherence to treatment rather than chelator pharmacology is the primary driver of our approach to iron chelation. The main goal is to arrive at an effective plan that the patient agrees to follow, even if the regimen is not ideal based on pharmacology.
In general, we use DFX first because of its long half-life and once daily administration. General dosing approaches are summarized in Table IV. Dividing the daily dose into two doses can help with gastrointestinal symptoms, may maintain higher blood levels over a longer time (Lu, et al 2015) and help to consistently reduce LPI. For patients with very high LIC (> 15–20 mg/g), we will often use DFX in combination with DFP. Currently, none of these agents are licensed for use in combination. However, combinations of these agents have been increasingly used and additional toxicities have not been identified (Aydinok, et al 2015, Cassinerio, et al 2012, Galanello and Origa 2010, Gomber, et al 2016, Grady, et al 2013, Kolnagou, et al 2010, Kolnagou and Kontoghiorghes 2010b, Lai, et al 2010, Maggio, et al 2011, Totadri, et al 2015, Voskaridou, et al 2011).
While we start chelation with the published dosing guidelines, the regimens are individually tailored for each patient to respect their life style and to minimise toxicity. As long as there is significant iron loading (LIC > 7 mg/g), we increase the chelator dose to near maximum over six to twelve months based on patient tolerance and toxicity. We generally do not reduce the dose until the LIC comes into the moderate range, even if LIC or ferritin starts dropping (Table IV). Chelator efficacy depends on transfusion load (Cohen, et al 2008) and specific chelator dosing regimens have been recommended based on transfusion rate, ferritin or LIC (Hoffbrand, et al 2012, Porter, et al 2013). These approaches are solidly based on retrospective response data, but these differences in dosing seem outweighed by unpredictability due to therapy adherence, thus we do not use such detailed dosing approaches. We base dosing on tolerance of the medication by the patients, LIC response and whether the patient is being regularly transfused or on RCE as noted in general guidelines in Table IV. If the patient is not on regular transfusion or is on exchange transfusions, there is increased risk of over chelation at the low end of the iron loading range. Likewise, if the patient is on regular simple transfusions, the chelation should not be totally stopped, except intermittently for toxicity.
While we measure serum ferritin with each transfusion, we caution our patients not to become fixated on ferritin levels. A monotonic decrease in ferritin can be encouraging to the patients and a monotonic increase over time may indicate problems. However, we use MRI as the basis for treatment decisions. Especially at low iron levels, there can be very a big discordance between the ferritin trends and change in the LIC (Puliyel, et al 2014). In general, we monitor LIC by MRI annually, and more often if the ferritin changes do not seem to accurately reflect the clinical situation (Table I).
Maintaining a very supportive and positive collaboration with the patient is a very critical part of the chelation success, in our opinion. The patient is unlikely to adhere to a treatment plan if the patient does not sense that the team thinks chelation is important. Thus, an experienced multidisciplinary team is critical.
During treatment, we monitor blood pressure, creatinine, creatinine clearance, neutrophil and platelet counts, and urine protein/creatinine at every transfusion visit. We also monitor liver functions and electrolytes at least every three months. Audiogram and eye examinations are done annually, although the utility of these tests for iron complications in patients who are not on DFO is questionable. Even though DFX safety has been well established in large studies, we remain concerned about kidney function in SCD patients who are at risk for renal disease independently of chelation. Proteinuria, which can result from SCD itself, is problematic when patients are on DFX, a nephrotoxic agent. We make sure that all patients know that they need to report any severe abdominal pain that may suggest bleeding, and to stop DFX immediately and to contact the centre. They are also instructed to stop DFP immediately and go to the emergency room for a blood test if they have any fever. The major toxicities and their management are noted in Table III.
We recognise that many providers do not have access to MRI and must manage chelation based on ferritin levels alone. Excellent guidelines have been published for chelator management using ferritin based on results in thalassaemia (Taher, et al 2015). While we feel that normalization of iron (LIC 0.8 to 1.5 mg/g) is an ideal goal, we would not advocate trying to achieve an LIC less than 3 to 5 mg/g in the absence of easy access to accurate MRI measures and to a team with significant experience with chelation therapy, especially in a patient who is not adherent to therapy and may not understand the possible complications.
Summary thoughts
SCD is associated with effective erythropoiesis and an inflammatory phenotype that results in higher levels of hepcidin and generally lower levels of reactive LPI. Thus, end organ toxicity in patients with SCD and severe iron overload seems to be significantly less than that seen in thalassaemia patients. However, severe complications of iron overload can occur in SCD. As severe complications of SCD are treated by transfusion, a correlation between organ damage and iron load is guaranteed. Nonetheless, we have seen a number of cases of significant iron-related organ damage in patients in their third and fourth decades. Therefore, we suspect that iron-related organ damage in SCD is under recognised, partly because the damage is often attributed to SCD itself and because of the lack of familiarity with iron overload by some providers.
While there are some differences in the biology of iron overload between SCD and thalassaemia or marrow failure, the pharmacological treatment is essentially the same. The incidence of severe iron cardiomyopathy is much lower in SCD, but the treatment is identical to that in thalassaemia.
We recommend treating iron overload in patients with SCD with the ideal intent of normalising plasma and tissue iron levels. As SCD subjects live longer, they will probably encounter complications of long-term iron exposure, and keeping their iron load as low as can be achieved safely would seem prudent. The success of chelation therapy is dominated by patient adherence to prescribed treatment; thus, adjustment of treatment regimens to increase the likelihood of adherence to treatment is fundamental.
Acknowledgments
This work was supported by funding from the NIH to J. C. Wood (1R01DK097115-01A1).
TDC wrote the manuscript, JCW edited sections related to monitoring with MRI. The authors recognize the critical contributions of Susan Carson RN who manages the patients. The general approach described here is the result of many discussions regarding patients by TDC, SC and JCW over many years.
The authors wish to thank Dr. Martine Torres for reviewing of the manuscript.
Footnotes
Conflict of Interest: Dr Coates is a consultant for Novartis, ApoPharma, Ionis Pharma, Celgene, Agios, and Prolong. Dr Wood is a consultant for ApoPharma, Ionis, Celgene, Vifor, WorldCare Clinical, BiomedInformatics, and AMAG
References
- Anderson LJ, Westwood MA, Holden S, Davis B, Prescott E, Wonke B, Porter JB, Walker JM, Pennell DJ. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haematol. 2004;127:348–355. doi: 10.1111/j.1365-2141.2004.05202.x. [DOI] [PubMed] [Google Scholar]
- Angelucci E, Brittenham GM, McLaren CE, Ripalti M, Baronciani D, Giardini C, Galimberti M, Polchi P, Lucarelli G. Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med. 2000;343:327–331. doi: 10.1056/NEJM200008033430503. [DOI] [PubMed] [Google Scholar]
- Aubart M, Ou P, Elie C, Canniffe C, Kutty S, Delos V, Graffigne C, de Montalembert M, Brousse V. Longitudinal MRI and Ferritin Monitoring of Iron Overload in Chronically Transfused and Chelated Children With Sickle Cell Anemia and Thalassemia Major. J Pediatr Hematol Oncol. 2016;38:497–502. doi: 10.1097/MPH.0000000000000595. [DOI] [PubMed] [Google Scholar]
- Aydinok Y, Kattamis A, Cappellini MD, El-Beshlawy A, Origa R, Elalfy M, Kilinc Y, Perrotta S, Karakas Z, Viprakasit V, Habr D, Constantinovici N, Shen J, Porter JB, Investigators H. Effects of deferasirox-deferoxamine on myocardial and liver iron in patients with severe transfusional iron overload. Blood. 2015;125:3868–3877. doi: 10.1182/blood-2014-07-586677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aygun B, Mortier NA, Kesler K, Lockhart A, Schultz WH, Cohen AR, Alvarez O, Rogers ZR, Kwiatkowski JL, Miller ST, Sylvestre P, Iyer R, Lane PA, Ware RE Stroke With Transfusions Changing to Hydroxyurea Trial, I. Therapeutic phlebotomy is safe in children with sickle cell anaemia and can be effective treatment for transfusional iron overload. Br J Haematol. 2015;169:262–266. doi: 10.1111/bjh.13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas SK. Iron overload is a determinant of morbidity and mortality in adult patients with sickle cell disease. Seminars in Hematology. 2001;38:30–36. doi: 10.1016/s0037-1963(01)90058-7. [DOI] [PubMed] [Google Scholar]
- Berdoukas V, Modell B. Transfusion-dependent thalassaemia: a new era. The Medical journal of Australia. 2008;188:68–69. doi: 10.5694/j.1326-5377.2008.tb01523.x. [DOI] [PubMed] [Google Scholar]
- Berdoukas V, Coates TD, Cabantchik ZI. Iron and oxidative stress in cardiomyopathy in thalassemia. Free Radic Biol Med. 2015;88:3–9. doi: 10.1016/j.freeradbiomed.2015.07.019. [DOI] [PubMed] [Google Scholar]
- Bernaudin F, Verlhac S, Arnaud C, Kamdem A, Vasile M, Kasbi F, Hau I, Madhi F, Fourmaux C, Biscardi S, Epaud R, Pondarre C. Chronic and acute anemia and extracranial internal carotid stenosis are risk factors for silent cerebral infarcts in sickle cell anemia. Blood. 2015;125:1653–1661. doi: 10.1182/blood-2014-09-599852. [DOI] [PubMed] [Google Scholar]
- Breda L, Rivella S. Modulators of erythropoiesis: emerging therapies for hemoglobinopathies and disorders of red cell production. Hematol Oncol Clin North Am. 2014;28:375–386. doi: 10.1016/j.hoc.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvaruso G, Vitrano A, Di Maggio R, Ballas S, Steinberg MH, Rigano P, Sacco M, Telfer P, Renda D, Barone R, Maggio A for the Investigators of the Multicenter Randomized Clinical Trial of Deferiprone versus Deferoxamine in SCD. Deferiprone versus deferoxamine in sickle cell disease: results from a 5-year long-term Italian multi-center randomized clinical trial. Blood Cells Mol Dis. 2014;53:265–271. doi: 10.1016/j.bcmd.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Camaschella C, Nai A. Ineffective erythropoiesis and regulation of iron status in iron loading anaemias. Br J Haematol. 2016;172:512–523. doi: 10.1111/bjh.13820. [DOI] [PubMed] [Google Scholar]
- Cassinerio E, Roghi A, Pedrotti P, Brevi F, Zanaboni L, Graziadei G, Pattoneri P, Milazzo A, Cappellini MD. Cardiac iron removal and functional cardiac improvement by different iron chelation regimens in thalassemia major patients. Ann Hematol. 2012;91:1443–1449. doi: 10.1007/s00277-012-1480-8. [DOI] [PubMed] [Google Scholar]
- Chirnomas D, Smith AL, Braunstein J, Finkelstein Y, Pereira L, Bergmann AK, Grant FD, Paley C, Shannon M, Neufeld EJ. Deferasirox pharmacokinetics in patients with adequate versus inadequate response. Blood. 2009;114:4009–4013. doi: 10.1182/blood-2009-05-222729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claster S, Wood JC, Noetzli L, Carson SM, Hofstra TC, Khanna R, Coates TD. Nutritional deficiencies in iron overloaded patients with hemoglobinopathies. Am J Hematol. 2009;84:344–348. doi: 10.1002/ajh.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates TD. Physiology and pathophysiology of iron in hemoglobin-associated diseases. Free Radic Biol Med. 2014;72:23–40. doi: 10.1016/j.freeradbiomed.2014.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates TD, Carson S, Wood JC, Berdoukas V. Management of iron overload in hemoglobinopathies: what is the appropriate target iron level? Ann N Y Acad Sci. 2016;1368:95–106. doi: 10.1111/nyas.13060. [DOI] [PubMed] [Google Scholar]
- Cohen AR, Glimm E, Porter JB. Effect of transfusional iron intake on response to chelation therapy in beta-thalassemia major. Blood. 2008;111:583–587. doi: 10.1182/blood-2007-08-109306. [DOI] [PubMed] [Google Scholar]
- Danielson CF. The role of red blood cell exchange transfusion in the treatment and prevention of complications of sickle cell disease. Ther Apher. 2002;6:24–31. doi: 10.1046/j.1526-0968.2002.00396.x. [DOI] [PubMed] [Google Scholar]
- Darbari DS, Kple-Faget P, Kwagyan J, Rana S, Gordeuk VR, Castro O. Circumstances of death in adult sickle cell disease patients. Am J Hematol. 2006;81:858–863. doi: 10.1002/ajh.20685. [DOI] [PubMed] [Google Scholar]
- de Montalembert M, Ferster A, Colombatti R, Rees DC, Gulbis B European Network for, R. & Congenital A. ENERCA clinical recommendations for disease management and prevention of complications of sickle cell disease in children. Am J Hematol. 2011;86:72–75. doi: 10.1002/ajh.21865. [DOI] [PubMed] [Google Scholar]
- de Swart L, Hendriks JC, van der Vorm LN, Cabantchik ZI, Evans PJ, Hod EA, Brittenham GM, Furman Y, Wojczyk B, Janssen MC, Porter JB, Mattijssen VE, Biemond BJ, MacKenzie MA, Origa R, Galanello R, Hider RC, Swinkels DW. Second international round robin for the quantification of serum non-transferrin-bound iron and labile plasma iron in patients with iron-overload disorders. Haematologica. 2016;101:38–45. doi: 10.3324/haematol.2015.133983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detterich JA, Kato RM, Rabai M, Meiselman HJ, Coates TD, Wood JC. Chronic transfusion therapy improves but does not normalize systemic and pulmonary vasculopathy in sickle cell disease. Blood. 2015;126:703–710. doi: 10.1182/blood-2014-12-614370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussiot M, Maciel TT, Fricot A, Chartier C, Negre O, Veiga J, Grapton D, Paubelle E, Payen E, Beuzard Y, Leboulch P, Ribeil JA, Arlet JB, Cote F, Courtois G, Ginzburg YZ, Daniel TO, Chopra R, Sung V, Hermine O, Moura IC. An activin receptor IIA ligand trap corrects ineffective erythropoiesis in beta-thalassemia. Nat Med. 2014 doi: 10.1038/nm.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmaki K, Tzoumari I, Pappa C, Chouliaras G, Berdoukas V. Normalisation of total body iron load with very intensive combined chelation reverses cardiac and endocrine complications of thalassaemia major. Br J Haematol. 2010;148:466–475. doi: 10.1111/j.1365-2141.2009.07970.x. [DOI] [PubMed] [Google Scholar]
- Fasano RM, Leong T, Kaushal M, Sagiv E, Luban NL, Meier ER. Effectiveness of red blood cell exchange, partial manual exchange, and simple transfusion concurrently with iron chelation therapy in reducing iron overload in chronically transfused sickle cell anemia patients. Transfusion. 2016;56:1707–1715. doi: 10.1111/trf.13558. [DOI] [PubMed] [Google Scholar]
- Fernandes JL, Loggetto SR, Veríssimo MPA, Fertrin KY, Baldanzi GR, Fioravante LAB, Tan DM, Higa T, Mashima DA, Piga A, Coelho OR, Costa FF, Saad ST. A randomized trial of amlodipine in addition to standard chelation therapy in patients with thalassemia major. Blood. 2016;128:1555–1561. doi: 10.1182/blood-2016-06-721183. [DOI] [PubMed] [Google Scholar]
- Frazer DM, Anderson GJ. The regulation of iron transport. Biofactors. 2014;40:206–214. doi: 10.1002/biof.1148. [DOI] [PubMed] [Google Scholar]
- Fung EB, Harmatz P, Milet M, Ballas SK, De Castro L, Hagar W, Owen W, Olivieri N, Smith-Whitley K, Darbari D, Wang W, Vichinsky E for the Multi-Center Study of Iron Overload Research Group. Morbidity and mortality in chronically transfused subjects with thalassemia and sickle cell disease: A report from the multi-center study of iron overload. Am.J Hematol. 2007;82:255–265. doi: 10.1002/ajh.20809. [DOI] [PubMed] [Google Scholar]
- Galanello R, Origa R. Beta-thalassemia. Orphanet J Rare Dis. 2010;5:11. doi: 10.1186/1750-1172-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93:1721–1741. doi: 10.1152/physrev.00008.2013. [DOI] [PubMed] [Google Scholar]
- Ganz T, Nemeth E. Iron metabolism: interactions with normal and disordered erythropoiesis. Cold Spring Harb Perspect Med. 2012;2:a011668. doi: 10.1101/cshperspect.a011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardenghi S, Marongiu MF, Ramos P, Guy E, Breda L, Chadburn A, Liu Y, Amariglio N, Rechavi G, Rachmilewitz EA, Breuer W, Cabantchik ZI, Wrighting DM, Andrews NC, de Sousa M, Giardina PJ, Grady RW, Rivella S. Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood. 2007;109:5027–5035. doi: 10.1182/blood-2006-09-048868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner K, Douiri A, Drasar E, Allman M, Mwirigi A, Awogbade M, Thein SL. Survival in adults with sickle cell disease in a high-income setting. Blood. 2016;128:1436–1438. doi: 10.1182/blood-2016-05-716910. [DOI] [PubMed] [Google Scholar]
- Gomber S, Jain P, Sharma S, Narang M. Comparative Efficacy and Safety of Oral Iron Chelators and their Novel Combination in Children with Thalassemia. Indian Pediatr. 2016;53:207–210. doi: 10.1007/s13312-016-0821-4. [DOI] [PubMed] [Google Scholar]
- Grady RW, Galanello R, Randolph RE, Kleinert DA, Dessi C, Giardina PJ. Toward optimizing the use of deferasirox: potential benefits of combined use with deferoxamine. Haematologica. 2013;98:129–135. doi: 10.3324/haematol.2012.070607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R, Charlton R, Seftel H, Bothwell T, Mayet F, Adams B, Finch C, Layrisse M. Body iron excretion in man: a collaborative study. Am J Med. 1968;45:336–353. doi: 10.1016/0002-9343(68)90069-7. [DOI] [PubMed] [Google Scholar]
- Habibi A, Arlet JB, Stankovic K, Gellen-Dautremer J, Ribeil JA, Bartolucci P, Lionnet F centre de reference maladies, r. [French guidelines for the management of adult sickle cell disease: 2015 update] La Revue de medecine interne / fondee … par la Societe nationale francaise de medecine interne. 2015;36:5S3–584. doi: 10.1016/S0248-8663(15)60002-9. [DOI] [PubMed] [Google Scholar]
- Hoffbrand AV, Taher A, Cappellini MD. How I treat transfusional iron overload. Blood. 2012;120:3657–3669. doi: 10.1182/blood-2012-05-370098. [DOI] [PubMed] [Google Scholar]
- Inati A, Musallam KM, Wood JC, Sheikh-Taha M, Daou L, Taher AT. Absence of cardiac siderosis by MRI T2* despite transfusion burden, hepatic and serum iron overload in Lebanese patients with sickle cell disease. Eur J Haematol. 2009;83:565–571. doi: 10.1111/j.1600-0609.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- Jordan L, Adams-Graves P, Kanter-Washko J, Oneal PA, Sasane M, Vekeman F, Bieri C, Magestro M, Marcellari A, Duh MS. Multicenter COMPACT study of COMplications in patients with sickle cell disease and utilization of iron chelation therapy. Curr Med Res Opin. 2015;31:513–523. doi: 10.1185/03007995.2014.998815. [DOI] [PubMed] [Google Scholar]
- Khamseekaew J, Kumfu S, Chattipakorn SC, Chattipakorn N. Effects of Iron Overload on Cardiac Calcium Regulation: Translational Insights Into Mechanisms and Management of a Global Epidemic. The Canadian journal of cardiology. 2016;32:1009–1016. doi: 10.1016/j.cjca.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Kim HC. Red cell exchange: special focus on sickle cell disease. Hematology Am Soc Hematol Educ Program. 2014;2014:450–456. doi: 10.1182/asheducation-2014.1.450. [DOI] [PubMed] [Google Scholar]
- Kirk P, Roughton M, Porter JB, Walker JM, Tanner MA, Patel J, Wu D, Taylor J, Westwood MA, Anderson LJ, Pennell DJ. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation. 2009;120:1961–1968. doi: 10.1161/CIRCULATIONAHA.109.874487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolnagou A, Kontoghiorghes GJ. Maintenance of normal range body iron store levels for up to 4.5 years in thalassemia major patients using deferiprone monotherapy. Hemoglobin. 2010a;34:204–209. doi: 10.3109/03630269.2010.485890. [DOI] [PubMed] [Google Scholar]
- Kolnagou A, Kontoghiorghes GJ. New golden era of chelation therapy in thalassaemia: the achievement and maintenance of normal range body iron stores. Br J Haematol. 2010b;150:489–490. doi: 10.1111/j.1365-2141.2010.08229.x. author reply 491. [DOI] [PubMed] [Google Scholar]
- Kolnagou A, Kleanthous M, Kontoghiorghes GJ. Reduction of body iron stores to normal range levels in thalassaemia by using a deferiprone/deferoxamine combination and their maintenance thereafter by deferiprone monotherapy. Eur J Haematol. 2010;85:430–438. doi: 10.1111/j.1600-0609.2010.01499.x. [DOI] [PubMed] [Google Scholar]
- Kontoghiorghes GJ. The 18th ICOC Proceedings in Athens, Greece: New breakthrough in thalassemia leading to the complete treatment of iron overload and to hundreds of patients achieving and maintaining normal body iron stores. Ethical questions on chelation therapy. Hemoglobin. 2010;34:199–203. doi: 10.3109/03630269.2010.484963. [DOI] [PubMed] [Google Scholar]
- Koren A, Fink D, Admoni O, Tennenbaum-Rakover Y, Levin C. Non-transferrin-bound labile plasma iron and iron overload in sickle-cell disease: a comparative study between sickle-cell disease and beta-thalassemic patients. Eur J Haematol. 2010;84:72–78. doi: 10.1111/j.1600-0609.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- Koskenkorva-Frank TS, Weiss G, Koppenol WH, Burckhardt S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med. 2013;65:1174–1194. doi: 10.1016/j.freeradbiomed.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Lai ME, Grady RW, Vacquer S, Pepe A, Carta MP, Bina P, Sau F, Cianciulli P, Maggio A, Galanello R, Farci P. Increased survival and reversion of iron-induced cardiac disease in patients with thalassemia major receiving intensive combined chelation therapy as compared to desferoxamine alone. Blood Cells Mol Dis. 2010;45:136–139. doi: 10.1016/j.bcmd.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Lu MY, Wang N, Wu WH, Lai CW, Kuo PH, Chiang PH, Lin KH, Wu TH. Simultaneous Determination of Plasma Deferasirox and Deferasirox-Iron Complex Using an HPLC-UV System and Pharmacokinetics of Deferasirox in Patients With beta-Thalassemia Major: Once-daily Versus Twice-daily Administration. Clin Ther. 2015;37:1751–1760. doi: 10.1016/j.clinthera.2015.05.506. [DOI] [PubMed] [Google Scholar]
- Lucania G, Vitrano A, Filosa A, Maggio A. Chelation treatment in sickle-cell-anaemia: much ado about nothing? Br J Haematol. 2011;154:545–555. doi: 10.1111/j.1365-2141.2011.08769.x. [DOI] [PubMed] [Google Scholar]
- Maggio A, Filosa A, Vitrano A, Aloj G, Kattamis A, Ceci A, Fucharoen S, Cianciulli P, Grady RW, Prossomariti L, Porter JB, Iacono A, Cappellini MD, Bonifazi F, Cassara F, Harmatz P, Wood J, Gluud C. Iron chelation therapy in thalassemia major: a systematic review with meta-analyses of 1520 patients included on randomized clinical trials. Blood Cells Mol Dis. 2011;47:166–175. doi: 10.1016/j.bcmd.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Makani J, Cox SE, Soka D, Komba AN, Oruo J, Mwamtemi H, Magesa P, Rwezaula S, Meda E, Mgaya J, Lowe B, Muturi D, Roberts DJ, Williams TN, Pallangyo K, Kitundu J, Fegan G, Kirkham FJ, Marsh K, Newton CR. Mortality in sickle cell anemia in Africa: a prospective cohort study in Tanzania. PLoS One. 2011;6:e14699. doi: 10.1371/journal.pone.0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni A, Puliyel M, Pepe A, Berdoukas V, Coates TD, Wood JC. Cardiac iron overload in sickle-cell disease. Am J Hematol. 2014;89:678–683. doi: 10.1002/ajh.23721. [DOI] [PubMed] [Google Scholar]
- Modell B, Letsky EA, Flynn DM, Peto R, Weatherall DJ. Survival and desferrioxamine in thalassaemia major. Br Med J (Clin Res Ed) 1982;284:1081–1084. doi: 10.1136/bmj.284.6322.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modell B, Khan M, Darlison M, Westwood MA, Ingram D, Pennell DJ. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008;10:42. doi: 10.1186/1532-429X-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CJ, Oudit GY. Iron-overload cardiomyopathy: pathophysiology, diagnosis, and treatment. J Card Fail. 2010;16:888–900. doi: 10.1016/j.cardfail.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Nemeth E. Hepcidin biology and therapeutic applications. Expert Rev Hematol. 2010;3:153–155. doi: 10.1586/ehm.10.1. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- Noetzli LJ, Papudesi J, Coates TD, Wood JC. Pancreatic iron loading predicts cardiac iron loading in thalassemia major. Blood. 2009;114:4021–4026. doi: 10.1182/blood-2009-06-225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noetzli LJ, Coates TD, Wood JC. Pancreatic iron loading in chronically transfused sickle cell disease is lower than in thalassaemia major. Br J Haematol. 2011;152:229–233. doi: 10.1111/j.1365-2141.2010.08476.x. [DOI] [PubMed] [Google Scholar]
- Noetzli LJ, Panigrahy A, Mittelman SD, Hyderi A, Dongelyan A, Coates TD, Wood JC. Pituitary iron and volume predict hypogonadism in transfusional iron overload. Am J Hematol. 2012;87:167–171. doi: 10.1002/ajh.22247. [DOI] [PubMed] [Google Scholar]
- Novelli EM, Gladwin MT. Crises in Sickle Cell Disease. Chest. 2016;149:1082–1093. doi: 10.1016/j.chest.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Origa R, Danjou F, Cossa S, Matta G, Bina P, Dessi C, Defraia E, Foschini ML, Leoni G, Morittu M, Galanello R. Impact of heart magnetic resonance imaging on chelation choices, compliance with treatment and risk of heart disease in patients with thalassaemia major. Br J Haematol. 2013;163:400–403. doi: 10.1111/bjh.12517. [DOI] [PubMed] [Google Scholar]
- Pennell DJ, Udelson JE, Arai AE, Bozkurt B, Cohen AR, Galanello R, Hoffman TM, Kiernan MS, Lerakis S, Piga A, Porter JB, Walker JM, Wood J. Cardiovascular function and treatment in beta-thalassemia major: a consensus statement from the American Heart Association. Circulation. 2013;128:281–308. doi: 10.1161/CIR.0b013e31829b2be6. [DOI] [PubMed] [Google Scholar]
- Perronne V, Roberts-Harewood M, Bachir D, Roudot-Thoraval F, Delord JM, Thuret I, Schaeffer A, Davies SC, Galacteros F, Godeau B. Patterns of mortality in sickle cell disease in adults in France and England. Hematol J. 2002;3:56–60. doi: 10.1038/sj.thj.6200147. [DOI] [PubMed] [Google Scholar]
- Piga A, Longo F, Musallam KM, Cappellini MD, Forni GL, Quarta G, Chiavilli F, Commendatore F, Mulas S, Caruso V, Galanello R. Assessment and management of iron overload in beta-thalassaemia major patients during the 21st century: a real-life experience from the Italian WEBTHAL project. Br J Haematol. 2013;161:872–883. doi: 10.1111/bjh.12340. [DOI] [PubMed] [Google Scholar]
- Porter JB, Garbowski M. The pathophysiology of transfusional iron overload. Hematol Oncol Clin North Am. 2014;28:683–701. doi: 10.1016/j.hoc.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Porter JB, Abeysinghe RD, Marshall L, Hider RC, Singh S. Kinetics of removal and reappearance of non-transferrin-bound plasma iron with deferoxamine therapy. Blood. 1996;88:705–713. [PubMed] [Google Scholar]
- Porter JB, Elalfy MS, Taher AT, Aydinok Y, Chan LL, Lee SH, Sutcharitchan P, Habr D, Martin N, El-Beshlawy A. Efficacy and safety of deferasirox at low and high iron burdens: results from the EPIC magnetic resonance imaging substudy. Ann Hematol. 2013;92:211–219. doi: 10.1007/s00277-012-1588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JB, El-Alfy M, Viprakasit V, Giraudier S, Chan LL, Lai Y, El-Ali A, Han J, Cappellini MD. Utility of labile plasma iron and transferrin saturation in addition to serum ferritin as iron overload markers in different underlying anemias before and after deferasirox treatment. Eur J Haematol. 2016;96:19–26. doi: 10.1111/ejh.12540. [DOI] [PubMed] [Google Scholar]
- Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005;84:363–376. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- Puliyel M, Mainous AG, 3rd, Berdoukas V, Coates TD. Iron toxicity and its possible association with treatment of Cancer: lessons from hemoglobinopathies and rare, transfusion-dependent anemias. Free Radic Biol Med. 2015;79:343–351. doi: 10.1016/j.freeradbiomed.2014.10.861. [DOI] [PubMed] [Google Scholar]
- Puliyel M, Sposto R, Berdoukas VA, Hofstra TC, Nord A, Carson S, Wood J, Coates TD. Ferritin trends do not predict changes in total body iron in patients with transfusional iron overload. Am J Hematol. 2014;89:391–394. doi: 10.1002/ajh.23650. [DOI] [PubMed] [Google Scholar]
- Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115:3447–3452. doi: 10.1182/blood-2009-07-233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivella S. Ineffective erythropoiesis and thalassemias. Curr Opin Hematol. 2009;16:187–194. doi: 10.1097/MOH.0b013e32832990a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlstedt L, Ebeling F, von Bonsdorff L, Parkkinen J, Ruutu T. Non-transferrin-bound iron during allogeneic stem cell transplantation. Br J Haematol. 2001;113:836–838. doi: 10.1046/j.1365-2141.2001.02820.x. [DOI] [PubMed] [Google Scholar]
- Savage WJ, Reddoch S, Wolfe J, Casella JF. Partial manual exchange reduces iron accumulation during chronic red cell transfusions for sickle cell disease. J Pediatr Hematol Oncol. 2013;35:434–436. doi: 10.1097/MPH.0b013e31829d470d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WR, Bovbjerg VE, Penberthy LT, McClish DK, Levenson JL, Roberts JD, Gil K, Roseff SD, Aisiku IP. Understanding pain and improving management of sickle cell disease: the PiSCES study. J Natl Med Assoc. 2005;97:183–193. [PMC free article] [PubMed] [Google Scholar]
- Smith WR, Penberthy LT, Bovbjerg VE, McClish DK, Roberts JD, Dahman B, Aisiku IP, Levenson JL, Roseff SD. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148:94–101. doi: 10.7326/0003-4819-148-2-200801150-00004. [DOI] [PubMed] [Google Scholar]
- Taher AT, Porter JB, Viprakasit V, Kattamis A, Chuncharunee S, Sutcharitchan P, Siritanaratkul N, Origa R, Karakas Z, Habr D, Zhu Z, Cappellini MD. Approaching low liver iron burden in chelated patients with non-transfusion-dependent thalassemia: the safety profile of deferasirox. Eur J Haematol. 2014;92:521–526. doi: 10.1111/ejh.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taher AT, Porter JB, Viprakasit V, Kattamis A, Chuncharunee S, Sutcharitchan P, Siritanaratkul N, Origa R, Karakas Z, Habr D, Zhu Z, Cappellini MD. Defining serum ferritin thresholds to predict clinically relevant liver iron concentrations for guiding deferasirox therapy when MRI is unavailable in patients with non-transfusion-dependent thalassaemia. Br J Haematol. 2015;168:284–290. doi: 10.1111/bjh.13119. [DOI] [PubMed] [Google Scholar]
- Totadri S, Bansal D, Bhatia P, Attri SV, Trehan A, Marwaha RK. The deferiprone and deferasirox combination is efficacious in iron overloaded patients with beta-thalassemia major: A prospective, single center, open-label study. Pediatr Blood Cancer. 2015;62:1592–1596. doi: 10.1002/pbc.25533. [DOI] [PubMed] [Google Scholar]
- Tsouana E, Kaya B, Gadong N, Hemmaway C, Newell H, Simmons A, Whitmarsh S, Telfer P. Deferasirox for iron chelation in multitransfused children with sickle cell disease; long-term experience in the East London clinical haemoglobinopathy network. Eur J Haematol. 2015;94:336–342. doi: 10.1111/ejh.12435. [DOI] [PubMed] [Google Scholar]
- Vekeman F, Sasane M, Cheng WY, Ramanakumar AV, Fortier J, Qiu Y, Duh MS, Paley C, Adams-Graves P. Adherence to iron chelation therapy and associated healthcare resource utilization and costs in Medicaid patients with sickle cell disease and thalassemia. J Med Econ. 2016;19:292–303. doi: 10.3111/13696998.2015.1117979. [DOI] [PubMed] [Google Scholar]
- Vichinsky E, Butensky E, Fung E, Hudes M, Theil E, Ferrell L, Williams R, Louie L, Lee PD, Harmatz P. Comparison of organ dysfunction in transfused patients with SCD or beta thalassemia. Am J Hematol. 2005;80:70–74. doi: 10.1002/ajh.20402. [DOI] [PubMed] [Google Scholar]
- Vichinsky EP, Ohene-Frempong K, Thein SL, Lobo CL, Inati A, Thompson AA, Smith-Whitley K, Kwiatkowski JL, Swerdlow PS, Porter JB, Marks PW. Transfusion and chelation practices in sickle cell disease: a regional perspective. Pediatr Hematol Oncol. 2011a;28:124–133. doi: 10.3109/08880018.2010.505506. [DOI] [PubMed] [Google Scholar]
- Vichinsky EP, Ohene-Frempong K, Transfusion C. Approaches to transfusion therapy and iron overload in patients with sickle cell disease: results of an international survey. Pediatr Hematol Oncol. 2011b;28:37–42. doi: 10.3109/08880018.2010.505497. [DOI] [PubMed] [Google Scholar]
- Vichinsky E, Bernaudin F, Forni GL, Gardner R, Hassell K, Heeney MM, Inusa B, Kutlar A, Lane P, Mathias L, Porter J, Tebbi C, Wilson F, Griffel L, Deng W, Giannone V, Coates T. Long-term safety and efficacy of deferasirox (Exjade) for up to 5 years in transfusional iron-overloaded patients with sickle cell disease. Br J Haematol. 2011c;154:387–397. doi: 10.1111/j.1365-2141.2011.08720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitrano A, Calvaruso G, Tese L, Gioia F, Cassara F, Campisi S, Butera F, Commendatore V, Rizzo M, Santoro V, Cigna V, Quota A, Bagnato S, Argento C, Fidone C, Schembari D, Gerardi C, Barbiera F, Bellisssima G, Giugno G, Polizzi G, Rosso R, Abbate G, Caruso V, Chiodi E, Gamberini MR, Giorgi B, Putti MC, Filosa A, De Ritis MR, Oliva E, Arcadi N, Fustaneo M, Mistretta L, Di Maggio R, Sacco M, Veronica DS, Giangreco A, Maggio A. Real-life experience with liver iron concentration R2 MRI measurement in patients with hemoglobinopathies: baseline data from LICNET. Eur J Haematol. 2016;97:361–370. doi: 10.1111/ejh.12740. [DOI] [PubMed] [Google Scholar]
- Voskaridou E, Christoulas D, Terpos E. Successful chelation therapy with the combination of deferasirox and deferiprone in a patient with thalassaemia major and persisting severe iron overload after single-agent chelation therapies. Br J Haematol. 2011;154:654–656. doi: 10.1111/j.1365-2141.2011.08626.x. [DOI] [PubMed] [Google Scholar]
- Walter PB, Fung EB, Killilea DW, Jiang Q, Hudes M, Madden J, Porter J, Evans P, Vichinsky E, Harmatz P. Oxidative stress and inflammation in iron-overloaded patients with beta-thalassaemia or sickle cell disease. Br J Haematol. 2006;135:254–263. doi: 10.1111/j.1365-2141.2006.06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware RE, Davis BR, Schultz WH, Brown RC, Aygun B, Sarnaik S, Odame I, Fuh B, George A, Owen W, Luchtman-Jones L, Rogers ZR, Hilliard L, Gauger C, Piccone C, Lee MT, Kwiatkowski JL, Jackson S, Miller ST, Roberts C, Heeney MM, Kalfa TA, Nelson S, Imran H, Nottage K, Alvarez O, Rhodes M, Thompson AA, Rothman JA, Helton KJ, Roberts D, Coleman J, Bonner MJ, Kutlar A, Patel N, Wood J, Piller L, Wei P, Luden J, Mortier NA, Stuber SE, Luban NL, Cohen AR, Pressel S, Adams RJ. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet. 2016;387:661–670. doi: 10.1016/S0140-6736(15)01041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JC. Impact of iron assessment by MRI. Hematology Am Soc Hematol Educ Program. 2011;2011:443–450. doi: 10.1182/asheducation-2011.1.443. [DOI] [PubMed] [Google Scholar]
- Wood JC. Guidelines for quantifying iron overload. Hematology Am Soc Hematol Educ Program. 2014;2014:210–215. doi: 10.1182/asheducation-2014.1.210. [DOI] [PubMed] [Google Scholar]
- Wood JC. Estimating tissue iron burden: current status and future prospects. Br J Haematol. 2015;170:15–28. doi: 10.1111/bjh.13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, Nelson MD, Coates TD. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106:1460–1465. doi: 10.1182/blood-2004-10-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JC, Pressel S, Rogers ZR, Odame I, Kwiatkowski JL, Lee MT, Owen WC, Cohen AR, St Pierre T, Heeney MM, Schultz WH, Davis BR, Ware RE for the TWiTCH Investigators. Liver iron concentration measurements by MRI in chronically transfused children with sickle cell anemia: baseline results from the TWiTCH trial. Am J Hematol. 2015;90:806–810. doi: 10.1002/ajh.24089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JC, Cohen AR, Pressel SL, Aygun B, Imran H, Luchtman-Jones L, Thompson AA, Fuh B, Schultz WH, Davis BR, Ware RE, Investigators TW. Organ iron accumulation in chronically transfused children with sickle cell anaemia: baseline results from the TWiTCH trial. Br J Haematol. 2016;172:122–130. doi: 10.1111/bjh.13791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawn BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH, Jordan L, Lanzkron SM, Lottenberg R, Savage WJ, Tanabe PJ, Ware RE, Murad MH, Goldsmith JC, Ortiz E, Fulwood R, Horton A, John-Sowah J. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033–1048. doi: 10.1001/jama.2014.10517. [DOI] [PubMed] [Google Scholar]