Abstract
Immunologic tolerance to solid organ and islet cell grafts has been achieved in various rodent models using antibodies directed at CD45RB and Tim-1. We have shown that this form of tolerance depends on regulatory B-cells. To elucidate further the mechanism by which Bregs induce tolerance we investigated the requirement of NK and NKT cells in this model. To do so, hyperglycemic B6, μMT, Beige or CD1d−/− mice received Balb/c islet grafts and treatment with the tolerance inducing regimen consisting of anti-CD45RB and anti –TIM1. B6 mice depleted of both NK and NKT cells by anti-NK1.1 antibody and mice deficient in NK activity (Beige) did not develop tolerance following dual antibody treatment. In contrast, transplant tolerance induction was successful in CD1d−/− recipients (deficient in NK-T cells), indicating that NK but not NKT cells are essential in B-cell dependent tolerance. In addition, reconstitution of Beige host with NK cells restored the ability to induce transplant tolerance with dual antibody treatment. Transfer of tolerance by B-cells from tolerant mice was also dependent on host Nk1.1+ cells. In conclusion, these results show that regulatory function of B-cells is dependent on NK cells in this model of transplantation tolerance.
Introduction
Several therapeutic antibodies have enabled transplantation tolerance in murine models. While most of these antibodies evoke well characterized pathways such as costimulatory blockade or cell adhesion to induce tolerance the mechanistic underpinning of other tolerance inducing antibodies is less clear. We and others have used an antibody binding CD45RB to induce immune tolerance to heterotopically transplanted allogeneic hearts and pancreatic islets (1, 2). More recently, we found that CD45RB acts synergistically with Tim-1 antibody that has been shown independently to induce tolerance to islet grafts(3). While the detailed mechanisms have yet to be identified, we and others have found that the tolerance induced by these antibodies is dependent on both regulatory B- and regulatory T-cells (Tregs). More specifically, while the adoptive transfer of Bregs alone is sufficient to induce antigen specific transplant tolerance it requires the presence of Tregs in the recipient(3, 4).
The biology of regulatory B-cells has been under intense investigation in recent years resulting in the emergence of a diversity of functional subsets and regulatory mechanisms(5, 6). A frequently described hallmark of Bregs, and the greatest common denominator of all subtypes, is the production of the immunomodulatory cytokine IL-10(5). However, it has become apparent that other mechanisms are at play and IL-10 is not always required for B-cells to exert immunoregulatory functions (7). However, the phenotypic diversity of Bregs appears to be greater than in Tregs and while Tregs are considered a distinct cell lineage, immune regulation may represent a functional state that many types of B-cells can acquire in the appropriate context(5). A unique and unifying transcription factor such as FoxP3 for Tregs has not been identified for Bregs (8), further lending to the hypothesis of Breg plasticity and functional diversity. Thus far the search for a Breg marker has been limited to its correlation with IL-10 expression in B-cells leading to the identification of a variety of putative Breg markers including Tim-1(9), CD9 (8) and CD1dhigh/CD5+ (10) among others(5).
We and others have previously shown that the induction of transplantation tolerance by B-cells is dependent on Tregs although it remains unclear how B cells cooperate with T-cells to promote tolerance (3). To better characterize their mechanism of action, we questioned whether cells other than B-cells and Tregs are critical to tolerance induction in our model. Since CD1d is highly expressed on IL-10+ B-cells(10), we reasoned that these Bregs might present lipid antigen to restricted invariant Natural Killer T-cells (iNKT). Herein, we assessed whether interactions between Bregs and iNKT cells are essential by depleting NK1.1 positive cells. While we found that Nk1.1+ cells are relevant, we discovered that the presence of NK rather than NKT is required for tolerance. In addition, the expression of CD1d on B-cells was not required to achieve tolerance.
Materials and Methods
Mice
Female BALB/c and male C57BL/6 (B6), B6μMT−/−(μMT – B cell deficient), B6.129S6-Del(3Cd1d2-Cd1d1)1Sbp/J (CD1d−/−) and B6.C3Rl-Lystbg/J (Beige – Nk cell deficient) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). All mice were housed under specific pathogen-free barrier conditions. All procedures were performed under the principles of laboratory animal care and approved by the IACUC committee at the Massachusetts General Hospital.
Hyperglycemia was induced in C57BL/6 mice or B6μMT−/− mice by intraperitoneal injection of 200 mg/kg Streptozotocin (STZ; Sigma-Aldrich, St. Louis, MO) and was defined as blood glucose levels >300 mg/dl for 3 consecutive days. Islets from BALB/c donors were isolated by the previously described technique of enzymatic digestion (Liberase DL, Roche, Indianapolis, IN) and density gradient purification (Euro-collins/Polysucrose; Cellgro, Mediatech, Manassas, VA). 300–400 fresh islets were transplanted under the kidney capsule of diabetic mice. Euglycemia was defined as a non-fasting blood glucose level <200 mg/dl. Islet graft rejection was diagnosed when animals became hyperglycemic again, with blood glucose >200 mg/dl for at least 3 consecutive days. Allograft function was confirmed by nephrectomy of the kidney containing the transplanted islets. All recipients with long term functional grafts became hyperglycemic within 48 h of nephrectomy (data not shown).
Antibody treatment
For induction of tolerance, 100 μg of anti-CD45RB mAb was given i.p. on days 0, 1, 3, 5 and 7 following transplant as well as antagonistic anti-TIM-1 mAb (RMT1–10) on day-1 (500 μg) and on days 0 and 5 (300 μg) post-transplant. Antibodies were purchased from BioXCell Inc. (West Lebanon, NH). Nk1.1+ cells were depleted with 200 μg of anti-NK1.1 (PK136, BioXCell) on days −7,1 and 8.
Cell sorting and transfer
B cells from C57BL/6 mice bearing long-term islet allografts (BALB/c) after combined antibody treatment were selected using Miltenyi anti-CD19 microbeads (Bergisch-Gladbach, Germany). Purity of the resulting B-cell population exceeded 95%. 5×106 B cells were then injected into B6μMT−/− mice that simultaneously received BALB/c islet transplants without antibody treatment. NK cells were isolated from naïve B6 mice using a negative selection kit (EasySep Mouse NK cell isolation kit, Stem cell technologies, Cambridge, MA) with NK cell purities approaching 90% (75–88%), and transferred by tail vein injection into Beige recipients of Balb/c islets.
Flow cytometry
Lymphocytes were prepared from spleen of mice that were transplanted and treated with combined antibody therapy. The following antibodies were used for staining: CD4−(PECy7), B220 (Pacific Blue), Foxp3 (APC), Tim-1/CD365 (clone RMT1-4; biotin), anti-CD49b (clone DX5), Foxp3 staining buffer kit (all from eBioscience, San Diego, CA), streptavidin-A750 (Invitrogen, Grand Island, NY, USA), NK1.1 (PK136, BioXcell, Lebanon, NH). Cells were analyzed on a LSRII cytometer (Becton Dickinson, San Diego, CA, USA), and the results were analyzed with FlowJo software (Treestar, Ashland, OR, USA).
Statistical analysis
For survival studies, Kaplan–Meier survival curves were generated and statistical analysis performed by use of the log-rank (Mantel-Cox) test. The t-test was used to test significance of quantitative differences in immune cell populations. All data were analyzed by the Prism 5 Program (Graph Pad, San Diego, CA, USA). A value of p < 0.05 was considered significant.
Results
Dual Antibody treatment causes quantitative shift in NK and NK-T cells
We observed that in dual antibody (anti-CD45RB, anti-TIM1) mediated islet transplant tolerance, the proportions of NK1.1+ cells are skewed in favor of NK-T cells (Figure 1). While we do not know if this shift is causally associated with tolerance, the expression of CD1d on regulatory B-cells(10) lead us to hypothesize that interactions between Bregs and CD1d restricted invariant NK-T cells are involved in the induction of tolerance.
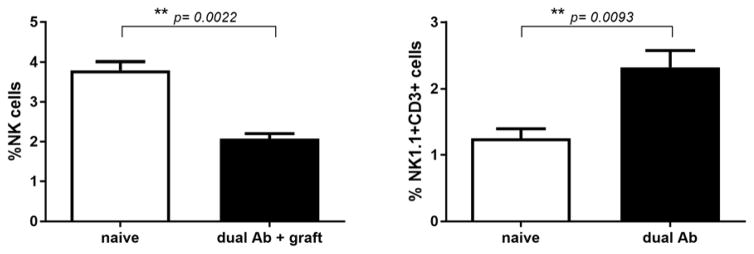
Figure 1. Antibody induced islet transplant tolerance is associated with skewing of NK1.1+ cells.
B6 recipients of Balb/c Islet grafts were rendered tolerant by dual antibody treatment (n=4). 16 days post-transplant, whole splenocytes were isolated for immunophenotyping by flow cytometry. The amount of NK cells was reduced (2.07% vs. 3.8% NK1.1+, DX5+, CD3−; p=0.0022) and proportion of NK-T cells increased by nearly 50 % as compared to naïve control animals (2.46% vs. 1.3% NK1.1+, CD3+; p=0.0093).
NK1.1+ cells are required for induction but not maintenance of islet transplant tolerance
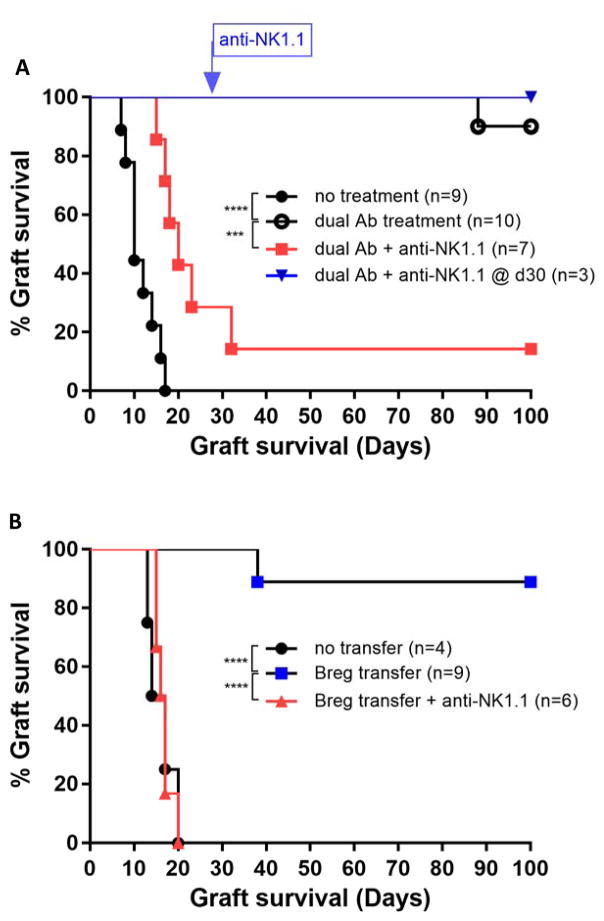
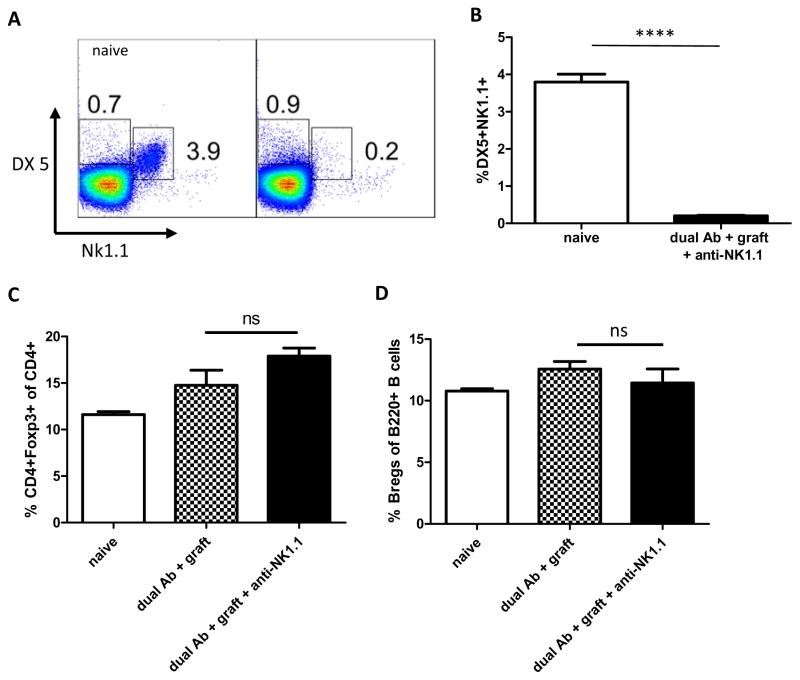
The high rate of long term immunologic tolerance to allogeneic islet grafts achieved by dual Antibody treatment (3), is completely abrogated when the recipient B6 mice are depleted of NK1.1+ cells prior to transplant (Figure 2A). The anti-NK1.1 antibody potently depletes Natural Killer T-cells (NKT) and Natural killer cells (NK) in B6 mice for at least 4 weeks after the injection of a single dose. Homeostatic NK cell repopulation following depletion has been shown to be incomplete (11–14) and does not appear to interfere with B-cell dependent transplant tolerance. Therefore, we assessed whether depletion of NK1.1+ cells abrogates tolerance after tolerance has been established with dual Ab treatment. Interestingly, depletion at 30 days post islet transplant did not affect transplant tolerance persistence (Figure 2A). Depletion of NK cells was confirmed by flow cytometry of recipient mice, 16 days post transplant (Figure 3A+B).
Figure 2. NK1.1+ cells are required for induction but not maintenance of islet transplant tolerance.
(A) Depletion of NK1.1+ cells prior to islet transplantation and dual Antibody treatment abrogates tolerance (p=0.0003,***). NK1.1 cell depletion on day 30 does not break tolerance (B) NK1.1+ cell depletion prevents transfer of islet transplant tolerance by adoptive transfer of Tim1+ Bregs from long-term tolerant islet recipients (p<0.0001.****).
Figure 3. Antibody depletes host NK-cells without altering Treg and Breg populations.
(A+B) NK1.1 Antibody treatment thoroughly depletes NK-cells in whole splenocytes (n=3 B6 mice). Splenocytes were harvested 16 days post Balb/c islet transplant and 8 days after last dose of anti-NK1.1 in mice receiving dual Ab treatment. (p<0.0001.****) (C) Dual Ab treatment and NK1.1 cell depletion does not alter regulatory T-cell (CD4+FoxP3+) quantities (n-3, NS) (D) nor the amount of regulatory B-cells (Bregs defined as B220+/Tim1+) (n=3, NS).
The depletion of NK 1.1+ cells does not result in quantitative changes of regulatory B cells (B220+/Tim1+) or regulatory T-cells (CD25+/FoxP3+) (Figure 3C+D).
Depletion of NK1.1+ cells prevents transfer of donor specific islet transplant tolerance
We have previously demonstrated that Tim1+ B-cells (B220+/Tim1+), from recipient B6 mice that were long term tolerant to Balb/c islet grafts after dual antibody treatment, can transfer tolerance to a syngeneic recipient in a islet donor strain dependent manner (3). When secondary islet recipient B6 mice were depleted of NK1.1+ cells with anti-NK1.1 mAb (PK136), transfer of Tim1+ B-cells from tolerant hosts did not prolong Balb/c islet graft survival (Figure 2B). This suggests that regulatory B-cells directly or indirectly interact with NK1.1+ cells to induce tolerance.
Invariant NK-T cells and B-cell CD1d signaling are dispensable for tolerance induction
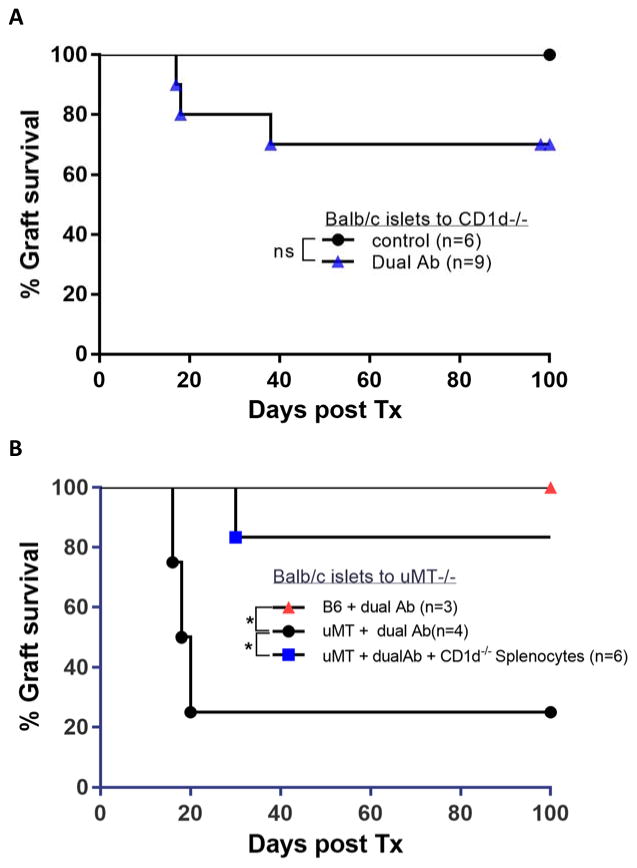
To evaluate whether all NK1.1+ cells or only NK or NK-T cell subsets are involved in tolerance induction, we transplanted CD1d knockout mice (lacking NK-T cells) with Balb/c islets. Antigen presenting cells lack CD1d protein and hence the ability to present lipid antigen. In addition, these mice exhibit low levels of NK-T cell derived cytokines such as IL-4, IL-2, INF-γ, TNF-α and GM-CSF owing to the complete absence of NK-T cell function(15). Unexpectedly, dual antibody treatment rendered >60 % of CD1d deficient recipients tolerant to an allogeneic Balb/c islet graft (Figure 4A). The reduced proportion of tolerant animals compared to wild-type B6 recipients may be attributable to the overall abnormal immune response in these animals, including a stronger Th1 response. The finding that a majority of mice acquired tolerance, albeit fewer than in wt mice, suggests that NKT cells are dispensable for tolerance induction but may have a supporting role.
Figure 4. NK-T cells and CD1d expression on B cells is not required for transplantation tolerance.
(A) Islet graft tolerance can be induced in CD1d−/− mice with dual antibody treatment, albeit with lower efficiency than in wt B6 mice. (p=0.156, NS) (B) B-cell dependent tolerance induction does not rely on the expression of CD1d on B-cells: B-cell deficient μMT mice were reconstituted with Splenocytes from CD1d−/− mice, rescuing islet transplantation tolerance induction with dual Antibody treatment (blue) approaching the 100 % tolerance rate in wt B6 mice (red). In contrast, dual antibody treatment fails to efficiently induce tolerance to allogeneic islet grafts in non-reconstituted, B cell deficient, uMT mice(black) (p=0.033,*).
Furthermore, to specifically probe the role of CD1d expression on regulatory B-cells, we transferred splenocytes from CD1d−/− mice to B-cell deficient mice and transplanted these reconstituted mice with Balb/c islets. Dual antibody treatment was able to induce tolerance in these mice, despite the lack of CD1d expression on B-cells (Figure 4B). Therefore, CD1d restricted lipid antigen presentation by Bregs to iNKT cells is not a requisite for the establishment of transplantation tolerance (Figure 4B).
NK cell deficiency prevents transplantation tolerance
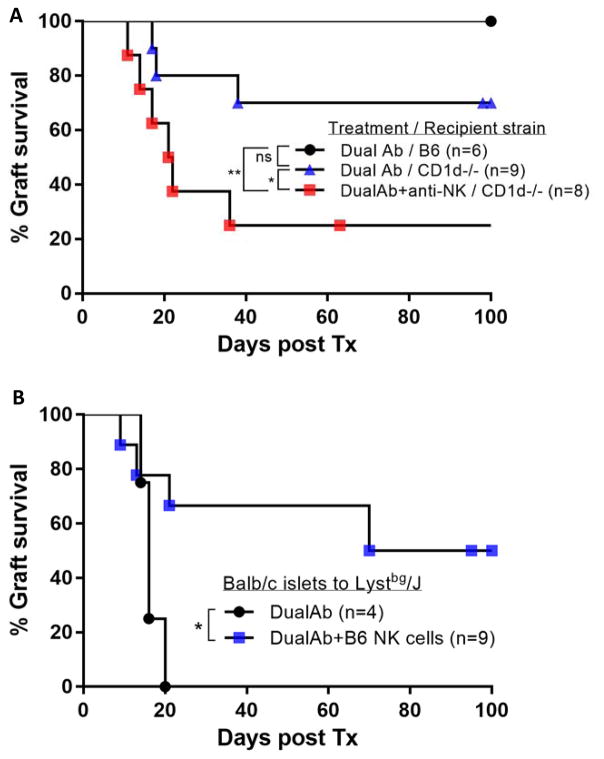
In the absence of NK-T cells in the CD1d−/− recipient model, we tested whether depletion of other NK1.1+ cells, specifically NK cells, would further abrogate tolerance. Although not statistically significant, depletion of NK cells with anti-NK1.1 antibody (clone Pk136), reduced the number of long term tolerant mice (Figure 5A).
Figure 5. NK cells are required for islet transplant tolerance.
(A) Depletion of Nk1.1+ cells in NK-T cell deficient CD1d−/− mice further reduces the amount of long term tolerant mice (dual Ab vs. dual Ab+aNK p=0.0428,*; control vs. dual Ab+aNK, p=0.0079,**) (B) Beige mice (Lystbg/J) are resistant to tolerance induction by dual antibody treatment. Transfer of functional NK cells, isolated from B6 Splenocytes, restores the tolerizing effect of dual antibody treatment (p=0.0342,*)
To further assess the requirement of NK-cells in this tolerance model, Beige mice on a B6 background were transplanted with Balb/c islets and treated with dual antibodies. Beige mice have a complex immune deficiency secondary to a lysosomal defect that prevents the formation of functional endosomes. However, a dominant defect is the lack of functional NK-cells in these mice making them a suitable model to study NK-cell function. In Beige mice, dual antibody treatment could not significantly prolong islet graft survival for more than a few days when compared to wt B6 mice (Figure 5B). Reconstitution of NK cells from wild-type B6 mice restores tolerance in these mice, further suggesting that it is the defect in NK cell function rather than the impaired function of other subsets that prevents the induction of transplant tolerance (Figure 5B).
Discussion
In our effort to elucidate the mechanism underlying Breg dependent transplantation tolerance, induced by treatment with the antibodies anti-CD45RB and anti-TIM1, we found that natural killer cells play an integral part in the induction of tolerance. We and others have previously shown that antibody induced tolerance models are dependent on regulatory B-cells (1, 3, 9). The preceding decade of tolerance research has revealed much about the biology of regulatory T-cells. However, as our capability of interrogating complex biological networks expands(16), it is becoming increasingly clear that many immune cells possess regulatory functions(17). Regulatory B-cells have recently emerged as a potent immunoregulatory element that is being studied intensively(5, 18). Natural killer cells have long been viewed as the only effector cells of the innate immune system, capable of distinguishing between self and non-self and attacking infected as well as transformed tumor cells. New subsets of innate effector cells have since been discovered and classified as innate lymphoid cells (ILC)(19). More recently it has become apparent that NK cells harbor many of the characteristics of the adaptive immune system, including clonal expansion and memory formation(20, 21), antigen specific receptors(22) and an MHC dependent education process(23). Furthermore, NK cells have previously been implicated in transplantation tolerance(24–26) and immune regulation. Even though the immune regulatory role of NK cells has been recognized over 10 years ago(13), the precise underlying mechanisms have not been well delineated.
Thus far our study has focused on interactions between regulatory B-cells and regulatory T-cells based on the observation that the dual antibody treatment induced tolerance is dependent on the presence of both(1, 3). The presented findings add another layer of complexity to the tolerance mechanism as it is dependent on a third cell type, NK1.1+ NK-cells. In B-cell deficient, but NK cell replete μMT mice, tolerance cannot be induced with dual AB treatment(3). In addition, NK-cell depletion in B-cell deficient μMT mice prevents tolerance induction by transferred tolerogenic B-cells, further emphasizing the interdependence of the two cell types(3). While Bregs are required for maintenance of tolerance beyond day 9 post-transplant (3), we found that depletion of NK-cells after 30 days does not thwart tolerance in this transplant model. Therefore, early post-transplant interactions of NK-cells with Bregs are required for tolerance induction but thereafter Bregs maintain tolerance without the need for NK cells. This may be interpreted in a way that Bregs depend on NK cell help for their development, but Bregs and not NK-cells actively suppress the allogeneic immune response. However, in other models of transplant tolerance and immunity, NK cells have been found to actively control the immune response by suppressing the activity of alloreactive CD8+ T-cells(27), monocytes and macrophages(12), CD4+ T-cells and follicular helper T-cells as well as B-cells (11). We did not observe a quantitative effect on Bregs or Tregs in NK1.1 depleted mice (Figure 3C,D), but the donor specific regulatory function may depend on them.
In tumor and infection models Bregs can suppress the immune responses of NK cells and CD8+ T-cells. Activated by CD40L on tumor cells, Bregs secrete IL-10 which suppresses Interferon-γ production by NK-cells(28). In an IL-10 independent mechanism, intratumoral B cells recruit and expand Tregs thereby preventing infiltration of CD49+ NK cells and CD8+ T-cells(29). Similarly, in an infection model, mice that have B-cells deficient in MyD88 and TLR2 exhibit a deficient IL-10 response in B-cells, the activity of NK-cells, neutrophils and T-cells is increased and a Salmonella typhi infection is cleared more effectively(30). In contrast to this Breg mediated restraint of NK-cell activity, the results presented here and by other investigators (12, 25, 27, 31) may suggest that Bregs and NK cells either act synergistically in regulating the allogeneic immune response or that NK cells provide help to Bregs.
A classical view of NK-cell functions involves the monitoring of the cell surface for markers of self-identity such as MHC Class I and others as well as markers for cellular stress and/or malignant transformation. In addition, a more complex regulation of their function has emerged in recent years; NK-cells exert a constant tension between engagement of a plethora of activating and inhibiting receptors(32). NK cells were found to exhibit remarkable phenotypical and functional diversity and dynamic plasticity. Apart from conventional NK cells (cNK) in the spleen, blood and other organs several characteristic tissue resident (trNK) cells have been identified (33). The environment is able to influence the development and phenotype of NK cells(34), possibly including the influence of Bregs. Observations in virus infection suggest that NK-cells may target and reduce the amount of follicular T-helper cells to regulate the activation of B-cells in the spleen(11, 35). Therefore, NK cells may not directly act to exert immunoregulatory function but may influence B-cell development, phenotype and activity.
Our finding of NK-cell involvement in dual antibody induced transplantation tolerance echoes a number of previous studies using a variety of antibodies to induce transplantation tolerance(12, 25, 26, 31). In these studies, it has been demonstrated that MHC class I expression and therefore missing-self mechanism is not required to induce tolerance with anti-CD40L or anti-LFA1 antibodies, while NK-cell perforin was essential(25). The target of NK cell toxicity had not been identified but it did not appear to be donor APCs(25). Using the immunologic more rigorous skin transplant model, two independent studies have suggested that NK cells mediate tolerance by targeting donor derived antigen presenting cells (APC) (26, 31). Other studies into the immunoregulatory function of NK-cells have demonstrated the involvement of cytokines secreted by NK cells (i.e. IFNγ, TNFα and GM-CSF). However, similar to Treg and Bregs, Il-10 has been shown to be secreted by NK cells with regulatory function(36). In turn it has also been shown that TGF-β secreted by regulatory T-cells can downregulate NK-cell cytotoxicity(37).
Finally, an additional NK1.1+ cell type has recently been identified, which exhibits hallmarks of both B-cells and NK-cells. These cells develop from B-cell precursors and lack the cytotoxic granules and receptor diversity of NK-cells. In mycobacterial infection, they activate NK cells and therefore are the earliest effector cells that activate the immune system towards invading pathogens. These so called NK like B-cells (NKB) may also be depleted in the islet recipient mice and may be required for the induction of allogeneic transplant tolerance(38). iNKT cells also exhibit considerable diversity reminiscent of TH1,2 and 17 cells, including a Nk1.1− population in B6 mice (39). We did not find convincing evidence that iNKT cells play a key role in this tolerance model.
While this and other studies, establish a significant role of NK-cells in transplantation tolerance, the findings are limited because the underlying mechanisms remain unclear. We cannot yet explain how NK-cells, Bregs and Tregs converge on regulating the alloimmune response. Furthermore, some of the animal models used in this work have complex immune alterations with potentially unaccounted effects on outcomes. Finally, it is unclear if the presented results in mice are relevant to human immunology.
In summary, we have identified an essential role for NK cells in B-cell dependent transplantation tolerance. The results presented herein suggest that the two cell types are interdependent in the induction but not maintenance of tolerance. We are currently working to elucidating the underlying mechanisms in greater detail. The recent appreciation of a remarkable functional diversity, plasticity and redundancy of both cell types in regulation of the immune response(5, 33) opens a number of possible mechanism that may be specific to each tolerance model or act simultaneously and interdependently.
Acknowledgments
This work was supported by the National Institutes of Health (NIH; RO1AI057851-12).
Abbreviations
- NK
Natural Killer cell
- APC
Antigen presenting cell
- Breg
regulatory B-cell
- Treg
regulatory T-cell
- CD
Cluster of differentiation
- STZ
Streptozotocin
- INF
Interferon
- TNF
Tumor necrosis factor
- cNK
conventional NK cell
- trNK
tissue resident NK cell
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Deng S, Moore DJ, Huang X, Lian MM, Mohiuddin M, Velededeoglu E, Lee MKt, Sonawane S, Kim J, Wang J, Chen H, Corfe SA, Paige C, Shlomchik M, Caton A, Markmann JF. Cutting edge: transplant tolerance induced by anti-CD45RB requires B lymphocytes. Journal of immunology. 2007;178(10):6028–6032. doi: 10.4049/jimmunol.178.10.6028. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z, Lazarovits A, Grant D, Garcia B, Stiller C, Zhong R. CD45RB monoclonal antibody induces tolerance in the mouse kidney graft, but fails to prevent small bowel graft rejection. Transplantation proceedings. 1996;28(5):2514. [PubMed] [Google Scholar]
- 3.Lee KM, Kim JI, Stott R, Soohoo J, O’Connor MR, Yeh H, Zhao G, Eliades P, Fox C, Cheng N, Deng S, Markmann JF. Anti-CD45RB/anti-TIM-1-induced tolerance requires regulatory B cells. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(8):2072–2078. doi: 10.1111/j.1600-6143.2012.04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee KM, Stott RT, Zhao G, SooHoo J, Xiong W, Lian MM, Fitzgerald L, Shi S, Akrawi E, Lei J, Deng S, Yeh H, Markmann JF, Kim JI. TGF-β-producing regulatory B cells induce regulatory T cells and promote transplantation tolerance. European journal of immunology. 2014;44(6):1728–1736. doi: 10.1002/eji.201344062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42(4):607–612. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Stolp J, Turka LA, Wood KJ. B cells with immune-regulating function in transplantation. Nature reviews Nephrology. 2014;10(7):389–397. doi: 10.1038/nrneph.2014.80. [DOI] [PubMed] [Google Scholar]
- 7.Kim JI, Rothstein DM, Markmann JF. Role of B cells in tolerance induction. Current opinion in organ transplantation. 2015;20(4):369–375. doi: 10.1097/MOT.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun J, Wang J, Pefanis E, Chao J, Rothschild G, Tachibana I, Chen JK, Ivanov II, Rabadan R, Takeda Y, Basu U. Transcriptomics Identify CD9 as a Marker of Murine IL-10-Competent Regulatory B Cells. Cell reports. 2015;13(6):1110–1117. doi: 10.1016/j.celrep.2015.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, Chalasani G, Sayegh MH, Najafian N, Rothstein DM. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. The Journal of clinical investigation. 2011;121(9):3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28(5):639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Rydyznski C, Daniels KA, Karmele EP, Brooks TR, Mahl SE, Moran MT, Li C, Sutiwisesak R, Welsh RM, Waggoner SN. Generation of cellular immune memory and B-cell immunity is impaired by natural killer cells. Nature communications. 2015;6:6375. doi: 10.1038/ncomms7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Touw W, Burrell B, Lal G, Bromberg JS. NK cells are required for costimulatory blockade induced tolerance to vascularized allografts. Transplantation. 2012;94(6):575–584. doi: 10.1097/TP.0b013e318264d3c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. The Journal of experimental medicine. 1997;186(10):1677–1687. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seaman WE, Sleisenger M, Eriksson E, Koo GC. Depletion of natural killer cells in mice by monoclonal antibody to NK-1.1. Reduction in host defense against malignancy without loss of cellular or humoral immunity. Journal of immunology. 1987;138(12):4539–4544. [PubMed] [Google Scholar]
- 15.Smiley ST, Kaplan MH, Grusby MJ. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 1997;275(5302):977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 16.Spitzer MH, Gherardini PF, Fragiadakis GK, Bhattacharya N, Yuan RT, Hotson AN, Finck R, Carmi Y, Zunder ER, Fantl WJ, Bendall SC, Engleman EG, Nolan GP. IMMUNOLOGY. An interactive reference framework for modeling a dynamic immune system. Science. 2015;349(6244):1259425. doi: 10.1126/science.1259425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bezie S, Picarda E, Ossart J, Martinet B, Anegon I, Guillonneau C. Compensatory Regulatory Networks between CD8 T, B, and Myeloid Cells in Organ Transplantation Tolerance. Journal of immunology. 2015;195(12):5805–5815. doi: 10.4049/jimmunol.1500473. [DOI] [PubMed] [Google Scholar]
- 18.Stolp J, Turka LA, Wood KJ. B cells with immune-regulating function in transplantation. Nature reviews Nephrology. 2014;10(7):389–397. doi: 10.1038/nrneph.2014.80. [DOI] [PubMed] [Google Scholar]
- 19.Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348(6237):aaa6566. doi: 10.1126/science.aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Sullivan TE, Sun JC, Lanier LL. Natural Killer Cell Memory. Immunity. 2015;43(4):634–645. doi: 10.1016/j.immuni.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, Yokoyama WM. Specific and nonspecific NK cell activation during virus infection. Nature immunology. 2001;2(10):951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 24.Benichou G, Yamada Y, Aoyama A, Madsen JC. Natural killer cells in rejection and tolerance of solid organ allografts. Current opinion in organ transplantation. 2011;16(1):47–53. doi: 10.1097/MOT.0b013e32834254cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beilke JN, Kuhl NR, Van Kaer L, Gill RG. NK cells promote islet allograft tolerance via a perforin-dependent mechanism. Nature medicine. 2005;11(10):1059–1065. doi: 10.1038/nm1296. [DOI] [PubMed] [Google Scholar]
- 26.Yu G, Xu X, Vu MD, Kilpatrick ED, Li XC. NK cells promote transplant tolerance by killing donor antigen-presenting cells. The Journal of experimental medicine. 2006;203(8):1851–1858. doi: 10.1084/jem.20060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lantow M, Eggenhofer E, Sabet-Baktach M, Renner P, Rovira J, Koehl GE, Schlitt HJ, Geissler EK, Kroemer A. CD27low natural killer cells prolong allograft survival in mice by controlling alloreactive CD8+ T cells in a T-bet-dependent manner. Transplantation. 2015;99(2):391–399. doi: 10.1097/TP.0000000000000585. [DOI] [PubMed] [Google Scholar]
- 28.Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer research. 2006;66(15):7741–7747. doi: 10.1158/0008-5472.CAN-05-3766. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Eliav Y, Shin SU, Schreiber TH, Podack ER, Tadmor T, Rosenblatt JD. B lymphocyte inhibition of anti-tumor response depends on expansion of Treg but is independent of B-cell IL-10 secretion. Cancer immunology, immunotherapy: CII. 2013;62(1):87–99. doi: 10.1007/s00262-012-1313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neves P, Lampropoulou V, Calderon-Gomez E, Roch T, Stervbo U, Shen P, Kuhl AA, Loddenkemper C, Haury M, Nedospasov SA, Kaufmann SH, Steinhoff U, Calado DP, Fillatreau S. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity. 2010;33(5):777–790. doi: 10.1016/j.immuni.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 31.Haspot F, Séveno C, Dugast A-SS, Coulon F, Renaudin K, Usal C, Hill M, Anegon I, Heslan M, Josien R, Brouard S, Soulillou J-PP, Vanhove B. Anti-CD28 antibody-induced kidney allograft tolerance related to tryptophan degradation and TCR class II B7 regulatory cells. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(10):2339–2348. doi: 10.1111/j.1600-6143.2005.01018.x. [DOI] [PubMed] [Google Scholar]
- 32.Martinet L, Smyth MJ. Balancing natural killer cell activation through paired receptors. Nature Reviews Immunology. 2015;15(4):243–254. doi: 10.1038/nri3799. [DOI] [PubMed] [Google Scholar]
- 33.Erick TK, Brossay L. Phenotype and functions of conventional and non-conventional NK cells. Current opinion in immunology. 2016;38:67–74. doi: 10.1016/j.coi.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cichocki F, Sitnicka E, Bryceson YT. NK cell development and function--plasticity and redundancy unleashed. Seminars in immunology. 2014;26(2):114–126. doi: 10.1016/j.smim.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Cook KD, Kline HC, Whitmire JK. NK cells inhibit humoral immunity by reducing the abundance of CD4+ T follicular helper cells during a chronic virus infection. Journal of leukocyte biology. 2015;98(2):153–162. doi: 10.1189/jlb.4HI1214-594R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maroof A, Beattie L, Zubairi S, Svensson M, Stager S, Kaye PM. Posttranscriptional regulation of II10 gene expression allows natural killer cells to express immunoregulatory function. Immunity. 2008;29(2):295–305. doi: 10.1016/j.immuni.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, Lecesne A, Robert C, Blay JY, Bernard J, Caillat-Zucman S, Freitas A, Tursz T, Wagner-Ballon O, Capron C, Vainchencker W, Martin F, Zitvogel L. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. The Journal of experimental medicine. 2005;202(8):1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang S, Xia P, Chen Y, Huang G, Xiong Z, Liu J, Li C, Ye B, Du Y, Fan Z. Natural Killer-like B Cells Prime Innate Lymphocytes against Microbial Infection. Immunity. 2016;45(1):131–144. doi: 10.1016/j.immuni.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 39.Georgiev H, Ravens I, Benarafa C, Forster R, Bernhardt G. Distinct gene expression patterns correlate with developmental and functional traits of iNKT subsets. Nature communications. 2016;7:13116. doi: 10.1038/ncomms13116. [DOI] [PMC free article] [PubMed] [Google Scholar]