Abstract
Chimpanzees (Pan troglodytes) demonstrate much cultural diversity in the wild, yet a majority of novel behaviours do not become group-wide traditions. Since many such novel behaviours are introduced by low-ranking individuals, a bias toward copying dominant individuals (‘rank-bias’) has been proposed as an explanation for their limited diffusion. Previous experimental work showed that chimpanzees (Pan troglodytes) preferentially copy dominant over low-rank models. We investigated whether low ranking individuals may nevertheless successfully seed a beneficial behaviour as a tradition if there are no ‘competing’ models. In each of four captive groups, either a single high-rank (HR, n=2) or a low-rank (LR, n=2) chimpanzee model was trained on one method of opening a two-action puzzle-box, before demonstrating the trained method in a group context. This was followed by eight hours of group-wide, open-access to the puzzle-box. Successful manipulations and observers of each manipulation were recorded. Barnard’s exact tests showed that individuals in the LR groups used the seeded method as their first-choice option at significantly above chance levels, whereas those in the HR groups did not. Furthermore, individuals in the LR condition used the seeded method on their first attempt significantly more often than those in the HR condition. A network-based diffusion analysis revealed that the best supported statistical models were those in which social transmission occurred only in groups with subordinate models. Finally, we report an innovation by a subordinate individual that built cumulatively on existing methods of opening the puzzle-box and was subsequently copied by a dominant observer. These findings illustrate that chimpanzees are motivated to copy rewarding novel behaviours that are demonstrated by subordinate individuals and that, in some cases, social transmission may be constrained by high-rank demonstrators.
Keywords: Social learning, rank, dominance, chimpanzee, culture
INTRODUCTION
It is now generally accepted that social learning is widespread in the animal kingdom and that socially transmitted traditions (‘cultures’) are found in a wide range of vertebrates [Whiten, 2005; Laland & Janik, 2006; Laland & Galef, 2009]. However, the processes by which a novel behaviour propagates to become a group-wide tradition remain unclear [Rendell et al., 2011]. Indiscriminately copying the behaviours of conspecifics is often not an optimal strategy, as the learner runs the risk of copying costly behaviours or wasting energy on those that are not productive [Kendal, Coolen, van Bergen & Laland, 2005; Rendell et al., 2010]. Accordingly, a number of adaptive ‘biases’ in social learning have been proposed as possible influences on whether individuals choose to utilise social information and who they get it from, for example ‘when uncertain, copy the majority’ [Henrich & McElreath, 2003; Laland, 2004; Claidière & Whiten, 2012; van Leeuwen & Haun, 2014]. Due to their cultural diversity [Whiten et al. 1999] and propensity for social learning, chimpanzees have been a favoured model species for studying these social learning biases. Chimpanzees also present an interesting paradox in that although innovations are not an uncommon occurrence, at one field site where researchers made an attempt to quantify their fate it was found that a majority of innovations failed to become group-wide traditions [Nishida, Matsusaka & McGrew, 2009]. The factors that determine whether a novel behaviour diffuses throughout a group or remains limited to one or a minority of individuals are largely unknown. The direct pay-off of a behaviour does not seem sufficient to explain this, given reported instances of the spread of ‘arbitrary’ traditions with no apparent functional benefits. A striking example of this is described by van Leeuwen, Cronin & Haun [2014], who report a single chimpanzee placing a piece of grass in its ear to no discernible benefit - a ‘fashion’ which was soon adopted by the rest of the group. Conversely, Hopper et al. [2011] found in a token-exchange task that most chimpanzees chose the same tokens as those selected by a trained model, even when the alternative token choice resulted in a more preferred food reward, presenting an interesting example of copying a behaviour which is visibly less beneficial than alternatives.
Many novel behaviours enter both wild and captive chimpanzee communities through the lower end of the dominance hierarchy – whether this be from subordinate innovators [Reader & Laland, 2001] or migrant females importing their native behavioural repertoire to their host group [Nakamura & Uehara, 2004; O’Malley, Wallauer, Murray & Goodall, 2012]. A bias toward copying dominant over subordinate individuals has been shown and proposed to explain the relative rarity of these novel behaviours becoming traditions [Kendal et al., 2015]. One might suppose that this would occur for strategic reasons (dominant individuals are successful, so copying them might be an adaptive option), due to normative effects (copying the dominant individual facilitates social cohesion) or simply as a result of an attentional bias towards these individuals (e.g. dominant individuals are central in the social network). In capuchin monkeys it has been found that subordinate individuals tend not to demonstrate acquired token-exchange behaviours in a group context [Addessi et al. 2011] or in the presence of a dominant individual [Lonsdorf et al., 2016], which means there is an inherent rank-bias in the source of social information available to observers. Although it has also been found that capuchins preferentially observe older, more dominant and more proficient nut-crackers in the wild, suggesting a more active learning bias [Coelho et al., 2015]. One or all of these may play a part in restricting the flow of social information from subordinate individuals and cause a group-wide convergence on the behaviour of dominant individuals. To date, two studies have offered evidence for a rank-bias in chimpanzees. Kendal et al. [2015] seeded a method of opening a two-action puzzle box into two groups of chimpanzees using mid-ranking female models (and allowed two other groups to explore the task without trained models), and through complex analysis of attention states during demonstrations found evidence that individuals preferentially attend to dominant and/or knowledgeable demonstrators. Horner et al. [2010] also concluded that when presented with demonstrations from both a ‘high prestige’ (high rank and track record as a model) and ‘low prestige’ (low rank) individual on a token-exchange task, chimpanzees preferentially copied the method demonstrated by the high prestige individual [Horner et al., 2010]. However, there remains the question of whether or not low-ranking individuals, who demonstrate a productive novel behaviour, will be copied if there are no more dominant models available. This question is important for our understanding of how innovations become traditions, and how traditions proliferate across communities.
Accordingly, we compared the diffusion of alternative methods of opening a two-action puzzle-box seeded by either a low- (female) or high-ranking (male) individual in four different groups of chimpanzees. In this context, based on prior work indicating a rank-bias in chimpanzee social learning, we predicted that either (a) social transmission of the seeded method will only occur in the groups with high-ranking models (we shall call this the ‘hard rank-bias hypothesis’), or (b) behaviour will be socially transmitted in both conditions, but the effect will be stronger in groups with high-ranking models (‘soft rank-bias hypothesis’).
METHODS
Study Site
This study was carried out at the National Center for Chimpanzee Care (NCCC) located at the Michale E. Keeling Center for Comparative Medicine and Research of The University of Texas MD Anderson Cancer Center in Bastrop (UTMDACC), Texas. Data was collected between April and August, 2015. A total of 38 chimpanzees (21 female) participated in the study, aged from 13 to 53 years of age. Most individuals were captive-born, but some (n=5) were wild-born. All individuals have participated in a wide range of previous behavioural research studies, some of which included puzzle-box tasks, but we have designed our apparatus to require different manipulations to those of earlier studies, as noted below. The participants include both nursery-reared and mother-reared individuals. Following previous studies [Horner et al. 2010, Kendal et al., 2015; Hopper et al., 2015a], the social rank of each individual was determined by surveying the judgments of 5 staff members (behavioural researchers, trainers and management) who had been working with these animals for at least 5 years each. Freeman et al. [2013] found that human assessment of dominance in chimpanzees has good predictive validity for relevant behavioural measures of dominance such as aggression and displacement. Each staff member was asked to rank the individuals in the group linearly from ‘1’ (highest rank) to N (lowest rank) without discussing their rankings with other staff. Agreement between observers was high (>80%), but where disagreements occurred the mode rank for each individual was used. From these rankings we determined the ‘alpha’ male for each group in the HR condition and chose a subordinate (averaging in the lower third of the hierarchy) female to act as the model for each group in the LR condition. All groups have access to two or more den areas (14m2 each) and either an outdoor habitat or dome (dome: 90m2, habitat: 400m2) with a range of enrichment devices and activities, and a variety of climbing and swinging structures to promote species-typical behaviours. Testing generally occurred indoors, but access to outdoor enclosures was not restricted. The full demographic and housing information for each participating individual can be found in Table 1. Ethical approval for this study was granted by the School of Psychology & Neuroscience at the University of St Andrews and the IACUC of UTMDACC, adhering to all the legal requirements of US law and the American Society of Primatologists’ principles for the ethical treatment of non-human primates. All subjects voluntarily participated in the testing procedures.
Table 1.
Demographic information for all participating individuals. Condition: HR = High rank model, LR = Low-rank model. Asterisk next to name indicates individual was the trained model for their group.
| ID | CONDITION | SEX | WILD BORN? | DOB | REARING | HOUSING |
|---|---|---|---|---|---|---|
| RAD | LR (1) | M | N | 14/01/1990 | MOTHER | HABITAT |
| ANG* | LR (1) | F | Y | 01/01/1975 | UNKNOWN | HABITAT |
| CHE | LR (1) | F | N | 09/12/1990 | NURSERY | HABITAT |
| KIH | LR (1) | F | N | 06/08/1988 | NURSERY | HABITAT |
| MAH | LR (1) | M | N | 26/10/1988 | MOTHER | HABITAT |
| NAH | LR (1) | F | N | 04/07/1990 | NURSERY | HABITAT |
| AKI | LR (2) | M | N | 09/02/1980 | NURSERY | DOME |
| CEC* | LR (2) | F | N | 24/02/1991 | MOTHER | DOME |
| HAA | LR (2) | M | N | 30/12/1991 | MOTHER | DOME |
| MAR | LR (2) | F | Y | 01/01/1966 | UNKNOWN | DOME |
| MART | LR (2) | F | Y | 01/01/1965 | UNKNOWN | DOME |
| TAS | LR (2) | F | N | 18/11/1992 | MOTHER | DOME |
| ZOE | LR (2) | F | N | 13/04/2002 | MOTHER | DOME |
| BRI | HR (1) | F | N | 31/08/1995 | MOTHER | DOME |
| CHI | HR (1) | M | N | 25/08/1988 | MOTHER | DOME |
| MAG | HR (1) | M | N | 24/07/1992 | MOTHER | DOME |
| MAN | HR (1) | F | N | 08/09/1984 | MOTHER | DOME |
| NIC* | HR (1) | M | N | 07/04/1988 | MOTHER | DOME |
| BER | HR (2) | F | N | 18/02/1978 | NURSERY | DOME |
| JUD* | HR (2) | M | N | 26/08/1990 | NURSERY | DOME |
| KOB | HR (2) | M | Y | 01/01/1972 | UNKNOWN | DOME |
| QUI | HR (2) | F | Y | 01/01/1971 | UNKNOWN | DOME |
| TUL | HR (2) | F | N | 01/05/1980 | MOTHER | DOME |
| TOD | CONTROL | F | Y | 01/01/1971 | UNKNOWN | DOME |
| SAB | CONTROL | F | Y | 01/01/1968 | UNKNOWN | DOME |
| PEP | CONTROL | F | Y | 01/01/1967 | UNKNOWN | DOME |
| ALP | CONTROL | F | N | 08/11/1984 | MOTHER | DOME |
| BET | CONTROL | F | N | 23/06/1994 | MOTHER | DOME |
| BIL | CONTROL | M | N | 16/06/1993 | MOTHER | HABITAT |
| BO | CONTROL | M | N | 16/05/1993 | MOTHER | HABITAT |
| JOE | CONTROL | M | Y | 01/01/1972 | UNKNOWN | DOME |
| MAY | CONTROL | F | Y | 01/01/1965 | UNKNOWN | DOME |
| MOO | CONTROL | M | Y | 01/01/1971 | UNKNOWN | DOME |
| GRE | CONTROL | M | Y | 01/01/1970 | UNKNOWN | DOME |
| AJA | CONTROL | M | N | 01/01/1978 | UNKNOWN | DOME |
| LUL | CONTROL | F | N | 16/01/1982 | MOTHER | DOME |
| TAB | CONTROL | M | N | 25/08/1991 | MOTHER | DOME |
| KUD | CONTROL | M | N | 07/12/1982 | MOTHER | DOME |
Apparatus
This study employed a two-action, sliding-door puzzle-box (the ‘Vert’, see Figure 1), a vertical variation we designed to require different actions to those common to earlier social learning studies [Aplin et al., 2015; Hopper, Lambeth, Schapiro & Whiten, 2008; Kendal et al. 2015].
Figure 1.
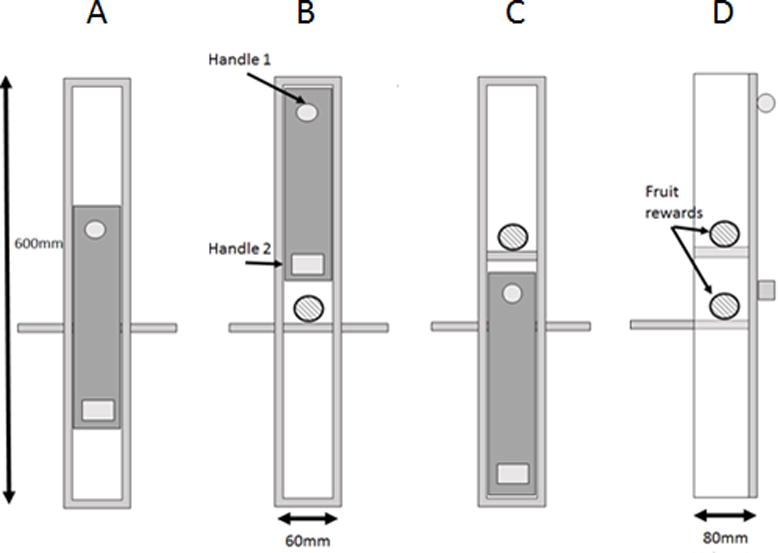
The ‘vertical artificial vegetable’ (the ‘Vert’) could be opened to reveal a food reward either by sliding the door entirely upwards (B) or entirely downwards (C). The resting position on presentation is shown in (A). The side-profile is shown in (D). Upon a completed opening, the door locked so as to restrict access to the alternative reward. The anchor platform was attached to a trolley with vice clamps.
Sessions were recorded using a Panasonic HC-X920 video camera. All videos were coded using BORIS, version 2.05 (www.http://penelope.unito.it/boris). All analyses were carried out using R Statistical Package Version 3.2.3 [2015] with R Studio Version 0.99.491 [2015].
Procedure
For Condition LR (low-rank), in two groups (n=6, 7), a low-ranking female individual was voluntarily separated and trained to open the door by either sliding the door up or down. Likewise in the HR (high-rank) condition, the dominant male of each of two groups (n = 5, 5) was trained on a method of opening the Vert. Females were used for Condition LR and males for Condition HR to maximise the rank disparity between these individuals. Since males are almost always of higher social rank than females in chimpanzee communities, in some groups it would not have been possible to select a high-ranking female to act as a model. However, Kendal et al. [2015] found no bias in whether male or female chimpanzees were preferentially attended to during their experiments, so we would not anticipate sex acting as a confound here. Nevertheless, below we include an analysis of audience sizes during demonstrations of the present study in order to explore whether males and females may differently tolerate observers. LR and HR conditions differed only in the choice of model.
Training began by presenting the baited Vert to the test subject with one of the slide-directions locked so it could not be used. Once a reward had been retrieved successfully 10 times in a row, the alternative method was unlocked and baited for all further trials. Models were considered to be ‘trained’ once they completed a total of 30 sequential uses of the trained method without deviation.
After being trained, the model was reintroduced to the group and given access to the Vert in a group context. Two 20-minute demonstration sessions were carried out on subsequent days, during which only the model had access to the box. The Vert was gently pulled out of reach if another individual displaced the model. This was to ensure a roughly equal number of demonstrations between dominant and subordinate models and make the methods comparable with previous work on rank-bias and social learning [Horner et al. 2010; Kendal et al. 2015; Hopper et al. 2015b].
The demonstration period was followed by 8 hours of open-diffusion in which unrestricted access to the Vert was provided. Open-diffusion occurred across multiple sessions, typically of 60 minutes but varying between 45 minutes (due to unforeseen interruptions) and 120 minutes in length (group HR2 had an unavoidably condensed test period, resulting in longer sessions to make up time).
Once any individual in the demonstration or open-diffusion phase had retrieved a reward, the Vert was withdrawn one metre, the door was reset and the reward chamber re-baited. When re-setting the door, the Vert was covered with a cloth to avoid possible directional cues from the experimenter.
To determine whether an inherent directional bias may have influenced which method individuals from experimental groups chose to use, 15 individuals were selected from non-experimental groups to participate in an asocial control condition. Individuals were selected based on advice from care staff about their willingness to voluntarily separate from the group and engage with research procedures. Individuals separated voluntarily from their group and were then presented with the Vert for a period of 20 minutes each. Both reward chambers were baited and both methods of opening the door were unlocked. If an individual completed a successful manipulation of the Vert, the Vert was reset and baited as described above.
Statistical analyses
We used binomial tests to determine whether the number of individuals in the control condition to use each method on their first trial differed significantly from chance (50%), which would indicate an inherent directional bias that would have acted as a confound. We then used Barnard’s exact test, an alternative to Fisher’s exact test with greater power for small sample sizes [Mehta & Senchaudhuri, 2003], to test whether individuals from high or low rank conditions were significantly more likely to use the seeded method on their first successful trial. Binomial tests were subsequently used to determine whether the proportion of individuals in each condition who used the seeded method on their first successful trial differed significantly from chance (50%). Finally, we applied the same tests to a more conservative, truncated form of the experimental data set. In order to mitigate the possibility that individuals had learned from individuals not of direct interest to the research question, for example a dominant female who had asocially learned the same method as the subordinate model, we only analysed data (for this analysis only) from individuals in both conditions who had only observed their group’s model demonstrating. This resulted in 11 individuals being excluded from this model, leaving n=8. We also carried out Bayesian equivalents of the analyses described above, which can be found in the Supplemental Material by an interested reader and which were consistent with the findings reported below.
Network-based diffusion analysis
Network-based diffusion analysis (NBDA) is a powerful method of determining whether an observed pattern of acquisition of behaviours is consistent with the predictions of a group’s social network [Franz & Nunn, 2009; Hoppitt, Boogert & Laland., 2010; Allen, Weinrich, Hoppitt & Rendell, 2013; Hobaiter, Poisot, Zuberbuhler, Hoppitt & Gruber, 2014]. In this case, the social network was created using the number of times Individual A observed Individual B using the seeded method before Individual A first demonstrated this method. Because we were able to record the exact times at which an individual first used the method, we used the Time of Acquisition Diffusion Analysis (TADA) variant of NBDA [Hoppitt et al., 2010]. Times entered into the model were the number of seconds which the group had been exposed to the Vert before a given individual first opened it using the seeded method.
We used an information theoretic approach [Burnham & Anderson, 2002], using Akaike’s information criterion corrected for sample size (AICc) from which total Akaike weights (Σwi) for each model were calculated. Total Akaike Weights were then used to create model averaged estimates for the factor by which individuals’ learning rates are increased per observation of the seeded method. Models were constructed based on the predictions outlined by the rank-bias hypothesis and the necessary conditions for refutation (above).
This analysis was carried out using the NBDA R Script Version 1.2.11 (available at http://lalandlab.st-andrews.ac.uk/freeware/).
Generalised linear mixed effects models
We used two sets of generalised linear mixed effects models (GLMMs) to determine whether the sex of a demonstrator was a useful predictor in determining how many individuals were likely to be in proximity (<3m) on any given trial. The first set of models considered audience size as an absolute value, whereas the second considered it as a proportion of group size. In all models, ‘individual’ was fit with random intercepts and random slopes to account for multiple measurements from each individual. We took an information theoretic approach to inference, using akaike’s information criterion corrected for small sample sizes (AICc) to estimate model fit. From this we calculated total akaike weights (Σwi) and use these to compute model-averaged estimates of parameter coefficients, allowing us to estimate the effect of a parameter while taking into account model uncertainty. Due to the use of model-averaging, rather than use p-values to determine whether a parameter had an important effect on the output variable, this was established according to whether its 95% confidence intervals overlapped with 0.
Video Coding
The method used by any individual who successfully opened the box was coded, as well as the identities of any individual within 3 meters. Any individuals within 3m whose heads were oriented towards the Vert and did not have their view obstructed was recorded as having observed the opening. Videos were coded by SKW. Inter-observer reliability was carried out with RAH on the method (‘up’ or ‘down’) used and who was observing each demonstration in 30 clips of individuals opening the Vert, with 100% agreement.
RESULTS
Although the raw data from the control condition (Figure 2) are somewhat suggestive of a greater tendency for pushing down than lifting up the door, the number of individuals who chose either method did not differ significantly from chance (Up: n = 3, P = 0.343, 95% CI = 0.07–0.65; Down: n = 7, P = 0.343, 95% CI: 0.35–0.93). Nevertheless, the direction of the seeded method was counterbalanced across groups in the experimental condition. Furthermore, five out of 15 control individuals failed to open the box at all, from which we may infer that the two methods of opening the door were not so salient that every chimpanzee was easily capable of opening it without the use of social information.
Figure 2.
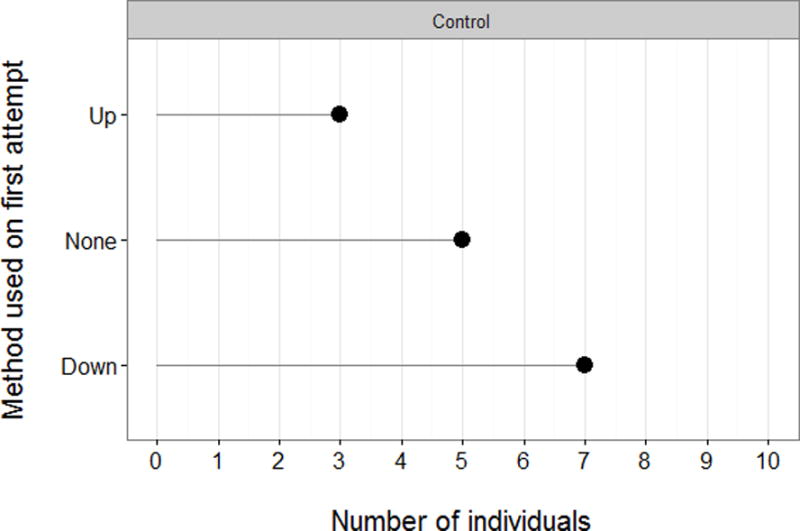
Method used on first opening of the Vert by individuals in the control condition, and number of individuals who failed to open the box.
A Barnard’s exact test found that individuals in the low-rank condition used the seeded method on their first successful trial significantly more often than individuals in the high-rank condition (X2 = 2.09, N=19, P=0.048, see Figure 3). Exact binomial tests found that individuals in the low-rank condition used the seeded method significantly more often than chance (n = 11, P=0.032, 95% CI=0.53–1.0) but high-rank condition did not (n=8, P=0.855, 95% CI=0.111 – 1.0).
Figure 3.
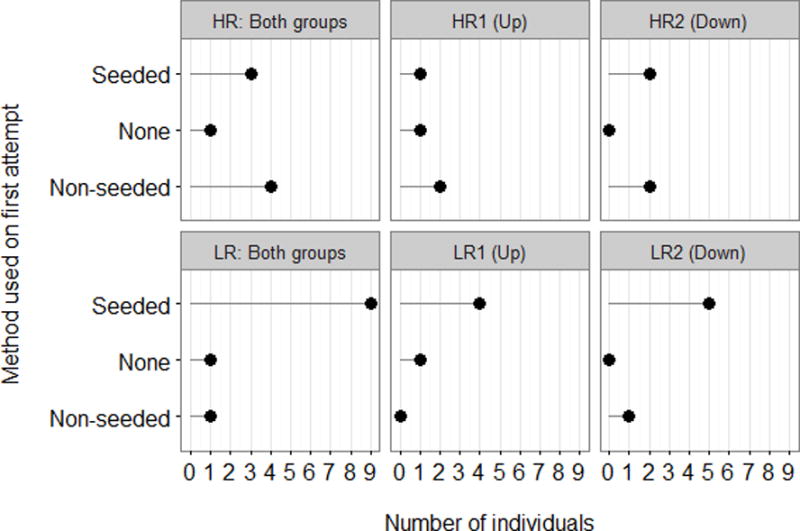
Column 1 - Methods used on first opening of the box for each condition. Columns 2 and 3 - Methods used on first opening of the box in each group. Directionality of trained method indicated for each group in brackets.
Using a truncated data set (Figure 4: procedure and rationale for exclusion detailed above), there remained a significant difference between low and high-rank conditions in the number of individuals who used the seeded method on their first trial (Barnard’s exact test: X2=2.19, n=8, P=0.047). However, it is worth noting that two of the four individuals in HR condition (see HR2 in Table 2) who first used the non-seeded method later switched to consistently use the seeded method. No other individuals in any group persistently switched to a method other than their first-learned, with the exception of the innovation described in detail below. Both individuals who did not solve the task were males. Neither individual tried any other method of interacting with the door (e.g. hitting, pushing, pulling, etc), indicating that they lacked the motivation to engage with the task.
Figure 4.
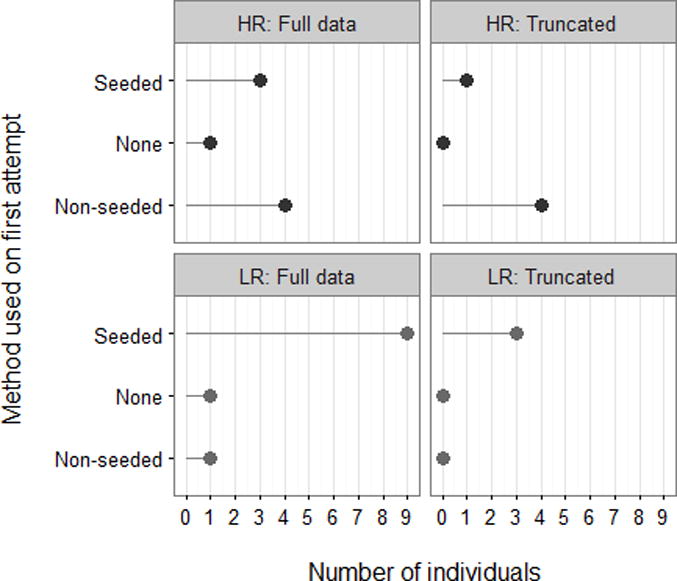
Counts for methods used on first opening of the box in the original ‘full’ data set, side-by-side with ‘truncated’ data set.
Table 2.
Summary table of each individual’s interactions with the puzzle box.
| ID | Group | First method as seeded? | Total trials | Total trials as seeded |
|---|---|---|---|---|
| JUD | HR1 | Y | 730 | 720 |
| BER | HR1 | N | 69 | 0 |
| TUL | HR1 | Y | 234 | 214 |
| QUI | HR1 | N | 1 | 0 |
| KOB | HR1 | n/a | 0 | 0 |
| NIC | HR2 | Y | 535 | 463 |
| CHI | HR2 | N | 109 | 108 |
| MAN | HR2 | N | 54 | 48 |
| MAG | HR2 | Y | 106 | 66 |
| BRI | HR2 | Y | 9 | 6 |
| CEC | LR1 | Y | 170 | 170 |
| MAY | LR1 | Y | 185 | 184 |
| ZOE | LR1 | Y | 123 | 121 |
| AKI | LR1 | N | 171 | 3 |
| TAS | LR1 | Y | 166 | 163 |
| MAR | LR1 | Y | 34 | 34 |
| HAA | LR1 | Y | 138 | 138 |
| ANG | LR2 | Y | 146 | 146 |
| CHE | LR2 | Y | 115 | 108 |
| KIH | LR2 | Y | 326 | 133 |
| NAH | LR2 | Y | 188 | 162 |
| RAD | LR2 | Y | 13 | 13 |
| MAH | LR2 | n/a | 0 | 0 |
Network-based Diffusion Analysis
There was most support for models (Table 3) in which there was an effect of social transmission (S) in the LR condition but not HR, with S varying between groups (Σwi = 0.75). Model-averaged estimates for S indicate that each observation increased an average individual’s learning rate by 3% in LR1 and 15% in LR2. Model averaged estimates for S indicate that each observation increased an average individual’s learning rate in HR1 and HR2 by 0.1% per observation. Models based on the hard rank-bias hypothesis were not well supported (Σwi = 0.002 and Σwi = 0.009). A model allowing for the soft rank-bias hypothesis had some support (Σwi = 0.078), but contrary to the predictions of this hypothesis, the effect of S was estimated as being greater in the LR condition (S = 0.08) than HR (S = 0.00). Individual-level variables (sex, age and rearing history) were added to the best fitting model, but there was little support for any of them improving the model (Table 4).
Table 3.
AICc, delta AICc and Total Akaike Weights (Σwi) for each model. ‘S’ = social transmission.
| Model | AICc | Delta AICc | Total weighted AICc (Σwi) |
|---|---|---|---|
| *S only in HR, varies between HR groups | 334.13 | 11.91 | 0.002 |
| S in all groups | 330.96 | 8.74 | 0.009 |
| *S only in HR, constant between HR groups | 330.96 | 8.74 | 0.009 |
| S varies between all groups | 328.6 | 6.38 | 0.027 |
| No S in any group | 328.27 | 6.05 | 0.036 |
| **S varies between LR and HR | 326.75 | 4.53 | 0.078 |
| S only in LR, constant between LR groups | 326.5 | 4.28 | 0.088 |
| S only in LR, varies between LR groups | 322.22 | 0 | 0.75 |
‘Hard’ rank bias hypothesis
‘Soft’ rank-bias hypothesis candidate
Table 4.
AICc, delta AICc and Total Akaike Weights (Σwi) for the best fitting model from Table 3 with additional individual-level variables.
| Asocial variable | df | AICc | Delta AICc | Total weighted AICc |
|---|---|---|---|---|
| Sex | 4 | 325.73 | 3.51 | 0.07 |
| Rearing | 4 | 323.13 | 0.91 | 0.25 |
| Age | 4 | 323.08 | 0.86 | 0.26 |
| None | 3 | 322.22 | 0 | 0.41 |
GLMMs
A model-averaged estimate (Table 5) of the coefficient for the effect of demonstrator sex on audience size when counting absolute number of individuals within 3m was 0.14 (95% CI: -0.23, 0.51), and when considering audience size as a proportion of total group size was 0.02 (95% CI: -0.05, 0.08). We may infer that Sex did not have an important effect as the 95% confidence intervals did not overlap with zero. Furthermore, as seen in Table 5, adding Sex to the models resulted in a considerably higher AICc and therefore poorer fit.
Table 5.
Model comparison summary statistics for two sets of GLMMs. Sex = Sex of demonstrator. 1|ID = Random intercepts for individual. Sex|ID = random slopes and intercepts for Individual. K = number of effective parameters.
| GLMM Set 1: Audience = Number of individuals < 3m from demonstrator | ||||
|---|---|---|---|---|
| Model | K | AICc | Delta AICc | Total AICc weight |
| Audience ~ 1|ID | 3 | 9178.19 | 0 | 0.94 |
| Audience ~ Sex + Sex|ID | 6 | 9183.63 | 5.44 | 0.06 |
|
| ||||
| GLMM Set 2: Audience = Proportion of group < 3m from demonstrator | ||||
| Model | K | AICc | Delta AICc | Total AICc weight |
| Audience ~ 1|ID | 3 | −2114.53 | 0 | 0.94 |
| Audience ~ Sex + Sex|ID | 6 | −2108.95 | 5.58 | 0.06 |
An Innovation
Finally, we report an innovation which occurred in one of the high-rank condition groups. After 7 hours of open-diffusion, a subordinate individual (TUL) discovered a narrow window of motion in which the door can be opened using ‘Up’, so that a reward can be retrieved, but the locking mechanism is not activated. This allowed her to then also use ‘Down’ to move the door a second time and obtain a second reward. TUL had not used ‘Down’ prior to this discovery, but had observed two other females in her group using it on multiple occasions. This suggests TUL combined her first-learned method with previously acquired social information about that used by others to generate a more productive method, although asocial learning cannot be ruled out. Despite the innovator being of low rank, after 11 observations of this improved method the dominant male (JUD) of the group, who to this point had exclusively used the ‘Up’ method, also began to use the combined form. A similar pattern was observed in a second group. Again, the first individual was a subordinate female (CHE) and the method was subsequently used by two higher ranking females (KIH, NAH). Due to the limited data available, it is not possible to carry out any formal analyses of these events, but we present them as ‘naturally’ occurring examples of subordinates’ innovations achieving limited diffusion through their groups.
DISCUSSION
Rank-bias has been proposed as a way to account for the relatively rare adoption of innovations to produce traditions within chimpanzee communities [Horner et al., 2010; Kendal et al., 2015]. Based on this ‘rank-bias hypothesis’, we predicted that novel behaviours seeded by subordinates either fail to spread, or motivate a considerably lesser degree of social learning than novel behaviours seeded by dominant individuals. In our study, not only were the group-mates of low-ranking models more likely to use the seeded rather than non-seeded method on their first opening of the box, but they were also substantially more likely to do so than individuals in groups with high-rank models. Furthermore, a NBDA showed greatest support for models in which social transmission of the seeded method was present only in the low-rank condition. Finally, we reported innovations developed by two subordinate chimpanzees in separate groups which built on pre-existing methods and were subsequently used by more dominant individuals, likely as a result of social learning. While one must be cautious in interpreting isolated events, these instances are striking in their pertinence to our research question and in how they contrast with the predictions of the rank-bias hypothesis.
We conclude these findings strongly suggest that the rank-bias identified by previous studies [Kendal et al. 2015; or ‘prestige-bias’ in Horner et al., 2010], which occurred when observers had a choice between models of various ranks, does not prohibit the successful emergence of group-wide behaviour patterns from subordinate models or innovators when no competing model is present. As well as a rank-bias, Kendal et al. [2015] identified a bias towards copying ‘knowledgeable’ individuals, which our results suggest to be the case even when demonstrators are of low social rank. This may make adaptive sense, since if one observes an individual doing something that is rewarding, it is counterintuitive to ignore this information simply on the basis of the demonstrator’s low social status. However, this does not preclude the indirect importance of rank in more natural settings. For example, if recent immigrants tend to be spatially peripheral to the group, this would reduce the number of individuals in close enough proximity to observe (and copy) any novel behaviours being used, functionally resulting in a rank bias. By contrast, individuals in the present study could only carry out the behaviour when performing it in a central, commonly used space where the researcher and experiment were set up, making them readily visible to their group. Furthermore, while we did identify comprehensive diffusion of methods seeded subordinate models, it is important to note the difference in group size between the relatively small groups studied here (between 6 and 8 individuals) and wild chimpanzee communities which can have anything from 20 to 150 members [Goodall 1986; Nishida 1990; Boesch & Boesch-Achermann 2000]. Communities of larger scale, as well as the presence of fission-fusion social dynamics, may present additional obstacles for behavioural diffusion.
Being raised in captivity and participating in behavioural research for so many years [e.g. Brosnan et al., 2007; Hopper, Lambeth, Schapiro & Whiten, 2008; Dean, Kendal, Schapiro, Thierry & Laland, 2012; Kendal et al., 2015] may also have shaped the study population to be more ready social learners [Carpenter & Tomasello, 1995], further mediating the effects of rank-bias. The influence of such developmental, cultural, environmental and individual differences on social learning are difficult to examine in such long-lived species, but are likely to be critical in our understanding of cultural transmission [Mesoudi, Chang, Dall & Thornton, 2016]. Nevertheless, this would not explain why there was a greater effect of social transmission in the low-rank condition than in our high-rank condition.
These results contrast with prior studies [Horner et al. 2010; Kendal et al. 2015] in that the effect of social transmission was found to be stronger in our low-rank condition, and a greater proportion of individuals in the LR condition used the seeded method on their first trial than those in HR. One methodological difference between the current study and previous work that might explain this discrepancy is that our high-ranking models were dominant males rather than dominant females. This was an intentional design choice, as males are almost always dominant relative to females, and it was desirable to maximise the rank disparity between model types. However, this may have introduced additional confounds. While males were successfully used as models in Price, Lambeth, Schapiro & Whiten [2009], the study used video demonstrations and observers were not always from the same group as the model, and were therefore unaware of their rank. Wrangham et al. [2016] found that in a community of chimpanzees where multiple grooming techniques were in use, individuals tended to converge on the method primarily used by their matriline, potentially hinting at a sex bias in chimpanzee social learning. However, the only systematically documented example of an incipient tradition diffusing through a wild chimpanzee community originated in a male chimpanzee [Hobaiter et al. 2014], indicating that males can also make effective models. Furthermore, in a series of GLMM’s we examined whether the number of individuals in proximity or attending to an individual’s demonstrations could be predicted by that demonstrator’s sex, and this was not found to be the case (Table 5). From this we may infer that our use of differently sexed models did not introduce an important confound with respect to social tolerance that would explain the contrast between effects of high versus low rank models in our study. In any case, the key finding in our results is not so much the contrast between effects of high versus low ranked models, but that the low ranked female provided an adequate model whose preferred behavioural option was copied by others.
There is already good evidence for an attentional bias toward dominant individuals [Kendal et al. 2015], but it is unclear to what extent this may be vigilance rather than active social learning. Spatial tolerance between demonstrators and observers is also likely to be crucial in facilitating social learning [van Schaik, Fragaszy & Perry, 2003], which may be confounded when highly dominant demonstrators monopolise a resource. The difficulty associated with faithfully copying a socially intolerant individual may explain why two observers in the HR condition first discovered the non-seeded method and then switched to consistently use the seeded method for the remainder of testing. Based on previous work [Hrubesch, Preuschoft & van Schaik, 2009] we would expect such individuals to fixate on their first-learned method, since the alternative did not provide a greater payoff [van Leeuwen et al., 2013]. It may be that, in this case, the first-used method was an ‘accidental’ discovery on the route to learning the seeded method.
As previously discussed, capuchin monkeys inhibit demonstration of known behaviours while in the presence of dominant males [Lonsdorf et al. 2016]. If the same is true of chimpanzees, then non-dominant individuals having to wait for an appropriate social context to interact with the task may have introduced additional demands on memory that would interfere with accurate copying models in the HR condition. In our experiment, the fact that we removed the Vert when models were displaced in the demonstration phase meant that the resource could not be immediately monopolised. The reason for this was to remain methodologically consistent with prior work on rank-bias [Horner et al. 2010; Kendal et al. 2015], as well as to directly examine the motivation of observers to learn from subordinate models rather than the effects of resource-monopolisation on the diffusion of novel behaviours. Competition over resources remains an unexamined and potentially important influence on the diffusion of chimpanzee traditions.
While this study has shown that chimpanzees are motivated to learn novel methods of accessing a resource from subordinate individuals, it is possible this is not true of forms of imitative behaviour that are thought to be normatively motivated and therefore, perhaps particularly directed toward important social partners. Examples of this include the fashion of putting grass in one’s ear, invented by a high-ranking female, described by van Leeuwen et al. [2014] or vocal convergence resulting from close social affiliation [Fedurek et al. 2013; Watson et al. 2015]. Further examination of context-specific qualities, such as behavioural-domain, extrinsic motivators (e.g food or social benefits), ease of monopolisation and how these inhibit or promote particular learning biases, may be a fruitful area of research.
Supplementary Material
Research highlights.
-
-
Behaviours seeded by subordinate (but not dominant) chimpanzees were copied by observers.
-
-
This finding contrasts with prior work suggesting social learning bias towards dominant chimpanzees.
Acknowledgments
SKW, GV, RAH and AW are grateful for the support of the John Templeton Foundation, grant ID40128, ‘Exploring the evolutionary foundations of cultural complexity, creativity and trust’ to AW and Kevin Laland, which funded this project. Thanks to Will Hoppitt and Charlotte Brand for statistical discussion. Thanks also to the anonymous reviewers whose suggestions greatly improved this manuscript. The National Center for Chimpanzee Care is supported by NIH Cooperative Agreement U42 OD-011197.
References
- Allen J, Weinrich M, Hoppitt W, Rendell L. Network-based diffusion analysis reveals cultural transmission of lobtail feeding in humpback whales. Science. 2013;340:485–488. doi: 10.1126/science.1231976. [DOI] [PubMed] [Google Scholar]
- Addessi E, Mancini A, Crescimbene L, Visalberghi E. How social context, token value, and time course affect token exchange in capuchin monkeys (Cebus apella) International Journal of Primatology. 2011;32:83–98. [Google Scholar]
- Aplin LM, Farine DR, Morand-Ferron J, Cockburn A, Thornton A, Sheldon BC. Experimentally induced innovations lead to persistent culture via conformity in wild birds. Nature. 2015;518:538–541. doi: 10.1038/nature13998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesch C, Boesch-Achermann H. The chimpanzees of the Taï Forest: Behavioural ecology and evolution. Oxford University Press; USA: 2000. [Google Scholar]
- Brosnan SF, Jones OD, Lambeth SP, Mareno MC, Richardson AS, Schapiro SJ. Endowment effects in chimpanzees. Current Biology. 2007;17:1704–1707. doi: 10.1016/j.cub.2007.08.059. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Multimodel inference understanding AIC and BIC in model selection. Sociological methods & research. 2004;33:261–304. [Google Scholar]
- Carpenter M, Tomasello M. Joint attention and imitative learning in children, chimpanzees, and enculturated chimpanzees. Social Development. 1995;4:217–237. [Google Scholar]
- Claidière N, Whiten A. Integrating the study of conformity and culture in humans and nonhuman animals. Psychological bulletin. 2012;138:126. doi: 10.1037/a0025868. [DOI] [PubMed] [Google Scholar]
- Coelho CG, Falótico T, Izar P, Mannu M, Resende BD, Siqueira JO, Ottoni EB. Social learning strategies for nut-cracking by tufted capuchin monkeys (Sapajus spp.) Animal cognition. 2015;18:911–9. doi: 10.1007/s10071-015-0861-5. [DOI] [PubMed] [Google Scholar]
- Dean LG, Kendal RL, Schapiro SJ, Thierry B, Laland KN. Identification of the social and cognitive processes underlying human cumulative culture. Science. 2012;335:1114–1118. doi: 10.1126/science.1213969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz M, Nunn CL. Network-based diffusion analysis: a new method for detecting social learning. Proceedings of the Royal Society of London B: Biological Sciences. 2009;276:1829–1836. doi: 10.1098/rspb.2008.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedurek P, Machanda ZP, Schel AM, Slocombe KE. Pant hoot chorusing and social bonds in male chimpanzees. Animal Behaviour. 2013;86:189–196. [Google Scholar]
- Friard O, Gamba M. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods in ecology and Evolution. 2016 doi: 10.1111/2041-210X.12584. [DOI] [Google Scholar]
- Goodall J. The chimpanzees of Gombe: Patterns of behavior 1986 [Google Scholar]
- Henrich J, McElreath R. The evolution of cultural evolution. Evolutionary Anthropology: Issues, News, and Reviews. 2003;12:123–135. [Google Scholar]
- Hobaiter C, Poisot T, Zuberbühler K, Hoppitt W, Gruber T. Social network analysis shows direct evidence for social transmission of tool use in wild chimpanzees. PLoS Biol. 2014;12:e1001960. doi: 10.1371/journal.pbio.1001960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper LM, Schapiro SJ, Lambeth SP, Brosnan SF. Chimpanzees’ socially maintained food preferences indicate both conservatism and conformity. Animal Behaviour. 2011;81:1195–1202. doi: 10.1016/j.anbehav.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper LM, Lambeth SP, Schapiro SJ, Whiten A. Observational learning in chimpanzees and children studied through ‘ghost’ conditions. Proceedings of the Royal Society of London B: Biological Sciences. 2008;275:835–840. doi: 10.1098/rspb.2007.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper LM, Kurtycz LM, Ross SR, Bonnie KE. Captive chimpanzee foraging in a social setting: a test of problem solving, flexibility, and spatial discounting. PeerJ. 2015a;17(3):e833. doi: 10.7717/peerj.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper LM, Lambeth SP, Schapiro SJ, Whiten A. The importance of witnessed agency in chimpanzee social learning of tool use. Behavioural processes. 2015b;112:120–9. doi: 10.1016/j.beproc.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppitt W, Boogert NJ, Laland KN. Detecting social transmission in networks. Journal of Theoretical Biology. 2010;263:544–555. doi: 10.1016/j.jtbi.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Horner V, Proctor D, Bonnie KE, Whiten A, de Waal FB. Prestige affects cultural learning in chimpanzees. PLoS One. 2010;5:e10625. doi: 10.1371/journal.pone.0010625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrubesch C, Preuschoft S, van Schaik C. Skill mastery inhibits adoption of observed alternative solutions among chimpanzees (Pan troglodytes) Animal cognition. 2009;12:209–216. doi: 10.1007/s10071-008-0183-y. [DOI] [PubMed] [Google Scholar]
- Kendal RL, Coolen I, Van Bergen Y, Laland KN. Trade-offs in the adaptive use of social and asocial learning. Advances in the Study of Behavior. 2005;35:333–379. [Google Scholar]
- Kendal R, Hopper LM, Whiten A, Brosnan SF, Lambeth SP, Schapiro SJ, Hoppitt W. Chimpanzees copy dominant and knowledgeable individuals: implications for cultural diversity. Evolution and Human Behavior. 2015;36:65–72. doi: 10.1016/j.evolhumbehav.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laland KN. Social learning strategies. Animal Learning & Behavior. 2004;32:4–14. doi: 10.3758/bf03196002. [DOI] [PubMed] [Google Scholar]
- Laland KN, Janik VM. The animal cultures debate. Trends in Ecology & Evolution. 2006;21:542–547. doi: 10.1016/j.tree.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Laland KN, Galef BG. The question of animal culture. Harvard University Press; 2009. [Google Scholar]
- Lonsdorf EV, Bonnie KE, Grim M, Krupnick A, Prestipino M, Whyte J. Seeding an arbitrary convention in capuchin monkeys: the effect of social context. Behaviour. 2016;153:633–54. [Google Scholar]
- Mehta CR, Senchaudhuri P. Conditional versus unconditional exact tests for comparing two binomials. Cytel Software Corporation. 2003;675 [Google Scholar]
- Mesoudi A, Chang L, Dall SR, Thornton A. The Evolution of Individual and Cultural Variation in Social Learning. Trends in Ecology & Evolution. 2016;31(215):225. doi: 10.1016/j.tree.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Uehara S. Proximate Factors of Different Types of Grooming Hand-Clasp in Mahale Chimpanzees: Implications for Chimpanzee Social Customs 1. Current Anthropology. 2004;45:108–114. [Google Scholar]
- Nishida T. Chimpanzees of the Mahale Mountains. University of Tokyo Press; 1990. [Google Scholar]
- Nishida T, Matsusaka T, McGrew WC. Emergence, propagation or disappearance of novel behavioral patterns in the habituated chimpanzees of Mahale: a review. Primates. 2009;50:23–36. doi: 10.1007/s10329-008-0109-y. [DOI] [PubMed] [Google Scholar]
- O’Malley RC, Wallauer W, Murray CM, Goodall J. The appearance and spread of ant fishing among the Kasekela chimpanzees of Gombe: a possible case of intercommunity cultural transmission. Current anthropology. 2012;53:650. doi: 10.1086/666943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R. RStudio, Inc; Boston, MA: 2015. URL http://www.rstudio.com/ [Google Scholar]
- Price EE, Lambeth SP, Schapiro SJ, Whiten A. A potent effect of observational learning on chimpanzee tool construction. Proceedings of the Royal Society of London B: Biological Sciences. 2009:rspb20090640. doi: 10.1098/rspb.2009.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader SM, Laland KN. Primate innovation: sex, age and social rank differences. International Journal of Primatology. 2001;22:787–805. [Google Scholar]
- Rendell L, Boyd R, Cownden D, Enquist M, Eriksson K, Feldman MW, Fogarty L, Ghirlanda S, Lillicrap T, Laland KN. Why copy others? Insights from the social learning strategies tournament. Science. 2010;328:208–13. doi: 10.1126/science.1184719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell L, Fogarty L, Hoppitt WJ, Morgan TJ, Webster MM, Laland KN. Cognitive culture: theoretical and empirical insights into social learning strategies. Trends in cognitive sciences. 2011;15:68–76. doi: 10.1016/j.tics.2010.12.002. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2015. Vienna, Austria. URL http://www.R-project.org/ [Google Scholar]
- Van Leeuwen EJ, Cronin KA, Haun DB. A group-specific arbitrary tradition in chimpanzees (Pan troglodytes) Animal cognition. 2014;17:1421–1425. doi: 10.1007/s10071-014-0766-8. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen EJ, Haun DB. Conformity without majority? The case for demarcating social from majority influences. Animal Behaviour. 2014;96:187–194. [Google Scholar]
- Van Schaik CP, Fragaszy D, Perry S. Local traditions in orangutans and chimpanzees: social learning and social tolerance. The biology of traditions: Models and evidence. 2003:297–328. [Google Scholar]
- Watson SK, Townsend SW, Schel AM, Wilke C, Wallace EK, Cheng L, West V, Slocombe KE. Vocal learning in the functionally referential food grunts of chimpanzees. Current Biology. 2015;25:495–499. doi: 10.1016/j.cub.2014.12.032. [DOI] [PubMed] [Google Scholar]
- Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin CEG, Wrangham RW, Boesch C. Cultures in chimpanzees. Nature. 1999;399:682–685. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- Whiten A. The second inheritance system of chimpanzees and humans. Nature. 2005;437:52–55. doi: 10.1038/nature04023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


