Abstract
Background
Patients with aneurysmal subarachnoid hemorrhage (SAH) often develop hydrocephalus requiring an external ventricular drain (EVD). The best available evidence suggests that a rapid EVD wean and intermittent CSF drainage is safe, reduces complications, and shortens ICU and hospital length of stay as compared to a gradual wean and continuous drainage. However, optimal EVD management remains controversial and the baseline practice among neurological ICUs is unclear. Therefore, we sought to determine current institutional practices of EVD management for patients with aneurysmal SAH.
Methods
An email survey was sent to attending intensivists and neurosurgeons from 72 neurocritical care units that are registered with the Neurocritical Care Research Network or have been previously associated with existing literature on the management of EVDs in critically ill patients. Only one response was counted per institution.
Results
There were 45 out of 72 institutional responses (63%). The majority of responding institutions (80%) had a single predominant EVD management approach. Of these, 78% favored a gradual EVD weaning strategy. For unsecured aneurysms, 81% kept the EVD continuously open and 19% used intermittent drainage. For secured aneurysms, 94% kept the EVD continuously open and 6% used intermittent drainage. Among continuously drained patients, the EVD was leveled at 18 (unsecured) and 11 cm H2O (secured) (p<0.0001). When accounting for whether the EVD strategy was to enhance or minimize CSF drainage, there was a significant difference in the management of unsecured vs. secured aneurysms with 42% using an enhance drainage approach in unsecured patients and 92% using an enhance drainage approach in secured patients (p<0.0001).
Conclusion
Most institutions utilize a single predominant EVD management approach, with a consensus towards a continuously open EVD to enhance CSF drainage in secured aneurysm patients coupled with a gradual weaning strategy. This finding is surprising given that the best available evidence suggests that the opposite approach is safe and can reduce ICU and hospital length of stay. We recommend a critical reassessment of the approach to the management of EVDs. Given the potential impact on patient outcomes and length of stay more research needs to be done to reach a threshold for practice change, ideally via multicenter and randomized trials.
Keywords: intracranial aneurysm, subarachnoid hemorrhage, hydrocephalus, catheters, postoperative complications, length of stay
Introduction
Hydrocephalus requiring an external ventricular drain (EVD) is a frequent requirement after aneurysmal SAH1. It has been suggested that EVD management influences rates of delayed cerebral ischemia, drain complications, ventriculo-peritoneal shunt (VPS) placement, ICU and hospital length of stay (LOS), and cognitive outcome among survivors of SAH. Randomized controlled trials in this population suggest that a rapid EVD wean is associated with shorter ICU and hospital LOS2 and that continuous (compared to intermittent) drainage is associated with more EVD complications3. The Neurocritical Care Society recently released a consensus statement encouraging an EVD wean “as quickly as is clinically feasible,”4 but in the absence of high-level evidence there is little definitive advice about how clinicians can accomplish this. Therefore, the optimal EVD approach remains controversial with no specific guidelines available to practitioners who care for this patient population5.
Surveys of EVD management have disclosed wide practice variation6–8. However, it remains unknown whether the degree of variance reflects inter-institutional practice versus intra-institutional practice variance6. We sought to investigate practice variance in the management of EVDs in patients with SAH using the novel approach of surveying at the institutional level rather than individual practitioners.
Materials and Methods
An 8-question email survey (Supplemental Data S1) was used to determine EVD management practices at the institutional level in neurocritical care units in the United States. Respondents were asked if their institution: (1) had a single predominant approach or multiple approaches to EVD management, (2) used a rapid or gradual wean, (3) used which units for their EVD level (cm H2O or mm Hg or not sure), (4) at default kept the EVD open or closed in unsecured aneurysms and (5) at which level from the level of the tragus is the EVD intermittently or continuously open, and (6) at default kept the EVD open or closed in secured aneurysms and (7) at which level. Finally, (8) we asked respondents to optionally further describe their EVD wean.
The research protocol was reviewed and an exemption granted by the Partners in Healthcare Institutional Review Board Committee. The survey was administered between May 2015 – September 2015 in the form of an email to practitioners working in neurocritical care units within the United States. Follow up emails were sent to non-respondents and if there was no response, attempts were made for up to 2 alternate practitioners from that institution. Implied consent was obtained by taking part in the survey. Permission was obtained to list participating institution name, but links to answers and individual participants were kept anonymous.
Care was taken to count only one response per institution. Institutions were primarily identified based on registration with the Neurocritical Care Society’s Neurocritical Care Research Network. Additional sites were identified based on association with contributions to the existing literature on EVD management. Individuals within these institutions were recruited and contacted based on (A) their directory listing from the Neurocritical Care Society or the American Academy of Neurology, (B) their institution’s website, or (C) prior personal communication with one of the authors. A total of 72 institutions were identified and sent a survey with the following United States Census Bureau-designated regional distribution: Northeast (31%, 22/72), Midwest (25%, 18/72), South (24%, 17/72), and West (21%, 15/72). Data were analyzed using Microsoft Excel and GraphPad Prism.
Results
There was a 63% response rate (45 of 72 institutions, Supplemental Data S2). All respondents were attending physicians. The majority were neurology-trained intensivists, followed by anesthesiology, neurosurgery, and internal medicine (Table). Most responses were from the Northeast (40%, 18/45), followed by Midwest (24%, 11/45), South (22%, 10/45), and West (13%, 6/45). Of respondents, 80% (36/45) reported that their institution had a single predominant EVD approach. All 36 of the survey responses from centers with a single EVD management approach had complete answers. These responses were analyzed further.
Table.
Characteristics of respondents and summary of survey results
| Variable | n | % |
|---|---|---|
| Responses | 45/72 | 63% |
| Respondents | ||
| Neurology | 35/45 | 78% |
| Anesthesiology | 5/45 | 11% |
| Neurosurgery | 4/45 | 9% |
| Internal Medicine | 1/45 | 2% |
| Region of United States | ||
| Northeast | 18/45 | 40% |
| South | 10/45 | 22% |
| Midwest | 11/45 | 24% |
| West | 6/45 | 13%* |
| Single institutional approach | 36/45 | 80% |
| Unsecured | ||
| Continuous | 29/36 | 81% |
| Intermittent | 7/36 | 19% |
| Enhance drainage | 15/36 | 42% |
| Minimize drainage | 21/36 | 58% |
| Secured | ||
| Continuous | 34/36 | 94% |
| Intermittent | 2/36 | 6% |
| Enhance drainage | 33/36 | 92% |
| Minimize drainage | 3/36 | 8% |
| Gradual wean | 28/36 | 78% |
| Rapid wean | 8/36 | 22% |
Regions do not add up to 100% due to rounding.
Intermittent vs. Continuous Drainage
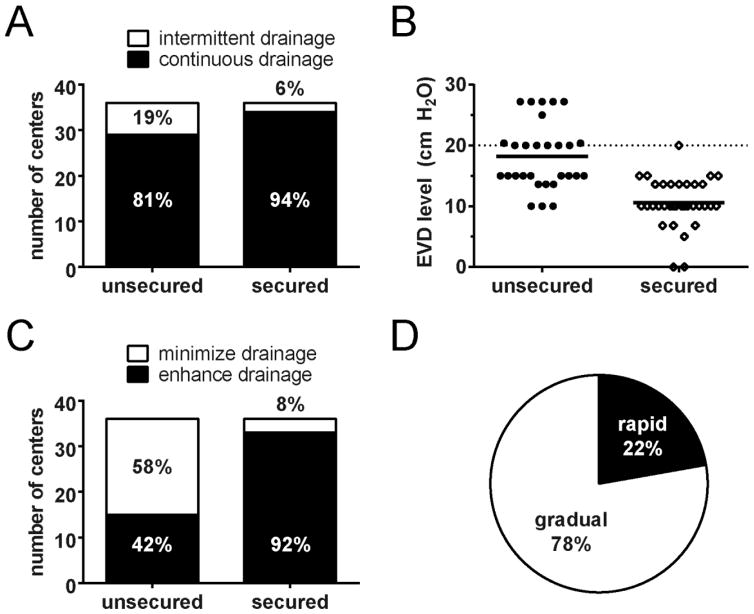
Prior to securing of the aneurysm by endovascular coiling or open surgical clipping, 19% (7/36) favored intermittent drainage and 81% (29/36) favored continuous drainage. Once the aneurysm was secured, 6% (2/36) favored intermittent drainage and 94% (34/36) favored continuous drainage (p=0.15, Fisher’s exact test, Figure 1A). There were 26 centers which used units of cm H2O and 10 centers which used units of mm Hg for their EVD level. We converted mm Hg to cm H2O to make the data comparable.
Figure 1.
EVD management strategies for institutions with a single approach. (A) There is no difference in use of intermittent vs. continuous drainage for unsecured and secured aneurysms (p=0.15). (B) Default EVD levels for institutions using a continuous drainage strategy. Mean level 18 cm H2O for unsecured (N=29) and 11 cm H2O for secured (N=34, p<0.0001). Dotted line at 20 cm H2O. (C) Use of a minimize vs. enhance drainage strategy is significantly different between unsecured and secured aneurysms (p<0.0001). Minimize drainage group includes intermittent plus continuous with the EVD ≥ 20 cm H2O. Enhance drainage only includes continuous with EVD <20 cm H2O. (D) Proportion of rapid (8/36, 22%) and gradual (28/36, 78%) weans. N=36 centers for (A), (C), and (D).
For continuously-drained patients, we determined the default level at which the EVD was kept. The mean EVD level between unsecured (n=29) and secured patients (n=34) was significantly different: 18 vs. 11 cm H2O (p<0.0001 unpaired two-tailed t test with Welch’s correction, Figure 1B). To ascertain whether the EVD strategy was to enhance CSF drainage or minimize CSF drainage, a cutoff of 20 cm H2O was established and the data dichotomized into minimize drainage (defined as intermittent plus continuous with EVD ≥ 20 cm H2O) and enhance drainage (defined as continuous with EVD <20 cm H2O). For unsecured, 42% (15/36) were enhance drainage compared to 58% (21/36) minimize drainage whereas for secure almost all were enhance drainage (p<0.0001, Fisher’s exact test, Figure 1C).
Rapid vs. Gradual Wean
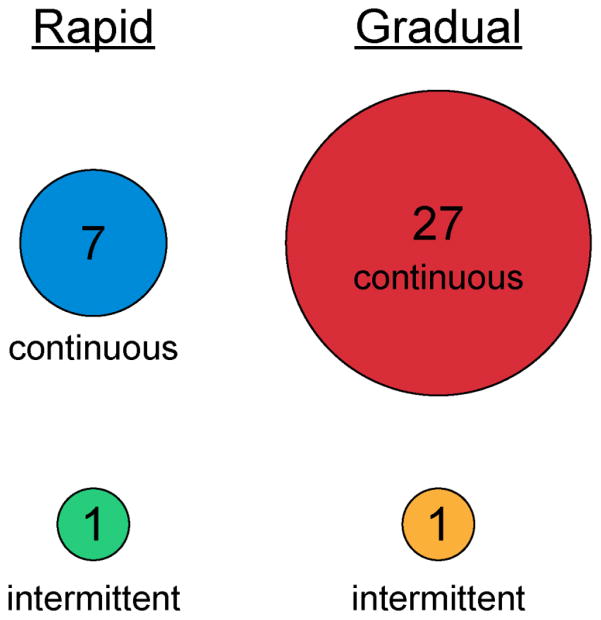
Once the aneurysm was secured, 22% (8/36) favored a rapid EVD wean and 78% (28/36) a gradual wean (Figure 1D). Continuous drainage with a gradual wean was overwhelmingly the most common strategy, followed by continuous drainage with a rapid wean (Figure 2). There were only 2 centers that used an intermittent drainage strategy once the EVD was secured: 1 stated that they used a gradual wean and 1 used a rapid wean.
Figure 2.
Bubble plot for secured aneurysms showing number of institutions following combinations of intermittent vs. continuous drainage and rapid vs. gradual weans.
Centers with Multiple EVD Approaches
There were 9 centers that reported multiple EVD approaches. All but one of the responses were incomplete. Furthermore, it was not possible to ascertain how prevalent each individual EVD approach was at each center (i.e. if there were 2 approaches, it was not possible to tell if they were used equally or not). Therefore, these data were excluded from the single center analysis.
The following is a summary of incompletely reported individual practices from the 9 centers with multiple approaches. Prior to securing of the aneurysm, 40% (4/10) favored intermittent drainage and 60% (6/10) favored continuous drainage. Once the aneurysm was secured, 18% (2/11) favored intermittent drainage and 82% (9/11) favored continuous drainage. For continuously-drained patients, the mean default level at which the EVD was kept was 17 cm H2O for unsecured (n=6) and 9 cm H2O for secured (n=9) patients. For unsecured patients, 30% (3/10) took an enhance drainage approach and 70% (7/10) a minimize drainage approach. For secured patients, 82% (9/11) took an enhance drainage approach and 18% (2/11) a minimize drainage approach. Finally, 31% (4/13) favored a rapid EVD wean and 69% (9/13) a gradual wean. A summary of the individual responses are included in Supplemental Data S3.
Discussion
Our study shows that there is general concordance in EVD drainage strategy with most centers favoring a gradual wean with continuously open drains once the aneurysm is secured. The results are noteworthy because the best available evidence suggests that the opposite approach may result in fewer drain complications and more timely weans.
Furthermore, our findings are in contrast to previous surveys which detected much wider practice variation6–8. The largest of these prior surveys of EVD management included 241 practitioners and found that 54% of respondents agreed to do continuous CSF drainage for SAH without elevated ICP; however, only 24% disagreed7. The remainder did not answer the question or stated that they were neutral. There was greater consensus in their study in the setting of elevated ICP in SAH with 74% of respondents saying they agreed to continuously drain, while only 13% disagreed. These results are disparate from our results, likely related to differences in how the data were collected.
An important limitation of the survey conducted by Olsen et al. is that they did not analyze how many discrete neurological ICUs were represented in their study. As a result, they did not report institutional practices or limit the number of responses per institution. Responses were obtained from attending physicians, fellows, residents, staff nurses, advance practice nurses, and a substantial number of respondents that did not indicate their professional category. Therefore, the variability they reported may have reflected both practitioner and practice variability which could have obscured any national consensus.
We sought to control for this confounder by only analyzing one response per institution and analyzing only those 80% of institutional respondents with a single predominant approach to EVD management. Notably, all of our respondents were attending physicians. Almost all of the institutions by default sought to enhance CSF drainage with a continuously open EVD once the aneurysm was secured. In addition, 3 times as many centers favored a gradual EVD weaning strategy over a rapid wean. Even if we assume that the 20% of centers with multiple approaches have diametrically opposed strategies, our results show a clear preference of centers in the United States to keep the EVD open and to perform a gradual wean.
This preference is contrary to the best available evidence. There have been recent single center prospective observational and randomized controlled studies comparing intermittent vs. continuous EVD management3,9. Although the observational study was small, the investigators found more EVD complications in the continuous drainage group. The follow up randomized controlled trial at the same institution was stopped early by the Data Safety and Monitoring Board due to increased complications in the continuous group. The complications mostly consisted of an increased rate of ventriculitis and EVD malfunction. There have otherwise been no prospectively collected data showing an effect on rates of delayed cerebral ischemia or VPS placement. As for rapid vs. gradual weaning, as pointed out in a recent review commissioned by the Neurocritical Care Society4, there is a single randomized controlled trial which showed that a rapid EVD wean is safe and results in shorter ICU length of stay and decreased EVD days with no effect on functional outcome2. Therefore, although the data are limited, the best available evidence suggests that intermittent drainage coupled with a rapid EVD wean is a safe strategy that could result in decreased ICU and EVD days.
The discrepancy between the available evidence and clinical practice may be a result of the nature and paucity of data available. The threshold for evidence to produce a practice change may yet to be reached. Importantly, the studies discussed above are from single centers, represent relatively weak evidence, and have received a significant amount of criticism. For example, in the continuous vs. intermittent drainage study the continuous drainage group had a higher rate of EVDs that lost patency compared to the intermittent group3. This may have led to more EVD flushing or EVD replacement which in turn may have led to higher ventriculitis rates due to loss of sterility to the system. Therefore, many centers may not follow the results of the study because of a different experience with their own continuous drainage approach.
Additionally, the historical use of the gradual weaning strategy, which has been previously discussed2, may play an outsized role in its continued use today. The use of continuous vs. intermittent drainage is also likely influenced by historical approaches. EVDs were first employed in the management of hydrocephalus after subarachnoid hemorrhage in the 1950s but it was not until the 1970s that they gained widespread use in the acute phase10. The data from this era reflects EVD management in the setting of unsecured aneurysms, as the predominant practice had been to delay surgery for approximately a week after rupture11. The approach to keep the EVD at a relatively high level in unsecured aneurysms is borne out of a fear of causing aneurysm rebleeding and comes primarily from two studies. Nornes described a series of 29 SAH patients and based on a data trend taken from epidural screw intracranial pressure monitors (now no longer in use due to poor reliability) identified low pressures as associated with aneurysm re-rupture12. Subsequently, Sundbärg and Pontén described in a series of 127 patients with the EVD set at 15 mm Hg (corresponding to ~20 cm H2O) a rebleed rate of 16% which was about average for that time13. These results formed a cornerstone in the continuous drainage approach—that when kept at a relatively high level, continuous drainage is safe with regard to rebleeding risk in unsecured aneurysmal SAH patients14.
However, since the advent of early microsurgical and endovascular approaches to aneurysm treatment, the risk of aneurysm re-rupture has been mitigated. Consequently, in patients with secured aneurysms, a strategy of enhancing CSF drainage has been taken due to a belief that clearing subarachnoid blood and enhancing cerebral perfusion pressure through low intracranial pressure could improve rates of delayed cerebral ischemia and long term cognitive outcomes. This rationale has not been adequately tested and the practice has continued despite newer data suggesting that continuous drainage could be potentially harmful for reasons unrelated to aneurysmal re-rupture3,9. Furthermore, when coupled with a gradual wean starting at a lower EVD level, continuous drainage could unnecessarily increase EVD and ICU days. Therefore, more data are needed to address the theoretical benefits and potential harm in using continuous drainage and gradual EVD weans.
Our study is limited by response bias inherent to all survey studies; however, our high response rate helps to mitigate this confounder (63% compared to 10–30% typical of the surveys in this field6,15,16). Because responses from centers with multiple approaches were incomplete we were not able to fully analyze their approaches or appropriately compare their practice with that of centers with a single institutional approach. Regardless, the raw analysis of incomplete responses from the centers with multiple approaches approximated the results from single approach centers. A future study could focus on centers with multiple approaches. Another limitation is the short length of our survey as we had to constrain the amount of data obtained to enhance response rate. One of the ways that we achieved a short length is that we asked for a qualitative assessment of rapid vs. gradual weans; therefore, we effectively dichotomized the data and had respondents chose a “best” answer based on an example of either type of wean on the survey form (Supplemental Data S1). An important limitation is that we were not able to quantify how many centers had a practice that was in between the examples. Additionally, there was a skew in both our survey distribution and response rate towards Northeastern centers. Finally, ICU size was not ascertained and we did not distinguish between academic and community practice.
Conclusions
Our study found a previously unreported national consensus in EVD management approaches after SAH with almost all responding centers reporting a continuously open EVD to enhance CSF drainage and most centers preferring gradual EVD weans after securing of the ruptured aneurysm. We address many of the limitations cited by prior studies including low response rate and assessment of inter-institutional vs. intra-institutional variance. Given the widespread use of EVDs, the potential impact on complications, VPS rate, ICU and hospital LOS, patient outcomes, and the discrepancy between our findings and the currently available evidence there is no question that we need a multicentered study to balance confounds inherent in existing single center studies.
Supplementary Material
Acknowledgments
We thank all the participating centers who shared their management strategies. Additionally, we would like to thank Stephan Mayer, Jonathan Rosand, and Leigh Hochberg for helpful comments, Mabel Chung for critical review of the manuscript, and Eric Rosenthal and Abby Cohen for assistance with IRB preparation. Dr. Chung is supported through NINDS R25 NS065743.
References
- 1.Gigante P, Hwang BY, Appelboom G, Kellner CP, Kellner MA, Connolly ES. External ventricular drainage following aneurysmal subarachnoid haemorrhage. Br J Neurosurg. 2010;24:625–32. doi: 10.3109/02688697.2010.505989. [DOI] [PubMed] [Google Scholar]
- 2.Klopfenstein JD, Kim LJ, Feiz-Erfan I, et al. Comparison of rapid and gradual weaning from external ventricular drainage in patients with aneurysmal subarachnoid hemorrhage: a prospective randomized trial. Journal of Neurosurgery. 2004;100:225–9. doi: 10.3171/jns.2004.100.2.0225. [DOI] [PubMed] [Google Scholar]
- 3.Olson DM, Zomorodi M, Britz GW, Zomorodi AR, Amato A, Graffagnino C. Continuous cerebral spinal fluid drainage associated with complications in patients admitted with subarachnoid hemorrhage. Journal of Neurosurgery. 2013;119:974–80. doi: 10.3171/2013.6.JNS122403. [DOI] [PubMed] [Google Scholar]
- 4.Fried HI, Nathan BR, Rowe AS, et al. The Insertion and Management of External Ventricular Drains: An Evidence-Based Consensus Statement : A Statement for Healthcare Professionals from the Neurocritical Care Society. Neurocrit Care. 2016;24:61–81. doi: 10.1007/s12028-015-0224-8. [DOI] [PubMed] [Google Scholar]
- 5.Connolly ES, Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke; a journal of cerebral circulation. 2012;43:1711–37. doi: 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- 6.Olson DM, Batjer HH, Abdulkadir K, Hall CE. Measuring and monitoring ICP in Neurocritical Care: results from a national practice survey. Neurocrit Care. 2014;20:15–20. doi: 10.1007/s12028-013-9847-9. [DOI] [PubMed] [Google Scholar]
- 7.Olson DM, Lewis LS, Bader MK, et al. Significant practice pattern variations associated with intracranial pressure monitoring. J Neurosci Nurs. 2013;45:186–93. doi: 10.1097/JNN.0b013e3182986400. [DOI] [PubMed] [Google Scholar]
- 8.Allan D. Intracranial pressure monitoring: a study of nursing practice. Journal of advanced nursing. 1989;14:127–31. doi: 10.1111/j.1365-2648.1989.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 9.Amato A, Britz GW, James ML, et al. An observational pilot study of CSF diversion in subarachnoid haemorrhage. Nurs Crit Care. 2011;16:252–60. doi: 10.1111/j.1478-5153.2010.00444.x. [DOI] [PubMed] [Google Scholar]
- 10.Kusske JA, Turner PT, Ojemann GA, Harris AB. Ventriculostomy for the treatment of acute hydrocephalus following subarachnoid hemorrhage. Journal of Neurosurgery. 1973;38:591–5. doi: 10.3171/jns.1973.38.5.0591. [DOI] [PubMed] [Google Scholar]
- 11.Macdonald RL. Origins of the Concept of Vasospasm. Stroke; a journal of cerebral circulation. 2016;47:e11–5. doi: 10.1161/STROKEAHA.114.006498. [DOI] [PubMed] [Google Scholar]
- 12.Nornes H. The role of intracranial pressure in the arrest of hemorrhage in patients with ruptured intracranial aneurysm. Journal of Neurosurgery. 1973;39:226–34. doi: 10.3171/jns.1973.39.2.0226. [DOI] [PubMed] [Google Scholar]
- 13.Sundbärg G, Pontén U. ICP and CSF Absorption Impairment After Subarachnoid Hemorrhage. In: Beks JWF, Bosch DA, Brock M, editors. Intracranial Pressure III. Berlin, Heidelberg: Springer Berlin Heidelberg; 1976. pp. 139–46. [Google Scholar]
- 14.Voldby B, Enevoldsen EM. Intracranial pressure changes following aneurysm rupture. Part 3: Recurrent hemorrhage. Journal of Neurosurgery. 1982;56:784–9. doi: 10.3171/jns.1982.56.6.0784. [DOI] [PubMed] [Google Scholar]
- 15.O’Neill BR, Velez DA, Braxton EE, Whiting D, Oh MY. A survey of ventriculostomy and intracranial pressure monitor placement practices. Surgical neurology. 2008;70:268–73. doi: 10.1016/j.surneu.2007.05.007. discussion 73. [DOI] [PubMed] [Google Scholar]
- 16.Rehman T, Rehman A-u, Rehman A, et al. A US-based survey on ventriculostomy practices. Clin Neurol Neurosurg. 2012;114:651–4. doi: 10.1016/j.clineuro.2011.12.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.