Abstract
Objective
Sensitive, objective and easily applied methods for evaluating disease progression and response to therapy are needed for clinical trials in Duchenne muscular dystrophy (DMD). In this study, we evaluated whether electrical impedance myography (EIM) could serve this purpose.
Methods
In this non-blinded study, 36 boys with DMD and 29 age-similar healthy boys underwent multifrequency EIM measurements for up to 2 years on 6 muscles unilaterally along with functional assessments. A linear mixed-effects model with random intercept and slope terms was used for the analysis of multifrequency EIM values and functional measures. Seven DMD boys were initiated on corticosteroids; these data were analyzed using a piecewise linear mixed-effects model.
Results
In boys >7.0 years, a significant difference in the slope of EIM phase-ratio trajectories in the upper extremity was observed by 6 months of -0.074/month, p=0.023, 95% confidence interval (CI)[−0.013,−0.14]); at two years, this difference was −0.048/month, p<0.0001 95%CI[−0.028,−0.068]. In boys ≤7.0 years, differences appeared at 6 months in gastrocnemius (EIM phase-slope −0.83°/kHz-month, p=0.007 95%CI[−0.26,−1.40]). EIM outcomes showed significant differences earlier than functional tests. Initiation of corticosteroids significantly improved the slope of EIM phase-ratio (0.057/month, p=0.00019 95%CI[0.028,0.086]) and EIM phase-slope (0.14°/kHz-month, p=0.013 95%CI[0.028,0.25]), consistent with corticosteroids’ known clinical benefit.
Interpretation
EIM detects deterioration in muscles of both younger and older boys by 6 months; it also identifies the therapeutic effect of corticosteroid initiation. Since EIM is rapid to apply, painless, and requires minimal operator training, the technique deserves to be further evaluated as a biomarker in DMD clinical therapeutic trials.
Keywords: Muscular dystrophy, Electrical impedance, Biomarker
INTRODUCTION
Clinical trials in Duchenne Muscular Dystrophy (DMD) rely on measures of function and strength, such as the 6-minute walk test (6MWT), to assess outcome.1-3 While practical, such tools have substantial limitations, including requiring boys to be cooperative and ambulatory. Interpretation of functional outcomes over time in young children with DMD is also complicated by age-related improvements in strength and function.2, 4 In fact, recent studies employing the 6MWT enroll only ambulant children over age 7 years in whom repeatable data can be obtained and in whom a measurable rate of decline is anticipated.5, 6
Researchers continue to seek more effective tools for evaluating the effect of therapy in DMD, regardless of age. For example, biopsies evaluating dystrophin expression can provide evidence of drug effect at the cellular level.7 Magnetic resonance imaging (MRI) has also been used to evaluate the health of DMD muscle.8-10 Importantly, a recent longitudinal study demonstrated MRI's high sensitivity to disease progression as measured by the amount of fat deposition using the Dixon technique, even in boys younger than 7 years.10 However, MRI is relatively expensive, requires identical acquisition protocols across centers, is restricted to mainly lower extremity muscles in this population, and can be difficult to perform in younger children, those with behavioral problems, or those with advanced disease.
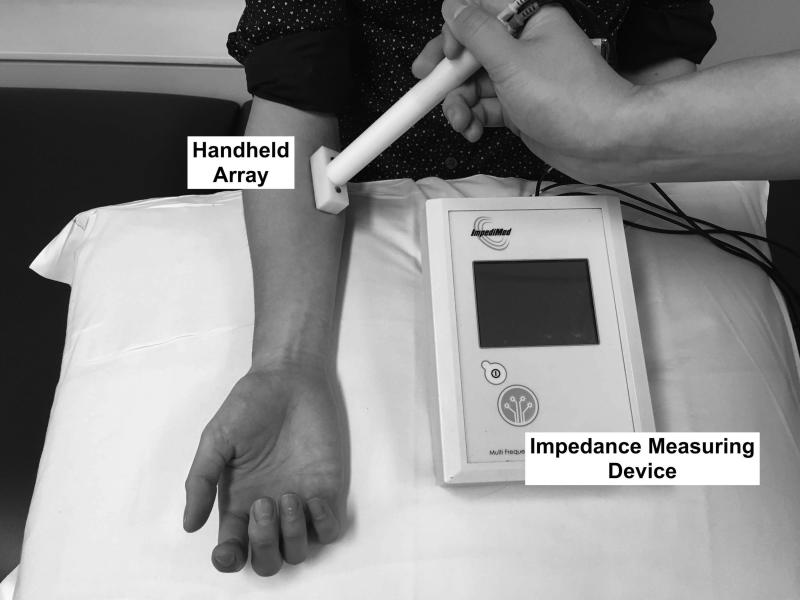
Electrical impedance myography (EIM) represents another potential method for assessing the effect of therapy in DMD that does not have these limitations.11 In EIM, the evaluator places a small 4-electrode array over a muscle of interest connected to a multifrequency impedance measuring device (Figure 1).12 A very low intensity alternating current at a range of frequencies (approximately 1 kHz to 1MHz) is passed across the outer two electrodes; the inner two electrodes measure the resulting voltages. Alterations in the muscle's structure and composition, including myocyte hypertrophy and atrophy, inflammation, edema, and connective tissue and fat deposition will impact the measured impedances.13-15 Our underlying hypothesis is that EIM is sensitive to changes in the biophysical properties of muscle due to DMD progression as well as to the effects of therapy. EIM has several additional potential advantages, including its being entirely painless, rapid to apply to a variety of upper and lower body muscles at the bedside, and its providing numerical data that do not require the complex image analysis. Evaluator training is also straightforward, and high reproducibility of measurements is possible.16 Importantly, cross-sectional data obtained in both mdx and wild-type mice as well as in DMD and healthy boys demonstrates marked differences in EIM values between healthy and dystrophin-deficient muscle.11, 13
Figure 1.
EIM being performed on anterior forearm with a custom-designed handheld array and the commercial bioimpedance device used in this study.
In this 2-year non-blinded, longitudinal study, we evaluated alterations in EIM values in a group of boys with DMD and compared changes to those of aged-matched healthy boys. Functional measures as well as quantitative ultrasound data (discussed in our companion paper)17 were also obtained. Our goal was to identify the character of EIM change in healthy children versus those with DMD. In addition, we separately evaluated alterations in a subset of DMD boys initiated on corticosteroids to determine whether EIM was sensitive to the therapeutic impact of that class of medications.
METHODS
Boys with DMD and healthy volunteers
Boston Children's Hospital Institutional Review Board approved the protocol, and parents and children provided written informed consent and verbal assent, respectively. Boys with DMD aged 2-14 years were recruited through the neuromuscular clinic at Boston Children's Hospital, as part of our Quantitative ultrasound and EIM in DMD (QED) study (the ultrasound results are reported in the companion paper17). All boys with DMD had genetic confirmation of disease or had a brother with genetically confirmed DMD and a characteristic clinical picture. DMD boys were excluded if they were enrolled in a therapeutic clinical trial or had a concomitant condition that substantially impacted health. Boys were enrolled and followed regardless of corticosteroid use. Healthy boys had no history of neuromuscular disease or any other disorder that would affect muscle health and were recruited via advertisement and word-of-mouth.
Study design
After enrollment, subjects had full assessment visits at baseline, 3-7 days (for reproducibility assessment; intra-examiner repeatability was assessed on all muscles and inter-examiner repeatability was assessed on biceps and quadriceps only), 1, 2, 3, 6, 9, 12, 18, and 24 months. All subjects had EIM performed at all visits as well as weight and height. Age- and ability-appropriate motor function tests were also performed (see below). We aimed to enroll 35 healthy boys and 35 boys with DMD, anticipating a 15% attrition rate; this was based on a power analysis using limited preliminary data.
EIM measurements
EIM measurements were obtained with the Imp SFB7® (Impedimed, Sydney, Australia) attached via cables to a custom-designed handheld array as previously described (Figure 1).11 As with any electrophysiological test, electrode size and positioning will impact the results; details regarding probe size, angle, depth of penetration, and device specifications are discussed in several previous articles.18-20 Given that the children ranged in age, 3 different sized electrode arrays were employed consistently on a given subject throughout the study. Measurements were performed on six muscles or muscle groups (deltoid, biceps, anterior forearm, rectus femoris, tibialis anterior, and medial gastrocnemius) on the dominant side; if dominance could not be established, testing was performed on the right side. The probe was placed over the bulk of the muscle using measurement paradigms based on boney prominences and other landmarks. Measurements were performed with the array placed longitudinally (current flow parallel to the muscle fibers) and transversely (current flow perpendicular to the major muscle fiber direction).
Functional measurements
The timed supine-to-stand test was administered to all boys who could directions and perform the test safely;15 the outcome was time to complete the test. Additionally, the 6-minute walk test (6MWT) was administered to boys ≥5 years if they were able to reliably and safely perform the test;14 the outcome was total distance walked.
Data analysis
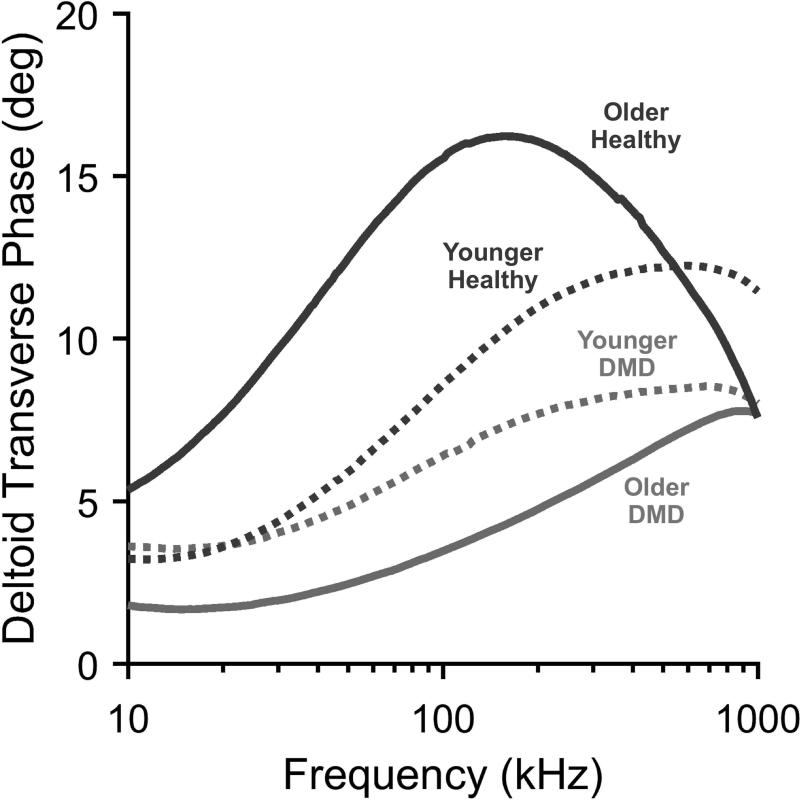
Statistical analyses were completed using MATLAB (Mathworks, Natick MA) and SAS Version 9.4 (SAS Institute, Inc, Cary NC). Basic analyses were performed separately for boys ≤7.0 years and those >7.0 years at the baseline visit since DMD clinical trials generally only enroll ambulatory children in the older group, where clear, predictable clinical deterioration is observed. A second reason for doing so was that our previous cross-sectional EIM analysis revealed a change in EIM values mirroring the clinical status at about 7 years of age.1 Figure 2 demonstrates typical baseline data obtained in 4 boys, two healthy and two with DMD, showing the marked differences in impedance spectral characteristics.
Figure 2.
Example of impedance spectra comparing older and younger boys: two healthy and two with DMD (Young DMD, 3. 7 years, young control, 3.9 years; older DMD 9.0 years, older control 8.7 Years). Young DMD and controls’ spectra are relatively close together, but the older boys’ are much further apart.
The three major basic impedance variables are the resistance (a measure of difficulty passing current through the tissue, generally increasing with disease progression), the reactance (a measure of the capacitive effects of the cell membranes, generally decreasing with disease progression), and the phase (equal to the arctan (reactance/resistance), also decreasing with disease progression). (Magnetic inductive effects are not believed to contribute to the impedance in biological systems.21) In this study, we analyzed phase values since it is less impacted by inter-electrode distance variation than reactance and resistance. In much of our work to date, we have utilized single frequency values, including in studies of DMD11 as well as in amyotrophic lateral sclerosis22 and spinal muscular atrophy.23 However, multifrequency rather than single-frequency outputs are often employed in the bioimpedance field since they provide a richer portrait of tissue condition.2 Indeed, we have also identified that multifrequency values, including a two-frequency phase ratio (EIM-PR),24 and average EIM-phase slope (EIM-PS) estimated using a linear model23 are both more sensitive to disease progression and can help reduce the impact of the subcutaneous fat on the obtained data (since muscle and fat have distinct frequency-dependent impedance characteristics).24 These measures in the form of a 100 kHz /300 kHz ratio and a least-squares fit of the EIM data between 100-500 kHz were the primary outcomes employed here. (Note that the previous study reported on the 50/200 ratio;24 however, subsequent unpublished work has supported that this latter ratio is more robust.) In addition, previous work in both animals and our cross-sectional analysis in humans suggested that the transverse (across-fiber) values would be more useful in DMD boys than the longitudinal (along-fiber) values, and thus we employed only transverse values in this analysis (the longitudinal data being obtained for future analysis of muscle electrical anisotropy).13, 25 We evaluated single muscle, composite upper and lower extremity values (3 muscles/limb), and whole-body measures (all 6 muscles).
Baseline demographic comparisons were performed using unpaired tests, 2-tailed, alpha=0.05. Intraclass correlation coefficients were used to evaluate reproducibility between baseline and the 3-7 day visit. Spearman correlation was used to relate changes in EIM metrics to functional change. Longitudinal analysis was performed using a linear mixed-effects model for each muscle's EIM and functional data with random intercept and slope terms to account for within-subject correlations and between-subject variability under the missing-at-random assumption. Outcomes at three time points were calculated in order to assess validity of results at standard clinical trial lengths (6 and 12 months) as well as the general robustness of the measure in the natural course of disease progression over 2 years. For these analyses, the main result of interest was the slope difference since it has the most direct relevance to clinical trials. Missing data were not imputed, but all measurements were included, except for those from boys who were initiated on corticosteroids during the study, post-corticosteroid initiation. The baseline age effect was estimated using the healthy control data that was subsequently removed from both populations prior to the analysis. Prior to analysis, given their small numerical values, the EIM-PR and EIM-PS were multiplied by scaling factors of 10 and 1000, respectively, to avoid rounding-off errors in SAS and to improve presentation in figures and tables. The final model selection was performed using Akaike Information criterion (AIC). Likelihood ratio test (LRT) for the nested models was also used to test the significance of variance covariance parameters of the random-effects. In addition, we applied the Benjamini-Hochberg false discovery rate procedure,26, 27 which controls for expected proportion of false discoveries relative to total discoveries, in order to control for multiple comparisons across all times and muscles/muscle groups analyzed. This approach was chosen over the more conservative Bonferonni correction since 1) the total number of comparisons performed (N=36) is large 2) individual muscle and time point data are not truly independent of one another; for this analysis, we accepted the proportion of false discoveries to be 10% (i.e., q=0.10), implying that 1 in 10 significant findings were actually true nulls. Finally, sample size estimates for a potential clinical trial were obtained using the effect sizes observed in our current study. Specifically, effect size was computed as the (mean slope difference) /(slope difference standard deviation) where the difference is between the healthy and DMD boys. The standard deviation of the slope difference is obtained as the square root of sum of two terms: the residual variance divided by a factor dependent on the linear trend of the design matrix (within-subject variance) and the random slope variance (between-subject variance). The factor for the residual variance in our model is the sum of squared deviations of the time-points of measurements from the average measurement period. This estimate of standard deviation can also be obtained easily from the estimated slope difference standard error from the mixed-effects model. For our sample size calculation, we have assumed that the measurements are obtained at every month (which is different from our current study design) with 80% power.
In order to evaluate the impact of corticosteroids, we compared post-steroid initiation data to values from those same boys pre-initiation combined with those boys who were not on steroids at any time, estimating slope differences using a piecewise linear mixed effects model.
RESULTS
Subjects
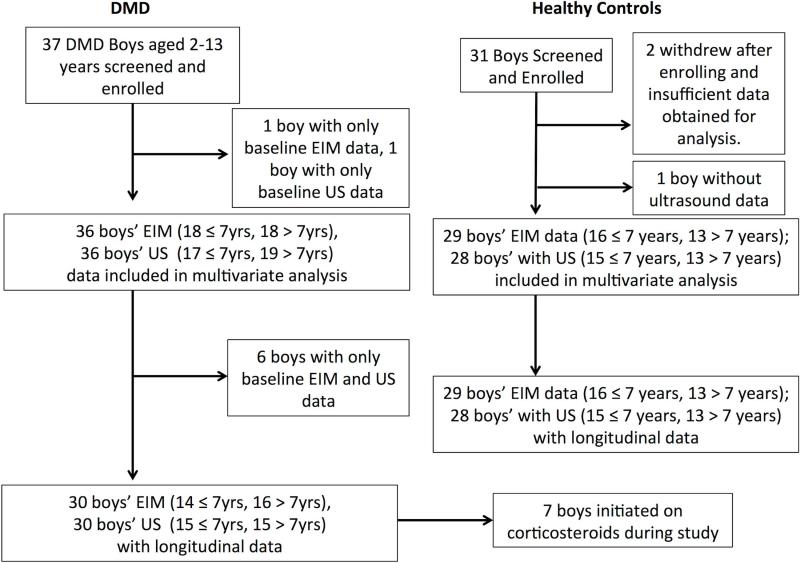
Figure 3, a Consort flow chart, summarizes the overall enrollment for the study (for both the EIM and quantitative ultrasound data collected, the latter being analyzed and discussed in the companion paper). For EIM, a total of 37 DMD boys and a total of 31 healthy controls were initially screened and enrolled. Of these, one DMD and 2 healthy controls were excluded due to insufficient data. Thus, a total 36 boys with DMD, mean age 7.3 years (range 2.2-13.1) and a total of 29 healthy controls, mean age 7.1 years (range 2.0 to 14.6 years) had data that were included in the analysis; these ages were comparable (p=0.85). Whereas all healthy controls returned for at least 1 follow-up visit, 6 of these DMD boys did not return for follow-up visits; nonetheless, all data in both groups was included in the analyses performed (see Supplementary Table 1 for detailed breakdown of participation by visit). Sixteen boys with DMD were taking corticosteroids throughout the study, 7 initiated corticosteroids during the study, and 13 were not treated with corticosteroids. Of the total EIM measurements made, 4.5% were excluded from the analysis due to poor technical quality. There were a total of 252 healthy subject and 217 DMD patient EIM assessment sessions over the 2-year period included in the analysis. Supine-to-stand test was performed a total of 110 times in 23 DMD boys and 189 times in 29 healthy controls; 6MWT test was performed 62 times in 16 DMD boys and 155 times in 24 healthy controls.
Figure 3.
Consort flow chart incorporating both the EIM and quantitative ultrasound aspects of the QED study.
Intra- and inter-examiner repeatability.
Repeatability data between the two first visits, spaced no more than 1-week apart, for both the EIM-PR and EIM-PS are presented in Table 1. Both intra- and inter-examiner repeatability was good to excellent in both healthy and DMD boys.
Table 1.
Reproducibility assessments at 3-7 days versus baseline
| EIM Measure and muscle(s) | Healthy Controls | DMD | EIM Measure and muscle(s) | Healthy Controls | DMD |
|---|---|---|---|---|---|
| Intra-rater intraclass correlation coefficient values | |||||
| EIM-PR Six-muscle | 0.87 | 0.94 | EIM-PS Six-muscle | 0.73 | 0.87 |
| EIM-PR Upper | 0.85 | 0.93 | EIM-PS Upper | 0.66 | 0.83 |
| EIM-PR Lower | 0.81 | 0.89 | EIM-PS Lower | 0.69 | 0.80 |
| EIM-PR Biceps | 0.85 | 0.88 | EIM-PS Biceps | 0.78 | 0.75 |
| EIM-PR Quads | 0.76 | 0.881 | EIM-PS Quads | 0.65 | 0.71 |
|
Inter-rater intraclass correlation coefficient values | |||||
| EIM-PR Biceps | 0.94 | 0.94 | EIM-PS Biceps | 0.89 | 0.82 |
| EI-PR Quads | 0.78 | 0.88 | EIM-PS Quads | 0.56 | 0.81 |
EIM change in boys >7.0 years of age
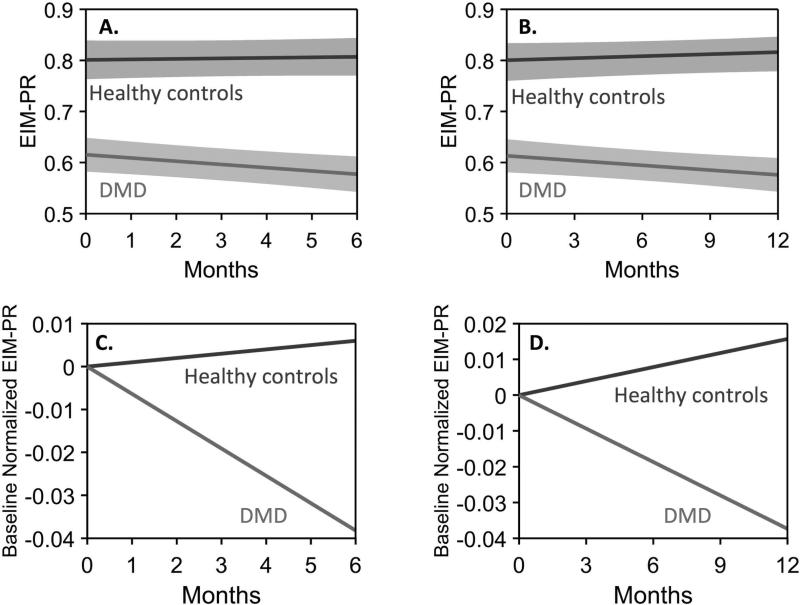
The EIM-PR in the upper extremities showed a significant difference between healthy boys and those with DMD at 6 months of age and the significance of this difference increased out to 2 years, with slope differences ranging from −0.074/month, p=0.023 95%CI[−0.013,−0.14] at 6 months to −0.048/month, p=<0.0001 95%CI[−0.028,−0.068] at 2 years; in the lower extremities this became significant only at 24 months and in the combined 6-muscle average metric at 12 months (see Figure 4 and Table 2). The EIM-PS measure also showed significant change at 12 months onward, mainly in the anterior forearm (see Supplemental Table 1). False discovery rate analysis appropriately reduced the number of significant findings. These upper versus lower extremity differences may represent a floor effect in lower extremity muscles; see the discussion section for additional detail on this point.
Figure 4.
Example of 6-month and 1-year data from longitudinal mixed-effects model in boys > 7 years with and without DMD. A, B. Averaged upper extremity EIM-PR mean trajectory for each cohort along with its respective 95% confidence interval. C, D. show the models with the baseline differences removed to emphasize the observed longitudinal changes.
Table 2.
Summary of Data
| N | 6 months | 12 months | 24 months | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age >7.0 EIM-PR* | Slope Diff | 95% CI | p-value | Slope Diff | 95% CI | p-value | Slope Diff | 95% CI | p-value | |
| EIM | 18,13 | |||||||||
| Upper ext. avg. | −0.074 | [−0.013,−0.135] | 0.023 | −0.044 | [−0.013,−0.075] | 0.0098§ | −0.048 | [−0.028,−0.068] | <0.00010§ | |
| Anterior | −0.10 | [−0.018,−0.182] | 0.017 | −0.080 | [−0.047,−0.113] | <0.00010§ | −0.061 | [−0.034,−0.088] | 0.00020§ | |
| Lower ext. avg. | −0.029 | [0.036,−0.094] | 0.38 | −0.0059 | [0.025,−0.037] | 0.72 | −0.028 | [−0.004,−0.052] | 0.026 | |
| Gastrocnemius | −0.064 | [0.044,−0.172] | 0.25 | −0.024 | [0.027,−0.075] | 0.34 | −0.029 | [0.004,−0.062] | 0.099 | |
| Six-muscle avg. | −0.052 | [−0.001,−0.103] | 0.055 | −0.029 | [−0.002,−0.056] | 0.041 | −0.038 | [−0.019,−0.057] | 0.00030§ | |
| 6-MWT | 6, 13 | 11.9 | [−5.152,28.952] | 0.18 | 6.13 | [−4.885,17.145] | 0.29 | −1.74 | [3.02,−6.50] | 0.48 |
| STST | 7, 13 | 0.84 | [−0.16,1.840] | 0.11 | 0.38 | [−0.365,1.125] | 0.33 | 0.40 | [−0.07,0.87] | 0.12 |
| Age ≤7.0 EIM-PS† | ||||||||||
| EIM | 18,16 | |||||||||
| Upper ext. avg. | −0.27 | [0.16,−0.70] | 0.22 | −0.039 | [0.13,−0.21] | 0.66 | 0.10 | [−0.02,0.22] | 0.090 | |
| Deltoid | −0.20 | [0.23,−0.63] | 0.37 | 0.017 | [−0.18,0.21] | 0.87 | 0.10 | [−0.037,0.24] | 0.15 | |
| Lower ext avg. | −0.48 | [−0.088,−0.87] | 0.021 | −0.16 | [−0.001,−0.319] | 0.063 | −0.013 | [0.10,−0.13] | 0.82 | |
| Gastrocnemius | −0.83 | [−0.26,−1.40] | 0.0068§ | −0.24 | [−0.024,−0.46] | 0.032 | −0.042 | [0.097,−0.18] | 0.56 | |
| Six-muscle avg. | −0.38 | [0.012,−0.77] | 0.063 | −0.096 | [0.061,−0.25] | 0.24 | 0.042 | [−0.064,0.15] | 0.44 | |
| 6-MWT | 10,11 | 1.56 | [−15.94,19.06] | 0.86 | 1.24 | [−9.56,12.04] | 0.82 | −1.13 | [3.18,−5.44] | 0.61 |
| STST | 16,15 | 0.088 | [−0.62,0.79] | 0.81 | 0.19 | [−0.34,0.72] | 0.49 | 0.062 | [−0.25,0.38] | 0.71 |
Significant values are in bold font. Slope difference refers to the difference (DMD-control) in the slope of a given parameter over time. EIM-PR and -PS, electrical impedance myography phase ratio and phase slope. N, Number of DMD boys, Number of healthy controls included in analysis; upper ext. avg., upper extremity average; lower ext. avg., lower extremity average; 6-MWT, 6-minute walk test; STST, supine-to-stand test
EIM-PR units are (months−1)
FDR q-value < 0.1.
EIM-PS units are Deg-kHz−1-month−1
6MWT feet/month; supine-to-stand seconds/month. Values are mean (+/− standard error). N.B. Estimates of slope differences for EIM-PR incorporate a multiplicative scaling factor of 10; estimates for EIM-PS incorporate a factor of 1000; these are included for numerical stability and presentation.
EIM changes in boys ≤7.0 years
As anticipated, the alteration in values in younger boys was subtler. Whereas the EIM-PR showed no significant difference in upper or lower extremities, the EIM-PS measure showed a significant change in gastrocnemius as early as 6 months (−0.83°/kHz-month, p=0.0068 95%CI[−0.26,−1.40]) and remained significant out to 18 months (−0.18°/kHz-month, p=0.044 95%CI[−0.006,−0.35]); the lower extremity average was significant at 6 and 12 months (Table 1). Again, as expected the false discovery rate analysis reduced the significance of findings to just the gastrocnemius values at 6 months. These changes may also reflect a floor effect observed in the lower extremity muscles and in the gastrocnemius specifically and participant drop out over longer study periods.
Correlation to standard functional measures over time
In younger DMD boys, the changes in EIM-PS in lower extremities correlated with the change in the supine-to-stand test time (rho=0.62, p=0.0081). In older boys, changes in upper extremity EIM-PR had a similar strength correlation to 6MWT (rho=0.55) although significance was not reached (p=0.082).
Effect sizes and clinical trial sample size estimations
Based on the rates of progression, we calculated effect sizes and sample sizes for hypothetical clinical trials that utilized these EIM metrics as outcome markers of drug efficacy in the older boys using the EIM-PR and the EIM-PS in the younger boys, assuming both 100% and a 50% treatment effects in the assessed values. Table 3 shows these values for the younger and older boys based on our analyses and in comparison to the two standard functional measures obtained (the timed floor-to-stand in the younger boys and the 6MWT in the older boys) with studies of 6 and 12 months’ duration, assuming 80% power, two-tailed. As can be seen, the EIM indices showed smaller sample sizes than using the functional assessments for both age groups. The effect size values are of a similar order to those recently reported for MRI.10
Table 3.
Effect size and sample size estimations based on estimate of slope differences
| Study Length | 6 months | 12 months | |||
|---|---|---|---|---|---|
| Boys > 7.0 years | Treatment effect | Effect size | Sample size | Effect size | Sample size |
| EIM Upper extremity average | 100% | 0.88 | 21 | 1.00 | 16 |
| 50% | 82 | 64 | |||
| EIM Forearm | 100% | 0.97 | 17 | 1.76 | 6 |
| 50% | 67 | 21 | |||
| 6MWT | 100% | 0.73 | 30 | 0.69 | 33 |
| 50% | 117 | 131 | |||
| Supine-to-stand test | 100% | 0.87 | 21 | 0.44 | 82 |
| 50% | 83 | 325 | |||
| Boys ≤ 7.0 years | |||||
| EIM Lower extremity average | 100% | 0.84 | 23 | 0.67 | 36 |
| 50% | 89 | 141 | |||
| EIM Gastrocnemius | 100% | 0.98 | 17 | 0.76 | 27 |
| 50% | 65 | 108 | |||
| 6MWT | 100% | 0.096 | 1702 | 0.089 | 1963 |
| 50% | 6805 | 7851 | |||
| Supine-to-stand test | 100% | 0.091 | 1878 | 0.27 | 223 |
| 50% | 7510 | 892 | |||
These results assume 80% power and monthly evaluations. EIM, electrical impedance myography. 6MWT, 6-minute walk test
Effect of corticosteroids
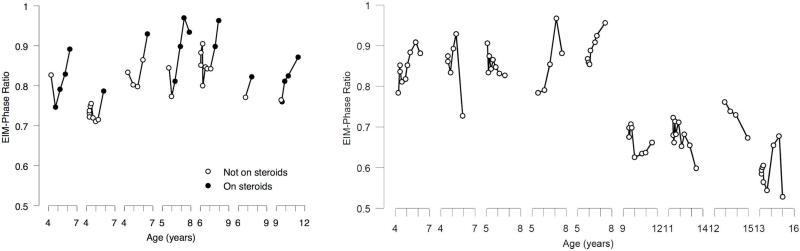
Seven boys (mean age 5.8 years) were placed on corticosteroids during the study. These drugs included prednisone, prednisolone, and deflazacort, the last obtained from abroad, at varying doses. Figure 5A summarizes the EIM-PR 6-muscle data for these 7 boys from baseline to their last corticosteroid dose; Figure 5B summarizes the same data for the 9 boys of similar age who were not on steroids at any time. As can be seen, in all boys in whom corticosteroids were initiated, there was an increase in values shortly after the initiation, These post-steroid slopes were significantly different compared to their pre-steroid slopes combined with those of boys who were not on corticosteroids throughout the study as assessed by the piecewise model with slope difference for EIM-PR of 0.057/month, p=0.00019 95%CI[0.028,0.086]; EIM-PS was also significant, with slope difference of 0.14°/kHz-month p=0.013 95%CI[0.028,0.25]. Upper extremity EIM PR also showed a significant change (0.055/month, p=0.0016 95%CI[0.021,0.089]) as did lower extremity EIM-PS°/kHz-month (0.17, p=0.0096 95%CI[0.043,0.30]).
Figure 5.
Corticosteroid effect. (A.) Shows the impact of corticosteroids on the 6-muscle EIM-PR in each of the 7 boys in whom corticosteroids was initiated during the study; open circles, pre-corticosteroids; closed circles, post-corticosteroid initiation. (B.) Analogous data for the group of 9 boys who remained off steroids throughout the entire study.
DISCUSSION
These results show that EIM has the potential of identifying progression of DMD in boys within 6 months, a length of time comparable to that achieved with MRI and that it may also be sensitive to the beneficial effect of corticosteroids. We believe that taken together, these data support that EIM may be able to serve as a biomarker in DMD clinical trials, especially since the technology is convenient to apply and demands relatively minimal training. However, it is important to emphasize that this study also raises new uncertainties as to exactly how EIM would be employed since different metrics were effective in different muscles in younger as compared to older boys.
In this analysis we have focused on multifrequency impedance values, both a two-frequency ratio and a least-squares fit of the multifrequency data.24, 28 The use of multifrequency metrics is the underlying principle of the field of bioimpedance spectroscopy since it has long been recognized that single frequency data offer limited insight into tissue condition. And, indeed, the approach of reducing the impedance spectrum to a single value is commonly employed.21 In the case of DMD, multifrequency data are anticipated to be more sensitive to myofiber diameter, since the entire impedance spectrum shifts to the left (to lower frequencies) as myofibers enlarge;21 capturing this effect is not possible using single frequency data. Second, the impedance characteristics of fat are considerably different from those of muscle.29 By using multifrequency values, we have been able to show that the impact of subcutaneous fat on the measurements can be greatly reduced while still maintaining a strong correlation to function.24 As a post-hoc analysis, we also evaluated single frequency values (e.g. 50 kHz transverse phase). As anticipated, these were less effective at identifying differences over time; for example, the 50 kHz transverse phase only showed a difference in upper extremity values at 24 months (p=0.032). This finding of reduced sensitivity is consistent with our spinal muscular atrophy results.3
What do these multifrequency EIM changes represent? In boys with DMD, increasing fat and connective tissue and associated myofiber loss and atrophy likely cause a shift in the frequency spectrum, with increasing values at higher frequencies and reductions at lower frequencies (see Figure 2). Stated another way, a gradual reduction in the 100 kHz value and increase in the high frequency values decreases the calculated ratio; the phase slope metric captures an analogous change. In healthy muscle, normal growth and maturation and increasing muscle fiber diameter causes an increase in at 100 kHz but a reduction at 300 kHz, thus producing a greater ratio and a corresponding increase in EIM-PS.
Like much research assessing new concepts and technologies, this study answers some questions but raises new ones. For example, why do upper extremity muscles appear to show larger/more consistent changes in older boys? And why would the lower extremity muscles of younger boys be more sensitive to change? It is possible that by the time a boy with DMD reaches age 10 or 11, his lower extremity muscles are already showing considerable disease. It may be easier to detect alterations in less affected upper extremity muscles. In younger boys, in contrast, the lower extremities would be expected to show the first evidence of disease progression since this is the region first typically affected.
One of our decisions in this study prior to analysis was to separate the younger boy data from the older boy data. The main reason for doing so was that while some younger DMD boys lose motor function or remain stable, many gain motor milestones up until the age of approximately 7 years at which point nearly all DMD boys begin to lose function.30 Since we have previously shown in a cross-sectional analysis that basic EIM parameters appear to correlate positively with age in younger boys and negatively correlate in older boys,11 this seemed a reasonable approach to consider. And, in fact, many clinical trials employ strict age cut-offs. Nonetheless, such limitations may not be necessary as our corticosteroid initiation data demonstrate. Indeed, our analysis suggests the identification of a treatment effect regardless of age, the boys ranging from approximately 4-10 years at the time of corticosteroid initiation.
Indeed, the observed rapid alteration in EIM values in the subgroup of DMD boys initiated on corticosteroids further supports EIM's potential utility since corticosteroids generally improve function in the months following initiation.31 We can only speculate as to the reasons underlying the often abrupt and marked impedance change. A possible explanation is that EIM is detecting a reduction in ongoing inflammation/muscle fiber breakdown or a restoration of more normal myocyte growth. However, it seems unlikely that it represents a reduction in fat or connective tissue deposition, since those tissues would likely not be impacted positively by steroids in the short term; moreover, if those effects were present, we may have seen a corresponding change in the quantitative ultrasound data, which was not apparent, as described in our companion paper.17
Despite the presence of an observed effect of corticosteroids and differences in trajectories for the EIM parameters in healthy versus DMD boys, there clearly remain challenges to the application of this technique in a clinical trial. For example, would different outcome measures or different body regions need to be studied? And what threshold for EIM improvement would be needed to support an actual functional improvement? While these questions are important, it is also essential to remember that currently a biomarker such as EIM would likely only be used in clinical trial research to assist in early go/no-go decisions regarding further therapy evaluation. Ultimately, for regulatory approval, an actual functional improvement would still be required.
In addition to our other analyses, we also completed a false discovery rate analysis given the large number of comparisons that were made. While we believe that this more conservative interpretation of the data is necessary in order to be complete, it is important recognize that the nature of this work is as much hypothesis generating as it is hypothesis testing. Identifying novel metrics of disease progression using a new technology may require a less-restrictive perspective than say for a technology such as magnetic resonance imaging that has been studied and developed by countless investigators over many decades. In short, much remains unknown about EIM and its relationship to DMD. It is very possible, for example, that other still more sensitive and effective EIM metrics will be identified as the technology continues to mature.
It is unlikely that any future therapy, no matter how effective, will put the muscle of boys with DMD on a normal trajectory of growth and maturation. In fact, in most instances, therapies will likely transform DMD into a milder Becker muscular dystrophy-like phenotype and not provide complete disease reversal. Thus, the sample size analysis must be interpreted conservatively, since it is more likely that at best, only a partial response is to be anticipated. Accordingly, in our sample size estimations we included a 50% treatment effect size as well as the more typically reported 100% effect. Of note, effect sizes for the EIM measures in Table 2 are in the range of those sought in several double-blind placebo controlled clinical trials that relied on functional outcome measures (effect sizes of 0.5 to 1.29).32-35
The results we have observed here mirror findings in mdx mice,13 in which EIM phase values were lower in 2-year-old than 6-month-old mice. EIM values also correlated to both cell size and connective tissue deposition, supporting the technique's construct validity. Also, marked differences compared to wild type animals were already present at 6-months, a time when very little fat, edema, or connective tissue infiltration is present. This supports that observed EIM differences can be due to primary cellular morphology effects.
There are a number of limitations to this study. First, the technology being used here incorporated a system not specifically designed for muscle assessment. The handheld arrays also did not use optimized designs. For example, recent work has shown that positioning of the current emitting electrodes further from the voltage measuring electrodes affords improved sensitivity to muscle condition and reduces the impact of subcutaneous fat.18 Second, there are a variety of potential EIM parameters to evaluate. Whereas work has demonstrated that various multifrequency metrics might improve upon standard single frequency parameters in assessing progression across a variety of disorders, including spinal muscular atrophy,28 disuse atrophy,36 and amyotrophic lateral sclerosis,37 its application to DMD is new. Clearly, the results will thus require validation in separate studies. Third, this is a single site investigation, and it would be important to replicate these findings in a multicenter cohort. Fourth, we have only examined non-linearity visually in our outcomes. Further work using non-parametric statistical approaches may lead to a better understanding of trajectories over longer durations. However, we are restricted from doing so here by missing data and the sample size in this study. Fifth, a much greater loss of follow up visits in DMD patients over time may have reduced the significance of findings over longer lengths of time. Similarly, there are relatively fewer data points for the 6MWT and supine-to-stand test likely reducing significance of those functional measures as well. Finally, the sample size comparisons are meaningful for that measure only. In other words, detecting a 50% treatment effect using an EIM measure may not necessarily translate into a 50% functional improvement. Nonetheless, we did observe a significant relationship between change in 6MWT distance and EIM metrics.
This study supports the basic concept that EIM is sensitive to disease progression in both younger and older boys with DMD and can also detect a corticosteroid treatment effect. Given its extreme ease of application, both from evaluator and patient standpoints, further study of this novel tool in DMD clinical therapeutic trials should be pursued.
Supplementary Material
Acknowledgements
This study was funded in its entirety by NIH grant R01AR060850.
Footnotes
Clinicaltrials.gov identifier: NCT01491555
Author contributions: SBR, BTD, KK, JSW and CZ were responsible for study concept and design, data analysis, and drafting the manuscript and figures. SY, A Pacheck, A Pasternak, LM, HS, TH were responsible for data acquisition and analysis.
Potential conflicts of interest: Dr. Rutkove has equity in, and serves a consultant and scientific advisor to, Skulpt, Inc. a company that designs impedance devices for clinical and research use; he is also a member of the company's Board of Directors. The company also has an option to license patented impedance technology of which Dr. Rutkove is named as an inventor. This study, however, did not employ any relevant company or patented technology.
References
- 1.Mayhew JE, Florence JM, Mayhew TP, et al. Reliable surrogate outcome measures in multicenter clinical trials of Duchenne muscular dystrophy. Muscle Nerve. 2007;35:36–42. doi: 10.1002/mus.20654. [DOI] [PubMed] [Google Scholar]
- 2.Mazzone E, Vasco G, Sormani MP, et al. Functional changes in Duchenne muscular dystrophy: a 12-month longitudinal cohort study. Neurology. 2011;77:250–6. doi: 10.1212/WNL.0b013e318225ab2e. [DOI] [PubMed] [Google Scholar]
- 3.McDonald CM, Henricson EK, Han JJ, et al. The 6-minute walk test as a new outcome measure in Duchenne muscular dystrophy. Muscle Nerve. 2010;41:500–10. doi: 10.1002/mus.21544. [DOI] [PubMed] [Google Scholar]
- 4.Connolly AM, Florence JM, Cradock MM, et al. One year outcome of boys with Duchenne muscular dystrophy using the Bayley-III scales of infant and toddler development. Pediatric neurology. 2014;50:557–63. doi: 10.1016/j.pediatrneurol.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazzone ES, Pane M, Sormani MP, et al. 24 month longitudinal data in ambulant boys with Duchenne muscular dystrophy. PloS one. 2013;8:e52512. doi: 10.1371/journal.pone.0052512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald CM, Henricson EK, Abresch RT, et al. The 6-minute walk test and other clinical endpoints in duchenne muscular dystrophy: Reliability, concurrent validity, and minimal clinically important differences from a multicenter study. Muscle Nerve. 2013 doi: 10.1002/mus.23905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendell JR, Rodino-Klapac LR, Sahenk Z, et al. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann Neurol. 2013;74:637–47. doi: 10.1002/ana.23982. [DOI] [PubMed] [Google Scholar]
- 8.Wary C, Azzabou N, Giraudeau C, et al. Quantitative NMRI and NMRS identify augmented disease progression after loss of ambulation in forearms of boys with Duchenne muscular dystrophy. NMR Biomed. 2015;28:1150–62. doi: 10.1002/nbm.3352. [DOI] [PubMed] [Google Scholar]
- 9.Bonati U, Hafner P, Schadelin S, et al. Quantitative muscle MRI: A powerful surrogate outcome measure in Duchenne muscular dystrophy. Neuromuscul Disord. 2015;25:679–85. doi: 10.1016/j.nmd.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Willcocks RJ, Rooney WD, Triplett WT, et al. Multicenter prospective longitudinal study of magnetic resonance biomarkers in a large duchenne muscular dystrophy cohort. Ann Neurol. 2016 doi: 10.1002/ana.24599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutkove SB, Geisbush TR, Mijailovic A, et al. Cross-sectional evaluation of electrical impedance myography and quantitative ultrasound for the assessment of Duchenne muscular dystrophy in a clinical trial setting. Pediatric neurology. 2014;51:88–92. doi: 10.1016/j.pediatrneurol.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutkove SB. Electrical Impedance Myography: Background, Current State, and Future Directions. Muscle Nerve. 2009;40:936–46. doi: 10.1002/mus.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Geisbush TR, Rosen GD, Lachey J, Mulivor A, Rutkove SB. Electrical impedance myography for the in vivo and ex vivo assessment of muscular dystrophy (mdx) mouse muscle. Muscle Nerve. 2014;49:829–35. doi: 10.1002/mus.24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahad M, Rutkove SB. Electrical impedance myography at 50 kHz in the rat: technique, reproducibility, and the effects of sciatic injury and recovery Clinical Neurophysiology. Clinical Neurophys. 2009;120:1534–8. doi: 10.1016/j.clinph.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahad MA, Fogerson PM, Rosen GD, Narayanswami P, Rutkove SB. Electrical characteristics of rat skeletal muscle in immaturity, adulthood, and after sciatic nerve injury and their relation to muscle fiber size. Physiol Meas. 2009;30:1415–27. doi: 10.1088/0967-3334/30/12/009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaidman CM, Wang LL, Connolly AM, et al. Electrical impedance myography in Duchenne muscular dystrophy and healthy controls: A multicenter study of reliability and validity. Muscle Nerve. 2015;52:592–7. doi: 10.1002/mus.24611. [DOI] [PubMed] [Google Scholar]
- 17.Zaidman C, Wu J, Kapur K, et al. Quantitative Muscle Ultrasound Detects Disease Progression in Duchenne Muscular Dystrophy. Submitted. [DOI] [PMC free article] [PubMed]
- 18.Jafarpoor M, Li J, White JK, Rutkove SB. Optimizing electrode configuration for electrical impedance measurements of muscle via the finite element method. IEEE Trans Biomed Eng. 2013;60:1446–52. doi: 10.1109/TBME.2012.2237030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sung M, Spieker AJ, Narayanaswami P, Rutkove SB. The effect of subcutaneous fat on electrical impedance myography when using a handheld electrode array: the case for measuring reactance. Clin Neurophysiol. 2013;124:400–4. doi: 10.1016/j.clinph.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narayanaswami P, Spieker AJ, Mongiovi P, Keel JC, Muzin SC, Rutkove SB. Utilizing a handheld electrode array for localized muscle impedance measurements. Muscle Nerve. 2012;46:257–63. doi: 10.1002/mus.23307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimnes S, Martinsen OG. Bioimpedance and Bioelectricity Basics. Second ed. Academic press; London: 2008. [Google Scholar]
- 22.Rutkove SB, Caress JB, Cartwright MS, et al. Electrical impedance myography as a biomarker to assess ALS progression. Amyotroph Lateral Scler. 2012;13:439–45. doi: 10.3109/17482968.2012.688837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rutkove SB, Shefner JM, Gregas M, et al. Characterizing spinal muscular atrophy with electrical impedance myography. Muscle Nerve. 2010;42:915–21. doi: 10.1002/mus.21784. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz S, Geisbush TR, Mijailovic A, Pasternak A, Darras BT, Rutkove SB. Optimizing electrical impedance myography measurements by using a multifrequency ratio: a study in Duchenne muscular dystrophy. Clin Neurophysiol. 2015;126:202–8. doi: 10.1016/j.clinph.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutkove SB, Darras BT. Electrical impedance myography for the assessment of children with muscular dystrophy: a preliminary study. Journal of physics Conference series. 2013:434. doi: 10.1088/1742-6596/434/1/012069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc, Series B. 1995;57:289–300. [Google Scholar]
- 27.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–5. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rutkove SB, Gregas MC, Darras BT. Electrical impedance myography in spinal muscular atrophy: A longitudinal study. Muscle Nerve. 2012;45:642–7. doi: 10.1002/mus.23233. [DOI] [PubMed] [Google Scholar]
- 29.Gabriel C, Gabriel S, Corthout E. The dielectric properties of biological tissues: I. Literature survey. Physics in medicine and biology. 1996;41:2231–49. doi: 10.1088/0031-9155/41/11/001. [DOI] [PubMed] [Google Scholar]
- 30.Bushby K, Connor E. Clinical outcome measures for trials in Duchenne muscular dystrophy: report from International Working Group meetings. Clin Investig (Lond) 2011;1:1217–35. doi: 10.4155/cli.11.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griggs RC, Moxley RT, 3rd, Mendell JR, et al. Prednisone in Duchenne dystrophy. A randomized, controlled trial defining the time course and dose response. Clinical Investigation of Duchenne Dystrophy Group. Arch Neurol. 1991;48:383–8. doi: 10.1001/archneur.1991.00530160047012. [DOI] [PubMed] [Google Scholar]
- 32.Campbell C, McMillan HJ, Mah JK, et al. Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: Results of a randomized, placebo-controlled clinical trial. Muscle Nerve. 2016 doi: 10.1002/mus.25268. [DOI] [PubMed] [Google Scholar]
- 33.Escolar DM, Zimmerman A, Bertorini T, et al. Pentoxifylline as a rescue treatment for DMD: a randomized double-blind clinical trial. Neurology. 2012;78:904–13. doi: 10.1212/WNL.0b013e31824c46be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirschner J, Schessl J, Schara U, et al. Treatment of Duchenne muscular dystrophy with ciclosporin A: a randomised, double-blind, placebo-controlled multicentre trial. Lancet Neurol. 2010;9:1053–9. doi: 10.1016/S1474-4422(10)70196-4. [DOI] [PubMed] [Google Scholar]
- 35.Escolar DM, Buyse G, Henricson E, et al. CINRG randomized controlled trial of creatine and glutamine in Duchenne muscular dystrophy. Ann Neurol. 2005;58:151–5. doi: 10.1002/ana.20523. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Spieker AJ, Rosen GD, Rutkove SB. Electrical impedance alterations in the rat hind limb with unloading. J Musculoskelet Neuronal Interact. 2013;13:37–44. [PMC free article] [PubMed] [Google Scholar]
- 37.Wang LL, Spieker AJ, Li J, Rutkove SB. Electrical impedance myography for monitoring motor neuron loss in the SOD1 G93A amyotrophic lateral sclerosis rat. Clin Neurophysiol. 2011;122:2505–11. doi: 10.1016/j.clinph.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.