Abstract
Liver endothelial cell (LEC) damage is essential in the pathogenesis of ischemia-reperfusion injury (IRI) in transplant recipients. We analyzed the mechanism of LEC resistance against IRI by using a novel recombinant soluble form of PSGL-1 (Tandem P-Selectin Glycoprotein Ligand-Immunoglobulin; TSGL-Ig) in a mouse model of hepatic cold preservation (4°C in UW for 20h) and syngeneic orthotopic liver transplantation (OLT). Unlike in controls, TSGL-Ig protected OLT against IR-stress, evidenced by depressed sALT levels, well-preserved hepatic architecture, and improved survival (42% vs. 92%). TSGL-Ig suppressed neutrophil/macrophage sequestration and pro-inflammatory cytokine/chemokine programs in OLT. Treatment with TSGL-Ig mitigated LEC activation (P-/E-selectin, VCAM-1/ICAM-1 expression). In parallel in vitro studies, TSGL-Ig diminished cellular damage in H2O2-stressed LEC cultures (LDH/ALT levels). Increased Trx, GCL, NQO1, and HIF-1α expression, along with transcription factor Nrf2, implied TSGL-Ig exerts anti-oxidant functions in IR-stressed OLT and H2O2-stressed LECs. Indeed, Nrf2-deficient livers suffered fulminant IRI, compared with WT despite concomitant TSGL-Ig therapy. Thus, TSGL-Ig is not only acting as competitive antagonist blocking leukocyte migration into IR-stressed liver, but it may also act directly as an agonist stimulating Nrf2-mediated cytoprotection in LECs. This study supports the role of P-selectin signaling in hepatic homeostasis in OLT, with broad implications for tissue damage conditions.
Introduction
Local sterile inflammation represents an innate immune signature of hepatic ischemia-reperfusion injury (IRI), an inevitable event in organ procurement, transplantation, partial hepatectomy, trauma or shock. The IRI often results in hepatic non-function or dysfunction at early post-transplant phase, and may lead to increased incidence of acute and chronic rejection [1, 2]. In addition, IRI contributes to donor organ shortage as marginal livers are particularly susceptible to the ischemic insult. However, despite recent progress, more studies are needed to further elucidate mechanisms of innate immunity-driven liver inflammation, and to develop much needed new therapeutic concepts against IR-triggered hepatocellular damage.
Liver endothelial cells (LECs) form the vascular wall of the hepatic sinusoid. With no organized basal membrane, microvascular endothelium allows macrophage and neutrophil infiltration causing inflammatory reactions [3–5]. LECs play protective roles controlling vascular homeostasis, inflammation, vascular tone, and toxicants clearance. Insult to hepatic endothelium during cold preservation contributes to liver IRI, bringing out low graft microcirculation, upregulation of adhesion molecule expression, oxidative stress, and further augmenting neutrophil infiltration and hepatocyte necrosis [4, 5].
The selectins, a family of lectin-domain glycoproteins expressed on endothelial cells, platelets and leukocytes mediate the initial capture and support leukocyte rolling on sinusoidal endothelial cells in the initial phase of IRI [6]. The principal selectin ligand, P-selectin glycoprotein ligand-1 (PSGL-1, CD162) is the only known high-affinity ligand for P-selectin in the cell adhesion cascade [7]. We have reported that treatment with recombinant PSGL-Ig ameliorated hepatic IRI in rat liver models of cold ischemia followed by ex-vivo isolated perfusion or syngeneic orthotopic liver transplantation (OLT) [8, 9]. Moreover, in a recent clinical trial at UCLA, a short-term rPSGL-Ig therapy proved effective in deceased-donor liver transplant patients with 6-month follow-up [10].
The oxidative stress caused by excessive production of reactive oxygen species (ROS), is vital for hepatocellular insult in the pathophysiology of liver IRI [1, 2, 11]. The Keap1-Nrf2-ARE signaling axis represents an essential trigger for adaptive oxidative stress response, in processing excessive ROS production, as well as maintaining the cellular redox balance and metabolism [12, 13]. This pathway consists of three components: Kelch-like ECH-associated protein 1 (Keap1), nuclear factor erythroid 2-related factor 2 (Nrf2), and antioxidant response elements (AREs). The transcription factor Nrf2 belongs to the cap ‘n’ collar family with a conserved basic leucine zipper structure. It may protect cells against oxidative stress [13] and maintain the cellular redox balance by regulating endogenous antioxidants, phase II detoxification enzymes, and defensive proteins via AREs [14]. On basal conditions, Nrf2 is associated in the cytoplasm by Keap1, ubiquitinated by the Cul3-Keap1 E3 ligase complex, and degraded by the proteasome pathway [15]. Keap1-mediated ubiquitination of Nrf2 is disrupted in response to oxidative stress, leading to Nrf2 stabilization. The level of Nrf2 protein is elevated correspondingly and the transcription factor translocates into the nucleus, where it binds ARE-containing promoters. This triggers ARE-controlled expression of cytoprotective oxidative stress response enzymes, such as thioredoxin (Trx), glutamate-cysteine ligase (GCL) and NAD(P)H dehydrogenasequinine-1 (NQO1) [16].
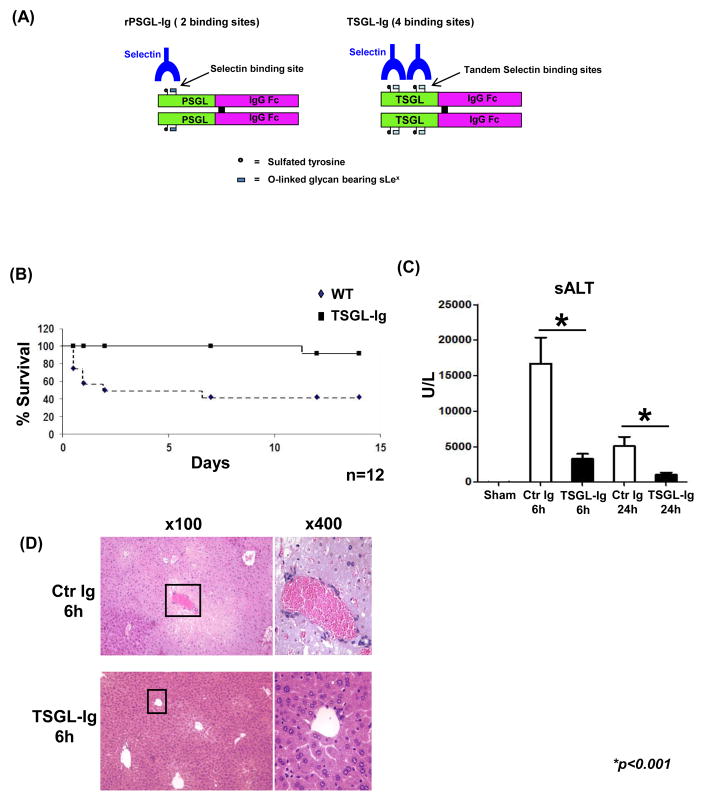
To gain further insight into putative role of adhesion molecule antagonism in hepatic inflammation and the acquisition of intracellular redox homeostasis, we have constructed a new soluble recombinant Ig fusion molecule, designated “TSGL-Ig” where the “T” stands for “tandem”. In the TSGL-Ig construct, two P-selectin sulfated glycopeptide binding domains from human PSGL-1 [17, 18] are combined in a tandem configuration on a single polypeptide chain and fused to an inactivated human IgG1 Fc domain (Fig. 1A). The TSGL-Ig construct enhances the selectin binding properties of the previously described rPSGL-Ig, that has been widely studied and shown to act as a competitive antagonist for leukocyte binding to selectins both in vitro and in vivo [8–10].
Figure 1.
(A) The structure of TSGL-Ig. (B – D) TSGL-Ig treatment ameliorated IRI in OLT. Livers from B6 mice, stored 20h at 4°C, were transplanted to syngeneic recipients. TSGL-Ig or control Ig was infused at donor liver harvest and prior to OLT reperfusion (100ug/mice via portal vein). (B) OLT survival (n=12/group). (C) sALT levels at 6h and 24h post-OLT (n=6/group). Means ± SD are shown; (*p<0.001). (D) OLT histology at 6h. Representative of n=4–6/group is shown (H&E staining; magnification x100 and x400).
The present study examines therapeutic effects and mechanisms by which TSGL-Ig molecule targets hepatic IRI in a clinically-relevant mouse liver transplant model subjected to prolonged period of cold storage. Anticipating that TSGL-Ig would bind to selectins on activated endothelial cells, which play a key role in IRI, we tested whether TSGL-Ig homeostatic function also involves Nrf2 translocation/activation in LECs. Our novel findings suggest that TSGL-Ig binding to selectins on endothelial cells may mitigate cellular damage in IR-stressed mouse liver grafts by exerting direct cytoprotection on LECs in Nrf2-dependent manner.
Materials and Methods
Animals
Male 8–12 weeks old wild-type (WT) mice (Jackson Laboratory, Bar Harbor, ME) and Nrf2-deficient mice (breeding pairs provided by Dr. T. Kensler, The Johns Hopkins University, Baltimore, MD) on a C57BL/6 background (backcrossed for at least twelve generations) were used. Mice were housed in the UCLA animal facility under specific pathogen-free conditions and received humane care, as per the criteria outlined in Guide for the Care and Use of Laboratory Animals (prepared by the National Academy of Sciences; NIH publication 86–23, revised 1985).
OLT Model
Livers from WT or Nrf2 KO mice were stored for 20 h at 4°C in UW solution prior to transplantation into syngeneic hosts [19]. Three groups of WT recipients were treated with TSGL-Ig, rPSGL-Ig, or control Ig (100ug/mouse via portal vein) at the time of liver harvest and immediately prior to reperfusion. The OLT and serum samples were collected at 6h and 24h post-transplant. The severity of liver IRI was graded with modified Suzuki’s histological criteria in which sinusoidal congestion, hepatocyte necrosis, and ballooning degeneration are graded from 0 to 4 [20–22].
Warm Liver IRI Model
In a mouse model of segmental (70%) hepatic warm IRI, the arterial/portal venous blood supply to the cephalad lobes was interrupted by an atraumatic clip for 90 min [22]. WT and Nrf2 KO mice were infused 1h prior to the onset of liver ischemia with TSGL-Ig (100ug i.v.) or control Ig. Mice were sacrificed at 6h of reperfusion; liver and serum samples were collected. Sham-operated mice underwent the same procedure, but without vascular occlusion.
Hepatocellular Function Assay
Serum alanine transaminase (sALT) levels were measured in blood samples by IDEXX Laboratories (Sacramento, CA, USA).
Histopathology
Liver specimens (4μm), stained with hematoxylin and eosin (H&E), were analyzed blindly [21, 22]. Primary mAb against mouse neutrophils (Ly-6G) (1A8; BD Biosciences, San Jose, CA) and macrophages (CD68) (FA-11; AbD Serotec, Raleigh, NC) were used. Labeled cells were counted in 10 high-power fields (HPF). Superoxide levels in hepatic tissue were detected with ROS-sensing dye dihydroethidium (DHE; AnaSpec, Fremont, CA).
Myeloperoxidase Activity Assay
The presence of myeloperoxidase (MPO) was utilized as an index of neutrophil accumulation in the liver [23]. An absorbance unit (U) of MPO activity was defined as the quantity of enzyme degrading 1mol peroxide/min at 25°C/gram of tissue.
Quantitative RT-PCR Assay
Quantitative PCR was used with platinum SYBR green quantitative PCR kit (Invitrogen, Carlsbad, CA) with the Chromo 4 detector (MJ Research, Waltham, MA). Primers to amplify specific gene fragments were published [24] or shown in Table S1. Target gene expressions were calculated by their ratios to the housekeeping gene hypoxanthine-guanine phosphoribosyl transferase (HPRT).
Western Blot Analysis
Cytosolic and nuclear proteins were isolated from OLT samples by NE-PER™ Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific, Waltham, MA). Equal amounts of cytosolic and nuclear extracts were electrophoresed, blotted, and incubated with primary Nrf2 mAb (CST, Beverly, MA), secondary HRP-conjugated Ab, and developed. Beta-actin was used as a protein marker.
Cell Cultures
Mouse LECs were isolated by in situ two-stage collagenase perfusion and cultured with complete DMEM medium (Life Technologies, Grand Island, NY) for 24h [25]. LEC viability was 95–99%, and the purity was 97–99% (minimal contamination with ICAM-1 negative hepatocytes), assessed by ICAM-1 staining (HA58, eBioscience, San Diego, CA) (Figure S1A). Hydrogen peroxide (H2O2, 0.2mM, Sigma-Aldrich) was used to trigger LEC necrosis, as described by us in primary murine hepatocyte cultures [26]. TSGL-Ig or control Ig (10 or 20ug/ml) were added 1h prior to H2O2-induced stress. At the conclusion of 6h culture, LECs were stained with anti-rabbit Nrf2 mAb (CST) and donkey anti-rabbit Alexa Fluor 594 (Jackson ImmunoResearch Laboratories, West Grove, PA) as secondary Ab. Alexa Fluor 488 actin conjugate (Life Technologies, Waltham, MA) was used to stain cellular skeleton.
LDH/ALT Release Assay
LDH/ALT release into culture medium was measured by LDH/ALT kit (Stanbio Laboratory, Boerne, TX) [24].
Statistical Analysis
All values are expressed as mean ± standard deviation (SD). Data were analyzed with an unpaired, two-tailed Student’s-t test; p<0.05 was considered statistically significant.
Results
TSGL-Ig Improves OLT Survival and Ameliorates Hepatic IRI
First, we determined whether treatment with TSGL-Ig (100ug/mouse via portal vein at liver harvest and reperfusion) promotes survival in mouse recipients of syngeneic OLT subjected to prolonged ex vivo cold storage (20h at 4 °C). Compared with 40% survival in control Ig-treated group (5 of 12), over 90% of OLT recipients conditioned with TSGL-Ig (11 of 12) remained alive at day 14 post-transplant (Fig. 1B; p<0.001).
Then, we analyzed the hepatocellular function in IR-stressed OLT recipients by screening sera and liver graft samples (6h and day 1; n=6/group). Unlike in controls, mice given TSGL-Ig were IR-resistant, evidenced by reduced sALT levels (U/L; Fig. 1C: [6h]: 16,694±3,670 vs. 3,300±711, respectively; [24h]: 5,081±1,291 vs. 1,041±328, respectively; p<0.001). The H&E staining at 6h post-OLT has revealed well-preserved hepatic architecture (minimal sinusoidal congestion, no lobular edema, vacuolization, or necrosis) in TSGL-Ig as compared with Ig-treatment controls (Fig. 1D). These data correlated with Suzuki’s histological grading of hepatocellular damage at 6h (2.8±0.4 vs. 0.7±0.5, respectively; p<0.001; not shown).
In parallel, we have set up separate groups of IR-stressed OLT recipients, which were conditioned with original rPSGL-Ig (100ug/mouse via portal vein at liver harvest and reperfusion). By 6h of reperfusion, sALT levels (U/L) were decreased (p<0.01) in the treatment group (7,218±2471; n=5) as compared with controls (14,493±4,675; n=4), implying the TSGL-Ig molecule was somewhat more effective than rPSGL-Ig in targeting IRI and protecting the hepatocellular function (Figure S2A).
TSGL-Ig Depresses Neutrophil and Macrophage Sequestration in IR-stressed OLT
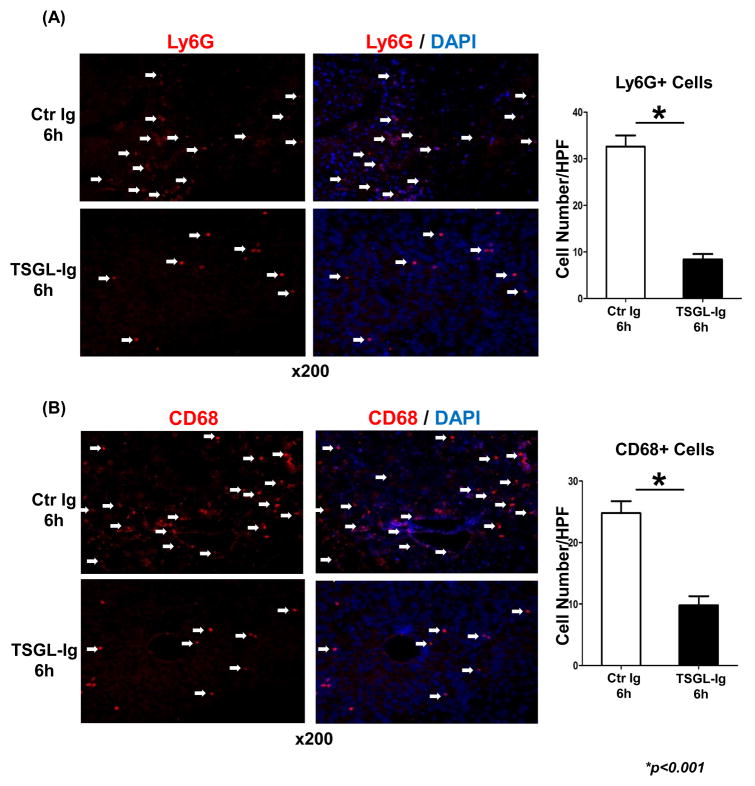
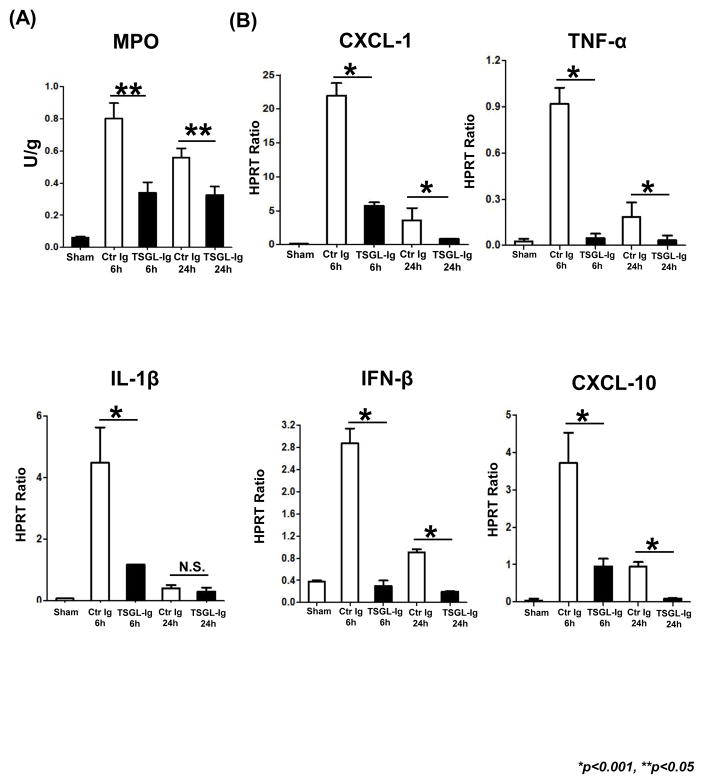
We used immunohistology to analyze and quantitate cell trafficking patterns at 6h post-OLT. P-selectin blockade with TSGL-Ig markedly decreased neutrophil (Fig. 2A; 6h: 8.40±1.14 vs. 32.60±2.41; p<0.001) and macrophage (Fig. 2B; 6h: 9.80±1.48 vs. 24.80±1.92; p<0.001) recruitment in ischemic OLT. Consistent with immunostaining data, MPO assay, reflecting hepatic neutrophil activity (U/g), was decreased in TSGL-Ig treated OLT, as compared with controls (Fig. 3A: [6h]: 0.34±0.07 vs. 0.80±0.10, respectively; [24h]: 0.33±0.05 vs. 0.56±0.06, respectively; p<0.05).
Figure 2.
TSGL-Ig treatment suppressed trafficking of neutrophils (A; Ly-6G+) and macrophages (B; CD68+) into IR-stressed livers (6h post-OLT). TSGL-Ig or control Ig was infused at donor liver harvest and prior to OLT reperfusion (100ug/mice via portal vein). Representative of 4–6 experiments/group is shown (magnification x200).
Figure 3.
TSGL-Ig treatment depressed neutrophil and macrophage function in IR-stressed OLT (6h post-transplant). (A) MPO-based liver neutrophil activity; (B) Quantitative RT-PCR-assisted detection of proinflammatory cytokine/chemokine programs. Data normalized to HPRT gene expression. Means ± SD are shown (**p<0.05, *p<0.001, n=4–6/group).
TSGL-Ig Depresses IR-Induced Liver Cytokine/Chemokine Programs
To analyze immune regulatory functions of TSGL-Ig, we screened for chemokine and cytokine expression patterns in IR-stressed OLT. By 6h post-transplant, neutrophil/monocyte-derived pro-inflammatory chemokine (CXCL-1, CXCL-10) and cytokine (TNF-α, IL-1β and IFN-β) programs were uniformly suppressed (p<0.001) in TSGL-Ig treatment group as compared to Ig-control (Fig. 3B).
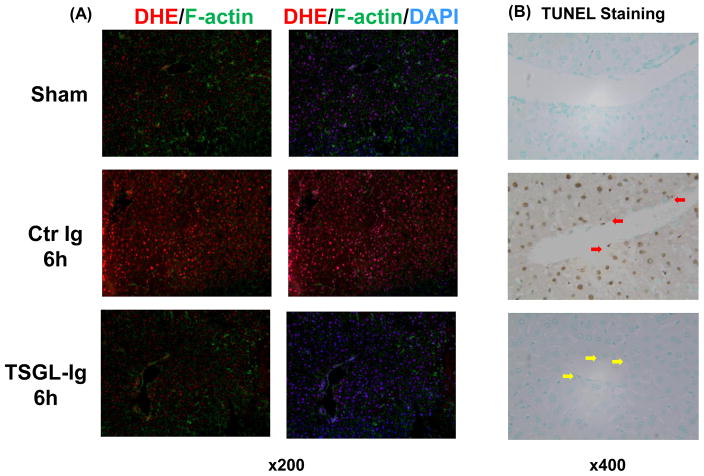
TSGL-Ig Decreases IR-Induced Liver Oxidative Stress and Necrosis/Apoptosis
Unlike IR-stressed control OLT characterized by abundant ROS levels, those treated with TSGL-Ig showed weak DHE staining, barely oxidized by superoxide (Fig. 4A: massive red fluorescence vs. light red fluorescence, respectively). TSGL-Ig diminished otherwise abundant hepatocellular necrosis and apoptosis, evidenced by decreased frequency of TUNEL+ cells (Fig. 4B). In addition, TSGL-Ig therapy protected LECs (yellow arrow) against IR-triggered necrosis/apoptosis (red arrow) (Fig. 4B).
Figure 4.
TSGL-Ig diminished IR-induced liver oxidative stress (A; dihydroethidium (DHE staining) and necrosis/apoptosis (B; TUNEL assay). Red arrows indicate necrotic/apoptotic endothelial cells. Representative of 4–6 experiments/group is shown (magnification x200).
TSGL-Ig Differentially Regulates IR-induced LEC Activation
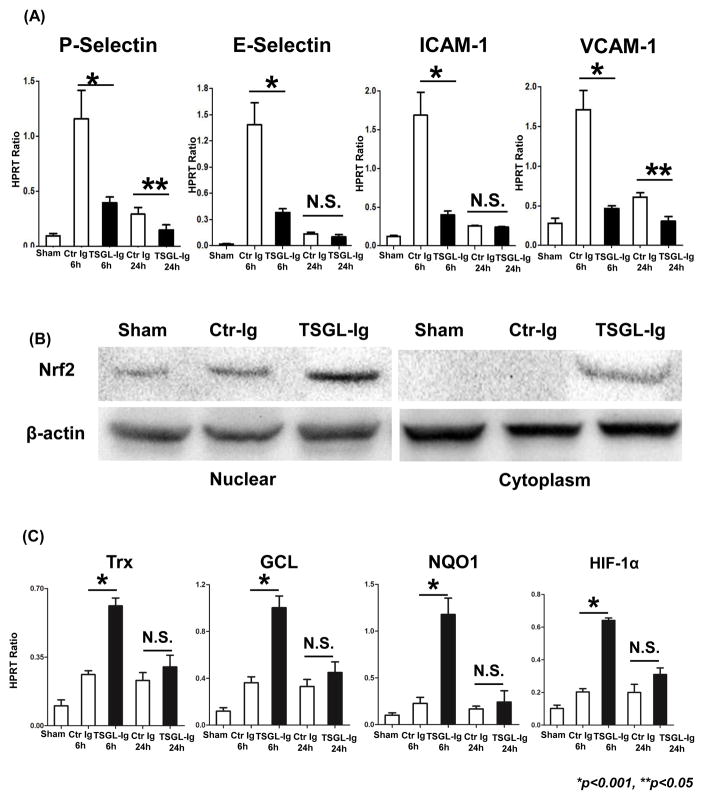
To determine LEC activation in vivo, we assessed mRNA levels coding for selectin and cell adhesion proteins in IR-stressed OLT (Fig. 5A). The qRT-PCR-assisted analysis of control grafts by 6h or reperfusion has revealed strong gene induction of P-/E-selectin, VCAM-1/ICAM-1, which declined thereafter. TSGL-Ig abolished (p<0.001) the expression of LEC-associated cell adhesion genes in IR-resistant OLT.
Figure 5.
Treatment with TSGL-Ig mitigated LEC activation in vivo (A; qRT-PCR-assisted P-Selectin/E-Selectin and ICAM-1/VCAM-1 at 6h and 24h); enhanced cytoplasm/nuclear Nrf2 levels (B; Western blots; representative at 6h); and promoted Nrf2 downstream genes (C; qRT-PCR-assisted Trx, GCL, NQO1, and HIF-1α at 6h and 24h) in IR-stressed OLT. Data normalized to HPRT gene expression. Means ± SD are shown (**p<0.05, *p<0.001, n=4–6/group).
TSGL-Ig Mediates LEC Cytoprotection in Nrf2-Dependent Manner
Western blots have revealed that treatment with TSGL-Ig elevated both cytosolic and nuclear Nrf2 in IR-stressed OLT (Fig. 5B). Unlike in control OLT, TSGL-Ig increased the expression of Nrf2 downstream target genes, including Trx, GCL, NQO1, and HIF-1α (p<0.001) (Fig. 5C).
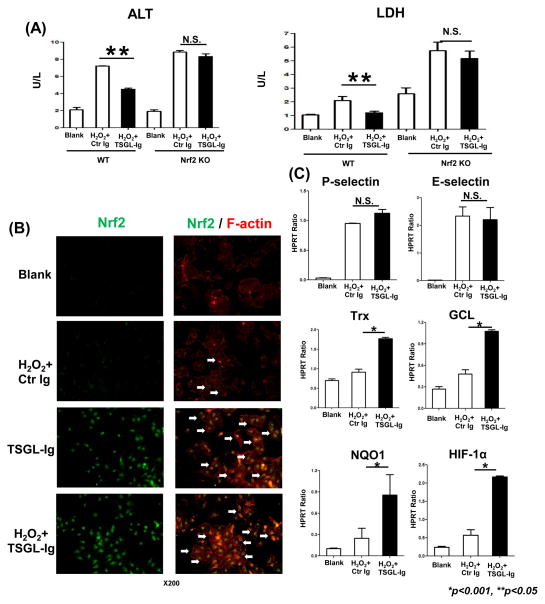
To test molecular mechanisms of TSGL-Ig direct P-selectin binding, primary LECs separated from WT livers were cultured with H2O2, an in vitro setting that mimics ROS-mediated ischemia insult in liver grafts [26]. H2O2-stress augmented LEC damage, measured by ALT and LDH release into the culture medium, was consistently suppressed (p<0.05) after TSGL-Ig was added to the culture (Fig. 6A).
Figure 6.
The cytoprotective effects of TSGL-Ig upon LECs in vitro. Primary mouse liver endothelial cells from WT or Nrf2 KO donors were subjected to H2O2-stress with TSGL-Ig or control Ig supplement (20ug/ml at -1h) in 6h cultures. (A) ALT and LDH release; (B) Immunostaining of Nrf2 in LECs. White arrows indicate translocation of Nrf2 from cytoplasm to nucleus (Representative of n=3; magnification x200); (C) Quantitative RT-PCR-assisted detection of P-/E-selectin, Trx, GCL, NQO1, and HIF-1α. Data normalized to HPRT gene expression. Means ± SD are shown (**p<0.05, *p<0.001; n=4–6/group).
By employing immunofluorescence, we detected TSGL-Ig triggered translocation of Nrf2 transcriptional activator into LEC nucleus, with or without concomitant H2O2 (Fig. 6B, white arrows). Consistent with the ability of Nrf2 to regulate the induction of ARE target gene promoter regions, TSGL-Ig increased the expression of Trx, GCL, NQO1, and HIF-1α in H2O2-stressed LECs (p<0.001) as compared with controls (Fig. 6C).
We then employed primary LECs from Nrf2 KO mice to analyze mechanism of TSGL-Ig mediated cytoprotection. Indeed, compared with Nrf2-proficient LEC cultures, those with disrupted Nrf2 signaling were more susceptible to H2O2-stress, as measured by ALT/LDH release (Fig. 6A). Pretreatment with TSGL-Ig failed to protect Nrf2-deficient LECs against H2O2-stress, consistent with comparable ALT and LDH release in WT and Nrf2 KO cell culture groups (Fig. 6A).
Nrf2 Signaling is Required for TSGL-Ig Hepatoprotection Against IR-Stress
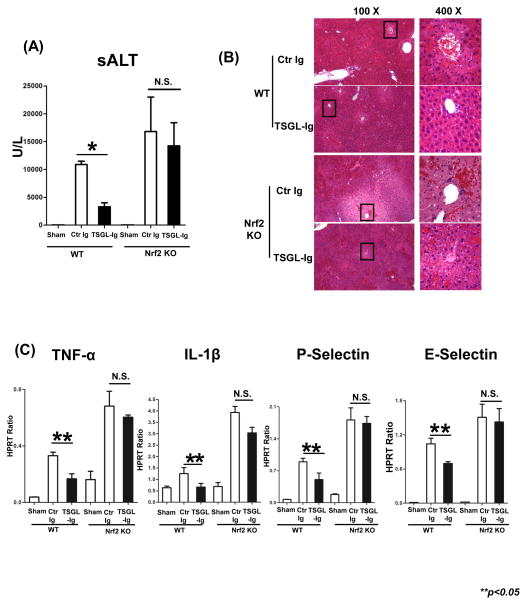
We analyzed whether Nrf2 dependence of TSGL-Ig protection against H2O2-stress, as seen in LEC cultures, may translate into in vivo settings. First, we employed Nrf2 KO mice as liver donors in our IRI-OLT model (20 h of cold storage). Indeed, unlike in WT, TSGL-Ig failed to protect Nrf2 deficient OLT against IRI, as measured by sALT (U/L) at 6h of reperfusion (n=5–6/gr): WT→WT=10,886±597 vs. 3,300±711 [p<0.001]; Nrf2 KO→WT=16,807±6,179 vs. 14,236±4,154 [N.S.]) (Fig. 7A). In addition, TSGL-Ig failed to mitigate IR-triggered hepatocellular damage after disruption of Nrf2 signaling, as documented by liver H&E staining (Fig. 7B); LEC activation (P-/E-selectin); and macrophage cytokine programs (TNF-α, IL-1β; Fig. 7C).
Figure 7.
The cytoprotective effects of TSGL-Ig in cold-stored OLT were Nrf2 dependent. Livers from WT or Nrf2 KO donors, preserved for 20h at 4°C, were transplanted to syngeneic WT recipients. TSGL-Ig or control Ig was infused during liver harvesting and prior to graft reperfusion (100ug/mice via portal vein). (A) sALT levels at 6h post-OLT (n=4/group). Means ± SD are shown; (*p<0.001). (B) OLT histology (Representative of n=3; H&E staining; magnification x100 and x400); (C) qRT-PCR-assisted detection of TNF-α, IL-1β, P-/E-selectin. Data normalized to HPRT gene expression. Means ± SD are shown (**p<0.05, n=3/group).
We used murine model of hepatic partial warm IRI to confirm that TSGL-Ig protection against IR stress is Nrf2-dependent. WT and Nrf2 KO mice subjected to 90 min of hepatic warm ischemia were pre-treated with TSGL-Ig or control Ig (at -1 hr i.v.); sera and liver samples were collected at 6h of reperfusion, the peak of hepatocellular damage [22]. WT mice given TSGL-Ig were IRI-resistant, as compared to controls, evidenced by decreased sALT levels (Figure S3A; p<0.05); well-preserved hepatic architecture (Figure S3B); as well as depressed LEC (P-/E-selectin); and macrophage (TNF-α, IL-1β) activation (Figure S3C; p<0.05). Consistent with our findings in cold-stored OLT, TSGL-Ig failed to abolish IR-mediated hepatocellular damage in Nrf2-deficient warm IR-stressed livers, as documented by sALT levels (U/L; Figure S3A); liver H&E staining (Figure S3B); and hepatic IRI cytokine signature/selectin expression patterns (Figure S3C).
Discussion
Previously, we and others have found that rPSGL-Ig, acts as a competitive inhibitor of the initial tethering of leukocytes to activated platelets and endothelium, by binding to selectins [8, 9, 27, 28]. Blockade of leukocyte tethering via the P-selectin–PSGL-1 axis prevented hepatocellular damage in normal [8] and steatotic [9] rat liver models of extended cold storage and reperfusion ex vivo on isolated perfusion machine or syngeneic OLT. Furthermore, our recent clinical phase II study in deceased-donor liver transplant recipients with 6-month follow-up, documented rPSGL-Ig’s safety and beneficial impact on liver transplantation IRI, supported by therapeutic modulation of hepatic IRI biomarkers [10]. These findings were confirmed when rPSGL-Ig was used in renal transplant patients, when a robust increase in inflammatory gene transcript levels during kidney transplantation IRI was reduced by inhibition of leukocyte adhesion [29]. In agreement with our clinical findings [10], we now confirm that rPSGL-Ig protected mouse syngeneic OLT against IRI, as evidenced by decreased sALT levels (Figure S2A); reduced ROS production (Figure S2B); enhanced Nrf2 nuclear translocation (Figure S2C); and increased expression of Trx, GCL, NQO1, and HIF-1α, i.e., Nrf2 downstream activation genes (Figure S2D).
Our current study is the first to utilize a newly generated TSGL-Ig molecule in a clinically relevant murine model of syngeneic OLT with prolonged period (20h) of cold ischemia. Preliminary surface plasmon resonance binding analysis indicates that TSGL-Ig has improved P-selectin binding properties, as compared with rPSGL-Ig (GDS, unpublished). While this improved binding would be expected to translate in vivo, the protective activity of TSGL-Ig in an experimental organ IRI model required confirmation. Here, we have shown that: 1) TSGL-Ig improved OLT survival and ameliorated hepatic IRI; 2) depressed neutrophil and macrophage sequestration along with proinflammatory cytokine and chemokine programs in IR-stressed OLT; 3) diminished some aspects of LEC activation; and 4) conferred cytoprotection against IR-stress by acting directly as an agonist to stimulate Nrf2-mediated cytoprotection in liver endothelial cells. The new TSGL-Ig was somewhat more effective than the original rPSGL-Ig drug molecule in its ability to blunt IRI in OLT and improve the hepatocellular function.
In the pathogenesis of liver IRI, the endothelium damage determines poor graft microcirculation, platelet activation, persistent vasoconstriction, up-regulation of adhesion molecules, oxidative stress, Kupffer cell activation, neutrophil infiltration, and eventually hepatocyte death [1, 2, 5]. The principal selectin ligand, PSGL-1, mediates the capturing and rolling of leukocytes on LECs in the initial phase of IRI [7, 30, 31]. The newly constructed TSGL-Ig has enhanced selectin binding activity as compared with the corresponding soluble form of native human rPSGL-Ig. We asked whether TSGL-Ig would attenuate liver IRI and if so, how it may preserve LEC integrity as direct binding is essential to promote OLT survival. In a mouse model of liver extended cold storage (4°C UW solution for 20h) and syngeneic OLT, TSGL-Ig treatment group showed improved survival rates, preservation of hepatic architecture, and minimal overall liver damage. Hence, TSGL-Ig is effective for combating hepatic IRI and to maintain homeostasis in IR-stressed mouse OLT.
In the initial IR-mediated inflammation phase, we found increased activation and recruitment of CD68+ macrophages, consistent with preferential proinflammatory chemotactic gene expression pattern in IR-stressed livers [8, 9, 19, 21, 22]. As rPSGL-Ig therapy suppressed macrophage function in rats [8, 9], we expected that TSGL-Ig may work in a similar fashion in IR-stressed mouse livers. Indeed, TSGL-Ig treatment decreased CD68+ macrophage infiltration and diminished their activation/function, evidenced by immunohistology and expression of IRI signature markers, including TNF-α, IL-1β, IFN-β and CXCL-10.
Activated neutrophils generate ROS to promote IR-stressed hepatocellular damage [11, 12]. Indeed, unlike sham controls, Ly-6G+ neutrophil infiltration and MPO activity increased in control Ig-treated IRI. By contrast, livers in TSGL-Ig-conditioned mice were characterized by decreased neutrophil infiltration/MPO activity and depressed CXCL-1 levels, the key neutrophil chemoattractant. Since neutrophil activation and target tissue sequestration can be enhanced by macrophage-derived inflammatory cytokines, TSGL-Ig can exert its regulatory influence during liver IRI through cytokine and chemokine networks.
Both necrosis and apoptosis are responsible for IR-hepatocyte damage (22). ROS promote mitochondrial permeability transition, leading to hepatocellular swelling, rupture of the plasma membrane, release of cytochrome C, ultimately resulting in ATP depletion-dependent oncotic necrosis and caspase-dependent apoptosis. Our present data shows TSGL-Ig suppressed ROS production (DHE staining) and inhibited oncotic necrosis/apoptosis in hepatocytes and endothelial cells in IR-stressed OLT.
We have previously shown that rPSGL-Ig competitively bound P-selectin to block leukocytic PSGL-1 – P-selectin interactions and to prevent hepatocellular injury [8–10]. In the recent clinical trial, rPSGL-Ig improved early liver allograft function particularly in patients with donor risk index above the study-average [10], implying it has direct effect on the marginal liver graft. As TSGL-Ig should directly bind selectins on the surface of LECs, we asked what effects TSGL-Ig treatment would have on LEC viability, activation, and function. As LECs serve as the first line of defense against ROS oxidation, we employed H2O2 to mimic in vivo ROS-mediated necrotic stress in LEC cultures. We found that addition TSGL-Ig (20ug/ml) but not a control Ig into the culture, an hour before the H2O2 challenge, ameliorated ALT/LDH release and prevented LEC death. This indicates that TSGL-Ig can act directly on cultured LECs to induce an Nrf2-mediated stress protective response. The qRT-PCR data indicates that mRNA for both P-selectin and E-selectin is actively expressed in LEC cultures. Therefore, it is plausible that TSGL-Ig is binding and signaling via selectins expressed on the LEC surface. Transient increases in cytosolic calcium, required for PMN transit across the EC barrier, have previously been observed following treatment of endothelial cells with anti-selectin antibodies, indicating that binding to selectins can initiate signaling events in endothelial cells [32]. However, confirmation that TSGL-Ig agonistic activity is solely mediated via selectin binding will require further investigation.
By regulating cellular resistance against oxidative stress, the transcription factor Nrf2 has emerged as a “master switch” of intracellular redox homeostasis [12, 13]. We have shown that mouse liver grafts with hepatocyte-specific Keap1 deficiency were IRI resistant [33]. The deficiency of Keap1-mediated ubiquitination of Nrf2 resulted in elevated hepatocyte Nrf2 activation. As Nrf2 deficiency accelerated hepatocellular damage in IR-stressed livers, we postulated that Nrf2 should be considered as a denominator of donor liver quality. Western blot analysis of IRI-OLT and immunostaining in primary LEC cultures showed TSGL-Ig triggered translocation of Nrf2 transcriptional activator into the nucleus. After its binding to AREs promoter, the gene inductions of Trx, GCL, NQO1, and HIF-1α were all enhanced to exert cytoprotective and antioxidant functions. Furthermore, we determined whether TSGL-Ig may function through ROS-Nrf2-ARE pathway by employing Nrf2 KO mice. Consistent with increased ALT/LDH release levels, TSGL-Ig failed to protect Nrf2-deficient LECs from H2O2-inflicted stress in vitro. The failure of TSGL-Ig to ameliorate liver IRI in Nrf2 KO mice highlights a previously unrecognized role of selectins in anti-oxidant function by directly regulating LEC viability and promoting Nrf2-dependent cytoprotection.
The observed TSGL-Ig protection mediated via anti-oxidant Nrf2 in IRI cascade in the present study, along with a recent identification of PSGL-1 as a key immune checkpoint regulator of T cell exhaustion [34], highlight multifaceted functions of P-selectin signaling beyond cell trafficking, of direct relevance in organ transplantation.
Supplementary Material
Figure S1: (A) Cultured LEC purity (Representative ICAM-1 staining; x400); (B) H2O2 dose response in LEC culture: ALT and LDH release. Means ± SD are shown (**p<0.05 when respective H2O2/TSGL-Ig doses are compared; n=4/group).
Figure S2: rPSGL-Ig treatment ameliorated IRI in OLT. Livers from B6 mice, stored 20h at 4°C, were transplanted to syngeneic recipients. rPSGL-Ig or control Ig was infused at donor liver harvest and prior to OLT reperfusion (100ug/mice via portal vein). (A) sALT levels were measured at 6h post-OLT. (B) PSGL-Ig diminished IR-induced liver oxidative stress (dihydroethidium [DHE] staining), (C) enhanced cytoplasm/nuclear Nrf2 levels (Western blot); and (D) promoted Nrf2 downstream target gene expression (Trx, GCL, NQO1, HIF-1α) in IR-stressed OLT (6h). Data normalized to HPRT gene expression. Means ± SD are shown (*p<0.001, n=4–5/group).
Figure S3: The cytoprotective effects of TSGL-Ig in livers subjected to warm ischemia were Nrf2-dependent. Groups of WT and Nrf2 KO mice preconditioned with TSGL-Ig of control Ig (-1h) were analyzed at 6h of reperfusion after 90min of warm hepatic IR-insult. (A) sALT levels; (B) liver histology (Representative of n=3; H&E staining; magnification x100 and x400); (C) Quantitative RT-PCR-assisted detection of TNF-α, IL-1β, P-/E-selectin. Data normalized to HPRT gene expression. Means ± SD are shown (**p<0.05, n=3/group).
Table S1: Primers used for qRT-PCR analyses.
Acknowledgments
This study was funded by NIH Grants RO1 R01DK102110, RO1DK062357, R01DK107533 (JWKW); R21 AI122155 (H.J); W.M. Keck Foundation; and The Dumont Research Foundation.
Abbreviations
- ARE
antioxidant response element
- IRI
ischemia-reperfusion injury
- Keap1
Kelch-like ECH-associated protein 1
- LDH
lactic acid dehydrogenase
- MPO
myeloperoxidase
- Nrf2
nuclear factor erythroid 2-related factor 2
- OLT
orthotopic liver transplantation
- PSGL-1
P-selectin glycoprotein ligand-1
- ROS
reactive oxygen species
- rPSGL-Ig
recombinant P-selectin glycoprotein ligand Immunoglobulin
- sALT
serum alanine aminotransferase
- TSGL-Ig
tandem P-Selectin glycoprotein ligand Immunoglobulin
Footnotes
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79–89. doi: 10.1038/nrgastro.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transplant. 2011;11:1563–1569. doi: 10.1111/j.1600-6143.2011.03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaeschke H, Smith CW. Cell adhesion and migration. III. Leukocyte adhesion and transmigration in the liver vasculature. Am J Physiol. 1997;273:1169–1173. doi: 10.1152/ajpgi.1997.273.6.G1169. [DOI] [PubMed] [Google Scholar]
- 4.Peralta C, Jimenez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol. 2013;59:1094–1106. doi: 10.1016/j.jhep.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Marrone G, Shah VH, Gracia-Sancho J. Sinusoidal communication in liver fibrosis and regeneration. J Hepatol. doi: 10.1016/j.jhep.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones RT, Toledo-Pereyra LH, Quesnelle KM. Selectins in Liver Ischemia and Reperfusion Injury. J Invest Surg. 2015;28:292–300. doi: 10.3109/08941939.2015.1056920. [DOI] [PubMed] [Google Scholar]
- 7.Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood. 2011;118:6743–6751. doi: 10.1182/blood-2011-07-343566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dulkanchainun TS, Goss JA, Imagawa DK, Shaw GD, Anselmo DM, Kaldas F, et al. Reduction of hepatic ischemia/reperfusion injury by a soluble P-selectin glycoprotein ligand-1. Ann Surg. 1998;227:832–840. doi: 10.1097/00000658-199806000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amersi F, Farmer DG, Shaw GD, Kato H, Coito AJ, Kaldas F, et al. P-selectin glycoprotein ligand-1 (rPSGL-Ig)-mediated blockade of CD62 selectin molecules protects rat steatotic liver grafts from ischemia/reperfusion injury. Am J Transplant. 2002;2:600–608. doi: 10.1034/j.1600-6143.2002.20704.x. [DOI] [PubMed] [Google Scholar]
- 10.Busuttil RW, Lipshutz GS, Kupiec-Weglinski JW, Ponthieux S, Gjertson DW, Cheadle C, et al. rPSGL-Ig for Improvement of Early Liver Allograft Function: A Double-Blind, Placebo-Controlled, Single-Center Phase II Study. Am J Transplant. 2011;11:786–797. doi: 10.1111/j.1600-6143.2011.03441.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, et al. ROS and ROS-Mediated Cellular Signaling. Oxid Med Cell Longev. 2016;2016:4350965. doi: 10.1155/2016/4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 13.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 15.Furukawa M, Xiong Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Mol Cell Biol. 2005;25:162–171. doi: 10.1128/MCB.25.1.162-171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes & development. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Somers WS, Tang J, Shaw GD, Camphausen RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P-and E-selectin bound to SLe(X) and PSGL-1. Cell. 2000;103:467–479. doi: 10.1016/s0092-8674(00)00138-0. [DOI] [PubMed] [Google Scholar]
- 18.Leppänen A, White SP, Helin J, McEver RP, Cummings RD. Binding of glycosulfopeptides to P-selectin requires stereospecific contributions of individual tyrosine sulfate and sugar residues. J Biol Chem. 2000;275:39569–39578. doi: 10.1074/jbc.M005005200. [DOI] [PubMed] [Google Scholar]
- 19.Shen XD, Gao F, Ke B, Zhai Y, Lassman CR, Tsuchihashi S, et al. Inflammatory responses in a new mouse model of prolonged hepatic cold ischemia followed by arterialized orthotopic liver transplantation. Liver Transpl. 2005;11:1273–1281. doi: 10.1002/lt.20489. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Ji H, Zhang Y, Shen XD, Gao F, Nguyen TT, et al. Negative CD4 + TIM-3 signaling confers resistance against cold preservation damage in mouse liver transplantation. Am J Transplant. 2015;15:954–964. doi: 10.1111/ajt.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji H, Liu Y, Zhang Y, Shen XD, Gao F, Busuttil RW, et al. T-cell immunoglobulin and mucin domain 4 (TIM-4) signaling in innate immune-mediated liver ischemia-reperfusion injury. Hepatology. 2014;60:2052–2064. doi: 10.1002/hep.27334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985;14:157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- 24.Ji H, Zhang Y, Shen XD, Gao F, Huang CY, Abad C, et al. Neuropeptide PACAP in mouse liver ischemia and reperfusion injury: Immunomodulation by the cAMP-PKA pathway. Hepatology. 2013;57:1225–1237. doi: 10.1002/hep.25802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braet F, De Zanger R, Sasaoki T, Baekeland M, Janssens P, Smedsrød B, et al. Assessment of a method of isolation, purification, and cultivation of rat liver sinusoidal endothelial cells. Lab Invest. 1994;70:944–952. [PubMed] [Google Scholar]
- 26.Liu Y, Ji H, Zhang Y, Shen X, Gao F, He X, et al. Recipient T cell TIM-3 and hepatocyte galectin-9 signalling protects mouse liver transplants against ischemia-reperfusion injury. J Hepatol. 2015;62:563–572. doi: 10.1016/j.jhep.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Théorêt JF, Chahrour W, Yacoub D, Merhi Y. Recombinant P-selectin glycoprotein-ligand-1 delays thrombin-induced platelet aggregation: a new role for P-selectin in early aggregation. Br J Pharmacol. 2006;148:299–305. doi: 10.1038/sj.bjp.0706734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newman CM, Crosdale DJ, Fisher KD, Briggs SS, Norman KE, Seymour LW, et al. P-selectin dependent targeting to inflamed endothelium of recombinant P-selectin glycoprotein ligand-1 immunoglobulin chimera-coated poly[N-(2-hydroxypropyl) methacrylamide]-DNA polyplexes in vivo visualised by intravital microscopy. J Gene Med. 2009;11:326–334. doi: 10.1002/jgm.1294. [DOI] [PubMed] [Google Scholar]
- 29.Cheadle C, Watkins T, Ehrlich E, Barnes K, Gaber AO, Hemmerich S, et al. Effects of anti-adhesive therapy on kidney biomarkers of ischemia reperfusion injury in human deceased donor kidney allografts. Clin Transplant. 2011;25:766–75. doi: 10.1111/j.1399-0012.2010.01365.x. [DOI] [PubMed] [Google Scholar]
- 30.Tsuchihashi S, Fondevila C, Shaw GD, Lorenz M, Marquette K, Benard S, et al. Molecular Characterization of Rat Leukocyte P-Selectin Glycoprotein Ligand-1 and Effect of Its Blockade: Protection from Ischemia-Reperfusion Injury in Liver Transplantation. J Immunol. 2005;76:616–624. doi: 10.4049/jimmunol.176.1.616. [DOI] [PubMed] [Google Scholar]
- 31.Yadav SS, Howell DN, Steeber DA, Harland RC, Tedder TF, Clavien PA. P-Selectin mediates reperfusion injury through neutrophil and platelet sequestration in the warm ischemic mouse liver. Hepatology. 1999;29:1494–1502. doi: 10.1002/hep.510290505. [DOI] [PubMed] [Google Scholar]
- 32.Lorenzon P, Vecile E, Nardon E, Ferrero E, Harlan JM, Tedesco F, et al. Endothelial cell E-and P-selectin and vascular cell adhesion molecule-1 function as signaling receptors. J Cell Biol. 1998;142:1381–1391. doi: 10.1083/jcb.142.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ke B, Shen XD, Zhang Y, Ji H, Gao F, Yue S, et al. KEAP1-NRF2 complex in ischemia-induced hepatocellular damage of mouse liver transplants. J Hepatol. 2013;59:1200–1207. doi: 10.1016/j.jhep.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tinoco R, Carrette F, Barraza ML, Otero DC, Magana J, Bosenberg MW, et al. PSGL-1 is an immune checkpoint regulator that promotes T cell exhaustion. Immunity. 2016;44:1190–203. doi: 10.1016/j.immuni.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: (A) Cultured LEC purity (Representative ICAM-1 staining; x400); (B) H2O2 dose response in LEC culture: ALT and LDH release. Means ± SD are shown (**p<0.05 when respective H2O2/TSGL-Ig doses are compared; n=4/group).
Figure S2: rPSGL-Ig treatment ameliorated IRI in OLT. Livers from B6 mice, stored 20h at 4°C, were transplanted to syngeneic recipients. rPSGL-Ig or control Ig was infused at donor liver harvest and prior to OLT reperfusion (100ug/mice via portal vein). (A) sALT levels were measured at 6h post-OLT. (B) PSGL-Ig diminished IR-induced liver oxidative stress (dihydroethidium [DHE] staining), (C) enhanced cytoplasm/nuclear Nrf2 levels (Western blot); and (D) promoted Nrf2 downstream target gene expression (Trx, GCL, NQO1, HIF-1α) in IR-stressed OLT (6h). Data normalized to HPRT gene expression. Means ± SD are shown (*p<0.001, n=4–5/group).
Figure S3: The cytoprotective effects of TSGL-Ig in livers subjected to warm ischemia were Nrf2-dependent. Groups of WT and Nrf2 KO mice preconditioned with TSGL-Ig of control Ig (-1h) were analyzed at 6h of reperfusion after 90min of warm hepatic IR-insult. (A) sALT levels; (B) liver histology (Representative of n=3; H&E staining; magnification x100 and x400); (C) Quantitative RT-PCR-assisted detection of TNF-α, IL-1β, P-/E-selectin. Data normalized to HPRT gene expression. Means ± SD are shown (**p<0.05, n=3/group).
Table S1: Primers used for qRT-PCR analyses.