Abstract
Background
Advanced cholangiocarcinoma carries a poor prognosis, and no standard treatment exists beyond the first-line gemcitabine/platinum-based chemotherapy. We performed a single arm phase II and biomarker study of cabozantinib, a multikinase inhibitor with potent activity against VEGFR2 and MET, in patients with advanced refractory cholangiocarcinoma.
Methods
Previously treated patients with unresectable or metastatic cholangiocarcinoma received cabozantinib 60 mg orally daily continuously. The primary endpoint was progression free survival (PFS). Tumor MET expression and plasma biomarkers were evaluated.
Results
The study enrolled 19 patients with cholangiocarcinoma (female 68%; median age 67yo; intra- vs extrahepatic, 84% vs 16%). The median PFS was 1.8 months (95%CI; 1.6, 5.4), and the median overall survival was 5.2 months (95%CI; 2.7, 10.5). Grade 3/4 adverse events occurred in 89% of patients, and included neutropenia (5%), hyperbilirubinemia (5%), epistaxis (5%), bowel perforation (5%), enterocutaneous fistula (5%), and hypertension (11%). One patient with 3+ MET expression in the tumor stayed on treatment for 278 days, but MET expression did not correlate with outcomes in the overall study population. Plasma VEGF, PlGF and SDF1α increased and soluble VEGFR2 and Ang2 decreased after treatment (all p<0.01). Plasma TIMP-1 inversely correlated with PFS, and soluble MET (sMET) and IL-6 inversely correlated with OS.
Conclusions
In unselected patients with cholangiocarcinoma, cabozantinib demonstrated limited activity and significant toxicity. In the first clinical trial to assess the role of MET inhibition in cholangiocarcinoma, one patient with a MET-high tumor had prolonged benefit from treatment. Baseline plasma sMET was associated with OS. Any further development of this drug in cholangiocarcinoma should include dose reduction and a biomarker-driven approach.
Keywords: Cholangiocarcinoma, cabozantinib, soluble MET, VEGFR2, phase II
INTRODUCTION
Cholangiocarcinoma is a rare malignancy of intra- and extrahepatic bile ducts with a poor prognosis. Recent data have shown that intrahepatic cholangiocarcinoma (ICC) is rising in incidence globally1. While surgery can be curative if the tumor is detected early, recurrence rates are as high as 70–75%, and five-year overall survival (OS) rates are as low as 5–10%.1 The standard first-line treatment for unresectable/advanced disease consists of gemcitabine and platinum chemotherapy, and offers a median OS of approximately one year.2 No standard therapy exists beyond the first line, and no targeted therapies are approved in this disease. Use of other regimens in the second-line, such as fluoropyrimidine- or taxane-based regimens, is associated with a median progression free survival of 2.8 months and OS of 7.5 months.3
The vascular endothelial growth factor (VEGF) receptor 2 (VEGFR2) and hepatocyte growth factor (HGF) receptor MET signaling are often upregulated in cholangiocarcinoma and promote carcinogenesis by increasing angiogenesis and invasion. VEGF is expressed in approximately 50% of ICCs and 32–59% of extrahepatic cholangiocarcinomas (ECCs)4,5, and is associated with metastasis, and recurrence and poor OS in biliary tract cancer.5,6 Similarly, MET is expressed in 20–58% of ICCs and 0–68% of ECCs7–10. Moreover, MET overexpression is seen in 12% of ICCs and 16% of ECCs, and correlates with decreased 5-year OS.9
Cabozantinib is an orally bioavailable multikinase inhibitor whose targets include VEGFR2 (IC50=0.035nM) and MET (IC50=1.3nM). Dual inhibition of these two receptors has been shown to have synergistic anti-tumor effects because of the intricate crosstalk between the VEGF/VEGFR2 and HGF/MET pathways11. For example, over time VEGFR2 inhibition may cause an increase in tumor hypoxia, which can upregulate MET and enhance cancer cell invasion and promote treatment resistance12.
In the first clinical trial to address the role of MET inhibition in cholangiocarcinoma, we evaluated cabozantinib in a phase II study of patients with advanced refractory cholangiocarcinoma. The primary objective of the study was to evaluate the progression-free survival (PFS) of these patients on cabozantinib 60mg orally daily on a continuous schedule. The correlative studies included evaluation of tissue MET expression and plasma molecules as potential biomarkers of response to cabozantinib.
PATIENTS AND METHODS
Study Population
Patients with histologically proven, measurable, unresectable or metastatic cholangiocarcinoma were eligible. Other inclusion criteria included progressive disease after one or two prior lines of systemic therapy for advanced cholangiocarcinoma; age ≥18 years; ECOG performance status ≤ 1; life expectancy of ≥ 3 months; white blood cells ≥1,500/μL, platelet count ≥100,000/μL; hemoglobin ≥ 9 g/dL; serum creatinine ≤1.5 mg/dL or GFR ≥ 60 mL/min/1.73m2; total bilirubin <2 × upper limit of normal (ULN), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤5 × ULN; lipase <2.0 × ULN; serum phosphorus, calcium, magnesium, and potassium ≥ lower limit of normal (LLN); albumin ≥2.8 g/dL; PT/PTT ≤ 1.5 × ULN; urine protein: creatinine ratio ≤ 1.
Key exclusion criteria included prior treatment with cabozantinib or other VEGFR-targeted therapies; gallbladder carcinoma or periampullary tumors; chemotherapy within the prior 4 weeks; radiation to the thorax, abdomen, or pelvis in the prior 3 months or to bone or brain metastases in the prior 14 days; sustained BP > 140 mm Hg systolic, or > 90 mm Hg diastolic despite optimal antihypertensive treatment within the prior 7 days; QTc > 500 milliseconds; class III or IV congestive heart failure; prior history of pulmonary embolism or deep venous thrombosis; unstable angina, clinically significant cardiac arrhythmia, stroke, myocardial infarction, thromboembolic event requiring therapeutic anticoagulation, venous filter, or clinically significant gastrointestinal bleeding within the previous 6 months; gastrointestinal disorders associated with a high risk of perforation or fistula formation; severely impaired lung function or history of interstitial lung disease; history of hepatic encephalopathy; active brain or epidural metastases; history of organ transplant; major surgery within prior 3 months or minor surgery within the prior 1 month (biliary stenting was not considered minor surgery and did not require delay); concomitant use of therapeutic anticoagulation or strong CYP3A4 inducers or inhibitors; treatment with another investigational agent within the prior 28 days; uncompensated hypothyroidism; concurrent malignancy; and pregnancy or lactation. All patients provided written informed consent before study participation. The protocol was approved by the Partners Institutional Review Board.
Study Design
In this single arm phase II study, patients received cabozantinib 60mg orally daily continuously for repeating 28 day cycles. Dose reductions were permitted down to two dose levels, 40mg orally daily and 20mg orally daily. The primary endpoint was PFS. Secondary endpoints included objective response rate (ORR), OS, safety and tolerability, and a pre-planned post-hoc analysis of PFS, ORR, and OS by tumor MET expression. Plasma biomarkers were assessed as potential biomarkers of response.
Patients were evaluated for response serologically by CA19–9 levels and radiographically with computed tomography or magnetic resonance imaging every 8 weeks. Response was determined by an independent radiologic review using RECIST criteria version 1.1,13 and patients continued treatment until disease progression, unacceptable toxicity, or withdrawal of consent. Patients were monitored for safety weekly in cycle 1, every other week in cycle 2, and monthly thereafter. Safety evaluations included vital signs, physical exam, performance status evaluation, complete blood count, blood chemistries, coagulation studies, thyroid function tests, urinalyses, and electrocardiograms. Adverse events were assessed according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI CTCAE) Version 4.0. Safety data were monitored on an ongoing basis by an independent Data and Safety Monitoring Committee (DSMC).
Correlative Studies
Plasma biomarkers
Exploratory analyses of potential plasma biomarkers of cabozantinib were performed by measuring proteins in the plasma at baseline (one day prior to the first dose of cabozantinib) and their changes after 1 week (range 3–6 weeks) and 2 weeks (range 13–18 weeks) of treatment, to accommodate weekends and holidays. Plasma analysis was carried out for a panel of circulating pro-angiogenic and pro-inflammatory biomarkers: VEGF, placental growth factor (PlGF), soluble (s)VEGFR1/FLT-1, basic fibroblast growth factor (bFGF), VEGF-C, VEGF-D, TIE-2, interleukin (IL)-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α using multiplex protein array plates from Meso-Scale Discovery (Gaithersburg, MD); and HGF, sVEGFR2, angiopoietin-1 (Ang-1), Ang-2, tissue inhibitor on matrix metalloproteinase (TIMP)-1, carbonic anhydrase (CA)-IX, free insulin-like growth factor (IGF)-I and stromal-derived factor (SDF1)-α from R&D Systems (Minneapolis, MN), and sMet from Life Technologies/Invitrogen (Carlsbad, CA). All samples were run in duplicate.
Tissue biomarkers
Immunohistochemical (IHC) evaluation of total MET was performed using the anti-total MET rabbit monoclonal primary antibody (SP44; Ventana Medical Systems, Tucson, AZ). The active fraction of the MET protein was determined using an anti-phospho-MET (Tyr1349) rabbit monoclonal antibody from Millipore (EP2367Y; Billerica, MA). IHC analysis was performed on the Ventana BenchMark XT platform according to standard protocols, and samples were processed in batch to minimize inter-run variability. The IHC scoring was done by a trained GI pathologist (VN). As defined in the OAM4558g MetMAb phase II study,14 the scoring criteria for MET positivity was ≥50% of tumor cells positive for target immunostaining with a moderate or strong intensity (≥2+).
Statistical Methods
The sample size was based on a Simon two-stage design with 80% power to reject the null hypothesis of a 16-week PFS rate of 36% if the true PFS of cabozantinib was 54%. Given these criteria, the target accrual for stage I was 20 patients, and at least 9 patients needed to be progression-free at 16 weeks before the second stage could proceed. If this criterion was met, an additional 24 patients were to be enrolled in Stage II. At least 20 of the 44 patients enrolled in Stage I and II needed to be progression-free at 16 weeks to warrant further development of this agent in this setting.
All patients who received at least one dose of cabozantinib were included for safety and efficacy analysis. PFS was defined as the time from trial registration to evidence of radiographic progression as defined by RECIST criteria or death from any cause without evidence of disease progression, whichever occurred first. OS was defined as the time from trial registration to death from any cause. Cases with incomplete follow up or without adequate disease evaluations were censored at date last documented to be progression free. Kaplan-Meier estimates of median PFS and OS were calculated along with their corresponding 95% confidence intervals. For biomarker changes over time, we used the Wilcoxon signed rank test. Given the exploratory nature of these analyses, we did not correct for multiple comparisons. Cox proportional hazards regression model was used to assess the effect of cabozantinib and biomarkers on PFS and OS. The point estimate in the Cox regression model with a 95% confidence interval are presented. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
Between October 2013 and June 2014, 19 patients were registered and received at least one dose of cabozantinib. The median age of the patients was 67 years old, and most patients had intrahepatic cholangiocarcinoma (84%). Fifty-three percent of patients had progressed on only one previous line of therapy, and 47% had progressed on two (see Table 1).
Table 1.
Patient Baseline Characteristics (n=19)
| Characteristic | Value |
|---|---|
| Median age, y (range) | 67y (45–74y) |
| Age, (N, %) | |
| ≤ 49 | 1 (5%) |
| 50–69 | 13 (68%) |
| ≥ 70 | 5 (26%) |
| Sex, (N, %) | |
| Male | 6 (32%) |
| Female | 13 (68%) |
| Race, (N, %) | |
| White | 18 (95%) |
| Black | 0 |
| Hispanic | 0 |
| Asian | 1 (5%) |
| Other | 0 |
| ECOG Performance Status | |
| 0 | 7 (37%) |
| 1 | 12 (63%) |
| Type of Cholangiocarcinoma | |
| Intrahepatic | 16 (84%) |
| Extrahepatic | 3 (16%) |
| Presentation | |
| Primary Metastatic | 15 (79%) |
| Recurrent Metastatic | 4 (21%) |
| Locally Advanced | 0 |
| Histology | |
| Well differentiated | 0 |
| Moderately differentiated | 10 (53%) |
| Poorly differentiated | 7 (37%) |
| AJCC Stage, (N, %) | |
| III | 0 |
| IV | 19 (100%) |
| Prior Local Therapy | |
| Surgery | 4 (21%) |
| Chemoembolization | 4 (21%) |
| Radioembolization | 2 (11%) |
| Liver Radiation | 0 |
| Prior Systemic Therapy | |
| 1 line of chemotherapy | 11 (58%) |
| 2 lines of chemotherapy | 8 (42%) |
Patients received a median of 2 cycles (range 1–10 cycles) of treatment. Dose reductions to 40mg and 20mg were required in 11 (57.9%) and 1 (5.3%) patients, respectively, all due to toxicity. The median duration on therapy was 57 days (range, 8–278 days). At the time of analysis, all 19 patients had discontinued cabozantinib treatment: 11 due to radiological progression, 5 due to clinical progression with stable disease by RECIST criteria, 2 due to toxicities, and 1 due to death. The toxicities that led to discontinuation were a grade 3 enterocutaneous fistula and a grade 3 gastrointestinal perforation. The patient who had an enterocutaneous fistula healed after a treatment break and went on to subsequent treatment. The patient who had a gastrointestinal perforation passed away 3 weeks later. The death that occurred while a subject was on study was recorded within 30 days of study treatment; the patient was admitted for abdominal pain in the setting of progression of peritoneal metastases and died 3 days later. Following withdrawal for progression, 6 of 16 (38%) patients received further systemic therapy including 5-fluorouracil (5-FU)/leucovorin (n=1), 5-FU/leucovorin/oxaliplatin (n=2), erlotinib (n=1), the PD-L1 inhibitor MPDL280A on a clinical trial (n=1), and the stem cell kinase inhibitor BBI503 on a clinical trial (n=1).
Safety
The most common drug-related adverse events of any grade were fatigue (74%), elevated AST (74%), and thrombocytopenia (63%), and most of these were grade 1 or 2 (see Table 2). Toxicities potentially related to VEGF inhibition and attributed to therapy included fatigue (74%), hypertension (37%), diarrhea (26%), mucositis (37%), palmar plantar erythrodysesthesia (21%), and minor bleeding (5%). Grade 3 and 4 adverse events occurred in 89% of patients and included but were not limited to neutropenia (5%), hyperbilirubinemia (5%), epistaxis (5%), bowel perforation (5%), enterocutaneous fistula (5%), hypertension (11%) (see Table 3). No patients experienced liver failure, and no treatment-related deaths occurred. The death discussed above was attributed by the investigator to complications of disease progression.
Table 2.
Most Common (≥5%) Adverse Events (n=19)
| Adverse Event | Any AE | Drug Related AE | ||
|---|---|---|---|---|
|
| ||||
| # of Pts | % | # of Pts | % | |
|
| ||||
| Hematologic | ||||
|
| ||||
| Thrombocytopenia | 13 | 68% | 12 | 63% |
| Leukopenia | 11 | 58% | 11 | 58% |
|
| ||||
| Anemia | 8 | 42% | 6 | 32% |
| Neutropenia | 7 | 37% | 7 | 37% |
|
| ||||
| Non-Hematologic | ||||
|
| ||||
| Fatigue | 17 | 89% | 14 | 74% |
| Abdominal pain | 13 | 68% | 2 | 11% |
|
| ||||
| Nausea | 8 | 42% | 7 | 37% |
| Anorexia | 8 | 42% | 4 | 21% |
|
| ||||
| Diarrhea | 7 | 37% | 5 | 26% |
| Mucositis | 7 | 37% | 7 | 37% |
|
| ||||
| HTN | 7 | 37% | 7 | 37% |
| Hypothyroidism | 7 | 37% | 7 | 37% |
|
| ||||
| Constipation | 6 | 32% | 2 | 11% |
| Vomiting | 4 | 21% | 4 | 21% |
|
| ||||
| Asthenia | 4 | 21% | 1 | 5% |
| Dyspepsia/Heartburn | 4 | 21% | 4 | 21% |
|
| ||||
| Rash | 4 | 21% | 2 | 11% |
| Palmar Plantar Erythrodysesthesia | 4 | 21% | 4 | 21% |
|
| ||||
| Abdominal distension | 4 | 21% | 0 | 0% |
| Pyrexia | 3 | 16% | 0 | 0% |
|
| ||||
| Dry Skin | 3 | 16% | 2 | 11% |
| Weight loss | 3 | 16% | 1 | 5% |
|
| ||||
| Peripheral edema | 2 | 11% | 0 | 0% |
| Foot pain | 2 | 11% | 1 | 5% |
|
| ||||
| Insomnia | 2 | 11% | 1 | 5% |
| Peripheral sensory neuropathy | 2 | 11% | 1 | 5% |
|
| ||||
| Peripheral motor neuropathy | 2 | 11% | 0 | 0% |
| Hyperhidrosis | 2 | 11% | 0 | 0% |
|
| ||||
| Flatulence | 2 | 11% | 1 | 5% |
| Cough | 2 | 11% | 0 | 0% |
|
| ||||
| Back Pain | 2 | 11% | 0 | 0% |
| Psychiatric d/o | 2 | 11% | 0 | 0% |
|
| ||||
| Dizziness | 2 | 11% | 1 | 5% |
| Bleeding | 2 | 11% | 1 | 5% |
|
| ||||
| Laboratory Abnormalities | ||||
|
| ||||
| Elevated AST | 16 | 84% | 14 | 74% |
| Elevated Alkaline phosphatase | 14 | 74% | 5 | 26% |
|
| ||||
| Hyperglycemia | 13 | 68% | 1 | 5% |
| Elevated ALT | 12 | 63% | 9 | 47% |
|
| ||||
| Hyponatremia | 12 | 63% | 6 | 32% |
| Hypomagnesemia | 7 | 37% | 4 | 21% |
|
| ||||
| Hyperbilirubinemia | 4 | 21% | 1 | 5% |
| Hypophosphatemia | 4 | 21% | 2 | 11% |
|
| ||||
| Hypoalbuminemia | 4 | 21% | 1 | 5% |
| Hypokalemia | 2 | 11% | 0 | 0% |
|
| ||||
| Hyperkalemia | 2 | 11% | 0 | 0% |
AEs: Adverse Events; AST: Aspartate aminotranferase; ALT: Alanine aminotransferase Drug related AE = attribution of possibly, probably, or definitely
Table 3.
Most Common (≥5%) Grade 3 and 4 Adverse Events (n=19)
| Grade 3 | Grade 4 | Total | |
|---|---|---|---|
| # of Pts (%) |
# of Pts (%) |
# of Pts (%) |
|
| Adverse Event | |||
| Hematologic | |||
| Neutropenia | 1 (5%) | 1 (5%) | |
| Thrombocytopenia | 1 (5%) | 1 (5%) | |
| Lymphopenia | 2 (11%) | 2 (11%) | |
| Non-Hematologic Toxicities | |||
| Gastrointestinal perforation | 1 (5%) | 1 (5%) | |
| Hypertension | 2 (11%) | 2 (11%) | |
| Abdominal pain | 1 (5%) | 1 (5%) | |
| Fatigue | 1 (5%) | 1 (5%) | |
| Mucositis | 1 (5%) | 1 (5%) | |
| Gastrointestinal fistula | 1 (5%) | 1 (5%) | |
| PPE | 1 (5%) | 1 (5%) | |
| Epistaxis | 1 (5%) | 1 (5%) | |
| Dizziness | 1 (5%) | 1 (5%) | |
| Laboratory Abnormalities | |||
| AST increased | 4 (31%) | 4 (31%) | |
| ALT increased | 1 (5%) | 1 (5%) | |
| Serum ALKP increased | 2 (11%) | 2 (11%) | |
| Hyperbilirubinemia | 1 (5%) | ||
| Lipasemia | 1 (5%) | 1 (5%) | 1 (5%) |
| Hyponatremia | 4 (31%) | 4 (31%) | |
| Hypophosphatemia | 2 (11%) | 2 (11%) | |
Efficacy
After 12 patients failed to be progression-free at 16 weeks, the study was terminated as it was determined that the criterion for proceeding to stage 2 could not be met. With a median follow-up of 5.2 months, the median PFS was 1.8 months (95% CI, 1.6–5.4), and median OS was 5.2 months (95% CI, 2.7–10.5). No objective responses were seen. Five patients (24%) had stable disease at 16 weeks.
Correlative Studies
Plasma VEGF, PlGF, VEGF-D and SDF1α increased and Ang-2, TIMP-1 and TNF-α decreased at both time-points (1 week and 2 weeks) after treatment (Table 4). Plasma IL-10 and IFN-γ transiently decreased during week 1, and IGF-1 and sTie-2 increased and sVEGFR2, HGF and IL-8 decreased only after 2 weeks of treatment (all p<0.05). Of note, previous PK studies have shown that steady state is only achieved after 2 weeks of daily dosing of cabozantinib15,16. There were no significant changes detected in plasma sMET, CAIX, sVEGFR1, VEGF-C, Ang-1, bFGF, IL-1β, IL-2, IL-6 or IL-12 p70 at these time-points. Of the baseline biomarkers, low plasma TIMP-1 was associated with longer PFS (p=0.029), and low IL-6 (p=0.021) and high sMET (p=0.0027) were correlated with longer OS.
Table 4.
Change in plasma biomarkers (significance changes highlighted in grey).
| Plasma biomarker | Baseline | Week 1 | Week 2 |
|---|---|---|---|
| Median [interquartile ranges]; n (patients) | |||
| VEGF (pg/ml) | 68.8 [56.8, 104.2] n=17 |
109.8 [73.4, 178.2] n=19 |
211.2 [155.4, 323.4] n=17 |
| P value* | N/A | 0.0026 | 0.0004 |
|
| |||
| PlGF (pg/ml) | 43.4 [38.5, 50.8] n=17 |
58.3 [45.6, 80.7] n=19 |
97.4 [79.8, 130.2] n=17 |
| P value | N/A | <0.0001 | <0.0001 |
|
| |||
| SDF1α (pg/ml) | 2089 [1882, 2395] n=17 |
2265 [1991, 2602] n=19 |
2337 [2203, 2464] n=17 |
| P value | N/A | 0.0001 | 0.0006 |
|
| |||
| VEGF-D (pg/ml) | 1098.4 [907.5, 1823.2] n=17 |
1414.7 [1203.8, 2404.1] n=19 |
1820.8 [1459.9, 2720.2] n=17 |
| P value | N/A | <0.0001 | <0.0001 |
|
| |||
| TIMP-1 (ng/ml) | 229.0 [171.6, 338.5] n=17 |
160.9 [126.5, 214.4] n=19 |
149.6 [131.7, 190.9] n=17 |
| P value | N/A | <0.0001 | <0.0001 |
|
| |||
| Ang-2 (pg/ml) | 3423.3 [2838.6, 4534.1] n=17 |
3277.4 [2356.9, 4326.9] n=19 |
2792.1 [2200.2, 3458.8] n=17 |
| P value | N/A | 0.0067 | 0.0015 |
|
| |||
| TNF-α (pg/ml) | 2.82 [2.10, 3.67] n=17 |
2.59 [1.83, 3.13] n=19 |
2.45 [1.69, 3.14] n=17 |
| P value | N/A | 0.0046 | 0.0002 |
|
| |||
| IFN-γ (pg/ml) | 9.2 [6.5, 19.9] n=17 |
7.6 [6.1, 13.2] n=19 |
14.9 [8.8, 19.7] n=17 |
| P value | N/A | 0.013 | 0.33 |
|
| |||
| IL-10 (pg/ml) | 0.47 [0.38, 0.77] n=17 |
0.42 [0.35, 0.65] n=19 |
0.45 [0.35, 0.58] n=17 |
| P value | N/A | 0.022 | 0.094 |
|
| |||
| Free IGF-I (ng/ml) | 0.664 [0.517, 0.811] n=17 |
0.633 [0.511, 0.967] n=19 |
0.991 [0.677,1.329] n=17 |
| P value | N/A | 0.2247 | 0.0004 |
|
| |||
| sTie-2 (pg/ml) | 5950.7 [5398.5, 6580.7] n=17 |
6333.3 [5567.8, 6834.6] n=19 |
6225.3 [5468.2, 7995.5] n=17 |
| P value | N/A | 0.051 | 0.026 |
|
| |||
| sVEGFR-2 (pg/ml) | 7903 [6091, 8683] n=17 |
7961 [5974, 8905] n=19 |
6375 [5182, 6751] n=17 |
| P value | N/A | 0.38 | 0.0026 |
|
| |||
| HGF (pg/ml) | 1490.0 [1242.5, 1959.8] n=17 |
1289.7 [1030.1, 2328.5] n=19 |
1254.3 [1065.5, 1582.6] n=17 |
| P value* | N/A | 0.16 | 0.030 |
|
| |||
| IL-8 (pg/ml) | 42.6 [20.6, 145.0] n=17 |
29.8 [15.5, 71.5] n=19 |
21.0 [11.8, 43.2] n=17 |
| P value | N/A | 0.051 | 0.0004 |
|
| |||
| sMET (ng/ml) | 1348.4 [1287.5, 1597.6] n=16 |
1479.3 [1305.1, 1772.7] n=19 |
1519.4 [1323.5, 1901.2] n=17 |
| P value | N/A | 0.16 | 0.11 |
|
| |||
| CAIX (pg/ml) | 208.2 [92.6, 251.3] n=9 |
205.6 [129.4, 255.8] n=10 |
205.6 [129.4, 255.8] n=10 |
| P value | N/A | 0.30 | 0.65 |
|
| |||
| Ang-1 (pg/ml) | 1030.7 [62.5, 1328.6] n=17 |
62.5 [62.5, 1277.8] n=19 |
62.5 [62.5, 1341.4] n=17 |
| P value | N/A | 0.92 | 0.34 |
|
| |||
| bFGF (pg/ml) | 13.3 [5.5, 17.3] n=17 |
13.8 [8.5, 17.3] n=19 |
14.5 [8.1, 22.7] n=17 |
| P value | N/A | 0.96 | 0.52 |
|
| |||
| sVEGFR-1 (pg/ml) | 105.9 [71.4, 206.3] n=17 |
74.9 [55.7, 257.6] n=19 |
74.2 [58.3, 173.8] n=17 |
| P value | N/A | 0.12 | 0.72 |
|
| |||
| VEGF-C (pg/ml) | 83.0 [83.0, 83.0] n=17 |
83.0 [83.0, 105.9] n=19 |
83.0 [83.0, 94.7] n=17 |
| P value | N/A | 0.22 | 0.50 |
|
| |||
| IL-12 p70 (pg/ml) | 0.48 [0.48, 0.56] n=17 |
0.48 [0.48-0.48] n=19 |
0.48 [0.48, 0.56] n=17 |
| P value | N/A | 0.81 | 0.22 |
|
| |||
| IL-2 (pg/ml) | 0.69 [0.69, 0.71] n=17 |
0.69 [0.69, 0.76] n=19 |
0.69 [0.69, 0.80] n=17 |
| P value | N/A | 0.30 | 0.95 |
|
| |||
| IL-6 (pg/ml) | 3.16 [2.01, 5.83] n=17 |
2.81 [1.24, 3.66] n=19 |
3.04 [2.13, 4.70] n=17 |
| P value | N/A | 0.089 | 0.52 |
Median and IQR for IL-1β and IL-4 were not tabulated because the majority of them had median values under the detectable threshold.
P values were from Wilcoxon signed rank test.
Abbreviations: Ang, angiopoietin; bFGF, basic fibroblast growth factor; CAIX, carbonic anhydrase IX; HGF, hepatocyte growth factor; IFN-γ, interferon gamma; IGF, insulin-like growth factor; IL, interleukin; IQR, interquartile range; PlGF, placental growth factor; SDF1α, stromal cell-derived factor 1 alpha; sVEGFR, soluble vascular endothelial growth factor receptor; sMET, soluble MET; TIMP, tissue inhibitor of matrix metalloproteinase; TNF-α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor.
Tumor MET overexpression (estimated as 2+ or 3+ by IHC) was seen in 4 of 10 patients evaluated (see Figure 1). The remainder of the patients (n=9) had insufficient tissue for testing. The patient with the longest disease control (9.2 months) had 3+ tumor MET expression, but MET expression status by IHC did not correlate with PFS (p=0.38) or OS (p=0.17) in the 10 patients evaluated.
Figure 1. Total MET immunohistochemistry.
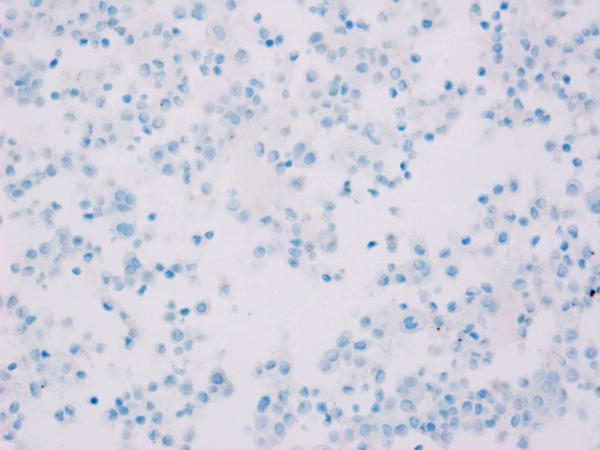
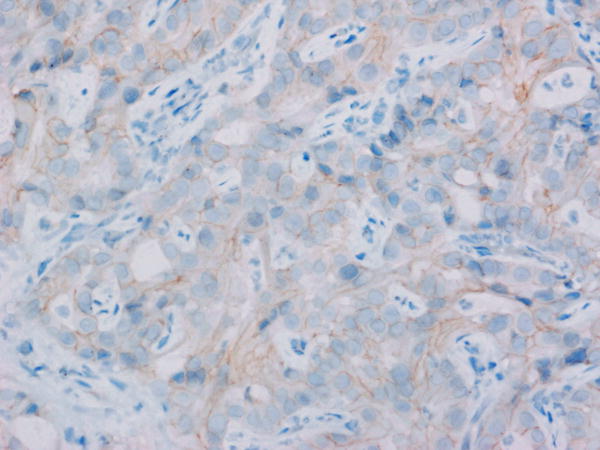
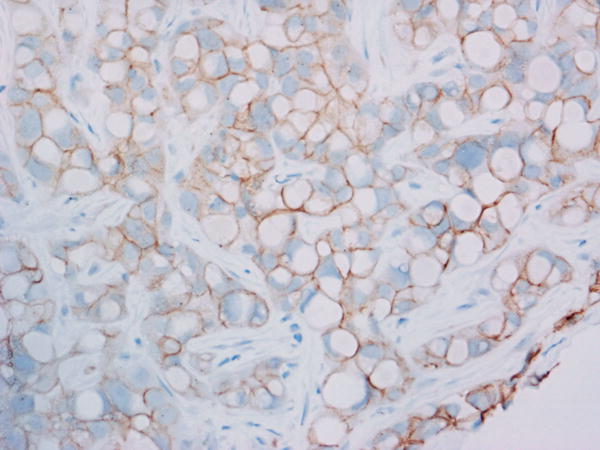
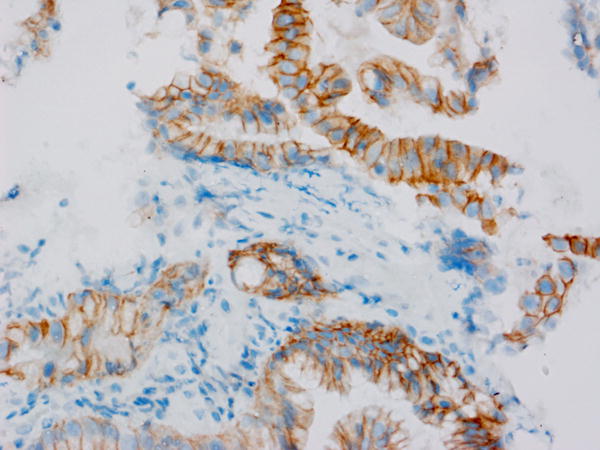
Levels of total MET protein were evaluated in patient archived pre-treatment FFPE tumor tissue. Shown are representative photomicrographs captured using a 40X objective across the IHC scores of A) 0 (study ID #3), B) 1+ (study ID #11), C) 2+ (study ID #14) and D) 3+ (study ID #5).
DISCUSSION
The current study is the first clinical trial to evaluate the role of MET inhibition in patients with cholangiocarcinoma. As an oral once-daily multi-kinase inhibitor, cabozantinib targets VEGFR2, MET, RET, KIT, AXL, FLT3 and TIE-2. In this trial, cabozantinib demonstrated limited clinical activity in an unselected population of patients with advanced refractory cholangiocarcinoma. However, one patient with 3+ MET overexpression in the tumor stayed on treatment for 278 days. This observation suggests that any path forward for this agent in cholangiocarcinoma would involve a biomarker-driven approach. Our study included exploratory biomarker studies using a panel of plasma and tissue biomarkers to examine cabozantinib’s impact on pro-angiogenic and immune pathways as well as the HGF/MET pathway in cholangiocarcinoma.
The correlative studies confirmed pharmacodynamic biomarker changes consistent with antiangiogenic effects of cabozantinib. Treatment was associated with significant increases in VEGF, PlGF, SDF1α and decreases in Ang-2 and sVEGFR2, as seen with cabozantinib and other VEGFR2 kinase inhibitors in liver cancer and other other solid tumors17–21. In addition, and consistent with data in breast cancer patients, cabozantinib treatment was associated with an increase in plasma VEGF-D, IGF-I and sTIE-2. As also seen in breast cancer, cabozantinib treatment led to a decrease in plasma HGF and TIMP-1, both of which are associated with stellate cell activation and fibrosis, as well as a decrease in the cytokines IFN-γ, TNF-α, IL-8 and IL-10.17 These data indicate that the agent also has potential anti-fibrotic and anti-inflammatory activity in cholangiocarcinoma. Further to this point, plasma TIMP-1 and IL-6, biomarkers known to be associated with liver fibrosis and immunosuppression in cholangiocarcinoma22–27, were associated in this study with PFS and OS, respectively. Plasma TIMP-1 is an established biomarker of liver fibrogenesis, but has not been yet used as a biomarker of tumor fibrosis in a highly desmoplastic disease such as CCA. While the exact effects of cabozantinib on the cholangiocarcinoma microenvironment and systemic immunity remain to be established, the drug exerts intriguing changes on the immune system17,28,29, and these mechanisms may be relevant for designing future combinations with other agents such as immunotherapy. However, these biomarker analyses were not adjusted for multiple comparisons and therefore are hypothesis-generating only.
Despite the intriguing response seen in the patient whose tumor had MET overexpression, in the overall study population high MET expression in tumor tissue did not appear to correlate with cabozantinib activity. This lack of correlation was based on limited number of cases and the lack of pretreatment biopsies, but this finding is consistent with the data in renal cell carcinoma and breast cancer17,30. High pretreatment plasma sMET levels, however, did correlate with longer OS in the overall study population, and this result is also consistent with the cabozantinib experience in advanced triple-negative breast cancer, where higher sMET levels were significantly associated with better outcomes17. While hypothesis generating in nature, these results warrant further evaluations of tissue MET and plasma sMET in larger studies in patients receiving cabozantinib.
Despite close monitoring, a high rate of ≥ grade 3 toxicities was observed at 60 mg daily dosing. The majority (72%) of the grade 3 and 4 toxicities were laboratory abnormalities, and none led to drug discontinuation and many were in patients who had abnormal baseline liver function tests or blood counts. While some patients with ICC can have underlying cirrhosis31–33, only one patient in this trial had a history of cirrhosis upon reviewing the imaging and clinical history of all patients. The rate of dose reductions (63%) was similar to the rate seen a phase III trial in advanced renal cell carcinoma (62%)30 but higher than that seen in the phase III trial in metastatic castrate resistant prostate cancer (33%)34 and a phase II trial in breast cancer (34%)17, all of which evaluated the 60mg daily dosing. Patients with advanced refractory cholangiocarcinoma may be more sensitive to drug doses tolerated by other solid tumor patient populations, and therefore, close monitoring remains critical for these patients in early phase clinical trials.
This study has limitations including the single-arm design and the small sample size. In addition, tissue for biomarker analysis was available in only 54% of patients. Tissue was prioritized for clinical testing including tumor genetic profiling, so minimal or no tissue was available for correlative research studies in some patients. Given that majority of patients with cholangiocarcinoma are diagnosed by fine needle aspirate or core liver biopsy, the paucity of tissue is a common occurrence that hinders tissue biomarker discovery in this disease. If additional tissue was available, analysis of VEGF expression and MET amplification could also have been conducted, although neither of these has consistently been found to predict response to anti-angiogenic drugs or MET inhibitors.
Given the intriguing preclinical data and clinical correlative results suggesting immunomodulatory effects of cabozantinib, future studies of cabozantinib in cholangiocarcinoma may include combinations with chemotherapy or immune checkpoint blockers. A randomized phase II study of gemcitabine and cisplatin with and without the potent type II ATP competitive MET kinase inhibitor merestinib, which also inhibits at least 13 other receptor tyrosine kinases, is currently ongoing in patients with advanced biliary tract cancer (NCT02711553). A trial of cabozantinib plus nivolumab alone or with ipilimumab is currently underway in patients with metastatic genitourinary cancers (NCT02496208) and may lead to development in other solid tumors if tolerable and effective. Ultimately, a strong focus on correlative studies in these trials and others will hopefully pave the path forward for selecting subpopulations likely to benefit from a strategy of MET inhibition in cholangiocarcinoma.
Acknowledgments
Funding: L.G. received the NIH Loan Repayment Program Award. Exelixis provided funding for this trial.
Footnotes
Disclaimers: MBY receives research funding from Myriad Genetic Laboratories, Inc. JMC reports research funding to his institution from Merrimack Pharmaceuticals, Taiho Oncology, Merck, Roche, Abbvie, Precision Biologics, and Bristol Myers Squib. He received a consulting honorarium from Agios Pharmaceuticals. DRB is a paid consultant for Bio-Reference Laboratories, Inc (Licensee of SNaPshot). DGD receives research funding from Merrimack, Bayer and Bristol Meyer Squibb. RKJ received consultant fees from Enlight, Noxxon, Zyngenia and WebMD. RKJ owns equity in Enlight, SynDevRx and XTuit, and serves on the Board of Directors of XTuit and Boards of Trustees of H&Q Healthcare Investors and H&Q Life Sciences Investors. No reagents or funding from these companies were used in these studies.
All other authors declare no conflicts of interest.
ClinicalTrials.gov Identifier: NCT01954745
References
- 1.Nagino M, Ebata T, Yokoyama Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013;258(1):129–140. doi: 10.1097/SLA.0b013e3182708b57. [DOI] [PubMed] [Google Scholar]
- 2.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 3.Walter T, Horgan AM, McNamara M, et al. Feasibility and benefits of second-line chemotherapy in advanced biliary tract cancer: a large retrospective study. Eur J Cancer. 2013;49(2):329–335. doi: 10.1016/j.ejca.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Yoshikawa D, Ojima H, Iwasaki M, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98(2):418–425. doi: 10.1038/sj.bjc.6604129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hida Y, Morita T, Fujita M, et al. Vascular endothelial growth factor expression is an independent negative predictor in extrahepatic biliary tract carcinomas. Anticancer Res. 1999;19(3B):2257–2260. [PubMed] [Google Scholar]
- 6.Park BK, Paik YH, Park JY, et al. The clinicopathologic significance of the expression of vascular endothelial growth factor-C in intrahepatic cholangiocarcinoma. Am J Clin Oncol. 2006;29(2):138–142. doi: 10.1097/01.coc.0000204402.29830.08. [DOI] [PubMed] [Google Scholar]
- 7.Nakazawa K, Dobashi Y, Suzuki S, Fujii H, Takeda Y, Ooi A. Amplification and overexpression of c-erbB-2, epidermal growth factor receptor, and c-met in biliary tract cancers. J Pathol. 2005;206(3):356–365. doi: 10.1002/path.1779. [DOI] [PubMed] [Google Scholar]
- 8.Aishima SI, Taguchi KI, Sugimachi K, Shimada M, Tsuneyoshi M. c-erbB-2 and c-Met expression relates to cholangiocarcinogenesis and progression of intrahepatic cholangiocarcinoma. Histopathology. 2002;40(3):269–278. doi: 10.1046/j.1365-2559.2002.00353.x. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto M, Ojima H, Iwasaki M, et al. Prognostic significance of overexpression of c-Met oncoprotein in cholangiocarcinoma. Br J Cancer. 2011;105(1):131–138. doi: 10.1038/bjc.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum Pathol. 1998;29(2):175–180. doi: 10.1016/s0046-8177(98)90229-5. [DOI] [PubMed] [Google Scholar]
- 11.Sulpice E, Ding S, Muscatelli-Groux B, et al. Cross-talk between the VEGF-A and HGF signalling pathways in endothelial cells. Biol Cell. 2009;101(9):525–539. doi: 10.1042/BC20080221. [DOI] [PubMed] [Google Scholar]
- 12.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3(4):347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Spigel DR, Edelman MJ, Mok T, et al. Treatment Rationale Study Design for the MetLung Trial: A Randomized, Double-Blind Phase III Study of Onartuzumab (MetMAb) in Combination With Erlotinib Versus Erlotinib Alone in Patients Who Have Received Standard Chemotherapy for Stage IIIB or IV Met-Positive Non-Small-Cell Lung Cancer. Clin Lung Cancer. 2012;13(6):500–504. doi: 10.1016/j.cllc.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen L, Holland J, Miles D, et al. Pharmacokinetic (PK) drug interaction studies of cabozantinib: Effect of CYP3A inducer rifampin and inhibitor ketoconazole on cabozantinib plasma PK and effect of cabozantinib on CYP2C8 probe substrate rosiglitazone plasma PK. J Clin Pharmacol. 2015;55(9):1012–1023. doi: 10.1002/jcph.510. [DOI] [PubMed] [Google Scholar]
- 16.Kurzrock R, Sherman SI, Ball DW, et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29(19):2660–2666. doi: 10.1200/JCO.2010.32.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tolaney SM, Ziehr DR, Guo H, et al. Phase II and Biomarker Study of Cabozantinib in Metastatic Triple-Negative Breast Cancer Patients. Oncologist. 2016 Oct 27; doi: 10.1634/theoncologist.2016-0229. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu AX, Duda DG, Sahani DV, Jain RK. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol. 2011;8(5):292–301. doi: 10.1038/nrclinonc.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu-Emerson C, Duda DG, Emblem KE, et al. Lessons from anti-vascular endothelial growth factor and anti-vascular endothelial growth factor receptor trials in patients with glioblastoma. J Clin Oncol. 2015;33(10):1197–1213. doi: 10.1200/JCO.2014.55.9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duda DG. Molecular Biomarkers of Response to Antiangiogenic Therapy for Cancer. ISRN Cell Biol. 2012;2012 doi: 10.5402/2012/587259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu AX, Ancukiewicz M, Supko JG, et al. Efficacy, safety, pharmacokinetics, and biomarkers of cediranib monotherapy in advanced hepatocellular carcinoma: a phase II study. Clin Cancer Res. 2013;19(6):1557–1566. doi: 10.1158/1078-0432.CCR-12-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yokomuro S, Tsuji H, Lunz JG, 3rd, et al. Growth control of human biliary epithelial cells by interleukin 6, hepatocyte growth factor, transforming growth factor beta1, and activin A: comparison of a cholangiocarcinoma cell line with primary cultures of non-neoplastic biliary epithelial cells. Hepatology. 2000;32(1):26–35. doi: 10.1053/jhep.2000.8535. [DOI] [PubMed] [Google Scholar]
- 23.Sripa B, Thinkhamrop B, Mairiang E, et al. Elevated plasma IL-6 associates with increased risk of advanced fibrosis and cholangiocarcinoma in individuals infected by Opisthorchis viverrini. PLoS Negl Trop Dis. 2012;6(5):e1654. doi: 10.1371/journal.pntd.0001654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prakobwong S, Pinlaor S, Yongvanit P, Sithithaworn P, Pairojkul C, Hiraku Y. Time profiles of the expression of metalloproteinases, tissue inhibitors of metalloproteases, cytokines and collagens in hamsters infected with Opisthorchis viverrini with special reference to peribiliary fibrosis and liver injury. Int J Parasitol. 2009;39(7):825–835. doi: 10.1016/j.ijpara.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Ince AT, Yildiz K, Gangarapu V, et al. Serum and biliary MMP-9 and TIMP-1 concentrations in the diagnosis of cholangiocarcinoma. Int J Clin Exp Med. 2015;8(2):2734–2740. [PMC free article] [PubMed] [Google Scholar]
- 26.Goydos JS, Brumfield AM, Frezza E, Booth A, Lotze MT, Carty SE. Marked elevation of serum interleukin-6 in patients with cholangiocarcinoma: validation of utility as a clinical marker. Ann Surg. 1998;227(3):398–404. doi: 10.1097/00000658-199803000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheon YK, Cho YD, Moon JH, et al. Diagnostic utility of interleukin-6 (IL-6) for primary bile duct cancer and changes in serum IL-6 levels following photodynamic therapy. Am J Gastroenterol. 2007;102(10):2164–2170. doi: 10.1111/j.1572-0241.2007.01403.x. [DOI] [PubMed] [Google Scholar]
- 28.Kwilas AR, Donahue RN, Tsang KY, Hodge JW. Immune consequences of tyrosine kinase inhibitors that synergize with cancer immunotherapy. Cancer Cell Microenviron . 2015;2(1) doi: 10.14800/ccm.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwilas AR, Ardiani A, Donahue RN, Aftab DT, Hodge JW. Dual effects of a targeted small-molecule inhibitor (cabozantinib) on immune-mediated killing of tumor cells and immune tumor microenvironment permissiveness when combined with a cancer vaccine. J Transl Med. 2014;12:294. doi: 10.1186/s12967-014-0294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):917–927. doi: 10.1016/S1470-2045(16)30107-3. [DOI] [PubMed] [Google Scholar]
- 31.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48(1):308–321. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128(3):620–626. doi: 10.1053/j.gastro.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 33.Choi SH, Lee SS, Kim SY, et al. Intrahepatic Cholangiocarcinoma in Patients with Cirrhosis: Differentiation from Hepatocellular Carcinoma by Using Gadoxetic Acid-enhanced MR Imaging and Dynamic CT. Radiology. 2016:160639. doi: 10.1148/radiol.2016160639. [DOI] [PubMed] [Google Scholar]
- 34.Smith M, De Bono J, Sternberg C, et al. Phase III Study of Cabozantinib in Previously Treated Metastatic Castration-Resistant Prostate Cancer: COMET-1. J Clin Oncol. 2016;34(25):3005–3013. doi: 10.1200/JCO.2015.65.5597. [DOI] [PubMed] [Google Scholar]


