Abstract
Rett syndrome (RTT) is caused by MECP2 mutations, resulting in various neurological symptoms. Prolonged corrected QT interval (QTc) is also reported and is a speculated cause of sudden death in RTT. The purpose of this study was to correlate QTc in RTT patients with age, clinical severity, and genotype. 100 RTT patients (98 females, 2 males) with MECP2 mutations underwent baseline neurological evaluation (KKI-RTT Severity Scale) and QTc measurement (standard 12 lead electrocardiogram) as part of our prospective natural history study. Mean QTc of the cohort was 422.6 msec, which did not exceed the normal values for age. 7/100 patients (7%) had QTc prolongation (>450 msec). There was a trend for increasing QTc with age and clinical severity (p=0.09). No patients with R106C, R106W, R133C, R168*, R270*, R294*, R306C, R306H, and R306P mutations demonstrated QTc prolongation. There was a relatively high proportion of QTc prolongation in patients with R255* mutations (2/8, 25%) and large deletions (1/4, 25%). The overall presence of QTc prolongation did not correlate with mutation category (p=0.52). Our findings demonstrate that in RTT, the prevalence of QTc prolongation is lower than previously reported. Hence, all RTT patients warrant baseline ECG; if QTc is prolonged, then cardiac followup is warranted. If initial QTc is normal, then annual ECGs, particularly in younger patients, may not be necessary. However, larger sample sizes are needed to solidify the association between QTc and age and clinical severity. The biological and clinical significance of mild QTc prolongation above the normative data remains undetermined.
Keywords: Rett syndrome, QTc prolongation, genotype
Introduction
Rett syndrome (RTT) is an X-linked dominant neurological disorder, associated with mutations in methy-CpG-binding protein 2 (MECP2) gene located in the Xq28 region [Amir et al., 1999]. RTT is characterized by apparent normal development until 6 to 18 months of age followed by regression and later plateau of symptoms. Common manifestations include decelerated head growth, psychomotor retardation, stereotyped hand movements with loss of purposeful hand use, impaired language, gait apraxia, irregular respiration in the wake period, and electroencephalogram abnormalities with seizures [Christodoulou and Ho, 1993; Hagberg et al., 1983]. The condition is more often seen in girls due to de novo mutations arising almost exclusively in the paternal X chromosome [Zhang et al., 2012; Thomas, 1996]. The rare affected males, who have maternally inherited mutations, demonstrate a greater degree of encephalopathy from infancy and have shorter life spans than their female counterparts [Schüle et al., 2008].
In addition to experiencing neurological symptoms, individuals with RTT also have reported electrocardiogram and rhythm abnormalities, including prolonged corrected QT interval (QTc) [McCauley et al., 2011; Guideri et al., 1999, 2001; Ellaway et al., 1999; Sekul et al., 1994], T-wave abnormalities [Sekul et al., 1994], and reduced heart-rate variability [Guideri et al., 1999, 2001]. In rare cases, sinus bradycardia [Madan et al., 2004], asymptomatic sinoatrial block [Guideri and Acampa, 2005], and ventricular tachycardia [Guideri and Acampa, 2005] can occur in affected individuals. In RTT, cardiac imaging studies do not suggest any morphologic or functional changes, but there is report of subtle echocardiographic features of systolic and diastolic dysfunction [De Felice et al., 2012].
Compared to children and adolescents in the general population, individuals with RTT have a higher incidence of sudden, unexpected death (0.3% versus 0.0001%) [Kerr et al., 1997; Driscoll and Edwards, 1985]. Possible causes of sudden death in RTT include sudden unexpected death in epilepsy (SUDEP), respiratory failure, aspiration, acute gastric perforation, and cardiac arrhythmias [Byard, 2006]. One of the most studied cardiac risk factors for sudden death is prolonged QTc. The percentage of patients with prolonged QTc in RTT reportedly ranges from 18.5%–55% [Sekul et al., 1994; Guideri et al., 1999; Ellaway et al., 1999; Guideri et al., 2001; McCauley et al., 2011]. QTc prolongation is associated with RTT clinical severity/advanced stages of the disease in some studies [Guideri et al., 2001, 1999; Sekul et al., 1994] but not others [Ellaway et al., 1999]. A common manifestation of RTT is dysautonomia, which is associated with reduced heart-rate variability [Dotti et al., 2004; Guideri et al., 2001, 1999; Acampa et al., 2008] that may be a potential cause of prolonged QTc. Cardiomyocytes from Mecp2 deficient mice demonstrate persistently abnormal sodium current [McCauley et al., 2011]; however, there is no direct metabolic evidence correlating MECP2 mutations and QTc prolongation.
To our knowledge, there are no studies that have evaluated the relationship between QTc and mutation type in RTT. In this study, we investigated QTc in 100 RTT patients, analyzing its relationship to age, clinical severity, and mutation type.
Patients and Methods
Participants
One hundred MECP2 mutation positive RTT patients were evaluated at the Pediatric Clinical Research Unit of Johns Hopkins Hospital as part of a prospective Natural History study of RTT. Prior to entry into the study, MECP2 mutation analysis was performed by sequencing as described previously [Hoffbuhr et al., 2001] or by commercial laboratories. If sequencing was normal, including exon 1, and the index of suspicion was high, advanced studies were performed at the Greenwood Genetics Center (Greenwood, South Carolina), using multiplex ligation dependent probe amplification analysis to identify large deletions or duplications. Study protocols were approved by the Institutional Review Board at Johns Hopkins Medical Institution, and informed consent was obtained from parents.
Clinical Assessment
To assess clinical severity for each participant at the beginning of the study, standard neurological examinations and a modified version of the Kennedy Krieger Institute Rett Syndrome Severity Scale (RSSS) [Hoffbuhr et al., 2001] were used. The revised RSSS rates 7 categories (seizures, gait, scoliosis, respiratory irregularity, hand use, speech, and sleep) on a scale from 0 (normal/absent) to 3 (most severe), and a total score is generated by summing the values (possible values range from 0–21) (Table S1). Mild clinical severity corresponds to a total score between 0–7; moderate clinical severity corresponds to a total score between 8–14; and severe clinical severity corresponds to a total score between 15–21.
Electrocardiograms
Standard 12-lead electrocardiograms (ECGs) were obtained at baseline upon entry into the research study. The QT interval was hand measured twice by a single pediatric cardiologist (JB), blinded to clinical severity and genotype, using a standard approach. The observed QT (QTo) was corrected for heart rate by Bazett’s correction utilizing the preceding RR interval such that . For consistency across all ages, prolongation was defined as QTc greater than 450 msec. Medical charts were reviewed for medication use at the time of ECG.
Subgroups
Patients were grouped by age groups, clinical severity groups, and genotypes at baseline. Participants were divided into three age groups because of the clinical changes with age as noted previously [Hagberg and Witt-Engerström, 1986; Trevathan and Naidu, 1988]. The first, second, and third groups included patients who were 1 to < 7 years old, 7 to < 13 years old, and 13 to < 19 years old, respectively. Clinical severity groups were based on the RSSS categories of mild, moderate, and severe. The genotypes consisted of each individual common mutation (defined as a missense or nonsense mutation at a position that is affected > 1 individual in the sample), small deletions, large deletions, and uncommon mutations (defined as missense or nonsense mutations each of which had a prevalence in our sample of n = 1).
Data Analysis
Data was analyzed using STATA 9.0 (Stata Corporation, College Station, Texas, USA). Chi-squared test was used to assess the association between mutation category and having prolonged QTc. Nonparametric Kruskal-Wallis test was used to compare the mean QTc values across the mutation categories. Linear regression model was fit to assess the relationship between QTc and patient characteristics: age and clinical severity (RSSS total score). All statistical tests were two-sided and performed at 5% statistical significance.
Results
Demographics
The study cohort consisted of 98 females and 2 males. Ages ranged from 1 to 17 years (mean 6.19 ± 3.60 years).
QTc Overview
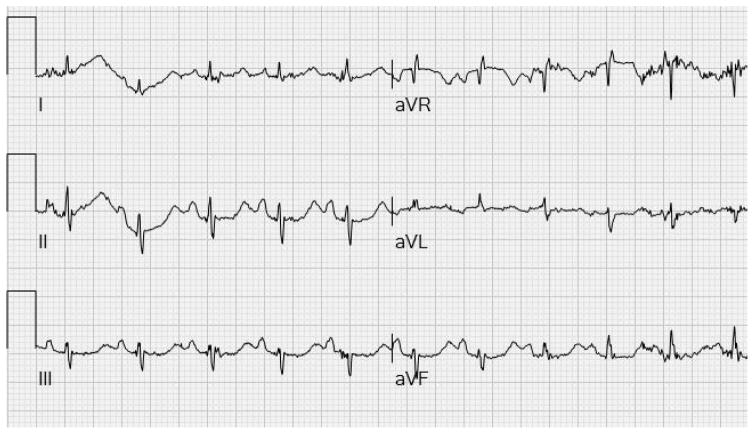
Among 100 patients, the average QTc was 422.60 ± 21.11 msec. The mean QTc interval overall and for each age group was within normal limits (Table 1). QTc prolongation occurred in 7 of the 100 patients (7%). The longest QTc of 483 msec was seen in a 7-year-old girl with an R255* mutation (Figure 1). This patient had a composite clinical severity score of 19, in the severe category. She had a family history of long QT syndrome, but in one of her family members who underwent genotyping for genetic variants associated with long QT syndrome, results were negative.
Table 1.
Demographics and QTc values of the cohort, stratified by age groups.
| 1.0 to < 7 years n = 60 |
7 to < 13 years n = 33 |
13 to < 19 years n = 7 |
Entire cohort n = 100 |
|
|---|---|---|---|---|
| Number female | 58 | 33 | 7 | 98 |
| Number male | 2 | 0 | 0 | 2 |
| Average age (years) | 3.80 ± 1.48 | 8.78 ± 1.58 | 14.57 ± 1.51 | 6.19 ± 3.60 |
| QTc (msec) | 419.95 ± 20.25 | 425.61 ± 22.34 | 431.14 ± 21.39 | 422.60 ± 21.11 |
Figure 1.
Portion of electrocardiogram of RTT patient with prolonged QTc of 483 msec.
QTc Versus Age
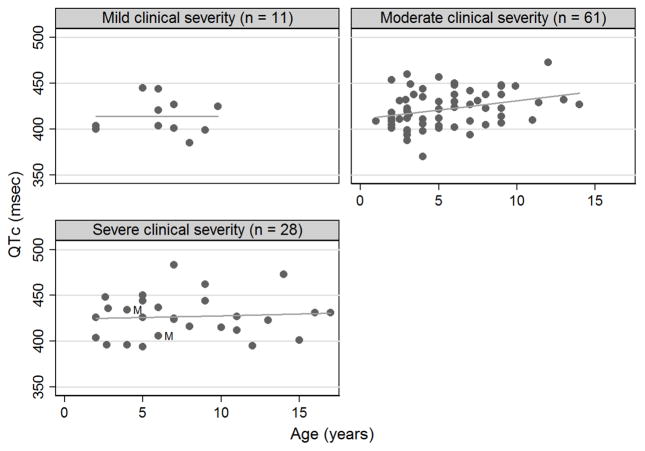
With respect to age, QTc prolongation occurred in 3/60 (5%) of patients aged 1 to < 7 years, 3/33 (9.1 %) of patients aged 7 to < 13 years, and 1/7 (14.3 %) of patients aged 13 to < 19 years. There was a positive relationship between QTc and age after adjusting for total clinical severity score (beta = 0.17; 95% confidence interval −0.17 to 2.18), but this linear relationship did not reach statistical significance (p = 0.09). When the data was stratified by clinical severity group, this association between QTc and age was significant for the moderate clinical severity group (linear regression; p = 0.01) but not for mild clinical severity (linear regression; p = 0.98) or severe clinical severity group (linear regression; p = 0.72) (Figure 2). Excluding the two male subjects did not affect the statistical significance of these findings.
Figure 2.
QTc (msec) of individual participants versus age (years). Graphs are by categorical clinical severity. Circles represent raw data. Males are denoted by the label “M”.
QTc Versus Clinical Severity
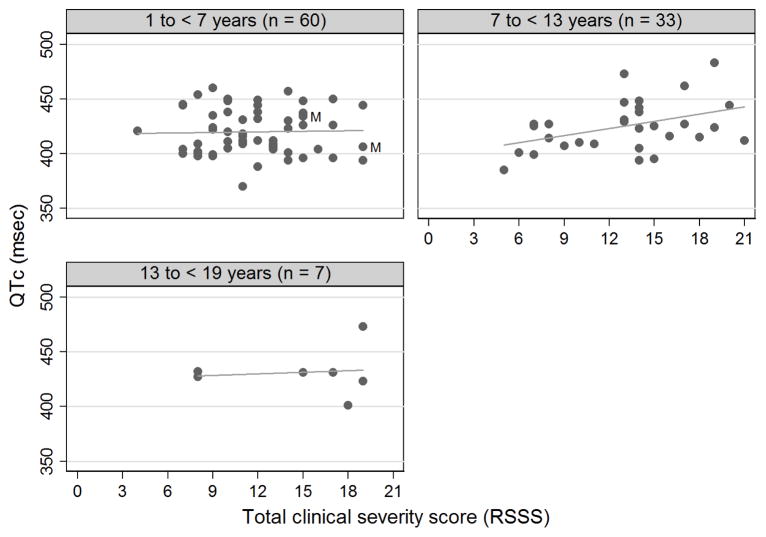
With respect to clinical severity, QTc prolongation occurred in 0/11 (0%) of mildly affected patients, 4/61 (6.5%) of moderately affected patients, and 3/28 (10.7%) of severely affected patients. There was a positive relationship between QTc and clinical severity score after adjusting age (beta = 0.17; 95% confidence interval −0.16 to 2.04), but this linear relationship did not reach statistical significance (p = 0.10). When the data was stratified by age group, this association between QTc and clinical severity score was significant for the 7 to < 13 years age group (linear regression; p = 0.02) but not for the youngest (linear regression; p = 0.85) or oldest age group (linear regression; p = 0.82) (Figure 3). As with QTc versus age, excluding the two male subjects did not affect the statistical significance of these findings.
Figure 3.
QTc (msec) of individual participants versus total clinical severity score (based on RSSS). Graphs are by age group. Circles represent raw data. Males are denoted by the label “M”.
Interestingly, the two severely affected males in the study, aged 4 years (mutation Q83*) and 6 years (mutation R133C), both had neonatal encephalopathy, respiratory and gastrointestinal compromise, and intermittent bradycardia, but normal QTc.
QTC Versus Genotype
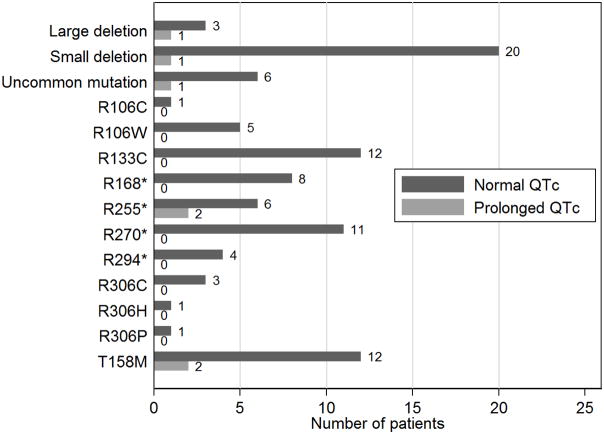
QTc status was examined by mutation (Figure 4). There were no instances of QTc prolongation in patients with the following common mutations: R106C (n = 1), R106W (n = 5), R133C (n = 12), R168* (n = 8), R270* (n = 11), R294* (n = 4), R306C (n = 3), R306H (n = 1), and R306P (n = 1). There was a relatively low proportion of QTc prolongation in patients with T158M mutations (2/14, 14.3%), small deletions (1/21, 4.8%), and uncommon mutations (1/7, 14.3%), and a relatively high proportion of QTc prolongation in patients with R255* mutations (2/8, 25%) and large deletions (1/4, 25%). The category of uncommon mutations included the following mutations (each with n = 1): K266*, Q83*, P322A, Y141*, D156E, Q244*, D134C. The proportion of QTc prolongation among missense and nonsense mutations (including uncommon mutations) was 7.5% (3/40) and 5.7% (2/35), respectively (chi2(1) = 0.10, p = 0.76).
Figure 4.
Number of patients with either normal or prolonged QTc stratified by mutation.
There was no correlation between presence of QTc prolongation versus mutation category (missense mutations, nonsense mutations, small deletions, and large deletions) (chi2(3) = 2.26, p = 0.52). The patients with large deletions (n = 4) had a higher mean QTc value (437.3 ± 25.4 msec) than those with missense mutations (n= 40; 423.8 ± 20.0 msec), nonsense mutations (n = 35; 420.1 ± 21.1 msec), and small deletions (n = 21; 421.7 ± 22.7 msec); however, there was no statistical difference in the QTc distribution across these mutation groups (p = 0.46).
Additional Factors
Nine patients were taking potentially QTc prolonging medications (see Table 2). Of these, only one had QTc prolongation. This patient was taking azithromycin, a potent QTc prolonging agent. In this cohort, there were two known deaths related to aspiration pneumonia but no sudden deaths, though no systematic follow-up data on patient outcomes outside of the study were collected.
Table 2.
List of nine patients taking medications known to cause QTc prolongation at time of electrocardiogram generating QTc values.
| Patient | Potentially QTc prolonging medications | QTc (msec) |
|---|---|---|
| 1 | azithromycin | 473 |
| 2 | famotidine, erythromycin | 437 |
| 3 | escitalopram | 444 |
| 4 | famotidine | 396 |
| 5 | erythromycin | 409 |
| 6 | erythromycin | 409 |
| 7 | escitalopram | 438 |
| 8 | erythromycin | 423 |
| 9 | sertraline | 410 |
Discussion
We report a lower prevalence of prolonged QTc in RTT patients compared to other studies [Sekul et al., 1994; Guideri et al., 1999; Ellaway et al., 1999; Guideri et al., 2001; McCauley et al., 2011]. Of the seven patients with prolonged QTc, one had a family history of prolonged QTc (without identified etiology on genetic testing) and another was on a potentially QTc prolonging medication, leaving five with no known predisposition. The ECG measurements in our cohort are likely valid based on the following two lines of reasoning. First, all ECGs were performed on an outpatient basis, during periods of relative stability for the patients. Second, each QT interval was hand-measured twice by an experienced pediatric cardiologist. One possible explanation for the relatively lower prevalence of QTc prolongation in our cohort is the age distribution; there were only three individuals (all female) > 14 years old. Girls 14–18 years-old generally have greater QTc intervals compared to boys [Pearl, 1996], and hence this age group was underrepresented in our sample compared to other studies in RTT patients.
The second finding of this report is that there was a positive relationship between QTc and age, which reached statistical significance only in the medium severity group. Similar, statistically significant positive relationship was observed between QTc and clinical severity score among 7 to <13 year olds. The slight increase of QTc with age in our overall sample may parallel what occurs in the normative population. These findings are intriguing, but in light of the relatively fewer numbers of individuals in the other subgroups (particularly the mild clinical severity group and oldest age group as shown in Figures 2–3), this analysis in these groups may be underpowered and potentially more data points may be needed before a definite conclusion is drawn whether the positive linear relationships are limited to certain subgroups. In the literature, data is mixed regarding the association of prolonged QTc with age and clinical severity in RTT. In one study, patients with classic RTT had lower heart rate variability and longer QTc compared to patients with the preserved speech variant RTT [Guideri et al., 2001]. In two studies, QTc prolongation significantly correlated with advancing clinical stages of the syndrome [Sekul et al., 1994; Guideri et al., 1999], whereas another study found no correlation with either clinical stage or age [Ellaway et al., 1999]. Hence, there is need for a large scale prospective longitudinal cohort study to determine the precise relationship between QTc and age/severity.
The final finding of this report is that there was no genotype which predicted QTc prolongation. One explanation is that perhaps there is an association, but a larger number of individuals in each mutation are needed for an effect to become apparent. Another possibility is the presence of non-genetic/epigenetic factors (such as health status) modulating the QTc interval. Our study, though limited, is the first to demonstrate no relationship of specific mutations in MECP2 with QTc prolongation in RTT.
In RTT, the significance of QTc prolongation in relation to sudden death remains unknown, but one possible explanation pertains to induction of life threatening arrhythmias. Some investigators suggest that the sudden deaths in these patients may be due to long QTc related ventricular arrhythmias [Guideri and Acampa, 2005; McCauley et al., 2011]. However, the prevalence of such arrhythmias seems to be low [Acampa and Guideri, 2006]; in one study, out of 214 patients with RTT who underwent ECG screening, there was only one individual who had findings of ventricular tachycardia preceding sudden death [Guideri and Acampa, 2005]. Moreover, there is very little published data about sudden deaths in RTT patients, including information on related biochemical, electrophysiological, or pathological changes.
Though human data on QTc and sudden death in RTT is lacking, there is relevant animal model data that offer more insight. Mice with a Mecp2 deletion specific to the nervous system had an age-related increase in the QTc and had a high incidence of inducible ventricular arrhythmias during programmed electrical stimulation [McCauley et al., 2011]. Since the ventricular tachycardia induced in these mice by programmed stimulation was actually monomorphic and not polymorphic, the correlation with long QT, which is characterized by polymorphic torsade de pointes [Bhandari et al., 1985], is unclear, and actually suggests another mechanism of sudden death, such as alterations in sympathetic tone, known to exist in RTT [Weese-Mayer et al., 2008; Acampa et al., 2008; Rohdin et al., 2007; Guideri et al., 2001, 1999, 2005]. Sympathetic disturbances have also been associated with QTc prolongation in other disease states [Asai et al., 2007].
The mild degree of QTc prolongation in RTT patients seen by us and by other investigators [McCauley et al., 2011; Guideri et al., 1999, 2001; Ellaway et al., 1999; Sekul et al., 1994] does not support a direct causal relationship between prolonged QTc and sudden death in RTT. However, one can speculate that poor heart rate variability and autonomic dysfunction, combined with seizure activity possibly acutely prolonging the QTc interval, could create a “perfect storm” to increased susceptibility to ventricular arrhythmia and sudden cardiac death. In support of this notion is data that secondarily generalized tonic-clonic seizures, the predominant type of epilepsy in RTT syndrome, can lead to persistent tachycardia and abnormal QTc shortening [Surges et al., 2010]. Therapy to prevent sudden death in these patients thus may need to include management of issues other than the QTc, especially in the area of autonomic dysfunction. The findings of an antiarrhythmic effect of phenytoin but not propranolol in the RTT mouse model [McCauley et al., 2011; Herrera et al., 2015] and that chronic oral acetyl-L carnitine administration in RTT patients can improve heart rate variability [Guideri et al., 2005] are noteworthy. Moreover, according to a retrospective analysis, RTT patients treated with anticonvulsants which block Na+ channels demonstrated improvements in QT interval [Herrera et al., 2015].
In terms of clinical surveillance, screening for prolonged QTc with ECG should be part of the initial evaluations after a diagnosis of RTT [Christodoulou and Ho, 1993]. We recommend a detailed family history in all RTT patients with observed QTc prolongation to exclude a possible inherited cause, along with a thorough review of the medication history for medications that may prolong the QTc. A recent study revealed that one third of pediatric patients presenting to the emergency department with syncope/presyncope showed borderline QTc prolongation (QTc ≥ 440 msec) which tends to normalize in subsequent ECGs [Dorn et al., 2011]. Hence, a follow up evaluation is recommended for all patients with QTc prolongation seen on first-time ECG, especially if the initial ECG was obtained during an acute illness. For persistent QTc prolongation, possible treatment options include beta-blockers, but but this should be reserved for patients who are determined by a pediatric cardiologist to have long QT, and the best treatment approach is routine follow up and avoidance of QTc-prolonging medications. In RTT, if the first-time ECG does not show QTc prolongation, then periodic (e.g., yearly) ECG has been a recommendation [Christodoulou and Ho, 1993]. However, the utility of annual ECGs in RTT patients with normal baseline QTc has not been established, and may not be warranted, particularly in younger patients. Surveillance with 24-hour ECG monitoring or implantable loop recorders could be useful as research tools to enhance our understanding of QTc prolongation and arrhythmia risk in RTT.
The main limitation of our study is the cross-sectional design and a lack of longitudinal follow-up data in the study patients. Not all of the 100 patients evaluated in this study have been followed longitudinally at our institution, so it is possible that cardiac-related deaths may have occurred of which we are unaware. The second limitation lies within the subgroup analyses: though the findings reached statistical significance in some groups, we may have had low power to detect similar associations in the other groups, especially most severe and older patients, due to small sample size.
Conclusions
Our findings demonstrate that in RTT, the prevalence of QTc prolongation is lower than previously reported. There is a positive relationship between QTc with age and clinical severity in some subgroups, but larger sample sizes are needed to determine whether this association is consistent in other subgroups as well. In our sample, there was no association between QTc and genotype. Until the mechanisms of sudden death and the basis for the QTc prolongation in a few RTT subjects are better understood, caution is advised when attempting to predict risk of sudden death in individuals with MECP2 mutations based on minimally increased QTc interval.
Supplementary Material
Acknowledgments
We would like to thank the families for their participation. We would also like to thank the following for their help with the paper: Sankar R. Chirumamilla, Brandon M. Metcalf, Charles A. Rohde, and Mary Beth E. Yablonski. Dr. Naidu was supported by an NIH grant, PO1 HDO24448. Dr. Yenokyan and Ms. Sanyal are supported by a grant 1U54HD079123-01A1, Intellectual and Development Disabilities Centers 2014. Dr. Srivastava is supported by an NIH grant, 4T32GM007748-38.
Footnotes
Disclosures
The authors have no conflicts of interest.
References
- Acampa M, Guideri F. Cardiac disease and Rett syndrome. Arch Dis Child. 2006;91:440–443. doi: 10.1136/adc.2005.090290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acampa M, Guideri F, Hayek J, Blardi P, De Lalla A, Zappella M, Auteri A. Sympathetic overactivity and plasma leptin levels in Rett syndrome. Neurosci Lett. 2008;432:69–72. doi: 10.1016/j.neulet.2007.12.030. [DOI] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Asai H, Hirano M, Udaka F, Shimada K, Oda M, Kubori T, Nishinaka K, Tsujimura T, Izumi Y, Konishi N, Matsumoto S, Kameyama M, Ueno S. Sympathetic disturbances increase risk of sudden cardiac arrest in sporadic ALS. J Neurol Sci. 2007;254:78–83. doi: 10.1016/j.jns.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Bhandari AK, Shapiro WA, Morady F, Shen EN, Mason J, Scheinman MM. Electrophysiologic testing in patients with the long QT syndrome. Circulation. 1985;71:63–71. doi: 10.1161/01.cir.71.1.63. [DOI] [PubMed] [Google Scholar]
- Byard RW. Forensic issues and possible mechanisms of sudden death in Rett syndrome. J Clin Forensic Med. 2006;13:96–99. doi: 10.1016/j.jcfm.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Christodoulou J, Ho G. MECP2-Related Disorders. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJ, Bird TD, Fong C-T, Mefford HC, Smith RJ, Stephens K, editors. GeneReviews(®) Seattle (WA): University of Washington, Seattle; 1993. [Google Scholar]
- De Felice C, Maffei S, Signorini C, Leoncini S, Lunghetti S, Valacchi G, D’Esposito M, Filosa S, Della Ragione F, Butera G, Favilli R, Ciccoli L, Hayek J. Subclinical myocardial dysfunction in Rett syndrome. Eur Heart J Cardiovasc Imaging. 2012;13:339–345. doi: 10.1093/ejechocard/jer256. [DOI] [PubMed] [Google Scholar]
- Dorn CSV, Johnson JN, Taggart NW, Thorkelson L, Ackerman MJ. QTc Values Among Children and Adolescents Presenting to the Emergency Department. Pediatrics. 2011;128:e1395–e1401. doi: 10.1542/peds.2010-1513. [DOI] [PubMed] [Google Scholar]
- Dotti MT, Guideri F, Acampa M, Orrico A, Battisti C, Federico A. Autonomic dysfunction in mental retardation and spastic paraparesis with MECP2 mutation. J Child Neurol. 2004;19:964–966. doi: 10.1177/08830738040190121001. [DOI] [PubMed] [Google Scholar]
- Driscoll DJ, Edwards WD. Sudden unexpected death in children and adolescents. J Am Coll Cardiol. 1985;5:118B–121B. doi: 10.1016/s0735-1097(85)80540-4. [DOI] [PubMed] [Google Scholar]
- Ellaway CJ, Sholler G, Leonard H, Christodoulou J. Prolonged QT interval in Rett syndrome. Arch Dis Child. 1999;80:470–472. doi: 10.1136/adc.80.5.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guideri F, Acampa M. Sudden death and cardiac arrhythmias in Rett syndrome. Pediatr Cardiol. 2005;26:111. doi: 10.1007/s00246-004-0701-x. [DOI] [PubMed] [Google Scholar]
- Guideri F, Acampa M, DiPerri T, Zappella M, Hayek Y. Progressive cardiac dysautonomia observed in patients affected by classic Rett syndrome and not in the preserved speech variant. J Child Neurol. 2001;16:370–373. doi: 10.1177/088307380101600512. [DOI] [PubMed] [Google Scholar]
- Guideri F, Acampa M, Hayek G, Zappella M, Di Perri T. Reduced heart rate variability in patients affected with Rett syndrome. A possible explanation for sudden death. Neuropediatrics. 1999;30:146–148. doi: 10.1055/s-2007-973480. [DOI] [PubMed] [Google Scholar]
- Guideri F, Acampa M, Hayek Y, Zappella M. Effects of acetyl-L-carnitine on cardiac dysautonomia in Rett syndrome: prevention of sudden death? Pediatr Cardiol. 2005;26:574–577. doi: 10.1007/s00246-005-0784-z. [DOI] [PubMed] [Google Scholar]
- Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: report of 35 cases. Ann Neurol. 1983;14:471–479. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- Hagberg B, Witt-Engerström I. Rett syndrome: a suggested staging system for describing impairment profile with increasing age towards adolescence. Am J Med Genet Suppl. 1986;1:47–59. doi: 10.1002/ajmg.1320250506. [DOI] [PubMed] [Google Scholar]
- Herrera JA, Ward CS, Pitcher MR, Percy AK, Skinner S, Kaufmann WE, Glaze DG, Wehrens XHT, Neul JL. Treatment of cardiac arrhythmias in a mouse model of Rett syndrome with Na+-channel-blocking antiepileptic drugs. Dis Model Mech. 2015;8:363–371. doi: 10.1242/dmm.020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffbuhr K, Devaney JM, LaFleur B, Sirianni N, Scacheri C, Giron J, Schuette J, Innis J, Marino M, Philippart M, Narayanan V, Umansky R, Kronn D, Hoffman EP, Naidu S. MeCP2 mutations in children with and without the phenotype of Rett syndrome. Neurology. 2001;56:1486–1495. doi: 10.1212/wnl.56.11.1486. [DOI] [PubMed] [Google Scholar]
- Kerr AM, Armstrong DD, Prescott RJ, Doyle D, Kearney DL. Rett syndrome: analysis of deaths in the British survey. Eur Child Adolesc Psychiatry. 1997;6(Suppl 1):71–74. [PubMed] [Google Scholar]
- Madan N, Levine M, Pourmoghadam K, Sokoloski M. Severe sinus bradycardia in a patient with Rett syndrome: a new cause for a pause? Pediatr Cardiol. 2004;25:53–55. doi: 10.1007/s00246-003-0341-6. [DOI] [PubMed] [Google Scholar]
- McCauley MD, Wang T, Mike E, Herrera J, Beavers DL, Huang T-W, Ward CS, Skinner S, Percy AK, Glaze DG, Wehrens XHT, Neul JL. Pathogenesis of lethal cardiac arrhythmias in Mecp2 mutant mice: implication for therapy in Rett syndrome. Sci Transl Med. 2011;3:113ra125. doi: 10.1126/scitranslmed.3002982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl W. Effects of gender, age, and heart rate on QT intervals in children. Pediatr Cardiol. 1996;17:135–136. doi: 10.1007/BF02505201. [DOI] [PubMed] [Google Scholar]
- Rohdin M, Fernell E, Eriksson M, Albåge M, Lagercrantz H, Katz-Salamon M. Disturbances in cardiorespiratory function during day and night in Rett syndrome. Pediatr Neurol. 2007;37:338–344. doi: 10.1016/j.pediatrneurol.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Schüle B, Armstrong DD, Vogel H, Oviedo A, Francke U. Severe congenital encephalopathy caused by MECP2 null mutations in males: central hypoxia and reduced neuronal dendritic structure. Clin Genet. 2008;74:116–126. doi: 10.1111/j.1399-0004.2008.01005.x. [DOI] [PubMed] [Google Scholar]
- Sekul EA, Moak JP, Schultz RJ, Glaze DG, Dunn JK, Percy AK. Electrocardiographic findings in Rett syndrome: an explanation for sudden death? J Pediatr. 1994;125:80–82. doi: 10.1016/s0022-3476(94)70128-8. [DOI] [PubMed] [Google Scholar]
- Surges R, Scott CA, Walker MC. Enhanced QT shortening and persistent tachycardia after generalized seizures. Neurology. 2010;74:421–426. doi: 10.1212/WNL.0b013e3181ccc706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GH. High male:female ratio of germ-line mutations: an alternative explanation for postulated gestational lethality in males in X-linked dominant disorders. Am J Hum Genet. 1996;58:1364–1368. [PMC free article] [PubMed] [Google Scholar]
- Trevathan E, Naidu S. The clinical recognition and differential diagnosis of Rett syndrome. J Child Neurol. 1988;3(Suppl):S6–16. doi: 10.1177/0883073888003001s03. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, Ramirez JM. Autonomic dysregulation in young girls with Rett Syndrome during nighttime in-home recordings. Pediatr Pulmonol. 2008;43:1045–1060. doi: 10.1002/ppul.20866. [DOI] [PubMed] [Google Scholar]
- Zhang J, Bao X, Cao G, Jiang S, Zhu X, Lu H, Jia L, Pan H, Fehr S, Davis M, Leonard H, Ravine D, Wu X. What does the nature of the MECP2 mutation tell us about parental origin and recurrence risk in Rett syndrome? Clin Genet. 2012;82:526–533. doi: 10.1111/j.1399-0004.2011.01838.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.