Abstract
Bile duct obstruction is a potent stimulus for cholangiocyte proliferation, especially for large cholangiocytes. Our previous studies reported that conjugated bile acids (CBAs) activate the AKT and ERK1/2 signaling pathways via the sphingosine 1-phosphate receptor 2 (S1PR2) in hepatocytes and cholangiocarcinoma cells. It also has been reported that taurocholate (TCA) promotes large cholangiocyte proliferation and protects cholangiocytes from bile duct ligation (BDL)-induced apoptosis. However, the role of S1PR2 in bile acid-mediated cholangiocyte proliferation and cholestatic liver injury has not been elucidated. Here we report that S1PR2 is the predominant S1PR expressed in cholangiocytes. Both TCA- and S1P-induced activation of ERK1/2 and AKT were inhibited by JTE-013, a specific antagonist of S1PR2, in cholangiocytes. In addition, TCA- and S1P-induced cell proliferation and migration were inhibited by JTE-013 and a specific shRNA of S1PR2 as well as chemical inhibitors of ERK1/2 and AKT in mouse cholangiocytes. In BDL mice, the expression of S1PR2 was upregulated in whole liver and cholangiocytes. S1PR2 deficiency significantly reduced BDL-induced cholangiocyte proliferation and cholestatic injury as indicated by significant reduction of inflammation and liver fibrosis in S1PR2−/− mice. Treatment of BDL mice with JTE-013 significantly reduced total bile acid levels in the serum and cholestatic liver injury. This study suggests that the CBA-induced activation of S1PR2-mediated signaling pathways plays a critical role in obstructive cholestasis and may represent a novel therapeutic target for cholestatic liver diseases.
Keywords: Cholangiocytes, Bile acids, Sphingosine 1-phosphate receptor, Cholestasis, bile duct ligation
INTRODUCTION
Cholestasis represents the main clinical feature of cholangiopathies (1). Impaired bile formation or bile flow results in hepatic injury, fibrosis and eventually cirrhosis. Liver transplantation is the only therapy for patients with end-stage cholestatic diseases (2). Although tremendous efforts have been put into studying the pathophysiology of cholestatic disease progression over the past two decades, the underlying cellular/molecular mechanisms remain largely unknown.
Cholangiocytes are the major target cells involved in cholestatic liver diseases. Previous studies suggest that cholangiocyte proliferation may be linked to hepatic fibrosis (3). There is growing evidence to indicate that cholangiocyte proliferation is regulated by multiple biological mediators including neuropeptides, neurotransmitters and hormones (4). Bile duct obstruction (BDO) is a potent stimulus for cholangiocyte proliferation, especially for large cholangiocytes (5). It is well-known that bile acids are important signaling molecules, which play a critical role in regulating hepatic metabolic pathways under physiological and pathological conditions (6). Bile acids not only activate multiple protein kinase signaling pathways, such as PKA/C and MAPKs, but also activate several nuclear receptors [farnesoid X receptor (FXR), pregnane x receptor (PXR), vitamin D receptor] and G protein coupled receptors (GPCR), such as TGR5 and sphingosine 1-phosphate receptor 2 (S1PR2) (7, 8). However, we do not have a complete understanding of how bile acid signals are integrated to regulate key events involved in cholangiocyte proliferation under cholestatic conditions.
S1P is an important bioactive lipid molecule involved in the regulation of fundamental cellular responses (9). S1P can directly act as an intracellular signaling molecule or function as the natural ligand of five different GPCRs (S1PR1-5)(10). S1P and S1PRs have been shown to have differential roles in the regulation of cell proliferation, migration and survival in different tissues and cells (11–14). It has been reported that S1P/S1PR3-signaling is involved in cholestasis-induced liver fibrosis (15) and S1P mediates bone marrow-derived macrophage migration via activation of S1PR2 and S1PR3 in the BDL mouse model (16). In addition, antagonism of S1PR2 selectively reduces portal vein pressure in BDL rodents (17). Our previous studies showed that S1PR2 is highly expressed in the liver followed by S1PR1 and S1PR3 and the expression levels of S1PR4 and S1PR5 are too low to be detected. We also discovered that CBAs activated the ERK1/2 and AKT signaling pathways via S1PR2 in rodent hepatocytes (18). Our recent studies further identified a critical role of S1PR2 in regulating sphingosine kinase 2 (SphK2) expression and expression of multiple hepatic genes (19). Furthermore, we also showed that S1PR2 is responsible for CBA-mediated invasive growth, bile duct proliferation and upregulation of cyclooxygenase 2 (COX-2) in cholangiocarcinoma cells (20, 21).
In the current study, we report that activation of S1PR2 plays an essential role in CBA-induced cholangiocyte proliferation under cholestatic conditions. Inhibition of S1PR2 activation using a specific chemical antagonist or knock down of S1PR2 expression using a gene-specific shRNA significantly inhibited TCA-induced cholangiocyte proliferation and migration. BDL-induced cholestatic liver injury was markedly reduced in S1PR2−/− mice. The chemical antagonist of S1PR2, JTE-013, also showed protective effect against BDL-induced liver injury. Our study suggests that targeting of CBA/S1PR2-mediated signaling pathways may have therapeutic potential for a subset of cholestatic liver diseases that currently lack effective therapies.
MATERIALS AND METHODS
Materials
S1P and JTE-013 were purchased from Cayman Chemical (Boston, MA). The Bio-Rad protein assay reagent, Precision Plus Protein Kaleidoscope Standards, and iQ™ SYBR Green Supermix were obtained from Bio-Rad (Hercules, CA). IRDye secondary antibody was from LI-COR (Lincoln, NE). FuGene HD transfection Reagent was from Promega (Madison, WI). Taurocholate (TCA) and other chemicals were from Sigma-Aldrich (St. Louis, MO).
Cell culture
The immortalized normal mouse small cholangiocytes (MSE) and large cholangiocytes (MLE) were kind gifts from Dr. Gianfranco Alpini (Texas A&M Health Science Center) and were cultured in minimal essential medium containing 10% fetal bovine serum (FBS), penicillin G (100 U/mL) and streptomycin (100 μg/mL) at 37°C with 5% CO2 in a humidified cell culture incubator. Mouse primary cholangiocytes were isolated by an immunoaffinity method using a rat monoclonal antibody against Ep-CAM (from DSHB, University of Iowa), an antigen expressed by all mouse cholangiocytes as described previously (22).
Animals
All experiments and procedures involving mice were approved by the institutional animal care and use committee of Virginia Commonwealth University and were conducted in accord with the Declaration of Helsinki, The Guide for the Care and Use of Animals (National Academies Press, Washington, DC, 1996), and all applicable regulations. S1PR2−/− mice were a kind gift from Dr. R. Proia (NIDDK). They have been well-characterized and used in a number of studies (19). All mice were bred in pathogen-free conditions with normal lighting and wild type and knockout mice were from the same litters. Both male and female mice (8–12 weeks) were used.
Bile duct ligation (BDL) mouse model
The standard partial common bile duct ligation was performed as we described previously (23). Briefly, cystic duct and the common bile duct were ligated using 7-0 nylon. Sham mice underwent similar laparotomy without BDL. At the end, the abdomen was closed by a 6-0 double-layer suture, and the mice were allowed to wake up on a heating pad. After 3 or 14 days, serum and bile were collected and liver tissues were harvested for isolation of primary cholangiocytes or processed for quantitative real-time RT-PCR, Western blot analysis, histology and immunohistochemistry analysis.
Cell invasion assays
MLE cells were seeded in the Matrigel-coated upper chamber. Cells were pretreated with JTE-013 (10 μM) or vehicle DMSO for 1 h, then treated with TCA (100 μM), S1P (100 nM), or vehicle DMSO and incubated at 37ºC for 24 h. The migrated cells were stained and analyzed as described previously (21).
Measurement of total bile acids
Mouse serum was collected at the end of the in vivo study. Liver tissues were homogenized in RIPA buffer. The amount of total bile acids was measured by using the total bile acids assay kit (Crystal Chem Inc., Downers Grove, IL) according to the manufacturer’s instructions. Total bile acids content in liver tissues was normalized with protein concentration and expressed as μmol/mg protein.
Measurement of liver functional enzyme activities
The activities of the plasma aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) were measured using assay kits from Sigma (St. Louis, MO).
Measurement of hepatic hydroxyproline
Liver hydroxyproline content was measured using the assay kit from Sigma (St. Louis, MO). The amount of hydroxyproline was measured according to the manufacturer’s instructions. The result was expressed as total hydroxyproline (μg) per mg liver sample.
Histopathology analysis and Immunohistochemistry
Liver tissue sections were collected and fixed in 4% paraformaldehyde in 0.1 M of PBS at room temperature overnight. Regions of specimens were standardized for all mice. The paraffin-embedded liver tissue was sectioned (≤5μm), and hematoxylin and eosin (H&E) and Masson’s trichrome staining were done by the histopathology core lab of Medical College Virginia Hospital. The expression of CK-19 was detected by immunohistochemistry staining as described previously (21).
Statistical analysis of data
All the experiments were repeated at least three times and the results were expressed as mean ± SE. One-way analysis of variance (ANOVA) was employed to analyze the differences between sets of data using GraphPad Prism (Graph-Pad, San Diego, CA). A value of P < 0.05 was considered statistically significant (19).
Results
S1PR2 is the predominant S1PR expressed in cholangiocytes and upregulated by BDL
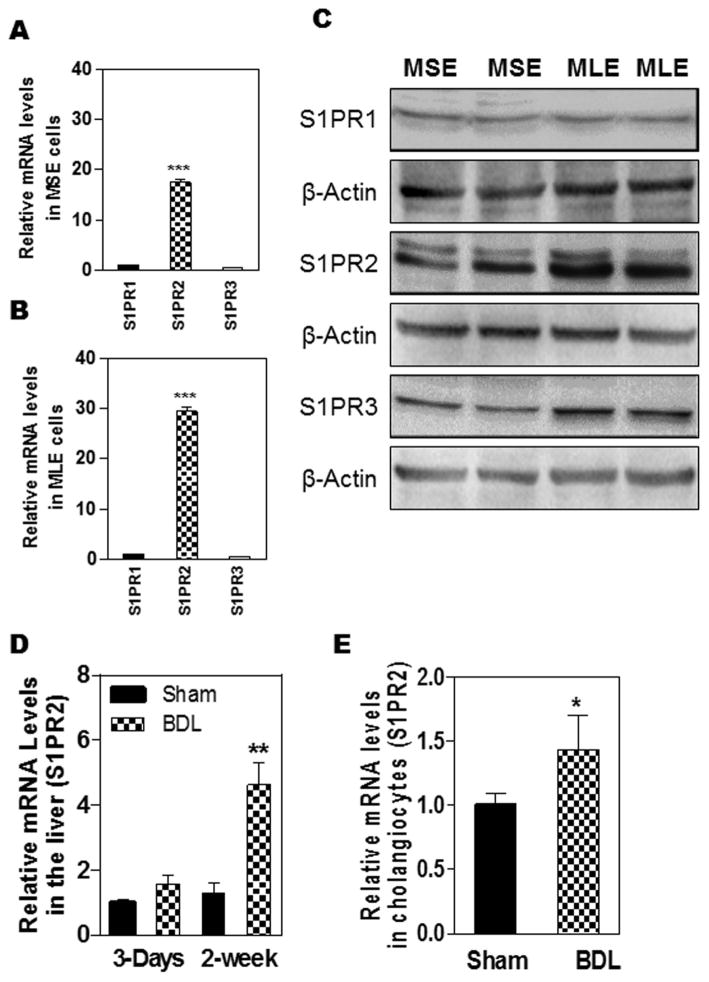
We have previously reported that S1PR2 is the predominant S1PR in hepatocytes and cholangiocarcinoma cells (18). CBA-induced activation of S1PR2 plays an important role in regulating hepatic lipid metabolism (19). Previous studies reported that taurocholic acid (TCA) protects cholangiocyte apoptosis and promotes large cholangiocyte proliferation (5, 24–26). To determine whether S1PR2 is involved in TCA-induced cholangiocyte proliferation, we first examined the expression levels of different S1PRs in normal mouse cholangiocytes. As shown in Fig. 1A and B, S1PR2 is the predominant S1PR in both mouse small cholangiocytes (MSE) and mouse large cholangiocytes (MLE). The expression level of S1PR2 in MLE is higher than that in MSE. Consistent with real-time RT-PCR results, Western blot analysis indicated that S1PR2 is the major S1PR in cholangiocytes (Fig. 1C). More interestingly, 3-day BDL had no significant effect on total hepatic S1PR2 expression, but significantly increased S1PR2 expression in mouse primary cholangiocytes (Fig. 1D and E). Furthermore, 2-week BDL not only markedly increased S1PR2 mRNA level, but also increased S1PR2 protein level in the liver (Fig. 1D and supplementary Fig. 1).
Figure 1. Expression of S1PR2 in mouse cholangiocytes and liver under cholestasis conditions.
(A–C): Normal mouse small cholangiocytes (MSE) and mouse large cholangiocytes (MLE) in culture were used to isolate total cellular RNA and total protein. (D). C57/BL6 wild type mice were subjected to sham operation or BDL for 3-days or two-weeks. Total RNA was isolated. (E). Mouse primary cholangiocytes were isolated from sham control or BDL mice (3-day). The mRNA levels of S1PR1, S1PR2 and S1PR3 were detected by real-time RT-PCR and normalized using GAPDH. The protein levels of S1P1, S1PR2 and S1PR3 were detected by Western Blot analysis. A and B: ***p<0.01, statistical significance relative to S1PR1, n=3; C: The representative images of the immunoblots for S1PR1-3 are shown. β-actin was used as a loading control. D and E: *p<0.05, **p<0.01, statistical significance relative to sham control, n=3–5.
The role of TCA-induced activation of S1PR2 in promoting cholangiocyte proliferation
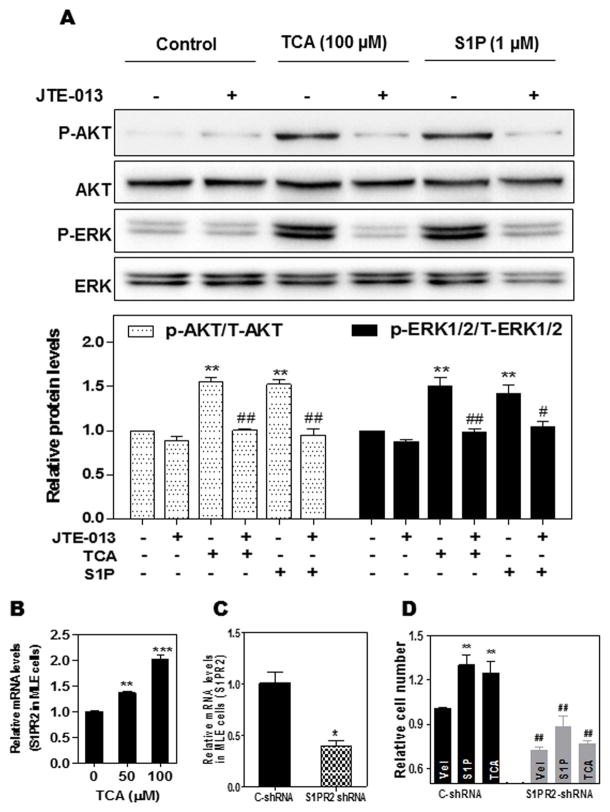
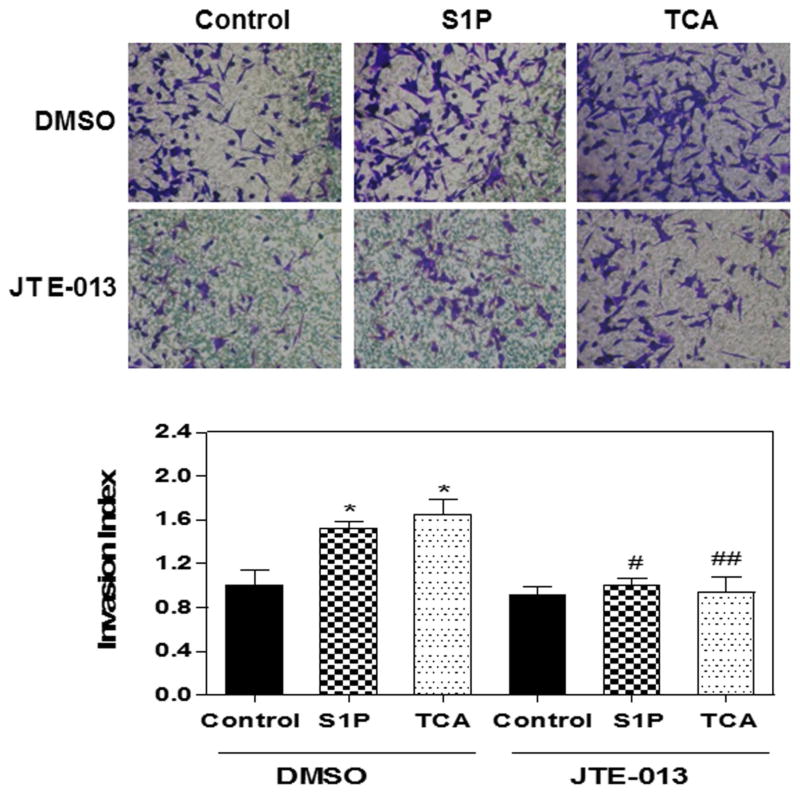
BDO is a strong stimulus that promotes cholangiocyte proliferation and represents one of the major causes of cholestatic liver diseases. It has been long believed that direct toxicity of bile acids causes cholestatic liver injury. However, recent study showed that the concentrations of unconjugated cytotoxic bile acids are too low to induce hepatocellular injury in BDL mice (27). TCA is the major bile acid identified in mouse serum and liver after BDL (27). These studies indicated that TCA may play a critical role in BDL-induced cholestatic liver injury. In order to determine the mechanism underlying TCA-induced cholestatic liver injury, we first examined whether TCA can activate S1PR2 and further induce activation of downstream ERK1/2 and AKT signaling pathways in MLE cells. As shown in Fig. 2A, both TCA and S1P significantly induced p-AKT and p-ERK1/2 phosphorylation, which were inhibited by the S1PR2 antagonist JTE-013. Real-time PCR analysis further showed that TCA dose-dependently increased S1PR2 mRNA expression levels in MLE (Fig. 2B). We have previously reported that TCA-induced S1PR2 activation contributes to invasive growth of cholangiocarcinoma cells (21). We further examined if TCA- and S1P-induced activation of S1PR2 is also involved in normal cholangiocyte proliferation. As shown in Fig. 2C and D, down-regulation of S1PR2 using a specific lentiviral shRNA of S1PR2, completely blocked TCA- and S1P-induced cell proliferation in MLE. In order to determine whether TCA- and S1P-induced activation of p-AKT and p-ERK1/2 signaling pathways is involved in cell proliferation, specific chemical inhibitors of ERK1/2 (U0126) and AKT (MK2206) were used. As shown in online supplementary Fig. 2, both TCA- and S1P-induced cell proliferation was blocked by U0126 or MK2206 in MLE cells. Similar to the findings in cholangiocarcinoma cells, both TCA- and S1P-induced cell migration and invasion were inhibited by down-regulation of S1PR2 expression using a specific shRNA (Supplementary Fig. 3) or inhibition of S1PR2 activation using JTE-013 (Fig. 3).
Figure 2. The role of S1PR2 in TCA/S1P-induced AKT and ERK1/2 activation and cholangiocyte proliferation.
(A). MLE were pretreated with JTE-013 (10 μM) for 30 min, then treated with vehicle control (DMSO), TCA (100 μM) or S1P (1 μM) for 30 minutes. The protein levels of phosphorylated AKT (p-AKT), total AKT, p-ERK1/2 and T-ERK1/2 were determined by Western Blot analysis. The representative images of the immunoblots for p-AKT, T-AKT, p-ERK and T-ERK are shown. The relative density of p-ERK/T-ERK and p-AKT/T-AKT were determined by Image J software. **p<0.01, statistical significance relative to control; ##p<0.01, statistical significance relative to the corresponding treatment group with and without JTE-013 (n=3). (B). MLE cells were treated with different amounts of TCA (0, 50, and 100 μM) for 24 h. The mRNA levels of S1PR2 were detected by real-time RT-PCR and normalized with GAPDH as an internal control. The relative amount of S1PR2 mRNA is shown. (C). MLE cells were transduced with purified lentiviral control shRNA (C-shRNA) or S1PR2 shRNA for 48 h. The S1PR2 mRNA was measured by real-time RT-PCR and normalized with GAPDH as an internal control. (D). MLE cells were transduced with lentiviral C-shRNA or S1PR2 shRNA for 36 h, then treated with vehicle control, TCA (100 μM) or S1P (1 μM) for 48 h. Viable cells were analyzed using a Cellometer Vision CBA automatic cell counter. Relative cell number, compared to vehicle control group, is shown. Values represent the mean ± S.E. of three independent experiments. Statistical significance relative to the vehicle control group (designated=1): *p<0.05, **p<0.01, ***p<0.001; Statistical significance relative to the C-shRNA group with the same treatment group, ##p<0.01.
Figure 3. Effect of JTE-013 on TCA- and S1P-induced cell invasion in MLE Cells.
MLE cells were plated in the upper transwell inserts. Cells were pretreated with JTE-013 (10 μM) for 30min then treated with TCA (100 μM) or S1P (100 nM) or vehicle control (DMSO) for 24h. At the end of treatment, the number of invasion cells on the lower surface of inserts and the invasion index were analyzed as described in Methods. (A) Representative images for each treatment are shown. (B) Invasion index. *p<0.05 compared to vehicle control; #p<0.05, ##p<0.01 compared to the same treatment group without JTE-013; n=3.
S1PR2−/− mice have lower levels of bile acids in serum and liver after BDL
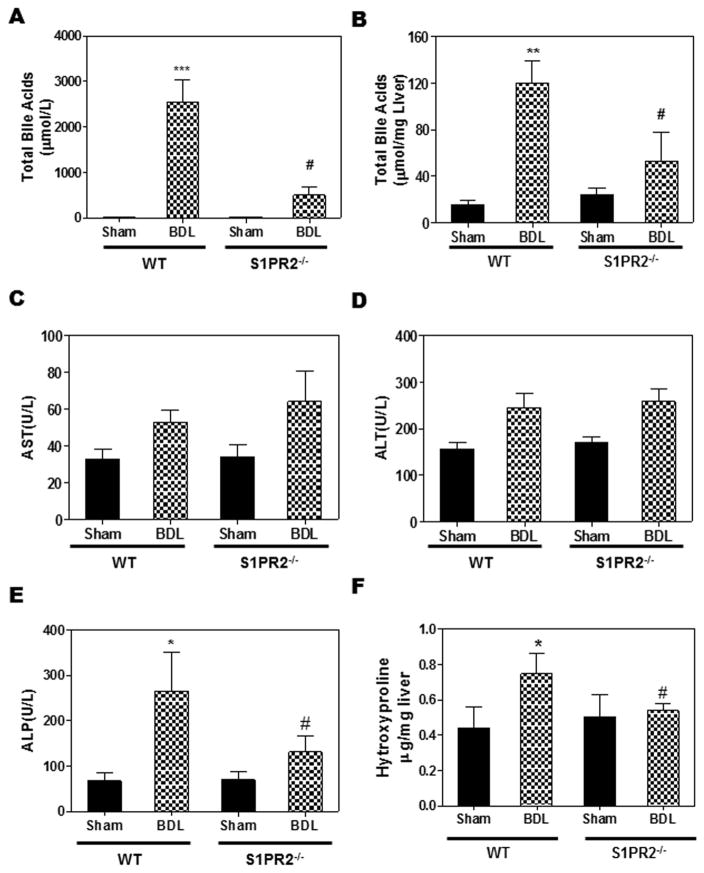
Previous studies have shown that BDL markedly increased the bile acid levels in serum and liver (27). In BDL mice, the S1PR2 expression levels in liver and cholangiocytes were significantly increased (Fig.1). Next, we wanted to determine if there was any link between S1PR2 and bile acid levels after BDL. Both wild type and S1PR2−/− mice were subjected to a sham operation or BDL. After two weeks, serum and livers were harvested and total bile acid levels were measured using a commercial kit. As shown in Fig. 4A, BDL dramatically increased serum total bile acid levels as compared to sham control wild type mice. However, in S1PR2−/− mice, total bile acid levels in serum were significantly reduced compared to wild type mice after BDL. Similarly, BDL-induced increase of hepatic bile acid levels was significantly reduced in S1PR2−/− mice (Fig. 4B).
Figure 4. The role of S1PR2 in BDL-induced increase of bile acid levels and fibrotic liver injury.
Wild type and S1PR2−/− mice were subjected to 2-week BDL or sham operation as described in “Methods”. The bile acids, AST, ALT, ALP and hydroxyproline levels in serum and liver were measured using commercial kits according to the manufacturer’s instructions. (A) Total bile acids in serum. (B) Total bile acids in liver, normalized with liver weight and expressed as μM/mg. (C) AST activity in serum. (D) ALT activity in Serum. (E) ALP activity in serum. (F) Hydroxyproline in liver. Statistical significance relative to sham control, *p<0.05, **p<0.01, ***p<0.001, n=5–7; Statistical significance relative to WT BDL, *p<0.05, n=5–7.
S1PR2−/− mice were protected from cholestasis-induced liver injury
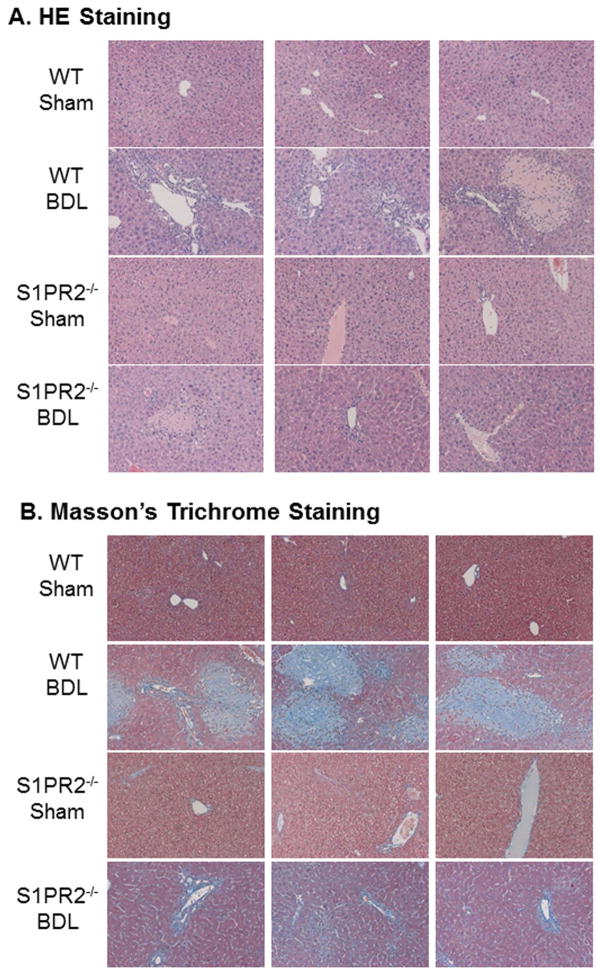
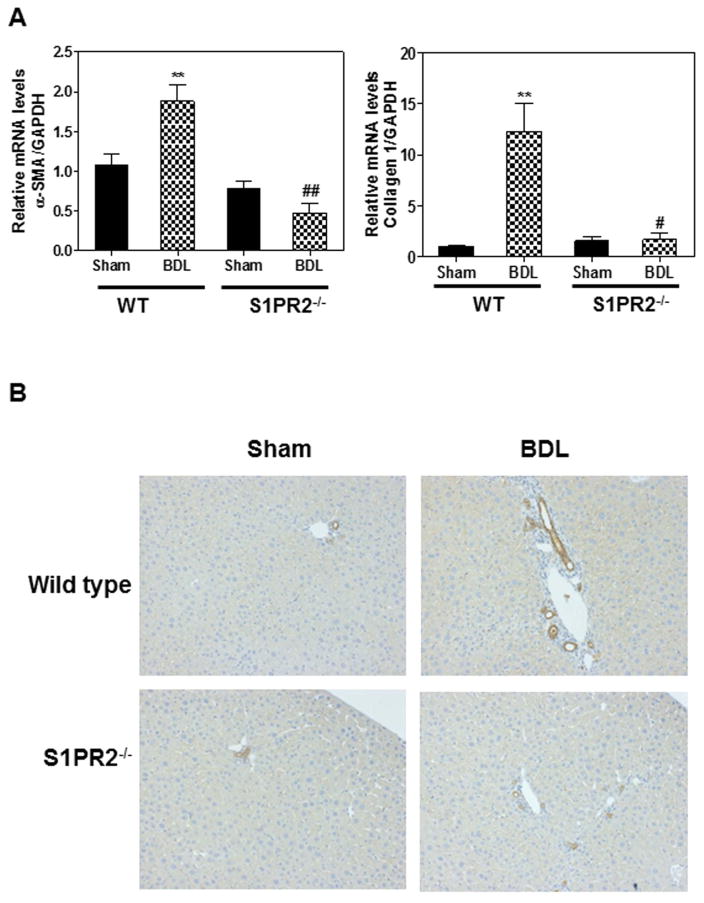
Cholestatic liver injury is mainly induced by CBAs in the BDL mouse model (27). S1PR2 deficiency prevented bile acid accumulation in the liver and serum after a two-week BDL. In order to further determine whether S1PR2−/− mice were protected from cholestasis-induced liver injury, we measured the liver functional enzyme activities. As shown in Fig. 4C–D, AST and ALT levels were not changed significantly by BDL in either wild type or S1PR2−/− mice. However, the ALP activity was significantly increased after a two-week BDL in wild type mice. S1PR2 deficiency markedly reduced BDL-induced increase of ALP (Fig. 4E). In addition, liver weight and spleen weight were increased by BDL, but not significantly different between wild type and S1PR2−/− mice (Supplementary Fig.4). To examine the role of S1PR2 in cholestasis-induced liver fibrosis, collagen content was determined by measurement of hydroxyproline content in livers. As shown in Fig.4F, the hepatic hydroxyproline level was increased in wild type mice after BDL, but not in S1PR2−/− mice, indicating that S1PR2 deficiency prevented BDL-induced liver fibrosis. To confirm this observation, we examined liver histology using H&E staining and Masson’s Trichrome staining. As shown in Fig. 5 and Supplementary Fig.5–6, two-week BDL significantly induced hepatic injury and liver fibrosis in wild type mice, but had less impact in S1PR2−/− mice. Consistently, real-time RT-PCR results showed that BDL significantly increased mRNA expression levels of collagen type I and α-smooth actin (α-SMA) in the liver of wild type mice, but not in that of S1PR2−/− mice (Fig. 6A). Similarly, both collagen 1 and α-SMA mRNA levels were significantly increased in primary mouse cholangiocytes isolated from wild type BDL mice, not from S1PR2−/− BDL mice (Supplementary Fig. 7). To further examine the effect of BDL on cholangiocyte proliferation, the intrahepatic bile duct mass was evaluated by CK-19 immunohistochemistry in paraffin-embedded liver sections. As shown in Fig. 6B, BDL-induced cholangiocyte proliferation was significantly reduced in S1PR2−/− mice.
Figure 5. The role of S1PR2 in BDL-induced cholestatic liver injury.
Wild type and S1PR2−/− mice were subjected to 2-week BDL or sham operation as described in “Methods”. The liver sections were stained with H&E or Masson’s Trichrome. The images were taken with an Olympus microscope equipped with an image recorder using a 200 × lens. Representative images are shown. (A). H&E staining; (B) Masson’s Trichrome staining.
Figure 6. The role of S1PR2 in BDL-induced cholangiocyte proliferation and fibrosis.
Wild type and S1PR2−/− mice were subjected to 2-week BDL or sham operation as described in “Methods”. (A) The mRNA levels of α-SMA and collagen type I were determined by real-time RT-PCR and normalized with GAPDH as an internal control. Statistical significance relative to sham control, **p<0.01, n=5–7; Statistical significance relative to WT BDL, #p<0.05, ##p<0.01, n=5–7. (B) Representative images of immunohistochemistry staining of CK-19.
In order to test whether inhibition of S1PR2 activation using JTE-013 protected mice from BDL-induced cholestatic liver injury, wild type sham-operated or BDL mice were treated with JTE-013 or vehicle control via ip injection for two weeks. As shown in Supplementary Fig. 8, JTE-013 significantly reduced BDL-induced increase of ALT, ALP and total bile acid levels in the serum. In addition, JTE-013 also reduced BDL-induced CK-19, Ki67 and collagen I expression (Supplementary Fig.9).
S1PR2−/− mice were protected from cholestasis-induced inflammation
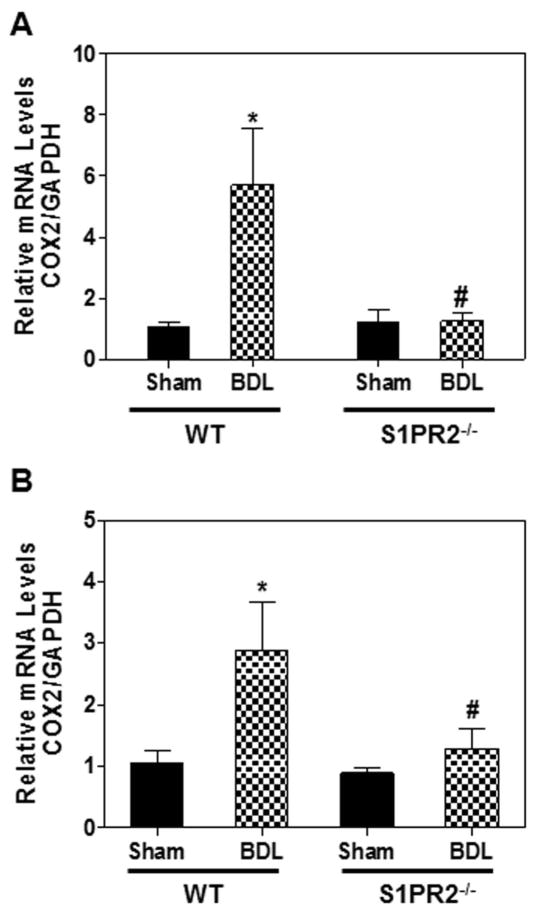
Inflammation plays a more important role than the direct cytotoxic effects of bile acids on hepatocytes in BDL-induced cholestatic liver injury (27). BDL dramatically increased bile acid levels in liver and serum (Fig. 4A & B). Our recent studies reported that TCA activated the ERK1/2-NF-κB-COX-2 signaling pathway via S1PR2 in human cholangiocarcinoma cells (20). To identify the potential mechanisms underlying CBA-mediated cholangiocyte proliferation under cholestasis conditions, we examined the COX-2 expression levels in the liver and primary cholangiocytes after BDL. As shown in Fig. 7, BDL significantly upregulated COX-2 expression in the liver and cholangiocytes of the wild type mice, but not in those of S1PR2−/− mice. In addition, BDL significantly promoted bile duct proliferation (Supplementary Fig. 10A) and markedly down-regulated expression of the bile acid transporter apical sodium-dependent bile acid transporter (ASBT) and FXR-α in the cholangiocytes of wild type mice (Supplementary Fig.10B).
Figure 7. The role of S1PR2 in BDL-induced expression of COX-2.
(A) Wild type and S1PR2−/− mice were subjected to 2-week BDL or sham operation as described in “Methods”. (B) Mouse primary cholangiocytes were isolated from wild type and S1PR2−/− mice after 3 days of BDL. The mRNA levels of COX-2 in mouse liver were determined by real-time RT-PCR and normalized using GAPDH as an internal control. Statistical significance relative to sham control, *p<0.05, n=5–7; Statistical significance relative to WT BDL, #p<0.05, n=5–7.
Discussion
We previously reported that TCA-mediated activation of S1PR2 contributed to invasive growth of cholangiocarcinoma cells (21). Our recent study further indicated that TCA-induced activation of S1PR2 promoted an inflammatory response by activating the ERK1/2/AKT-NF-kB signaling cascade, resulting in upregulation of COX-2 expression and prostaglandin E2 (PGE2) production in human cholangiocarcinoma cells (20). It has been previously shown that cholestasis-induced liver injury is mainly due to inflammation and not the direct toxic effect of bile acids (27). In the mouse BDL cholestasis model, more than 99% of bile acids in liver and serum are CBAs, mainly TCA and TβMCA. Animals with histopathologic signs of bile duct hyperplasia also have elevated serum levels of CBAs (TCA and GCA) (28). Metabolomics studies further showed that an altered bile acid profile was a key characteristic of the liver injury (29, 30). These studies suggest that CBAs may play a critical role in cholestasis-induced liver injury. We previously showed that CBAs activate the S1PR2 in primary hepatocytes and in vivo using the chronic bile fistula rat model (18). Recent studies further indicated that S1PR2 plays an important role in regulating hepatic metabolism via activation of SphK2 (19). However, the physiological and pathological relevance of TCA-induced activation of S1PR2 in cholangiocyte proliferation and cholestasis-induced liver injury is not clear. We propose that S1PR2 plays an essential role in cholestasis-induced liver injury by promoting an inflammatory response.
Similar to our previous findings in cholangiocarcinoma cells, the current study demonstrated that S1PR2 is the predominant S1PR in mouse cholangiocytes. The mRNA level of S1PR2 was markedly increased in whole liver and primary cholangiocytes under cholestasis conditions (Fig.1). Our current in vitro studies further demonstrated that TCA dose-dependently increased S1PR2 expression (Fig.2B). TCA-induced ERK1/2 and AKT activation was correlated with cell proliferation and migration, which was blocked by inhibition of S1PR2 activation using a specific chemical antagonist or down-regulation of S1PR2 expression using a specific shRNA in MLE cells (Fig.2 &3 and Supplementary Fig.3). BDL-induced cholestatic liver injury was significantly reduced in S1PR2−/− mice compared to wild type mice as assessed by ALP level, hepatic hydroxyproline level and liver histology (Fig.4 & 5). Proliferation of cholangiocytes is a major characteristic of cholestatic liver diseases. BDL of wild type mice significantly induced cholangiocyte proliferation as indicated by an increase of CK-19-positive bile ducts (Fig. 6B). However, knock out of the gene encoding S1PR2 protected the mice from BDL-induced bile duct proliferation and liver fibrosis (Fig. 5B and 6A). Moreover, BDL significantly induced COX-2 expression in the liver and cholangiocytes of wild type mice, but not in those of S1PR2−/− mice (Fig.7). Our data suggest that CBAs-induced activation of S1PR2 and subsequent induction of an inflammatory response represents an important cellular mechanism underlying bile duct obstruction-induced cholestatic liver injury. Our preliminary studies indicated that treatment of BDL mice with JTE-013 had some protective effect against cholestatic injury (Supplementary Fig. 8–9). However, due to poor solubility of JTE-013, the bioavailability was not consistent. It will be necessary to develop a better delivery system for JTE-013 in order to test its therapeutic potential of this S1PR2 antagonist in in vivo animal models.
During the last two decades, extensive studies have been done to elucidate the functions of bile acids as important signaling molecules in regulating hepatic metabolism, initiating an inflammatory response and inducing systemic functions (6, 7, 31–33). Identification of bile acid-activated GPCRs (TGR5 and S1PR2) opened new directions of bile acid research for the development of novel therapeutic targets of various liver diseases (34–36). Recent studies by Reich, R et al. reported that TGR5 is essential for bile acid-dependent cholangiocyte proliferation both in cholangiocytes and in in vivo BDL mice (37). In this study, BDL-induced biliary hyperplasia within the portal tracts was significantly reduced in TGR5−/− mice as indicated by reduced CK-19 positive bile ducts and lower numbers of Ki67-positive cholangiocytes. TGR5 expression was also upregulated in human cholangiocarcinoma tissue. However, there was no data reporting whether BDL-induced liver fibrosis was also reduced in TGR5−/− mice. TGR5 is mainly activated by the secondary bile acids and is coupled to the cAMP signaling pathway. Under physiological or cholestatic conditions, the levels of secondary bile acids in liver are very low, while conjugated primary bile acid levels are much higher. In addition, TGR5 is not expressed in hepatocytes. Recent studies also reported that TGR5-mediated activation resulted in cholangiocyte hyperproliferation and cyst growth in an animal model of polycystic liver disease (34). Although TGR5 is expressed in cholangiocytes, its expression level is much lower than that of S1PR2 (Supplementary Fig. 11). Moreover, it has been shown that TGR5-mediated cholangiocyte proliferation in the BDL mouse model was independent of activation of the adenylyl cyclase/cAMP signaling pathway (37). In our studies, we found that TGR5 expression was not affected by BDL or TCA, but S1PR2 expression is induced by BDL and TCA (Fig. 1 & 2). A recent study reported that activation of TGR5 attenuated inflammation and renal fibrosis in diabetic nephropathy via inhibiting S1PR2 expression and promoting S1PR2 internalization in glomerular mesangial cells (38). It would be of interest to determine if S1PR2 expression in cholangiocytes is altered in TGR5−/− mice. Previous studies have reported that bile acids activated EGFR-ERK1/2 signaling pathway in hepatocytes in the absence of TGR5 (39). Our studies in human and rat cholangiocarcinoma cells as well as mouse cholangiocytes demonstrated that bile acid-induced ERK1/2 activation is linked to S1PR2 (20, 21). In current studies we also showed that TCA-induced cell proliferation was inhibited by an ERK1/2 inhibitor (Supplementary Fig.2). Our unpublished data in Mdr2−/− mice, a well-established mouse model for primary sclerosing cholangitis, indicated that increased hepatic S1PR2 expression level was correlated to the severity of cholestasis-induced liver fibrosis, but TGR5 expression level was decreased as fibrosis progress (data not shown). In total, these studies suggest that S1PR2 is an important player in cholestatic liver injury.
Inflammation is an important contributor to cholestasis-induced liver injury (40). S1P and S1PRs have been identified as key players in various fibrotic diseases including liver fibrosis (16, 41–45). Recent studies reported that S1PR2/3-mediated homing of bone marrow-derived immune cells contributes to cholestasis-induced liver fibrosis (13, 16, 46). Bile acid-induced ERK1/2 activation not only up-regulates pro-inflammatory cytokines, such as macrophage inflammatory protein 2 (MIP2), but also increases expression of IL-23, a key regulator of IL-17A production. Activation of IL-23/IL-17A further enhances bile acid-mediated hepatic inflammation during obstructive cholestasis (47). If and how S1PR2 is involved in communication among cholangiocytes, immune cells and hepatocytes under cholestatic conditions remains to be further examined.
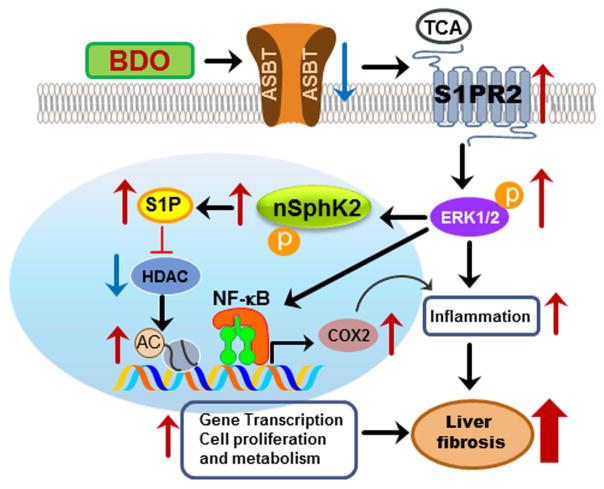
In summary, our current results suggest that S1PR2 is a crucial GPCR that mediates CBA-induced liver injury under cholestasis conditions. As illustrated in Fig. 8, accumulation of CBAs due to BDO with subsequent inflammation results in down regulation of ASBT and activation of S1PR2, which further activates the ERK1/2 signaling pathway. Our recent study reported that TCA/S1PR2-mediated ERK1/2 activation phosphorylates nuclear SphK2 (nSphK2), which controls nuclear S1P levels (19). Nuclear S1P is a powerful inhibitor of histone deacetylases HDAC1 and HDAC2 (48, 49). Increase of histone acetylation will promote the transcriptional activity of key genes involved in regulating cell growth and hepatic lipid metabolism. Activation of ERK1/2 also can activate NF-κB, which induces expression of various inflammatory genes including COX-2. Both cell proliferation and inflammation are key contributors to promoting fibrosis under cholestasis conditions (Fig.8).
Figure 8. Model of bile duct obstruction-induced liver fibrosis.
Bile duct obstruction (BDO) induces inflammation and CBAs, mainly TCA, in the liver. BDO-induced inflammation down-regulates apical sodium dependent bile acid transporter (ASBT) and upregulates S1PR2 in cholangiocytes. Extracellular TCA activates S1PR2, which further activates the ERK1/2-SphK2 signaling pathway. Activation of nuclear SphK2 increases the levels of S1P in the nucleus, which further inhibits specific histone deacetylases (HDACs) resulting in an increase in acetylation of histones and up-regulation of genes involved in cell proliferation and metabolism. Activation of ERK1/2 also induces activation of NF-κB and increases COX-2 expression. CBA-induced S1PR2 activation plays a critical role in cholestasis-induced liver injury.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R01 DK104893 (to HZ and PBH), R01DK-057543-11 (to PBH and HZ), R01CA160688 (to KT); VA Merit Award I01BX001390 (to HZ); National Natural Science Foundation of China Grants 81320108029 (to L. Z.), 81572448, 81070245 and 81270489 (to H. Z.); Massey Cancer Center pilot grant (to HZ and PBH). Microscopy was performed at the VCU Microscopy Facility, supported in part by funding from NIH-NCI Cancer Center Grant P30 CA016059. Specific Fund for Public Interest Research of Traditional Chinese Medicine, Ministry of Finance, China (201507004-002). National “Major Scientific and Technological Special Project for Significant New Drugs Creation” Project (2015ZX09501004-002-004).
Abbreviations
- BDL
bile duct ligation
- CBA
conjugated bile acid
- MSE
mouse small cholangiocyte
- MLE
mouse large cholangiocyte
- SphK1
sphingosine kinase 1
- SphK2
sphingosine kinase 2
- S1P
sphingosine-1-phosphate
- S1PR2
sphingosine 1-phosphate receptor 2
- S1PR2−/−
sphingosine 1-phosphate receptor 2 knock out
- TCA
taurocholate
- TβMCA
tauro-β-muricholic acid
Footnotes
Competing Financial interest: The authors declare no competing financial interest.
Contributions: PBH, WMP, KT and HZ conceived the original ideas, designed the study, analyzed the data and wrote the manuscript; HA and YW did BDL surgery; YW, JY, KP, RL, XL, XQ carried out the real-time PCR analysis, measurement of serum and liver bile acids, ALT, AST, ALP and hydroxyproline analysis, western blot analysis and data analysis. LS did immunohistochemistry. GL helped by doing histology analysis. ECG did animal breeding and genotyping.
References
- 1.Hirschfield GM, Heathcote EJ. Cholestasis and cholestatic syndromes. Curr Opin Gastroenterol. 2009;25:175–179. doi: 10.1097/mog.0b013e32832914b4. [DOI] [PubMed] [Google Scholar]
- 2.O’Leary JG, Pratt DS. Cholestasis and cholestatic syndromes. Curr Opin Gastroenterol. 2007;23:232–236. doi: 10.1097/MOG.0b013e3280d942d8. [DOI] [PubMed] [Google Scholar]
- 3.Glaser SS, Gaudio E, Miller T, Alvaro D, Alpini G. Cholangiocyte proliferation and liver fibrosis. Expert Rev Mol Med. 2009;11:e7. doi: 10.1017/S1462399409000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall C, Sato K, Wu N, Zhou T, Kyritsi T, Meng F, Glaser S, et al. Regulators of Cholangiocyte Proliferation. Gene Expr. 2016 doi: 10.3727/105221616X692568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glaser S, Onori P, Gaudio E, Ueno Y, Pannarale L, Franchitto A, Francis H, et al. Taurocholic acid prevents biliary damage induced by hepatic artery ligation in cholestatic rats. Dig Liver Dis. 2010;42:709–717. doi: 10.1016/j.dld.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. Bile acids as regulatory molecules. J Lipid Res. 2009;50:1509–1520. doi: 10.1194/jlr.R900007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou H, Hylemon PB. Bile acids are nutrient signaling hormones. Steroids. 2014;86:62–68. doi: 10.1016/j.steroids.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagahashi M, Yuza K, Hirose Y, Nakajima M, Ramanathan R, Hait NC, Hylemon PB, et al. The roles of bile acids and sphingosine-1-phosphate signaling in the hepato-biliary diseases. J Lipid Res. 2016 doi: 10.1194/jlr.R069286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takabe K, Spiegel S. Export of sphingosine-1-phosphate and cancer progression. J Lipid Res. 2014;55:1839–1846. doi: 10.1194/jlr.R046656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malik FA, Meissner A, Semenkov I, Molinski S, Pasyk S, Ahmadi S, Bui HH, et al. Sphingosine-1-Phosphate Is a Novel Regulator of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Activity. PLoS One. 2015;10:e0130313. doi: 10.1371/journal.pone.0130313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao CG, Qin J, He XJ, Guan YC, Jia Y, Lei W. Sphingosine-1-phosphate is a possible fibrogenic factor in gluteal muscle fibrosis. Physiol Res. 2013;62:691–699. doi: 10.33549/physiolres.932441. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Zheng S, You H, Liu X, Lin M, Yang L, Li L. Sphingosine 1-phosphate (S1P)/S1P receptors are involved in human liver fibrosis by action on hepatic myofibroblasts motility. J Hepatol. 2011;54:1205–1213. doi: 10.1016/j.jhep.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda H, Watanabe N, Ishii I, Shimosawa T, Kume Y, Tomiya T, Inoue Y, et al. Sphingosine 1-phosphate regulates regeneration and fibrosis after liver injury via sphingosine 1-phosphate receptor 2. J Lipid Res. 2009;50:556–564. doi: 10.1194/jlr.M800496-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C, Jiang X, Yang L, Liu X, Yue S, Li L. Involvement of sphingosine 1-phosphate (SIP)/S1P3 signaling in cholestasis-induced liver fibrosis. Am J Pathol. 2009;175:1464–1472. doi: 10.2353/ajpath.2009.090037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, Han Z, Tian L, Mai P, Zhang Y, Wang L, Li L. Sphingosine 1-Phosphate Receptor 2 and 3 Mediate Bone Marrow-Derived Monocyte/Macrophage Motility in Cholestatic Liver Injury in Mice. Sci Rep. 2015;5:13423. doi: 10.1038/srep13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kageyama Y, Ikeda H, Watanabe N, Nagamine M, Kusumoto Y, Yashiro M, Satoh Y, et al. Antagonism of sphingosine 1-phosphate receptor 2 causes a selective reduction of portal vein pressure in bile duct-ligated rodents. Hepatology. 2012;56:1427–1438. doi: 10.1002/hep.25780. [DOI] [PubMed] [Google Scholar]
- 18.Studer E, Zhou X, Zhao R, Wang Y, Takabe K, Nagahashi M, Pandak WM, et al. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology. 2012;55:267–276. doi: 10.1002/hep.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagahashi M, Takabe K, Liu R, Peng K, Wang X, Wang Y, Hait NC, et al. Conjugated bile acid-activated S1P receptor 2 is a key regulator of sphingosine kinase 2 and hepatic gene expression. Hepatology. 2015;61:1216–1226. doi: 10.1002/hep.27592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu R, Li X, Qiang X, Luo L, Hylemon PB, Jiang Z, Zhang L, et al. Taurocholate Induces Cyclooxygenase-2 Expression via the Sphingosine 1-phosphate Receptor 2 in a Human Cholangiocarcinoma Cell Line. J Biol Chem. 2015;290:30988–31002. doi: 10.1074/jbc.M115.668277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu R, Zhao R, Zhou X, Liang X, Campbell DJ, Zhang X, Zhang L, et al. Conjugated bile acids promote cholangiocarcinoma cell invasive growth through activation of sphingosine 1-phosphate receptor 2. Hepatology. 2014;60:908–918. doi: 10.1002/hep.27085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glaser SS, Gaudio E, Rao A, Pierce LM, Onori P, Franchitto A, Francis HL, et al. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest. 2009;89:456–469. doi: 10.1038/labinvest.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aoki H, Aoki M, Yang J, Katsuta E, Mukhopadhyay P, Ramanathan R, Woelfel IA, et al. Murine model of long-term obstructive jaundice. Journal of Surgical Research. 2016;206:118–125. doi: 10.1016/j.jss.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marzioni M, Ueno Y, Glaser S, Francis H, Benedetti A, Alvaro D, Venter J, et al. Cytoprotective effects of taurocholic acid feeding on the biliary tree after adrenergic denervation of the liver. Liver Int. 2007;27:558–568. doi: 10.1111/j.1478-3231.2007.01443.x. [DOI] [PubMed] [Google Scholar]
- 25.Marucci L, Alpini G, Glaser SS, Alvaro D, Benedetti A, Francis H, Phinizy JL, et al. Taurocholate feeding prevents CCl4-induced damage of large cholangiocytes through PI3-kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2003;284:G290–301. doi: 10.1152/ajpgi.00245.2002. [DOI] [PubMed] [Google Scholar]
- 26.Marzioni M, LeSage GD, Glaser S, Patel T, Marienfeld C, Ueno Y, Francis H, et al. Taurocholate prevents the loss of intrahepatic bile ducts due to vagotomy in bile duct-ligated rats. Am J Physiol Gastrointest Liver Physiol. 2003;284:G837–852. doi: 10.1152/ajpgi.00398.2002. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Hong JY, Rockwell CE, Copple BL, Jaeschke H, Klaassen CD. Effect of bile duct ligation on bile acid composition in mouse serum and liver. Liver Int. 2012;32:58–69. doi: 10.1111/j.1478-3231.2011.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo L, Schomaker S, Houle C, Aubrecht J, Colangelo JL. Evaluation of serum bile acid profiles as biomarkers of liver injury in rodents. Toxicol Sci. 2014;137:12–25. doi: 10.1093/toxsci/kft221. [DOI] [PubMed] [Google Scholar]
- 29.Yamazaki M, Miyake M, Sato H, Masutomi N, Tsutsui N, Adam KP, Alexander DC, et al. Perturbation of bile acid homeostasis is an early pathogenesis event of drug induced liver injury in rats. Toxicol Appl Pharmacol. 2013;268:79–89. doi: 10.1016/j.taap.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 30.Lin Y, Si D, Zhang Z, Liu C. An integrated metabonomic method for profiling of metabolic changes in carbon tetrachloride induced rat urine. Toxicology. 2009;256:191–200. doi: 10.1016/j.tox.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 31.Arab JP, Karpen SJ, Dawson PA, Arrese M, Trauner M. Bile acids and nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Hepatology. 2016 doi: 10.1002/hep.28709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Pan Y, Lin C, Zheng Y, Sun H, Zhang H, Wang J, et al. Bile acids evoke placental inflammation by activating Gpbar1/NF-kappaB pathway in intrahepatic cholestasis of pregnancy. J Mol Cell Biol. 2016 doi: 10.1093/jmcb/mjw025. [DOI] [PubMed] [Google Scholar]
- 33.Zhu C, Fuchs CD, Halilbasic E, Trauner M. Bile acids in regulation of inflammation and immunity: friend or foe? Clin Exp Rheumatol. 2016;34:25–31. [PubMed] [Google Scholar]
- 34.Masyuk TV, Masyuk AI, LaRusso NF. TGR5 in the Cholangiociliopathies. Dig Dis. 2015;33:420–425. doi: 10.1159/000371696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keitel V, Reich M, Haussinger D. TGR5: pathogenetic role and/or therapeutic target in fibrosing cholangitis? Clin Rev Allergy Immunol. 2015;48:218–225. doi: 10.1007/s12016-014-8443-x. [DOI] [PubMed] [Google Scholar]
- 36.Pean N, Doignon I, Tordjmann T. Bile acids and liver carcinogenesis: TGR5 as a novel piece in the puzzle? Clin Res Hepatol Gastroenterol. 2013;37:226–229. doi: 10.1016/j.clinre.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Reich M, Deutschmann K, Sommerfeld A, Klindt C, Kluge S, Kubitz R, Ullmer C, et al. TGR5 is essential for bile acid-dependent cholangiocyte proliferation in vivo and in vitro. Gut. 2016;65:487–501. doi: 10.1136/gutjnl-2015-309458. [DOI] [PubMed] [Google Scholar]
- 38.Yang Z, Xiong F, Wang Y, Gong W, Huang J, Chen C, Liu P, et al. TGR5 activation suppressed S1P/S1P2 signaling and resisted high glucose-induced fibrosis in glomerular mesangial cells. Pharmacol Res. 2016;111:226–236. doi: 10.1016/j.phrs.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 39.Rao YP, Studer EJ, Stravitz RT, Gupta S, Qiao L, Dent P, Hylemon PB. Activation of the Raf-1/MEK/ERK cascade by bile acids occurs via the epidermal growth factor receptor in primary rat hepatocytes. Hepatology. 2002;35:307–314. doi: 10.1053/jhep.2002.31104. [DOI] [PubMed] [Google Scholar]
- 40.Kosters A, Karpen SJ. The role of inflammation in cholestasis: clinical and basic aspects. Semin Liver Dis. 2010;30:186–194. doi: 10.1055/s-0030-1253227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yaghobian D, Don AS, Yaghobian S, Chen X, Pollock CA, Saad S. Increased sphingosine 1-phosphate mediates inflammation and fibrosis in tubular injury in diabetic nephropathy. Clin Exp Pharmacol Physiol. 2016;43:56–66. doi: 10.1111/1440-1681.12494. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Hong Q, Wang Z, Yu Y, Zou X, Xu L. Transforming growth factor-beta-sphingosine kinase 1/S1P signaling upregulates microRNA-21 to promote fibrosis in renal tubular epithelial cells. Exp Biol Med (Maywood) 2016;241:265–272. doi: 10.1177/1535370215605586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang LS, Natarajan V. Sphingolipids in pulmonary fibrosis. Adv Biol Regul. 2015;57:55–63. doi: 10.1016/j.jbior.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang L, Yue S, Yang L, Liu X, Han Z, Zhang Y, Li L. Sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis is involved in liver fibrosis-associated angiogenesis. J Hepatol. 2013;59:114–123. doi: 10.1016/j.jhep.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 45.Shiohira S, Yoshida T, Sugiura H, Nishida M, Nitta K, Tsuchiya K. Sphingosine-1-phosphate acts as a key molecule in the direct mediation of renal fibrosis. Physiol Rep. 2013;1:e00172. doi: 10.1002/phy2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li C, Kong Y, Wang H, Wang S, Yu H, Liu X, Yang L, et al. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J Hepatol. 2009;50:1174–1183. doi: 10.1016/j.jhep.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 47.O’Brien KM, Allen KM, Rockwell CE, Towery K, Luyendyk JP, Copple BL. IL-17A synergistically enhances bile acid-induced inflammation during obstructive cholestasis. Am J Pathol. 2013;183:1498–1507. doi: 10.1016/j.ajpath.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hait NC, Bellamy A, Milstien S, Kordula T, Spiegel S. Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. J Biol Chem. 2007;282:12058–12065. doi: 10.1074/jbc.M609559200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.