Abstract
IMPORTANCE
The Zika virus (ZIKV) has rapidly reached epidemic proportions, especially in northeastern Brazil, and has rapidly spread to other parts of the Americas. A recent increase in the prevalence of microcephaly in newborn infants and vision-threatening findings in these infants is likely associated with the rapid spread of ZIKV.
OBJECTIVE
To evaluate the ocular findings in infants with microcephaly associated with presumed intrauterine ZIKV infection in Salvador, Bahia, Brazil.
DESIGN, SETTING, AND PARTICIPANTS
Case series at a tertiary hospital. Twenty-nine infants with microcephaly (defined by a cephalic circumference of ≤32 cm) with a presumed diagnosis of congenital ZIKV were recruited through an active search and referrals from other hospitals and health unities. The study was conducted between December 1 and December 21, 2015.
INTERVENTIONS
All infants and mothers underwent systemic and ophthalmic examinations from December 1 through December 21, 2015, in the Roberto Santos General Hospital, Salvador, Brazil. Anterior segment and retinal, choroidal, and optic nerve abnormalities were documented using a wide-field digital imaging system. The differential diagnosis included toxoplasmosis, rubella, cytomegalovirus, herpes simplex virus, syphilis, and human immunodeficiency virus, which were ruled out through serologic and clinical examinations.
MAIN OUTCOMES AND MEASURES
Ocular abnormalities associated with ZIKV.
RESULTS
Twenty-three of 29 mothers (79.3%) reported suspected ZIKV infection signs and symptoms during pregnancy, 18 in the first trimester, 4 in the second trimester, and 1 in the third trimester. Of the 29 infants (58 eyes) examined (18 [62.1%] female), ocular abnormalities were present in 17 eyes (29.3%) of 10 children (34.5%). Bilateral findings were found in 7 of 10 patients presenting with ocular lesions, the most common of which were focal pigment mottling of the retina and chorioretinal atrophy in 11 of the 17 eyes with abnormalities (64.7%), followed by optic nerve abnormalities in 8 eyes (47.1%), bilateral iris coloboma in 1 patient (2 eyes [11.8%]), and lens subluxation in 1 eye (5.9%).
CONCLUSIONS AND RELEVANCE
Congenital infection due to presumed ZIKV exposure is associated with vision-threatening findings, which include bilateral macular and perimacular lesions as well as optic nerve abnormalities in most cases.
The Zika virus (ZIKV) is anarbovirus that carries the name of a forest near Kampala, Uganda. The virus was first identified in rhesus monkeys in 1947 through a sylvatic yellow fever surveillance network in Uganda1 and was further isolated in humans in Uganda and Tanzania in 1952.2 The transmission is mostly vectorial by mosquitoes from the Culicidae family and the Aedes genus, especially Aedes aegypti (urban transmission). The virus usually is transmitted to hematophagous arthropods during their blood meal and reproduces in the host vector without affecting it, remaining in the insect during its entire life span. The virus is transmitted to reservoir animals during the next blood meal.3 Direct interhuman transmission, most likely by sexual intercourse, also has been described,4 and perinatal transmission of arbovirus has been reported for dengue virus (DENV),5–8 chikungunya virus,9,10 West Nile virus (WNV),11 and yellow fever virus.12 Both DENV8 and West Nile virus reportedly have been transmitted in breast milk.13 There is also some evidence of perinatal and breast milk transmission of ZIKV.14,15
Few human cases were reported until 2007, when a ZIKV outbreak occurred in Yap, Micronesia, even though ZIKV activity had been reported in Africa and Asia through virologic surveillance and entomologic studies.15 At the beginning of 2015, several cases of DENV and chikungunya virus infections were reported in Brazil.16 The number of cases of ZIKV reported in Brazil is uncertain because of problems in detection without the commercial availability of a serologic test.
In addition, the signs and symptoms in patients infected with the 3 distinct viruses, ie, DENV, chikungunya virus, and ZIKV, are similar. Moreover, it was estimated that 80% of patients with ZIKV infection are asymptomatic or oligosymptomatic. Finally, ZIKV infection occurs in demographic areas in which A aegypti is endemic and where the mosquito is the biological vector of the 3 distinct viral diseases, and coinfection with these viruses is not uncommon.17
Transmission to the Americas appears to have originated in the Pacific Islands.18 The Brazilian state of Bahia was the first to identify cases in Brazil, although the state of Pernambuco has a larger number of notifications.19 By December 10, 2015, ZIKV had spread to 18 other Brazilian states and rapidly became an epidemic, especially in northeastern Brazil.20,21 Circulating ZIKV in Brazil was identified as an Asian genotype through phylogenetic analyses of the envelope region in the 2 cases from Bahia.18
The only method to diagnose ZIKV infection is by real-time polymerase chain reaction, which is useful to detect the virus only in the first days of an acute infection. Real-time polymerase chain reaction is not helpful for confirming the infection in infants. Therefore, for now, ZIKV-related microcephaly is diagnosed clinically.22–25 The frequency of cross reactions with other flaviviruses (DENV, yellow fever virus)may make diagnosis difficult.
Since April 2015, an epidemic of ZIKV has been occurring in Brazil. It is estimated that more than 1 million Brazilians have had ZIKV infections in 2015, reflecting the virus’ capacity to cause large-scale outbreaks where the biological vector is present.25,26 Six months after the onset of the ZIKV outbreak in Brazil, there was an unusual increase in newborns with microcephaly. In 2015, 1248 new suspected cases were registered, corresponding to a prevalence of 99.7 per 100000 live births and representing a 20-fold increase compared with recent years.27 On January 4, 2016, the Brazilian Ministry of Health reported 3174 microcephalic newborns, the majority of whom were in Pernambuco and almost all in northeastern Brazil.28
Key Points.
Question
Are there ocular abnormalities in infants with microcephaly associated with presumed Zika virus congenital infection in Brazil?
Findings
Congenital infection due to presumed Zika virus exposure was associated with vision-threatening findings; the majority of cases had bilateral macular and perimacular lesions as well as optic nerve abnormalities.
Meaning
These data suggest that clinicians should consider ophthalmologic evaluations of newborns from regions in the Americas where Zika virus transmission has spread rapidly to identify lesions associated with this presumed Zika virus congenital infection.
The recent increase in the prevalence of microcephaly in several northeastern Brazilian states has been strongly suspected of being associated with ZIKV congenital infection, with the virus found in the amniotic fluid of 2 pregnant women whose newborns presented with a reduced head circumference.29 This serious effect of the ZIKV infection in fetuses is unsurprising considering the perinatal transmission reported for 2 women from French Polynesia and the strong neurotropism of the virus.1,14 In January 2016, Ventura et al30 published the first report of possible congenital ocular lesions in 3 infants from Recife, Pernambuco, Brazil. Recife is experiencing an outbreak of ZIKV infection as well as an increase in the number of newborns with microcephaly. The cases reported by Ventura and colleagues do not compose this case series.
The aim of this study was to describe the ocular findings in 29 infants with microcephaly from mothers presumably infected with ZIKV during pregnancy, during an outbreak in Salvador, Bahia, Brazil.
Methods
Study Site
This study was conducted between December 1 and December 21, 2015. All children and their mothers were evaluated at the Roberto Santos General Hospital, Salvador, Brazil, a tertiary care hospital. Ophthalmologists who are experts in medical/surgical retina, uveitis, and neuro-ophthalmology evaluated the patients.
Thirty-one infants with microcephaly due to a possible ZIKV congenital infection and their mothers were referred for ophthalmologic evaluation from other hospitals and health unities.
In December 2015, the Brazilian Ministry of Health revised the definition for microcephaly in newborn term infants. The head circumference criterion was reduced from a threshold of 33 cmor less to 32 cmor less,31 which was the criterion used in this study.
A direct parent (mother or father) provided written informed consent. The Ethics Committee of the Federal University of São Paulo previously approved the study protocol, which followed the tenets of the Declaration of Helsinki.32
A detailed clinical history was obtained, including the prenatal and postnatal history and maternal systemic history.
Patients
Inclusion Criteria of the Mothers and Infants
Infants with a cephalic circumference of 32 cmor less at birth were included. Other congenital infections included in the differential diagnosis were toxoplasmosis, rubella, cytomegalovirus, herpes simplex virus, syphilis, and human immunodeficiency virus (HIV), which were ruled out through serologic examinations. In all cases, the specific tests performed were part of the routine screening and also included serology in the mothers. All of the cases had negative serology results for syphilis and HIV. None presented with high titers of IgG antibodies or the presence of IgM antibodies specific to toxoplasmosis, cytomegalovirus, herpes simplex virus, or rubella.
The suspected diagnosis of congenital ZIKV was also based on clinical features of the mothers during pregnancy, including cutaneous rash, fever, arthralgia, headache, itch, and malaise.
Exclusion Criteria of the Infants
Newborn infants were excluded if they had a cephalic diameter that exceeded 33 cm, evidence for another congenital infection such as toxoplasmosis, rubella, cytomegalovirus, herpes simplex virus, or HIV, or a familial history of microcephaly, or if the mother had a history of alcohol or illicit drug use during pregnancy.
Ocular Examination
All mothers underwent an external ocular examination, biomicroscopy, and dilated indirect ophthalmoscopy. All infants underwent an ocular external examination and indirect ophthalmoscopy. Optic nerve, retinal, and choroidal abnormalities were documented with a wide-field digital imaging system(RetCam Shuttle; Clarity Medical Systems) after pupillary dilation.
Owing to the small number of patients included in this case series, a descriptive report was conducted and no statistical analysis was performed.
Results
Thirty-one infants aged 1 to 6 months had a confirmed diagnosis of microcephaly. Two patients were excluded from the study owing to impossibility of evaluating the results of their serology tests for other infections, as they did not return for blood collection.
Of the 29 children, 18 (62.1%)were female. Twenty-three of the 29 mothers (79.3%) had ZIKV signs and symptoms during pregnancy, which included cutaneous rash (21 of 29 mothers [72.4%]), fever (13 mothers [44.8%]), arthralgia (11 mothers [37.9%]), headache (5 mothers [17.2%]), and itch (4 mothers [13.8%]). All mothers denied signs or symptoms of conjunctivitis. Among 23 mothers who reported symptoms during pregnancy, 18(78.3%)reported ZIKV symptoms during the first trimester of pregnancy, 4 (17.4%) during second trimester, and 1 (4.3%) during the third trimester. No mothers had signs of active or previous uveitis and all had normal findings on ocular examination.
Ocular abnormalities were observed in 10 of the 29 patients with microcephaly (34.5%) who were examined. Seventeen of 20 eyes (85.0%) in 10 children had ophthalmoscopic abnormalities. Bilateral findings were found in 7 of 10 patients (70.0%)presenting with ocular lesions, the most common of which were focal pigment mottling and chorioretinal atrophy in 11 of the 17 eyes with abnormalities (64.7%) (Figure 1, Figure 2, Figure 3, and Figure 4). Severe chorioretinal atrophy also was observed and involved the macula in 3 eyes (Figure 1B and Figure 3A), the nasal retina in 3 eyes (Figure 5), and the paramacular area in 5 eyes (Figure 2 and Figure 3B). Two distinct components were observed: (1) circumscribed areas of chorioretinal atrophy, which in some areas had no choroidal vessels visible at all (Figure 1B, Figure 2, Figure 3B, Figure 4, and Figure 5); and (2) circumscribed areas of pigmentary clumping (Figure 2, Figure 3B, and Figure 4). Some patients had chorioretinal atrophy surrounded by a hyperpigmented halo and also showed the hyperpigmented mottling (Figure 2, Figure 3B, and Figure 4).
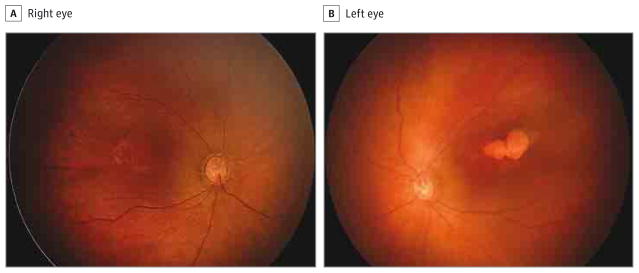
Figure 1. Fundus Photographs of a 2-Month-Old Girl.
The right eye has granular, pigmentary mottling in the macula (A), and the left eye has a chorioretinal lobulated atrophic lesion and slight pigmentary mottling (B).
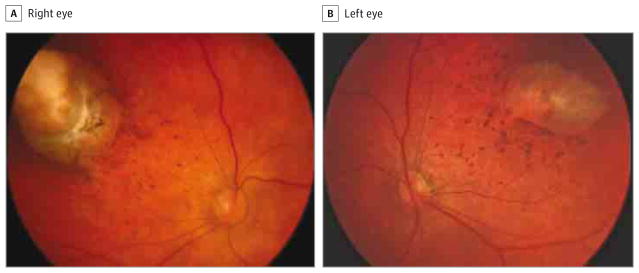
Figure 2. Fundus Photographs of a 1-Month-Old Boy.
The right (A) and left (B) eyes have paramacular superotemporal round chorioretinal atrophy surrounded by a hyperpigmented halo and hyperpigmented mottling.
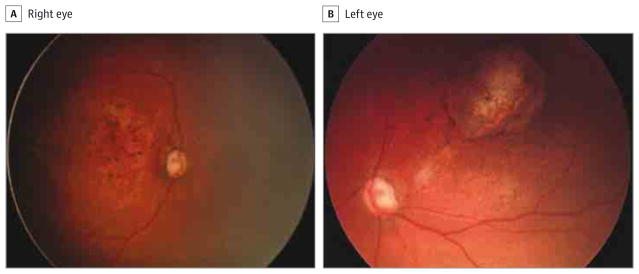
Figure 3. Fundus Photographs of a 1-Month-Old Infant.
The right eye has an enlarged cup-disc ratio and macular pigmentary mottling (A), and the left eye has a roundish macular chorioretinal atrophic lesion with a hyperpigmented halo and pigmentary mottling, as well as an enlarged cup-disc ratio of the optic nerve.
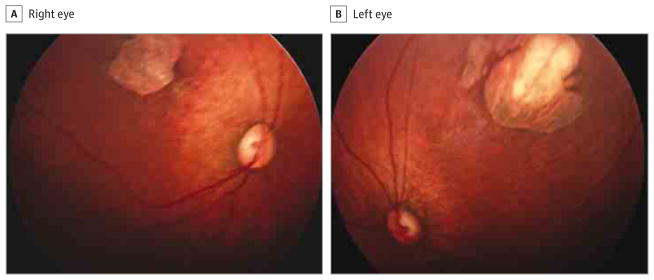
Figure 4. Fundus Photographs of a 1-Month-Old Infant.
The right eye has a superotemporal perimacular chorioretinal scar with perilesional pigmentary mottling (A), and the left eye has similar findings (B).
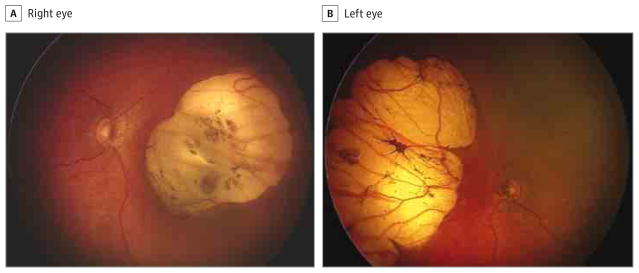
Figure 5. Fundus Photographs of a 20-Day-Old Infant.
The right eye has optic disc hypoplasia, peripapillary nasal atrophy, and an excavated nasal round lesion with a hyperpigmented halo, with a colobomatous-like aspect (A), and the left eye has optic disc hypoplasia, peripapillary nasal atrophy, and a retinal nasal lesion with a similar pattern (B).
Other ocular findings included optic nerve abnormalities in 8 eyes (47.1%; 2 cases of hypoplasia and 6 cases of severe optic disc cupping), lens subluxation in 1 eye (5.9%), and bilateral iris coloboma (2 eyes [11.8%]) in the same patient with lens subluxation. Data are summarized in the Table. No infants had vasculitis or active uveitis.
Table.
Ocular Findings in Infants With Microcephaly and Presumed Zika Virus Congenital Infection
| Ocular Finding | Patient No. | Affected Eyes, No. (%) (n = 17) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Focal pigment mottling | OU | OD | OU | No | OU | OD | No | OU | OS | No | 11 (64.7) |
| Chorioretinal atrophy | OU | OS | OU | OU | OS | No | OS | OU | No | No | 11 (64.7) |
| Optic nerve abnormalities | OU | No | No | OU | OU | No | No | No | No | OU | 8 (47.1) |
| Iris coloboma | No | No | No | No | No | OU | No | No | No | No | 2 (11.8) |
| Lens subluxation | No | No | No | No | No | OS | No | No | No | No | 1 (5.9) |
| Signs of active uveitis | No | No | No | No | No | No | No | No | No | No | 0 |
| Vasculitis | No | No | No | No | No | No | No | No | No | No | 0 |
Abbreviations: OD, right eye; OS, left eye; OU, each eye.
Discussion
Findings from this case series demonstrate that 10 of 29 infants (34.5%) born with presumed ZIKV-associated microcephaly have vision-threatening lesions. In 2015, Salvador, Bahia, and several other cities in northeastern Brazil had an unprecedented ZIKV outbreak. This virus became a major public health problem in a short period owing to its association with severe neurologic malformations such as microcephaly and possibly related ocular diseases in newborn infants.14,28
Because the rate of ocular abnormalities is very high in this age group and the retinal lesions differ from other congenital infections described previously in the literature, we hypothesized that a cause and effect relationship between ZIKV infection and ocular abnormalities was possible.
The ocular lesions consisted of focal pigment mottling and chorioretinal atrophy with a predilection for the posterior pole, especially the macular area, as well as optic disc abnormalities. No signs of active uveitis or vasculitis were observed.
The current data suggest the possibility that even oligosymptomatic or asymptomatic pregnant patients presumably infected with ZIKV may have microcephalic newborns with ophthalmoscopic lesions. An important question is whether patients without microcephaly will need to be screened to identify these ocular lesions.
Our study has limitations including generalizability of our findings (because it was conducted at 1 hospital), a small sample, and a lack of prior research studies on the topic. The convenience sample and lack of statistical analysis do not allow us to affirm whether percentages or findings are unique to these data or could be generalizable to presumed ZIKV infection.
The growing number of cases, rapid disease dissemination, and epidemic pattern observed in Brazil may indicate the risk of a new pandemic disease throughout the Americas, especially given that the biological vector A aegypti is endemic in Latin America, the Caribbean, and some regions in the United States, where cases have already been reported.25,26,33 These regions where the virus is spreading can likely experience an outbreak with the same types of birth defects including the ophthalmologic lesions observed in this study.
Unlike the findings by Duffy et al34 reported in 2009, the current study did not identify conjunctivitis as a frequent finding in ZIKV infection. No mothers reported conjunctivitis during pregnancy when specifically asked about it. Our findings suggest that conjunctivitis may not be an important clinical finding in the differential diagnosis of ZIKV.
Conclusions
In summary, 10 of 29 infants with microcephaly (34.5%) had severe ocular abnormalities; these infants were born after a ZIKV outbreak in the state of Bahia in Brazil. The posterior ocular findings were focal pigment mottling and chorioretinal atrophy with a predilection for the macular area, as well as optic disc abnormalities. These findings can contribute to the diagnosis of ZIKV congenital infection in children with congenital microcephaly, although the retinal lesions found in this sample may resemble West Nile virus or toxoplasmosis retinochoroiditis.35 Advances in serologic ZIKV tests and additional studies are necessary to confirm such findings.
This study can help guide clinical management and practice, as we observed that a high proportion of the infants with microcephaly had ophthalmologic lesions. Infants with microcephaly should undergo routine ophthalmologic evaluations to identify such lesions. In high-transmission settings, such as South America, Central America, and the Caribbean, ophthalmologists should be aware of the risk of congenital ZIKV-associated ophthalmologic sequelae.
Acknowledgments
Funding/Support: Hospital Geral Roberto Santos provided the site and materials for ophthalmologic examination. Federal University of São Paulo (Paulista Medical School), Vision Institute (IPEPO), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) provided funds for travel expenses as well as the materials necessary for the ocular examinations.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Role of the Funder/Sponsor: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author Contributions: Dr de Paula Freitas had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: de Paula Freitas, de Oliveira Dias, Prazeres, Maia, Belfort.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: de Paula Freitas, de Oliveira Dias, Prazeres, Sacramento, Ko, Maia.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: de Paula Freitas.
Obtained funding: Belfort.
Administrative, technical, or material support: de Paula Freitas, Prazeres, Sacramento, Maia, Belfort.
Study supervision: de Paula Freitas, de Oliveira Dias, Sacramento, Maia, Belfort.
References
- 1.Dick GW, Kitchen SF, Haddow AJ. Zika virus, I: isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46(5):509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 2.Dick GW. Zika virus, II: pathogenicity and physical properties. Trans R Soc Trop Med Hyg. 1952;46(5):521–534. doi: 10.1016/0035-9203(52)90043-6. [DOI] [PubMed] [Google Scholar]
- 3.Ioos S, Mallet HP, Leparc Goffart I, Gauthier V, Cardoso T, Herida M. Current Zika virus epidemiology and recent epidemics. Med Mal Infect. 2014;44(7):302–307. doi: 10.1016/j.medmal.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–882. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan PC, Rajasingam G, Devi S, Omar SZ. Dengue infection in pregnancy: prevalence, vertical transmission, and pregnancy outcome. Obstet Gynecol. 2008;111(5):1111–1117. doi: 10.1097/AOG.0b013e31816a49fc. [DOI] [PubMed] [Google Scholar]
- 6.Pouliot SH, Xiong X, Harville E, et al. Maternal dengue and pregnancy outcomes: a systematic review. Obstet Gynecol Surv. 2010;65(2):107–118. doi: 10.1097/OGX.0b013e3181cb8fbc. [DOI] [PubMed] [Google Scholar]
- 7.Adam I, Jumaa AM, Elbashir HM, Karsany MS. Maternal and perinatal outcomes of dengue in PortSudan, Eastern Sudan. Virol J. 2010;7:153. doi: 10.1186/1743-422X-7-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barthel A, Gourinat AC, Cazorla C, Joubert C, Dupont-Rouzeyrol M, Descloux E. Breast milk as a possible route of vertical transmission of dengue virus? Clin Infect Dis. 2013;57(3):415–417. doi: 10.1093/cid/cit227. [DOI] [PubMed] [Google Scholar]
- 9.Fritel X, Rollot O, Gerardin P, et al. Chikungunya-Mere-Enfant Team. Chikungunya virus infection during pregnancy, Reunion, France, 2006. Emerg Infect Dis. 2010;16(3):418–425. doi: 10.3201/eid1604.091403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gérardin P, Barau G, Michault A, et al. Multidisciplinary prospective study of mother-to-child chikungunya virus infections on the island of La Réunion. PLoS Med. 2008;5(3):e60. doi: 10.1371/journal.pmed.0050060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart RD, Bryant SN, Sheffield JS. West Nile virus infection in pregnancy. Case Rep Infect Dis. 2013;2013:351872. doi: 10.1155/2013/351872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bentlin MR, de Barros Almeida RA, Coelho KI, et al. Perinatal transmission of yellow fever, Brazil, 2009. Emerg Infect Dis. 2011;17(9):1779–1780. doi: 10.3201/eid1709.110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Possible West Nile virus transmission to an infant through breast-feeding: Michigan, 2002. MMWR Morb Mortal Wkly Rep. 2002;51(39):877–878. [PubMed] [Google Scholar]
- 14.Besnard M, Lastère S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19(13):20751. [PubMed] [Google Scholar]
- 15.Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14(8):1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ministério da Saúde, Secretaria de Vigilância em Saúde. Monitoramento dos casos de dengue e febre de chikungunya até a Semana Epidemiológica 30, 2015. Boletim Epidemiológico. 2015;46(24):1–8. http://portalsaude.saude.gov.br/images/pdf/2015/setembro/03/2015-029---SE-30.pdf. [Google Scholar]
- 17.Dupont-Rouzeyrol M, O’Connor O, Calvez E, et al. Co-infection with Zika and dengue viruses in 2 patients, New Caledonia, 2014. Emerg Infect Dis. 2015;21(2):381–382. doi: 10.3201/eid2102.141553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zanluca C, de Melo VC, Mosimann AL, Dos Santos GI, Dos Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015;110(4):569–572. doi: 10.1590/0074-02760150192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campos GS, Bandeira AC, Sardi SI. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis. 2015;21(10):1885–1886. doi: 10.3201/eid2110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Centre for Disease Prevention and Control. Zika Virus Epidemic in the Americas: A Potential Association With Microcephaly and Guillain-Barré Syndrome. Stockholm, Sweden: European Centre for Disease Prevention and Control; 2015. [Google Scholar]
- 21.Musso D. Zika virus transmission from French Polynesia to Brazil. Emerg Infect Dis. 2015;21(10):1887. doi: 10.3201/eid2110.151125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuno G, Chang GJ. Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch Virol. 2007;152(4):687–696. doi: 10.1007/s00705-006-0903-z. [DOI] [PubMed] [Google Scholar]
- 23.Kutsuna S, Kato Y, Takasaki T, et al. Two cases of Zika fever imported from French Polynesia to Japan, December 2013 to January 2014 [corrected] [published correction appears in Euro Surveill. 2014;19(5):20694] Euro Surveill. 2014;19(4):20683. doi: 10.2807/1560-7917.es2014.19.4.20683. [DOI] [PubMed] [Google Scholar]
- 24.Hayes EB. Zika virus outside Africa. Emerg Infect Dis. 2009;15(9):1347–1350. doi: 10.3201/eid1509.090442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. [Accessed December 17, 2015];Epidemiological alert: neurological syndrome, congenital malformations, and Zika virus infection: implications for public health in the Americas. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32405&lang=em.
- 26.Ministério da Saúde, Secretaria de Vigilância em Saúde. [Accessed December 26, 2015];Protocolo de vigilância e resposta à ocorrência demicrocefalia relacionada à infecção pelo vírus Zika. http://portalsaude.saude.gov.br/images/pdf/2015/dezembro/09/Microcefalia---Protocolo-de-vigil--ncia-e-resposta---vers--o-1----09dez2015-8h.pdf.
- 27.Centro de Operações de Emergências em Saúde Pública sobre Microcefalias. [Accessed December 26, 2015];Monitoramento dos casos de microcefalias no Brasil. http://portalsaude.saude.gov.br/images/pdf/2015/novembro/30/coes-microcefalias---informe-epidemiol--gico---se-47.pdf.
- 28.Ministério da Saúde. [Accessed January 9, 2016];Microcefalia: 3174 casos são investigados em 20 estados e no Distrito Federal. http://portalsaude.saude.gov.br/index.php/o-ministerio/principal/secretarias/job/webradio/21527-microcefalia-3174-casos-sao-investigados-em-20-estados-e-no-distrito-federal.
- 29.Ministério da Saúde. [Accessed December 26, 2015];Ministério da Saúde confirma relação entre vírus Zika e microcefalia. http://portalsaude.saude.gov.br/index.php/cidadao/principal/agencia-saude/21014-ministerio-da-saude-confirma-relacao-entre-virus-zika-e-microcefalia.
- 30.Ventura CV, Maia M, Bravo-Filho V, Góis AL, Belfort R., Jr Zika virus in Brazil and macular atrophy in a child with microcephaly [published online January 7, 2016] Lancet. doi: 10.1016/S0140-6736(16)00006-4. [DOI] [PubMed] [Google Scholar]
- 31.Ministério da Saúde. [Accessed December 26, 2015];Nota sobre medida do perímetro cefálico para diagnóstico de bebês com microcefalia relacionada ao vírus Zika. http://www.blog.saude.gov.br/50426-nota-sobre-medida-do-perimetro-cefalico-para-diagnostico-de-bebescom-microcefalia-relacionada-ao-virus-zika.
- 32.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 33.Kelly JC. First Zika virus case in continental United States confirmed in Texas. Medscape. 2016 Jan 11; [Google Scholar]
- 34.Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 35.Alpert SG, Fergerson J, Noël LP. Intrauterine West Nile virus: ocular and systemic findings. Am J Ophthalmol. 2003;136(4):733–735. doi: 10.1016/s0002-9394(03)00452-5. [DOI] [PubMed] [Google Scholar]