Abstract
Objective
Obesity in adults is associated with inflammation and oxidative stress. Whether or not this phenotype is reflected in human milk (HM) composition, or may impact infant growth remains unknown. We investigated whether HM from overweight/obese (OW/Ob) mothers exhibited higher concentrations of inflammatory cytokines and markers of oxidative stress. We also correlated these bioactive components with infant growth patterns.
Methods
This was an observational cohort of 56 breastfeeding mothers and their infants (33 normal weight (NW) and 23 OW/Ob). Infants were followed until 6 months of age and HM collected at 2-weeks and 4-months.
Results
Markers of oxidative stress, 8-hydroxy-deoxyguanosine (8OHdG) and 4-hydroxynonenol (HNE), decreased in HM over time (p<0.001) and did not differ between NW and OW/Ob women. Concentrations of inflammatory cytokines, IL-6, IL-8, and TNF-α, were all inter-correlated (p<0.001) but did not differ between NW and OW/Ob women. HM fat, protein, lactose, and total calories did not differ between NW and OW/Ob women. Infant growth patterns did not differ by group. In a model of infant weight-for-length-Z score trajectory, there was a significant interaction between both lactose and 8OHdG with maternal group: HM lactose and 8OHdG concentrations were both positively associated with increases in WLZ trajectory only among infants breastfed by OW/Ob mothers.
Conclusions for Practice
HM composition was relatively stable between NW and OW/Ob women. In exclusively breastfed infants, HM concentrations of lactose and 8OHdG, a marker of oxidative stress, may contribute to regulation of infant weight gain, especially among infants of OW/Ob women.
Keywords: Oxidative Stress, Infant Growth, Human Milk Composition, Maternal Obesity, Breastfeeding
Introduction
The first 6 months of life are a critical period of development when nutritional exposures can exert long-lasting programming effects (Young, Johnson, & Krebs, 2012). Rapid and excess weight gain during this time is consistently identified as a predictor of later obesity, and obesity-related co-morbidities (Monteiro & Victora, 2005; Taveras et al., 2011; Young et al., 2012). Maternal obesity is independently associated with both elevated infant growth rates (Druet et al., 2012), and later obesity risk (Boney, Verma, Tucker, & Vohr, 2005; Whitaker, 2004). While exclusive breastfeeding may impart a modest protective effect against later obesity (Beyerlein & von Kries, 2011) rapid weight gain is still associated with risk of overweight, even among breastfed infants.
Human milk (HM) is a dynamic and complex substance which delivers large doses of bioactive components (including hormones, cytokines, and prebiotics) that support infant development and optimize health (Garofalo, 2010; Groer & Shelton, 2009). Evidence from animal models suggests that transmission of obesity may be partially explained by alterations in the milk of mothers with metabolic dysfunction. Milk of obese or overnourished dams imparts deleterious programming effects to offspring, predisposing them to obesity, inflammation and components of metabolic syndrome (Du et al., 2012; Gorski, Dunn-Meynell, Hartman, & Levin, 2006; Oben et al., 2010). Data from humans is contradictory. While one epidemiological study reported no difference in pediatric obesity risk among children breastfed by normal weight vs obese mothers (Mayer-Davis et al., 2006), others suggest the protective effect of breastfeeding may even be more profound among obese mothers (Buyken et al., 2008; Li et al., 2005).
Maternal BMI may impact characteristics of HM such as % fat, leptin, adiponectin, and insulin (Ahuja et al., 2011; Andreas et al., 2014; Barbosa, Butte, Villalpando, Wong, & Smith, 1997). However, less is known about the oxidative stress and inflammatory profile of HM and how it may differ in overweight/obese (OW/Ob) mothers. F2-isoprostanes and 8-hydroxy-2-deoxy-guanosine (8-OH-dG), two marker of oxidative stress, are found in HM (Lam et al., 2012; Szlagatys-Sidorkiewicz et al., 2012). TNF-α, IL-6, and IL-8 are all inflammatory cytokines found in HM at widely varied concentrations (Groer & Shelton, 2009). Maternal obesity is characterized by chronic systemic low grade inflammation and oxidative stress; elevations of which are associated with development of the hallmarks of metabolic syndrome in mice and adults (Kirchner, Kieu, Chow, Casey, & Blumberg, 2010; Samuel & Shulman, 2012). Cytokines and markers of oxidative stress in the infant diet may directly affect cell signaling of enterocytes. Additionally, due to the permeability of the young infant gut, these bioactive compounds in HM may induce systemic effects by entering the circulation. Both of these mechanisms - alone or in concert - could theoretically affect infant nutrient utilization and adipogenesis.
Given these gaps in current understanding, this study had two aims: 1) determine if maternal OW/Ob was associated with alterations in HM macronutrient composition or concentration of inflammatory cytokines or markers of oxidative stress; and 2) determine whether variation in these HM characteristics were associated with rapid growth patterns that place the infant at risk for later obesity.
Methods
Participants
All participants provided informed consent for all study procedures, and all aspects of the study were approved by the institutional review board of Cincinnati Children's Hospital Medical Center. Participants in this cohort were a subset of the Research Human Milk Bank-Longitudinal Cohort in Cincinnati, OH (described in: (Geraghty et al., 2005; Martin et al., 2006)). Participants were recruited during pregnancy or immediately after delivery between 2002-2005. To participate, women needed to be carrying a singleton pregnancy, have no other medical complications (such as gestational diabetes or pre-eclampsia), and intending to breastfeed for ≥ 6 months. Participants needed to live in the surrounding metro-area of Cincinnati Children's Hospital, and were recruited through interaction with the University of Cincinnati Medical Center and Cincinnati Children's Hospital system (mostly surrounding their antenatal care). A single research nurse consented all women, and conducted all study visits. Women who delivered before 37 weeks or delivered an infant <2.5 kg were excluded. Birth weight was obtained by maternal report.
After delivery, participants were seen in their home every week for the first month, and every month thereafter until 6 months of age. At each visit, study personnel measured infant weight in a clean diaper using a Baby Checker Scale (Medela, McHenry, IL), and supine length using a folding lightweight measuring board (Hopkins Medical Products, Baltimore, MD), and head circumference. All measures were performed in triplicate. Infant weight-for-age, length-for-age, and weight-for-length Z-score (WAZ, LAZ and WLZ) were calculated at each time point according to WHO growth standards for breastfed infants (Prevention, 2010). Infants who exhibited an increase in WLZ >0.66 units were considered “over-gainers”.
At the first visit, maternal height was measured to the nearest 0.1 cm with participants standing (without shoes) against a wall with heels together and toes apart at 45° angle, and head in the Frankfort horizontal plane. Maternal height was marked on the wall, and distance from the floor measured. At every monthly visit, maternal weight was measured (in street clothes without shoes) to the nearest 0.1 kg using an E-Z Carry Portable Digital Scale (Hopkins Medical Products, Baltimore, MD) that was calibrated regularly.
Collection of Feeding Data and Classification of Breastfeeding Exposure
At each visit mothers were administered a questionnaire that queried about infant feeding mode (breastmilk vs formula and breast vs bottle feeding), intake of any solid food, infant health over the previous week and maternal mastitis. Breastfeeding exposure at each visit was calculated as the percentage of total breastmilk and formula feeds in the previous 24 hours that were mother's milk. Total Breastfeeding Exposure over the 6-month study period was calculated as the weighted mean of each study visit exposure and expressed as a percentage where 100% indicates only mother's milk (and no formula) was fed over the 6 month study period (Crume et al., 2012). For example, a Total Breastfeeding Exposure of 50% could indicate either 50% of all feeds were breastmilk over the first 6 months; or exclusive breastfeeding for the first 3 months followed by exclusive formula feeding for months 3 – 6. Total Breastfeeding Exposure ≥50% is defined as “Predominant Breastfeeding”. Total Breastfeeding Exposure does not reflect intake from solid foods. No infants were introduced to solid foods before the 4-month visit.
Milk Collection & Analysis
Breast milk samples were collected from mothers at 2-weeks and 4-months of age in the home by a single study nurse. Mothers were asked to completely empty one breast using an electric pump between 10:00h and 13:00h. Milk was immediately placed on ice and transported back to the laboratory. Whole milk aliquots were frozen at -800 until analysis. HM percent fat was measured by creamatocrit (Lawrence, 2011). Milk was skimmed by centrifugation at 10,000 rcf for 10 minutes at 40C, followed by the removal of the fat layer. Lactose was measured by enzymatic digestion and spectrophotometric detection of galactose (BioVision, Inc. Milpitas, CA), and protein were measured using a modification to the Bradford Method (BioRad Inc. Hercules, CA). HM caloric density was calculated as follows:
4-hydroxynonenol (HNE) (ug/mg BSA standard) in HM was measured using an adduct competitive ELISA (Cell BioLabs Inc, SanDiego, CA) as follows: skim milk samples were diluted 1:5 in distilled water in order to dilute out inhibitors, and then 25µg/mL of HNE/BSA standard was added to each sample in order to recover in the linear range of the standard curve. HNE was only measured in a subset of samples (n=51) due to alterations in the kit formulation after that point. 8-hydroxy-deoxyguanosine (8OHdG) was measured in skim milk samples using a commercial EIA (Cayman Chemical, Ann Arbor, MI). HM IL-6, IL-8, IL-10 and TNF-α were measured simultaneously using a magnetic bead Milliplex Assay (Millipore, Billerica, MA). Inflammatory cytokine “load” was calculated as the sum of the standardized mean of IL-6, IL-8, and TNF- α at both 2-weeks and 4-months.
Statistical Analysis
All variables were tested for normality using the Shapiro-Wilks test. Differences in maternal and infant characteristics, and HM composition between normal weight (NW) and OW/Ob mothers were tested using a t-test or Wilcoxon test.
Simple linear regression was used to determine predictors of HNE and 8OHdG in HM and to test cross-sectional relationships between milk characteristics and infant WLZ at 2-weeks and 4-months. Non-normally distributed variables were log-transformed to ensure normality of the model's residuals.
When constructing a longitudinal model of WLZ trajectory using all of the infant anthropometric measures collected; 6 initial model forms were considered: a linear model with a random linear effect, quadratic models with random linear and quadratic effects, and cubic models with random linear, quadratic, and cubic effects. All covariates of interest were included in these candidate models. After the final model form was chosen, backwards selection was used to identify the covariates that would remain in the final model. Covariates were considered significant in the model with a parameter-estimate p<0.15. The initial covariates considered were: maternal BMI group (NW vs OW/Ob), maternal age, parity, delivery mode, infant sex, birth weight, breastfeeding exposure, age of introduction of solid food, total solid food intake by 6-months, and the average of the 2-week and 4-month (mean) HM components: macronutrient, calories, individual cytokines, cytokine “load”, 8OHdG, and HNE. Interactions between maternal BMI group and HM characteristics were also considered. All statistical analyses were performed using SAS (SAS Institute Inc., Cary, NC).
Results
Participant Characteristics
The Research Human Milk Bank-Longitudinal Cohort recruited at total of 61 mother/infant dyads. This study includes a final sample size of 56 dyads, from whom milk samples were available at both 2-weeks and 4-months. The five maternal/infant dyads that were excluded lacked either adequate follow up (to 6-months) or adequate milk volume for testing.
Maternal characteristics are presented in Table 1. Over 90% of the mothers identified as non-Hispanic White. Pre-pregnancy BMI was unavailable in these women and thus mothers were categorized based on their BMI at the 6-month visit, as postpartum weight loss is likely to have plateaued at this time. At that time, maternal BMI ranged from 19.8 to 34.8 kg/m2 with 51 % of the cohort classified as normal weight (BMI < 25 kg/m2), 27% as overweight (BMI ≥ 25 and < 30 kg/m2), and 22% as obese (BMI ≥ 30 kg/m2). All infant birth weights were normal (between 2500 and 4500g). Changes in infant anthropometric z-scores are presented in Figure 1.
Table 1. Maternal and Infant Characteristics1.
| All | NW (n=33) | OW/Ob (n=23) | p-value2 | |
|---|---|---|---|---|
| Maternal Age (y) | 31.7±4.5 | 31.1 ±4.4 | 32.5±4.6 | 0.27 |
| Maternal BMI (kg/m2) | 24.5±3.6 | 22.1±1.4 | 27.8±3.0 | <0.0001 |
| Parity | 1.0±1.0 | 0.8±0.8 | 1.3±1.3 | 0.08 |
| Gestational age (wks) | 39.6±1.2 | 39.5±1.2 | 39.9±1.2 | 0.16 |
| Infant Sex (male) | 48% | 52% | 43% | 0.96 |
| Delivery Type (Vaginal) | 77% | 76% | 78% | 0.83 |
| Birth Weight (g) | 3,407±417 | 3,374±392 | 3,455±457 | 0.48 |
| Total Breastfeeding Exposure (%)3 (not including solids) | 93.7±9.7 | 94.4±9.1 | 92.8±10.7 | 0.55 |
| % of Infants consuming Solid Food3 Age of introduction (wks) | 67% 21.6 ± 2.5 | 69% 21.3 ± 2.6 | 65% 22.1 ± 2.4 | 0.78 0.39 |
Presented as mean ± SD
P-value for comparison between groups
At the final 6-month visit
NW = Normal weight mothers; OW/Ob= Overweight/Obese mothers
Figure 1.
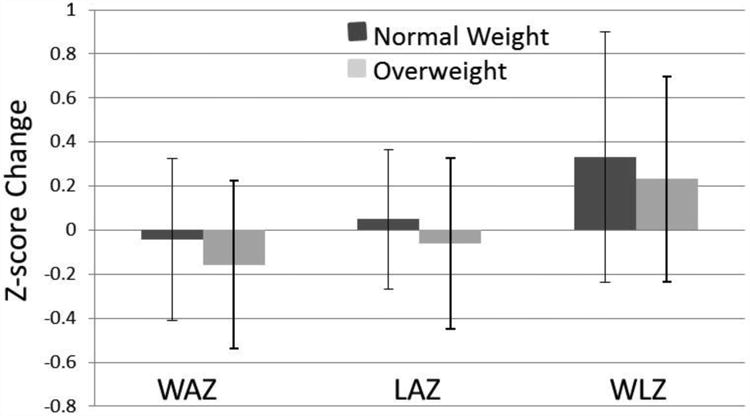
Change in infant anthropometric Z-scores from 2-weeks to 4-months of life. WAZ = weight-for-age Z-score LAZ = length-for-age Z-score; WLZ = weight-for-length Z-score. Data are presented as mean ± SD.
The 2-week visit occurred at 15.0 ± 2.6 days; the 4-month and 6-month visit at 16.6 ± 1.2 and 25.8 ± 1.9 weeks, respectively. There were no differences in WAZ, LAZ and WLZ between infants of NW and overweight/obese (OW/Ob) women at any time point. Average Total Breastfeeding Exposure was 94% with a minimum of 66%. Thus, all infants were either exclusively or predominantly breastfed.
Differences in HM Characteristics
Milk macronutrients and concentrations of cytokines and markers of oxidative stress are shown in Table 2. Protein concentrations were lower in HM of OW/Ob women at 2-weeks (p=0.04); lactose was lower in HM of OW/Ob women at 4-months (p=0.02), and HNE concentrations were higher in HM of OW/Ob women at 4-months (p=0.03). No consistent differences between groups were observed at both time points. In the cohort as a whole, milk fat and caloric density did not change over time. Protein concentrations decreased from 2-weeks to 4-months by an average of 0.33 mg/100mL (p<0.0001) and lactose concentrations increased by an average of 0.79 mg/100mL (p<0.0001). Lactose concentrations at 2-weeks were predictive of lactose concentrations at 4-months (p=0.005) whereas protein concentrations and caloric density at 2-weeks were unrelated to those at 4-months.
Table 2. Human Milk Composition.
| 2-weeks | 4-months | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HM Analyte1 | All | NW | OW/Ob | p-value3 | All | NW | OW/Ob | p-value3 | p-value4 |
| Fat (g/100mL) | 3.6 ±1.2 | 3.8 ±1.2 | 3.4 ±1.3 | 0.20 | 3.4 ±1.3 | 3.2 ±1.3 | 3.7 ±1.3 | 0.17 | 0.42 |
| Protein (g/100mL) | 1.1 ±0.2 | 1.1 ± 0.2 | 1.0 ±0.2 | 0.05 | 0.8 ±0.2 | 0.8 ±0.2 | 0.8 ±0.2 | 0.28 | <0.0001 |
| Lactose (g/100mL) | 6.5 ±0.9 | 6.5 ±0.7 | 6.6 ±1.0 | 0.46 | 7.3 ±0.8 | 7.5 ±0.7 | 7.0 ±0.9 | 0.02 | <0.0001 |
| Calories per 100mL | 63.0 ±10.9 | 64.4 ±10.4 | 60.8 ±11.6 | 0.23 | 62.3 ±12.1 | 61.9 ±11.9 | 64.4 ±12.5 | 0.46 | 0.99 |
| 8OHdG (ng/mL) | 18.1 ±9.5 | 18.4 ±8.9 | 17.6 ±10.6 | 0.76 | 13.4 ±8.2 | 14.6 ±9.5 | 11.6 ±5.4 | 0.17 | <0.0001 |
| HNE-BSA (ug/mL)* | 115.1 ±46.8 | 120.0 ±48.3 | 107.8 ±44.8 | 0.38 | 101 ±137 | 92.5 ±36.6 | 114.9 ±30.0 | 0.03 | 0.004 |
| IL-6 (pg/mL) | 9.6 ±19.9 | 12.1 ±25.3 | 6.0 ±5.8 | 0.26 | 3.0 ±6.2 | 3.4 ±7.4 | 2.3 ±3.7 | 0.50 | <0.00015 |
| IL-8 (pg/mL) | 97.0 ±137. 5 | 111.5 ±190. 8 | 98.3 ±146.0 | 0.78 | 67.0 ±92.9 | 74.2 ±112. 9 | 56.1 ±50.7 | 0.48 | 0.0685 |
| TNF-α (pg/mL) | 4.3 ±2.9 | 4.3 ±3.1 | 4.4 ±2.6 | 0.85 | 3.0 ±2.4 | 2.9 ±2.5 | 3.0 ±2.2 | 0.88 | 0.0125 |
| Inflammator y “load” 2 | -0.00 ±2.6 | 0.14 ±3.07 | -0.20 ±1.85 | 0.62 | -0.00 ±2.6 | 0.13 ±3.13 | -0.20 ± 1.61 | 0.61 | 0.99 |
Presented as Mean ± SD
Calculated as the sum of the standardized mean of IL-6, IL-8, and TNF-α
p-value for comparison between groups.
p-value for change over time.
p-value for change over time controlling for the decrease in total protein.
n=51
NW = Normal weight mothers; OW/Ob= Overweight/Obese mothers
The concentrations of cytokines in HM were widely variable in each group (Table 2). Concentrations of IL-6 and TNF-α in HM decreased over time (p<0.0001). When controlling for the decrease in total protein, the decrease in IL-6 (p<0.0001), IL-8 (p=0.068), and TNF-α (p=0.012) remained at least borderline significant. These decreases corresponded to 6% of original concentrations of IL-6; 69% of original concentrations of IL-8; and a 15% of original concentrations of TNF-α. Controlling for the decrease in total protein, concentrations of IL-6, IL-8, and TNF-α at 2-weeks were all significantly correlated with the concentration at 4-months (p<0.0001, R2=0.31; p=0.004, R2=0.15; p<0.0001, R2=0.41, respectively). Furthermore, the concentration of these three cytokines were all inter-correlated with each other at both 2-weeks and 4-months (p<0.0001, R2 ≥ 0.3).
Both 8OHdG and HNE (controlled for total protein) decreased over time (p<0.0001, n=56 and p=0.03, n=46 respectively). Concentrations of 8OHdG and HNE at 2-weeks was positively correlated with concentrations at 4-months (HNE: p=0.030, R2 = 0.10, n =46, Figure 2A; and 8OHdG: p<0.0001, R2=0.39, n=56, Figure 2B). HNE and 8OHdG were not correlated with each other at either time point. Neither individual macronutrients, caloric density nor inflammatory cytokines were related to either 8OHdG or HNE at any time. Maternal BMI, age, type of delivery, and infant sex were all unrelated to HM HNE and 8OHdG. Maternal parity was positively related to both HNE and 8OHdG at 2-weeks (p=0.04, n=48 and p=0.007, n=56). Only one HM sample was collected from a mother with mastitis, at 2-weeks. While macronutrients and inflammatory cytokines in this sample were normal, concentrations of 8OHdG were the highest observed (45.5 ng/mL), but returned to normal by 4-months.
Figure 2.
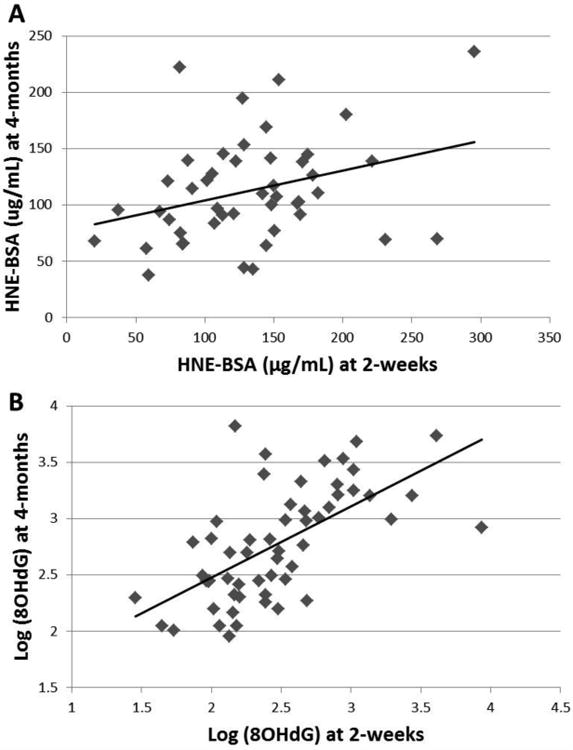
Morning breast milk samples (full breast collection) were obtained from women at 2-weeks and 4-months postpartum. Markers of oxidative stress, HNE and 8OHdG were measured in all samples via ELISA. A) HNE in HM at 2-weeks was positively correlated with concentrations in HM at 4-months: p=0.030, R2 = 0.10, n =46. B) 8OHdG in HM at 2-weeks was positively correlated with concentrations in HM at 4-months: p<0.0001, R2=0.39, n=56.
Introduction of Solid Foods
None of the infants were introduced to solid foods before the 4-month visit. 62% of the infants began consuming solid foods before the 6-month time point; 38% of infants were not consuming any solids at the conclusion of the study (Table 1). Among the 63% consuming complementary food, the average age of introduction was 21.6 ± 2.5 weeks (~4.8 months). In this subset of infants, solid foods were offered to infants on average 2.0 ± 0.8 times per day at the final 6-month visit. Neither solid food intake at 6 months (as feedings per day) nor age of introduction of solids were related to any markers of growth. Neither complementary food parameter differed between over- vs. normal-gainers, or between maternal BMI groups, and neither correlated with Total Breastmilk Exposure. Intake of solids at 6-months and age of introduction of solids were also not significant in models of WLZ trajectory. These results together suggest that solid food intake was not contributing a large relative proportion of caloric intake over the course of the study period.
Relationships with Infant Growth Characteristics
By 4-months, 30% of the infants had exhibited increases in WLZ > 0.66 (from 2-weeks to 4-months). By 6-months, 42% of the infants had exhibited increases in WLZ > 0.66 (from 2-weeks to 6-months). The average weight gain per day among these “over-gainers” from 2-weeks to 6 months was 26 g/day (n=23) compared with 21 g/day (n=32) (p<0.0001). The proportion of infants exhibiting this rapid weight gain did not differ between NW and OW/Ob women. Additionally, degree of formula supplementation (Total Breastfeeding Exposure) was not different between over- vs. normal-gainers. The total protein content of HM at 4-months was lower among over-gainers (0.84±0.19 vs 0.70±0.15, p=0.006). However, no HM components differed between over- vs normal-gainers at either time point.
In the cohort as a whole, neither individual HM macronutrients, caloric density, cytokines, nor HNE were related to infant WLZ at either 2-weeks or 4-months. 8OHdG in HM at 2-weeks was positively related to infant WLZ at 2-weeks (p=0.007, R2=0.13, n=56, Figure 3A) and at 4-months (p=0.04, R2=0.08, n=56, Figure 3B). There was a trend for a positive relationship between 8OHdG in HM at 4-months and infant WLZ at 6-months (p=0.06, R2=0.07, n=55).
Figure 3.
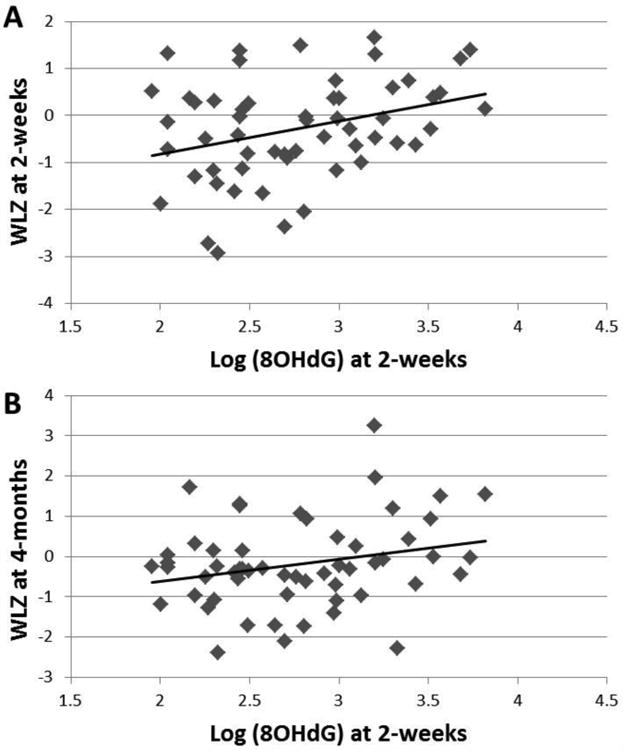
In this cohort of exclusively breastfed infants, HM 8OHdG concentrations at 2-weeks were positively correlated with A) concurrent infant weight-for-length Z-scores (WLZ) at 2-weeks (p=0.007, R2=0.13, n=56) as well as with B) prospective WLZ at 4-months (p=0.04, R2=0.08, n=56).
When modeling WLZ trajectory over the first 6-months, a linear repeated measures model was the best fit for the longitudinal WLZ data; final model results are presented in Table 3. Infant birth weight, HM lactose, HM 8OHdG, and interactions between maternal group and HM composition remained significant statistical predictors of infant WLZ trajectory.
Table 3.
Model of infant WLZ trajectory from 2-weeks to 6-months of age.
| Parameter | Estimate | Std Error | p-value2 |
|---|---|---|---|
| Intercept | -1.5059 | 1.9356 | 0.4401 |
| Time (weeks) | 0.0046 | 0.0059 | 0.4384 |
| Group (NW = 0) | -6.0999 | 2.1646 | 0.0051 |
| Birth Weight (g) | 0.5422 | 0.2636 | 0.0404 |
| 8OHdG1 | 0.0172 | 0.0167 | 0.3038 |
| 8OHdG*Group (NW = 0) | 0.07600 | 0.02975 | 0.0111 |
| Lactose1 | -0.1153 | 0.2291 | 0.6150 |
| Lactose*Group (NW = 0) | 0.7354 | 0.3086 | 0.0177 |
Mean value of 2-week and 4-month concentrations
Parameter estimate p-value
NW = Normal weight mothers
Discussion
In this longitudinal cohort of predominantly breastfed infants, we fail to find any evidence that maternal overweight is associated with alterations in HM macronutrient composition or in inflammatory or oxidative stress characteristics. We did detect a novel, significant interaction between 8OHdG concentrations and maternal BMI category on infant WLZ trajectory. While 8OHdG and HNE have been previously detected in HM (Michalski, Calzada, Makino, Michaud, & Guichardant, 2008), this is the first longitudinal characterization of concentrations and predictors of these markers of oxidative stress in HM.
The most well-characterized marker of lipid peroxidation in HM, F2-isoprostanes, decreases over time as lactation progresses (Szlagatys-Sidorkiewicz et al., 2012). This temporal trend is similar to our finding that concentrations of HNE and 8OHdG in HM decrease from 2-weeks to 4-months of lactation. The reason for this decline is unknown. However, the decrease in HM may potentially be attributed to the powerful metabolic demand placed on the mammary gland early in lactation during secretory activation in addition to the metabolic stress and turbulence that results from active maternal healing during the peri-partum period. Our data from one mother with mastitis at 2-weeks suggests that local infection in the mammary gland does increase 8OHdG (but not HNE) concentrations in HM.
Mechanisms whereby markers of oxidative stress consumed by the infant may impact infant WLZ are speculative at this point but likely multifactorial in nature. 8OHdG has never been studied in the context of HM and so its exact activity in the infant remains unknown. The newborn gut is relatively “leaky” as tight junctions are not fully closed. As a result, relatively large molecules can enter the infant circulation intact and exert hormonal effects. Biological activity of the molecule 8OHdG has been demonstrated in murine brain microglial cells and macrophages where it's positive effects include reduction of LPS-induced reactive oxidative species (ROS) and nitric oxide (NO) production, inhibition of Rac1 signaling and reduction of TNF-α, IL-6, and IFN-γ expression (D. H. Kim et al., 2006; H. S. Kim et al., 2006; Lee et al., 2009). Oral administration of 8OHdG, in a murine model of atherosclerosis, exerted powerful antiinflammatory effects which reduced plaque formation via these mechanisms in neutrophils, macrophages, and vascular smooth muscle cells (the only cell types studied) (Huh et al., 2012). Interestingly, the oral dose of 8OHdG administered to rats in that study was only 12 times greater than the estimated average dose received by an exclusively breastfed infant at 4 months of age (based on average concentration of 8OHdG detected in this study, and average milk consumption of infants at this age (Huh et al., 2012)). These studies suggest that oral 8OHdG consumed by the infant via HM may indeed alter signaling pathways in various cell types in the infant.
It is also possible that 8OHdG exerts effects on the development and/or function of enterocytes or immune cells in the intestinal mucosa. 8OHdG has been shown to affect murine macrophage signaling and function both in-vitro and in-vivo (Huh et al., 2012; Lee et al., 2009), giving credence to the hypothesis that this molecule could also affect the local environment of the infant intestine, theoretically impacting WLZ downstream.
Lastly, it is possible that 8OHdG in HM simply serves as a proxy for some unidentified exposure. All of these potential mechanisms remain speculative and should be the focus of future research.
The interaction between HM 8OHdG concentrations and maternal BMI group on infant WLZ trajectory is of particular note. The nature of the interaction suggests that 8OHdG in HM has a positive impact on WLZ trajectory only among infants of OW/Ob women. A positive WLZ trajectory is equivalent to an infant crossing growth curve percentiles in an upwards fashion. Sustained upwards growth in that manner during the first 6 months of life places an infant at risk for later obesity (Taveras et al., 2011; Young et al., 2012). Our models suggest that 8OHdG in HM may either contribute to accelerated weight gain, or limited linear growth, resulting in increased WLZ. The fact that this relationship occurs only among infants of OW/Ob women is reminiscent of the “double hit hypothesis” which theorizes that exposure to maternal obesity in-utero results in malprogramming effects that predispose offspring to later obesity especially/only in the context of a second risk factor.
The nature of the interaction between HM lactose and maternal BMI group on infant WLZ trajectory is similar: lactose concentrations were positively related to increases in WLZ trajectories (which is a profile that may place infants at risk for later overweight), but only among infants of OW/Ob women. In addition to providing energy to the infant as the main carbohydrate source in HM, lactose plays additional roles in the large intestine. A small amount of lactose arrives in the large intestine undigested, serving as a prebiotic for resident bacteria that ferment lactose and produce short chain fatty acids (SCFA) (Alexandre et al., 2013) which are utilized as an energy source by colonocytes (He et al., 2006). Additionally, lactose induces expression of the antimicrobial peptide, cathelicidin in colonic epithelial cells, indicating a role for lactose in development of the innate immune system and intestinal microbiota in infants (Cederlund et al., 2013). As the microbial communities that settle in the intestine during infancy contribute to the regulation and rate of weight gain and body composition later in childhood, modulation of this early colonization may affect growth trajectory (Kalliomaki, Collado, Salminen, & Isolauri, 2008). In the context of the double-hit hypothesis, higher doses of lactose may contribute to increased weight gain only among infants who acquired predisposition for metabolic disease in utero via exposure to maternal obesity.
The role of HM cytokines in the development of the innate immune system of the immature infant gut is relatively well established (Garofalo, 2010). Furthermore elevated circulating inflammatory cytokines (especially TNF-α and IL-6) can result in suppressed bone and linear growth (Sederquist, Fernandez-Vojvodich, Zaman, & Savendahl, 2014). We failed to detect any differences in HM cytokines based on maternal BMI group. We did detect strong inter-relationships between all cytokines measured and over time. This means that certain infants were exposed to relatively high concentrations of all of these cytokines consistently throughout the entire 4-months of lactation. Given this exposure, the complete details of the role of HM cytokines in infant growth and development should be further studied.
Previously, comprehensive characterization of HM that includes markers of oxidative stress and inflammation by maternal phenotype has been unavailable. Our novel data indicate that milk composition (both macronutrient profile and bioactive components) is not affected by maternal overweight, which corroborates recommendations to exclusively breastfeed regardless of maternal size. The novelty of our assessment of markers of oxidative stress along with inflammatory cytokines in HM improves to our understanding of the complex and dynamic nature of HM, and suggests that maternal BMI phenotype has limited impact on these bioactive components of HM. Our cohort of predominantly breastfed infants allowed us to address infant growth parameters while controlling for feeding mode, the most potent confounder of infant growth. Our study was not without limitations. This was a secondary analysis of the Research Human Milk Bank-Longitudinal Cohort of Cincinnati, OH and thus no a-priori sample size calculation was performed and we may be underpowered to detect relationship between other HM components that have smaller effect sizes. Furthermore, this cohort was relatively homogeneous in nature, and thus results may not be generalizable to populations that are more racially and ethnically diverse, and have a higher degree of formula supplementation.
Lack of pre-pregnant BMI is a noted limitation. We chose to classify women by their 6-month BMI, as some women would have been actively losing weight at 4-months. It is likely that the physiological state of active weight loss would have more impact on mothers' metabolic status and milk composition than her static BMI at that time. Thus, classifying women by her more stable weight status at 6-months provided the most clinically relevant approach. Additionally, while the range in maternal BMI in this cohort was extensive, we did not have a large proportion of obese women, which reduced our power to detect differences in milk composition in the highest risk women. However, no additional differences in milk composition or infant growth were detected when OW and Ob mothers were analyzed separately (data not shown). Additionally, we did not collect quantitative data on milk volume, and thus are unable to make inferences about total infant caloric intake or total daily exposure to these bioactive components.
This is the first comprehensive study to characterize the predictors of both HNE and 8OHdG in HM. Our inability to detect differences in the milk of OW/Ob vs NW women demonstrates that maternal phenotype does not seem to impact components of HM that are characteristically associated with obesity. The tight correlations between concentrations of several inflammatory cytokines and markers of oxidative stress over time highlights the large range of exposure habitually experienced by healthy breastfed infants. We did, however, establish that 8OHdG and lactose in HM are associated with a positive infant WLZ trajectory only among infants born to OW/Ob women, perhaps reflecting differential susceptibility to postnatal exposures following different in-utero environments. Future research should focus on the mechanism whereby markers of oxidative stress in HM may contribute to the regulation of infant weight gain among breastfed infants.
Significance Statement.
What is already known about this topic?
Human milk (HM) is full of bioactive components that may impact infant growth. Maternal overweight/obesity impacts some hormones in HM, but whether it affects cytokines and markers of oxidative stress remains unknown.
What this study adds?
We demonstrate that macronutrients and concentrations of inflammatory cytokines and 2 markers of oxidative stress (HNE and 8OHdG) do not differ in HM of normal weight vs overweight women. 8OHdG and lactose were associated with an upwards weight-for-length z-score trajectory only among infants breastfed by overweight mothers.
Acknowledgments
We wish to thank the mothers and infants who participated in this research. This work was supported in part by: National Institute of Health: NIDDK T32: DK007658-21 and NIDDK K24: DK083772 and NICHD P01: HD1302; Colorado Clinical & Translational Sciences Institute (CCTSI) Child and Maternal Health Award and with the Development and Informatics Service Center (DISC) grant support (NIH/NCRR Colorado CTSI Grant Number UL1 RR025780); and Cincinnati Clinical & Translational Sciences & Training (CCTST): Grant ULRR026314
List of Abbreviations
- WAZ
weight for age Z-score
- LAZ
length for age Z-score
- WLZ
weight for length Z-score
- HM
human milk
- 8OHdG
8-hydroxy-deoxyguanosine
- HNE
4-hydroxynonenol
Footnotes
Clinical Trials Registry: This study was approved prior to 2008 so no clinical trials number was assigned.
Contributor Information
Bridget E. Young, University of Colorado School of Medicine - Department of Pediatrics Section of Nutrition, Aurora, CO.
Zachary W. Patinkin, University of Colorado School of Medicine - Department of Pediatrics Section of Nutrition, Aurora, CO.
Laura Pyle, University of Colorado School of Medicine - Department of Pediatrics Section of Nutrition, Aurora, CO; University of Colorado School of Public Health - Department of Biostatistics and Informatics, Aurora, CO.
Becky de la Houssaye, University of Colorado School of Medicine - Department of Pediatrics Section of Neonatology, Aurora, CO.
Barbara S. Davidson, Cincinnati Children's Hospital Medical Center - Department of Pediatrics, Cincinnati, OH.
Sheela Geraghty, Cincinnati Children's Hospital Medical Center - Center for Breastfeeding Medicine, Cincinnati, OH.
Ardythe L. Morrow, Cincinnati Children's Hospital Medical Center - Department of Pediatrics, Cincinnati, OH.
Nancy Krebs, University of Colorado School of Medicine - Department of Pediatrics Section of Nutrition, Aurora, CO.
References
- Ahuja S, Boylan M, Hart S, Roman-Shriver C, Spallholz J, Pence B, et al. Sawyer B. Glucose and Insulin Levels are Increased in Obese and Overweight Mothers' Breast-Milk. Food and Nutrition Sciences. 2011;2:201–206. [Google Scholar]
- Alexandre V, Even PC, Larue-Achagiotis C, Blouin JM, Blachier F, Benamouzig R, et al. Davila AM. Lactose malabsorption and colonic fermentations alter host metabolism in rats. British Journal of Nutrition. 2013;110(4):625–631. doi: 10.1017/S0007114512005557. [DOI] [PubMed] [Google Scholar]
- Andreas NJ, Hyde MJ, Gale C, Parkinson JR, Jeffries S, Holmes E, et al. Modi N. Effect of maternal body mass index on hormones in breast milk: a systematic review. PloS One. 2014;9(12):e115043. doi: 10.1371/journal.pone.0115043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa L, Butte NF, Villalpando S, Wong WW, Smith EO. Maternal energy balance and lactation performance of Mesoamerindians as a function of body mass index. The American Journal of Clinical Nutrition. 1997;66(3):575–583. doi: 10.1093/ajcn/66.3.575. [DOI] [PubMed] [Google Scholar]
- Beyerlein A, et al. von Kries R. Breastfeeding and body composition in children: will there ever be conclusive empirical evidence for a protective effect against overweight? American Journal of Clinical Nutrition. 2011;94(6 Suppl):1772S–1775S. doi: 10.3945/ajcn.110.000547. [DOI] [PubMed] [Google Scholar]
- Boney CM, Verma A, Tucker R, et al. Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- Buyken AE, Karaolis-Danckert N, Remer T, Bolzenius K, Landsberg B, et al. Kroke A. Effects of breastfeeding on trajectories of body fat and BMI throughout childhood. Obesity (Silver Spring) 2008;16(2):389–395. doi: 10.1038/oby.2007.57. [DOI] [PubMed] [Google Scholar]
- Cederlund A, Kai-Larsen Y, Printz G, Yoshio H, Alvelius G, Lagercrantz H, et al. Agerberth B. Lactose in human breast milk an inducer of innate immunity with implications for a role in intestinal homeostasis. PloS One. 2013;8(1):e53876. doi: 10.1371/journal.pone.0053876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crume TL, Ogden LG, Mayer-Davis EJ, Hamman RF, Norris JM, Bischoff KJ, et al. Dabelea D. The impact of neonatal breast-feeding on growth trajectories of youth exposed and unexposed to diabetes in utero: the EPOCH Study. Int J Obes (Lond) 2012 doi: 10.1038/ijo.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druet C, Stettler N, Sharp S, Simmons RK, Cooper C, Smith GD, et al. Ong KK. Prediction of childhood obesity by infancy weight gain: an individual-level meta-analysis. Paediatr Perinat Epidemiol. 2012;26(1):19–26. doi: 10.1111/j.1365-3016.2011.01213.x. [DOI] [PubMed] [Google Scholar]
- Du Y, Yang M, Lee S, Behrendt CL, Hooper LV, Saghatelian A, Wan Y. Maternal western diet causes inflammatory milk and TLR2/4-dependent neonatal toxicity. Genes and Development. 2012;26(12):1306–1311. doi: 10.1101/gad.191031.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo R. Cytokines in human milk. The Journal of Pediatrics. 2010;156(2 Suppl):S36–S40. doi: 10.1016/j.jpeds.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Geraghty SR, Davidson BS, Warner BB, Sapsford AL, Ballard JL, List BA, et al. Morrow AL. The development of a research human milk bank. J Hum Lact. 2005;21(1):59–66. doi: 10.1177/0890334404273162. [DOI] [PubMed] [Google Scholar]
- Gorski JN, Dunn-Meynell AA, Hartman TG, et al. Levin BE. Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2006;291(3):R768–778. doi: 10.1152/ajpregu.00138.2006. [DOI] [PubMed] [Google Scholar]
- Groer MW, Shelton MM. Exercise is associated with elevated proinflammatory cytokines in human milk. J Obstet Gynecol Neonatal Nurs. 2009;38(1):35–41. doi: 10.1111/j.1552-6909.2008.00303.x. [DOI] [PubMed] [Google Scholar]
- He T, Priebe MG, Harmsen HJ, Stellaard F, Sun X, Welling GW, Vonk RJ. Colonic fermentation may play a role in lactose intolerance in humans. Journal of Nutrition. 2006;136(1):58–63. doi: 10.1093/jn/136.1.58. [DOI] [PubMed] [Google Scholar]
- Huh JY, Son DJ, Lee Y, Lee J, Kim B, Lee HM, et al. Chung MH. 8-Hydroxy-2-deoxyguanosine prevents plaque formation and inhibits vascular smooth muscle cell activation through Rac1 inactivation. Free Radical Biology and Medicine. 2012;53(1):109–121. doi: 10.1016/j.freeradbiomed.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliomaki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. The American Journal of Clinical Nutrition. 2008;87(3):534–538. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- Kim DH, Cho IH, Kim HS, Jung JE, Kim JE, Lee KH, et al. Chung MH. Anti-inflammatory effects of 8-hydroxydeoxyguanosine in LPS-induced microglia activation: suppression of STAT3-mediated intercellular adhesion molecule-1 expression. Experimental and Molecular Medicine. 2006;38(4):417–427. doi: 10.1038/emm.2006.49. [DOI] [PubMed] [Google Scholar]
- Kim HS, Ye SK, Cho IH, Jung JE, Kim DH, Choi S, et al. Chung MH. 8-hydroxydeoxyguanosine suppresses NO production and COX-2 activity via Rac1/STATs signaling in LPS-induced brain microglia. Free Radical Biology and Medicine. 2006;41(9):1392–1403. doi: 10.1016/j.freeradbiomed.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Kirchner S, Kieu T, Chow C, Casey S, Blumberg B. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Molecular endocrinology (Baltimore, Md) 2010;24(3):526–539. doi: 10.1210/me.2009-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam PM, Mistry V, Marczylo TH, Konje JC, Evans MD, Cooke MS. Rapid measurement of 8-oxo-7,8-dihydro-2′-deoxyguanosine in human biological matrices using ultra-high-performance liquid chromatography-tandem mass spectrometry. Free Radic Biol Med. 2012;52(10):2057–2063. doi: 10.1016/j.freeradbiomed.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence RAL, R M. Breastfeeding - A Guide for the Medical Professional. 7. Elsevier Mosby; 2011. [Google Scholar]
- Lee SH, Taek Han S, Choi SW, Sung SY, You HJ, Ye SK, et al. Chung MH. Inhibition of Rac and Rac-linked functions by 8-oxo-2′-deoxyguanosine in murine macrophages. Free Radical Research. 2009;43(1):78–84. doi: 10.1080/10715760802609432. [DOI] [PubMed] [Google Scholar]
- Li C, Kaur H, Choi WS, Huang TT, Lee RE, et al. Ahluwalia JS. Additive interactions of maternal prepregnancy BMI and breast-feeding on childhood overweight. Obesity Research. 2005;13(2):362–371. doi: 10.1038/oby.2005.48. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Woo JG, Geraghty SR, Altaye M, Davidson BS, Banach W, et al. Morrow AL. Adiponectin is present in human milk and is associated with maternal factors. The American Journal of Clinical Nutrition. 2006;83(5):1106–1111. doi: 10.1093/ajcn/83.5.1106. [DOI] [PubMed] [Google Scholar]
- Mayer-Davis EJ, Rifas-Shiman SL, Zhou L, Hu FB, Colditz GA, Gillman MW. Breastfeeding and risk for childhood obesity: does maternal diabetes or obesity status matter? Diabetes Care. 2006;29(10):2231–2237. doi: 10.2337/dc06-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalski MC, Calzada C, Makino A, Michaud S, Guichardant M. Oxidation products of polyunsaturated fatty acids in infant formulas compared to human milk--a preliminary study. Mol Nutr Food Res. 2008;52(12):1478–1485. doi: 10.1002/mnfr.200700451. [DOI] [PubMed] [Google Scholar]
- Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life--a systematic review. Obes Rev. 2005;6(2):143–154. doi: 10.1111/j.1467-789X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- Oben JA, Mouralidarane A, Samuelsson AM, Matthews PJ, Morgan ML, McKee C, et al. Taylor PD. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol. 2010;52(6):913–920. doi: 10.1016/j.jhep.2009.12.042. [DOI] [PubMed] [Google Scholar]
- Prevention, C. f. D. C. a. Growth Charts. WHO Growth Standards Are Recommended for Use in the U S for Infants and Children 0 to 2 Years of Age. 2010 Retrieved from http://www.cdc.gov/growthcharts/who_charts.htm.
- Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148(5):852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederquist B, Fernandez-Vojvodich P, Zaman F, et al. Savendahl L. Recent research on the growth plate: Impact of inflammatory cytokines on longitudinal bone growth. Journal of Molecular Endocrinology. 2014;53(1):T35–44. doi: 10.1530/JME-14-0006. [DOI] [PubMed] [Google Scholar]
- Szlagatys-Sidorkiewicz A, Zagierski M, Jankowska A, Luczak G, Macur K, Baczek T, et al. Kaminska B. Longitudinal study of vitamins A, E and lipid oxidative damage in human milk throughout lactation. Early Human Development. 2012;88(6):421–424. doi: 10.1016/j.earlhumdev.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Taveras EM, Rifas-Shiman SL, Sherry B, Oken E, Haines J, Kleinman K, et al. Gillman MW. Crossing growth percentiles in infancy and risk of obesity in childhood. Archives of Pediatrics and Adolescent Medicine. 2011;165(11):993–998. doi: 10.1001/archpediatrics.2011.167. [DOI] [PubMed] [Google Scholar]
- Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114(1):e29–36. doi: 10.1542/peds.114.1.e29. [DOI] [PubMed] [Google Scholar]
- Young BE, Johnson SL, et al. Krebs NF. Biological determinants linking infant weight gain and child obesity: current knowledge and future directions. Advances in Nutrition. 2012;3(5):675–686. doi: 10.3945/an.112.002238. [DOI] [PMC free article] [PubMed] [Google Scholar]


