Abstract
Background
Patients with AML without complete remission (CR) or in first relapse (relapse1) can have extended leukemia control and survival following allogeneic hematopoietic cell transplantation (HCT). Transplantation in relapse1 or primary induction failure (PIF) versus treatment to achieve CR2 and subsequent HCT might yield similar outcomes, but available comparative data are scarce.
Methods
We studied 4682 HCT recipients, analyzing survival by disease status: PIF (N=1440), relapse1 (failing ≥1 reinduction, N=1256) and CR2 (N=1986).
Results
Patient, disease and transplantation characteristics were similar except CR2 patients more often had performance scores of 90-100, de novo AML and longer duration of CR1. Adverse cytogenetics were more common in PIF patients. 5-year survival adjusted for performance score, cytogenetic risk and donor type for CR2 was 39% (95% CI 37-41) compared to 18% (95% CI 16-20) for HCT in relapse1 and 21% (95% CI 19-23) in PIF, p<0.0001.
Conclusion
Although survival is superior for HCT in CR2, transplantation for selected patients in relapse1 or PIF may still be valuable. These data can guide decision-making about additional salvage therapy versus prompt HCT for patients not in CR, but also highlight intrinsically more treatable AML who have favorable risk cytogenetics, longer CR1 duration and younger patients with better performance status.
Keywords: Acute Myeloid Leukemia, Allogeneic transplantation, Complete remission, Primary induction failure, Relapse
Introduction
For patients with acute myeloid leukemia (AML) failing to achieve complete remission (CR) or in relapse after initial remission, allogeneic hematopoietic cell transplantation (HCT) can produce leukemia control and extended survival (1-4). Some reports suggest that immediate transplantation, if an available donor can be quickly identified, is a best strategy for primary induction failure (PIF) or at first relapse (relapse1) (5-8). Other data argue that additional therapy to achieve remission – whether a difficult-to-achieve CR1 or CR2, yields favorable outcomes and superior survival (9-19) noting that achieving CR may indicate intrinsically more responsive leukemia. Recognizing the limitation that our transplant registry data cannot address outcomes for those who never receive a transplant, we analyzed the comparative survival for patients proceeding to allogeneic transplantation in PIF, in relapse1 or in CR2. These data can guide decision-making about the utility of additional salvage therapy versus an alternative strategy of prompt transplant therapy, even for patients not in CR.
Subjects and Methods
Data Source
Data were obtained from the Center for International Blood Marrow Transplant Research (CIBMTR) a voluntary working group of over 350 transplant centers that report their consecutive transplantations to the CIBMTR. Data are collected on standardized data collection forms pre-transplant, at 3-, 6- and 12-months and annually thereafter until death or loss to follow-up. Compliance is ensured by on-site audits. Patients provide written informed consent and the study was approved by the National Marrow Donor Program's Institutional Review Board.
Patients
Eligibility criteria included the following: patients aged 18 years and older with AML, in second complete remission (CR2; n=1986), first relapse (relapse1; n=1256) and primary induction failure (PIF; n=1440). Patients transplanted in relapse1 had either failed at least 1 re-induction chemotherapy cycle (n=254), were untreated (n=331; 10 treatment status unknown) while those in PIF had failed at least two induction cycles. Donor sources included HLA-matched sibling, other relative (including haploidentical), HLA-matched and mismatched unrelated donor. Recipients of cord blood grafts and second or subsequent allogeneic transplants were excluded. All transplants occurred between 2000 and 2013.
Endpoints
The primary outcome was overall survival. Death from any cause was considered an event and surviving patients were censored at last follow-up. Relapse was defined as morphologic recurrence of leukemic blasts in marrow or blood. Patients transplanted in relapse1 or PIF who relapsed within 3 months after transplantation were considered as having persistent leukemia. Measures of pre-HCT residual disease (flow cytometry, FISH or molecular) were not consistently available for the CR2 patients and were not analyzed. Non-relapse mortality (NRM) was defined as death not attributed to relapse or persistent leukemia.
Statistical Methods
Demographic, leukemia and transplant characteristics were compared using the Chi square statistic for categorical variables. The probability of overall survival was calculated using the Kaplan Meier estimator with 95% confidence limits derived from the standard error. The duration of survival was defined as interval between date of transplantation and date of last contact. Multivariate analysis for overall mortality was performed using Cox regression model (20). Estimation of relapse and NRM were limited to patients who survived at least 100 days post-transplantation with documented CR (as a day +100 landmark analysis). The subsequent incidences of relapse and NRM were calculated using the cumulative incidence estimator and considering competing risks (21). Duration of post-transplant remission or time to death not attributed to relapse was defined as interval between day 100 post transplantation and date of event (relapse or death). Multivariate analyses for relapse and NRM were performed using the Fine and Grey regression model (22).
Factors tested in multivariate models include: disease status at HCT (CR2 versus relapse1 versus PIF) and the duration of CR1 for CR2 and relapse1, age (decade), time (in months from relapse to HCT (for relapse1 and CR2), time from diagnosis to HCT (for PIF), number of induction (or reinduction) cycles prior to HCT, performance score (90-100 versus <90), recipient cytomegalovirus (CMV) serostatus (positive versus negative), AML type (de novo versus secondary), white blood count at diagnosis (<30 versus 30-100 versus >100 × 109/L), cytogenetic risk (favorable versus intermediate versus poor risk according to the SWOG classification as previously reported (23)), conditioning intensity (myeloablative versus reduced intensity), donor/HLA match (HLA-matched sibling versus other related (including haploidentical) versus HLA-matched unrelated donor (URD) versus partially matched URD), graft type (peripheral blood versus bone marrow), GVHD prophylaxis and transplant period (2000-2005 versus 2006-2013). Molecular data to define other high risk subsets were not routinely available. Flt3 ligand mutation was positive in only 3% of each HCT cohort; but was missing in the majority. Adjusted probabilities for survival, relapse and NRM were calculated considering the significant factors identified in final multivariate regression models and using left-truncation of time from diagnosis (for PIF), or from relapse (for relapse1 or CR2) to HCT. All factors met the assumptions of proportionality and there were no first order interactions between disease status at transplantation and other factors held in final multivariate models. A p-value <0.01 was considered statistically significant. The previously reported (15) CIBMTR score for HCT during relapse was also analyzed, but circulating and marrow blast counts were not reported for all patients, but the analysis was performed using all available information. All analyses were performed with SAS version 9.3 (Cary, NC).
Results
The characteristics of patients, their disease and transplant are shown in Table 1. Of the 4682 patients who met eligibility criteria 1986 (36%) were in CR2, 1256 (27%) in relapse1, and 30% in PIF at HCT. Seven percent of patients in CR2 and relapse1 had received a prior autologous HCT compared to 3% of patients in PIF. The median age in the three groups was 47, 49 and 52 years, respectively. Patient age, sex and CMV serostatus were similar across the three groups, but patients transplanted in relapse1 or PIF were more likely reported with performance scores less than 90. HCT co-morbidity index (HCT-CI) score was available after 2008; there were no differences between the groups. There were differences in disease characteristics between the groups. Patients in CR2 were less likely to have secondary AML (therapy-related or evolved from myelodysplastic or myeloproliferative syndrome) or poor risk cytogenetics compared to those in relapse1 or PIF. The duration of CR1 was < 6 months for 50% of patients transplanted in relapse1 compared to 18% for those transplanted in CR2. The duration of CR1 was > 12 months for a third of patients in CR2. Most transplants in PIF (73%) occurred within 6 months from diagnosis. The median times from relapse to HCT in relapse1 or CR2 were similar (median of 2 and 4 months, respectively) and their number of cycles from relapse to HCT (or from diagnosis to HCT for PIF) were also similar. There were no differences in regards to donor type between the groups; a third of transplants used an HLA-matched sibling donor, 40% a suitably matched URD and the remaining, mismatched URD. Only a few received non-sibling related donors (including haploidentical). There were no differences in the intensity of transplant-conditioning regimen and GVHD prophylaxis between the groups. Peripheral blood was the most common graft type. About 80% of HCTs occurred in the United States. The median follow up of patients was 5 years for patients in CR2, 7 years for those in relapse1 and 5 years for those in PIF.
Table 1. Patient, disease and transplant characteristics.
| Variable | CR2 N (%) | Relapse1 N (%) | PIF N (%) |
|---|---|---|---|
| Number of patients | 1986 | 1256 | 1440 |
| Patient age at transplant, in years | |||
| 18-29 | 360 (18) | 172 (14) | 163 (11) |
| 30-39 | 317 (16) | 200 (16) | 184 (13) |
| 40-49 | 483 (24) | 292 (23) | 289 (20) |
| 50-59 | 539 (27) | 351 (28) | 466 (32) |
| 60-69 | 264 (13) | 218 (17) | 307 (21) |
| ≥70 | 23 (1) | 23 (2) | 31 (2) |
| Gender | |||
| Male | 1050 (53) | 641 (51) | 814 (57) |
| Female | 936 (47) | 615 (49) | 626 (43) |
| Recipient CMV status | |||
| Negative | 711 (36) | 400 (32) | 481 (33) |
| Positive | 1261 (63) | 848 (68) | 947 (66) |
| Missing | 14 (<1) | 8 (<1) | 12 (<1) |
| Performance score | |||
| <90% | 531 (27) | 566 (45) | 696 (48) |
| ≥90% | 1337 (67) | 616 (49) | 672 (47) |
| Missing | 118 (6) | 74 (6) | 72 (5) |
| HCT-CI | |||
| 0 | 426 (21) | 215 (17) | 327 (23) |
| 1 | 197 (10) | 86 (7) | 99 (7) |
| 2 | 21 (1) | 12 (<1) | 15 (1) |
| 3 | 3 (<1) | 2 (<1) | 2 (<1) |
| Missing or not collected (before 2008) | 1339 (67) | 941 (75) | 997 (69) |
| White blood cell count at diagnosis, × 109/L | |||
| <30 | 1007 (51) | 724 (58) | 955 (66) |
| 30-100 | 414 (21) | 225 (18) | 207 (14) |
| >100 | 203 (10) | 120 (10) | 111 (8) |
| Missing | 362 (18) | 187 (15) | 167 (12) |
| Cytogenetic risk group | |||
| Favorable | 307 (15) | 65 (5) | 21 (1) |
| Intermediate | 1187 (60) | 766 (61) | 796 (55) |
| Poor/adverse | 222 (11) | 280 (22) | 449 (31) |
| Not tested/missing | 270 (14) | 145 (12) | 174 (12) |
| Time from relapse to HCT (CR2 and Rel1 only) | |||
| <2 months | 141 (7) | 595 (47) | N/A |
| 2-4 months | 769 (39) | 394 (31) | |
| 4-6 months | 582 (29) | 143 (11) | |
| ≥6 months | 258 (18) | 109 (9) | |
| Missing | 136 (7) | 15 (1) | |
| Time from diagnosis to HCT (PIF only) | |||
| <3 months | N/A | N/A | 372 (26) |
| 3-6 months | 670 (47) | ||
| ≥6 months | 395 (27) | ||
| Missing | 3 (<1) | ||
| Duration of CR1 (CR2 and Rel1 only) | |||
| <6 months | 357 (18) | 632 (50) | N/A |
| 6-12 months | 511 (26) | 281 (22) | |
| >12 months | 759 (38) | 173 (14) | |
| Missing | 359 (18) | 170 (14) | |
| Number of cycles of induction/reinduction | |||
| ≤2 | 1053 (53) | 623 (50) | 403 (28) |
| >2 | 54 (3) | 49 (4) | 81 (6) |
| Missing | 120 (6) | 92 (7) | 473 (33) |
| Not collected* | 759 (38) | 492 (39) | 483 (34) |
| Number of cycles of consolidation | |||
| 0 | 39 (2) | 92 (7) | N/A |
| 1 | 496 (25) | 307 (24) | |
| ≥2 | 461 (23) | 185 (15) | |
| Missing | 231 (12) | 180 (14) | |
| Not collected* | 759 (38) | 492 (39) | |
| Extramedullary disease any time pre-HCT | |||
| No | 1919 (97) | 1168 (93) | 1367 (95) |
| Yes | 67 (3) | 88 (7) | 73 (5) |
| Prior autologous HCT** | |||
| No | 1855 (93) | 1167 (93) | 1400 (97) |
| Yes | 131 (7) | 89 (7) | 40 (3) |
| Conditioning regimen intensity | |||
| Myeloablative | 1362 (69) | 871 (69) | 978 (68) |
| RIC/NMA | 623 (31) | 381 (30) | 458 (32) |
| Missing | 1 (<1) | 4 (<1) | 4 (<1) |
| Donor-recipient gender match | |||
| Female donor, male recipient | 340 (17) | 241 (19) | 261 (18) |
| Other | 1557 (78) | 958 (76) | 1108 (77) |
| Missing | 89 (4) | 57 (5) | 71 (5) |
| Donor/recipient CMV serostatus | |||
| +/+ | 636 (32) | 412 (33) | 473 (33) |
| +/- | 229 (12) | 119 (9) | 148 (10) |
| -/+ | 609 (31) | 414 (33) | 450 (31) |
| -/- | 468 (24) | 275 (22) | 326 (23) |
| Missing | 44 (2) | 36 (3) | 43 (3) |
| Donor | |||
| HLA identical sibling | 536 (27) | 377 (30) | 455 (32) |
| Other relative | 77 (4) | 50 (4) | 67 (5) |
| Well-matched unrelated or 8/8 allele matched | 935 (47) | 548 (44) | 643 (45) |
| Partially-matched unrelated or 7/8,6/8 | 349 (18) | 223 (18) | 205 (14) |
| Mismatched unrelated or 5/8 | 80 (4) | 55 (4) | 64 (4) |
| Unrelated (matching uncertain) | 9 (<1) | 3 (<1) | 6 (<1) |
| Graft source | |||
| Bone marrow | 508 (26) | 309 (25) | 298 (21) |
| Peripheral blood | 1478 (74) | 947 (75) | 1142 (79) |
| GVHD prophylaxis | |||
| Tacrolimus containing | 1042 (52) | 709 (56) | 786 (55) |
| Cyclosporine containing | 749 (38) | 427 (34) | 491 (34) |
| CD34 selection or ex-vivo T-cell depletion | 131 (7) | 80 (6) | 97 (7) |
| Othera | 43 (2) | 24 (2) | 42 (3) |
| Missing | 21 (1) | 16 (1) | 24 (2) |
| ATG/Alemtuzumab | |||
| Yes | 1315 (66) | 824 (66) | 889 (62) |
| No | 561 (28) | 329 (26) | 428 (30) |
| Missing | 110 (6) | 103 (8) | 123 (9) |
| Year of transplant | |||
| 2000-2005 | 962 (48) | 704 (56) | 697 (48) |
| 2006-2013 | 1024 (52) | 552 (44) | 743 (52) |
| Median follow-up of survivors (range), months | 72 (2-174) | 83 (1-170) | 71 (3-171) |
Other GVHD prophylaxis: Cyclophosphamide (n=59), MTX + MMF (n=1), MTX ± others (n=19), MMF ± others (n=21), palifermin (n=1), sirolimus alone (n=2), ATG alone (n=1), corticosteroids alone (n=4), other not specified (n=1)
Not collected – for URD before 2008;
autologous transplant for disease other than AML
Overall Survival
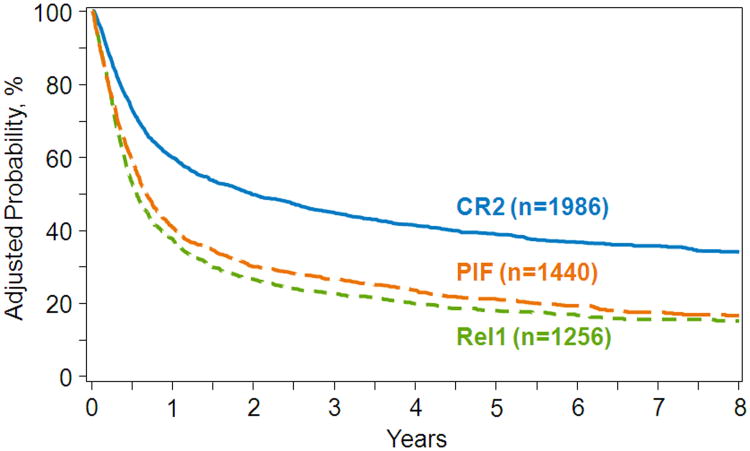
At a median of 5 years of follow-up, 42% of CR2, 17% of relapse1, and 20% of PIF patients are alive. The unadjusted 2-year overall survival for patients transplanted in CR2, relapse1 and PIF were 50% (95% CI 48-52), 27% (95% CI 24-29) and 29% (95% CI 27-32), respectively. The results of multivariate analysis are shown in Table 2. Compared to transplants in CR2, mortality risks were higher for transplants in relapse1 and PIF, regardless of the duration of CR1, Marrow blast % (for PIF or relapse1), or the number of cycles of induction or reinduction prior to HCT did not influence mortality risks (data not shown). The outcome of transplants in relapse1 versus PIF varied by duration of CR1 for relapse1. Mortality risks were lower for transplants in PIF than for relapse1, but only if the duration of CR1 was less than 6 months. Mortality risks were higher for transplants in PIF if the duration of CR1 was greater than 6 months for relapse1 transplants, but mortality risks were similar PIF and relapse1 when the duration of CR1 was between 6-12 months. Other factors associated with overall mortality were age (50 years or older), poor performance score (<90), intermediate and poor cytogenetic risk and mismatched related or URD transplant. The 5-year probability of overall survival after transplants in CR2, relapse1 and PIF adjusted for age, performance score, cytogenetic risk and donor type were 39% (95% CI 37-41), 18% (95% CI 16-20) and 21% (95% CI 19-23), respectively (Figure 1).
Table 2. Factors associated with overall survival.
| N | Hazard Ratio (95% confidence interval) | P-value | |
|---|---|---|---|
|
| |||
| Disease status at transplantation | |||
|
| |||
| Relapse1 vs. CR2 | 1256 vs. 1986 | 1.65 (1.51 – 1.81) | <0.0001 |
|
| |||
| PIF vs. CR2 (CR1 <6 months) | 1440 vs. 357 | 1.26 (1.13 – 1.41) | <0.0001 |
| PIF vs. CR2 (CR1 6-12 months) | 1440 vs. 511 | 1.60 (1.43 – 1.80) | <0.0001 |
| PIF vs. CR2 (CR1 >12 months) | 1440 vs. 759 | 2.24 (2.01 – 2.50) | <0.0001 |
|
| |||
| PIF vs. Relapse1 (CR1 <6 months) | 1440 vs. 632 | 0.76 (0.70 – 0.84) | <0.0001 |
| PIF vs. Relapse1 (CR1 6-12 months) | 1440 vs. 281 | 0.97 (0.86 – 1.09) | 0.60 |
| PIF vs. Relapse1 (CR1 >12 months) | 1440 vs. 173 | 1.36 (1.20 – 1.54) | <0.0001 |
|
| |||
| Age: ≥50 vs. 18 – 49 years | 2222 vs. 2460 | 1.15 (1.07 – 1.23) | <0.0001 |
|
| |||
| Performance Score: 90-100 | 2625 | 1.00 | <0.0001 |
| <90 | 1793 | 1.28 (1.19 – 1.37) | <0.0001 |
|
| |||
| Cytogenetic risk: Favorable | 393 | 1.00 | <0.0001 |
| Intermediate/Poor | 3700 | 1.50 (1.29 – 1.74) | <0.0001 |
|
| |||
| Donor type: HLA-matched sibling | 1368 | 1.00 | <0.0001 |
| Other Relative | 194 | 1.52 (1.27 – 1.81) | <0.0001 |
| Matched Unrelated | 2126 | 1.05 (0.97 – 1.14) | 0.24 |
| Mismatched Unrelated | 976 | 1.31 (1.19 – 1.45) | <0.0001 |
Figure 1.
The 5-year probability of overall survival by disease status at transplantation, adjusted for age, performance score, cytogenetic risk and donor type.
Overall there were 1159 (58%) deaths among patients transplanted in CR2, 1046 (83%) deaths among patients transplanted in relapse1 and 1145 (80%) deaths among patients transplanted in PIF. Recurrent or persistent leukemia was the most common cause of death occurring in 485 of 1159 (42%) of patients transplanted in CR2 and 567 of 1046 (54%) and 603 of 1145 (53%) of patients transplanted in relapse1 and PIF, respectively. Other predominant causes of death include GVHD, infection and organ failure.
Duval et al (15) previously reported a scoring system to predict relapse following myeloablative HCT for AML with active disease. Survival was inferior in patients with short initial remission, circulating blasts, donors other that HLA-identical siblings, performance score <90% and poor risk cytogenetics. While data was incomplete (circulating and marrow blasts were incompletely reported) a higher score was associated with inferior survival (Supplemental table S1).
Relapse and Non-relapse Mortality
The population considered for analyses of relapse and NRM are shown in Table 3. We noted in this overall high risk population that leukemia relapse was common within 100 days after HCT in CR2 (n=221; 11%) and persistent disease noted prior to day +100 was very common after HCT in relapse1 (n=577; 46%) and PIF (n=706; 49%). Thus the analyses of relapse and NRM were limited to a landmark analysis including only those patients who were alive and in CR at day 100 post transplantation. Analysis of factors associated with early (before day +100) death or relapse/persistent leukemia identified that disease status at transplant (CR2 vs. Rel1 vs. PIF) was a significant risk factor for this early treatment failure. Additionally, adverse cytogenetics, short duration of CR1 and GVHD prophylaxis also indicated higher risks of early treatment failure in all 3 cohorts while in Rel1 and PIR patients a lower KPS and year of HCT were also important (data not shown).
Table 3. Survival and Disease status by day 100 post transplantation.
| Disease status at transplantation | |||
|---|---|---|---|
| CR2 | Relapse1 | PIF | |
| Number Evaluable | |||
| At transplant | 1986 | 1256 | 1440 |
| Persistent disease after HCT | -- | 534 | 660 |
| Relapse within 100 days after HCT | 221 | 43 | 46 |
| Death within 100 days after HCT | 254 | 154 | 153 |
| Follow up < 100 days | 15 | -- | -- |
| Alive and in CR by 100 days after HCT | 1496 (75%) | 525 (42%) | 581 (40%) |
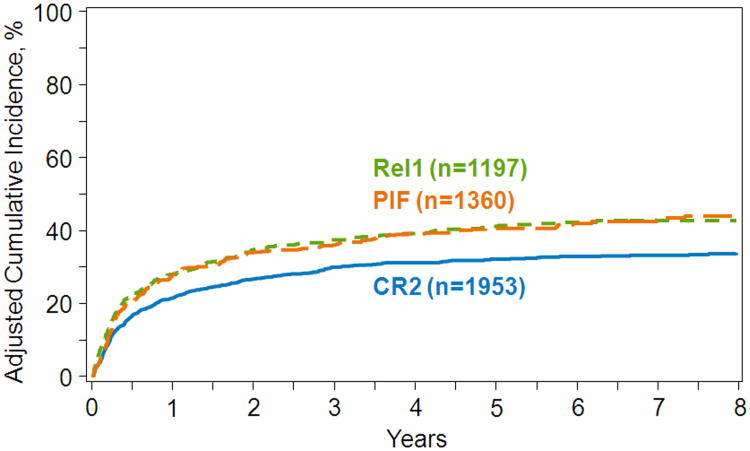
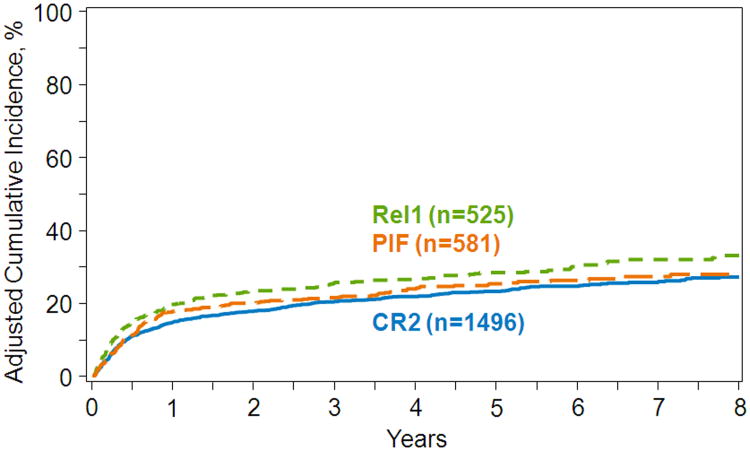
The results of these post day+100 landmark multivariate analyses are shown in Table 4. Compared to transplants in CR2, post day +100 relapse risks were higher for transplants in relapse1, regardless of the duration of CR1. Risks of relapse were also higher in PIF compared to relapse1 with a CR1 duration greater than 12 months, but not with shorter CR1 duration where there were no differences in relapse risks after transplantation in either relapse1 or PIF. Compared to transplants in CR2, NRM risks were higher for transplants in relapse1, regardless of the duration of CR1. However, there were no significant differences in NRM risks following transplants in CR2 or relapse1 compared to transplants in PIF. Other factors associated with higher relapse and NRM risks were age (50 years or older), poor performance score (<90), intermediate and poor cytogenetic risk and mismatched related or URD transplant. The 5-year adjusted probabilities of relapse after transplants in CR2, relapse1 and PIF adjusted for cytogenetic risk were 32% (95% CI 29-34), 41% (95% CI 36-45) and 40% (95% CI 36-44), respectively (Figure 2). The corresponding probabilities for NRM were 23% (95% CI 21-25), 28% (95% CI 24-32) and 25% (95% CI 21-29) (Figure 3).
Table 4. Factors associated with Non-relapse mortality and Relapse.
| Non-relapse mortality | N | Hazard Ratio (95% confidence interval) | P-value |
|---|---|---|---|
|
| |||
| Disease status at transplantation | |||
|
| |||
| Relapse1 vs. CR2 | 525 vs. 1496 | 1.32 (1.09 – 1.61) | 0.005 |
| PIF vs. CR2 (CR1 < 6 months) | 581 vs. 237 | 0.99 (0.76 – 1.27) | 0.92 |
| PIF vs. CR2 (CR1 6–12 months) | 581 vs. 379 | 1.18 (0.91 – 1.52) | 0.21 |
| PIF vs. CR2 (CR1 >12 months) | 581 vs. 615 | 1.30 (1.04 – 1.63) | 0.02 |
| PIF vs. Relapse1 (CR1 < 6 months) | 581 vs. 215 | 0.75 (0.58 – 0.96) | 0.02 |
| PIF vs. Relapse1 (CR1 6–12 months | 581 vs. 133 | 0.89 (0.68 – 1.17) | 0.40 |
| PIF vs. Relapse1 (CR1 >12 months) | 581 vs. 103 | 0.98 (0.75 – 1.29) | 0.91 |
|
| |||
| Age: | |||
| 18– 49 years | 1422 | 1.00 | <0.0001 |
| 50+ years | 1180 | 1.48 (1.27 – 1.73) | <0.0001 |
|
| |||
| Performance score: | |||
| 90-100% | 1605 | 1.00 | 0.002 |
| <90% | 834 | 1.30 (1.10 – 1.53) | 0.002 |
|
| |||
| Cytogenetic risk: | |||
| Favorable | 300 | 1.00 | 0.10 |
| Intermediate/Poor | 1986 | 1.34 (1.01 – 1.78) | 0.05 |
|
| |||
| Donor type: | |||
| HLA-identical sibling | 715 | 1.00 | 0.004 |
| Other relative | 75 | 1.42 (0.88 – 2.29) | 0.15 |
| Matched Unrelated | 1266 | 1.14 (0.94 – 1.38) | 0.19 |
| Mismatched Unrelated | 538 | 1.51 (1.22 – 1.89) | 0.0002 |
|
| |||
| Relapse | N | Hazard Ratio (95% confidence interval) | P-value |
|
| |||
| Disease status at transplantation | |||
|
| |||
| Relapse1 vs. CR2 | 525 vs. 1496 | 1.34 (1.13 – 1.58) | 0.001 |
| PIF vs. CR2 (CR1 < 6 months) | 581 vs. 237 | 1.11 (0.90 – 1.38) | 0.33 |
| PIF vs. CR2 (CR1 6–12 months) | 581 vs. 379 | 1.18 (0.96 – 1.45) | 0.11 |
| PIF vs. CR2 (CR1 >12 months) | 581 vs. 615 | 2.01 (1.64 – 2.46) | <0.0001 |
| PIF vs. Relapse1 (CR1 < 6 months) | 581 vs. 215 | 0.83 (0.67 – 1.03) | 0.09 |
| PIF vs. Relapse1 (CR1 6–12 months) | 581 vs. 133 | 0.89 (0.71 – 1.11) | 0.29 |
| PIF vs. Relapse1 (CR1 >12 months) | 581 vs. 103 | 1.50 (1.19 – 1.91) | 0.001 |
|
| |||
| Age: | |||
| 18 – 49 years | 1422 | 1.00 | |
| ≥50 years | 1180 | 1.09 (0.96 – 1.25) | 0. 19 |
|
| |||
| Performance score: | |||
| 90-100% | 1605 | 1.00 | |
| <90% | 834 | 1.14 (0.99 – 1.32) | 0.06 |
|
| |||
| Cytogenetic risk: | |||
| Favorable | 300 | 1.00 | 0.03 |
| Intermediate/Poor | 1986 | 1.38 (1.09 – 1.76) | 0.008 |
|
| |||
| Donor type: | |||
| HLA-identical sibling | 715 | 1.00 | 0.13 |
| Other relatives | 75 | 1.62 (1.13 – 2.32) | 0.009 |
| Matched Unrelated | 1266 | 1.09 (0.93 – 1.27) | 0.31 |
| Mismatched Unrelated | 538 | 1.07 (0.88 – 1.30) | 0.48 |
Figure 2.
Amongst 100 day survivors in CR, the 5-year probability of relapse by disease status at transplantation, adjusted for age, performance score, cytogenetic risk and donor type.
Figure 3.
Amongst 100 day survivors in CR, the 5-year probability of non-relapse mortality (NRM) by disease status at transplantation, adjusted for age, performance score, cytogenetic risk and donor type.
Discussion
Allogeneic transplantation in later stage AML can still yield long term disease control and improved survival for sizable fractions of patients, but this analysis strongly suggests that transplantation during CR2 is preferred over other approaches, including transplantation in relapse1 or PIF. This analysis has substantial, and to a large extent unquantifiable, selection bias due to inclusion of only those deemed fit for HCT during relapse and who were referred to a center accepting them for HCT. Nonetheless, the large numbers of cases, the multivariable regression adjustments and the international experience likely represent valid outcomes for patients transplanted in these three clinical situations who are selected for HCT. The favorable outcomes with HCT during CR2 suggest that additional salvage therapy to induce remission for patients in PIF or first relapse may yield a selected group who can have favorable post-transplant outcomes and improved survival. Since attaining remission with additional therapy may only identify patients with biologically less aggressive leukemia rather than rescue the outcome of those with resistant disease, the risks and benefits of repetitive remission-induction attempts must be carefully considered, particularly as new agents and reinduction regimens are being developed and tested.
Beyond disease status, outcomes were superior for those with intrinsically more treatable leukemia, including those who have favorable risk cytogenetics and longer duration of CR1 prior to relapse. Additionally, patient characteristics including age younger than 50 and good performance status (90 or 100) also led to better survival. Transplant was recognized as useful and yielding extended disease control in this broad array of patients with persistent, relapsed, or second-remission AML. This echoes reports from the earliest experience of transplant where a fraction of advanced AML patients could be salvaged using myeloablative conditioning and allografts (7-15). Several decades ago, untreated first relapse and second remission patients were reported as having similar outcomes after allogeneic transplantation (9, 10). In more recent series, including an earlier report from the CIBMTR, disease burden manifest as bone marrow blast percentage or circulating blasts, high-risk phenotype (adverse cytogenetics), as well as age and performance status influenced the outcome of transplantation with active disease (11-15). Molecular or flow cytometric measures of detectable disease, even for those in CR prior to HCT (24) might further refine the prognosis following HCT although such data were not available for this analysis.
Limited more recent experience incorporating re-induction with fludarabine, cytarabine and amsacrine followed immediately by a reduced intensity allotransplant yielded reasonable outcomes for a fraction of patients with otherwise persistent disease (19). Novel approaches however are still required. More effective, yet less toxic conditioning regimens to deplete detectable residual tumor (25, 26), enhanced immunologic surveillance of residual disease (27) or post-transplant maintenance therapy (28) may all be required to produce effective leukemia control for such high-risk patients.
Additionally, measures of physiologic fitness to tolerate intensive conditioning and transplantation are needed. Patients may be particularly vulnerable to complications after extended, though unsuccessful therapy to induce remission, along with its attendant period of extended neutropenia. This will require careful patient selection, aggressive supportive care techniques and vigorous infection prophylaxis to identify patients most likely to benefit from transplantation with active disease. Early referral and prompt donor identification remain essential to avoid delays which put patients at risk for early disease progression or relapse or clinical deterioration further compromising post-HCT outcomes. Nonetheless, these data, even recognizing the limits of selection evident in a transplant registry-based report, still demonstrate that inherent leukemic sensitivity and achievement of second remission by conventional or novel approaches still yields better and curative outcomes for a larger fraction of patients. Thus, if added therapy can attain remission without compromising HCT eligibility, transplantation during remission remains a preferred goal.
Supplementary Material
Acknowledgments
The authors are grateful to the patients and to the medical and data management staff at the transplant centers who provided the data for this analysis.
CIBMTR Support List: The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-15-1-0848 and N00014-16-1-2020 from the Office of Naval Research; and grants from Alexion; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; AstraZeneca; Be the Match Foundation; *Bluebird Bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cellular Dynamics International, Inc.; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Genentech, Inc.; Genzyme Corporation; *Gilead Sciences, Inc.; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Jeff Gordon Children's Foundation; The Leukemia & Lymphoma Society; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Merck & Co, Inc.; Mesoblast; MesoScale Diagnostics, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Otsuka Pharmaceutical Co, Ltd. – Japan; PCORI; Perkin Elmer, Inc.; Pfizer, Inc; *Sanofi US; *Seattle Genetics; *Spectrum Pharmaceuticals, Inc.; St. Baldrick's Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; University of Minnesota; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
The study was performed at the CIBMTR with partial financial support from Sunesis who had no independent part in the data collection, analysis or publication. One co-author (P. Hyare) is a Sunesis employee.
Footnotes
All others report no conflicts of interest in the study design or analysis.
Author Contributions: See attached list of roles
References
- 1.Dombret H, Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood. 2016;127(1):53–61. doi: 10.1182/blood-2015-08-604520. Epub2015 Dec 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127(1):62–70. doi: 10.1182/blood-2015-07-604546. Epub 2015 Dec 10. [DOI] [PubMed] [Google Scholar]
- 3.Forman SJ, Schmidt GM, Nademanee AP, Amylon MD, Chao NJ, Fahey JL, et al. Allogeneic bone marrow transplantation as therapy for primary induction failure for patients with acute leukemia. J of Clin Oncol. 1991;9(9):1570–1574. doi: 10.1200/JCO.1991.9.9.1570. [DOI] [PubMed] [Google Scholar]
- 4.Mehta J, Powles R, Horton C, Milan S, Treleaven J, Tait D, et al. Bone marrow transplantation for primary refractory acute leukaemia. Bone Marrow Transplant. 1994;14(3):415–418. [PubMed] [Google Scholar]
- 5.Estey EH. Treatment of relapsed and refractory acute myelogenous leukemia. Leukemia. 2000;14(3):476–479. doi: 10.1038/sj.leu.2401568. [DOI] [PubMed] [Google Scholar]
- 6.Ravandi F, Cortes J, Faderl S, O'Brien S, Garcia-Manero G, Verstovesk S, et al. Characteristics and outcome of patients with acute myeloid leukemia refractory to 1 cycle of high-dose cytarabine-based induction chemotherapy. Blood. 2010;116(26):5818–5823. doi: 10.1182/blood-2010-07-296392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zander AR, Dicke KA, Keating M, Vellekoop L, Culbert S, Spitzer G, et al. Allogeneic bone marrow transplantation for acute leukemia refractory to induction chemotherapy. Cancer. 1985;56(6):1374–1379. doi: 10.1002/1097-0142(19850915)56:6<1374::aid-cncr2820560626>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Craddock C, Labopin M, Pillai S, Finke J, Bunjes D, Greinix H, et al. Factors predicting outcome after unrelated donor stem cell transplantation in primary refractory acute myeloid leukaemia. Leukemia. 2011;25(5):808–813. doi: 10.1038/leu.2011.13. [DOI] [PubMed] [Google Scholar]
- 9.Clift RA, Buckner D, Appelbaum FR, Schoch G, Petersen FB, Bensinger WI, et al. Allogeneic marrow transplantation during untreated first relapse of acute myeloid leukemia. J Clin Oncol. 1992;10(11):1723–1729. doi: 10.1200/JCO.1992.10.11.1723. [DOI] [PubMed] [Google Scholar]
- 10.Greinix HT, Reiter E, Keil F, Fischer G, Lechner K, Dieckmann K, et al. Leukemia-free survival and mortality in patients with refractory or relapsed acute leukemia given marrow transplants from sibling and unrelated donors. Bone Marrow Transplant. 1998;21(7):673–678. doi: 10.1038/sj.bmt.1701152. [DOI] [PubMed] [Google Scholar]
- 11.Armistead PM, de Lima M, Pierce S, Qiao W, Wang X, Thail PF, et al. Quantifying the survival benefit for allogeneic hematopoietic stem cell transplantation in relapsed acute myelogenous leukemia. Biol Blood Marrow Transplant. 2009;15(11):1431–1438. doi: 10.1016/j.bbmt.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oyekunle AA, Kroger N, Zabelina T, Ayuk F, Schieder H, Renges H, et al. Allogeneic stem-cell transplantation in patients with refractory acute leukemia: a long-term follow-up. Bone Marrow Transplant. 2006;37(1):45–50. doi: 10.1038/sj.bmt.1705207. [DOI] [PubMed] [Google Scholar]
- 13.Michallet M, Thomas X, Vernant JP, Juentz M, Socie G, Esperou-Bourdeau H, et al. Long-term outcome after allogeneic hematopoietic stem cell transplantation for advanced stage acute myeloblastic leukemia: a retrospective study of 379 patients reported to the Societe Francaise de Greffe de Moelle (SFGM) Bone Marrow Transplant. 2000;26(11):1157–1163. doi: 10.1038/sj.bmt.1702690. [DOI] [PubMed] [Google Scholar]
- 14.Wong R, Shahjahan M, Wang X, Thall PF, deLima M, Khouri I, et al. Prognostic factors for outcomes of patients with refractory or relapsed acute myelogenous leukemia or myelodysplastic syndromes undergoing allogeneic progenitor cell transplantation. Biol Blood Marrow Transplant. 2005;11(2):108–114. doi: 10.1016/j.bbmt.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28(23):3730–3738. doi: 10.1200/JCO.2010.28.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rambaldi A, Bacigalupa A, Fanin R, Cicero F, Bonifazi F, Falda M, et al. Outcome of patients activating an unrelated donor search: the impact of transplant with reduced intensity conditioning in a large cohort of consecutive high-risk patients. Leukemia. 2012;26(8):1770–1885. doi: 10.1038/leu.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohty M, de Lavallade H, Ladaique P, Faucher C, Vey N, Coso D, et al. The role of reduced intensity conditioning allogeneic stem cell transplantation in patients with acute myeloid leukemia: a donor vs. no donor comparison. Leukemia. 2005;19(6):916–920. doi: 10.1038/sj.leu.2403770. [DOI] [PubMed] [Google Scholar]
- 18.Jabbour E, Daver N, Champlin R, Mathisen M, Oran B, Ciurea S, et al. Allogeneic stem cell transplantation as initial salvage for patients with acute myeloid leukemia refractory to high-dose cytarabine-based induction chemotherapy. Am J Hematol. 2014;89(4):395–398. doi: 10.1002/ajh.23655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D, et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood. 2006;108(3):1092–1099. doi: 10.1182/blood-2005-10-4165. [DOI] [PubMed] [Google Scholar]
- 20.Cox DR. Regression model and life tables. J R Stat Soc Series B. 1972;34(2):187–200. [Google Scholar]
- 21.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 23.Slovak ML, Kpoecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96(13):4075–4083. [PubMed] [Google Scholar]
- 24.Araki D, Wood BL, Othus M, Radich JP, Halper AB, Zhou Y, et al. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: time to move toward a minimal residual disease-based definition of complete remission? J Clin Oncol. 2016;34(4):329–36. doi: 10.1200/JCO.2015.63.3826. Epub 2015 Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbui T, Carobbio A, Finazzi G, Guglielmelli P, Salmoiraghi S, Rosti V, et al. The CIBMTR score predicts survival of AML patients undergoing allogeneic transplantation with active disease after a myeloablative or reduced intensity conditioning: a retrospective analysis of the Gruppo Italiano Trapianto Di Midollo Osseo. Leukemia. 2013;27(10):2086–2091. doi: 10.1038/leu.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oyekunle AA, Kroger N, Zabelina T, Ayuk F, Schieder H, Renges H, et al. Allogeneic stem-cell transplantation in patients with refractory acute leukemia: a long-term follow-up. Bone Marrow Transplant. 2006;37(1):45–50. doi: 10.1038/sj.bmt.1705207. [DOI] [PubMed] [Google Scholar]
- 27.Giralt S, Estey E, Albitar M, van Besien K, Rondón G, Anderlini P, et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: Harnessing graft-versus-leukemia without myeloablative therapy. Blood. 1997;89(12):4531–4536. [PubMed] [Google Scholar]
- 28.de Lima M, Giralt S, Thall PF, de Padua Silva L, Jones RB, Komanduri K, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010;116(23):5420–5431. doi: 10.1002/cncr.25500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.