Abstract
The Immune Development in Pediatric Transplantation (IMPACT) study was conducted to evaluate relationships between alloimmunity, protective immunity, immune development, physical parameters and clinical outcome in children undergoing kidney transplantation. We prospectively evaluated biopsy-proven acute rejection (BPAR), de novo donor specific antibody (dnDSA) formation, viremia, viral infection, T cell immunophenotyping and BMI/weight Z scores in the first year post-transplant in 106 pediatric kidney transplant recipients. Outcomes were excellent with no deaths and 98% graft survival. Rejection and dnDSA occurred in 24% and 22% respectively. Pre-transplant CMV and EBV serologies and subsequent viremia were unrelated to BPAR or dnDSA. Viremia occurred in 73% of children (EBV, 34%; CMV, 23%; BK, 23%; and JC, 21%). Memory lymphocyte phenotype at baseline was not predictive of alloimmune complications. Patients who developed viral infection had lower weight (−2.1) (p=0.028) and BMI (−1.2) (p=0.048) Z scores at transplant. The weight difference persisted to 12 months compared to patients without infection (p=0.038). These data indicate that there is a high prevalence of viral disease following pediatric kidney transplantation, and underweight status at transplant appears to be a risk factor for subsequent viral infection. The occurrence of viremia/viral infection is not associated with alloimmune events.
Introduction
Kidney transplantation is the optimal treatment for children with end-stage renal disease (ESRD). (1-3) Nonetheless, significant morbidities still exist, primarily related to dependence on non-specific immunosuppression. Immunosuppressive agents impair protective immunity against infection while simultaneously yielding only partial protection from rejection (4).
In addition to the obvious changes in physical development, childhood and adolescence are times of both dynamic immunological evolution and exposure/response to viral pathogens. During this time, the adaptive cellular immune system of childhood changes from an exclusively naïve to a “memory” repertoire (5-7). At the same time, children encounter innumerable viruses, developing immunity against some and establishing latency with others (e.g., herpesviruses) (8, 9). The impact of these changes in viral status and immune cell maturation on transplant outcomes has not been prospectively examined.
In solid organ transplantation, viral infections have been linked to adverse clinical alloimmune outcomes. A number of mechanisms have been proposed whereby viral infections might mediate allograft dysfunction and/or rejection (10). These include direct virus-mediated inflammation and graft injury (11), acute rejection mediated by T cells with cross reactivity between donor alloantigens and pathogen-associated antigens (termed heterologous immunity) (12), by-stander activation of resting T cells driven by pro-inflammatory signals initiated in response to a post-transplant viral infection (13), and rejection related to the heightened alloimmune reactivity when immunosuppression is decreased in the face of viral infection(14-16). In pediatric transplantation, children and adolescents exist in a theoretically precarious balance, particularly in the first post-transplant year, when both immunosuppressive medication doses and the danger of rejection are highest. Insufficient immunosuppression risks alloimmune responses to the graft, including rejection and alloantibody formation. Excessive immunosuppression increases the incidence of opportunistic infection, often viral in nature. Few prospective data exist to establish relationships between ongoing immune maturation, infection and adverse alloimmune events in children after kidney transplant. It is also unclear which factors independently influence the incidence of viral infection, and whether these are the same factors that predict rejection or generation of donor specific alloantibody (DSA).
The Immune Development in Pediatric Transplantation (IMPACT) study was a prospective, multi-center, observational study of pediatric kidney transplant recipients designed to examine the natural history of pediatric kidney transplantation in the first post-transplant year, and to explicitly evaluate relationships between alloimmunity, protective immunity, viremia, immune development, physical development and clinical outcome in children undergoing kidney transplantation. The study focused on pre-transplant herpesvirus experience (using donor and recipient serostatus), post-transplant viral acquisition (using comprehensive, longitudinal viral PCR-based viral monitoring), and the immunophenotyping of T cell memory. We examined the relationship of these factors to clinical outcomes including symptomatic viral infection, biopsy-proven acute allograft rejection (BPAR), and de novo DSA (dnDSA) formation. We surveyed a broader range of viruses than have previously been examined, and combined this with a validated set of surface antigen markers for T cell memory. In this report, we address the following questions: Does viral status (prior viral exposure, viremia or clinical viral infection) influence alloimmune outcomes? Can clinical risk be anticipated based on pre-transplant memory T cell acquisition or CMV/EBV seropositivity (17)? And do physical parameters before or during transplantation influence viral or alloimmune outcomes?
Materials and Methods
Patients
This was a multi-center, prospective, observational cohort study (Clinicaltrials.gov NCT00951353) in pediatric kidney transplant recipients conducted at three institutions. Eligible patients were between 1 and 20 years of age at enrollment and undergoing their first kidney transplant. Recipients of multiple organ transplants were excluded. Informed consent was obtained from the patient’s parent/legal guardian or the subject (≥18 years old). The Institutional Review Boards of each institution approved the study protocol.
We serially examined post-transplant alloimmune events (BPAR and DSA), T cell immunophenotyping, and viremia using PCR testing for 9 different viruses. We then examined the relationship of these parameters to pre-transplant viral exposure and to post-transplant indices of growth and nutrition. To assess the patients’ viral antigen exposure, subjects were categorized based on pre-enrollment CMV and EBV serology testing. These viruses were chosen as surrogates for broader immune exposure based on the ubiquitous nature of their testing pre-transplant. Enrolled children were placed in one of three groups: viral seropositive (seropositive for both CMV and EBV), viral seronegative (seronegative for both CMV and EBV), or split serology (positive for either CMV or EBV, but not both).
The trial was powered on a preliminary hypothesis that the pre-transplant seropositivity might be associated with an increase in the risk of BPAR (17) . The target accrual was 100 patients, to be followed for one year. The study had 86% power to detect the difference between a rejection rate of 35% in CMV / EBV seropositive patients and versus 5% in viral naïve (i.e., seronegative) patients.
Trial conduct
Transplants and subsequent immunosuppressive management were performed according to the clinical standard at each site (Table 1). The majority of patients (n=103; 97%) received anti-CMV prophylaxis: intravenous ganciclovir transitioned to oral valgancyclovir. Valgancyclovir was administered for an average 93 days [range 1 - 358 days, median =79 days]. Study visits occurred at months 1, 3, 6, 9, and 12 after transplant. (See Supplementary Data), at which time children were assessed for Alloimmune Failure (BPAR and/or DSA), and Protective Immune Failure (all cause infection, viral infection and/or viremia). Estimated glomerular filtration rates (eGFR) were determined using the updated Schwartz formula (18).
Table 1.
Baseline Characteristics for all 106 transplanted patients
| Characteristics | Total Transplanted (N=106) |
|---|---|
|
| |
| Demographics | |
|
| |
| Age (year) | |
| Mean ± SD | 11.9 ± 6.20 |
|
| |
| Male Gender | 70 (66.0) |
|
| |
|
| |
| Race | |
|
| |
| White | 77 (72.6) |
|
| |
| Black or African American | 14 (13.2) |
|
| |
| Asian | 8 (7.5) |
|
| |
| Other | 2 (1.9) |
|
| |
| Unknown or Not Reported | 5 (4.7) |
|
| |
|
| |
| Ethnicity | |
|
| |
| Hispanic or Latino | 47 (44.3) |
|
| |
| Not Hispanic or Latino | 53 (50.0) |
|
| |
| Unknown or Not Reported | 6 (5.7) |
|
| |
|
| |
| Height and Weight | |
|
| |
| Height Z scores | |
|
| |
| Mean ± SD | −1.2 ± 1.70 |
|
| |
|
| |
| Weight Z scores | |
|
| |
| Mean ± SD | −0.5 ± 1.55 |
|
| |
|
| |
|
| |
| BMI Z scores | |
|
| |
| Mean ± SD | −0.2 ± 4.19 |
|
| |
|
| |
| Use of Induction Therapy | |
|
| |
| Basiliximab | 36 (34.0) |
|
| |
| Daclizumab | 45 (42.5) |
|
| |
| Thymoglobulin | 27 (25.5) |
|
| |
|
| |
| Use of Maintenance Therapy | |
|
| |
| Tacrolimus | 105 (99.1) |
|
| |
| MMF | 104 (98.1) |
|
| |
| Prednisone | 63 (59.4) |
|
| |
| Sirolimus | 20 (18.9) |
|
| |
| Azathioprine | 7 (6.6) |
|
| |
| Cyclosporine | 2 (1.9) |
|
| |
| Leflunomide | 1 (0.9) |
|
| |
|
| |
| Clinical Infection | |
|
| |
| Bacterial | 22 (20.8) |
|
| |
| Fungal | 3 (2.8) |
|
| |
| Viral | 27 (25.5) |
|
| |
|
| |
| Protozoal | 2 (1.9) |
|
| |
| Parasitic | 1 (0.9) |
|
| |
|
| |
| Viremia by PCR | |
|
| |
|
| |
| CMV+ by Local or Central PCR | 24 (22.6) |
|
| |
| EBV+ by Local or Central PCR | 36 (34.0) |
|
| |
| BKV+ by Local or Central PCR | 24 (22.6) |
|
| |
| Other+ by Central PCR [1] | 39 (36.8) |
|
| |
|
| |
| Clinical Outcomes | |
|
| |
| Alloimmune Failure | 40 (37.7) |
|
| |
| BPAR incl Borderline | 24 (22.6) |
|
| |
| denovo DSA | 23 (21.7) |
|
| |
| Viremia | 77 (72.6) |
|
| |
|
| |
Other viruses include adeno, HHV, and JCV.
Diagnosis of biopsy-proven acute rejection (BPAR)
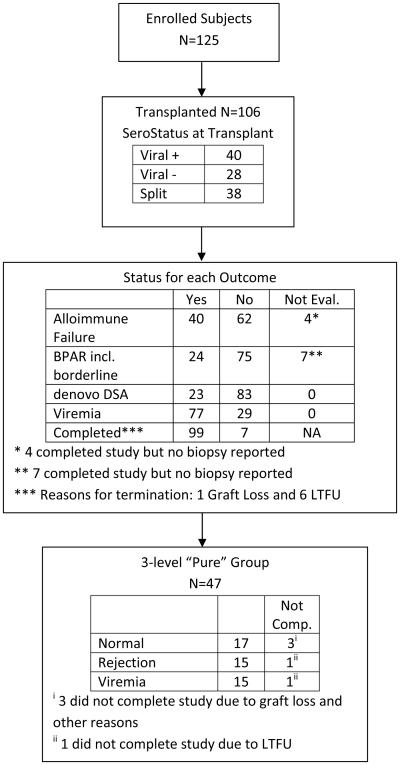
Surveillance biopsies were performed at 6 months and 1 year. Clinically indicated biopsies were performed at the discretion of the treating physician. Treatment was based on the local biopsy interpretation. A designated central pathologist (R.S.), blinded to the clinical circumstance, also read slides retrospectively. A study diagram is shown in Figure 1.
Figure 1.
Consort Diagram
Assessment of alloantibodies
(See Supplementary Data) HLA typing for HLA-A, B, DRB1, DRB3/4/5, and DQB1 loci was performed using molecular methods. Additional typing for HLA-C, DQA1, DPA1, DPB1 and MICA was performed as needed to assign DSA. HLA and MICA antibodies were considered positive when the normalized MFI value was >1000, with the exception of antibodies to HLA-Cw antigens (positive >2000) (19).
Infection assessment
Patients were assessed for bacterial, viral, fungal and parasitic infection at each visit and as clinically indicated using the local laboratories. Infections were considered clinically significant if they were culture or PCR positive and treated. Viremia was also surveyed centrally at each study visit using a protocol PCR-based assay for BKV, CMV, EBV, Adenovirus (AV), JC virus (JC), Human Herpesvirus (HHV) 6, 7, and 8. (See Supplementary Data).
Flow cytometry
At baseline, and at months 1, 2, 6, 9 and 12, peripheral blood was drawn into Cyto-chex tubes (Streck Laboratories, Omaha, NE). T cells were segregated based on CD3 expression, divided into CD4 and CD8 expressing subsets, then further segregated into naïve (CD45RA+ CD197+), central memory (CD45RA−CD197+), effector memory (CD45RA− CD197−) or terminal effectors (CD45RA+ CD197−) (5) (See Supplementary Data).
Clinical Endpoints
Alloimmune Failure (AF) was defined as the presence of either BPAR or dnDSA. For the purposes of maximal sensitivity analysis, BPAR assessed centrally included all Banff grades plus those biopsies read as “borderline”. Viremia was identified by central or local laboratory PCR when there were ≥100 copies /mL.
From the total of 106 patients, we subclassified patients into three distinct, “phenotypically pure” groups. These were defined as 1) patients who during their follow-up had no BPAR, viremia or viral infection; 2) patients who had either viral infection by local assessment or viremia with >10,000 copies with no BPAR; or 3) patients who had only BPAR confirmed by the central pathologist.
Statistical Analysis
Continuous variables were summarized with the means and standard deviations and categorical variables with counts and percentages. Patient characteristics were compared using chi-square, Fisher’s Exact, or Cochran-Mantel-Haenszel tests for categorical variables, and t-tests for continuous variables. Univariate and multivariable logistic regression models were used to describe relationships between clinical outcomes and patient characteristics. Selected baseline characteristics and on-study covariates meeting the p=0.1 criterion stayed in the model and were used to construct the final multivariable logistic regression model presented. F-tests from a generalized linear model were used for the height, weight and BMI Z score analyses. A generalized multivariate mixed model was used to analyze the relationship between the repeated flow cytometry measures of CD4 and CD8 and clinical outcomes. Log10 transformations were applied as necessary to satisfy normal distribution assumptions. All statistical analyses were performed using SAS Version 9.3 (SAS Institute, Inc., Cary, NC).
Results
Demographics
Between July 2009 through October 2011, 125 patients were enrolled and 106 proceeded to transplantation. Ninety-nine (93%) patients completed one year of follow-up. Of the 106 patients, 4 were not evaluable for Alloimmune Failure and 7 were not evaluable for BPAR (Figure 1). Eighty of the 106 (75%) received deceased donor transplants. The mean age of the 106 transplanted patients was 11.9 ± 6.20 years; 66% were male (Table 1). Compared to the 2014 NAPRTCS database, African Americans were underrepresented (13 % vs. approximately 18%), while Hispanics were overrepresented (44% vs. approximately 18%). (https://web.emmes.com/study/ped/annlrept/annualrept2014.pdf)
Immunosuppression
All patients received immediate induction therapy. Rabbit polyclonal anti-thymocyte globulin (Thymoglobulin) was used in 26%. Seventy-seven percent received an anti-CD25 monoclonal antibody; 34% received the chimeric antibody basiliximab and 43% were given the humanized antibody daclizumab, which was given for a mean of 2.6 doses and an average total duration of 26 days per patient. No patient received daclizumab for more than 5.6 months. Two patients received both an anti-CD 25 monoclonal antibody and a short course of anti-thymocyte globulin. One patient received one perioperative dose of daclizumab, but was converted to a 3 day course of anti-thymocyte globulin because of temporary delayed graft function and a clinical decision to delay initiation of oral tacrolimus. The other patient was begun on anti-thymocyte globulin but received one dose of basiliximab because of a clinical concern about a failure to deplete CD3+ T cells adequately. During the one year of follow-up, 99% of patients received tacrolimus, 98% mycophenolate mofetil, 59% prednisone, 19% sirolimus, 7% azathioprine, 2% cyclosporine and 1% leflunomide (Table 1).
Clinical Outcomes
All transplants were technically successful. No patient had delayed graft function, defined as the need for dialysis in the first week. The mean eGFR at 1, 6 and 12 months was 72±26, 74±21 and 74±25 mL/min/1.73 m2, respectively. There were no deaths; two graft losses occurred, yielding an actual one-year graft survival of 98%.
Biopsy-Proven Acute Rejection (BPAR)
In the 99 patients with evaluable BPAR data, 24 (24%) patients developed a total of 30 BPAR episodes. Acute cellular rejection (ACR) as a single entity represented 93% of all rejection episodes. While all 24 patients with rejection had ACR, two of these patients also developed concurrent antibody medicated rejection (AMR). Fourteen of the 24 (58%) patients had biopsy changes classified as “Borderline”. No patient developed isolated or chronic AMR. There were no significant differences in demographic data, physical parameters, HLA matching, or type of immunosuppressive medications between those with and without BPAR. Specifically, there were no significant associations between the diagnosis of BPAR and the use of either tacrolimus, mycophenolate mofetil or sirolimus. There was a nonsignificant association between the use of corticosteroids as maintenance immunosuppression and the diagnosis of BPAR. (p=0.077) (Table 2.) Similarly, there were no associations between the specific induction antibodies used and the diagnosis of BPAR. There was a nonsignificant trend towards increased BPAR in patients who manifested any baseline sensitization, i.e., PRA>0% (p=0.070) (Table 2). Using multivariable logistic regression, there was no association with BPAR and either pre-transplant CMV/EBV serostatus or post-transplant development of viral infection or viremia. We also found no association with any of the above parameters when patients with borderline changes were removed from the analyses (data not shown).
Table 2.
Subject Baseline Characteristics for Central BPAR, denovo DSA, and Alloimmune Failure,
| Characteristics | Central BPAR = Yes (N=24) |
Central BPAR = No (N=75) |
p-val | denovo DSA = Yes (N=23) |
denovo DSA = No (N=83) |
p-val | Alloimmune Failure = Yes (N=40) |
Alloimmune Failure = No (N=62) |
p-val |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Demographics | |||||||||
|
| |||||||||
| Age (year) | |||||||||
|
| |||||||||
| Mean ± SD | 12.8 ± 5.48 | 12.2 ± 6.41 | 0.665 | 11.1 ± 6.33 | 12.2 ± 6.19 | 0.485 | 12.0 ± 5.86 | 12.3 ± 6.42 | 0.828 |
|
| |||||||||
|
| |||||||||
| Male Gender | 18 (75.0) | 48 (64.0) | 0.320 | 16 (69.6) | 54 (65.1) | 0.686 | 28 (70.0) | 39 (62.9) | 0.461 |
|
| |||||||||
|
| |||||||||
| Race | |||||||||
|
| |||||||||
| White | 18 (75.0) | 55 (73.3) | 0.800 | 17 (73.9) | 60 (72.3) | 0.660 | 30 (75.0) | 45 (72.6) | 0.720 |
|
| |||||||||
| Black or African American | 3 (12.5) | 9 (12.0) | 3 (13.0) | 11 (13.3) | 5 (12.5) | 8 (12.9) | |||
|
| |||||||||
| Asian | 1 (4.2) | 6 (8.0) | 3 (13.0) | 5 (6.0) | 3 (7.5) | 4 (6.5) | |||
|
| |||||||||
| Other | 0 | 2 (2.7) | 0 | 2 (2.4) | 0 | 2 (3.2) | |||
|
| |||||||||
| Unknown or Not Reported | 2 (8.3) | 3 (4.0) | NA | 0 | 5 (6.0) | NA | 2 (5.0) | 3 (4.8) | NA |
|
| |||||||||
| Ethnicity | |||||||||
|
| |||||||||
| Hispanic or Latino | 10 (41.7) | 37 (49.3) | 0.313 | 7 (30.4) | 40 (48.2) | 0.070 | 14 (35.0) | 33 (53.2) | 0.021 |
|
| |||||||||
| Not Hispanic or Latino | 14 (58.3) | 32 (42.7) | 16 (69.6) | 37 (44.6) | 26 (65.0) | 23 (37.1) | |||
|
| |||||||||
| Unknown or Not Reported | 0 | 6 (8.0) | NA | 0 | 6 (7.2) | NA | 0 | 6 (9.7) | NA |
|
| |||||||||
| Height and Weight | |||||||||
|
| |||||||||
| Height Z scores | |||||||||
|
| |||||||||
| Mean ± SD | −0.9 ± 1.35 | −1.2 ± 1.81 | 0.375 | −0.6 ± 2.52 | −1.3 ± 1.36 | 0.065 | −0.8 ± 2.13 | −1.4 ± 1.38 | 0.088 |
|
| |||||||||
| Weight Z scores | |||||||||
|
| |||||||||
| Mean ± SD | −0.4 ± 2.10 | −0.6 ± 1.40 | 0.621 | −0.3 ± 1.51 | −0.6 ± 1.56 | 0.430 | −0.5 ± 1.85 | −0.6 ± 1.38 | 0.959 |
|
| |||||||||
|
| |||||||||
| BMI Z scores | |||||||||
|
| |||||||||
| Mean ± SD | −0.1 ± 1.95 | −0.3 ± 4.85 | 0.850 | −1.4 ± 8.59 | 0.1 ± 1.45 | 0.125 | −1.1 ± 6.69 | 0.3 ± 1.28 | 0.119 |
|
| |||||||||
|
| |||||||||
| Positive PRA (>0%) | 12 (50.0) | 22 (29.3) | 0.070 | 5 (21.7) | 30 (36.1) | 0.311 | 14 (35.0) | 21 (33.9) | 0.690 |
|
| |||||||||
|
| |||||||||
| Delayed Graft Function | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 | NA |
|
| |||||||||
|
| |||||||||
| HLA A, B, DR Mismatch | |||||||||
|
| |||||||||
| Mean ± SD | 4.3 ± 1.31 | 3.9 ± 1.64 | 0.219 | 4.9 ± 1.29 | 3.7 ± 1.60 | 0.002 | 4.5 ± 1.38 | 3.7 ± 1.63 | 0.018 |
|
| |||||||||
| Use of Induction Therapy | |||||||||
|
| |||||||||
| Basiliximab | 9 (37.5) | 22 (29.3) | 0.453 | 8 (34.8) | 28 (33.7) | 0.925 | 16 (40.0) | 17 (27.4) | 0.185 |
|
| |||||||||
| Daclizumab | 10 (41.7) | 33 (44.0) | 0.841 | 11 (47.8) | 34 (41.0) | 0.556 | 16 (40.0) | 28 (45.2) | 0.607 |
|
| |||||||||
| Thymoglobulin | 5 (20.8) | 22 (29.3) | 0.416 | 4 (17.4) | 23 (27.7) | 0.315 | 8 (20.0) | 19 (30.6) | 0.234 |
|
| |||||||||
|
| |||||||||
| Use of Maintenance Therapy | |||||||||
|
| |||||||||
| Steroid | 18 (75.0) | 41 (54.7) | 0.077 | 13 (56.5) | 50 (60.2) | 0.748 | 26 (65.0) | 34 (54.8) | 0.309 |
|
| |||||||||
|
| |||||||||
| eGFR | |||||||||
|
| |||||||||
|
| |||||||||
| Mean ± SD at M1 | 70.9 ± 23.18 | 72.8 ± 27.49 | 0.769 | 64.9 ± 23.03 | 74.5 ± 26.03 | 0.113 | 68.9 ± 21.31 | 74.7 ± 28.80 | 0.284 |
|
| |||||||||
|
| |||||||||
| Mean ± SD at M6 | 71.1 ± 21.77 | 74.0 ± 20.60 | 0.587 | 74.0 ± 26.28 | 73.8 ± 19.31 | 0.971 | 72.6 ± 22.86 | 74.7 ± 19.86 | 0.638 |
|
| |||||||||
| Mean ± SD at M12 | 67.1 ± 21.17 | 75.3 ± 24.96 | 0.193 | 70.6 ± 22.18 | 75.6 ± 25.28 | 0.414 | 69.2 ± 20.06 | 76.9 ± 26.61 | 0.150 |
|
| |||||||||
|
| |||||||||
| Sero Status at Transplant | |||||||||
|
| |||||||||
| CMV+/EBV+ | 10 (41.7) | 28 (37.3) | 0.463 | 6 (26.1) | 34 (41.0) | 0.386 | 14 (35.0) | 24 (38.7) | 0.931 |
|
| |||||||||
|
| |||||||||
| CMV−/EBV− | 4 (16.7) | 22 (29.3) | 8 (34.8) | 20 (24.1) | 11 (27.5) | 16 (25.8) | |||
|
| |||||||||
|
| |||||||||
| Split Serology | 10 (41.7) | 25 (33.3) | 9 (39.1) | 29 (34.9) | 15 (37.5) | 22 (35.5) | |||
|
| |||||||||
|
| |||||||||
| Donor CMV/EBV Status | |||||||||
|
| |||||||||
| Donor CMV Status | |||||||||
|
| |||||||||
| Positive | 18 (75.0) | 47 (62.7) | 0.268 | 19 (82.6) | 51 (61.4) | 0.058 | 30 (75.0) | 37 (59.7) | 0.112 |
|
| |||||||||
| Negative | 6 (25.0) | 28 (37.3) | 4 (17.4) | 32 (38.6) | 10 (25.0) | 25 (40.3) | |||
|
| |||||||||
|
| |||||||||
|
| |||||||||
| Donor EBV Status | |||||||||
|
| |||||||||
| Positive | 22 (91.7) | 65 (86.7) | >0.999 | 22 (95.7) | 72 (86.7) | 0.680 | 38 (95.0) | 52 (83.9) | 0.306 |
|
| |||||||||
| Negative | 2 (8.3) | 7 (9.3) | 1 (4.3) | 8 (9.6) | 2 (5.0) | 7 (11.3) | |||
|
| |||||||||
| Not Done | 0 | 3 (4.0) | NA | 0 | 3 (3.6) | NA | 0 | 3 (4.8) | NA |
|
| |||||||||
|
| |||||||||
|
| |||||||||
| Clinical Outcome | |||||||||
|
| |||||||||
| Viremia by PCR (Local or Central) | 18 (75.0) | 54 (72.0) | 0.774 | 16 (69.6) | 61 (73.5) | 0.708 | 27 (67.5) | 46 (74.2) | 0.464 |
|
| |||||||||
Alloantibodies
At transplantation, 21 of 106 (20%) patients had pre-formed HLA-specific antibodies, although only 4 had donor-HLA-specific antibodies (DSA). None had pre-formed MICA antibodies. Twenty-three (22%) of 106 patients developed DSA during the study and 21 of the 23 patients (91%) developed HLA-specific dnDSA. Of the 23 patients, 5 (22%) developed Class I, 15 (65%) Class II, and 1 (4%) developed both Class I and II specific antibodies. Two patients (9%) generated MICA-specific dnDSA. The presence of alloantibody or DSA prior to transplantation was not associated with a risk of subsequent dnDSA development. Similarly, there was no association between the generation of dnDSA and the incidence of BPAR; only 7 of the 24 patients who developed BPAR also developed dnDSA.
There was a strong correlation between the generation of HLA-specific dnDSA and the number of HLA mismatches present (p=0.002). There were no associations between dnDSA and any demographic features, post-transplant physical parameters or type of immunosuppressive medications (induction, maintenance or steroid vs. steroid-free). There was a borderline association between donor CMV seropositivity and the development of dnDSA (p=0.058). However, we found no association between either recipient CMV serostatus or the development of CMV viremia / viral infection and the generation of dnDSA.
Alloimmune Failure (AF)
Of 102 patients in whom all data were available, 40 (39%) patients experienced AF (BPAR or dnDSA). Twenty-three patients developed dnDSA and 24 patients developed BPAR, with 7 patients having both.
A significantly lower proportion of Hispanic/Latino patients had AF (p=0.021) (Table 2). There were no other associations between the demographic characteristics, or a specific immunosuppressive regimen and AF. There was a significant association observed between HLA matching and AF (p=0.018), but this was due to the significant association between HLA matching and dnDSA (see above) (p=0.002); there was no demonstrable relationship between HLA matching and BPAR (p=0.219) (Table 2). Importantly, there were no associations of AF with either viremia or clinical viral infection (Table 2). When we examined the temporality of viral infection/viremia, we found that preceding clinical viral infection or viremia was not associated with subsequent AF, BPAR or DSA. Similarly, we observed no associations between AF or eGFR and the pre-transplant CMV or EBV serostatus.
Pre-transplant Serology and Outcomes
At enrollment, patients were classified as either seropositive for both CMV and EBV (+/+) (n=40; 38%), seronegative for both (−/−) (n=28; 26%) or “split serology” (CMV+/EBV- or CMV-/EBV+) (n=38; 36%) (Table 3). As expected, patients negative for both serologies were significantly younger than those in the other groups (p=<0.001). Patients who were seropositive for both CMV and EBV were the least likely to have pre-transplant sensitization, i.e., PRA > 0% (p=0.022) (Table3).
Table 3.
Subject Baseline Characteristics for Serology Status at Transplant and Viremia Status
| Characteristics | CMV+/EBV+ (N=40) |
CMV−/EBV− (N=28) |
Split Serology (N=38) |
p-val | Viremia = Yes (N=77) |
Viremia = No (N=29) |
p-val |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Demographics | |||||||
|
| |||||||
| Age (year) | |||||||
|
| |||||||
| Mean ± SD | 14.8 ± 4.57 | 6.6 ± 5.86 | 12.8 ± 5.57 | <0.001 | 11.9 ± 6.25 | 12.1 ± 6.18 | 0.864 |
|
| |||||||
|
| |||||||
| Male Gender | 21 (52.5) | 21 (75.0) | 28 (73.7) | 0.074 | 53 (68.8) | 17 (58.6) | 0.322 |
|
| |||||||
| Race | |||||||
|
| |||||||
| White | 29 (72.5) | 21 (75.0) | 27 (71.1) | 0.958 | 53 (68.8) | 24 (82.8) | 0.205 |
|
| |||||||
| Black or African American | 5 (12.5) | 4 (14.3) | 5 (13.2) | 11 (14.3) | 3 (10.3) | ||
|
| |||||||
| Asian | 2 (5.0) | 2 (7.1) | 4 (10.5) | 8 (10.4) | 0 | ||
|
| |||||||
| Other | 1 (2.5) | 0 | 1 (2.6) | 2 (2.6) | 0 | ||
|
| |||||||
| Unknown or Not Reported | 3 (7.5) | 1 (3.6) | 1 (2.6) | NA | 3 (3.9) | 2 (6.9) | NA |
|
| |||||||
|
| |||||||
| Ethnicity | |||||||
|
| |||||||
| Hispanic or Latino | 26 (65.0) | 4 (14.3) | 17 (44.7) | <0.001 | 32 (41.6) | 15 (51.7) | 0.545 |
|
| |||||||
| Not Hispanic or Latino | 12 (30.0) | 21 (75.0) | 20 (52.6) | 39 (50.6) | 14 (48.3) | ||
|
| |||||||
| Unknown or Not Reported | 2 (5.0) | 3 (10.7) | 1 (2.6) | NA | 6 (7.8) | 0 | NA |
|
| |||||||
|
| |||||||
| Height and Weight | |||||||
|
| |||||||
| Height Z scores | |||||||
|
| |||||||
| Mean ± SD | −1.2 ± 1.29 | −0.9 ± 2.32 | −1.3 ± 1.57 | 0.660 | −1.2 ± 1.77 | −1.1 ± 1.49 | 0.717 |
|
| |||||||
|
| |||||||
| Weight Z scores | |||||||
|
| |||||||
| Mean ± SD | −0.5 ± 1.33 | −0.5 ± 1.48 | −0.6 ± 1.82 | 0.969 | −0.7 ± 1.51 | −0.2 ± 1.64 | 0.182 |
|
| |||||||
|
| |||||||
| BMI Z scores | |||||||
|
| |||||||
| Mean ± SD | 0.1 ± 1.15 | −1.4 ± 8.45 | 0.2 ± 1.54 | 0.288 | −0.4 ± 4.76 | 0.4 ± 1.38 | 0.399 |
|
| |||||||
|
| |||||||
| Positive PRA (>0%) | 7 (17.5) | 11 (39.3) | 17 (44.7) | 0.022 | 24 (31.2) | 11 (37.9) | 0.545 |
|
| |||||||
|
| |||||||
| Delayed Graft Function | 0 | 0 | 0 | NA | 0 | 0 |
|
|
| |||||||
|
| |||||||
| Use of Induction Therapy | |||||||
|
| |||||||
| Basiliximab | 13 (32.5) | 10 (35.7) | 13 (34.2) | 0.962 | 26 (33.8) | 10 (34.5) | 0.945 |
|
| |||||||
| Daclizumab | 18 (45.0) | 13 (46.4) | 14 (36.8) | 0.681 | 36 (46.8) | 9 (31.0) | 0.144 |
|
| |||||||
| Thymoglobulin | 10 (25.0) | 5 (17.9) | 12 (31.6) | 0.451 | 17 (22.1) | 10 (34.5) | 0.191 |
|
| |||||||
|
| |||||||
| Use of Maintenance Therapy | |||||||
|
| |||||||
| Steroid | 25 (62.5) | 16 (57.1) | 22 (57.9) | 0.882 | 48 (62.3) | 15 (51.7) | 0.321 |
|
| |||||||
|
| |||||||
| Infection | |||||||
|
| |||||||
| Clinical Infection | |||||||
|
| |||||||
| Bacterial | 9 (22.5) | 8 (28.6) | 5 (13.2) | 0.298 | 14 (18.2) | 8 (27.6) | 0.287 |
|
| |||||||
| Fungal | 1 (2.5) | 1 (3.6) | 1 (2.6) | 0.962 | 1 (1.3) | 2 (6.9) | 0.181 |
|
| |||||||
| Viral | 11 (27.5) | 11 (39.3) | 5 (13.2) | 0.053 | 25 (32.5) | 2 (6.9) | 0.007 |
|
| |||||||
| Protozoal | 0 | 0 | 2 (5.3) | 0.164 | 2 (2.6) | 0 | >0.999 |
|
| |||||||
|
| |||||||
| Parasitic | 0 | 0 | 1 (2.6) | 0.409 | 1 (1.3) | 0 | >0.999 |
|
| |||||||
|
| |||||||
| Viremia by PCR (Local or Central) | |||||||
|
| |||||||
| CMV+ by Local or Central PCR | 7 (17.5) | 6 (21.4) | 11 (28.9) | 0.389 | 24 (31.2) | 0 | |
|
| |||||||
| EBV+ by Local or Central PCR | 12 (30.0) | 9 (32.1) | 15 (39.5) | 0.381 | 36 (46.8) | 0 | |
|
| |||||||
| BKV+ by Local or Central PCR | 11 (27.5) | 6 (21.4) | 7 (18.4) | 0.772 | 24 (31.2) | 0 | |
|
| |||||||
| Other+ by Central PCR [1] | 11 (27.5) | 12 (42.9) | 16 (42.1) | 0.306 | 39 (50.6) | 0 | |
|
| |||||||
|
| |||||||
| eGFR | |||||||
|
| |||||||
| Mean ± SD at M1 | 65.5 ± 18.87 | 82.8 ± 31.94 | 71.6 ± 24.51 | 0.025 | 72.4 ± 26.10 | 72.1 ± 24.66 | 0.967 |
|
| |||||||
| Mean ± SD at M6 | 69.9 ± 18.13 | 81.9 ± 25.13 | 72.5 ± 20.01 | 0.082 | 74.3 ± 20.24 | 72.4 ± 23.75 | 0.692 |
|
| |||||||
| Mean ± SD at M12 | 69.0 ± 21.42 | 77.5 ± 15.15 | 78.3 ± 31.20 | 0.242 | 73.6 ± 21.53 | 76.8 ± 31.78 | 0.588 |
|
| |||||||
|
| |||||||
|
| |||||||
| Donor CMV/EBV Status | |||||||
|
| |||||||
| Donor CMV Status | |||||||
|
| |||||||
| Positive | 31 (77.5) | 12 (42.9) | 27 (71.1) | 0.009 | 54 (70.1) | 16 (55.2) | 0.147 |
|
| |||||||
| Negative | 9 (22.5) | 16 (57.1) | 11 (28.9) | 23 (29.9) | 13 (44.8) | ||
|
| |||||||
|
| |||||||
| Donor EBV Status | |||||||
|
| |||||||
| Positive | 34 (85.0) | 27 (96.4) | 33 (86.8) | 0.418 | 69 (89.6) | 25 (86.2) | 0.701 |
|
| |||||||
| Negative | 5 (12.5) | 1 (3.6) | 3 (7.9) | 6 (7.8) | 3 (10.3) | ||
|
| |||||||
| Not Done | 1 (2.5) | 0 | 2 (5.3) | 2 (2.6) | 1 (3.4) | NA | |
|
| |||||||
|
| |||||||
|
| |||||||
| Clinical Outcomes | |||||||
|
| |||||||
| Alloimmune Failure | 14 (35.0) | 11 (39.3) | 15 (39.5) | 0.931 | 27 (35.1) | 13 (44.8) | 0.464 |
|
| |||||||
| BPAR incl Borderline | 10 (25.0) | 4 (14.3) | 10 (26.3) | 0.463 | 18 (23.4) | 6 (20.7) | 0.774 |
|
| |||||||
| denovo DSA | 6 (15.0) | 8 (28.6) | 9 (23.7) | 0.386 | 16 (20.8) | 7 (24.1) | 0.708 |
|
| |||||||
Other viruses include adeno, HHV, and JCV.
There were no associations between pre-transplant serostatus and BPAR or dnDSA (see above). In addition, post-transplant viremia rates were similar by pre-transplant serostatus. The eGFR levels were not significantly different between serology groups at 6 and 12 months.
Post-Transplant Viremia
Of a total of 106 patients, 77 (73%) developed viremia, as defined by either the central or local laboratory assessments. The most common viruses were EBV (n=36, 34%), CMV (n=24, 23%), BK (n=24, 23%) and JC (n=22, 21%) (Table 3). Seven of the 22 patients with JC viremia showed PCR results of >1000 copies/mL and 2 had levels exceeding 100,000 copies/mL. Other viruses were detected sporadically (Table 3). There were 30 instances of patients having 2 or more viremias, not all concurrent.
As expected, there was a significant association between the presence of viremia as documented by PCR and the development of viral infection (p=0.007) (Table 3). Nevertheless, only 1/3 of patients who had viremia by PCR actually demonstrated clinical signs of viral infection. By univariate analysis, we observed no relationships between the development of viremia with either the type of induction therapy used, thymoglobulin use, prior anti-rejection therapy, or any specific immunosuppression regimen including corticosteroid use. There were no associations between viremia and age, race, ethnicity or other patient characteristics. There were no differences in mean eGFR values when comparing patients with or without viremia (Table 3).
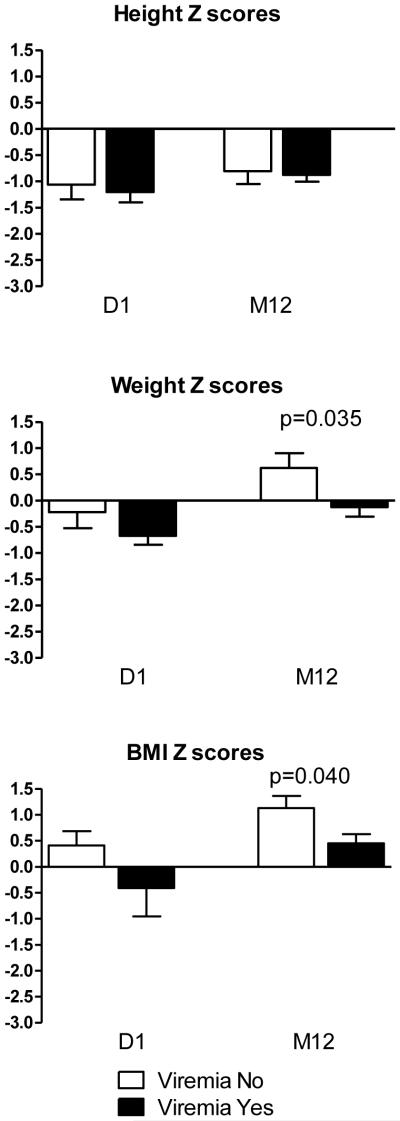
Patients who developed viremia did not differ from non-viremic patients with regard to height, weight, or BMI Z scores at transplant. However, at one year post-transplant, viremic patients weighed significantly less (p=0.035) and had significantly lower BMI (p=0.040). The mean and median BMI Z scores in viremic patients at 12 months were 0.5 and 1 respectively, while in non-viremic patients, the mean and median were 1.1 and 2 (Figure 2), suggesting either increased viral susceptibility in those with more slowly increasing BMI or a significant metabolic demand associated with viremia and/or control thereof.
Figure 2. Growth Z scores (mean with SE) by Viremia Status.
Shown are parameters of growth (height/ length) and nutritional status (weight and BMI) for children undergoing kidney transplantation. Children without viremia (white bar) and children experiencing viremia (black bar).
Clinical Viral Infection
Among the 106 patients studied, there were 54 instances of locally diagnosed clinical infection (Table 1). Viral infections were most numerous (n=27), followed by bacterial infections (n=22). When clinical infection was diagnosed, each center adjusted maintenance immunosuppression in accord with center-specific practice. As a group, first infections post-transplant were most frequently viral (n=21). Compared to patients receiving induction and/or maintenance with daclizumab, those receiving thymoglobulin had 68% lower odds of developing clinical viral infection (OR=0.32; 95% CI [0.10,1.02]), while those patients receiving basiliximab had 82% lower odds (OR=0.18; 95% CI [0.05,0.60]). When we added the transplant center to the covariates, these conclusions did not change. Other factors not related to clinical viral infection included age, pre-transplant CMV / EBV seropositivity, BPAR, dnDSA and HLA mismatch (≥2 vs. <2).
“Pure Group” Analysis
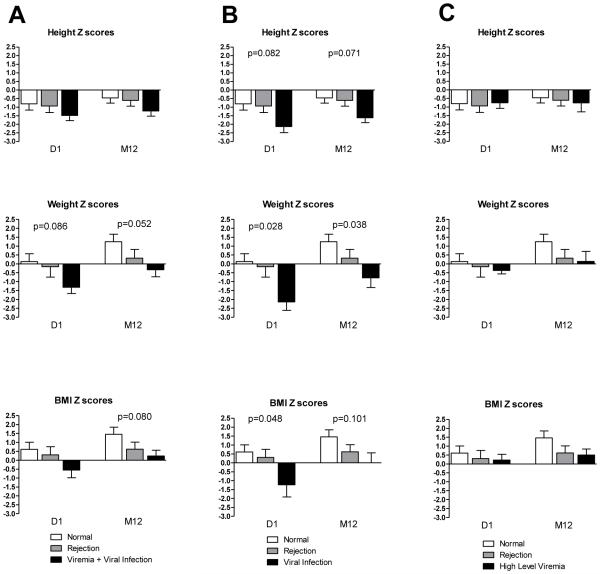
A number of the patients exhibited both rejection and viremia at different times, confounding the analyses of these distinct conditions. Thus, we defined three “phenotypically pure” groups to more precisely explore differences between these conditions of interest (Figures 1 and 3). Patients were characterized as those who experienced rejection-only (N=15); viremia-only (N=15); or neither rejection nor viremia (N=17). For some analyses, we further divided the viremia-only group into those with clinical viral infection (N=8) and those with high-level viremia (> 10,000 copies) only (N=7). We then analyzed the mean height, weight and BMI in each of the groups.
Figure 3. Growth and nutritional Z scores (mean with SE) in pure groups.
Shown are parameters of growth (height/ length) and nutritional status (weight and BMI) for children undergoing kidney transplantation segregated by assignment to pure outcome groups as defined in the Methods. Children without rejection or viremia (Normal; white bar); children experiencing rejection (gray bar); children experiencing viremia + viral infection (column A, black bar), children experiencing viral infection (column B, black bar), children experiencing high level viremia (column C, black bar).
The no-rejection/no-viremia group had the highest weight and BMI Z scores at baseline and month 12. Patients with viremia showed a mean weight Z score at baseline of −1.3, which was numerically lower (p=0.086) than in the other two pure groups; this borderline association continued to 12 months when the mean weight Z score was −0.3. (p=0.052). Mean BMI Z scores were also numerically lower in the viremia group at baseline, although the difference only reached borderline significance at 12 months (p=0.080) (Figure 3). However, upon further analysis, when we separated patients into those with clinical viral infection versus high level viremia-only, we noted that those with isolated high-level viremia showed no associations with low weight and BMI Z scores at any time point. On the other hand, patients who developed clinical viral infection had at baseline significantly lower Z scores for weight (−2.1) (p=0.028) and BMI (−1.2) (p=0.048), and the weight difference persisted to 12 months (p=0.038) (Figure3). Thus, with precise stratification, low weight and BMI were preceding risk factors for subsequent clinical viral infection.
Flow cytometry analyses
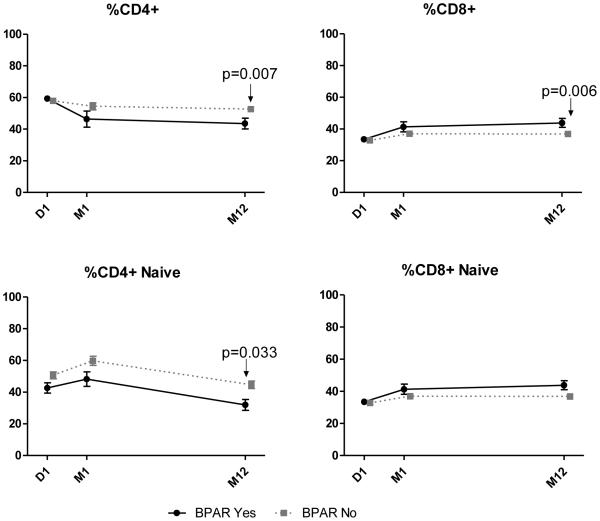
There were no significant differences in any memory T cell compartment at baseline that predicted BPAR or dnDSA (data not shown). At month 12, both the percentage and absolute numbers of CD4+ T cells were significantly decreased in rejecters compared to non-rejecters (p=0.007 and p=0.011 respectively (Figure 4). Similarly, there were significantly reduced percentages (Figure 4) and absolute numbers of naïve CD4+ T cells in rejecters compared to non-rejecters at month 12 (p=0.033 and p=0.0004 respectively). (Absolute numbers not shown.) The percentage of CD8+ T cells was significantly increased at 12 months in patients who experienced rejection (p=0.006).
Figure 4. Flow Cytometry by BPAR (mean with SE).
Shown are parameters of T-cells for children undergoing kidney transplantation. Children without BPAR (gray dot line) and children experiencing BPAR (solid black line).
As with BPAR, memory subsets at baseline were not related to the subsequent development of dnDSA. In patients with dnDSA, there was a decrease in the percent of CD4+ naïve cells from D1 to M12 (p=0.012), consistent with the requirement for T cell help for alloantibody formation. There were no differences in memory subsets prior to transplantation that signaled a risk for viremia and/or viral infection. Additionally, there were no memory subset patterns that related to viral disease over the first year, either in the entire study group or in the three “phenotypically pure” groups.
Discussion
The IMPACT study is the first to prospectively examine the relationships between viremia/viral infection, alloimmune events, BMI/weight, and T cell immunophenotyping in pediatric kidney transplant recipients. Our data indicate that viremia is very common, occurring in 73% of children regardless of immunosuppression or viral serostatus. We found no relationships between CMV and/or EBV serostatus or memory T cell phenotypes at the time of transplantation, and subsequent transplant outcome through one year of follow-up. An unexpected and potentially important observation is that clinical viral infection was significantly associated with baseline low BMI and weight. Moreover, both viremia and viral infection appeared to be associated with less weight gain, lower BMI gains over the first year, and lower BMI at 12 months after transplantation.
We found viremia in almost three quarters of our patients. The incidences of EBV, CMV and BK viremias were consistent with those in other reported pediatric series, as well as in one adult series (20-23). Previous large studies and surveys of post-transplant viral infection in pediatric kidney transplantation have examined only EBV, CMV and BK viremias. Ours is the first pediatric kidney transplant multicenter study that has looked for evidence not only of those 3 viruses, but also for HHV6, 7 and 8, adenovirus and JC virus. A surprising finding was the high prevalence of JC viremia, found in 21% of our patients. Previously, post-transplant JC viremia was generally assumed to be transient and infrequent (24-26). Primary JC virus infection is most common in childhood, adolescence and young adulthood. Approximately 50% of normal children are seropositive by ages 9–11years, and this number rises to 69% of all organ transplant recipients by age 29 (27). The finding of JC viremia in our immunosuppressed population warrants more investigation, since this virus has been associated with such long-term morbidities as progressive multifocal leukoencephalopathy (27), JC polyomavirus encephalopathy (28, 29), JC nephropathy (29) and CNS tumors (30).
Our study uncovered a new and potentially important interaction between children’s underweight status and clinical outcome. In our cohort, clinical viral infection was significantly more likely to occur in patients with low baseline weight and BMI Z scores. Malnutrition in children is associated with immune defects (31). It is therefore understandable that underweight pediatric transplant recipients may experience more viral infections. Underweight pediatric transplant recipients may suffer other morbidities as well. Ku et al have recently reported that pediatric transplant recipients classified as “underweight” have higher mortality rates than those with weight/BMI in the normal range (32). Protein energy wasting in children in the United States with chronic renal insufficiency ranges from 7–20% (33). Recent examinations of two large databases found that 6% and 10% of patients beginning renal replacement therapy were underweight in Europe and the United States respectively (32, 34). Children on maintenance dialysis for <1 year were more likely to be underweight than those who spent 1-3 years on dialysis (34). Thus, a small but important fraction of children with chronic kidney disease and ESRD appear to be undernourished, and this may have important clinical implications. Our results and those of Ku et al suggest that the effects of underweight status in pediatric transplant recipients deserve careful study.
The IMPACT Study sought to establish whether viral infection and/or viremia could influence alloimmune events and allograft outcome in the first post-transplant year. While some clinical studies have linked viral infection with allograft dysfunction and rejection, (17, 35-37) we observed no increase in BPAR with either viremia or clinical viral infection, including CMV or BK viremia. The lack of association between CMV viremia and rejection could be due in part to our use of valgancyclovir prophylaxis in almost every patient (10, 17). We also found no association between any viremia, and the generation of dnDSA. Prior exposure to the latent herpesviruses CMV and EBV, as denoted by pre-transplant seropositivity, also did not appear to influence the subsequent incidence of rejection or DSA formation. We did find a borderline association between donor CMV seropositivity and post-transplant DSA, but the relevance of this observation is unclear, given the absence of any association between DSA and recipient CMV serostatus or viremia. Taken together, our findings in this prospective study strongly suggest that viral infections do not directly lead to BPAR in children in the first post-transplant year. However, other studies suggest that viral infection may adversely affect long-term allograft outcome (38-40). For this reason, longer term follow-up of our patients and similar pediatric cohorts is warranted.
There are few data in pediatric kidney transplantation examining the clinical value of T cell immunophenotyping. Reports have been equivocal as to the importance of memory subset analysis as a prognostic tool (41). Our study prospectively assessed associations between bulk T cell memory and transplant outcomes. While bulk T cell phenotype at baseline did not anticipate rejection, rejection and/or its clinical treatment was associated with subsequent expansion of cytotoxic CD8+ T cell effectors and a reduction in antigen inexperienced CD4+ T cells. More in depth analyses to include regulatory, exhausted and senescent phenotypes are currently in progress.
Our study can reasonably be applied to today’s pediatric kidney transplant population. The immunosuppressive regimens were consistent with those used in the majority of pediatric transplant centers (42) . The graft and patient survivals were in keeping with expected modern results (2, 3, 43). The eGFR results in our patients were somewhat lower than those reported in the NAPRTCS database. (https://web.emmes.com/study/ped/annlrept/annualrept2014.pdf). However, we used the updated Schwartz formula which gives results that are somewhat lower than the older Schwartz formula that the NAPRTCS Registry used (18). The incidence of BPAR was similar to that in previous reports (42). Similarly, both the prevalence of dnDSA and the prominence of Class II DSA in our patients are similar to other reported series (4, 44-46). Similar to the findings of Kim et al (45), we did not find an association between BPAR and DSA. This is not surprising since previous pediatric studies, which did not include protocol biopsies, have found that it can take a year or more for patients who generate dnDSA to manifest biopsy changes of AMR (44). Most pediatric studies agree that dnDSA, and particularly Class II DSAs, are associated with deteriorating renal function (4, 45).
Our study has some important limitations. The sample size of 106 transplanted patients and the follow-up of only one year limit our ability to draw conclusions about such clinical questions as the relationship between dnDSA generation and late BPAR, and the long-term effects of viremia/viral infection on outcome. Moreover, the relatively small number of patients who were viremia-free limits our ability to categorically define the clinical correlates of freedom from viremia. Induction and maintenance immunosuppressive regimens were not harmonized between centers or patients, and the study was not powered to evaluate the effects of different immunosuppressive regimens. Finally, the racial / ethnic composition of our cohort limits the generalizability of our findings to the general pediatric kidney transplant population.
In summary, our study uncovers some important clinical interactions and associations between viremia, viral infection, indices of nutrition and T cell phenotyping. Further studies using the IMPACT database are in progress to examine and expand in greater detail the findings described in this report. These findings can serve to generate specific medical practices that will improve the outcome of pediatric kidney transplantation.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Tina Sledge, for her trial management support; Linda Stempora, for her flow cytometry expertise; Steve Kleiboeker, for his viral PCR analysis, ViraCor, Inc., for providing the viral PCR assays, and Ashley Morgan for proofreading the manuscript.
This work was funded by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health: U01 AI077821.
Abbreviations
- ACR
Acute Cellular Rejection
- AF
Alloimmune Failure
- AMR
Antibody Mediated Rejection
- BKV
BK Viremia
- BMI
body mass index
- BPAR
Biopsy-proven acute allograft rejection
- CMV
Cytomegalovirus
- DSA
Donor Specific Antibody
- ESRD
End Stage Renal Disease
- EBV
Epstein–Barr virus
- HHV
Human Herpesvirus
- IMPACT
Immune Development in Pediatric Transplantation
- JC
JC virus
- MICA
MHC class I polypeptide-related sequence A
- SDS
Standard Deviation Score
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary Materials and Methods
Appendix S1: Schedule of Events
References
- 1.Dharnidharka VR, Fiorina P, Harmon WE. Kidney transplantation in children. N Engl J Med. 2014;371(6):549–558. doi: 10.1056/NEJMra1314376. [DOI] [PubMed] [Google Scholar]
- 2.Van Arendonk KJ, Boyarsky BJ, Orandi BJ, James NT, Smith JM, Colombani PM, et al. National trends over 25 years in pediatric kidney transplant outcomes. Pediatrics. 2014;133(4):594–601. doi: 10.1542/peds.2013-2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Stewart DE, et al. OPTN/SRTR 2013 Annual Data Report: kidney. Am J Transplant. 2015;15(Suppl 2):1–34. doi: 10.1111/ajt.13195. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhuri A, Ozawa M, Everly MJ, Ettenger R, Dharnidharka V, Benfield M, et al. The clinical impact of humoral immunity in pediatric renal transplantation. J Am Soc Nephrol. 2013;24(4):655–664. doi: 10.1681/ASN.2012070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 6.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31(6):859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith C, Khanna R. Immune regulation of human herpesviruses and its implications for human transplantation. Am J Transplant. 2013;13(Suppl 3):9–23. doi: 10.1111/ajt.12005. quiz 23. [DOI] [PubMed] [Google Scholar]
- 8.Dowd JB, Palermo T, Brite J, McDade TW, Aiello A. Seroprevalence of Epstein-Barr virus infection in U.S. children ages 6-19, 2003-2010. PLoS One. 2013;8(5):e64921. doi: 10.1371/journal.pone.0064921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20(4):202–213. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- 10.Martin-Gandul C, Mueller NJ, Pascual M, Manuel O. The Impact of Infection on Chronic Allograft Dysfunction and Allograft Survival After Solid Organ Transplantation. Am J Transplant. 2015;15(12):3024–3040. doi: 10.1111/ajt.13486. [DOI] [PubMed] [Google Scholar]
- 11.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30(1):16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 12.Heutinck KM, Yong S, Tonneijck L, van den Heuvel H, van der Weerd NC, van der Pant KA, et al. Virus-specific CD8 T-cells cross-reactive to donor-alloantigen are transiently present in the circulation of kidney transplant recipients infected with CMV and/or EBV. Am J Transplant. 2015 doi: 10.1111/ajt.13618. [DOI] [PubMed] [Google Scholar]
- 13.Chong AS, Alegre ML. The impact of infection and tissue damage in solid-organ transplantation. Nat Rev Immunol. 2012;12(6):459–471. doi: 10.1038/nri3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Razonable RR, Paya CV. Infections and allograft rejection - intertwined complications of organ transplantation. Swiss Med Wkly. 2005;135(39-40):571–573. doi: 10.4414/smw.2005.10984. [DOI] [PubMed] [Google Scholar]
- 15.Dickenmann MJ, Cathomas G, Steiger J, Mihatsch MJ, Thiel G, Tamm M. Cytomegalovirus infection and graft rejection in renal transplantation. Transplantation. 2001;71(6):764–767. doi: 10.1097/00007890-200103270-00013. [DOI] [PubMed] [Google Scholar]
- 16.Nett PC, Heisey DM, Fernandez LA, Sollinger HW, Pirsch JD. Association of cytomegalovirus disease and acute rejection with graft loss in kidney transplantation. Transplantation. 2004;78(7):1036–1041. doi: 10.1097/01.tp.0000137105.92464.f3. [DOI] [PubMed] [Google Scholar]
- 17.Stern M, Hirsch H, Cusini A, van Delden C, Manuel O, Meylan P, et al. Cytomegalovirus serology and replication remain associated with solid organ graft rejection and graft loss in the era of prophylactic treatment. Transplantation. 2014;98(9):1013–1018. doi: 10.1097/TP.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blumberg JM, Gritsch HA, Reed EF, Cecka JM, Lipshutz GS, Danovitch GM, et al. Kidney paired donation in the presence of donor-specific antibodies. Kidney Int. 2013;84(5):1009–1016. doi: 10.1038/ki.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith JM, Corey L, Bittner R, Finn LS, Healey PJ, Davis CL, et al. Subclinical viremia increases risk for chronic allograft injury in pediatric renal transplantation. J Am Soc Nephrol. 2010;21(9):1579–1586. doi: 10.1681/ASN.2009111188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al Khasawneh E, Gupta S, Tuli SY, Shahlaee AH, Garrett TJ, Schechtman KB, et al. Stable pediatric kidney transplant recipients run higher urine indoleamine 2, 3 dioxygenase (IDO) levels than healthy children. Pediatr Transplant. 2014;18(3):254–257. doi: 10.1111/petr.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Chaudhuri A, Weintraub LA, Hsieh F, Shah S, Alexander S, et al. Subclinical cytomegalovirus and Epstein-Barr virus viremia are associated with adverse outcomes in pediatric renal transplantation. Pediatr Transplant. 2007;11(2):187–195. doi: 10.1111/j.1399-3046.2006.00641.x. [DOI] [PubMed] [Google Scholar]
- 23.Bamoulid J, Courivaud C, Coaquette A, Chalopin JM, Gaiffe E, Saas P, et al. Subclinical Epstein-Barr virus viremia among adult renal transplant recipients: incidence and consequences. Am J Transplant. 2013;13(3):656–662. doi: 10.1111/ajt.12009. [DOI] [PubMed] [Google Scholar]
- 24.Delbue S, Ferraresso M, Ghio L, Carloni C, Carluccio S, Belingheri M, et al. A review on JC virus infection in kidney transplant recipients. Clin Dev Immunol. 2013;2013:926391. doi: 10.1155/2013/926391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Razonable RR, Brown RA, Humar A, Covington E, Alecock E, Paya CV, et al. A longitudinal molecular surveillance study of human polyomavirus viremia in heart, kidney, liver, and pancreas transplant patients. J Infect Dis. 2005;192(8):1349–1354. doi: 10.1086/466532. [DOI] [PubMed] [Google Scholar]
- 26.Mengelle C, Kamar N, Mansuy JM, Sandres-Saune K, Legrand-Abravanel F, Miedouge M, et al. JC virus DNA in the peripheral blood of renal transplant patients: a 1-year prospective follow-up in France. J Med Virol. 2011;83(1):132–136. doi: 10.1002/jmv.21951. [DOI] [PubMed] [Google Scholar]
- 27.Antonsson A, Pawlita M, Feltkamp MC, Bouwes Bavinck JN, Euvrard S, Harwood CA, et al. Longitudinal study of seroprevalence and serostability of the human polyomaviruses JCV and BKV in organ transplant recipients. J Med Virol. 2013;85(2):327–335. doi: 10.1002/jmv.23472. [DOI] [PubMed] [Google Scholar]
- 28.Bialasiewicz S, Hart G, Oliver K, Agnihotri SP, Koralnik IJ, Viscidi R, et al. A Difficult Decision: Atypical JC Polyomavirus Encephalopathy in a Kidney Transplant Recipient. Transplantation. 2016 doi: 10.1097/TP.0000000000001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drachenberg CB, Hirsch HH, Papadimitriou JC, Gosert R, Wali RK, Munivenkatappa R, et al. Polyomavirus BK versus JC replication and nephropathy in renal transplant recipients: a prospective evaluation. Transplantation. 2007;84(3):323–330. doi: 10.1097/01.tp.0000269706.59977.a5. [DOI] [PubMed] [Google Scholar]
- 30.Eftimov T, Enchev Y, Tsekov I, Simeonov P, Kalvatchev Z, Encheva E. JC polyomavirus in the aetiology and pathophysiology of glial tumours. Neurosurg Rev. 2016;39(1):47–53. doi: 10.1007/s10143-015-0676-5. [DOI] [PubMed] [Google Scholar]
- 31.Peixoto Paes-Silva R, Correia de Macedo EM, Oliveira Tomiya MT, Machado Barbosa de Castro CM. Immune Response of Severe Malnutrition Children Treated According to the Protocol of the World Health Organization. Nutr Hosp. 2015;32(2):638–644. doi: 10.3305/nh.2015.32.2.9048. [DOI] [PubMed] [Google Scholar]
- 32.Ku E, Glidden DV, Hsu CY, Portale AA, Grimes B, Johansen KL. Association of Body Mass Index with Patient-Centered Outcomes in Children with ESRD. J Am Soc Nephrol. 2016;27(2):551–558. doi: 10.1681/ASN.2015010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abraham AG, Mak RH, Mitsnefes M, White C, Moxey-Mims M, Warady B, et al. Protein energy wasting in children with chronic kidney disease. Pediatr Nephrol. 2014;29(7):1231–1238. doi: 10.1007/s00467-014-2768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonthuis M, van Stralen KJ, Verrina E, Groothoff JW, Alonso Melgar A, Edefonti A, et al. Underweight, overweight and obesity in paediatric dialysis and renal transplant patients. Nephrol Dial Transplant. 2013;28(Suppl 4):iv195–iv204. doi: 10.1093/ndt/gft259. [DOI] [PubMed] [Google Scholar]
- 35.Weikert BC, Blumberg EA. Viral infection after renal transplantation: surveillance and management. Clin J Am Soc Nephrol. 2008;3(Suppl 2):S76–86. doi: 10.2215/CJN.02900707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sagedal S, Nordal KP, Hartmann A, Sund S, Scott H, Degre M, et al. The impact of cytomegalovirus infection and disease on rejection episodes in renal allograft recipients. Am J Transplant. 2002;2(9):850–856. doi: 10.1034/j.1600-6143.2002.20907.x. [DOI] [PubMed] [Google Scholar]
- 37.Jordan CL, Taber DJ, Kyle MO, Connelly J, Pilch NW, Fleming J, et al. Incidence, risk factors, and outcomes of opportunistic infections in pediatric renal transplant recipients. Pediatr Transplant. 2015 doi: 10.1111/petr.12625. [DOI] [PubMed] [Google Scholar]
- 38.Arthurs SK, Eid AJ, Pedersen RA, Kremers WK, Cosio FG, Patel R, et al. Delayed-onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin Infect Dis. 2008;46(6):840–846. doi: 10.1086/528718. [DOI] [PubMed] [Google Scholar]
- 39.Khan H, Mubarak M, Aziz T, Ahmed E, Fazal Akhter S, Kazi J, et al. Prevalence and risk factors for early chronic allograft nephropathy in a live related renal transplant program. J Nephropathol. 2014;3(2):69–79. doi: 10.12860/jnp.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dzabic M, Rahbar A, Yaiw KC, Naghibi M, Religa P, Fellstrom B, et al. Intragraft cytomegalovirus protein expression is associated with reduced renal allograft survival. Clin Infect Dis. 2011;53(10):969–976. doi: 10.1093/cid/cir619. [DOI] [PubMed] [Google Scholar]
- 41.Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5(3):465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 42.Smith JM, Martz K, Blydt-Hansen TD. Pediatric kidney transplant practice patterns and outcome benchmarks, 1987-2010: a report of the North American Pediatric Renal Trials and Collaborative Studies. Pediatr Transplant. 2013;17(2):149–157. doi: 10.1111/petr.12034. [DOI] [PubMed] [Google Scholar]
- 43.Muscheites J, Wigger M, Drueckler E, Klaassen I, John U, Wygoda S, et al. Estimated one-yr glomerular filtration rate is an excellent predictor of long-term graft survival in pediatric first kidney transplants. Pediatr Transplant. 2009;13(3):365–370. doi: 10.1111/j.1399-3046.2008.00976.x. [DOI] [PubMed] [Google Scholar]
- 44.Ginevri F, Nocera A, Comoli P, Innocente A, Cioni M, Parodi A, et al. Posttransplant de novo donor-specific hla antibodies identify pediatric kidney recipients at risk for late antibody-mediated rejection. Am J Transplant. 2012;12(12):3355–3362. doi: 10.1111/j.1600-6143.2012.04251.x. [DOI] [PubMed] [Google Scholar]
- 45.Kim JJ, Balasubramanian R, Michaelides G, Wittenhagen P, Sebire NJ, Mamode N, et al. The clinical spectrum of de novo donor-specific antibodies in pediatric renal transplant recipients. Am J Transplant. 2014;14(10):2350–2358. doi: 10.1111/ajt.12859. [DOI] [PubMed] [Google Scholar]
- 46.Athavale D, Worthington J, Webb NJ, Roberts D, Martin S, Shenoy M. Pediatric kidney recipients may benefit from monitoring for donor-specific antibodies. Pediatr Transplant. 2014;18(3):258–265. doi: 10.1111/petr.12247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.