Abstract
Objective
We assessed the degree of inter-individual responses in energy intake (EI) to an imposed sleep restriction versus habitual sleep duration protocol. We also investigated participant (age, sex, ethnicity and BMI) and study (study site, protocol order) characteristics as potential contributors to the variance in EI responses to sleep restriction between individuals.
Methods
Data from two randomized crossover trials were combined. All participants (n = 43; age: 31±7 years, BMI: 23±2 kg/m2) were free of medical/sleep conditions, non-smokers, reported not performing shift work, and having an average sleep duration of 7–9h/night. Ad libitum, 24h EI was objectively-assessed following sleep restriction (3.5–4h in bed/night) and habitual sleep (7–9h in bed/night) conditions.
Results
Large inter-individual variations in EI change (ΔEI) between restricted and habitual sleep conditions were noted (−813 to 1437 kcal/day). Only phase order was associated with ΔEI (β = −568 kcal/day, 95% CI for β = −921 to −215 kcal; P = 0.002); participants randomized to the habitual sleep condition first had greater increases in EI when sleep was restricted (P = 0.01).
Conclusions
Large inter-individual variations in ΔEI following sleep restriction were noted, suggesting that not all individuals were negatively impacted by the effects of sleep restriction.
Keywords: sleep restriction, energy intake, inter-individual variability
Introduction
Imposing sleep restriction up to five days can lead to short-term weight gain (1, 2, 3, 4). More specifically, these studies report mean increases in EI of 200–500 kcal/day following imposed sleep restriction compared to habitual sleep duration (1, 2, 3, 4), suggesting that increased EI may largely account for the weight gain observed following sleep restriction (5). In addition to these main effects, differences in EI responses to sleep loss according to certain participant characteristics, e.g. sex (4, 6, 7) and ethnicity (6), have been previously noted.
Although the abovementioned studies have consistently reported average increases in EI following imposed sleep restriction compared to habitual sleep duration, they also present large standard deviations for EI. Therefore, the range in EI responses to the same sleep restriction protocol may greatly vary between individuals.
The primary aim of this paper was to assess inter-individual responses in EI to an imposed partial sleep restriction protocol. Furthermore, we investigated participants’ age, sex, ethnicity, BMI, protocol order and study site as potential contributors to this degree of variance in EI responses to imposed sleep restriction between individuals.
Methods
Data from two randomized crossover sleep restriction interventions conducted at the University of Ottawa (Ottawa, Canada) (8) and St-Luke’s-Roosevelt Hospital/Columbia University (New York, USA) (7) were combined for this secondary analysis. Study protocols were approved by their Institution’s Ethics Committees (The University of Ottawa Ethics Committee; The Institutional Review Boards of St-Luke’s-Roosevelt Hospital Center and Columbia University), and participants provided informed consent. All participants were 18–45 years of age, free of neurological, metabolic and sleeping disorders, non-smokers and non-shift workers. Participants also reported sleeping on average 7–9 h/night, as verified with two weeks of accelerometry and sleep diary data in both studies.
The study conducted at St-Luke’s-Roosevelt Hospital/Columbia University included two sessions of five nights each: sleep restriction (4h in bed/night) and habitual sleep duration (9h in bed/night). At least four weeks separated each session. EI was standardized over the first 4 days of each session and ad libitum, 24h EI was assessed on day 5. Participants were able to self-select foods inside the research facility or purchase foods outside of the facility with a monetary allowance. All consumed food items were weighed and recorded by study staff. The study conducted at the University of Ottawa included three sessions of one night each: sleep restriction with advanced wake-time (3.5–4h in bed/night, remained awake during the second part of the night), sleep restriction with delayed bedtime (3.5–4h in bed/night, remained awake during the first part of the night) and habitual sleep duration (7–9h in bed/night). Advancing wake-time leads to selective reductions in rapid eye movement sleep (9); therefore, data from the sleep restriction with delayed bedtime condition was included as the “sleep restriction condition” in the present analysis. There was also no statistically significant difference in EI change (ΔEI) when comparing both sleep restriction conditions in this study (results not shown). At least seven days separated each session. Participants self-selected foods from a validated menu, which were served in ad libitum quantities. Study staff weighed and recorded all consumed food items. Despite differences in study protocol/intervention lengths between study sites, no statistically significant differences in ΔEI between studies were noted (Ottawa: 157±443 versus New York: 282±630 kcal/day; P = 0.48).
ΔEI for each participant was calculated by subtracting EI during the habitual sleep duration condition to EI during the sleep restriction condition. A multivariable, stepwise linear regression analysis was used to examine the strength of the associations between the participants’ age, sex (man or woman), ethnicity (white or other), BMI, protocol order (sleep restriction or habitual sleep duration first) and study site (New York or Ottawa) with ΔEI. A sample size of 43 participants with a pre-determined power of 0.80 and two-tailed alpha of 0.05 is estimated to provide a large effect size (Cohen’s f2 = 0.41) to detect significant associations with this regression model. Stratified analysis with an independent t-test were conducted if significant associations were noted with this regression model. Statistical analyses were performed using SPSS (version 19.0; SPSS, Chicago, IL, USA). Statistical significance was set at P<0.05.
Results
Details on mean differences in EI between sleep duration conditions for the studies presented herein are presented elsewhere (7, 8). Table 1 presents baseline characteristics for all participants, and according to phase order and study site.
Table 1.
Baseline characteristics for all participants, and according to phase order and study site
| All participants | Study site | Phase order | |||
|---|---|---|---|---|---|
|
| |||||
| n = 43 | Ottawa (n = 17) | New York (n = 26) | Habitual sleep first (n = 17) | Sleep restriction first (n = 26) | |
| Age (years); mean ± SD | 31±7 | 23±4 | 35±5 | 33±7 | 29±7 |
| BMI (kg/m2); mean ± SD | 23±2 | 23±3 | 24±1 | 24±2 | 23±2 |
| Sex; n (%) | |||||
| Men | 24 (55.8%) | 11 (64.7%) | 13 (50%) | 8 (47.1%) | 16 (61.5%) |
| Women | 19 (44.2%) | 6 (35.3%) | 13 (50%) | 9 (52.9%) | 10 (38.5) |
| Ethnicity; n (%) | |||||
| White | 27 (62.8%) | 15 (88.2%) | 12 (46.2%) | 11 (64.7%) | 16 (61.5%) |
| Other | 16 (37.2%) | 2 (11.8%) | 14 (53.8%) | 6 (35.3%) | 10 (38.5%) |
Note: BMI, body mass index; SD, standard deviation
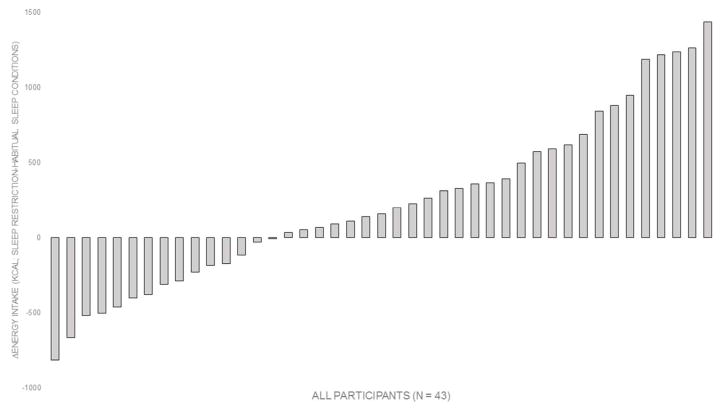
The range in ΔEI was large (−813 to 1437 kcal/day; Figure 1). In all participants, 41.9% had >300 kcal/day increase in EI during sleep restriction versus habitual sleep duration conditions, 39.5% had ≤300 kcal/day difference in EI between conditions, and 18.6% had >300 kcal/day decrease in EI following sleep restriction versus habitual sleep duration.
Figure 1.
Distribution of energy intake responses (ΔEI) to sleep restriction. ΔEI for each participant was calculated by subtracting EI during the habitual sleep duration condition to EI during the sleep restriction condition.
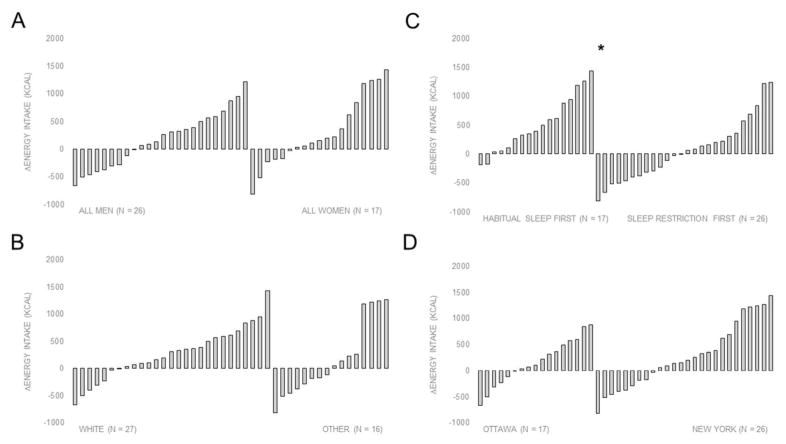
Figure 2 presents inter-individual variations in ΔEI according to sex, ethnicity, phase order and study site. Only phase order was significantly associated with ΔEI (β = −568 kcal/day, 95% CI for β = −921 to −215 kcal; P = 0.002) in the multivariable regression model. Post-hoc analysis revealed that participants randomized to the habitual sleep duration condition first had greater increases in EI when sleep was restricted versus habitual sleep duration (506±494 versus 54±537 kcal/day; P = 0.01).
Figure 2.
Distribution of energy intake responses (ΔEI) according to A) sex (man or woman), B) ethnicity (white or other), C) phase order (habitual sleep or sleep restriction conditions first) and D) study site (Ottawa or New York). *P = 0.01
Note: ΔEI for each participant was calculated by subtracting EI during the habitual sleep duration condition to EI during the sleep restriction condition.
Discussion
Our findings indicated large inter-individual variations in ΔEI in response to sleep restriction, suggesting that EI following similar degrees of imposed sleep restriction was highly variable between participants. Large inter-individual variations in weight loss following diet and/or exercise interventions have also been reported (10, 11, 12). Even though the trials presented herein used objective and precise methods to assess EI, accuracy and validity issues often arise as a result of large day-to-day variability in EI (11).
Spaeth et al. (13) also reported inter-individual differences in EI (−501 to 1178 kcal/day), and large differences in body weight change (−2.3 to 6.5 kg), between participants who took part in two identical sleep restriction conditions. There is evidence to suggest that trait-like differences between individuals may impact the degree of sensitivity to adverse cognitive effects of sleep loss (14, 15, 16, 17). Van Dongen et al. (14) were amongst the first to investigate inter-individual differences in sleepiness ratings and responses to psychomotor and cognitive tasks following total sleep deprivation, demonstrating large inter-, but not intra-, individual responses to sleep loss. Killgore et al. (15) later demonstrated that an extraversive personality trait was associated with greater declines in alertness and psychomotor vigilance following total sleep deprivation. Furthermore, individuals with lower cortical activity when rested (17) and/or greater ventrolateral prefrontal cortex activation following sleep loss (16) have been classified as being “resistant” to the effects of sleep loss.
No baseline participant characteristics (age, sex, ethnicity and BMI) included in our regression model were significantly associated with ΔEI between sleep conditions. Spaeth et al. (13) reported greater consistency in EI and body weight changes to consecutive sleep restriction interventions in men versus women, but no differences in body weight change to the sleep restriction intervention between normal-weight and overweight individuals. Only phase order was significantly associated with ΔEI in the present study, with participants randomized to the habitual sleep condition first having greater increases in EI when sleep was restricted versus habitual sleep duration. A similar order effect was previously reported by Markwald et al. (4), noting a reduction in EI and weight loss when participants transitioned from the sleep restriction to adequate sleep condition. Although a mere hypothesis, it is possible that participants were more cautious during the first sleep condition as a result of not knowing the randomization order and/or less familiarity with the laboratory settings and measurement procedures, compared to the second session. Conversely, the novelty associated with ad libitum access to food may lead to greater EI in some participants during the first session, independently of the study condition. Studies are needed to explore this hypothesis within the contexts of EI research.
Strengths of this paper include the combination of data from two different sleep restriction trials to increase the number of participants and better illustrate inter-individual variability in EI responses to imposed sleep restriction. Additionally, these studies included objective measurements of sleep and EI under strict laboratory conditions. Limitations include the measurement of EI over a single 24h period and the recruitment of healthy, young individuals with good sleep quality only, which limits generalizability of study findings to other populations (e.g. individuals with sleep disorders). Although no statistical difference in ΔEI was noted between study sites, additive effects of sleep restriction on EI may have occurred in one study imposing five nights of sleep restriction, but not the other which only imposed one night of sleep restriction, for each condition.
In conclusion, we demonstrated large inter-individual variations in EI responses to imposed sleep restriction, suggesting that not all individuals may be negatively impacted by the effects of sleep restriction. Future studies are needed to identify contributing behavioral (e.g. physical activity participation) and physiological (e.g. resting metabolic rate, (an)orexigenic hormonal variations) factors to the inter-individual responses in EI to imposed sleep restriction in order to better characterize those individuals who are “resistant” to the effects of partial sleep restriction on EI.
Study Importance Questions.
Consistent increases in energy intake have been reported following imposed sleep restriction compared to habitual sleep duration.
This study reports large inter-individual variations in energy intake responses to sleep restriction compared to habitual sleep duration.
Baseline participant characteristics were not statistically associated with inter-individual variations in energy intake changes between sleep conditions.
Acknowledgments
Funding: This study was supported in part by R01 HL091352 (St-Onge), P30 DK26687, and Columbia University’s CTSA grant No. UL1 TR000040 from NCAT/NIH.
We would like to thank the participants for taking part in these studies, as well as students for their involvement in data collection. We would also like to thank Amy Roberts for conducting the research at St-Luke’s-Roosevelt Hospital/Columbia University. Lastly, we thank Drs. Éric Doucet and Geneviève Forest for their involvement in the design and conduct of the study at the University of Ottawa.
Footnotes
Trial Registration: clinicaltrials.gov identifier: NCT00935402 (St-Luke’s-Roosevelt Hospital/Columbia University).
Disclosure: The authors declared no conflict of interest.
References
- 1.Calvin AD, Carter RE, Adachi T, Macedo PG, Albuquerque FN, van der Walt C, et al. Effects of experimental sleep restriction on caloric intake and activity energy expenditure. Chest. 2013;144:79–86. doi: 10.1378/chest.12-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, Hitze B, Later W, Wilms B, et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obesity facts. 2008;1:266–273. doi: 10.1159/000158874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spaeth AM, Dinges DF, Goel N. Effects of Experimental Sleep Restriction on Weight Gain, Caloric Intake, and Meal Timing in Healthy Adults. Sleep. 2013;36:981–990. doi: 10.5665/sleep.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5695–5700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.St-Onge MP, Bormes A, Salazar I. The role of sleep duration on energy balance: an update. Curr Nutr Rep. 2016;5:278. [Google Scholar]
- 6.Spaeth AM, Dinges DF, Goel N. Sex and race differences in caloric intake during sleep restriction in healthy adults. The American journal of clinical nutrition. 2014;100:559–566. doi: 10.3945/ajcn.114.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St-Onge MP, Roberts AL, Chen J, Kelleman M, O’Keeffe M, RoyChoudhury A, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. The American journal of clinical nutrition. 2011;94:410–416. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNeil J, Doucet E, Brunet JF, Hintze LJ, Chaumont I, Langlois E, et al. The effects of sleep restriction and altered sleep timing on energy intake and energy expenditure. Physiology & behavior. 2016;164:157–163. doi: 10.1016/j.physbeh.2016.05.051. [DOI] [PubMed] [Google Scholar]
- 9.Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. Journal of experimental psychology Applied. 2000;6:236–249. doi: 10.1037//1076-898x.6.3.236. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly JE, Honas JJ, Smith BK, Mayo MS, Gibson CA, Sullivan DK, et al. Aerobic exercise alone results in clinically significant weight loss for men and women: midwest exercise trial 2. Obesity (Silver Spring, Md) 2013;21:E219–228. doi: 10.1002/oby.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melanson EL, Keadle SK, Donnelly JE, Braun B, King NA. Resistance to exercise-induced weight loss: compensatory behavioral adaptations. Medicine and science in sports and exercise. 2013;45:1600–1609. doi: 10.1249/MSS.0b013e31828ba942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King NA, Hopkins M, Caudwell P, Stubbs RJ, Blundell JE. Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss. International journal of obesity (2005) 2008;32:177–184. doi: 10.1038/sj.ijo.0803712. [DOI] [PubMed] [Google Scholar]
- 13.Spaeth AM, Dinges DF, Goel N. Phenotypic vulnerability of energy balance responses to sleep loss in healthy adults. Scientific reports. 2015;5:14920. doi: 10.1038/srep14920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–433. [PubMed] [Google Scholar]
- 15.Killgore WD, Richards JM, Killgore DB, Kamimori GH, Balkin TJ. The trait of Introversion-Extraversion predicts vulnerability to sleep deprivation. Journal of sleep research. 2007;16:354–363. doi: 10.1111/j.1365-2869.2007.00611.x. [DOI] [PubMed] [Google Scholar]
- 16.Chuah YM, Venkatraman V, Dinges DF, Chee MW. The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:7156–7162. doi: 10.1523/JNEUROSCI.0906-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caldwell JA, Mu Q, Smith JK, Mishory A, Caldwell JL, Peters G, et al. Are individual differences in fatigue vulnerability related to baseline differences in cortical activation? Behavioral neuroscience. 2005;119:694–707. doi: 10.1037/0735-7044.119.3.694. [DOI] [PubMed] [Google Scholar]