Abstract
Introduction
Hypoalbuminemia is a predictor of poor outcomes in dialysis patients. Among hemodialysis patients, there has not been prior study of whether residual kidney function or decline over time impacts serum albumin levels. We hypothesized that a decline in residual kidney function is associated with an increase in serum albumin levels among incident hemodialysis patients.
Methods
In a large national cohort of 38,504 patients who initiated hemodialysis during 1/2007–12/2011, we examined the association of residual kidney function, ascertained by urine volume and renal urea clearance, with changes in serum albumin over five years across strata of baseline residual kidney function, race, and diabetes using case-mix adjusted linear mixed effects models.
Findings
Serum albumin levels increased over time. At baseline, patients with greater urine volume had higher serum albumin levels: 3.44±0.48, 3.50±0.46, 3.57±0.44, 3.59±0.45, and 3.65±0.46g/dL for urine volume groups of <300, 300-<600, 600-<900, 900-<1200, and ≥1200 mL/day, respectively (Ptrend<0.001). Over time, urine volume and renal urea clearance declined and serum albumin levels rose, while the baseline differences in serum albumin persisted across groups of urinary volume. In addition, the rate of decline in residual kidney function was not associated with the rate of change in albumin.
Discussion
Hypoalbuminemia in hemodialysis patients is associated with lower residual kidney function. Among incident hemodialysis patients, there is a gradual rise in serum albumin that is independent of the rate of decline in residual kidney function, suggesting that preservation of residual kidney function does not have deleterious impact on serum albumin.
Keywords: Residual kidney function, hypoalbuminemia, urine volume, hemodialysis
Introduction
Protein-energy wasting and inflammation are common conditions in patients with chronic kidney disease, and due to their frequent coexistence are also referred to as the malnutrition-inflammation cachexia syndrome (MICS).1–3 In maintenance dialysis patients, the MICS and its markers such as hypoalbuminemia are strong predictors of adverse events including lower health-related quality of life, higher hospitalization and death risk. 4–7 However, serum albumin levels are also affected by protein loss into the urine, especially since many patients with chronic kidney disease also have significant degrees of albuminuria. A few studies have demonstrated that serum albumin levels tend to rise in the months immediately following hemodialysis initiation.8–10 However, it is unknown if this rise in albumin is attributed to improved nutritional status after dialysis initiation or due to loss of residual kidney function over time upon transition to dialysis therapy. Whereas preservation of residual kidney function may be associated with better outcomes,11–15 its impact upon serum albumin levels remains unclear.
We therefore investigated the trajectory of serum albumin levels over five years after initiation of hemodialysis therapy across different levels of residual kidney function, and hypothesized that a decline in residual kidney function is associated with an increase in serum albumin levels.
Methods
Patients
We retrospectively analyzed clinical data from all incident in-center hemodialysis patients aged 18 years or older who were treated in facilities operated by a large dialysis organization in the US from January 1, 2007 to December 31, 2011.16 During this time period, there were 208,820 patients who initiated dialysis treatment. Patient follow up time was divided into 20 consecutive patient-quarters (PQ; 91-day periods from date of first dialysis). Patients treated for at least 60 consecutive days were considered to be on maintenance dialysis therapy. We excluded patients who were ever treated with peritoneal dialysis, home hemodialysis, nocturnal hemodialysis, less-frequent hemodialysis, and frequent hemodialysis, and identified 133,148 incident dialysis patients who were treated only with conventional hemodialysis. Patients were also excluded if data on serum albumin and urine volume at baseline (i.e., the first quarter) were missing (n=23,068 and 71,576, respectively), resulting in a final cohort of 38,504 patients (urine volume analysis, Appendix-Figure S1). In these patients, renal urea clearance (KRU) analysis was conducted among 35,961 patients who had KRU data. Differences in baseline characteristics among included versus excluded patients are shown in Appendix-Table S1. The study was approved by the Institutional Review Committees of the University of California, Irvine and Los Angeles Biomedical Research Institute at Harbor-UCLA. Given the large sample size, anonymity of the patients studied, and nonintrusive nature of the research, the requirement for written consent was exempted.
Demographic, clinical measures and laboratory measures
Information on race/ethnicity, cause of end-stage renal disease, primary insurance, access type and presence of comorbidities at baseline were obtained from the large dialysis organization’s database. Most laboratory values were measured monthly, including serum albumin, creatinine, hemoglobin, peripheral white blood cell count, lymphocyte percentage, total iron binding capacity (TIBC), calcium, phosphorus, bicarbonate and alkaline phosphatase. Serum intact parathyroid hormone (PTH) and ferritin levels were usually measured at least once during each patient quarter. Most blood samples were collected before dialysis. The normalized protein catabolic rate (nPCR) was measured monthly as an indicator of daily protein intake. Dialysis dose was estimated by single pool Kt/V (spKt/V) using the urea kinetic model. The average serum urea concentration during the collection were assumed to be 90% of the pre-dialysis concentration according to the Daugirdas approach and thus renal urea clearance (KRU) were calculated as follows.17
KRU was adjusted for body surface area and expressed as mL/min/1.73m2.18, 19 To minimize measurement variability, all repeated measures for each patient during any given patient-quarter (91-day interval) were averaged and summary estimates were used in all analyses. Concerning residual kidney function, patients were categorized into five groups according to baseline urine volume (<300, 300-<600, 600-<900, 900-<1200, ≥1200 mL/day) and six groups according to baseline KRU (<1, 1-<2, 2-<3, 3<4, 4<6, ≥6 mL/min/1.73m2).
Statistical analysis
Baseline characteristics across urine volume strata were summarized as proportions, means (±standard deviation, SD) or medians (interquartile range, IQR). Test for trend analyses were used to quantify the relationship of baseline characteristics across urine volume groups. Changes in mean serum albumin, urine volume and KRU over time (up to a total of 20 patient-quarters (PQ1-PQ20)) were summarized and examined in linear mixed effect models with case-mix adjustment. Effect modification of serum albumin trajectories by baseline urine volume strata, race, and diabetes status were examined by creation of interaction terms (effect modifier variable-x-patient quarter follow up) and comparing model fit with and without interaction terms by Wald test. In order to examine associations between change in residual kidney function (urine volume and KRU) with change in serum albumin levels from the first to the fifth patient-quarter (i.e. one-year interval), we created scatter plots with accompanied Pearson correlation estimates and conducted case-mix adjusted linear regression models using serum albumin as the dependent variable and urine volume or KRU as the independent variable. Case-mix variables included age, sex, race/ethnicity, diabetes, primary insurance, vascular access type, spKt/V and 9 preexisting comorbidities (hypertension, atherosclerotic heart disease, congestive heart failure, cerebrovascular disease, other cardiovascular disease, history of cancer, human immunodeficiency virus, chronic obstructive pulmonary disease and dyslipidemia). Factors related to nutrition, infections and inflammatory status may be in the causal pathway in the relationship between residual kidney function and change in albumin, and were therefore not used as covariates in this analysis. All analyses were carried out using STATA MP Version 13.1 (StataCorp, College Station, TX).
Results
Baseline demographic and laboratory characteristics according to baseline urine volume category
In the analytical cohort of 38,504 incident hemodialysis patients, the mean±SD age was 62±15 years among whom 38% were female, 54% were non-Hispanic white, 28% were African-American, and 46% had diabetic nephropathy as the cause of end-stage renal disease (Table 1). There was a significant trend towards higher serum albumin across urine volume strata (Ptrend <0.001); 3.44±0.48g/dL, 3.50±0.46g/dL, 3.57±0.44g/dL, 3.59±0.45g/dL, and 3.65±0.46g/dL for <300, 300-<600, 600-<900, 900-<1200, and ≥1200 mL/day, respectively. Patients with lower baseline urine volume were more likely to be elderly, female, African-American; had a lower prevalence of diabetes; had higher prevalence of hypertension, congestive heart failure and cerebrovascular disease; were more likely to use a central venous catheter; had higher serum calcium, alkaline phosphatase, serum ferritin and bicarbonate levels; and had lower spKt/V, pre-dialysis systolic blood pressure, pre-dialysis diastolic blood pressure, body mass index, nPCR, lymphocyte percentage, hemoglobin, serum albumin, serum phosphorus, intact PTH, and TIBC levels.
Table 1.
Baseline characteristics of 38,504 incident hemodialysis patients according to baseline urine volume category
| Variable | Total (n=38,504) | UV<300 (n=5,659) | 300<=UV<600 (n=8,578) | 600<=UV<900 (n=7,192) | 900<=UV<1200 (n=5,474) | UV>=1200 (n=11,601) | p value |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean Urine Volume (mL/day) | 787 (450, 1300) | 150 (50, 200) | 450 (365, 500) | 700 (650, 800) | 1000 (950, 1100) | 1600 (1350, 2000) | <0.001 |
| KRU (mL/min/1.73m2) | 2.92 (1.54, 4.77) | 0.48 (0.07, 0.94) | 1.80 ( 1.24, 2.57) | 2.79 (2.01, 3.87) | 3.72 (2.74, 5.04) | 5.18 (3.81, 7.04) | <0.001 |
| Age (years) | 62.0 ± 14.9 | 65.5 ± 15.1 | 64.6 ± 14.9 | 63.0 ± 14.8 | 61.1 ± 14.7 | 58.0 ± 13.9 | <0.001 |
| Women (%) | 38 | 51 | 45 | 38 | 34 | 27 | <0.001 |
| Race / ethnicity (%) | <0.001 | ||||||
| Non-Hispanic white | 54 | 52 | 54 | 54 | 54 | 57 | |
| Non-Hispanic black | 28 | 34 | 30 | 28 | 27 | 23 | |
| Hispanic | 11 | 9 | 10 | 11 | 12 | 12 | |
| Asian | 3 | 2 | 3 | 4 | 4 | 4 | |
| Others | 3 | 3 | 3 | 3 | 4 | 4 | |
| ESRD Reason (%) | <0.001 | ||||||
| Diabetes | 46 | 42 | 45 | 46 | 47 | 49 | |
| Hypertension | 28 | 32 | 31 | 29 | 28 | 24 | |
| Glomerulonephritis | 10 | 10 | 10 | 10 | 10 | 11 | |
| Cystic Kidney Disease | 2 | 1 | 1 | 2 | 2 | 3 | |
| Others | 14 | 15 | 13 | 13 | 13 | 13 | |
| Insurance (%) | <0.001 | ||||||
| Medicare | 52 | 57 | 55 | 53 | 50 | 46 | |
| Medicaid | 6 | 6 | 6 | 7 | 6 | 7 | |
| Others | 42 | 36 | 39 | 40 | 44 | 47 | |
| Access type (%) | <0.001 | ||||||
| Central Venous Catheter | 74 | 80 | 78 | 74 | 73 | 70 | |
| AV Fistula | 18 | 11 | 14 | 18 | 19 | 23 | |
| AV Graft | 4 | 4 | 4 | 4 | 4 | 3 | |
| AV Other | 0 | 0 | 0 | 0 | 0 | 0 | |
| Unknown | 4 | 5 | 4 | 4 | 4 | 4 | |
| Comorbidity (%) | |||||||
| Hypertension | 51 | 53 | 53 | 52 | 50 | 48 | <0.001 |
| Atherosclerotic heart disease | 14 | 15 | 15 | 14 | 13 | 14 | 0.14 |
| Congestive Heart Failure | 38 | 39 | 39 | 38 | 37 | 37 | 0.008 |
| Cerebrovascular disease | 2 | 2 | 2 | 2 | 1 | 1 | 0.006 |
| Other cardiovascular disease | 15 | 17 | 16 | 15 | 15 | 14 | <0.001 |
| History of Cancer | 3 | 3 | 3 | 3 | 2 | 2 | 0.007 |
| HIV | 0 | 0 | 0 | 0 | 1 | 1 | 0.128 |
| COPD | 5 | 6 | 6 | 5 | 4 | 4 | <0.001 |
| Dyslipidemia | 26 | 25 | 26 | 26 | 26 | 26 | 0.79 |
| sp KT/V | 1.56 ± 0.36 | 1.43 ± 0.29 | 1.50 ± 0.30 | 1.55 ± 0.34 | 1.59 ± 0.36 | 1.66 ± 0.42 | <0.001 |
| Mean Pre SBP (mmHg) | 148 ± 19 | 143 ± 20 | 146 ± 19 | 148 ± 18 | 149 ± 18 | 150 ± 18 | <0.001 |
| Mean Pre DBP (mmHg) | 78 ± 12 | 74 ± 12 | 76 ± 12 | 78 ± 12 | 79 ± 11 | 80 ± 11 | <0.001 |
| Body Mass Index (kg/m2) | 28.8 ± 7.2 | 28.1 ± 7.4 | 28.2 ± 7.3 | 28.4 ± 7.0 | 28.7 ± 7.1 | 29.7 ± 7.3 | <0.001 |
| nPCR (g/kg/day) | 0.85 ± 0.23 | 0.75 ± 0.21 | 0.80 ± 0.21 | 0.84 ± 0.21 | 0.87 ± 0.22 | 0.92 ± 0.24 | <0.001 |
| Laboratories | |||||||
| WBC (1,000/μL) | 7.5 ( 6.1, 9.0) | 7.5 ( 6.1, 9.1) | 7.4 (6.1, 9.0) | 7.4 (6.1, 9.0) | 7.5 (6.2, 9.0) | 7.5 (6.2, 9.0) | 0.13 |
| Lymphocyte (%) | 21 ± 8 | 20 ± 8 | 20 ± 8 | 21 ± 8 | 21 ± 7 | 21 ± 7 | <0.001 |
| Hemoglobin (g/dL) | 11.2 ± 1.1 | 11.0 ± 1.1 | 11.2 ± 1.1 | 11.2 ± 1.1 | 11.3 ± 1.1 | 11.3 ± 1.1 | <0.001 |
| Albumin (g/dL) | 3.56 ± 0.46 | 3.44 ± 0.48 | 3.50 ± 0.46 | 3.57 ± 0.44 | 3.59 ± 0.45 | 3.65 ± 0.46 | <0.001 |
| Corrected Calcium (mg/dL) | 9.1 ± 0.5 | 9.1 ± 0.5 | 9.1 ± 0.6 | 9.1 ± 0.5 | 9.1 ± 0.5 | 9.1 ± 0.6 | <0.001 |
| Phophorus (mg/dL) | 5.0 ± 1.1 | 4.8 ± 1.2 | 4.9 ± 1.1 | 5.0 ± 1.1 | 5.0 ± 1.1 | 5.1 ± 1.1 | <0.001 |
| intact PTH (pg/mL) | 310 (199, 475) | 289 (181, 457) | 299 (192, 462) | 311 (203, 475) | 318 (208, 490) | 324 (208, 486) | <0.001 |
| ALP (IU/L) | 84 (67, 110) | 87 (69, 119) | 86 (68, 115) | 85 (67, 110) | 83 (66, 108) | 82 (65, 105) | <0.001 |
| Creatinine (mg/dL) | 5.8 ± 2.4 | 5.9 ± 2.5 | 5.7 ± 2.5 | 5.8 ± 2.4 | 5.8 ± 2.3 | 5.8 ± 2.2 | <0.001 |
| TIBC (mg/dL) | 229 (200, 260) | 217 (186, 249) | 223 (194, 254) | 229 (202, 259) | 232 (204, 260) | 239 (211, 268) | <0.001 |
| Iron Saturation (%) | 23 ± 9 | 23 ± 10 | 23 ± 9 | 23 ± 8 | 23 ± 8 | 23 ± 8 | 0.15 |
| Ferritin (ng/mL) | 271 (158, 456) | 320 (185, 544) | 285 (168, 482) | 273 (161, 449) | 257 (150, 434) | 244 (142, 411) | <0.001 |
| Bicarbonate (mEq/L) | 23.5 ± 2.6 | 24.1 ± 2.7 | 24.0 ± 2.6 | 23.5 ± 2.5 | 23.3 ± 2.5 | 22.9 ± 2.6 | <0.001 |
Abbreviations: KRU, renal urea clearance; AV Fistula, arteriovenous fistula; AV Graft, arteriovenous graft; HIV, human immunodeficiency virus; COPD, chronic obstructive pulmonary disease; spKt/V, single pool Kt/V; SBP, systolic blood pressure; DBP, diastolic blood pressure; nPCR, normalized protein catabolic rate; WBC, white blood cell; PTH, parathyroid hormone; ALP, alkaline phosphatase; TIBC, total iron binding capacity
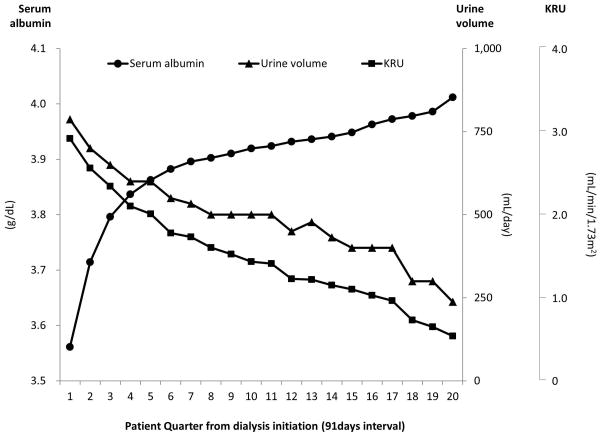
Serum albumin levels sharply increased over the first six patient quarters (i.e., 18 months) after initiating dialysis and then appeared to plateau (Figure 1). This trend was also observed among 71,576 patients who were excluded for missing baseline urine volume data and 44,064 patients who survived the first two years of dialysis treatment (Appendix-Figure S2). Conversely, urine volume and KRU showed a sustained decrease over time.
Figure 1. Mean serum albumin level, mean urine volume and mean renal urea clearance (KRU) per patient quarter in 38,504 incident hemodialysis patients.
Abbreviations: KRU, renal urea clearance.
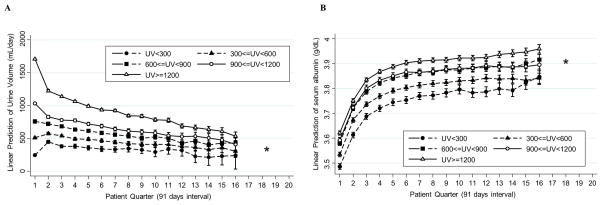
Trajectories of urine volume and serum albumin according to baseline urine volume strata
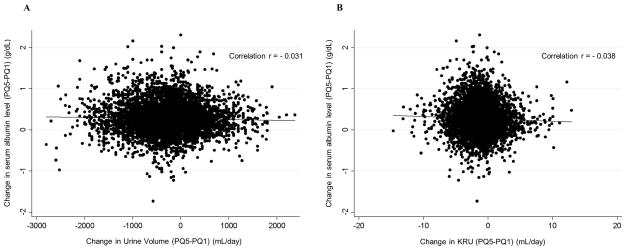
Across baseline urine volume strata, trajectories of urine volume were significantly different (PWald<0.001, Figure 2), while trajectories of serum albumin levels were similar (PWald=0.10). When compared to the middle baseline urine volume group (600 to <900 mL/day), relative changes in urine volume from baseline to PQ2 were −448 (95%CI, −476 to −421) mL/day, −166 (95%CI, −198 to −134) mL/day, +99 (95%CI, 70 to 129) mL/day, and +239 (95%CI, 206 to 272) mL/day in the urine volume groups of ≥1200 mL/day, 900-<1200 mL/day, 300-<600 mL/day, and <300 mL/day, respectively. The subsequent decrease in urine volume after PQ2 was also faster in the higher urine volume group (Ptrend<0.001). Patients with higher urinary volume not only had higher baseline serum albumin levels, but also maintained a higher serum albumin level over the follow up period (Figure 2(B)). The differences of baseline serum albumin according to urine volume strata persisted over the 14 patient quarters. This result is consistent among patients who survived more than 2 years (n=15,422) (Appendix-Figure S3). Across baseline KRU strata, serum albumin levels of all KRU groups increased in a similar fashion as among urine volume strata (Appendix-Figure S4). Patients with the lowest KRU levels had the lowest serum albumin levels over 12 patient quarters (i.e., 3 years). However, serum albumin levels in patients within other strata with KRU≥1 mL/min/1.73m2 exhibited no noticeable differences at baseline or over time. The association between annual change in urine volume and annual change in serum albumin level from dialysis start was statistically significant but clinically irrelevant (correlation r= −0.031, p=0.02, case-mix adjusted β= −0.0016 mg/dL per 100mL of urine volume, p=0.04, Figure 3(A)). Similarly, the association between annual change in KRU and annual change in serum albumin was also statistically significant but clinically irrelevant (correlation r= −0.038, p=0.003, case-mix adjusted β= −0.0064mg/dL per 1 mL/min/1.73m2 of KRU, p=0.002, Figure 3(B)).
Figure 2. Case-mix adjusted (A) mean urine volume and (B) mean serum albumin level per patient quarter over 5 years in 38,504 patients stratified by baseline urine volume groups.
Abbreviations: UV, urine volume.
*The results of the number of patients in either group <50 were not shown.
Figure 3. Correlation of change in serum albumin levels (PQ5-PQ1) with (A) change in urine volume (PQ5-PQ1), (B) change in KRU (PQ5-PQ1) in 6,010 patients.
Abbreviations: KRU, renal urea clearance.
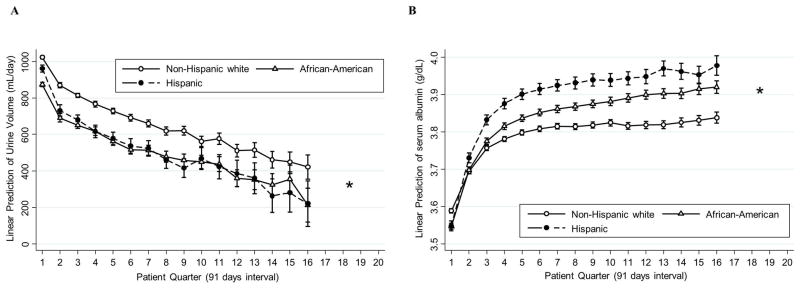
Trajectories of urine volume and serum albumin according to race and ethnicity
Trajectories of both urine volume and serum albumin levels differed by race (PWald <0.001 for both, Figure 4). Mean serum albumin levels at baseline were 3.57±0.45 g/dL, 3.56±0.48 g/dL, and 3.55±0.48g/dL in non-Hispanic white, African-American, and Hispanic patients, respectively. Compared to non-Hispanic white patients, urine volume declined faster in Hispanics by 77 (95%CI, 43–111) mL/day, 88 (95%CI, 51–126) mL/day, 96 (95%CI, 44–148) mL/day, and 139 (95%CI, −3–281) mL/day from baseline to PQ2, PQ4, PQ8, and PQ16, respectively. Compared to non-Hispanic white patients, Hispanics experienced 0.08 (95%CI, 0.07–0.09) g/dL, 0.14 (95%CI, 0.12–0.15) g/dL, 0.16 (95%CI, 0.14–0.17) g/dL, and 0.18 (95%CI, 0.15–0.21) g/dL greater rises in serum albumin from baseline to PQ2, PQ4, PQ8, and PQ16, respectively. Similarly, compared to non-Hispanic white patients, urine volume declined faster in African-Americans by 30 (95%CI, 2–57) mL/day at PQ2, however there were no significant differences in the decline of urine volume at PQ4, PQ8, and PQ16, respectively. Compared to non-Hispanic white patients, African-Americans experienced 0.05 (95%CI, 0.04–0.05) g/dL, 0.08 (95%CI, 0.07–0.08) g/dL, 0.09 (95%CI, 0.08–0.11) g/dL, and 0.12 (95%CI, 0.10–0.14) g/dL greater rises in serum albumin from baseline to PQ2, PQ4, PQ8, and PQ16, respectively.
Figure 4. Case-mix adjusted (A) mean urine volume and (B) mean serum albumin level per patient quarter over 5 years in 35,756 patients stratified by race.
*The results of the number of patients in either group <50 were not shown.
Trajectories of urine volume and serum albumin according to the causes of end-stage renal diseases
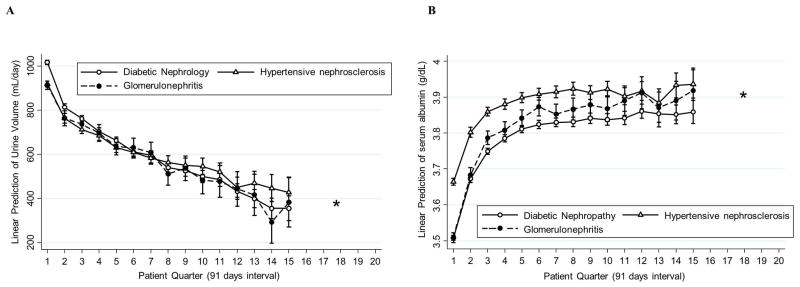
Trajectories of urine volume and serum albumin also differed across the causes of end-stage renal diseases: diabetic nephropathy (n=17,751), hypertensive nephrosclerosis (n=10,943), and glomerulonephritis (n=3,887) (PWald<0.001, Figure 5). Diabetic nephropathy patients had higher urine volume at baseline compared to the other groups, but the decline in urine volume over time among diabetic nephropathy patients was more rapid than that of other patients. The decline in urine volume in diabetic nephropathy patients, compared to hypertensive nephrosclerosis patients, was significantly greater by 48 (95%CI, 22–73) mL/day, 79 (95%CI, 52–106) mL/day, and 121 (95%CI, 83–158) mL/day at PQ2, PQ4, and PQ8, respectively. In contrast, patients with diabetic nephropathy and glomerulonephritis had lower serum albumin levels at baseline compared to those with hypertensive nephrosclerosis (3.50±0.44 mg/dL and 3.51±0.56 mg/dL vs. 3.65±0.44 mg/dL), but this difference in serum albumin level between glomerulonephritis and hypertensive nephrosclerosis was attenuated over time. However, patients with diabetic nephropathy had the lowest serum albumin levels over time among these three groups.
Figure 5. Case-mix adjusted (A) mean urine volume and (B) mean serum albumin level per patient quarter over 5 years in 32,581 patients stratified by the cause of end-stage renal diseases.
*The results of the number of patients in either group <50 were not shown
Discussion
In a longitudinal cohort of 38,504 incident hemodialysis patients who were followed for up to 5 years, we found that serum albumin levels rose over the first 6 patient quarters (18 months) after hemodialysis initiation. Patients with higher urine volume had higher baseline serum albumin levels and maintained higher serum albumin levels during follow up. Hispanic and diabetic patients showed a greater increase in serum albumin levels and faster decline in urine volume over time on dialysis. To our knowledge, this is the first study to examine the relationship between urine volume and serum albumin in a large cohort of incident hemodialysis patients with data starting at hemodialysis initiation and for up to 5 years of follow up.
Our finding that serum albumin levels tended to increase over time on dialysis is consistent with results from previous studies.8–10 Although we cannot exclude the possibility that this rise in serum albumin may in part be attributed to survivor bias (hypoalbuminemic patients are more likely to die earlier), our sensitivity analysis among 2-year survivors also showed a similar trend (Appendix-Figure S2). Conversely, in a study of prevalent hemodialysis patients by the Italian Cooperative Dialysis Study, a slight decline in serum albumin levels over time on dialysis was reported.20 However, the study only included 380 prevalent dialysis patients with only 24-months of follow up time. Suda et al. additionally reported that prevalent hemodialysis patients with greater residual kidney function had higher serum albumin levels.21 In their study, patients with residual kidney function experienced a rise in serum albumin over one year of follow up; while serum albumin levels among patients without residual kidney function remained the same. Differences in study population (i.e., incident versus prevalent hemodialysis patients) and improved standard treatments over time (i.e., 1990’s versus late 2000’s) may explain why results in a population who did not have substantial residual kidney function contrast with results found in our study.
The trajectories of serum albumin across urine volume strata and KRU strata were similar. However the incremental differences in baseline serum albumin and their trends over time were not observed across KRU strata. One potential reason could be that KRU is not a perfect indicator of the magnitude of albuminuria decline. Moreover, urine urea clearance does not account for tubular reabsorption of urea and may underestimate renal function. Conversely, urine creatinine clearance includes creatinine secreted by renal tubules and overestimates true renal function, especially in renal failure. Averaging urea and creatinine clearances may counteract their respective shortcomings but may misrepresent true innate renal function.22
We observed a rise in serum albumin over time independent of baseline residual kidney function (urine volume and KRU). Additionally, the rate of decline in residual kidney function was not associated with rise in albumin. The relationship between change in serum albumin with proteinuria remains controversial. Goldwasser et al 8 reported that the mean rate of increase in serum albumin significantly correlated with baseline proteinuria (r=0.49) among 115 incident hemodialysis patients. In contrast, Mehrotra et al 10 reported that the slope of change in serum albumin level was not associated with baseline 24-hour urine protein excretion among 62 incident hemodialysis patients. However, previous studies have demonstrated a rapid increase in dietary protein intake 10 and composite nutritional score including serum creatinine and serum phosphorus 23 after hemodialysis initiation. Our results also suggest that the rise in serum albumin may be mainly attributed to improvement in nutritional status due to hemodialysis initiation, and not to decline in urine volume or residual kidney function.
Several common mechanisms related to nutrition and protein metabolism may explain the positive association between residual kidney function and serum albumin levels and observed increases in serum albumin levels seen after dialysis initiation. In patients with advanced chronic kidney disease, metabolic acidosis is a common condition, known to stimulate protein degradation and branched-chain amino acid catabolism and to impair protein synthesis.24–26 Accumulation of uremic toxins as a result of the high rate of protein breakdown are postulated causes of poor appetite which may result in malnutrition and anorexia commonly seen in this population.27, 28 Therefore, attenuation of these conditions conferred by either residual kidney function or dialysis may lead to improved appetite, increased protein synthesis, reduced protein degradation, and subsequently higher albumin levels. Indeed, uremic patients with anorexia have been shown to regain appetite soon after dialysis initiation,29 and a previous study has also showed a link between residual kidney function and preserved appetite.30 This link may also be explained by better efficiency in solute clearance offered by residual kidney function due to its continuous nature, as compared to the intermittent dialysis treatment. Higher efficiency in solute clearance would lead to a greater reductions in inflammation, improvement in overall health status, and thereby improve patients’ appetites.21, 31, 32 In patients with some preserved renal function, the kidneys may still produce arginine and release it into the blood, with subsequent transport to skeletal muscle and increased protein synthesis.33 Improved nutritional status (higher albumin levels) seen in hemodialysis patients with higher residual kidney function may also serve to explain the better survival observed in these patients.11–15
In our study, African-American and Hispanic patients had both lower serum albumin levels and lower urine volume at dialysis initiation, compared to non-Hispanic whites. This finding in African-American patients is consistent with results from a prior study,34 which also showed that African-American patients tended to initiate dialysis later than white end-stage renal disease patients. A later dialysis start may explain why African-American hemodialysis patients have lower urine volume at dialysis initiation. Moreover, worse pre-dialysis access to health care and higher proteinuria during pre-dialysis kidney disease 35 may explain why African-Americans have lower levels of serum albumin at dialysis initiation. Conversely, Noori, et al 36 showed that Hispanics had approximately 0.1 g/dL higher serum albumin levels compared to both African American and non-Hispanic white hemodialysis patients. However, their study included prevalent hemodialysis patients (i.e., average vintage 28 months). In our study, Hispanic patients had initiated dialysis at lower levels of serum albumin, but due to more rapid rise of albumin levels over time on dialysis, Hispanic patients had notably higher levels of serum albumin compared to African-Americans and non-Hispanic whites after 1 year of treatment. In our study, rapid rises in serum albumin level observed for both Hispanic and African-American patients, compared to non-Hispanic whites was accompanied by a faster decline of urine volume over time on hemodialysis. These steeper changes in renal function may be a continuation of faster declines in urine volume observed in the pre-dialysis period.35
At baseline, patients with diabetic nephropathy had greater urine volume (or residual kidney function) than others, but also experienced faster decline in urine volume than other patients. Hypertensive nephrosclerosis patients had slower decline of urine volume. These findings correspond to the clinical course of chronic kidney diseases. Patients with diabetic nephropathy and glomerulonephritis had lower baseline serum albumin levels than those with hypertensive nephrosclerosis, which may due to a greater amount of proteinuria in diabetic nephropathy37 and glomerulonephritis patients. Nephrotic range proteinuria leads to hypoalbuminemia and is a strong risk factor for rapid decline in renal function in diabetic patients in the pre-dialysis period.38 Among hemodialysis patients, diabetes is reported to be a significant risk factor for low serum albumin (<3.8g/dL).39 It should be noted that patients with diabetic nephropathy had the lowest serum albumin levels over time. In diabetic patients, lower serum albumin levels observed at dialysis initiation may be due to both greater losses of albumin from higher rates of proteinuria in conjunction with higher rates of inflammation. At later stages of dialysis, inflammatory factors such as interleukin-6 40 may have important bearing on lower albumin levels seen in diabetic patients.
Several limitations of the present study should be noted. First, data on proteinuria, albuminuria, and direct inflammatory markers such as C-reactive protein and tumor necrosis factor-α were not available. Second, information about urine volume was not available for all patients and therefore results may not be representative of the entire cohort as shown in Appendix-Table S1 and may be subject to selection biases. However, analyses of serum albumin trajectories in both included and excluded patients were consistent. Third, linear mixed models did not account for patient censoring (kidney transplantation, transfer to a non-affiliated dialysis center, and death) and therefore patient numbers at later times of follow up may be lower and bias results. Nonetheless, changes in serum albumin levels were consistent with study findings in a subpopulation of 44,064 patients who survived the first 2 years of dialysis treatment (Appendix-Figure S2). Fourth, information on nutritional supplementation during the study period was not available. In two retrospective cohort studies among prevalent hemodialysis patients, oral intradialytic nutritional supplement use was associated with reduced mortality.41, 42 It is likely that patients with hypoalbuminemia used nutritional supplements, which might have influenced serum albumin trajectories. Lastly, in our analyses, information on insurance type served as a proxy for socio-economic status. Further details on patient socio-economic status (including change in access to health care from pre to post-dialysis initiation) were not available. Changes in patient access to care may impact health status and trajectories in serum albumin.
In conclusion, our study shows that a consistent rise in serum albumin occurs after transition to dialysis therapy which may not be fully explained by a gradual loss of residual kidney function. However, greater residual kidney function at baseline was associated with sustained higher serum albumin levels over time on hemodialysis. Further studies are required to examine how preserved residual kidney function contributes to better survival in hemodialysis patients, and whether strategies to preserve residual kidney function ultimately result in better clinical outcomes in hemodialysis patients.
Supplementary Material
Appendix-Figure S1. Algorithm (flow chart) of patient selection for the cohort
Appendix-Figure S2. Mean serum albumin levels per patient quarter (A) in 38,504 included patients and 71,576 excluded patients and (B) in 110,080 patients as total cohort and 44,064 who survived more than 2 years
Appendix-Figure S3. Case-mix adjusted mean serum albumin level per patient quarter in 15,422 patients who survived more than 2 years stratified by baseline urine volume groups.
Appendix-Figure S4. Case-mix adjusted mean serum albumin level per patient quarter over 5 years in 35,961 patients stratified by baseline KRU groups.
Abbreviations: KRU, renal urea clearance.
The results of the number of patients in either group <50 were not shown.
Acknowledgments
The study was supported by KKZ’s research grants from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institute of Health (R01-DK078106 and K24-DK091419), and philanthropic grants from Mr. Harold Simmons, Mr. Louis Chang and AVEO. YO has been supported by the Shinya Foundation for International Exchange of Osaka University Graduate School of Medicine Grant.
Footnotes
Potential Conflicts of Interest:
KKZ has received commercial honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, Astra-Zeneca, Aveo, Chugai, DaVita, Fresenius, Genentech, Haymarket Media, Hospira, Kabi, Keryx, Novartis, Pfizer, Relypsa, Resverlogix, Sandoz, Sanofi, Shire, Vifor, UpToDate, and ZS-Pharma. CPK has received honoraria from Abbott Nutrition, Relypsa, Sanofi-Aventis and ZS Pharma; and grant support from Shire. Funding from US government agencies (such as NIH) and non-for profit foundations or societies (such as NKF) are not listed.
References
- 1.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38(6):1251–1263. doi: 10.1053/ajkd.2001.29222. [DOI] [PubMed] [Google Scholar]
- 2.Kopple JD. Pathophysiology of protein-energy wasting in chronic renal failure. J Nutr. 1999;129(1S Suppl):247s–251s. doi: 10.1093/jn/129.1.247S. [DOI] [PubMed] [Google Scholar]
- 3.Lowrie EG. Acute-phase inflammatory process contributes to malnutrition, anemia, and possibly other abnormalities in dialysis patients. Am J Kidney Dis. 1998;32(6 Suppl 4):S105–112. doi: 10.1016/s0272-6386(98)70172-6. [DOI] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, McAllister CJ, Alcorn H, Jr, Kopple JD, Greenland S. Revisiting mortality predictability of serum albumin in the dialysis population: time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transplant. 2005;20(9):1880–1888. doi: 10.1093/ndt/gfh941. [DOI] [PubMed] [Google Scholar]
- 5.Bergstrom J. Nutrition and mortality in hemodialysis. J Am Soc Nephrol. 1995;6(5):1329–1341. doi: 10.1681/ASN.V651329. [DOI] [PubMed] [Google Scholar]
- 6.Iseki K, Kawazoe N, Fukiyama K. Serum albumin is a strong predictor of death in chronic dialysis patients. Kidney Int. 1993;44(1):115–119. doi: 10.1038/ki.1993.220. [DOI] [PubMed] [Google Scholar]
- 7.Owen WF, Jr, Lew NL, Liu Y, Lowrie EG, Lazarus JM. The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med. 1993;329(14):1001–1006. doi: 10.1056/NEJM199309303291404. [DOI] [PubMed] [Google Scholar]
- 8.Goldwasser P, Kaldas AI, Barth RH. Rise in serum albumin and creatinine in the first half year on hemodialysis. Kidney Int. 1999;56(6):2260–2268. doi: 10.1046/j.1523-1755.1999.00768.x. [DOI] [PubMed] [Google Scholar]
- 9.Parker TFWR, 3rd, Husni L, Ikizler TA, Parker RA, Hakim RM. Effect of membrane bioincompatibility on nutritional parameters in chronic hemodialysis patients. Kidney Int. 1996;49:551–556. doi: 10.1038/ki.1996.78. [DOI] [PubMed] [Google Scholar]
- 10.Mehrotra R, Berman N, Alistwani A, Kopple JD. Improvement of nutritional status after initiation of maintenance hemodialysis. Am J Kidney Dis. 2002;40(1):133–142. doi: 10.1053/ajkd.2002.33922. [DOI] [PubMed] [Google Scholar]
- 11.Brener ZZ, Thijssen S, Kotanko P, Kuhlmann MK, Bergman M, Winchester JF, Levin NW. The impact of residual renal function on hospitalization and mortality in incident hemodialysis patients. Blood Purif. 2011;31(4):243–251. doi: 10.1159/000322252. [DOI] [PubMed] [Google Scholar]
- 12.Termorshuizen F, Dekker FW, van Manen JG, Korevaar JC, Boeschoten EW, Krediet RT. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004;15(4):1061–1070. doi: 10.1097/01.asn.0000117976.29592.93. [DOI] [PubMed] [Google Scholar]
- 13.Shemin D, Bostom AG, Laliberty P, Dworkin LD. Residual renal function and mortality risk in hemodialysis patients. Am J Kidney Dis. 2001;38(1):85–90. doi: 10.1053/ajkd.2001.25198. [DOI] [PubMed] [Google Scholar]
- 14.Maiorca R, Brunori G, Zubani R, Cancarini GC, Manili L, Camerini C, et al. Predictive value of dialysis adequacy and nutritional indices for mortality and morbidity in CAPD and HD patients. A longitudinal study. Nephrol Dial Transplant. 1995;10(12):2295–2305. doi: 10.1093/ndt/10.12.2295. [DOI] [PubMed] [Google Scholar]
- 15.Obi Y, Streja E, Rhee CM, Ravel V, Amin A, Cupisti A, et al. Incremental hemodialysis, residual kidney function, and mortality risk in incident dialysis patients: A cohort study. Am J Kidney Dis. 2016 doi: 10.1053/j.ajkd.2016.01.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuttykrishnan S, Kalantar-Zadeh K, Arah OA, Cheung AK, Brunelli S, Heagerty PJ, et al. Predictors of treatment with dialysis modalities in observational studies for comparative effectiveness research. Nephrol Dial Transplant. 2015;30(7):1208–1217. doi: 10.1093/ndt/gfv097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daugirdas JT. Physiologic Principles and Urea Kinetic Modeling. In: Daugirdas JT, Blake PG, Ing TS, editors. Handbook of dialysis. 5. Philadelphia: Lippincott Williams & Wilkins; 2014. pp. 59–60. [Google Scholar]
- 18.Clinical practice guidelines for hemodialysis adequacy, update 2006. American Journal of Kidney Diseases. 2006;48(Supple 1):S2–90. doi: 10.1053/j.ajkd.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 19.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 20.Locatelli F, Mastrangelo F, Redaelli B, Ronco C, Marcelli D, La Greca G, Orlandini G. Effects of different membranes and dialysis technologies on patient treatment tolerance and nutritional parameters. The Italian Cooperative Dialysis Study Group. Kidney Int. 1996;50(4):1293–1302. doi: 10.1038/ki.1996.441. [DOI] [PubMed] [Google Scholar]
- 21.Suda T, Hiroshige K, Ohta T, Watanabe Y, Iwamoto M, Kanegae K, et al. The contribution of residual renal function to overall nutritional status in chronic haemodialysis patients. Nephrology Dialysis Transplantation. 2000;15(3):396–401. doi: 10.1093/ndt/15.3.396. [DOI] [PubMed] [Google Scholar]
- 22.Milutinovic J, Cutler RE, Hoover P, Meijsen B, Scribner BH. Measurement of residual glomerular filtration rate in the patient receiving repetitive hemodialysis. Kidney Int. 1975;8(3):185–190. doi: 10.1038/ki.1975.98. [DOI] [PubMed] [Google Scholar]
- 23.Thijssen S, Wong MM, Usvyat LA, Xiao Q, Kotanko P, Maddux FW. Nutritional Competence and Resilience among Hemodialysis Patients in the Setting of Dialysis Initiation and Hospitalization. Clin J Am Soc Nephrol. 2015;10(9):1593–1601. doi: 10.2215/CJN.08430814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.May RC, Masud T, Logue B, Bailey J, England B. Chronic metabolic acidosis accelerates whole body proteolysis and oxidation in awake rats. Kidney Int. 1992;41(6):1535–1542. doi: 10.1038/ki.1992.223. [DOI] [PubMed] [Google Scholar]
- 25.Ballmer PE, McNurlan MA, Hulter HN, Anderson SE, Garlick PJ, Krapf R. Chronic metabolic acidosis decreases albumin synthesis and induces negative nitrogen balance in humans. J Clin Invest. 1995;95(1):39–45. doi: 10.1172/JCI117668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hara Y, May RC, Kelly RA, Mitch WE. Acidosis, not azotemia, stimulates branched-chain, amino acid catabolism in uremic rats. Kidney Int. 1987;32(6):808–814. doi: 10.1038/ki.1987.280. [DOI] [PubMed] [Google Scholar]
- 27.Gil KM, Skeie B, Kvetan V, Friedman MI, Askanazi J. Parenteral nutrition and oral intake: effect of branched-chain amino acids. Nutrition. 1990;6(4):291–295. [PubMed] [Google Scholar]
- 28.Bossola M, Tazza L, Giungi S, Luciani G. Anorexia in hemodialysis patients: an update. Kidney Int. 2006;70(3):417–422. doi: 10.1038/sj.ki.5001572. [DOI] [PubMed] [Google Scholar]
- 29.Carrero JJ. Identification of patients with eating disorders: clinical and biochemical signs of appetite loss in dialysis patients. J Ren Nutr. 2009;19(1):10–15. doi: 10.1053/j.jrn.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Bergstrom J. Anorexia in dialysis patients. Semin Nephrol. 1996;16(3):222–229. [PubMed] [Google Scholar]
- 31.Bergstrom J, Furst P, Alvestrand A, Lindholm B. Protein and energy intake, nitrogen balance and nitrogen losses in patients treated with continuous ambulatory peritoneal dialysis. Kidney Int. 1993;44(5):1048–1057. doi: 10.1038/ki.1993.347. [DOI] [PubMed] [Google Scholar]
- 32.KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. Am J Kidney Dis. 2015;66(5):884–930. doi: 10.1053/j.ajkd.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 33.Morris SM., Jr Regulation of enzymes of urea and arginine synthesis. Annu Rev Nutr. 1992;12:81–101. doi: 10.1146/annurev.nu.12.070192.000501. [DOI] [PubMed] [Google Scholar]
- 34.Obrador GT, Arora P, Kausz AT, Ruthazer R, Pereira BJ, Levey AS. Level of renal function at the initiation of dialysis in the U.S. end-stage renal disease population. Kidney Int. 1999;56(6):2227–2235. doi: 10.1038/sj.ki.4491163. [DOI] [PubMed] [Google Scholar]
- 35.van den Beukel TO, de Goeij MC, Dekker FW, Siegert CE, Halbesma N. Differences in progression to ESRD between black and white patients receiving predialysis care in a universal health care system. Clin J Am Soc Nephrol. 2013;8(9):1540–1547. doi: 10.2215/CJN.10761012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noori N, Kovesdy CP, Dukkipati R, Feroze U, Molnar MZ, Bross R, et al. Racial and ethnic differences in mortality of hemodialysis patients: role of dietary and nutritional status and inflammation. Am J Nephrol. 2011;33(2):157–167. doi: 10.1159/000323972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jakuszewski P, Czerwienska B, Chudek J, Wiecek A. Which components of malnutrition-inflammation-atherosclerosis syndrome are more common in haemodialysis patients with diabetic nephropathy? Nephrology (Carlton) 2009;14(7):643–649. doi: 10.1111/j.1440-1797.2009.01096.x. [DOI] [PubMed] [Google Scholar]
- 38.Kitai Y, Doi Y, Osaki K, Sugioka S, Koshikawa M, Sugawara A. Nephrotic range proteinuria as a strong risk factor for rapid renal function decline during pre-dialysis phase in type 2 diabetic patients with severely impaired renal function. Clin Exp Nephrol. 2015;19(6):1037–1043. doi: 10.1007/s10157-015-1094-2. [DOI] [PubMed] [Google Scholar]
- 39.Sridhar NR, Josyula S. Hypoalbuminemia in hemodialyzed end stage renal disease patients: risk factors and relationships--a 2 year single center study. BMC Nephrol. 2013;14:242. doi: 10.1186/1471-2369-14-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abe M, Okada K, Maruyama T, Maruyama N, Matsumoto K, Soma M. Relationship between erythropoietin responsiveness, insulin resistance, and malnutrition-inflammation-atherosclerosis (MIA) syndrome in hemodialysis patients with diabetes. Int J Artif Organs. 2011;34(1):16–25. doi: 10.5301/ijao.2011.6314. [DOI] [PubMed] [Google Scholar]
- 41.Lacson E, Jr, Wang W, Zebrowski B, Wingard R, Hakim RM. Outcomes associated with intradialytic oral nutritional supplements in patients undergoing maintenance hemodialysis: a quality improvement report. Am J Kidney Dis. 2012;60(4):591–600. doi: 10.1053/j.ajkd.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 42.Weiner DE, Tighiouart H, Ladik V, Meyer KB, Zager PG, Johnson DS. Oral intradialytic nutritional supplement use and mortality in hemodialysis patients. Am J Kidney Dis. 2014;63(2):276–285. doi: 10.1053/j.ajkd.2013.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix-Figure S1. Algorithm (flow chart) of patient selection for the cohort
Appendix-Figure S2. Mean serum albumin levels per patient quarter (A) in 38,504 included patients and 71,576 excluded patients and (B) in 110,080 patients as total cohort and 44,064 who survived more than 2 years
Appendix-Figure S3. Case-mix adjusted mean serum albumin level per patient quarter in 15,422 patients who survived more than 2 years stratified by baseline urine volume groups.
Appendix-Figure S4. Case-mix adjusted mean serum albumin level per patient quarter over 5 years in 35,961 patients stratified by baseline KRU groups.
Abbreviations: KRU, renal urea clearance.
The results of the number of patients in either group <50 were not shown.