Abstract
A growing body of the literature supports the use of magnetic resonance imaging as a potential biomarker for disease severity in the hereditary myopathies. We performed a systematic review of the medical literature to evaluate patterns of fat infiltration observed in magnetic resonance imaging studies of muscular dystrophy and congenital myopathy. Searches were performed using MEDLINE, EMBASE, and grey literature databases. Studies that described fat infiltration of muscles in patients with muscular dystrophy or congenital myopathy were selected for full-length review. Data on preferentially involved or spared muscles were extracted for analysis. A total of 2172 titles and abstracts were screened, and 70 publications met our criteria for inclusion in the systematic review. There were 23 distinct genetic disorders represented in this analysis. In most studies, preferential involvement and sparing of specific muscles were reported. We conclude that magnetic resonance imaging studies can be used to identify distinct patterns of muscle involvement in the hereditary myopathies. However, larger studies and standardized methods of reporting are needed to develop imaging as a diagnostic tool in these diseases.
Keywords: Magnetic resonance imaging, Muscular dystrophy, Congenital myopathy, Distal myopathy
Introduction
Since the introduction of clinical magnetic resonance imaging (MRI), investigators in the radiographic sciences have noted the ability of MRI to produce high-resolution anatomic images of skeletal muscle. Unlike prior imaging modalities, MRI provides excellent contrast between various soft tissue structures, allowing for the examination of individual muscles in sharp contrast to adjacent fat [1]. In recent years, MRI of muscle has gained wider clinical use in the inflammatory myopathies, where the introduction of new immunosuppressive agents has created a need for tools that can accurately diagnose and monitor response to treatment. The ability of MRI to distinguish between acute inflammation and chronic fatty replacement in muscle also provides important prognostic information [2].
In the muscular dystrophies and other hereditary myopathies, the clinical use of MRI as a diagnostic modality has not entered into the standard of care for multiple reasons. First, genetic testing has become increasingly available and affordable, and it offers highly specific diagnostic information that cannot be achieved with muscle imaging. The relative absence of large studies in this diverse patient population also makes the interpretation of MRI scans difficult. There are also relatively few medical treatments for hereditary muscle disorders compared to the inflammatory myopathies and, subsequently, fewer ways in which MRI can influence medical decision-making.
Despite these known limitations, there is growing interest in using imaging (and MRI in particular) in research studies of hereditary muscle disease [3, 4]. Early trials in this disease population have highlighted the limitations of existing outcome measures for muscular dystrophy [5]. An objective, non-invasive measure that can be repeated many times could significantly improve the quality of trials in these diseases.
The purpose of this review is to examine the methods with which researchers have used MRI to study genetic myopathies and identify patterns of muscle involvement reported in the scientific literature. A systematic examination of the literature also provides an opportunity to identify obstacles that need to be addressed in the field of muscle imaging.
Methods
Literature searches
A search of the literature was performed in accordance with methods described by the Cochrane Handbook for Systematic Review of Interventions [6]. Controlled vocabulary and keyword searches were performed using the MEDLINE and EMBASE databases. Search terms were selected based on two concepts: genetic muscle disease (“muscular dystrophy” or “congenital myopathy”) and magnetic resonance imaging (“magnetic resonance imaging” or “MRI” or “magnetic resonance spectroscopy” or “MRS”). We did not restrict our searches with regard to publication date, allowing the search to include all articles published from the time of database inception to the date of the literature search (10/05/2016). We further evaluated reference lists from included articles and completed forward citation searching using the Web of Science database to identify additional citations. Abbreviated search strategies were used to search for relevant information in the Cochrane Library, OpenSIGLE (System for Information on Grey Literature in Europe), and the New York Academy of Medicine Grey Literature Report and Database.
Study selection
All titles and abstracts were reviewed, and studies were excluded based on the following criteria: (1) the study was not performed in humans; (2) the study did not analyze a majority of the skeletal muscles in at least one segment of one limb (such as the lower leg or thigh); (3) the study did not describe a genetically distinct muscular dystrophy, congenital myopathy, or distal myopathy; (4) the study did not isolate results of MRI studies from those of other imaging modalities (such as computed tomography); (5) the study described only a single kindred; (6) the study described fewer than four subjects; (7) the methods did not report or cite a system for scoring individual muscles based on fat infiltration; (8) the manuscript was a review or editorial publication that did not report primary research data; (9) study was not published in English; and (10) full-text versions of the article were not obtainable. The full-text versions of studies that met these criteria based on titles and abstracts were downloaded and reviewed. Studies that did not meet these inclusion criteria after full-text review were subsequently excluded.
Data extraction and synthesis
The studies that were selected for analysis based on full-text review underwent data extraction. The following information about each study was collected: first author, year of publication, study type, phenotype of study sample, genotype of study sample, number of MRI scans analyzed, regions imaged, and scoring system used for assessment of fat infiltration. In studies where multiple genetic diseases were described, each genetically distinct population was reported as a separate entry in the table. Data on muscles that were preferentially affected and spared in each study population were also collected. Muscles were considered preferentially affected if they were defined by the authors as the earliest, most severely, or most frequently infiltrated by fat across the study sample. Muscles were counted as preferentially spared if they were defined by the authors as relatively spared when compared to other muscles within an individual subject or were less frequently or severely involved across the study sample. The extracted data were stored in tabular form, with “?” signifying preferential involvement, “-” signifying sparing, “??” signifying the most preferentially involved muscle, and “-” signifying the most preferentially spared muscle. In instances where an entire muscle group was listed instead of an individual muscle, all muscles within that group were coded equally. However, if regions comprised of more than one muscle group were listed (for instance, “all pelvic muscles”) and the muscles within those regions were not explicitly defined, these groups were not coded. For studies where a list of muscles ranked in order of fat infiltration was provided without further description of preferential involvement, only the highest and lowest ranked muscles were coded.
The degree of heterogeneity between studies was assessed through subgroup analyses in which studies were stratified by disease phenotype, genotype, study type, scoring technique, sample size, and regions imaged.
Results
Study selection
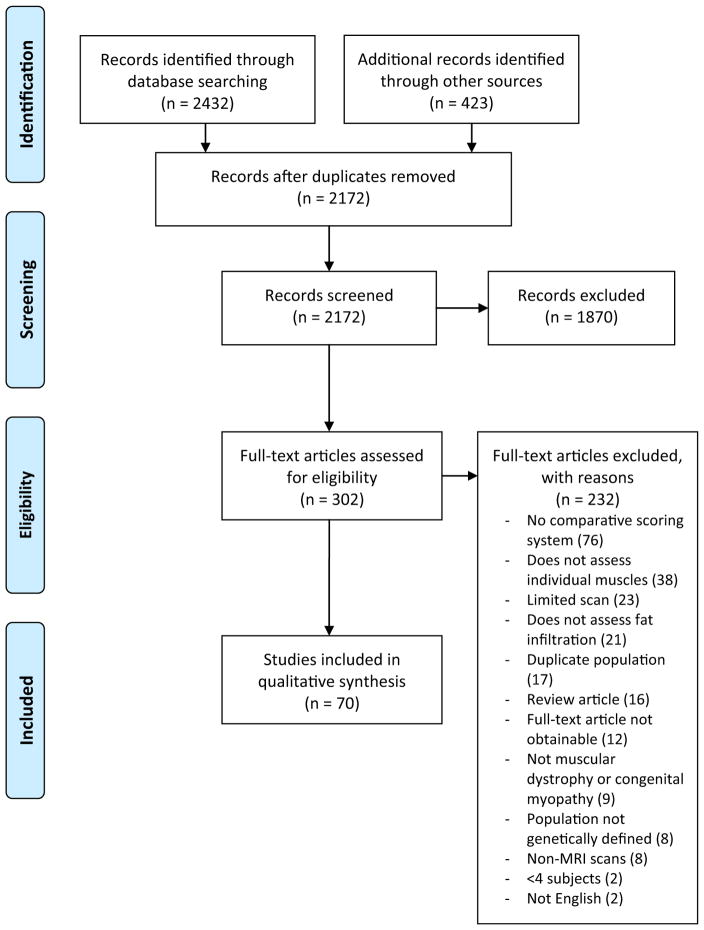
The search strategy identified 2172 unique citations (Fig. 1) [7]. Screening of titles and abstracts resulted in the exclusion of 1870 citations. A full-text review of the remaining 302 articles yielded 70 that were included in the final analysis. These studies were published between 1993 and 2016. Because some of these texts reported more than one genetic disease, there were 87 discrete disease populations (“cohorts”) included in the analysis. These cohorts included a total of 1918 MRI scans [8–77].
Fig. 1.
PRISMA (preferred reporting items for systematic reviews and meta-analyses)
Characteristics of included studies
The included studies reported data from 23 different genetic myopathies (Table 1). The number of studies for each disease varied widely (range 1–23), with the dystrophinopathies and facioscapulohumeral muscular dystrophy (FSHD) accounting for the largest numbers of studies (23 and 14 studies, respectively). The dystrophinopathies and FSHD each account for approximately one-third of the scans described, with the remaining 21 genetic diseases comprising the remaining third. The number of MRI scans analyzed in each study also varied widely (range 4–269). However, most cohorts were small (median 13, mean 22 scans), and only three included more than 100 scans.
Table 1.
Numbers of studies and scans for each genetic disorder included in systematic review
| Gene | Number of studies | Total number of scans | Percentage of studies | Percentage of scans |
|---|---|---|---|---|
| ACTA1 | 1 | 4 | 1.1 | 0.2 |
| COL6 | 5 | 58 | 5.7 | 3.0 |
| DES | 1 | 4 | 1.1 | 0.2 |
| DMPK | 7 | 99 | 8.0 | 5.2 |
| CNBP | 3 | 23 | 3.4 | 1.2 |
| DMD | 23 | 689 | 26.4 | 35.9 |
| Duchenne | 16 | 554 | 18.4 | 28.9 |
| Becker | 6 | 123 | 6.9 | 6.4 |
| Carrier | 1 | 12 | 1.1 | 0.6 |
| FSHD | 14 | 617 | 16.1 | 32.2 |
| CAPN | 3 | 22 | 3.4 | 1.1 |
| DYSF | 6 | 143 | 6.9 | 7.5 |
| FKRP | 3 | 51 | 3.4 | 2.7 |
| ANO5 | 2 | 30 | 2.3 | 1.6 |
| LMNA | 4 | 38 | 4.6 | 2.0 |
| MATR3 | 1 | 16 | 1.1 | 0.8 |
| MYOT | 2 | 13 | 2.3 | 0.7 |
| NEB | 1 | 6 | 1.1 | 0.3 |
| PABN | 2 | 18 | 2.3 | 0.9 |
| RYR1 | 2 | 15 | 2.3 | 0.8 |
| SEPN1 | 2 | 13 | 2.3 | 0.7 |
| TTN | 1 | 22 | 1.1 | 1.1 |
| TPM2 | 2 | 12 | 2.3 | 0.6 |
| TIA1 | 1 | 11 | 1.1 | 0.6 |
| MYH7 | 1 | 14 | 1.1 | 0.7 |
| Total | 87 | 1918 | 100.0 | 100.0 |
Most of the studies selected for review used a cross-sectional study design, meaning that enrolled subjects were scanned once. Only five studies included longitudinal follow-up imaging. Thirteen of the 70 articles reported the inclusion of healthy controls. Fourteen of the 70 articles reported the inclusion of diseased controls or multiple types of hereditary myopathy.
The majority of studies used a semi-quantitative scoring system with 4, 5, or 6 grades to rank individual muscles (Table 2). Higher numerical scores were used to signify more extensive fat infiltration. The specific muscle characteristics and cutoffs for each grade varied between studies; 25 cohorts were scored using a cutoff point of 50% fat infiltration (described by Jungbluth et al.) to denote changes in severity grade, and 34 cohorts were scored using cutoffs of 30 and 60% fat infiltration (described by Mercuri et al.) [64, 78]. Eight cohorts did not use specific cutoff percentages (the Lamminen scale) [79]. Only 20 of the 87 disease cohorts (13 dystrophinopathy, 4 FSHD, 2 myotonic dystrophy, and 1 MYH7-myopathy) were analyzed using alternative scoring systems. It is notable that of these 20, 15 were reported in articles published within the past five years and utilized fully quantitative methods of determining the amount of fat infiltration in muscle.
Table 2.
Majority of studies selected for review used a variant of one of the following three scoring systems
| Score | Lamminen [79] | Mercuri et al. [59, 78] | Jungbluth et al. [62, 64] |
|---|---|---|---|
| 0 | Normal | Normal | |
| 1 | Normal muscle signal intensity | Early moth-eaten appearance, with scattered small areas of increased density on T1 MRI | Mild with only traces of increased signal intensity |
| 2 | Slightly hyperintense, patchy intramuscular signal changes | A: Late moth-eaten appearance, with numerous discrete areas of increased density with beginning confluence, comprising less than 30% of the volume of the individual muscle B: Late moth-eaten appearance, with numerous discrete areas of increased density with beginning confluence, comprising 30–60% of the volume of the individual muscle |
Moderate with increased signal in less than 50% of affected muscle |
| 3 | Markedly hyperintense, patchy but widespread intramuscular changes | Washed-out appearance, fuzzy appearance due to confluent areas of increased density with muscle still present at the periphery | Severe with increased signal intensity in more than 50% of affected muscle |
| 4 | Total, homogeneous hyperintense signal change in whole muscle, equaling the signal intensity of adjacent subcutaneous or paramuscular fat | End-stage appearance, muscle replaced by increased density connective tissue and fat with only rim of fascia and neurovascular structures distinguishable | Entire muscle replaced by abnormal signal |
The majority of studies imaged the lower extremities, with 51 of the 87 cohorts exclusively imaging the lower extremities (lower leg, thigh, or pelvis). Thirty-two studies imaged the lower extremities and at least one other region of the body (trunk, shoulder, arm, or head/neck), and only four of the 87 cohorts reported imaging of the upper extremities only. Seventeen of the cohorts (described in 11 separate manuscripts) reported using whole-body MRI, or scanning of contiguous anatomic regions that included the arms, trunk, and legs. In 73 of the 87 cohorts (including all of the studies that used a semi-quantitative scoring system), analysis of fat infiltration was performed on T1-weighted images. Nine studies used Dixon sequences to calculate muscle fat fractions, and two used T2-based sequences to calculate fat fractions or ratios. Three studies used more than one type of sequence to analyze fat infiltration (T1-weighted imaging plus either proton density or Dixon imaging).
The dystrophinopathies
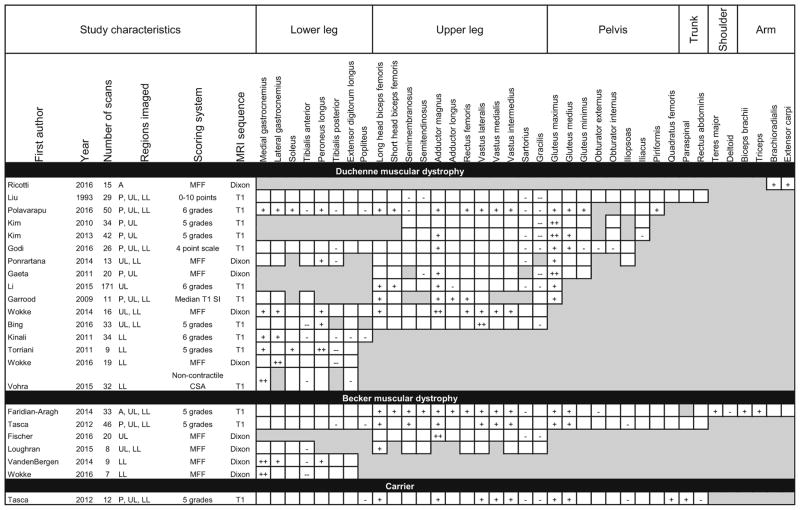
For the purposes of pattern analysis, dystrophinopathies were divided into three phenotypic subcategories (Duchenne, Becker, and carrier). While sharing a common genetic origin, the clinical characteristics and prognosis of these phenotypes vary widely. The Becker phenotype is milder than the Duchenne phenotype, while the majority of female carriers are asymptomatic. Despite these clinical disparities, the reported patterns of muscle involvement across all dystrophinopathies were similar. More than half of the studies that included imaging of the thigh reported preferential involvement of the gluteus maximus, gluteus medius, and adductor magnus with sparing of the gracilis and sartorius (Table 3). The dystrophinopathy studies included the widest variety of scoring techniques for fat infiltration, with only about half using a semi-quantitative scoring system. The remaining studies used fully quantitative techniques to characterize fat replacement. Nine of these studies quantified the muscle fat fraction, or the percentage of muscle replaced by fat.
Table 3.
Patterns of muscle involvement and sparing in MRI studies describing populations with mutations in dystrophin
Shaded boxes represent muscles that were not scanned or analyzed
MFF muscle fat fraction, CSA cross-sectional area, A arm, P pelvis, UL upper leg, LL lower leg
Facioscapulohumeral muscular dystrophy
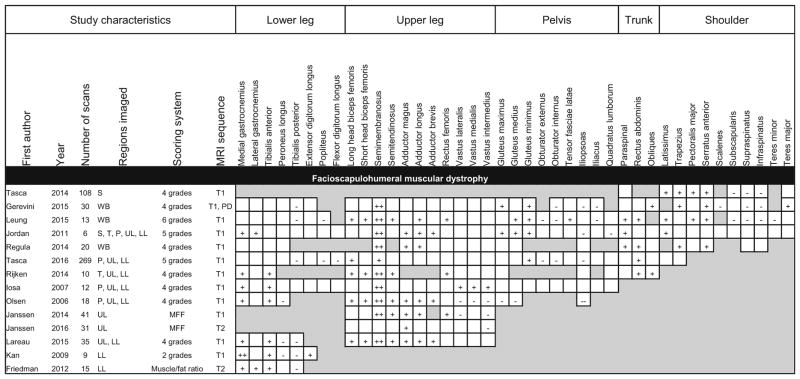
Fourteen studies reported MRI findings in individuals with FSHD (Table 4). This group of studies showed the greatest anatomic diversity, with almost half of the studies imaging regions other than the legs. MRI of the arms is technically more challenging than imaging of the legs, and the number of studies that included upper extremity imaging likely reflects the high prevalence of upper extremity involvement in FSHD. A consistent finding among the FSHD studies was the preferential involvement of the semimembranosus, which was reported to be the most severely involved muscle in 9 of the 11 studies that scanned the thigh. The medial gastrocnemius and tibialis anterior muscles were the most preferentially affected in the lower leg, while the tibialis posterior and peroneus were frequently spared. The hip flexors (iliopsoas and iliacus) were preferentially spared in multiple studies. However, in contrast to the dystrophinopathies and limb-girdle muscular dystrophies, the gracilis and sartorius were never reported to be preferentially spared. This may seem incongruous with prior observations of scans in which the gracilis and sartorius are spared relative to other muscles of the medial thigh (Supplemental Fig. 1). One possible explanation is that only a subset of patients with FSHD exhibits sparing of the gracilis and sartorius, and these muscles are not spared across the entire disease population.
Table 4.
Patterns of muscle involvement and sparing in MRI studies describing facioscapulohumeral muscular dystrophy
Shaded boxes represent muscles that were not scanned or analyzed
MFF muscle fat fraction, PD proton density, S shoulder, T trunk, P pelvis, UL upper leg, LL lower leg, WB whole-body
Observations across all studies
Most genes had too few studies or were too heterogeneous to discern specific patterns of involvement (Tables 5, 6, 7). However, evaluation of imaging patterns across all 87 cohorts yielded several notable observations. Several muscles in the thigh (long head biceps femoris, semimembranosus, and adductor magnus) and lower leg (medial gastrocnemius and soleus) were the most likely to be reported to be preferentially involved across all studies. The gracilis, sartorius, and tibialis posterior were most frequently reported as spared across all studies. The rectus femoris, adductor longus, peroneus longus, and tibialis anterior were found to have a mix of preferential involvement and sparing in different diseases. In some cases, the preferential involvement or sparing was disease-specific. For instance, in studies describing FSHD, the tibialis anterior was reported to be preferentially involved, while the peroneus longus was found to be preferentially spared. In the dystrophinopathies, the reverse was true, with multiple studies reporting sparing of the tibialis anterior and involvement of the peroneus longus.
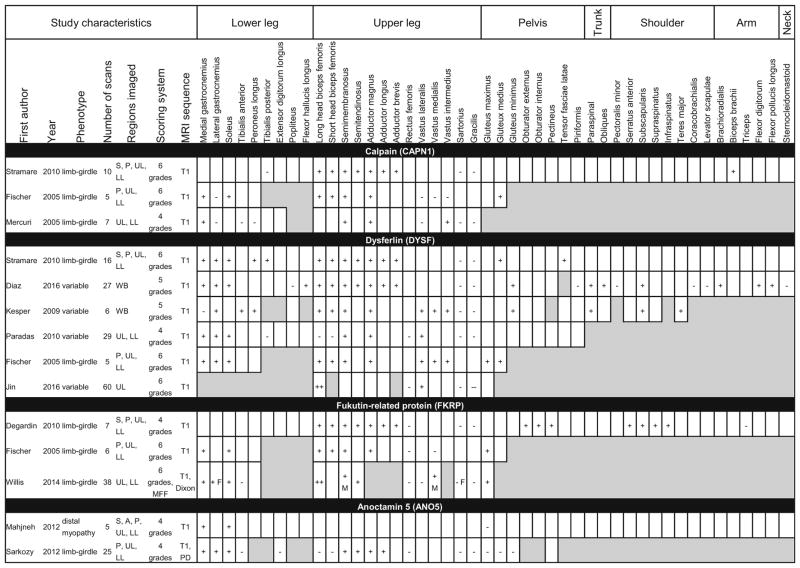
Table 5.
Patterns of muscle involvement and sparing in MRI studies describing limb-girdle muscular dystrophy
Shaded boxes represent muscles that were not scanned or analyzed. M and F denote findings seen only in males or females, respectively
MFF muscle fat fraction, A arm, S shoulder, P pelvis, UL upper leg, LL lower leg, WB whole-body
Table 6.
Patterns of muscle involvement and sparing in MRI studies describing congenital myopathy, congenital muscular dystrophy (CMD), Emery–Dreifuss muscular dystrophy (EDMD), distal myopathy, and oculopharyngeal muscular dystrophy (OPMD)
Shaded boxes represent muscles that were not scanned or analyzed. A * denotes fat replacement in the center of the muscle. A ± denotes fat replacement at the muscle periphery
T trunk, P pelvis, UL upper leg, LL lower leg, WB whole-body
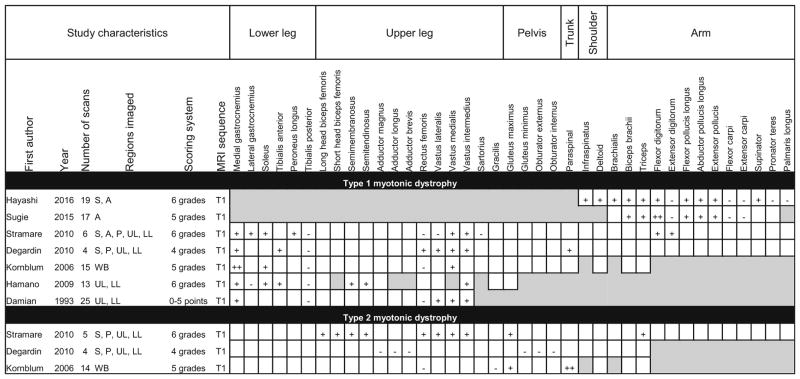
Table 7.
Patterns of muscle involvement and sparing in MRI studies describing myotonic dystrophy
Shaded boxes represent muscles that were not scanned or analyzed
A arm, S shoulder, P pelvis, UL upper leg, LL lower leg, WB whole-body
We also observed that the diseases with preferential involvement of the sartorius were either congenital myopathies or distal myopathies, while in the dystrophinopathies and limb-girdle muscular dystrophies, the sartorius was among the most frequently spared. Similarly, the adductor longus was much more frequently spared in the congenital myopathies and congenital muscular dystrophies compared to the dystrophinopathies or limb-girdle muscular dystrophies. In most other diseases and muscles, however, there were too few studies or scans to define a consistent pattern of involvement.
Some radiographic features that are specific to a particular disease were noted in this analysis. All the studies describing collagen VI disorders reported that the outer rim of the vastus lateralis was replaced by fat before the center of the muscle. The majority of these studies also reported a strip or notch of fat replacement in the center of the rectus femoris (Table 6). Multiple studies describing type 1 myotonic dystrophy reported a crescent-shaped region of involvement in the quadriceps resulting from preferential involvement of the vastus intermedius and vastus medialis compared to the vastus lateralis [50, 74, 80]. In the majority of studies, however, no similarly distinctive disease-defining features were reported.
Discussion
This review raises several important points about the role of MRI in the study of muscle disease. The identification of patterns of muscle involvement and sparing in different types of muscular dystrophy suggests that skeletal muscle imaging could be used in a diagnostic capacity. While MRI does not offer the specificity of gene testing and there is substantial overlap in the imaging findings in a number of myopathies, there are clinical scenarios in which MRI could be diagnostically useful. For instance, MRI may be helpful in distinguishing hereditary from acquired myopathies, which could impact medical management considerably. Muscle MRI could also have a role in determining the pathogenicity of variants of unknown significance identified through genetic testing.
The number of recent studies that used quantitative methods to evaluate fat infiltration may indicate a growing interest in using muscle MRI for research in hereditary muscle disease. Several characteristics of muscle MRI make it a promising a research tool for clinical trials and longitudinal studies. MRI scans are repeatable, non-invasive, and non-irradiating; furthermore, scanned images can be easily de-identified and stored for analysis by blinded reviewers. Compared to strength and function tests, MRI measurements may also be less vulnerable to confounding by the level of cooperation and effort on the part of the study subject. Although only one of the studies selected for this review was an interventional study [43], several clinical trials of novel drugs in muscular dystrophy have reported using MRI as a secondary outcome measure, and at least one trial has reported using MRI as a primary outcome measure (clinicaltrials.gov NCT02515669, NCT02310763, and NCT02927080) [81, 82].
The variability between studies of the same genetic disorder bears closer inspection. While the differences in reported patterns of muscle involvement are likely due in part to differences in scoring and reporting techniques, it is also possible that contradictory findings could reflect the presence of distinct subgroups within a disease population. The increasing use of genetic testing has shown that in some genetic diseases, phenotypic variability is wider than was previously suspected. A number of genes, such as collagen VI, are associated with more than one clinical phenotype, and genotyping studies in FSHD have shown that individuals who carry a disease-causing mutation can be asymptomatic or minimally affected [83, 84]. It is also worth noting that not all studies used genetic testing as a requirement for inclusion (the dystrophinopathies, for instance, may have been diagnosed through muscle biopsy), and there could be unrecognized genotypic variability within a study sample.
The results of this analysis also show that the field of MRI for muscular dystrophy is dominated by the dystrophinopathies and FSHD. This likely reflects not only the relative frequency of these disorders, but also the activity of subspecialty research centers, patient registries, and advocacy groups. The relative paucity of scans in other diseases compels caution in drawing conclusions about patterns of muscle involvement in these disorders, as small studies in selected populations may be subject to selection bias. Such bias could be further amplified by overlap in the study samples reported in multiple manuscripts from the same investigator group. In the extremely rare muscular dystrophies, the collection of sufficient numbers of scans to adequately characterize the disease population may only be achievable through collaborations between multiple centers.
There are some limitations to this qualitative review. First, we did not discriminate between different types of preferential involvement or sparing. Preferential involvement could mean the most frequently affected, the earliest affected, or the most severely affected muscles. Likewise, preferential sparing could mean that a muscle is universally spared across the cohort or relatively spared compared to other muscles in the same region. We relied on the authors to report preferentially involved or spared muscles in cases where the primary data were not provided in the manuscript text or figures, and the criteria for identifying these muscles was infrequently reported. For future observational studies and clinical trials, it will be essential to develop a uniform system of classifying and reporting imaging findings.
Our eligibility criteria for inclusion in the qualitative review were prospectively established with the purpose of minimizing risk of bias in the included studies. However, these criteria resulted in the exclusion of several important imaging studies that utilized computed tomography (CT) or a combination of CT and MRI [78, 85, 86]. While CT can identify fat infiltration in muscle, it has not been established that scoring results from MRI are exchangeable with those from CT. The eligibility criteria also excluded metabolic myopathies, mitochondrial myopathies, and non-dystrophic myotonias (the muscle ion channel disorders). While imaging studies have been performed in these disorders, fat replacement of muscle is not a universally reported feature in these diseases, and appropriate comparisons with regard to preferential muscle involvement could not be made [87–90].
Another potential limitation of this review is that we were unable to stratify imaging findings based on important confounders in muscular dystrophy, such as gender, age of onset (or duration of disease symptoms), age, distribution of weakness, or mutation type. At least one of the included studies reported that there were differences in the patterns of muscle involvement between males and females [51]. However, the reporting of these potential confounders was too inconsistent between manuscripts to allow substantive analysis of their impact. Our analysis also only included data on fat infiltration and did not include other features of muscle disease, such as edema-like changes, muscle hypertrophy, or atrophy. These features represent additional aspects of disease pathology that merit further characterization.
The results of this review underline several factors that should be considered in studies using MRI in muscle disease. First is the need for greater standardization across all stages of imaging, from the selection of participants and imaging sequences to the scoring and reporting of collected images. Standardized methodologies will facilitate the extraction and synthesis of findings across multiple investigator groups [91]. Second, the radiographic phenotype can differ considerably from clinical observations. For instance, several studies reported that the medial gastrocnemius muscle is as frequently or more frequently affected than the tibialis anterior in FSHD. However, foot drop is more frequently observed than calf weakness in this disease population. This may be due to the fact that there are multiple muscles involved in ankle plantarflexion, all of which are larger than the tibialis anterior. Extensive replacement of a single muscle may not be clinically apparent if other members of the same muscle group remain intact. It is also important to consider that many of the reviewed studies are fairly small case series in which a well-defined clinical population was selected for imaging. In these cases, we may expect imaging findings to be fairly homogeneous. As imaging studies expand to include more atypical cases from a greater number of centers, we would expect the radiographic phenotypes of these disorders to be more heterogeneous as well.
Supplementary Material
Acknowledgments
Funding DGL receives support from a Grant from the National Institutes of Health (5 K23 NS091379-02).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00415-016-8350-6) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflicts of interest The authors declare that they have no conflict of interest.
Ethical standards The manuscript does not contain clinical studies or patient data.
References
- 1.Murphy WA, Totty WG, Carroll JE. MRI of normal and pathologic skeletal muscle. AJR Am J Roentgenol. 1986;146(3):565–574. doi: 10.2214/ajr.146.3.565. [DOI] [PubMed] [Google Scholar]
- 2.Del Grande F, Carrino JA, Del Grande M, Mammen AL, Christopher Stine L. Magnetic resonance imaging of inflammatory myopathies. Top Magn Reson Imaging TMRI. 2011;22(2):39–43. doi: 10.1097/RMR.0b013e31825b2c35. [DOI] [PubMed] [Google Scholar]
- 3.Mercuri E, Pichiecchio A, Allsop J, Messina S, Pane M, Muntoni F. Muscle MRI in inherited neuromuscular disorders: past, present, and future. J Magn Reson Imaging JMRI. 2007;25(2):433–440. doi: 10.1002/jmri.20804. [DOI] [PubMed] [Google Scholar]
- 4.Wattjes MP, Kley RA, Fischer D. Neuromuscular imaging in inherited muscle diseases. Eur Radiol. 2010;20(10):2447–2460. doi: 10.1007/s00330-010-1799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tawil R, Padberg GW, Shaw DW, van der Maarel SM, Tapscott SJ, Participants FW. Clinical trial preparedness in facioscapulo-humeral muscular dystrophy: clinical, tissue, and imaging outcome measures 29–30 May 2015, Rochester, New York. Neuromuscul Disord NMD. 2016;26(2):181–186. doi: 10.1016/j.nmd.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Higgins JPT, Green S. Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. Wiley-Blackwell; Chichester: 2008. Cochrane book series. [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Faridian-Aragh N, Wagner KR, Leung DG, Carrino JA. Magnetic resonance imaging phenotyping of Becker muscular dystrophy. Muscle Nerve. 2014;50(6):962–967. doi: 10.1002/mus.24246. [DOI] [PubMed] [Google Scholar]
- 9.Tasca G, Iannaccone E, Monforte M, Masciullo M, Bianco F, Laschena F, Ottaviani P, Pelliccioni M, Pane M, Mercuri E, Ricci E. Muscle MRI in Becker muscular dystrophy. Neuromuscul Disord NMD. 2012;22(Suppl 2):S100–S106. doi: 10.1016/j.nmd.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Loughran T, Higgins DM, McCallum M, Coombs A, Straub V, Hollingsworth KG. Improving highly accelerated fat fraction measurements for clinical trials in muscular dystrophy: origin and quantitative effect of R2* changes. Radiology. 2015;275(2):570–578. doi: 10.1148/radiol.14141191. [DOI] [PubMed] [Google Scholar]
- 11.van den Bergen JC, Wokke BH, Janson AA, van Duinen SG, Hulsker MA, Ginjaar HB, van Deutekom JC, Aartsma-Rus A, Kan HE, Verschuuren JJ. Dystrophin levels and clinical severity in Becker muscular dystrophy patients. J Neurol Neurosurg Psychiatry. 2014;85(7):747–753. doi: 10.1136/jnnp-2013-306350. [DOI] [PubMed] [Google Scholar]
- 12.Tasca G, Monforte M, Iannaccone E, Laschena F, Ottaviani P, Silvestri G, Masciullo M, Mirabella M, Servidei S, Ricci E. Muscle MRI in female carriers of dystrophinopathy. Eur J Neurol. 2012;19(9):1256–1260. doi: 10.1111/j.1468-1331.2012.03753.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim HK, Laor T, Horn PS, Racadio JM, Wong B, Dardzinski BJ. T2 mapping in Duchenne muscular dystrophy: distribution of disease activity and correlation with clinical assessments. Radiology. 2010;255(3):899–908. doi: 10.1148/radiol.10091547. [DOI] [PubMed] [Google Scholar]
- 14.Gaeta M, Messina S, Mileto A, Vita GL, Ascenti G, Vinci S, Bottari A, Vita G, Settineri N, Bruschetta D, Racchiusa S, Minutoli F. Muscle fat-fraction and mapping in Duchenne muscular dystrophy: evaluation of disease distribution and correlation with clinical assessments preliminary experience. Skelet Radiol. 2012;41(8):955–961. doi: 10.1007/s00256-011-1301-5. [DOI] [PubMed] [Google Scholar]
- 15.Kim HK, Merrow AC, Shiraj S, Wong BL, Horn PS, Laor T. Analysis of fatty infiltration and inflammation of the pelvic and thigh muscles in boys with Duchenne muscular dystrophy (DMD): grading of disease involvement on MR imaging and correlation with clinical assessments. Pediatr Radiol. 2013;43(10):1327–1335. doi: 10.1007/s00247-013-2696-z. [DOI] [PubMed] [Google Scholar]
- 16.Liu GC, Jong YJ, Chiang CH, Jaw TS. Duchenne muscular dystrophy: MR grading system with functional correlation. Radiology. 1993;186(2):475–480. doi: 10.1148/radiology.186.2.8421754. [DOI] [PubMed] [Google Scholar]
- 17.Garrood P, Hollingsworth KG, Eagle M, Aribisala BS, Birchall D, Bushby K, Straub V. MR imaging in Duchenne muscular dystrophy: quantification of T1-weighted signal, contrast uptake, and the effects of exercise. J Magn Reson Imaging JMRI. 2009;30(5):1130–1138. doi: 10.1002/jmri.21941. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Zheng Y, Zhang W, Wang Z, Xiao J, Yuan Y. Progression and variation of fatty infiltration of the thigh muscles in Duchenne muscular dystrophy, a muscle magnetic resonance imaging study. Neuromuscul Disord NMD. 2015;25(5):375–380. doi: 10.1016/j.nmd.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Ponrartana S, Ramos-Platt L, Wren TAL, Hu HH, Perkins TG, Chia JM, Gilsanz V. Effectiveness of diffusion tensor imaging in assessing disease severity in Duchenne muscular dystrophy: preliminary study. Pediatr Radiol. 2014 doi: 10.1007/s00247-014-3187-6. ((Ponrartana S., sponrartana@chla.usc.edu; Wren T.A.L.; Hu H.H.; Gilsanz V.) Department of Radiology, Children’s Hospital Los Angeles, Los Angeles, United States) [DOI] [PubMed] [Google Scholar]
- 20.Wokke BH, van den Bergen JC, Versluis MJ, Niks EH, Milles J, Webb AG, van Zwet EW, Aartsma-Rus A, Verschuuren JJ, Kan HE. Quantitative MRI and strength measurements in the assessment of muscle quality in Duchenne muscular dystrophy. Neuromuscul Disord NMD. 2014;24(5):409–416. doi: 10.1016/j.nmd.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Kinali M, Arechavala-Gomeza V, Cirak S, Glover A, Guglieri M, Feng L, Hollingsworth KG, Hunt D, Jungbluth H, Roper HP, Quinlivan RM, Gosalakkal JA, Jayawant S, Nadeau A, Hughes-Carre L, Manzur AY, Mercuri E, Morgan JE, Straub V, Bushby K, Sewry C, Rutherford M, Muntoni F. Muscle histology vs MRI in Duchenne muscular dystrophy. Neurology. 2011;76(4):346–353. doi: 10.1212/WNL.0b013e318208811f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torriani M, Townsend E, Thomas BJ, Bredella MA, Ghomi RH, Tseng BS. Lower leg muscle involvement in Duchenne muscular dystrophy: an MR imaging and spectroscopy study. Skelet Radiol. 2012;41(4):437–445. doi: 10.1007/s00256-011-1240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vohra RS, Lott D, Mathur S, Senesac C, Deol J, Germain S, Bendixen R, Forbes SC, Sweeney HL, Walter GA, Vandenborne K. Magnetic resonance assessment of hypertrophic and pseudo-hypertrophic changes in lower leg muscles of boys with Duchenne muscular dystrophy and their relationship to functional measurements. PLoS One. 2015;10(6):e0128915. doi: 10.1371/journal.pone.0128915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricotti V, Evans MR, Sinclair CD, Butler JW, Ridout DA, Hogrel JY, Emira A, Morrow JM, Reilly MM, Hanna MG, Janiczek RL, Matthews PM, Yousry TA, Muntoni F, Thornton JS. Upper limb evaluation in Duchenne muscular dystrophy: fat-water quantification by mri, muscle force and function define endpoints for clinical trials. PLoS One. 2016;11(9):e0162542. doi: 10.1371/journal.pone.0162542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polavarapu K, Manjunath M, Preethish-Kumar V, Sekar D, Vengalil S, Thomas P, Sathyaprabha TN, Bharath RD, Nalini A. Muscle MRI in Duchenne muscular dystrophy: evidence of a distinctive pattern. Neuromuscul Disord NMD. 2016 doi: 10.1016/j.nmd.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Bing Q, Hu K, Tian Q, Zhao Z, Shen H, Li N, Hu J. Semi-quantitative assessment of lower limb MRI in dystrophinopathy. Int J Clin Exp Med. 2016;9(7):13723–13732. [Google Scholar]
- 27.Godi C, Ambrosi A, Nicastro F, Previtali SC, Santarosa C, Napolitano S, Iadanza A, Scarlato M, Natali Sora MG, Tettamanti A, Gerevini S, Cicalese MP, Sitzia C, Venturini M, Falini A, Gatti R, Ciceri F, Cossu G, Torrente Y, Politi LS. Longitudinal MRI quantification of muscle degeneration in Duchenne muscular dystrophy. Ann Clin Transl Neurol. 2016;3(8):607–622. doi: 10.1002/acn3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wokke BH, Van Den Bergen JC, Hooijmans MT, Verschuuren JJ, Niks EH, Kan HE. T2 relaxation times are increased in Skeletal muscle of DMD but not BMD patients. Muscle Nerve. 2016;53(1):38–43. doi: 10.1002/mus.24679. [DOI] [PubMed] [Google Scholar]
- 29.Fischer D, Hafner P, Rubino D, Schmid M, Neuhaus C, Jung H, Bieri O, Haas T, Gloor M, Fischmann A, Bonati U. The 6-minute walk test, motor function measure and quantitative thigh muscle MRI in Becker muscular dystrophy: a cross-sectional study. Neuromuscul Disord. 2016;26(7):414–422. doi: 10.1016/j.nmd.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Tasca G, Monforte M, Iannaccone E, Laschena F, Ottaviani P, Leoncini E, Boccia S, Galluzzi G, Pelliccioni M, Masciullo M, Frusciante R, Mercuri E, Ricci E. Upper girdle imaging in facioscapulohumeral muscular dystrophy. PLoS One. 2014;9(6):e100292. doi: 10.1371/journal.pone.0100292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jordan B, Eger K, Koesling S, Zierz S. Camptocormia phenotype of FSHD: a clinical and MRI study on six patients. J Neurol. 2011;258(5):866–873. doi: 10.1007/s00415-010-5858-z. [DOI] [PubMed] [Google Scholar]
- 32.Regula JU, Jestaedt L, Jende F, Bartsch A, Meinck HM, Weber MA. Clinical muscle testing compared with whole-body magnetic resonance imaging in facioscapulo-humeral muscular dystrophy. Clin Neuroradiol. 2015 doi: 10.1007/s00062-015-0386-y. [DOI] [PubMed] [Google Scholar]
- 33.Gerevini S, Scarlato M, Maggi L, Cava M, Caliendo G, Pasanisi B, Falini A, Previtali SC, Morandi L. Muscle MRI findings in facioscapulohumeral muscular dystrophy. Eur Radiol. 2015 doi: 10.1007/s00330-015-3890-1. ((Gerevini S., simonetta.gerevini@hsr.it; Caliendo G.; Falini A.) Neuroradiology Unit, Head and Neck Department, IRCCS San Raffaele Scientific Institute, Milan, Italy) [DOI] [PubMed] [Google Scholar]
- 34.Leung DG, Carrino JA, Wagner KR, Jacobs MA. Whole-body magnetic resonance imaging evaluation of facioscapulo-humeral muscular dystrophy. Muscle Nerve. 2015;52(4):512–520. doi: 10.1002/mus.24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rijken NH, van Engelen BG, de Rooy JW, Geurts AC, Weerdesteyn V. Trunk muscle involvement is most critical for the loss of balance control in patients with facioscapulohumeral muscular dystrophy. Clin Biomech (Bristol, Avon) 2014;29(8):855–860. doi: 10.1016/j.cl0inbiomech.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Olsen DB, Gideon P, Jeppesen TD, Vissing J. Leg muscle involvement in facioscapulohumeral muscular dystrophy assessed by MRI. J Neurol. 2006;253(11):1437–1441. doi: 10.1007/s00415-006-0230-z. [DOI] [PubMed] [Google Scholar]
- 37.Iosa M, Mazza C, Frusciante R, Zok M, Aprile I, Ricci E, Cappozzo A. Mobility assessment of patients with facioscapulohumeral dystrophy. Clin Biomech (Bristol, Avon) 2007;22(10):1074–1082. doi: 10.1016/j.clinbiomech.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Tasca G, Monforte M, Ottaviani P, Pelliccioni M, Frusciante R, Laschena F, Ricci E. Magnetic Resonance Imaging in a large cohort of facioscapulohumeral muscular dystrophy patients: pattern refinement and implications for clinical trials. Ann Neurol. 2016 doi: 10.1002/ana.24640. [DOI] [PubMed] [Google Scholar]
- 39.Janssen BH, Voet NB, Nabuurs CI, Kan HE, de Rooy JW, Geurts AC, Padberg GW, van Engelen BG, Heerschap A. Distinct disease phases in muscles of facioscapulohumeral dystrophy patients identified by MR detected fat infiltration. PLoS One. 2014;9(1):e85416. doi: 10.1371/journal.pone.0085416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lareau-Trudel E, Troter AL, Ghattas B, Pouget J, Attarian S, Bendahan D, Salort-Campana E. Muscle quantitative MR imaging and clustering analysis in patients with facioscapulo-humeral muscular dystrophy type 1. PLoS One. 2015;10(7):e0132717. doi: 10.1371/journal.pone.0132717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kan HE, Klomp DW, Wohlgemuth M, van Loosbroek-Wagemans I, van Engelen BG, Padberg GW, Heerschap A. Only fat infiltrated muscles in resting lower leg of FSHD patients show disturbed energy metabolism. NMR Biomed. 2010;23(6):563–568. doi: 10.1002/nbm.1494. [DOI] [PubMed] [Google Scholar]
- 42.Friedman SD, Poliachik SL, Carter GT, Budech CB, Bird TD, Shaw DW. The magnetic resonance imaging spectrum of facioscapulohumeral muscular dystrophy. Muscle Nerve. 2012;45(4):500–506. doi: 10.1002/mus.22342. [DOI] [PubMed] [Google Scholar]
- 43.Janssen B, Voet N, Geurts A, Van Engelen B, Heerschap A. Quantitative MRI reveals decelerated fatty infiltration in muscles of active FSHD patients. Neurology. 2016;86(18):1700–1707. doi: 10.1212/WNL.0000000000002640. [DOI] [PubMed] [Google Scholar]
- 44.Stramare R, Beltrame V, Dal Borgo R, Gallimberti L, Frigo AC, Pegoraro E, Angelini C, Rubaltelli L, Feltrin GP. MRI in the assessment of muscular pathology: a comparison between limb-girdle muscular dystrophies, hyaline body myopathies and myotonic dystrophies. Radiol Med (Torino) 2010;115(4):585–599. doi: 10.1007/s11547-010-0531-2. [DOI] [PubMed] [Google Scholar]
- 45.Fischer D, Walter MC, Kesper K, Petersen JA, Aurino S, Nigro V, Kubisch C, Meindl T, Lochmuller H, Wilhelm K, Urbach H, Schroder R. Diagnostic value of muscle MRI in differentiating LGMD2I from other LGMDs. J Neurol. 2005;252(5):538–547. doi: 10.1007/s00415-005-0684-4. [DOI] [PubMed] [Google Scholar]
- 46.Mercuri E, Bushby K, Ricci E, Birchall D, Pane M, Kinali M, Allsop J, Nigro V, Saenz A, Nascimbeni A, Fulizio L, Angelini C, Muntoni F. Muscle MRI findings in patients with limb girdle muscular dystrophy with calpain 3 deficiency (LGMD2A) and early contractures. Neuromuscul Disord NMD. 2005;15(2):164–171. doi: 10.1016/j.nmd.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Kesper K, Kornblum C, Reimann J, Lutterbey G, Schroder R, Wattjes MP. Pattern of skeletal muscle involvement in primary dysferlinopathies: a whole-body 3.0-T magnetic resonance imaging study. Acta Neurol Scand. 2009;120(2):111–118. doi: 10.1111/j.1600-0404.2008.01129.x. [DOI] [PubMed] [Google Scholar]
- 48.Diaz J, Woudt L, Suazo L, Garrido C, Caviedes P, Cardenas AM, Castiglioni C, Bevilacqua JA. Broadening the imaging phenotype of dysferlinopathy at different disease stages. Muscle Nerve. 2016 doi: 10.1002/mus.25045. [DOI] [PubMed] [Google Scholar]
- 49.Paradas C, Llauger J, Diaz-Manera J, Rojas-Garcia R, De Luna N, Iturriaga C, Marquez C, Uson M, Hankiewicz K, Gallardo E, Illa I. Redefining dysferlinopathy phenotypes based on clinical findings and muscle imaging studies. Neurology. 2010;75(4):316–323. doi: 10.1212/WNL.0b013e3181ea1564. [DOI] [PubMed] [Google Scholar]
- 50.Degardin A, Morillon D, Lacour A, Cotten A, Vermersch P, Stojkovic T. Morphologic imaging in muscular dystrophies and inflammatory myopathies. Skelet Radiol. 2010;39(12):1219–1227. doi: 10.1007/s00256-010-0930-4. [DOI] [PubMed] [Google Scholar]
- 51.Willis TA, Hollingsworth KG, Coombs A, Sveen ML, Andersen S, Stojkovic T, Eagle M, Mayhew A, de Sousa PL, Dewar L, Morrow JM, Sinclair CD, Thornton JS, Bushby K, Lochmuller H, Hanna MG, Hogrel JY, Carlier PG, Vissing J, Straub V. Quantitative magnetic resonance imaging in limb-girdle muscular dystrophy 2I: a multinational cross-sectional study. PLoS One. 2014;9(2):e90377. doi: 10.1371/journal.pone.0090377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahjneh I, Bashir R, Kiuru-Enari S, Linssen W, Lamminen A, Visser M. Selective pattern of muscle involvement seen in distal muscular dystrophy associated with anoctamin 5 mutations: a follow-up muscle MRI study. Neuromuscul Disord NMD. 2012;22(Suppl 2):S130–S136. doi: 10.1016/j.nmd.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 53.Sarkozy A, Deschauer M, Carlier RY, Schrank B, Seeger J, Walter MC, Schoser B, Reilich P, Leturq F, Radunovic A, Behin A, Laforet P, Eymard B, Schreiber H, Hicks D, Vaidya SS, Glaser D, Carlier PG, Bushby K, Lochmuller H, Straub V. Muscle MRI findings in limb girdle muscular dystrophy type 2L. Neuromuscul Disord NMD. 2012;22(Suppl 2):S122–S129. doi: 10.1016/j.nmd.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 54.Jin SQ, Yu M, Zhang W, Lyu H, Yuan Y, Wang ZX. Dysferlin gene mutation spectrum in a large cohort of chinese patients with dysferlinopathy. Chin Med J (Engl) 2016;129(19):2287–2293. doi: 10.4103/0366-6999.190671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mercuri E, Lampe A, Allsop J, Knight R, Pane M, Kinali M, Bonnemann C, Flanigan K, Lapini I, Bushby K, Pepe G, Muntoni F. Muscle MRI in Ullrich congenital muscular dystrophy and Bethlem myopathy. Neuromuscul Disord NMD. 2005;15(4):303–310. doi: 10.1016/j.nmd.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Quijano-Roy S, Avila-Smirnow D, Carlier RY group W-Mms. Whole body muscle MRI protocol: pattern recognition in early onset NM disorders. Neuromuscul Disord NMD. 2012;22(Suppl 2):S68–S84. doi: 10.1016/j.nmd.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 57.Mercuri E, Cini C, Pichiecchio A, Allsop J, Counsell S, Zolkipli Z, Messina S, Kinali M, Brown SC, Jimenez C, Brockington M, Yuva Y, Sewry CA, Muntoni F. Muscle magnetic resonance imaging in patients with congenital muscular dystrophy and Ullrich phenotype. Neuromuscul Disord NMD. 2003;13(7–8):554–558. doi: 10.1016/s0960-8966(03)00091-9. [DOI] [PubMed] [Google Scholar]
- 58.Gomez-Andres D, Dabaj I, Mompoint D, Hankiewicz K, Azzi V, Ioos C, Romero NB, Ben Yaou R, Bergounioux J, Bonne G, Richard P, Estournet B, Yves-Carlier R, Quijano-Roy S. Pediatric laminopathies: whole-body MRI fingerprint and comparison with SEPN1-myopathy. Muscle Nerve. 2015 doi: 10.1002/mus.25018. [DOI] [PubMed] [Google Scholar]
- 59.Mercuri E, Counsell S, Allsop J, Jungbluth H, Kinali M, Bonne G, Schwartz K, Bydder G, Dubowitz V, Muntoni F. Selective muscle involvement on magnetic resonance imaging in autosomal dominant Emery–Dreifuss muscular dystrophy. Neuropediatrics. 2002;33(1):10–14. doi: 10.1055/s-2002-23593. [DOI] [PubMed] [Google Scholar]
- 60.Carboni N, Mura M, Marrosu G, Cocco E, Marini S, Solla E, Mateddu A, Maioli MA, Piras R, Mallarini G, Mercuro G, Porcu M, Marrosu MG. Muscle imaging analogies in a cohort of patients with different clinical phenotypes caused by LMNA gene mutations. Muscle Nerve. 2010;41(4):458–463. doi: 10.1002/mus.21514. [DOI] [PubMed] [Google Scholar]
- 61.Hankiewicz K, Carlier RY, Lazaro L, Linzoain J, Barnerias C, Gomez-Andres D, Avila-Smirnow D, Ferreiro A, Estournet B, Guicheney P, Germain DP, Richard P, Bulacio S, Mompoint D, Quijano-Roy S. Whole-body muscle magnetic resonance imaging in SEPN1-related myopathy shows a homogeneous and recognizable pattern. Muscle Nerve. 2015;52(5):728–735. doi: 10.1002/mus.24634. [DOI] [PubMed] [Google Scholar]
- 62.Jungbluth H, Davis MR, Muller C, Counsell S, Allsop J, Chattopadhyay A, Messina S, Mercuri E, Laing NG, Sewry CA, Bydder G, Muntoni F. Magnetic resonance imaging of muscle in congenital myopathies associated with RYR1 mutations. Neuromuscul Disord NMD. 2004;14(12):785–790. doi: 10.1016/j.nmd.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 63.Jarraya M, Quijano-Roy S, Monnier N, Behin A, Avila-Smirnov D, Romero NB, Allamand V, Richard P, Barois A, May A, Estournet B, Mercuri E, Carlier PG, Carlier RY. Whole-Body muscle MRI in a series of patients with congenital myopathy related to TPM2 gene mutations. Neuromuscul Disord NMD. 2012;22(Suppl 2):S137–S147. doi: 10.1016/j.nmd.2012.06.347. [DOI] [PubMed] [Google Scholar]
- 64.Jungbluth H, Sewry CA, Counsell S, Allsop J, Chattopadhyay A, Mercuri E, North K, Laing N, Bydder G, Pelin K, Wallgren-Pettersson C, Muntoni F. Magnetic resonance imaging of muscle in nemaline myopathy. Neuromuscul Disord NMD. 2004;14(12):779–784. doi: 10.1016/j.nmd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 65.Schramm N, Born C, Weckbach S, Reilich P, Walter MC, Reiser MF. Involvement patterns in myotilinopathy and desminopathy detected by a novel neuromuscular whole-body MRI protocol. Eur Radiol. 2008;18(12):2922–2936. doi: 10.1007/s00330-008-1071-1. [DOI] [PubMed] [Google Scholar]
- 66.McNeill A, Birchall D, Straub V, Goldfarb L, Reilich P, Walter MC, Schramm N, Lochmuller H, Chinnery PF. Lower limb radiology of distal myopathy due to the S60F myotilin mutation. Eur Neurol. 2009;62(3):161–166. doi: 10.1159/000227266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muller TJ, Kraya T, Stoltenburg-Didinger G, Hanisch F, Korn-huber M, Stoevesandt D, Senderek J, Weis J, Baum P, Deschauer M, Zierz S. Phenotype of matrin-3-related distal myopathy in 16 German patients. Ann Neurol. 2014;76(5):669–680. doi: 10.1002/ana.24255. [DOI] [PubMed] [Google Scholar]
- 68.Mahjneh I, Lamminen AE, Udd B, Paetau AE, Hackman P, Korhola OA, Somer HV. Muscle magnetic resonance imaging shows distinct diagnostic patterns in Welander and tibial muscular dystrophy. Acta Neurol Scand. 2004;110(2):87–93. doi: 10.1111/j.1600-0404.2004.00283.x. [DOI] [PubMed] [Google Scholar]
- 69.Fischmann A, Gloor M, Fasler S, Haas T, Rodoni Wetzel R, Bieri O, Wetzel S, Heinimann K, Scheffler K, Fischer D. Muscular involvement assessed by MRI correlates to motor function measurement values in oculopharyngeal muscular dystrophy. J Neurol. 2011;258(7):1333–1340. doi: 10.1007/s00415-011-5937-9. [DOI] [PubMed] [Google Scholar]
- 70.Zhao J, Liu J, Xiao J, Du J, Que C, Shi X, Liang W, Sun W, Zhang W, Lv H, Yuan Y, Wang Z. Clinical and muscle imaging findings in 14 mainland chinese patients with oculopharyngodistal myopathy. PLoS One. 2015;10(6):e0128629. doi: 10.1371/journal.pone.0128629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fu J, Zheng YM, Jin SQ, Yi JF, Liu XJ, Lyn H, Wang ZX, Zhang W, Xiao JX, Yuan Y. “Target” and “sandwich” signs in thigh muscles have high diagnostic values for collagen VI-related myopathies. Chin Med J. 2016;129(15):1811–1816. doi: 10.4103/0366-6999.186638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fiorillo C, Astrea G, Savarese M, Cassandrini D, Brisca G, Trucco F, Pedemonte M, Trovato R, Ruggiero L, Vercelli L, D’Amico A, Tasca G, Pane M, Fanin M, Bello L, Broda P, Musumeci O, Rodolico C, Messina S, Vita GL, Sframeli M, Gibertini S, Morandi L, Mora M, Maggi L, Petrucci A, Massa R, Grandis M, Toscano A, Pegoraro E, Mercuri E, Bertini E, Mongini T, Santoro L, Nigro V, Minetti C, Santorelli FM, Bruno C. MYH7-related myopathies: clinical, histopathological and imaging findings in a cohort of Italian patients. Orphanet J Rare Dis. 2016;11(1):91. doi: 10.1186/s13023-016-0476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sugie K, Sugie M, Taoka T, Tonomura Y, Kumazawa A, Izumi T, Kichikawa K, Ueno S. Characteristic MRI findings of upper limb muscle involvement in myotonic dystrophy type 1. PLoS One. 2015;10(4):e0125051. doi: 10.1371/journal.pone.0125051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kornblum C, Lutterbey G, Bogdanow M, Kesper K, Schild H, Schroder R, Wattjes MP. Distinct neuromuscular phenotypes in myotonic dystrophy types 1 and 2: a whole body high-field MRI study. J Neurol. 2006;253(6):753–761. doi: 10.1007/s00415-006-0111-5. [DOI] [PubMed] [Google Scholar]
- 75.Damian MS, Bachmann G, Herrmann D, Dorndorf W. Magnetic resonance imaging of muscle and brain in myotonic dystrophy. J Neurol. 1993;240(1):8–12. doi: 10.1007/BF00838438. [DOI] [PubMed] [Google Scholar]
- 76.Hamano T, Kawamura Y, Mutoh T, Hirayama M, Kuriyama M. Muscle MRI in myotonic dystrophy type 1 with foot drop. Eur Neurol. 2010;63(3):144–148. doi: 10.1159/000280763. [DOI] [PubMed] [Google Scholar]
- 77.Hayashi K, Hamano T, Kawamura Y, Kimura H, Matsunaga A, Ikawa M, Yamamura O, Mutoh T, Higuchi I, Kuriyama M, Nakamoto Y. Muscle MRI of the upper extremity in the myotonic dystrophy type 1. Eur Neurol. 2016;76(1–2):87–94. doi: 10.1159/000448328. [DOI] [PubMed] [Google Scholar]
- 78.Mercuri E, Talim B, Moghadaszadeh B, Petit N, Brockington M, Counsell S, Guicheney P, Muntoni F, Merlini L. Clinical and imaging findings in six cases of congenital muscular dystrophy with rigid spine syndrome linked to chromosome 1p (RSMD1) Neuromuscul Disord NMD. 2002;12(7–8):631–638. doi: 10.1016/s0960-8966(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 79.Lamminen AE. Magnetic resonance imaging of primary skeletal muscle diseases: patterns of distribution and severity of involvement. Br J Radiol. 1990;63(756):946–950. doi: 10.1259/0007-1285-63-756-946. [DOI] [PubMed] [Google Scholar]
- 80.Stramare R, Beltrame V, Dal Borgo R, Gallimberti L, Frigo AC, Pegoraro E, Angelini C, Rubaltelli L, Feltrin GP. MRI in the assessment of muscular pathology: a comparison between limb-girdle muscular dystrophies, hyaline body myopathies and myotonic dystrophies. Radiologia Medica. 2010:1–15. doi: 10.1007/s11547-010-0531-2. ((Stramare R., roberto.stramare@unipd.it; Beltrame V.; Dal Borgo R.; Gallimberti L.; Rubaltelli L.; Feltrin G.P.) Department of Medical Diagnostic Sciences and Special Therapies, University of Padova, Padova, 35128, Italy) [DOI] [PubMed] [Google Scholar]
- 81.Wagner KR, Fleckenstein JL, Amato AA, Barohn RJ, Bushby K, Escolar DM, Flanigan KM, Pestronk A, Tawil R, Wolfe GI, Eagle M, Florence JM, King WM, Pandya S, Straub V, Juneau P, Meyers K, Csimma C, Araujo T, Allen R, Parsons SA, Wozney JM, Lavallie ER, Mendell JR. A phase I/IItrial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol. 2008;63(5):561–571. doi: 10.1002/ana.21338. [DOI] [PubMed] [Google Scholar]
- 82.Campbell C, McMillan HJ, Mah JK, Tarnopolsky M, Selby K, McClure T, Wilson DM, Sherman ML, Escolar D, Attie KM. Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: results of a randomized, placebo-controlled clinical trial. Muscle Nerve. 2016 doi: 10.1002/mus.25268. [DOI] [PubMed] [Google Scholar]
- 83.Sakellariou P, Kekou K, Fryssira H, Sofocleous C, Manta P, Panousopoulou A, Gounaris K, Kanavakis E. Mutation spectrum and phenotypic manifestation in FSHD Greek patients. Neuromuscul Disord NMD. 2012;22(4):339–349. doi: 10.1016/j.nmd.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 84.Lin F, Wang ZQ, Lin MT, Murong SX, Wang N. New Insights into genotype-phenotype correlations in Chinese facioscapulohumeral muscular dystrophy: a retrospective analysis of 178 patients. Chin Med J (Engl) 2015;128(13):1707–1713. doi: 10.4103/0366-6999.159336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.ten Dam L, van der Kooi AJ, van Wattingen M, de Haan RJ, de Visser M. Reliability and accuracy of skeletal muscle imaging in limb-girdle muscular dystrophies. Neurology. 2012;79(16):1716–1723. doi: 10.1212/WNL.0b013e31826e9b73. [DOI] [PubMed] [Google Scholar]
- 86.Díaz-Manera J, Alejaldre A, González L, Olivé M, Gómez-Andrés D, Muelas N, Vílchez JJ, Llauger J, Carbonell P, Márquez-Infante C, Fernández-Torrón R, Poza JJ, López de Munáin A, González-Quereda L, Mirabet S, Clarimon J, Gallano P, Rojas-García R, Gallardo E, Illa I. Muscle imaging in muscle dystrophies produced by mutations in the EMD and LMNA genes. Neuromuscul Disord. 2016;26(1):33–40. doi: 10.1016/j.nmd.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 87.Morrow JM, Matthews E, Raja Rayan DL, Fischmann A, Sinclair CD, Reilly MM, Thornton JS, Hanna MG, Yousry TA. Muscle MRI reveals distinct abnormalities in genetically proven non-dystrophic myotonias. Neuromuscul Disord NMD. 2013;23(8):637–646. doi: 10.1016/j.nmd.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kornblum C, Lutterbey GG, Czermin B, Reimann J, von Kleist-Retzow JC, Jurkat-Rott K, Wattjes MP. Whole-body high-field MRI shows no skeletal muscle degeneration in young patients with recessive myotonia congenita. Acta Neurol Scand. 2010;121(2):131–135. doi: 10.1111/j.1600-0404.2009.01228.x. [DOI] [PubMed] [Google Scholar]
- 89.Horvath JJ, Austin SL, Case LE, Greene KB, Jones HN, Soher BJ, Kishnani PS, Bashir MR. Correlation between quantitative whole-body muscle magnetic resonance imaging and clinical muscle weakness in Pompe disease. Muscle Nerve. 2015;51(5):722–730. doi: 10.1002/mus.24437. [DOI] [PubMed] [Google Scholar]
- 90.Olsen DB, Langkilde AR, Orngreen MC, Rostrup E, Schwartz M, Vissing J. Muscle structural changes in mitochondrial myopathy relate to genotype. J Neurol. 2003;250(11):1328–1334. doi: 10.1007/s00415-003-0206-1. [DOI] [PubMed] [Google Scholar]
- 91.Hollingsworth KG, de Sousa PL, Straub V, Carlier PG. Towards harmonization of protocols for MRI outcome measures in skeletal muscle studies: consensus recommendations from two TREAT-NMD NMR workshops, 2 May 2010, Stockholm, Sweden, 1–2 October 2009, Paris, France. Neuromuscul Disord NMD. 2012;22(Suppl 2):S54–S67. doi: 10.1016/j.nmd.2012.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.