Abstract
Purpose
Sperm-specific sodium-hydrogen exchanger (sNHE) is essential to maintain sperm normal function in mice; however, its role in human sperm has not been clarified to date. The aim of this study is to investigate the expression pattern of sNHE in human spermatozoa and its relationship with sperm functional parameters.
Method
Semen samples from 68 asthenozoospermic and 61 normozoospermic men were analyzed for sperm concentration, motility, and acrosome reaction, and high motile spermatozoa were collected by swim-up method. The expression of sNHE in spermatozoa was detected by Western blot and immunofluorescence staining. The relationship between sNHE expression and sperm parameters was assessed.
Results
We identified sNHE is mainly localized to the principal piece of the human sperm tail. The expression of sNHE was positively correlated with sperm concentration, total number, and progressive motility. Moreover, sNHE expression was upregulated in swim-up sperm and associated with most of sperm motility parameters including straight line velocity and curvilinear velocity. Our results also showed that sNHE expression is decreased in sperm from patients with asthenozoospermia compared with that from normal controls. However, no correlation was found between sNHE expression and acrosome reaction in spermatozoa.
Conclusions
The expression pattern of sNHE suggested that this protein may be involved in the regulation of sperm motility, and aberration of its expression in sperm may contribute to the pathogenesis of asthenozoospermia.
Keywords: Sodium-hydrogen exchanger, Human sperm, Motility parameters, Asthenozoospermia, Male infertility
Introduction
Infertility is considered to be a major health problem, affecting 10–15% of reproductive-age couples worldwide. In half of these cases, infertility is due to male factors [1]. In the clinic, individuals with a high proportion of poorly motile or immotile sperm, which is termed asthenozoospermia, are generally infertile or sterile without the aid of assisted reproductive techniques (ART). The pathogenesis of asthenozoospermia appears to be complicated and multifactorial, and it can be affected by both genetic and environmental factors [2]. A variety of causes, such as congenital or acquired urogenital abnormalities, varicocele, testicular infection or trauma, endocrine disturbances, and morphological defects of sperm, can give rise to asthenozoospermia. Despite these known etiologies, asthenozoospermia remains a description of clinical phenomenon, most of whose underlying molecular mechanism is still obscure.
The spermatozoa experience a series of functional transformations during their journey to achieve successful fertilization [3]. Motility is considered to be a fundamental characteristic of sperm and provides a reliable diagnostic and prognostic measure for male infertility. To complete fertilization, the sperm must travel from the vagina to the fallopian tubes, penetrate the cumulus oophorus surrounding the ovum, and fertilize the egg. All this relies on the capability of motility [3]. It is widely acknowledged that cAMP/protein kinase A (PKA)-dependent protein phosphorylation plays a crucial role in mediating the initiation and activation of sperm motility [4]. Moreover, the calcium signaling pathways are responsible for sperm motility [5]. The homeostasis between extra and intracellular calcium is required for development of both motility and hyperactivation of spermatozoa by activating the PKA or PKA-independent pathway. However, the regulation of sperm motility appears to be complicated and not fully elucidated. In view of the complexity of the regulatory pathways in sperm motility, it appears that any abnormalities of these pathways could contribute to impaired sperm motility. For example, mutation of a sperm-specific subunit of PKA does not affect the process of spermatogenesis, but impairs the capability of forward motility in mature sperm [6]; aberrated expression of the ion channel, such as with the voltage-dependent anion channel and chloride channels, could result in poor sperm motility and consequently asthenozoospermia [7, 8].
The sodium-hydrogen exchangers (NHEs), encoded by solute carrier family 9, are membrane cation-proton transporter proteins in mammalian cells [9]. They are selectively expressed in a variety of tissues and typically involved in the electroneutral exchange of intracellular H+ with extracellular Na+ according to the concentration gradient across the membrane. The activity of these transporters is required for diverse cellular physiological processes, including the regulation of intracelluar pH and cell volume, as well as cell growth and fluid absorption [10]. Several isoforms of NHE have been demonstrated to play a role in male reproduction. For example, disruption of NHE8 expression could result in impaired Leydig cell function and smaller testes, as well as fewer spermatozoa [11]. Interestingly, three NHEs have been identified in spermatozoa, namely NHE1, NHE5, and NHE10 (sperm-specific NHE isoform, sNHE) [12, 13]. Previous study found that normal sperm motility and fecundity are unaffected after elimination of NHE1 in male mice, suggesting this gene is male fertility independent [14]. However, sNHE is demonstrated to be exclusively expressed in spermatozoa and localized to the principal piece of their flagellum and involved in the regulation of sperm motility [15]. Disruption of the sNHE could seriously weaken sperm motility and capacitation, and consequently results in complete infertility [13].
The fact that the expression of sNHE is correlated with sperm function in animal models prompted us to study the characteristic of sNHE in human spermatozoa, which has not been determined to date. To better understand the role of sNHE in human sperm functions, we examined the expression and location of sNHE in human sperm and its relationship with sperm motility and acrosome reaction (RA). Moreover, we evaluated sNHE expression in high motility sperm portions selected by swim-up method and ejaculated spermatozoa of asthenozoospermia patients. Together, these data contribute to understanding the role of sNHE in the motility and fertilization of spermatozoa.
Materials and methods
Patients selection and semen analysis
The study was approved by the Ethical Committee and conducted according to the Helsinki Declaration. Semen samples were collected from men who were undergoing routine semen analysis for couple infertility from the andrology clinic. Every subject was fully informed of the purpose of the study and provided informed consent before the research. Donors with varicocele, infections, history of radiation and/or chemotherapy, abnormal autoimmune symptoms, endocrine abnormalities, or abnormal semen liquefaction were excluded. Asthenozoospermia was defined as progressive motility of <32% within 60 min of ejaculation. The seminal fluid was obtained by masturbation after 3 to 7 days of sexual abstinence. Collection and processing of semen samples were conducted in accordance with the criteria of the WorldHealth Organization (WHO) Laboratory guidelines (5th edition, 2010). A total of 68 patients with asthenozoospermia and 61 normal subjects were enrolled in this study, and the demographic characteristics are shown in Table 1. The parameters of sperm motility were analyzed by the computer-assisted semen analysis (CASA) system (Suiplus, China). The seminal volume, sperm concentration, progressive motility, and total motility of spermatozoa were examined. Average path velocity (VAP), straight line velocity (VSL), curvilinear velocity (VCL), amplitude of lateral head displacement (ALH), beat cross frequency (BCF), linearity of progression (LIN), path straightness (STR), and hyperactivated spermatozoa (HA) were recorded. The identification of HA cells was performed as previously described [16]. At least 200 spermatozoa and five fields were analyzed for each sample. All analyses were performed at 37 °C. In some normal samples, high motility sperm were acquired by swim-up method in human tubal fluid (HTF)-10% HSA medium. Semen samples were selected through 40% density gradient of PureSperm (Nidacon International, Molndal, Sweden) by centrifugation (500g, 30 min) at room temperature and washed with phosphate-buffered saline (PBS) for three times; the obtained spermatozoa were used for the following immunofluorescence experiments and Western blot analyses.
Table 1.
Demographic characteristics of normozoospermic (N) and asthenozoospermic (AS) men
| Parameters | N (n = 61) | AS (n = 68) | P value |
|---|---|---|---|
| Age | 29.8 (22–46) | 30.6 (24–51) | n.s. |
| Semen volume | 3.5 ± 1.3 | 3.1 ± 0.9 | <0.01 |
| Sperm concentration (×106/ml) | 50.2 (18.1–140.2) | 38.5 (13.1–112.9) | <0.01 |
| Total number (×106/ejaculate) | 165.1 (48.9–482.1) | 115.4 (36.7–248.3) | <0.01 |
| Progressive motility (%) | 43.5 ± 8.5 | 16.6 ± 6.2 | <0.05 |
| Immotile sperm (%) | 38.5 ± 10.9 | 76.8 ± 6.8 | <0.001 |
| Total motility (%) | 61.5 ± 10.9 | 23.2 ± 6.8 | <0.001 |
| Hyperactivated motility (%) | 2.7 (0–17.8) | 0.6 (0–3.8) | <0.001 |
Semen volume, progressive motility, immotile sperm, and total motility followed a normal distribution and were expressed as mean ± SD; statistical comparisons were assessed by t test. The other parameters did not follow a normal distribution and were expressed as median (minimum–maximum); statistical comparisons were assessed by Mann-Whitney U test
n.s. not significant
Immunofluorescence staining
For studies of the location of sNHE, spermatozoa were smeared and air-dried on polylysine-coated slides, then fixed in 4% paraformaldehyde for 30 min at room temperature. After washing with PBS for three times, they were permeabilized with 0.1% Triton X-100 in PBS for 5 min in ice and then blocked with 3% BSA in PBS for 30 min at room temperature. Subsequently, spermatozoa were incubated with goat anti-sNHE antibody (1:100 dilution, Santa Cruz, Dallas, TX, USA) or goat serum as a negative control at 4 °C overnight. Unbound antibodies were removed by washing with PBS for three times, and the slides were incubated with mouse anti-goat IgG-FITC antibody (1:100 dilution) in the dark for 60 min. After washing with PBS for three times, the spermatozoa were stained with 4′,6-diamidino-2-phenylindole (DAPI, 10 μg/ml, Sigma) in the dark for 5 min and finally examined by Zeiss LSM laser confocal microscope (Carl Zeiss, Thornwood, NY, USA).
Western blot analysis
The obtained sperm were washed twice with ice-cold PBS, then harvested with lysis buffer containing phosphatase and protease inhibitors. The protein concentration was quantified using the BCA Protein Assay. Forty micrograms total proteins in lysates for each sample was separated by SDS-PAGE, then transferred to nitrocellulose membrane, which was blocked with 5% bovine serum albumin at room temperature for 1 h, then incubated at 4 °C overnight with the primary antibodies goat anti-sNHE (1:1000 dilution, Santa Cruz, sc-99634), then corresponding IgG secondary antibody (1:10,000, LICOR, Lincoln, NE, USA). Membranes were scanned and analyzed using the Odyssey Infrared Imaging System.
Evaluation of the acrosome reaction
Sperm cells were incubated in HTF medium for 3 h at 37 °C and 5% CO2. After washed for three times, these spermatozoa were incubated with or without 10 μM of calcium ionophore A23187 for 15 min at 37 °C. Subsequently, the spermatozoa were smeared, air-dried onto the slides, and fixed with 100% methanol for 30 min at room temperature. The fixed spermatozoa were incubated with fluorescein isothyocianate-conjugated lectin from Pisum sativum (PSA-FITC, Sigma, St. Louis, MO, USA) in the dark for 20 min. The percentage of acrosome-reacted sperm was examined under fluorescent confocal microscopy. Then acrosome-intact spermatozoa are labeled with green fluorescence at the acrosomal region, whereas sperm with reacted acrosome display no staining in this region or very weak fluorescence over the head. At least 200 cells were counted for each sample by two independent researchers.
Statistical analysis
All data were analyzed by SPSS 18.0 (Chicago, IL, USA). The normal distribution was examined by Kolmogorov-Smirnov test. Data are reported as mean ± SD when normally distributed and as median with minimum and maximum values when non-normally distributed. The variables with normal distribution were analyzed by unpaired two-sided Student’s t test; the non-normally distributed variables were analyzed by Mann-Whitney non-parametric tests. Correlation between sNHE expression and parameters of sperm motility was performed by Pearson’s correlation tests for normally distributed values, and Spearman’s regression analysis for non-normally distributed values.
Results
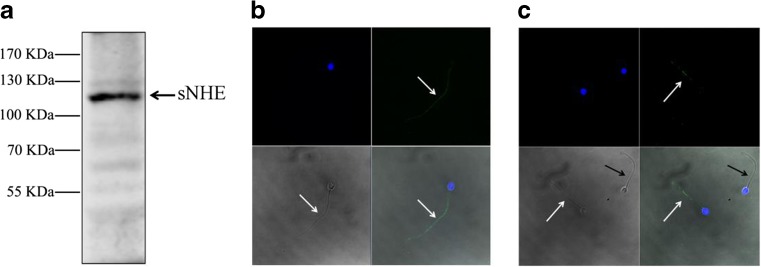
We detected the expression of sNHE in human spermatozoa using Western blot analysis and immunofluorescence staining. Western blot analysis of sperm protein extracts revealed the presence of a band at the molecular weight of 120 kDa (Fig. 1a). The expression of sNHE was also confirmed by the immunofluorescence approaches. As can be observed in Fig. 1b, c, there was a strong immunoreactivity in the principal piece of the tail of spermatozoa, which was consistent with that observed in mouse sperm; while the staining was not present in some sperm, suggesting inhomogeneous expression of sNHE channels in the ejaculate.
Fig. 1.
The expression and location of sNHE in human spermatozoa. a Western blot analysis of sNHE in human sperm protein lysis. b and c Immunofluorescence staining of sNHE protein in human spermatozoa. n = 3. Spermatozoa were stained with DAPI (blue, to detect the nuclei) and anti-sNHE (green). White arrow indicates sNHE-positive sperm, and black arrow indicates sperm negative to sNHE staining
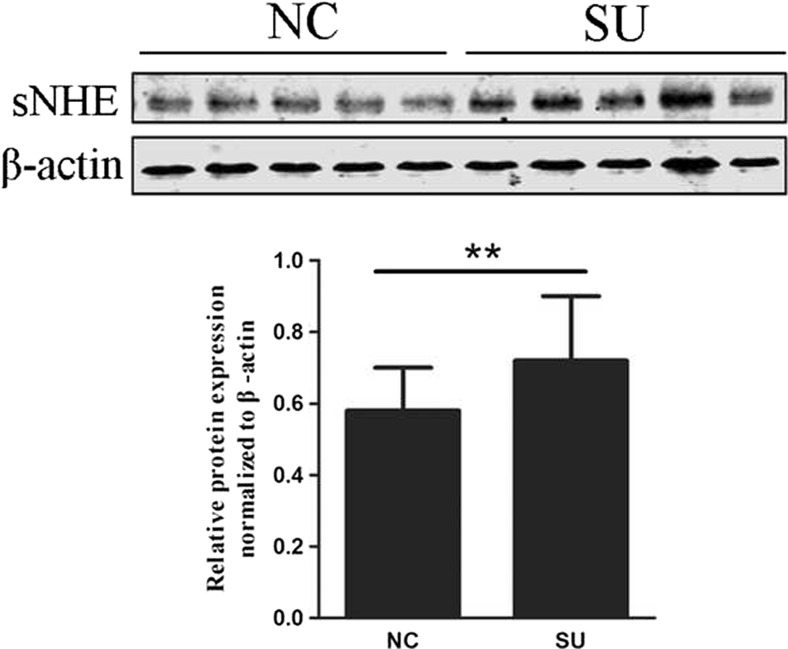
The location of sNHE in the tail of sperm indicates that it may be involved in the regulation of the motility. Therefore, we compared the expression of sNHE in the swim-up-selected sperm with that of the control donors. As shown in Fig. 2, there was a significant increase of the sNHE expression in these swim-up-selected sperm. Furthermore, we aimed to explore the relationship between the protein expression and seminal parameters. The expression of sNHE was positively associated with sperm concentration and total number (Table 2). Positive correlations between sNHE expression and progressive, total, and hyperactivated motility were also observed. However, no significant correlation was found between the protein expression and patients’ age and semen volume. In this way, we analyzed the correlation between the CASA parameter values and expression and observed similar tendencies. The expression of sNHE was positively correlated with most of sperm CASA parameters including VCL, VAP, BCF, ALH, VSL, and STR and negatively correlated with LIN (Table 3).
Fig. 2.
Comparison of the expression of sNHE expression in swim-up selected sperm (SU, n = 20) and normal donors (NC, n = 20). A significant increase of sNHE protein was observed in the group of SU compared with that of normal controls, and β-actin was used as an internal control for Western blot. **p < 0.01
Table 2.
Correlations between sNHE expression and semen parameter values (n = 129)
| Parameters | Correlation coefficients | P value |
|---|---|---|
| Age | −0.10 | n.s. |
| Semen volume | 0.13 | n.s. |
| Sperm concentration | 0.17 | <0.05 |
| Total number | 0.19 | <0.05 |
| Progressive motility | 0.37 | <0.001 |
| Immotile | −0.39 | <0.001 |
| Total motility | 0.39 | <0.001 |
| Hyperactivated motility | 0.33 | <0.001 |
Correlations of semen volume, progressive motility, immotile sperm and total motility were tested by Pearson’s correlation analysis; the other parameters were tested by Spearman’s correlation analysis
n.s. not significant
Table 3.
Correlations between sNHE expression and sperm motility parameter values (n = 129)
| Parameters | Correlation coefficients | P value |
|---|---|---|
| VSL | 0.383 | <0.001 |
| VAP | 0.394 | <0.001 |
| BCF | 0.364 | <0.001 |
| ALH | 0.395 | <0.001 |
| LIN | −0.306 | <0.001 |
| VCL | 0.365 | <0.001 |
| STR | 0.347 | <0.001 |
Correlations of VSL, VAP, BCF, ALH, and LIN were tested by Pearson’s correlation analysis; the parameters of VSL and STR were tested by Spearman’s correlation analysis
VSL straight line velocity, VAP average path velocity, BCF beat cross frequency, ALH amplitude of lateral head displacement, LIN linearity of progression, VCL,curvilinear velocity, STR path straightness
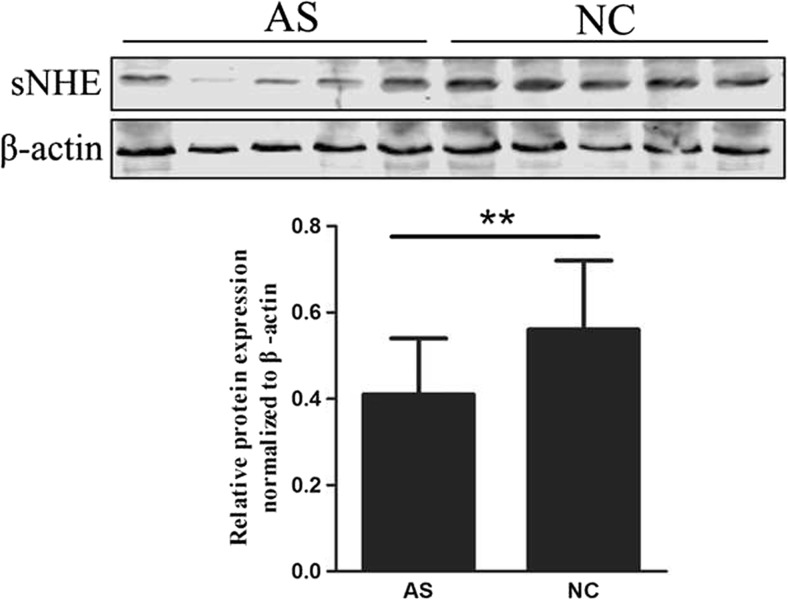
The fact that the expression of sNHE is associated with sperm motility indicates that it may be dysregulated in asthenozoospermia. To test this hypothesis, we examined the sNHE expression in sperm of asthenozoospermic and normozoospermic men. As presented in Fig. 3, sNHE expression was found to be significantly lower in sperm from asthenozoospermic men compared with those from the control group.
Fig. 3.
Western blot analysis of sNHE expression in samples from patients with asthenozoospermic men (AS, n = 68) and normal controls (NC, n = 61). Significant differences were noted between the group of AS and NC, and β-actin was used as an internal control. **p < 0.01
The involvement of sNHE in the AR was examined by investigating the relation of basal and calcium ionophore-stimulated AR with sNHE expression in spermatozoa. There was no significant correlation between sNHE protein expresssion and either basal (r = 0.18, p = 0.26) or calcium ionophore-stimulated AR (r = 0.20, p = 0.21).
Discussion
In this study, we identified the expression of sNHE in human spermatozoa and found the protein mainly localized to the principal piece of their tails. The expression of sNHE is upregulated in swim-up sperm and positive with sperm motility parameters. Interestingly, our results showed that sNHE expression is decreased in sperm from patients with asthenozoospermia compared with that of normal control subjects. The result of the present study indicates that sNHE is involved in the regulation of sperm motility and may contribute to the pathogenesis of asthenozoospermia.
The spermatozoa in the testis are immature and immotile. Unable to fertilize the oocytes, they must undergo several morphological and functional changes to travel through the female reproductive tract and implement fertilization [17]. The capacity of motility and fertilization is initiated in the epididymis and enhanced after ejaculation, which could be modulated by external and internal conditions. Intracellular pH has been considered to be an important element in the regulation of sperm function, and studies showed that ion channels are involved in intracellular pH during this process [18]. The sNHE protein is an important transporter and is responsible for exporting sodium and importing protons and regulating intracellular pH in spermatozoa [9]. In particular, the essential role of sNHE in mouse sperm fertility has been well investigated [13, 15, 19]. However, the role of sNHE in the pathophysiology of human sperm is still obscure. In the present study, we identified the expression of sNHE in human sperm by Western blot analysis and immunofluorescence staining, and found that the sNHE protein is mainly located in the principal piece of the spermatozoa tail. Moreover, immunofluorescence analysis showed that the protein of sNHE is dislocated in some spermatozoa, which indicates that sNHE expression is heterogeneous in human spermatozoa. Similar findings were observed in some other proteins such as CatSper and hCPβ3 in human spermatozoa [20, 21]. One possible reason is that these proteins including sNHE were vulnerable to adverse microenvironmental stimulus and degraded in the epididymis and semen, however further study is needed to explore the mechanism.
The location of sNHE in the principal piece of the sperm tail indicates that it may be associated with sperm motility. However, no studies so far have been conducted to examine the direct relationship between sNHE expression and sperm motility parameters. In our study, we found that the expression of sNHE is higher in swim-up-selected sperm compared with that of the normal control group, which indicates that the channel of sNHE could facilitate sperm migration to the upper medium. Recent studies suggested that NHE activity and normalized pH are required in the migration of various cells. Stimulation of NHE-1 promotes microglial migration via Na+ and Ca2+ signaling [22]; increased NHE activity contributes to proliferation and migration of smooth muscle cells in the model of pulmonary arterial hypertension [23]. Of interest, the results showed that sNHE expression is positively correlated with motility parameters. Sperm movement characteristics are demonstrated to be related with fertility outcome. Previous studies found that low fertilization rates are associated with reduced sperm VAP; Bergh et al. reported that patients with higher sperm VSL also have higher fertilization rate [24]. More important, our results showed that the expression of sNHE protein decreased in asthenozoospermia compared with that of normal cases, suggesting an involvement of these channels in the pathogenesis of asthenozoospermia. The possible mechanism is that abnormality of sNHE protein disturbs the equilibrium of intracellular pH and energy production in spermatozoa, resulting in motility dysfunction. The milieu of the epididymis is maintained at a relatively acidic level, and the sperm stay immotile primarily because of an acidic intracellular and extracellular pH [25, 26]. Then, the intracellular pH of sperm gradually increases after ejaculation, because the alkalinity of the seminal plasma and the female reproductive tract facilitates the alkalinization of the sperm cytoplasm and initiates the motility of sperm [17]. During the process of regulation of sperm pH, sNHE channel may play a crucial role. In the mouse model of sNHE knockout, although the testicular histology, sperm numbers, and morphology were normal, they were completely infertile with seriously impaired sperm motility, which could be partially reversed by ammonium chloride via raising intracellular pH [13].
It is generally accepted that hyperactivation is crucial to guarantee the ability of sperm to successfully reach the site of the oviduct ampulla and completes fertilization [3]. For example, hyperactivation could facilitate sperm to pass the highly visco-elastic oviduct and penetrate the layers surrounding the oocyte [27]. Some studies found that the percentage of hyperactivated sperm is tightly associated with the fertilization rate in vitro, and impaired hyperactivation may be one of etiologies of subfertility [28]. In this study, we found that the expression of sNHE is significantly related to the percentage of hyperactivated sperm. At the molecular level, initiation of capacitation requires a series of physiological processes that contain activation of cAMP-dependent phosphorylation, alteration of ion permeability, and hyperpolarization of the plasma membrane in spermatozoa [29]. The sNHE protein has been found to be required for the soluble adenylyl cyclase (sAC) signaling pathway and cAMP metabolism in mouse-capacitating sperm [19]. Acrosome reaction is another prerequisite step for sperm to digest the cumulus cells and penetrate the ovum [29]. Therefore, here we explore the relationship between sNHE expression and acrosome reaction in spermatozoa, and no statistical correlation was observed between them. Previous studies found that although NHE participate in the regulation of intracellular pH in capacitated human sperm, inhibition of NHE-dependent pH regulation is not adequate to prevent acrosome reaction triggered by progesterone [30], indicating that acrosome reaction may be modulated by other signal transduction events.
Sperm motility is the most primary characteristic of semen analysis and closely related to the fertility outcomes of both natural and assisted conception [31]. Many clinical studies showed that spermatozoa with low motility predict poor outcomes of fertilization rates, embryo qualities, and pregnancy rates, as well as subsequent frustrated ART outcomes [32, 33]. Thus, the study of the regulatory mechanism of motility in human spermatozoa will enable us to address the issue of male infertility. Several proteins, such as CatSper, have been demonstrated to be implicated in the regulation of motility [20]. Here, we found that the abnormal expression of sNHE is significantly associated with dysfunction of motility, indicating that this exchanger might be a target molecule of asthenozoospermia. In addition, Liu et al. reported that immunization of female mice with the sNHE DNA vaccine showed decreased fecundity, since the antiserum and vaginal fluid in these immunized mice could induce sperm agglutination and decrease sperm motility; chemical inhibitor of NHE, amiloride, also showed the potential of impeding sperm motility, suggesting that these inhibitors of sNHE applied as contraceptive needed to be further investigated [34, 35].
In conclusion, the present study demonstrated the sNHE mainly localized to the principal piece of the tails of human spermatozoa, and its expression is associated with most of the functional parameters of motility. Moreover, the expression of sNHE is downregulated in sperm from asthenozoospermic men, and the alterations of this protein may result in sperm dysfunction and consequent-impaired-fertilizing potential. This study will help to get a better understanding of the etiology of asthenozoospermia and provide new insight into potential therapeutic targets of male infertility and contraception.
Acknowledgments
This work was supported by Peking University 985 Clinical Hospital Cooperation Program to Hui Jiang.
Compliance with ethical standards
The study was approved by the Ethical Committee and conducted according to the Helsinki Declaration. Every subject was fully informed of the purpose of the study and provided informed consent before the research.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Skakkebaek NE, Jorgensen N, Main KM, Rajpert-De Meyts E, Leffers H, Andersson AM, et al. Is human fecundity declining? Int J Androl. 2006;29(1):2–11. doi: 10.1111/j.1365-2605.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 2.Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril. 2015;103(3):e18–25. doi:10.1016/j.fertnstert.2014.12.103. [DOI] [PubMed]
- 3.Yoshida M, Kawano N, Yoshida K. Control of sperm motility and fertility: diverse factors and common mechanisms. Cellular and molecular life sciences: CMLS. 2008;65(21):3446–3457. doi: 10.1007/s00018-008-8230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bajpai M, Doncel GF. Involvement of tyrosine kinase and cAMP-dependent kinase cross-talk in the regulation of human sperm motility. Reproduction (Cambridge, England) 2003;126(2):183–195. doi: 10.1530/rep.0.1260183. [DOI] [PubMed] [Google Scholar]
- 5.Singh AP, Rajender S. CatSper channel, sperm function and male fertility. Reprod BioMed Online. 2015;30(1):28–38. doi: 10.1016/j.rbmo.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Skalhegg BS, Huang Y, Su T, Idzerda RL, McKnight GS, Burton KA. Mutation of the Calpha subunit of PKA leads to growth retardation and sperm dysfunction. Molecular endocrinology (Baltimore, Md) 2002;16(3):630–639. doi: 10.1210/mend.16.3.0793. [DOI] [PubMed] [Google Scholar]
- 7.Liu SW, Li Y, Zou LL, Guan YT, Peng S, Zheng LX, et al. Chloride channels are involved in sperm motility and are downregulated in spermatozoa from patients with asthenozoospermia. Asian journal of andrology. 2016 doi: 10.4103/1008-682X.181816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu B, Wang P, Wang Z, Jia Y, Niu X, Wang W, et al. Analysis and difference of voltage-dependent anion channel mRNA in ejaculated spermatozoa from normozoospermic fertile donors and infertile patients with idiopathic asthenozoospermia. J Assist Reprod Genet. 2010;27(12):719–724. doi: 10.1007/s10815-010-9466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donowitz M, Ming Tse C, Fuster D. SLC9/NHE gene family, a plasma membrane and organellar family of Na+/H+ exchangers. Mol Asp Med. 2013;34(2–3):236–251. doi: 10.1016/j.mam.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuster DG, Alexander RT. Traditional and emerging roles for the SLC9 Na+/H+ exchangers. Pflugers Archiv: European journal of physiology. 2014;466(1):61–76. doi: 10.1007/s00424-013-1408-8. [DOI] [PubMed] [Google Scholar]
- 11.Xu H, Chen H, Li J, Zhao Y, Ghishan FK. Disruption of NHE8 expression impairs Leydig cell function in the testes. American journal of physiology Cell physiology. 2015;308(4):C330–C338. doi: 10.1152/ajpcell.00289.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo AL, James PF, Lingrel JB. Roles of the Na, K-ATPase alpha 4 isoform and the Na+/H+ exchanger in sperm motility. Mol Reprod Dev. 2002;62(3):348–356. doi: 10.1002/mrd.90002. [DOI] [PubMed] [Google Scholar]
- 13.Wang D, King SM, Quill TA, Doolittle LK, Garbers DL. A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat Cell Biol. 2003;5(12):1117–1122. doi: 10.1038/ncb1072. [DOI] [PubMed] [Google Scholar]
- 14.Bell SM, Schreiner CM, Schultheis PJ, Miller ML, Evans RL, Vorhees CV, et al. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Phys. 1999;276(4 Pt 1):C788–C795. doi: 10.1152/ajpcell.1999.276.4.C788. [DOI] [PubMed] [Google Scholar]
- 15.Liu T, Huang JC, Zuo WL, Lu CL, Chen M, Zhang XS, et al. A novel testis-specific Na+/H+ exchanger is involved in sperm motility and fertility. Frontiers in bioscience (Elite edition) 2010;2:566–581. doi: 10.2741/e115. [DOI] [PubMed] [Google Scholar]
- 16.Mortimer ST, Swan MA, Mortimer D. Effect of seminal plasma on capacitation and hyperactivation in human spermatozoa. Human reproduction (Oxford, England) 1998;13(8):2139–2146. doi: 10.1093/humrep/13.8.2139. [DOI] [PubMed] [Google Scholar]
- 17.Hamamah S, Gatti JL. Role of the ionic environment and internal pH on sperm activity. Human reproduction (Oxford, England) 1998;13(Suppl 4):20–30. doi: 10.1093/humrep/13.suppl_4.20. [DOI] [PubMed] [Google Scholar]
- 18.Darszon A, Trevino CL, Wood C, Galindo B, Rodriguez-Miranda E, Acevedo JJ, et al. Ion channels in sperm motility and capacitation. Society of Reproduction and Fertility supplement. 2007;65:229–244. [PubMed] [Google Scholar]
- 19.Wang D, Hu J, Bobulescu IA, Quill TA, McLeroy P, Moe OW, et al. A sperm-specific Na+/H+ exchanger (sNHE) is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase (sAC) Proc Natl Acad Sci U S A. 2007;104(22):9325–9330. doi: 10.1073/pnas.0611296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamburrino L, Marchiani S, Vicini E, Muciaccia B, Cambi M, Pellegrini S, et al. Quantification of CatSper1 expression in human spermatozoa and relation to functional parameters. Human reproduction (Oxford, England) 2015;30(7):1532–1544. doi: 10.1093/humrep/dev103. [DOI] [PubMed] [Google Scholar]
- 21.Soda T, Miyagawa Y, Ueda N, Takezawa K, Okuda H, Fukuhara S, et al. Systematic characterization of human testis-specific actin capping protein β3 as a possible biomarker for male infertility. Hum Reprod (Oxford, England) 2017 doi: 10.1093/humrep/dew353. [DOI] [PubMed] [Google Scholar]
- 22.Shi Y, Yuan H, Kim D, Chanana V, Baba A, Matsuda T, et al. Stimulation of Na+/H+ exchanger isoform 1 promotes microglial migration. PLoS One. 2013;8(8):e74201. doi: 10.1371/journal.pone.0074201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huetsch JC, Jiang H, Larrain C, Shimoda LA. The Na+/H+ exchanger contributes to increased smooth muscle proliferation and migration in a rat model of pulmonary arterial hypertension. Physiological reports. 2016;4:5. doi: 10.14814/phy2.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van den Bergh M, Emiliani S, Biramane J, Vannin AS, Englert Y. A first prospective study of the individual straight line velocity of the spermatozoon and its influences on the fertilization rate after intracytoplasmic sperm injection. Human reproduction (Oxford, England) 1998;13(11):3103–3107. doi: 10.1093/humrep/13.11.3103. [DOI] [PubMed] [Google Scholar]
- 25.Florman HM, Jungnickel MK, Sutton KA. Shedding light on sperm pHertility. Cell. 2010;140(3):310–312. doi: 10.1016/j.cell.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 26.Nishigaki T, Jose O, Gonzalez-Cota AL, Romero F, Trevino CL, Darszon A. Intracellular pH in sperm physiology. Biochem Biophys Res Commun. 2014;450(3):1149–1158. doi: 10.1016/j.bbrc.2014.05.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suarez SS. Control of hyperactivation in sperm. Hum Reprod Update. 2008;14(6):647–657. doi: 10.1093/humupd/dmn029. [DOI] [PubMed] [Google Scholar]
- 28.Wiser A, Sachar S, Ghetler Y, Shulman A, Breitbart H. Assessment of sperm hyperactivated motility and acrosome reaction can discriminate the use of spermatozoa for conventional in vitro fertilisation or intracytoplasmic sperm injection: preliminary results. Andrologia. 2014;46(3):313–315. doi: 10.1111/and.12068. [DOI] [PubMed] [Google Scholar]
- 29.Stival C, Puga Molina Ldel C, Paudel B, Buffone MG, Visconti PE, Krapf D. Sperm capacitation and acrosome reaction in mammalian sperm. Adv Anat Embryol Cell Biol. 2016;220:93–106. doi: 10.1007/978-3-319-30567-7_5. [DOI] [PubMed] [Google Scholar]
- 30.Garcia MA, Meizel S. Regulation of intracellular pH in capacitated human spermatozoa by a Na+/H+ exchanger. Mol Reprod Dev. 1999;52(2):189–195. doi: 10.1002/(SICI)1098-2795(199902)52:2<189::AID-MRD10>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 31.Tomlinson M, Lewis S, Morroll D. Sperm quality and its relationship to natural and assisted conception: British fertility society guidelines for practice. Human fertility (Cambridge, England) 2013;16(3):175–193. doi: 10.3109/14647273.2013.807522. [DOI] [PubMed] [Google Scholar]
- 32.Verheyen G, Tournaye H, Staessen C, De Vos A, Vandervorst M, Van Steirteghem A. Controlled comparison of conventional in-vitro fertilization and intracytoplasmic sperm injection in patients with asthenozoospermia. Human reproduction (Oxford, England) 1999;14(9):2313–2319. doi: 10.1093/humrep/14.9.2313. [DOI] [PubMed] [Google Scholar]
- 33.Ben-Chetrit A, Senoz S, Greenblatt EM, Casper RF. In vitro fertilization outcome in the presence of severe male factor infertility. Fertil Steril. 1995;63(5):1032–1037. doi: 10.1016/S0015-0282(16)57543-8. [DOI] [PubMed] [Google Scholar]
- 34.Liu T, Huang JC, Lu CL, Yang JL, Hu ZY, Gao F, et al. Immunization with a DNA vaccine of testis-specific sodium-hydrogen exchanger by oral feeding or nasal instillation reduces fertility in female mice. Fertil Steril. 2010;93(5):1556–1566. doi: 10.1016/j.fertnstert.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 35.Peralta-Arias RD, Vívenes CY, Camejo MI, Piñero S, Proverbio T, Martínez E, et al. ATPases, ion exchangers and human sperm motility. Reproduction. 2015;149(5):475–484. doi: 10.1530/REP-14-0471. [DOI] [PubMed] [Google Scholar]