Abstract
Protein secretion systems are vital for prokaryotic life, as they enable bacteria to acquire nutrients, communicate with other species, defend against biological and chemical agents, and facilitate disease through the delivery of virulence factors. In this review, we will focus on the recently discovered type IX secretion system (T9SS), a complex translocon found only in some species of the Bacteroidetes phylum. T9SS plays two roles, depending on the lifestyle of the bacteria. It provides either a means of movement (called gliding motility) for peace-loving environmental bacteria or a weapon for pathogens. The best-studied members of these two groups are Flavobacterium johnsoniae, a commensal microorganism often found in water and soil, and Porphyromonas gingivalis, a human oral pathogen that is a major causative agent of periodontitis. In P. gingivalis and some other periodontopathogens, T9SS translocates proteins, especially virulence factors, across the outer membrane (OM). Proteins destined for secretion bear a conserved C-terminal domain (CTD) that directs the cargo to the OM translocon. At least 18 proteins are involved in this still enigmatic process, with some engaged in the post-translational modification of T9SS cargo proteins. Upon translocation across the OM, the CTD is removed by a protease with sortase-like activity and an anionic LPS is attached to the newly formed C-terminus. As a result, a cargo protein could be secreted into the extracellular milieu or covalently attached to the bacterial surface. T9SS is regulated by a two-component system; however, the precise environmental signal that triggers it has not been identified. Exploring unknown systems contributing to bacterial virulence is exciting, as it may eventually lead to new therapeutic strategies. During the past decade, the major components of T9SS were identified, as well as hints suggesting the possible mechanism of action. In addition, the list of characterized cargo proteins is constantly growing. The actual structure of the translocon, situated in the OM of bacteria, remains the least explored area; however, new technical approaches and increasing scientific attention have resulted in a growing body of data. Therefore, we present a compact up-to-date review of this topic.
Keywords: secretion, T9SS, Porphyromonas gingivalis, pathogenesis, gliding motility, proteins, virulence
Introduction
Secretion of hemolysin A by E. coli, described four decades ago, was the first protein secretion system discovered in Gram-negative bacteria (diderm bacteria; Goebel and Hedgpeth, 1982). Since then, eight other protein secretion pathways have been characterized in these prokaryotes, which have a cell envelope consisting of the inner membrane (IM) and the outer membrane (OM) separated by the periplasm. They are now referred to as type x secretion systems (T1SS–T9SS; reviewed in Abdallah et al., 2007; Gerlach and Hensel, 2007; Remaut et al., 2008; Desvaux et al., 2009; Goyal et al., 2014; Costa et al., 2015; Abby et al., 2016). Secretion systems in diderm bacteria are considered gateways through the OM that transport cargo with the help of either dedicated IM and periplasmic proteins or the Sec, Tat, and holins systems that first transport cargo to the periplasm. In fact, the Sec, Tat, and holins pathways, which transport proteins across the cytoplasmic membrane, are universal among bacteria, eukaryotes, and even archaea (Hutcheon and Bolhuis, 2003; Denks et al., 2014; Berks, 2015; Saier and Reddy, 2015). Therefore, secretion may be either a single-step process in which substrates (proteins or DNA) are translocated through a designated cell envelope-spanning structure (T1SS, T3SS, T4SS, and T6SS) or a two-step process in which the substrates first cross the IM into the periplasm using the Sec/Tat/holins systems, then are directed to the OM translocon. The final destinations of secreted cargos are diverse: they may stay attached to the surface of the OM, be released into the extracellular milieu, or be injected into the cytoplasm of a target cell (Costa et al., 2015; Abby et al., 2016).
Secretion systems perform numerous physiological functions essential for cell propagation and fitness within a specific ecological niche. They facilitate nutrient acquisition, communication with the environment, attachment to various surfaces, defense against host antimicrobial systems, and delivery of virulence factors at a precise location such as a eukaryotic cell (Letoffe et al., 1994; Henke and Bassler, 2004; Gerlach and Hensel, 2007; Rondelet and Condemine, 2013; Gaytan et al., 2016; Hachani et al., 2016; Majerczyk et al., 2016). However, none of the above adaptations can be assigned solely to one type of secretion.
The presence of protein secretion systems varies among phylogenetic lineages of diderm bacteria. Proteobacteria encode the broadest range of described secretion types, whereas other clades have a strong preference for only one or two types (e.g., Fusobacteria possess only T5SS; Chlamydiae, T3SS and T5SS). The most widespread systems are T1SS and T5SS; conversely, T2SS is rarely detected outside Proteobacteria (Abby et al., 2016).
In this review, we will cover the current knowledge regarding the recently discovered type IX secretion system (T9SS), also known as the Por secretion system (PorSS) or PerioGate. T9SS is exclusively present in the Bacteroidetes phylum, in a majority of its species (62% out of 97 genomes available; Sato et al., 2010; McBride and Zhu, 2013; Abby et al., 2016).
Discovery of T9SS
Uncovering and characterizing this unique secretion system was a gradual process over the last two decades and originated from studies of the Gram-negative, non-motile, anaerobic bacterium Porphyromonas gingivalis. P. gingivalis is a human oral pathogen that is a major causative agent of periodontitis, and, along with two other bacteria, Tannerella forsythia and Treponema denticola, forms the so-called red complex (Hajishengallis, 2015). Besides being a key pathogen in periodontitis, P. gingivalis is implicated in many systemic illnesses such as atherosclerosis (Kebschull et al., 2010), aspiration pneumonia (Benedyk et al., 2016), rheumatoid arthritis (RA; Laugisch et al., 2016), and even cancer (Whitmore and Lamont, 2014; Gao et al., 2016).
An important initial finding was that P. gingivalis produces potent proteolytic enzymes called gingipains (Kgp, RgpA, and RgpB; discussed in more detail later in this review; Pike et al., 1994; Pavloff et al., 1995; Curtis et al., 1999). Gingipains are essential virulence factors responsible for corrupting host innate defense mechanisms (Potempa et al., 2003; Hajishengallis, 2015). They are secreted in large amounts and are mainly attached to the surface of the OM, but are also partially released in a soluble form into the extracellular milieu (Pike et al., 1994; Rangarajan et al., 1997). Because none of the genes associated with known protein secretion systems could be found in the P. gingivalis genome, it was suspected that this bacterium had developed a unique OM translocon.
The search for this novel secretion system was greatly facilitated by the observation that colonies of P. gingivalis deficient in gingipain activity lack black pigmentation while growing on blood agar plates (Figure 1; Okamoto et al., 1998; Shi et al., 1999). Colony pigmentation results from the accumulation of heme on the surface of P. gingivalis cells, a process dependent on the proteolytic activity and hemagglutinin- and heme/hemoglobin-binding activity of gingipains (Smalley et al., 1998; Sroka et al., 2001). Spontaneous white/beige mutants were occasionally observed, and this phenotype was associated with, among other things, decreased cell surface-associated proteolytic activity (McKee et al., 1988; Shah et al., 1989). The discovery of the essential role of secreted, cell-bound gingipains in heme acquisition meant that pigmentation could be used as an easy screening tool for mutations blocking gingipain secretion. Of note, as potent virulence factors, gingipains were of particular interest for elucidating the role of P. gingivalis in the development of periodontitis.
Figure 1.
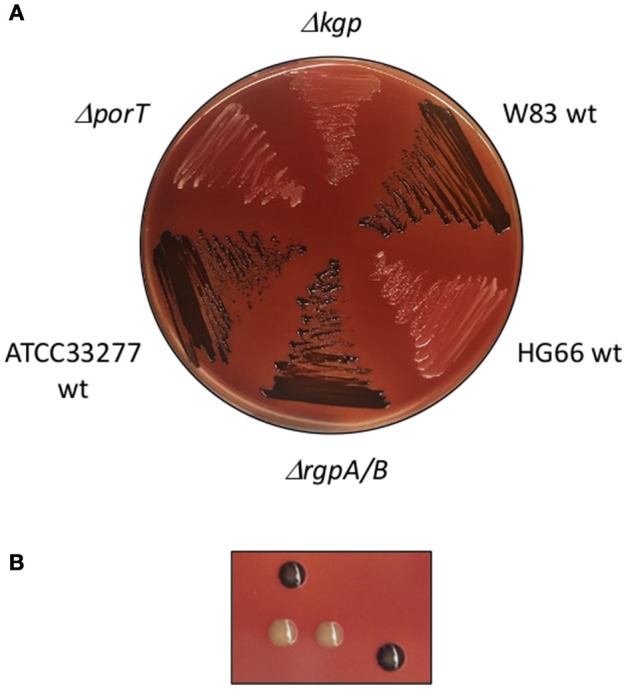
Pigmentation of various P. gingivalis W83 strains. (A) The wild-type P. gingivalis W83 and ATCC33277 strains grown anaerobically on blood agar plates present brown/black pigmentation due to heme accumulation. This phenotype is in a great part dependent on Kgp gingipain activity on the cell surface. P. gingivalis strains deficient in Kgp activity yield beige colonies which darken over the time. Arginine gingipains (RgpA/B) are not involved in this process and their deletion does not influence pigmentation. Strains impaired in T9SS e.g., ΔporT lack pigmentation which is never restored. Due to the absence of A-LPS in the P. gingivalis HG66 strain all gingipains and other T9SS cargo proteins are not associated with the cell membrane, but secreted into extracellular milieu resulting in white phenotype. (B) Single colonies of P. gingivalis strains grown for 7 days showing black or white pigmentation.
Several high-throughput transposon mutagenesis studies were performed, resulting in the characterization of various pigment-less clones. Early studies associated this phenotype with the impaired activity of trypsin-like proteases and diminished hemagglutination and heme acquisition by mutants (Hoover and Yoshimura, 1994; Genco et al., 1995). Later investigations found aberrations in polysaccharide synthesis and disruption of kgp (one of the gingipains; Simpson et al., 1999; Chen et al., 2000; Abaibou et al., 2001; Shoji et al., 2002). Finally, Sato et al. (2005) identified in their transposon study porT (PG0751/PGN_0778), the first gene encoding a protein involved in the secretion of gingipains. Their mutated, non-pigmented strain had impaired gingipain activity. Moreover, gingipains accumulated in the periplasm as enzymatically inactive proenzymes instead of being exported outside the cell. A database search (BLASTP) found that PorT is present only in some species of the Bacteroidetes phylum, such as Porphyromonas gingivalis, Cytophaga hutchinsonii, and Prevotella intermedia, and absent from many other phylum proteomes like Bacteroides thetaiotaomicron and Bacteroides fragilis (Sato et al., 2005). Two years later, another gene, sov (PG0809/PGN_0832), was implicated in the secretion of gingipains, showing a mutation phenotype identical to the one observed for the porT mutation (Saiki and Konishi, 2007).
Finally, the 2010 comparison of the porT-positive proteomes/genomes of C. hutchinsonii and P. gingivalis with the porT-negative species B. thetaiotaomicron resulted in a list of 55 genes (in addition to porT) potentially involved in the secretion mechanism. Subsequent isogenic mutagenesis of all selected genes resulted in the identification of 11 genes (including porT and sov) associated with gingipain transport across the OM and gingipain activation. Because these proteins do not have sequence similarity to components of any other known secretion system, it was assumed to be a novel secretion system and was originally called the Por secretion system (PorSS; Sato et al., 2010; Nakayama, 2015). To be consistent with the existing nomenclature of secretion systems in diderm bacteria, the system was later designated the type IX secretion system or T9SS.
New secretion system: A deadly weapon or a peaceful tool?
The comparative analysis of genomes carried out in a search for porT homologs revealed that T9SS is exclusively present in the Bacteroidetes phylum (Sato et al., 2005). Numerous studies on P. gingivalis show that T9SS is involved in virulence factor secretion, which damages human tissues and dysregulates immune responses (Potempa et al., 2003; Yoshimura et al., 2008; Sato et al., 2013; Bielecka et al., 2014; Taguchi et al., 2015). In addition, T. forsythia and Prevotella intermedia (another oral pathogenic bacteria) use this secretion pathway to disseminate their effector proteins (Nguyen et al., 2007; Veith et al., 2013; Narita et al., 2014; Tomek et al., 2014; Ksiazek et al., 2015b). Consequently, it is plausible that more pathogens from the Bacteroidetes phylum carrying porT homologs are utilizing this mechanism for virulence factor secretion. Although no experimental data are available to support this, it is likely that T9SS is a molecular weapon aimed at various host cells, similar to many other secretion systems (especially T3SS and T6SS).
Among Bacteroidetes' porT-positive species, there are many non-pathogenic environmental microorganisms such as C. hutchinsonii and F. johnsoniae. Both bacteria are aerobes ubiquitously distributed in soil and are capable of digesting macromolecules such as cellulose and chitin, respectively (Stanier, 1942, 1947). They are motile microorganisms that use a movement mechanism called gliding motility (Jarrell and McBride, 2008; Nakane et al., 2013). Surprisingly, the core T9SS genes are a subset of those necessary for gliding (gldK: ortholog of P. gingivalis porK, gldL/porL, gldM/porM, gldN/porN, sprA/sov, sprE/porW, and sprT/porT; Sato et al., 2010; McBride and Zhu, 2013; Shrivastava et al., 2013; McBride and Nakane, 2015). Moreover, secretion of chitinase and cellulase requires T9SS, meaning the system functions as a non-invasive tool used for movement and food acquisition in these bacteria (Kharade and McBride, 2014; Zhu and McBride, 2014; Yang et al., 2016).
The detailed mechanisms and regulation of T9SS in gliding motility and food scavenging are still under investigation and may reveal additional functions (even in non-gliding species).
Structural and functional components of P. gingivalis T9SS
Presently, 18 genes from a total of 29 candidates have been proven essential for proper T9SS function in P. gingivalis by deletion mutagenesis studies (Heath et al., 2016). Deletion of any of these genes results in the white pigmentation phenotype and accumulation of cargos (e.g., gingipains) in the periplasm. Some of these proteins build the core structures in the IM and OM, some play regulatory or accessory roles, and others are involved in post-translationally modifying cargo proteins (Table 1). Many aspects of their functions have yet to be discovered.
Table 1.
T9SS components.
| Locus Tag | Porphyromonas gingivalis W83 | |||||||
|---|---|---|---|---|---|---|---|---|
| W83 NC_002950.2 | ATCC33277 NC_010729.1 | Protein accession number | Protein description | Mol weight (kDa)a | Interactions bin vitrocin vivo | Homologs T. forsythia ATCC 43037–Tanf F. johnsoniae UW101-Fjoh | Referencesd | |
| CYTOPLASMIC AND INNER MEMBRANE COMPONENTS | ||||||||
| PG_RS04080 | PG0928 | PGN_1019 | WP_005875211.1 | PorX; chemotaxis protein CheY, cytoplasmic protein | 60.6 | PorYb, SigPb, PorLb | Tanf_12330 Fjoh_2906 |
Sato et al., 2010; Kadowaki et al., 2016; Vincent et al., 2016 |
| PG_RS00240 | PG0052 | PGN_2001 | WP_005873974.1 | PorY; sensor histidine kinase, inner membrane protein | 44.6 | PorXb | Tanf_13050 Fjoh_1592 |
|
| PG_RS01295 | PG0289 | PGN_1675 | WP_012458450.1 | PorL, inner membrane protein | 34.8 | PorMb,c | Tanf_02365 Fjoh_1854 (GldL) |
Sato et al., 2010; Gorasia et al., 2016; Kadowaki et al., 2016; Vincent et al., 2016, 2017 |
| PG_RS01300 | PG0290 | PGN_1674 | WP_005874203.1 | PorM, inner membrane protein | 56.4 | PorL/K/Nc | Tanf_02370 Fjoh_1855 (GldM) |
Sato et al., 2010; Gorasia et al., 2016; Kadowaki et al., 2016; Vincent et al., 2017 |
| PERIPLASMIC COMPONENTS | ||||||||
| PG_RS01305 | PG0291 | PGN_1673 | WP_005874243.1 | PorN | 41.3 | PorPb,PorK/L/Mc PG0189c | Tanf_02375 Fjoh_1856 (GldN) |
Sato et al., 2010; Gorasia et al., 2016; Kadowaki et al., 2016; Vincent et al., 2017 |
| PG_RS01290 | PG0288 | PGN_1676 | WP_043876477.1 | PorK; lipoprotein | 54.1 | PorNc, PorM/PbPG0189c | Tanf_02360 Fjoh_1853 (GldK) |
Sato et al., 2010; Gorasia et al., 2016; Kadowaki et al., 2016; Vincent et al., 2017 |
| PG_RS08590 | PG1947 | PGN_1877 | WP_005873869.1 | PorW; lipoprotein | 132.1 | n.d.e | Tanf_00060 Fjoh_1051 (SprE) |
Sato et al., 2010 |
| PG_RS04660 | PG1058 | PGN_1296 | WP_005873448.1 | Lipoprotein; TPRd, WD40d, CRDd, OmpA Family domain | 74.9 | n.d. | Tanf_02260 Fjoh_1647f |
Heath et al., 2016 |
| OUTER MEMBRANE AND SURFACE COMPONENTS | ||||||||
| PG_RS03550 | PG0809 | PGN_0832 | WP_012457811.1g | Sov; β-barrel protein | 281.1 | n.d. | Tanf_04410 Fjoh_1653 (SprA) |
Saiki and Konishi, 2007, 2010b; Sato et al., 2010; Kadowaki et al., 2016 |
| PG_RS02670 | PG0602 | PGN_0645 | WP_010956079.1 | PorQ; β-barrel protein | 37.9 | n.d. | Tanf_12465 Fjoh_2755 |
Sato et al., 2010 |
| PG_RS01285 | PG0287 | PGN_1677 | WP_005874180.1 | PorP; β-barrel protein | 35.0 | PorN/Kb | Tanf_02355 Fjoh_3477h |
Sato et al., 2010; Kadowaki et al., 2016; Vincent et al., 2017 |
| PG_RS03295 | PG0751 | PGN_0778 | WP_039417575.1 | PorT; β-barrel protein | 26.7 | n.d. | Tanf_10520 Fjoh_1466 (SprT) |
Sato et al., 2005, 2010; Nguyen et al., 2007; Kadowaki et al., 2016 |
| PG_RS00125 | PG0027 | PGN_0023 | WP_004583425.1 | PorV (LptO); β-barrel protein | 43.1 | PorUc | Tanf_04220 Fjoh_1555 |
Ishiguro et al., 2009; Sato et al., 2010; Chen et al., 2011; Glew et al., 2012; Saiki and Konishi, 2014 |
| PG_RS00870 | PG0189 | PGN_0297 | WP_005874727.1 | β-barrel protein | 25.6 | PorK/Nc | Tanf_09815 Fjoh_1692 |
Gorasia et al., 2016 |
| PG_RS02385 | PG0534 | PGN_1437 | WP_005875072.1 | TonB-dependent receptor; β-barrel protein | 92.3 | n.d. | Tanf_07980 Fjoh_0118 |
Saiki and Konishi, 2010a |
| PG_RS00885 | PG0192 | PGN_300 | WP_043876475.1 | Omp17; OmpH-like | 19.6 | n.d. | Tanf_09800 Fjoh_1689i |
Taguchi et al., 2015 |
| PG_RS00120 | PG0026 | PGN_0022 | WP_005874469.1 | PorU; surface C-terminal signal peptidase | 128.2 | PorV (LptO)c | Tanf_02580 Fjoh_1556 |
Sato et al., 2010; Glew et al., 2012; Saiki and Konishi, 2014; Gorasia et al., 2015 |
| PG_RS07070 | PG1604 | PGN_0509 | WP_010956350.1 | PorZ; surface B-propeller protein | 83.6 | n.d. | Tanf_12435 Fjoh_0707 |
Glew et al., 2014; Lasica et al., 2016 |
Calculated from amino acid sequence including a signal peptide.
In vitro experiments.
In vivo experiments.
References to original papers pertinent only to P. gingivalis T9SS. Proteomic papers are not cited in the table but they are referred in the text.
Not determined.
F. johnsoniae possesses 5 proteins homologous to PG1058, the one with the highest score is given in the table. All 5 F. johnsoniae proteins (Fjoh_1647, Fjoh_4540, Fjoh_3950, Fjoh_3973, Fjoh_3476) range between 26–29% identities with 90-98% coverage comparing to PG1058 (assessed by NCBI BLAST).
Accession number given in the table is for the Sov protein from P. gingivalis ATCC33277 due to miss-annotation in W83 genome as two separate ORFs (PG0809/PG0810; Saiki and Konishi, 2007).
F. johnsoniae possesses numerous homologous proteins to PG0287 (PorP), the one with the highest score is given in the table. Five proteins with the highest overall score (Fjoh_3477, Fjoh_3951, Fjoh_1646, Fjoh_4539, Fjoh_2274) range between 25–29% identities with 86–93% coverage and gaps 3–9% comparing to PG0287 (PorP). The most explored PorP-like protein of F. johnsoniae is Fjoh_0978 (SprF; Rhodes et al., 2011) however, it has lower scores comparing to the proteins mentioned above (22% identities, 83% coverage, 18% gaps) as assessed by NCBI BLAST.
The closest homolog in F. johnsoniae proteome is Fjoh_1689 is much larger protein than its equivalent in P. gingivalis (341 residues vs. 174; 32% identities, 95% PG0192 coverage assessed by NCBI BLAST).
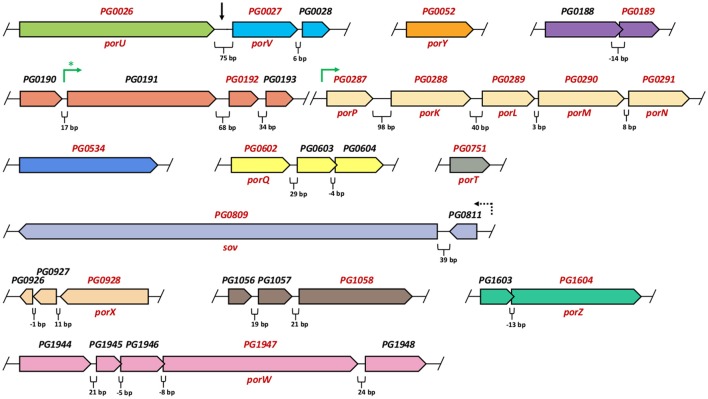
Genes encoding T9SS components are scattered around the P. gingivalis genome. The exception is a group of five genes, porP-porK-porL-porM-porN, that are co-transcribed (Vincent et al., 2016). In many other Bacteroidetes species, the operon structure of these genes is conserved [databases: STRING (Snel et al., 2000), DOOR (Dam et al., 2007; Mao et al., 2009), ProOpDB (Taboada et al., 2012), OperonDB (Pertea et al., 2009)]. Orthologs of the porP gene (sprP in some gliding motility bacteria) show the most variation, as the gene can be located in different genomic loci (e.g., F. johnsoniae Fjoh_3477 vs. gldK/Fjoh_1853), and, even if they precede porK, they remain as separate transcriptional units (e.g., C. hutchinsonii sprP/CHU_0170 and gldK/CHU_0171; Zhu and McBride, 2014). The rest of the P. gingivalis T9SS genes are either single units or predicted to be in 2–5 gene operons (Figure 2) with genes unrelated to T9SS structure and function. In addition, none of the adjacent genes encode T9SS cargo proteins.
Figure 2.
Arrangement of P. gingivalis W83 genes encoding T9SS components. Genes are grouped according to in silico operon predictions, reflecting direction of transcripts (Dam et al., 2007; Mao et al., 2009; Pertea et al., 2009; Taboada et al., 2012). Gaps in the genome are indicated by the slashes. Intervals between adjacent genes or overlapping regions (in base pairs-bp) are marked below each section. Each transcription unit is shown in different color. Genes encoding T9SS components are depicted in red font. Black vertical arrow shows continuous region (75 bp) between PG0026 (porU) and PG0027 (porV) but the two genes were predicted to transcribe independently. Green arrows indicate operons that were confirmed experimentally (Taguchi et al., 2015; Vincent et al., 2017). Green asterisk denotes proved single transcription unit for the PG0191-PG0192-PG0193 genes (in P. gingivalis ATCC33277 strain), however co-transcription of preceding the PG0190 gene (17 bp interval) was not investigated (Taguchi et al., 2015). The PG0809 (Sov) gene was re-sequenced and confirmed to consist of the two combined genes PG0809 and PG0810, mis-annotated in W83 genome as separate ORFs (Saiki and Konishi, 2007). A dashed arrow denotes indirect evidence that PG0809 (Sov) and PG0811 may be co-transcribed. It was shown that sigma factor SigP (regulator of other por genes) binds to the region preceding PG0811 but not the one before PG0809 (Kadowaki et al., 2016).
Cytoplasmic and IM components
Presently, there is only one known T9SS-related protein residing entirely in the cytoplasm: PorX (PG0928/PGN_1019). It is a response regulator (RR) of a two-component system (TCS) involved in regulating the expression of several T9SS genes. Its sensor kinase partner, PorY (PG0052/PGN_2001), is an IM-anchored protein containing two transmembrane (TM) helices and a large cytoplasmic domain (~222 aa; Sato et al., 2010; Vincent et al., 2016). Both proteins will be discussed in more detail in the Regulation Section.
Two other essential components of T9SS, PorL (PG0289 /PGN_1675) and PorM (PG0290/PGN_1674), are also anchored in the IM. PorL possesses two TM helices located between residues 17–48 and 48–74, with both N- and C-termini in the cytoplasm. The precise locations of the helices (the exact amino acids) have not been determined (Vincent et al., 2017). The large cytoplasmic C-terminal domain (~236 residues) interacts in vitro with PorX (Vincent et al., 2016); thus it may be involved in regulating T9SS function. Moreover, PorL cytoplasmic domain forms a homotrimer in E. coli cells and the full-length protein was found in a complex with PorM both in vitro (Gorasia et al., 2016; Vincent et al., 2017) and in vivo (Sato et al., 2010). PorM is anchored in the IM by a single TM helix at its N-terminus (between residues 9 and 41), with the remaining residues (475) forming a domain facing the periplasm. In E. coli cells, the periplasmic part of PorM dimerizes and interacts with two other core T9SS proteins: PorK and PorN (Vincent et al., 2017). The recombinant periplasmic domain (amino acid residues 36–516) was crystallized, presenting with tetragonal crystals, but automatic model building failed to provide a realistic structure, thus leaving the nature of interactions unknown (Stathopulos et al., 2015). Nevertheless, a possible function for PorL/PorM, apart from the regulatory implications for PorL, has been suggested.
It was proposed that the two proteins form an energy transducer complex to provide energy for T9SS assembly and substrate translocation. The idea came from F. johnsoniae, which utilizes a proton-motive force for gliding motility (Nakane et al., 2013; Gorasia et al., 2016). It was further noted that the hydrophobic TM helixes of GldL (PorL ortholog), PorL, and PorM possess conserved glutamate residues characteristic of known energy transducers (Shrivastava et al., 2013; Vincent et al., 2017). These assumptions need experimental verification; nevertheless, they are compatible with mechanisms used by other secretion systems to provide the energy needed to drive substrate transport such as hydrolysis of ATP, proton-motive force, low-energy assembly, and entropy gradient (Costa et al., 2015).
Periplasmic components
Four T9SS proteins are located in the periplasm: PorN (PG0291/PGN_1673), PorK (PG0288/PGN_1676), PorW (PG1947/PGN_1877), and PG1058/PGN_1296. All but one (PorN) are predicted or proven to be lipoproteins associated with membranes (Sato et al., 2010). PorW is the least investigated protein among the periplasmic elements of P. gingivalis secretion. Experimental work on PorW has only been performed on the F. johnsoniae PorW ortholog, SprE (Fjoh_1051), which is a predicted lipoprotein that localizes to a membrane fraction (most likely the OM). A mutant with a deleted sprE gene exhibits phenotypes in gliding bacteria typical of other T9SS function-deficient mutants, such as non-spreading colonies, defective gliding, and blocked secretion of chitinase (Rhodes et al., 2011; Kharade and McBride, 2015). Its subcellular localization and the effects of its mutation on the secretory/gliding phenotype suggest that SprE/PorW is yet another structural component of T9SS.
PG1058 is a multidomain protein necessary for T9SS function. The phenotype of P. gingivalis with an inactivated PG1058 gene is typical of other T9SS mutants: colonies on blood agar lack pigmentation and inactive, unprocessed gingipains accumulate in the periplasm. The PG1058 protein is anchored by its lipid modification to the periplasmic surface of the OM. The predicted structure suggests the presence of four structural domains: a tetratricopeptide repeat (TPR) domain, a β-propeller domain, a carboxypeptidase regulatory domain-like fold (CRD), and an OmpA_C-like putative peptidoglycan-binding domain. TPR and β-propeller domains are involved in protein-protein interactions; hence, together with the PG1058 mutant phenotype, it is plausible that PG1058 supports the T9SS translocon structure (Heath et al., 2016). Further, experiments are needed to verify this hypothesis.
PorN is a periplasmic protein that forms dimers in vitro and has the propensity to interact both in vitro and in vivo with IM protein PorM and periplasmic lipoprotein PorK (Gorasia et al., 2016; Vincent et al., 2017). The nature of the interaction with PorK is interesting, as both proteins form a ring-shaped structure with an external and internal diameter of 50 and 35 nm, respectively. It was proposed that they form a large complex in which PorN interacts in an almost 1:1 fashion (32–36 total subunits) with the PorK lipoprotein. The ring structure is anchored into the OM through the fatty acids of PorK. Consistent with detected interactions, PorN has a crucial role in stabilizing both PorL–PorM and PorN–PorK complexes, as deletion of the porN gene resulted in the degradation of PorL, PorM, and PorK in P. gingivalis cells. By contrast, deletion of either porL or porM does not interfere with the stability of the PorN/K complex (Gorasia et al., 2016).
Further, studies on PorK,L,M,N interactions suggest the existence of a PorK2L3M2N2 complex that likely oligomerizes to form a superstructure with a final molecular mass of over 1.2 MDa (Gorasia et al., 2016; Vincent et al., 2017). Such a large complex was originally reported by Sato and colleagues, who identified all four proteins in a single spot on a blue-native electrophoresis gel (Sato et al., 2010). However, additional elements of the complex were recently identified: PG0189 and PorP (PG0287/PGN_1677). Because they are predicted to be integral OM β-barrel proteins, they are discussed in more detail in the following section.
OM and surface components
The vast majority of T9SS components are confined to the OM. In addition to the peripheral OM-associated and periplasmic proteins delineated above, seven others (Sov, PorQ, PorP, PorT, PorV, PG0189, and PG0534) are predicted to be integral OM β-barrel proteins. Furthermore, two proteins, PorU and PorZ, are associated with the bacterial surface. In addition, PG0192 was found in a membrane fraction, but its association with the OM needs further verification.
PorT and Sov were the first proteins found to be essential for P. gingivalis protein secretion, and the discovery led to intense research on T9SS (see Discovery Section; Sato et al., 2005, 2010; Saiki and Konishi, 2007). Despite this, we still know very little about the structure and function of these proteins a decade later. PorT is predicted to have eight anti-parallel, membrane-traversing β-strands, with four large loops facing the environment, and this topology has been experimentally confirmed (Nguyen et al., 2009). Sov was also described as an integral OM protein with its C-terminal region likely exposed to the extracellular milieu (Saiki and Konishi, 2007, 2010b). However, the precise roles of both proteins in T9SS structure and function remain unknown. Even less information is available concerning PorQ (PG0602/PGN_0645) as a T9SS component (Sato et al., 2010). In the genome annotation, it is described as a hypothetical protein with a β-barrel structure belonging to the porin superfamily (Nelson et al., 2003); thus it is assumed to localize to the OM.
Similarly, little is known about PG0534/PGN_1437 as a protein essential for T9SS function (Saiki and Konishi, 2010a). Interestingly, PG0534 is upregulated in human gingival epithelial cells, suggesting its contribution to P. gingivalis eukaryotic cell invasion and/or intracellular survival (Park et al., 2004). In silico predictions run on the RaptorX server (Kallberg et al., 2012) modeled PG0534 as a β-barrel OM protein, with the pyochelin OM receptor FptA from Pseudomonas aeruginosa (Cobessi et al., 2005) as the best template (PDB: 1xkwA; p-value: 1.82e-23).
The next T9SS OM component, PG0192/PGN_300 (annotated as an OmpH-like protein), was found in the total membrane fraction. Due to its 17 kDa molecular mass, the protein is referred to as Omp17 (Taguchi et al., 2015). The best template prediction by the RaptorX server is a putative OM chaperone (OmpH-like) from Caulobacter crescentus (PDB: 4kqtA; p-value: 6.78e-04). The phenotypic effects of omp17 mutation are typical of other T9SS-defective mutants but with an interesting exception. The mutant is still able to secrete unprocessed T9SS cargo proteins, including pro-gingipains and CPG70, which accumulate in the periplasm in other secretion mutants (Taguchi et al., 2015). Of note, in the wild-type P. gingivalis, T9SS cargos remain attached to the bacterial surface through anionic lipopolysaccharide (A-LPS) anchoring (Shoji et al., 2002; Shoji and Nakayama, 2016). This modification is added by the surface-located PorU protein (Gorasia et al., 2015; for more details see the Mechanism Section). Taguchi and colleagues showed that A-LPS synthesis in the omp17 mutant was not affected, suggesting the impairment of PorU function. Consistent with that, PorU was not detected in the omp17− cell envelope fraction, but was found in the cytoplasm/periplasm fraction. Moreover, the omp17 mutant was less virulent than the wild type in the mouse subcutaneous model, which is consistent with the lack of gingipain activity (Taguchi et al., 2015).
As previously mentioned, PG0189 and PorP (a part of the porPKLMN operon) were detected in association with the PorKLMN complex. Specifically, a periplasmic loop of PG0189 interacts with both PorK and PorN, as shown by cross-linking experiments. Due to its low abundance, PG0189 is proposed to play an accessory role in secretion (Gorasia et al., 2016). The nature of the interaction of PorP with PorK and PorM is still enigmatic. The proteins co-precipitate in vitro; however, all tested proteins were produced in E. coli cells, and, so far, have not been detected in the native complex (Vincent et al., 2017).
Currently, the only OM β-barrel protein with an assigned function is PorV (PG0027/PGN_0023/LptO). The PorV-mutated strain retains inactive, unprocessed gingipains in the periplasm (Ishiguro et al., 2009) and fails to O-deacylate LPS, which might be a necessary step in post-translational processing during the secretion of cargo proteins (Chen et al., 2011; Glew et al., 2012). Yet another study indicated that PorV interacts in vivo with PorU (PG0026/PGN_0022), and it was proposed that PorV serves as an OM anchor for PorU (Saiki and Konishi, 2014). Indeed, PorU localizes to the surface of P. gingivalis cells and is involved in T9SS cargo processing (see the next section; Glew et al., 2012; Gorasia et al., 2015). Despite this relative abundance of knowledge on PorV, it remains unknown whether PorV is directly involved in LPS processing or if it is only an accessory protein for an unknown LPS O-deacylase. The secretion-deficient phenotype of the PorV mutant might be related to the lack of PorU immobilization on its surface.
The last known component of T9SS is a surface-located PorZ protein (PG1604/PGN_0509) recently characterized by our group (Lasica et al., 2016). The non-pigmented phenotype of the PorZ-mutant strain and its accumulation of unprocessed, inactive gingipains confirmed that PorZ is essential for the system. Interestingly, it was shown (through proteomics and mutagenesis studies) that PorZ is itself a cargo of T9SS and has the conserved C-terminal domain (CTD) (Glew et al., 2014; Lasica et al., 2016). The CTD works as a signal, directing T9SS cargo proteins to the OM translocon (see the next section; Shoji et al., 2011). However, unlike other cargos, the CTD of PorZ is not cleaved off upon secretion and the protein is not anchored in the OM in the same manner as other secreted proteins (Lasica et al., 2016). This phenomenon was observed for only one other protein, PorU, which is also both a functionally essential element and a cargo of T9SS (Glew et al., 2012). PorZ is currently the sole Por protein with a solved atomic structure. It is composed of two large β-propeller domains and a CTD, conforming to canonical β-sandwich architecture (de Diego et al., 2016; Lasica et al., 2016). Although the precise role of PorZ remains to be revealed, β-propeller domains are a good platform for protein-protein interactions and provide binding areas for small molecules (e.g., saccharides; Hunt et al., 1987; Zhang et al., 2014). Considering the structure and processing, we hypothesize that, like PorU, PorZ may be involved in post-translational maturation of T9SS cargo proteins during their translocation across the OM.
Mechanism of secretion
Protein secretion using T9SS is a two-step process. First, the cargo proteins are guided by a classical signal peptide to the Sec machinery in the IM. During translocation, the signal peptide is cleaved off by type I signal peptidase, and the cargo is released into the periplasm. Although, the Sec pathway has not been experimentally analyzed in P. gingivalis, the screening of Bacteroidetes genomes confirmed that the system is mostly conserved (McBride and Zhu, 2013). In the periplasm, transported proteins fold into a stable conformation, as indicated from the accumulation of their soluble forms in the periplasm of T9SS secretory mutants. Whether the cargo proteins require a chaperone(s) to assist in folding and/or guiding them to the OM translocon is still unknown.
A common feature of all T9SS cargo proteins is the conserved CTD that targets T9SS cargo proteins to the OM translocon. The function of the CTD was first recognized while studying the secretion and processing of the RgpB (PG0506/PGN_1466) gingipain. The protein without the C-terminal Ig-like domain of 72 amino acid residues was not secreted, but accumulated in the periplasm of the mutated P. gingivalis strain in its truncated form (Seers et al., 2006). A parallel study confirmed this observation, showing that the integrity of the CTD is essential for RgpB secretion, as even truncating the C-terminal by two residues hinders transport across the OM. The same effect is caused by mutating the highly conserved residues at the C-terminus of the CTD (Nguyen et al., 2007). The elegant follow-up investigations with CTDs from different P. gingivalis T9SS cargo proteins (HBP35/PG0616/PGN_0659, CPG70/PG0232/PGN_0335, P27/PG1795/no PGN, and RgpB) genetically fused to GFP found that GFP was secreted and post-translationally modified by P. gingivalis in the same way as the native T9SS cargos. The secretion/modification signal was narrowed down to the last 22 residues of the CTD domain (Shoji et al., 2011), and proteomic analysis revealed cleaved CTDs in the culture medium (Veith et al., 2013).
Taken together, these findings suggested the existence of a C-terminal-sorting peptidase responsible for the proteolytic removal of the CTD during the cargos' translocation across the OM. The postulated sortase was identified in P. gingivalis as PorU, a surface-located cysteine peptidase that shares significant sequence similarity with gingipains (see previous section; Glew et al., 2012). Analysis of the cleavage sites of T9SS cargos in P. gingivalis revealed a PorU preference toward polar or acidic amino acid residues (Ser, Thr, Asn, Asp) at the carbonyl site (P1′ position) and small amino acid residues (such as Gly, Ser, Ala) at the amide site (P1 position; Glew et al., 2012; Veith et al., 2013). This low specificity of PorU was confirmed when the amino acids surrounding the cleavage site (P1–P1′) in RgpB were mutated. Of note, this did not affect the secretion of the gingipain (Zhou et al., 2013).
Secretion signal for T9SS substrates is embedded in the secondary structure
Bioinformatic analysis of 21 fully sequenced genomes from the Bacteroidetes phylum revealed the presence of 663 predicted CTD-containing proteins (Veith et al., 2013). Alignment of the amino acid sequence of identified CTDs revealed up to five conserved sequential motifs (A–E) in different T9SS cargo proteins (Seers et al., 2006; Nguyen et al., 2007; Slakeski et al., 2011). Out of these, two sequential motifs, PxGxYVV and KxxxK, that reside in the last 22 amino acids of CTDs are the most conserved. This conservation is consistent with this fragment being sufficient for secretion in P. gingivalis (Shoji et al., 2011; Veith et al., 2013). Cumulatively, however, the limited sequence identity of CTDs suggests that the signal recognized by the T9SS machinery is not imprinted in the amino acid sequence but is formed by a specific fold of the CTD. This contention was confirmed by the atomic structure of the CTD from two P. gingivalis T9SS cargo proteins: RgpB and PorZ (de Diego et al., 2016; Lasica et al., 2016). Their CTDs consist of seven β-strands of similar length, generating a compact, sandwich-like fold typical of an immunoglobulin-superfamily (IgSF) domain. Analysis of the CTD of RgpB revealed a propensity of the protein to dimerize by swapping the last β-strand (de Diego et al., 2016). Of note, the last two β-strands overlap perfectly with the 22 amino acid residues essential for secretion of CTD proteins (Shoji et al., 2011). Despite the differences within the loops and the low amino acid sequence similarity, the PorZ-derived CTD structure is topologically equivalent to that of RgpB. This conclusion likely extends to the majority of identified CTDs, which share the fold of the IgSF domain. Therefore, the tertiary structure of the CTD, especially its two terminal β-strands, likely contains the signal recognized by the T9SS translocon (Lasica et al., 2016).
Secretion-associated modifications of T9SS cargo proteins
The characteristic feature of T9SS function is the retention of cargo proteins on the bacterial surface. SDS-PAGE analysis of OM-associated proteins produced diffuse bands about 20 kDa larger than that predicted from the primary structure of T9SS-secreted proteins (Veith et al., 2002). The difference is due to the presence of an A-LPS (Paramonov et al., 2005; Rangarajan et al., 2008) covalently attached to the cargo proteins imbedded into the OM, as indicated by western blot using specific antibodies (Abs). By contrast, the molecular mass of proteins accumulating in the periplasm of secretion mutants correlates well with the predicted molecular mass, and the proteins have no reactivity with anti-A-LPS Abs (Shoji et al., 2014). In addition, electron microscopy revealed that CTD-containing proteins (especially gingipains) form the electron-dense surface layer (EDSL) encapsulating P. gingivalis cells (Chen et al., 2011). Gorasia et al. (2015) found that the wbaP (PG1964/PGN_1896) mutant of P. gingivalis, which is defective in A-LPS synthesis, completely lacks the EDSL and releases T9SS cargos in soluble form into culture fluid. The proteins lack CTDs, suggesting normal PorU sortase activity, but are not A-LPS modified and therefore cannot be incorporated into the OM (Gorasia et al., 2015).
The mechanism of A-LPS attachment to CTD-containing proteins during secretion by T9SS is still unknown. The analysis of CTD proteins isolated from the growth media of the wbaP mutant revealed that peptides/amino acids derived from growth medium or glycine (if added in excess to the broth) were added to the proteins' C-termini via peptide bond. On the other hand, a 648 Da linker attached to C-termini by an isopeptide bond was identified in CTDs derived from the wild-type P. gingivalis strain (Gorasia et al., 2015). Such modification is reminiscent of a sortase-like mechanism of protein binding to peptidoglycan in Gram-positive bacteria. Sortases are cysteine proteases (C60 family) that have a catalytic Cys/His dyad, characteristic for many cysteine proteases, and possess a conserved Arg residue essential for sorting activity (Marraffini et al., 2004). This Arg is absent in gingipains, but is found in PorU sortase (Gorasia et al., 2015). All these findings suggest that PorU is a sortase, the first identified among Gram-negative bacteria. It cleaves the CTD and simultaneously attaches the A-LPS moiety to the newly generated C-terminus of a cargo protein via a linker of unknown structure. In this context, the T9SS mechanism resembles the covalent attachment of proteins to the cell wall in Gram-positive bacteria such as S. aureus (Schneewind and Missiakas, 2012).
Regulation
Essential T9SS genes, including porT, porV, sov, porP, porK, porL, porM, and porN, are regulated at the transcriptional level by a signaling pathway composed of the PorXY two-component system (TCS) and an extracytoplasmic function (ECF) sigma factor (SigP/PG0162/PGN_0274; Kadowaki et al., 2016). In contrast to the majority of TCSs, in which the components are encoded within the same operon, the porX and porY genes occur at separate loci within the P. gingivalis chromosome. Despite this unusual genomic organization, the activation of the PorXY TCS is canonical. PorY has a modular architecture typical for a histidine kinase (HK) and undergoes autophosphorylation at His193, as shown by radiolabeled [32P-γ]ATP. The phosphate group is then transferred to the conserved Asp58 residue in the receiver domain of PorX, which functions as the response regulator (RR). To compensate for the lack of a DNA-binding domain in the RR, PorX interacts with SigP, which directly binds the promotor regions of T9SS genes. The SigP protein level is very low in the porX-deletion mutant, suggesting a stabilizing function for PorX on SigP (Kadowaki et al., 2016). Disruption of the PorXY TCS results in the dysfunction of T9SS, which manifests as the decrease of Rgp and Kgp activity, as well as the impaired processing of gingipains (Sato et al., 2010).
PorX can also modulate the T9SS architecture directly by interacting with the cytoplasmic domain of PorL (Vincent et al., 2016). The N-terminal domain of PorX is similar to RRs belonging to the CheY family, which are involved in chemotaxis. After phosphorylation, the CheY protein binds to the C-ring of flagella, which changes the direction of flagellar movement (Roman et al., 1992; Sagi et al., 2003). Due to the fact that T9SS was proposed to be a rotary apparatus enabling the rotary movement of SprB adhesin in gliding bacteria (Shrivastava et al., 2015), it has been speculated that the PorX mechanism might be similar to that of CheY (Vincent et al., 2016). However, its role in P. gingivalis cells will likely be different as this bacterium is non-motile.
There are other studies reporting the changes in a T9SS protein's expression profile under specific circumstances. In a PorZ-deletion strain, some of the T9SS genes (including porT, porV, and porN), together with genes encoding CTD-cargo peptidases (RgpB, Kgp, and CPG70), are upregulated, whereas the expression of other T9SS genes (such as porQ, porW, sov, and porU) is not changed (Lasica et al., 2016). Additionally, the gliding motility protein GldN (orthologous of P. gingivalis PorN) of Flavobacterium psychrophilum is significantly upregulated under iron-limited growth conditions and in vivo (LaFrentz et al., 2009). The expression of T9SS proteins must be strictly regulated to fine-tune the energy-absorbing secretion of proteins into the environment. However, a precise environmental signal has not been identified and our knowledge about T9SS regulation is still limited.
Protein effectors in P. gingivalis
Only a few secretion systems are dedicated to carrying a single cargo protein; examples are HlyA in E. coli and HasA in S. marcescens for T1SS (Kanonenberg et al., 2013), and PulA in K. oxytoca and LT toxin in E. coli for T2SS (Rondelet and Condemine, 2013). The majority of secretion systems translocate many proteins of similar or diverse functions [e.g., T3SS; Gaytan et al., 2016]. In many respects, T9SS is one of the most robust secretion systems, which, in P. gingivalis alone, facilitates secretion of up to 35 cargos bearing the CTD (see Table 2), many of which are implicated in bacterial pathogenicity. In fact, experiments conducted to characterize the important virulence factors (the gingipains RgpA, RgpB, and Kgp) contributed to the discovery of T9SS. Below, we briefly describe only the most important cargos from the point of view of P. gingivalis virulence. References to other cargo proteins can be found in Table 2.
Table 2.
T9SS cargo proteins.
| Porphyromonas gingivalisa | |||||
|---|---|---|---|---|---|
| Locus Tag | |||||
| W83 NC_002950.2 | ATCC33277 NC_010729.1 | Protein accession number | Protein description | References | |
| PG_RS00120 | PG0026 | PGN_0022 | WP_005874469.1 | PorU; surface C-terminal sortase | Glew et al., 2012; Veith et al., 2013; Gorasia et al., 2015 |
| PG_RS00835 | PG0182 | PGN_0291 | WP_010955943.1 | Mfa5; VWA domain-containing protein [von Willebrand factor (vWF) type A domain] | Hasegawa et al., 2016 |
| PG_RS00840 | PG0183 | no PGN | WP_043876389.1 | Hypothetical protein containing VWA domain identical to that in PG0182 (circa 430 residues); lipoprotein | Found only by proteomic analysisa |
| PG_RS01060 | PG0232 | PGN_0335 | WP_005873522.1 | CPG70; zinc carboxypeptidase | Veith et al., 2004; Shoji et al., 2011; Zhou et al., 2013 |
| PG_RS01560 | PG0350 | PGN_1611 | WP_005873799.1 | Internalin; hypothetical protein; leucine-rich repeats (x8) | Found only by proteomic analysisa |
| PG_RS01820 | PG0410 | no PGN | WP_005873803.1 | Hypothetical gingipain-like peptidase C25 | |
| PG_RS01825 | PG0411 | PGN_1556 | WP_010956006.1 | T9SS C-terminal target domain-containing protein | Found only by proteomic analysisa |
| PG_RS02195 | PG0495 | PGN_1476 | WP_010956042.1 | T9SS C-terminal target domain-containing protein | Found only by proteomic analysisa |
| PG_RS02240 | PG0506 | PGN_1466 | WP_010956050.1 | RgpB; arginine specific gingipain B, cysteine protease | Pike et al., 1994; Seers et al., 2006; Guo et al., 2010; de Diego et al., 2016 |
| PG_RS02455 | PG0553 | PGN_1416 | WP_010956068.1 | PepK; lysine specific serine endopeptidase | Sato et al., 2013; Nonaka et al., 2014; Veith et al., 2014 |
| PG_RS02700 | PG0611 | PGN_0654 | WP_043876409.1 | Hypothetical protein | Found only by proteomic analysisa |
| PG_RS02710 | PG0614 | PGN_0657 | WP_005874506.1 | Hypothetical protein | Found only by proteomic analysisa |
| PG_RS02720 | PG0616 | PGN_0659 | WP_005874521.1 | HBP35 (hemin binding protein 35) | Shoji et al., 2010, 2011 |
| PG_RS02765 | PG0626 | no PGN | WP_005874512.1 | T9SS C-terminal target domain-containing protein | Found only by proteomic analysisa |
| PG_RS02890 | PG0654 | PGN_0693 | WP_005873571.1 | T9SS C-terminal target domain-containing protein | Found only by proteomic analysisa; Glew et al., 2012 |
| PG_RS03370 | PG0769 | PGN_0795 | WP_010956121.1 | Fibronectin; hypothetical proteinb | Found only by proteomic analysisa; Sato et al., 2013 |
| PG_RS03450 | PG0787 | PGN_0810 | WP_005873930.1 | T9SS C-terminal target domain-containing proteinc | Found only by proteomic analysisa |
| PG_RS04535 | PG1030 | PGN_1321 | WP_005874101.1 | T9SS C-terminal target domain-containing protein | Found only by proteomic analysisa |
| PG_RS05835 | PG1326 | PGN_1115 | WP_005875446.1 | Hemagglutinin | Found only by proteomic analysisa |
| PG_RS06055 | PG1374 | PGN_0852 | WP_005874331.1 | T9SS C-terminal target domain-containing protein, leucine-rich repeats (x7) | Found only by proteomic analysisa; Glew et al., 2012 |
| PG_RS06255 | PG1424 | PGN_0898 | WP_005873463.1 | PPAD; peptidylarginine deiminase | Sato et al., 2013; Koziel et al., 2014; Goulas et al., 2015 |
| PG_RS06260 | PG1427 | PGN_0900 | WP_005873781.1 | Periodontain; peptidase C10; PrtT-related | Nelson et al., 1999 |
| PG_RS06835 | PG1548 | PGN_0561 | WP_043876505.1 | PrtT; cystein protease (domain peptidase C10) | Madden et al., 1995; Gorasia et al., 2015 |
| PG_RS07070 | PG1604 | PGN_0509 | WP_010956350.1 | PorZ; surface B-propeller protein | Lasica et al., 2016 |
| PG_RS07920 | PG1795 | PGN_1770 | WP_005874140.1 | Hypothetical protein | Found only by proteomic analysisa |
| PG_RS07930 | PG1798 | PGN_1767 | WP_005874135.1 | T9SS C-terminal target domain-containing protein | Found only by proteomic analysisa |
| PG_RS08090 | PG1837 | PGN_1733 | WP_043876452.1 | HagA (hemagglutinin A, 8 HA domains) | Shi et al., 1999; Glew et al., 2012; Saiki and Konishi, 2014 |
| PG_RS08105 | PG1844 | PGN_1728 | WP_043876454.1 | Kgp; lysine specific gingipain, cysteine protease | Pike et al., 1994; Veith et al., 2002 |
| PG_RS08700 | PG1969d | no PGN | WP_010956456.1 | T9SS C-terminal target domain-containing protein | Found only by proteomic analysisa |
| PG_RS08940 | PG2024 | PGN_1970 | WP_010956476.1 | RgpA; arginine specific gingipain A; cysteine protease | Pike et al., 1994; Veith et al., 2002; Glew et al., 2012 |
| PG_RS09310 | PG2100 | no PGN | WP_005873768.1 | T9SS C-terminal target domain-containing protein; TapC | Kondo et al., 2010; Sato et al., 2013 |
| PG_RS09320 | PG2102 | PGN_0152 | WP_005873754.1 | T9SS C-terminal target domain-containing protein; TapA | Kondo et al., 2010; Glew et al., 2012; Sato et al., 2013 |
| PG_RS09640 | PG2172 | PGN_0123 | WP_005874973.1 | Hypothetical protein | Found only by proteomic analysisa; Glew et al., 2012 |
| PG_RS09755 | PG2198 | PGN_2065 | WP_005874281.1 | Hypothetical protein; peptidase | Found only by proteomic analysisa |
| PG_RS09850 | PG2216 | PGN_2080 | WP_010956525.1 | Hypothetical protein | Found only by proteomic analysisa; Glew et al., 2012 |
| Tannerella forsythia ATCC43037 | |||||
| Tanf_03370 | WP_046824918.1 | TfsA (surface layer protein A), classical CTD | Tomek et al., 2014 | ||
| Tanf_03375 | WP_046824919.1 | TfsB (surface layer protein B), classical CTD | Tomek et al., 2014 | ||
| Tanf_04820 | WP_046825062.1 | BspA, cell surface antigen, leucine rich protein, classical CTD | Veith et al., 2009; Friedrich et al., 2015 | ||
| Tanf_06225 | WP_046825275.1 | Forsilysin, metalloprotease, KLIKK-type CTD | Narita et al., 2014 | ||
| Tanf_00450 | WP_070098098.1 | Mirolysin, metalloprotease, KLIKK-type CTD | Karim et al., 2010; Ksiazek et al., 2015a,b; Koneru et al., 2017 | ||
| Tanf_06550 | D0EM77.2 | Karilysin, metalloprotease, KLIKK-type CTD | Karim et al., 2010; Narita et al., 2014; Ksiazek et al., 2015a; Koneru et al., 2017 | ||
| Tanf_00440 | AIZ49398.1 | Mirolase, serine protease, KLIKK-type CTD | Karim et al., 2010; Ksiazek et al., 2015a,b; Koneru et al., 2017 | ||
| Tanf_09450, Tanf_06530 (not merged in one contig) | AKG97061.1 | Miropsin-1, serine protease, KLIKK-type CTD | Ksiazek et al., 2015b | ||
| Tanf_06530 | WP_046825306.1 | Miropsin-2, serine protease KLIKK-type CTD | Narita et al., 2014 | ||
| F. johnsoniae UW101e | |||||
| Fjoh_4555 | WP_012026520.1 | ChiA, chitinase | Rhodes et al., 2010; Kharade and McBride, 2014 | ||
| Fjoh_0979 | WP_012023065.1 | SprB, surface adhesin, necessary for gliding motility | Rhodes et al., 2010; Shrivastava et al., 2013 | ||
| Fjoh_0808 | WP_052295174.1 | RemA, mobile surface adhesin, necessary for gliding motility | Shrivastava et al., 2012, 2013 | ||
All P. gingivalis cargo proteins excluding PG0410 (no PGN) and PG1548 (PGN_0561) were originally found by Veith et al. (2013).
PG0769 (PGN_0795) processing is unclear. Protein is devoid of N-terminal cleavage signal for periplasm transport (searched with SignalP and LipoP servers) as well as T9SS CTD domain.
PG0787 (PGN_0810) is a very small peptide (80 aa) devoid of N-terminal cleavage signal for periplasm transport (searched in SignalP and LipoP servers), however its last 66 aa constitute a classical T9SS CTD domain.
PG1969 processing is unclear. Protein is devoid of N-terminal cleavage signal for periplasm transport (searched with SignalP and LipoP servers) but contains T9SS CTD domain.
Proteins listed are the best studied among other identified T9SS cargos of F. johnsoniae. For more information please see Kharade and McBride (2015).
Gingipains and CPG70
There are three enzymes collectively termed gingipains: RgpA (PG2024/PGN_1970), RgpB (PG0506/PGN_1466), and Kgp (PG1844/PGN_1728). They are cysteine proteases that hydrolyze peptide bonds at the carboxyl group of arginine (RgpA/B: Arg–Xaa) or lysine residues (Kgp: Lys–Xaa; Pike et al., 1994). They are exported into the periplasm as inactive zymogens, with the N-terminal prodomain (NTP) functioning as a chaperone and maintaining the latency of the proteases (Mikolajczyk et al., 2003; Pomowski et al., 2017). After folding in the periplasm, they are transported to the bacterial surface, where they are subjected to extensive post-translational processing. The CTD is cleaved by PorU sortase during translocation, with the concomitant covalent attachment of A-LPS via an isopeptide bond to the newly formed carbonyl group (Glew et al., 2012; Gorasia et al., 2015). Then, the OM-anchored gingipains activate themselves by cleaving off the NTP. For RgpB, this is the end of processing, but the polypeptide chains of RgpA and Kgp are further fragmented to form a large, non-covalent complex of catalytic and hemagglutinin domains on the bacterial surface (Bhogal et al., 1997; Veith et al., 2002; Sztukowska et al., 2012). The activation and further processing are still not well-understood, and, in addition to trans- and cis-autoproteolysis, they also involve the removal of the C-terminal Arg and Lys residues by the Arg/Lys-specific carboxypeptidase CPG70 (PG0232/PGN_0335; Chen et al., 2002). Interestingly, CPG70 is a T9SS substrate itself (Veith et al., 2004; Zhou et al., 2013). Of note, the retention of gingipains, CPG70, and other T9SS cargos on the bacterial surface depends on the synthesis of A-LPS. The P. gingivalis strain HG66, which lacks the activity of an enzyme in the A-LPS synthesis pathway, secretes soluble gingipains into the media (Pike et al., 1994; Shoji et al., 2014; Siddiqui et al., 2014).
Gingipains are the most powerful weapon within the P. gingivalis arsenal of virulence factors, as they are responsible for nearly 85% of the total proteolytic activity (Potempa et al., 1997). They are responsible for a variety of pathogenic functions such as colonization, nutrition, neutralization of host defenses, and alteration of the inflammatory response, which all lead to massive oral tissue destruction called periodontitis during prolonged infection (reviewed in Guo et al., 2010; Bostanci and Belibasakis, 2012; Hajishengallis, 2015). However, gingipains are not only directed against host proteins, but are also involved in processing other P. gingivalis proteins [e.g., long fimbriae (FimA)] (Nakayama et al., 1996; Xu et al., 2016). Interestingly, gingipains' activities rely on their local concentration, resulting in either activation of some pathways at low concentrations (specifically human complement) or destroying them upon accumulation (Krauss et al., 2010). Moreover, despite the cleavage specificity to a single C-terminal Arg or Lys residue, they can act in a precise and fastidious manner or as unlimited shredders (Potempa et al., 2000; Sroka et al., 2001; Goulet et al., 2004).
Considering the broad range of activities combined with cell surface localization, it is not surprising that gingipains are a tempting target for designing periodontitis treatments as well as preventive strategies (inhibitors and vaccines; Olsen and Potempa, 2014; Inaba et al., 2016; Wilensky et al., 2016).
Porphyromonas peptidylarginine deiminase (PPAD)
Porphyromonas peptidylarginine deiminase (PPAD), encoded by PG1424/PGN_0898, is a unique enzyme among prokaryotes. It is the first and only bacterial peptidylarginine deiminase (PAD) identified, and, moreover, its presence is limited to a single species: P. gingivalis (McGraw et al., 1999; Gabarrini et al., 2015).
PADs are well-described eukaryotic enzymes functioning in vertebrates as post-translational modifiers of proteins. Specifically, they citrullinate internal arginine residues, which changes the fold, function, and half-life of proteins and peptides (Vossenaar et al., 2003; Gyorgy et al., 2006). Dysregulation of this process, particularly the accumulation of citrullinated proteins, leads to inflammatory disorders and has been associated with numerous diseases such as Alzheimer's disease, multiple sclerosis, psoriasis, fibrosis, cancer, and rheumatoid arthritis (RA) (Vossenaar et al., 2003; Chang and Han, 2006; Baka et al., 2012; Gudmann et al., 2015). The latter develops through an autoimmune response against citrullinated proteins and is enhanced by a combination of environmental and genetic factors (MacGregor et al., 2000; McInnes and Schett, 2011). Currently, periodontal disease is an acknowledged RA risk factor, and the discovery of PPAD uncovered a missing mechanistic link between the two illnesses (Wegner et al., 2010; Koziel et al., 2014; Quirke et al., 2015; Laugisch et al., 2016).
PPAD was identified as a T9SS substrate through proteomics studies of a porT mutant (Sato et al., 2013); however, the enzyme was characterized mostly in relation to its function rather than secretion. It citrullinates C-terminal arginine residues in a calcium-independent manner, whereas eukaryotic PADs are Ca2+-dependent (Takahara et al., 1986; McGraw et al., 1999; Abdallah et al., 2007; Wegner et al., 2010; Bielecka et al., 2014). Moreover, the C-terminal specificity of PPAD plays into the cleavage activities of RgpA/B (after Arg), which greatly enlarge the pool of citrullinated substrates from both bacterial and host origins as gingipains cleave numerous human proteins (Guo et al., 2010). Gingipain-null mutants (RgpA/B) are almost devoid of endogenous citrullination (Wegner et al., 2010). Furthermore, even the presence of PPAD (not only its activity) may elevate anti-citrullination immune responses, as it undergoes autocitrullination. Only this form triggers specific Abs in mice and was recognized by RA patients' sera (reviewed in Koziel et al., 2014).
Analysis of PPAD structure revealed that the enzyme is composed of four elements: a profragment, a catalytic domain (CD), an IgSF domain, and a CTD, resembling domains observed in gingipains. The CD has a flat 5-fold α/β-propeller architecture and includes a catalytic triad (C351-H236-N297) also conserved in human PADs (Goulas et al., 2015; Montgomery et al., 2016). The crystal structure of substrate-free and substrate-bound forms confirmed that PPAD is efficient in accommodating and processing C-terminally situated Arg residues regardless of total chain length (peptide or protein; Goulas et al., 2015).
The surface location of PPAD and the availability of its detailed structure, combined with its important role in two prevalent human diseases (periodontitis and RA), should make PPAD a good target for therapeutic strategies; however, no such experiments have been reported.
T9SS in T. forsythia
The mechanism of protein secretion by T9SS was mostly studied in P. gingivalis and gliding bacteria. Apart from a different subset of secreted proteins reflecting bacterial habitats, the mechanism of action is the same. Briefly, T9SS cargo proteins are directed to the T9SS machinery by the CTD, which is removed during secretion. Then, secreted proteins may be modified and attached to the surface by A-LPS (P. gingivalis), stay associated with the cell through polysaccharides, or be released (gliding bacteria; McBride and Nakane, 2015; Nakayama, 2015). However, analysis of T9SS in another member of the red complex, T. forsythia, revealed some interesting differences.
T. forsythia is covered with a two-dimensional crystalline surface (S-) layer that is thought to function as a protective coat, working as an external sieve and ion trap (Sleytr and Beveridge, 1999; Messner et al., 2010). It also mediates adhesion and subsequent invasion into human gingival epithelial cells (Sakakibara et al., 2007) and delays recognition of the bacterium by the host innate immune system (Sekot et al., 2012). The S-layer is composed of the glycosylated proteins TfsA (Tanf_03370) and TfsB (Tanf_03375). Deleting porU (Tanf_02580), porT (Tanf_10520), sov (Tanf_04410), or porK (Tanf_02360) results in the lack of an S-layer, which can be observed by transmission electron microscope (Narita et al., 2014; Tomek et al., 2014). In those mutants, both components of the S-layer are trapped within the periplasm, but, unlike in P. gingivalis, they are modified by O-glycosylation through the addition of multiple copies of a complex oligosaccharide using a general glycosylation pathway operating in Bacteroidetes (Coyne et al., 2013; Posch et al., 2013; Tomek et al., 2014). Nevertheless, TfsA and TfsB trapped in the periplasm are much smaller than both proteins in the wild-type cells, indicating that, upon secretion, both proteins are modified by a second glycan attachment in a manner different than O-glycosylation. It is speculated that, as in P. gingivalis, it could be a variant of LPS (Tomek et al., 2014).
T9SS cargo proteins in T. forsythia have two different types of CTD. The “classical” CTD associated with proteins from other Bacteroidetes species is found in TfsA, TfsB, and leucine rich protein BspA (Veith et al., 2009; Tomek et al., 2014). By contrast, a family of six proteases, three metalloproteases (karilysin, mirolysin, and forsilysin) and three serine proteases (mirolase, miropsin-1, and miropsin-2), bear a nearly identical CTD that shares very limited sequence similarity with the classical CTD. Because these six CTDs end with a KLIKK sequential motif, the enzymes are referred to as KLIKK proteases (Ksiazek et al., 2015b). The KLIKK proteases possess a unique structure and undergo extensive autoproteolytic processing (Cerda-Costa et al., 2011; Lopez-Pelegrin et al., 2015). Their activities, such as degrading complement proteins and LL-37 (the crucial antimicrobial peptide in the human oral cavity), may contribute to T. forsythia virulence through evading innate immunity (Jusko et al., 2015; Koneru et al., 2017).
In stark contrast to the other CTD proteins of T. forsythia, KLIKK proteases seem to be secreted directly into the extracellular medium, as shown for miropsin-2 (Tanf_06530), karilysin (Tanf_06550), and forsilysin (Tanf_06225) (Narita et al., 2014). Supporting this, proteomic analysis of the T. forsythia OM identified 13 of 26 proteins bearing the classical CTD, including TfsA, TfsB, and BspA (Tanf_04820), but none of the KLIKK proteases (Veith et al., 2009). Conversely, four KLIKK proteases, forsilysin, miropsin-2 (Friedrich et al., 2015), mirolase (Tanf_00440), and karilysin (Veith et al., 2015), were found in outer membrane vesicles (OMVs), although with a low Mascot score. This discrepancy could be explained by the transient presence of these proteases in the periplasm before they enter the OM translocon of T9SS. Interestingly, all three of the KLIKK proteases characterized thus far (karilysin, mirolase, and mirolysin) can remove the CTD during autoprocessing (Karim et al., 2010; Ksiazek et al., 2015a; Koneru et al., 2017). Collectively, the available data suggest that the KLIKK proteases are secreted into the extracellular milieu without removal of the CTD. This finding is similar to the secretion of PorU and PorZ from P. gingivalis, where the CTD is also not removed during secretion, although proteins stay associated with the cell surface (Lasica et al., 2016).
Concluding remarks
In this review, we summarized the biochemical and structural data concerning the recently discovered T9SS identified in a majority of the bacterial species belonging to the Bacteroidetes phylum (Sato et al., 2010; McBride and Zhu, 2013). The system has been investigated predominantly in human oral pathogens, such as P. gingivalis and T. forsythia, and environmental saprophytes, such as F. johnsoniae and C. hutchinsonii. It seems to be a major mechanism of protein secretion in these bacteria however, some families from Bacteroidetes were reported to possess other secretion systems e.g., T1SS or T6SS (Russell et al., 2014; Wilson et al., 2015; Abby et al., 2016; Chatzidaki-Livanis et al., 2016; Wexler et al., 2016; Ibrahim et al., 2017). Notably, both systems allow for direct substrate translocation from bacterial cytoplasm to the cell exterior, while T9SS cargos do not omit the periplasmic space during their secretion.
The role of T9SS is to ensure cell survival and fitness in response to the microorganisms' habitat by providing transportation of proteins necessary for, among other things, virulence, nutrition, and movement (gliding motility). Hence, the variety of secreted proteins even within a single species is large and comprises numerous adhesins and hydrolytic enzymes used for attachment and degradation of large organic compounds such as proteins, cellulose, and chitin (Guo et al., 2010; McBride and Nakane, 2015).
The cargo proteins of this system (Table 2) are equipped with the classical signal peptide for Sec-dependent translocation to the IM and the conserved CTD that directs them further to the secretion machinery in the OM. The recognition signal is mostly embedded within the IgSF-like tertiary structure of the CTD (de Diego et al., 2016; Lasica et al., 2016) and likely located within the 22 amino acid residues composing the sequential motifs of PxGxYVV and KxxxK in the two most C-terminal β-strands (Shoji et al., 2011; Veith et al., 2013).
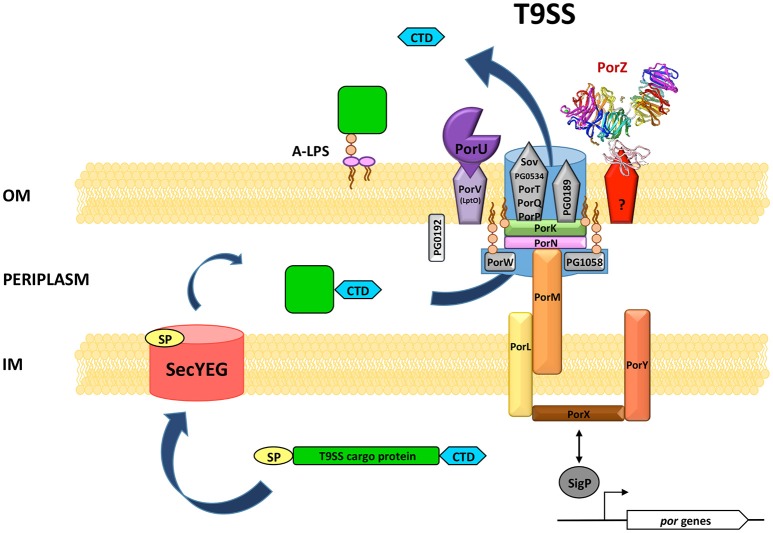
Currently, for P. gingivalis cells, there are 16 proteins recognized as the structural and/or functional components of the translocon and two additional elements involved in T9SS regulation (Table 1). None of these proteins are fully characterized, so their structure, mode of reciprocal interactions, and precise roles in secretion are still obscure. Nevertheless, a contemporary general concept of T9SS structure and function based on available data is presented in Figure 3. Verification of this model requires extensive structural and functional investigations to elucidate the mechanism of CTD recognition and cleavage, passage of cargos though the OM translocon, attachment of a glucan moiety, and anchoring of cargos onto the cell surface, their release into the environment, or their assembly into gliding motility machinery.
Figure 3.
Hypothetical model of the structure and function of P. gingivalis T9SS. The overall translocon structure and the protein(s) forming a pore in the OM (outer membrane) have not yet been characterized. Therefore, it is shown as a background blue shape accommodating known components. Interacting proteins are situated in close proximity. OM β-barrel proteins are depicted as pentagons. PorZ is presently the only T9SS protein with the known atomic structure. The mode of its association with the translocon is not yet defined. PorK, PorW, and PG1058 are lipoproteins anchored into the inner surface of the OM. PG0192 protein precise localization and possible interactions are not known. A T9SS cargo protein is equipped with two sorting signals: N-terminal signal peptide (SP) directing the protein to the general secretion system SecYEG and conserved C-terminal domain (CTD) recognized by T9SS. After translocation through the IM (inner membrane) most proteins acquire their proper fold in the periplasm. Next, CTD directs the protein for further translocation across the OM through T9SS. Finally, CTD is cleaved off by PorU sortase and a secreted protein is modified by attachment of A-LPS resulting in the anchorage of cargo protein to the cell surface. Two component system PorX/PorY and sigma factor SigP have regulatory effect on por genes. Although, they are not physical elements of T9SS, PorX was shown in vitro to interact with PorL.
Author contributions
AL analyzed literature, wrote the paper (excluding MK and MM sections), prepared the figures and tables. MK wrote the Mechanism of secretion and T9SS T. forsythia sections. MM wrote the Regulation section. JP edited the manuscript. All authors read and approved the full manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by National Science Centre (NCN)—2012/04/A/NZ1/00051 to JP and UMO-2015/19/N/NZ1/00322 to MM; Polish Ministry of Science and Higher Education—1306/MOB/IV/2015/0 (Mobilnosc Plus) to MK and K/DSC/003690 to MM and National Institutes of Health (NIH)—DE 022597 to AL and JP.
References
- Abaibou H., Chen Z., Olango G. J., Liu Y., Edwards J., Fletcher H. M. (2001). vimA gene downstream of recA is involved in virulence modulation in Porphyromonas gingivalis W83. Infect. Immun. 69, 325–335. 10.1128/IAI.69.1.325-335.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abby S. S., Cury J., Guglielmini J., Neron B., Touchon M., Rocha E. P. (2016). Identification of protein secretion systems in bacterial genomes. Sci. Rep. 6:23080. 10.1038/srep23080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah A. M., Gey van Pittius N. C., Champion P. A., Cox J., Luirink J., Vandenbroucke-Grauls C. M., et al. (2007). Type VII secretion–mycobacteria show the way. Nat. Rev. Microbiol. 5, 883–891. 10.1038/nrmicro1773 [DOI] [PubMed] [Google Scholar]
- Baka Z., Gyorgy B., Geher P., Buzas E. I., Falus A., Nagy G. (2012). Citrullination under physiological and pathological conditions. Joint Bone Spine 79, 431–436. 10.1016/j.jbspin.2012.01.008 [DOI] [PubMed] [Google Scholar]
- Benedyk M., Mydel P. M., Delaleu N., Plaza K., Gawron K., Milewska A., et al. (2016). Gingipains: critical factors in the development of aspiration pneumonia caused by Porphyromonas gingivalis. J. Innate Immun. 8, 185–198. 10.1159/000441724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berks B. C. (2015). The twin-arginine protein translocation pathway. Annu. Rev. Biochem. 84, 843–864. 10.1146/annurev-biochem-060614-034251 [DOI] [PubMed] [Google Scholar]
- Bhogal P. S., Slakeski N., Reynolds E. C. (1997). A cell-associated protein complex of Porphyromonas gingivalis W50 composed of Arg- and Lys-specific cysteine proteinases and adhesins. Microbiology 143(Pt 7), 2485–2495. 10.1099/00221287-143-7-2485 [DOI] [PubMed] [Google Scholar]
- Bielecka E., Scavenius C., Kantyka T., Jusko M., Mizgalska D., Szmigielski B., et al. (2014). Peptidyl arginine deiminase from Porphyromonas gingivalis abolishes anaphylatoxin C5a activity. J. Biol. Chem. 289, 32481–32487. 10.1074/jbc.C114.617142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostanci N., Belibasakis G. N. (2012). Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen. FEMS Microbiol. Lett. 333, 1–9. 10.1111/j.1574-6968.2012.02579.x [DOI] [PubMed] [Google Scholar]
- Cerda-Costa N., Guevara T., Karim A. Y., Ksiazek M., Nguyen K. A., Arolas J. L., et al. (2011). The structure of the catalytic domain of Tannerella forsythia karilysin reveals it is a bacterial xenologue of animal matrix metalloproteinases. Mol. Microbiol. 79, 119–132. 10.1111/j.1365-2958.2010.07434.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X., Han J. (2006). Expression of peptidylarginine deiminase type 4 (PAD4) in various tumors. Mol. Carcinog. 45, 183–196. 10.1002/mc.20169 [DOI] [PubMed] [Google Scholar]
- Chatzidaki-Livanis M., Geva-Zatorsky N., Comstock L. E. (2016). Bacteroides fragilis type VI secretion systems use novel effector and immunity proteins to antagonize human gut Bacteroidales species. Proc. Natl. Acad. Sci. U.S.A. 113, 3627–3632. 10.1073/pnas.1522510113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Dong H., Yong R., Duncan M. J. (2000). Pleiotropic pigmentation mutants of Porphyromonas gingivalis. Microb. Pathog. 28, 235–247. 10.1006/mpat.1999.0338 [DOI] [PubMed] [Google Scholar]
- Chen Y. Y., Cross K. J., Paolini R. A., Fielding J. E., Slakeski N., Reynolds E. C. (2002). CPG70 is a novel basic metallocarboxypeptidase with C-terminal polycystic kidney disease domains from Porphyromonas gingivalis. J. Biol. Chem. 277, 23433–23440. 10.1074/jbc.M200811200 [DOI] [PubMed] [Google Scholar]
- Chen Y. Y., Peng B., Yang Q., Glew M. D., Veith P. D., Cross K. J., et al. (2011). The outer membrane protein LptO is essential for the O-deacylation of LPS and the co-ordinated secretion and attachment of A-LPS and CTD proteins in Porphyromonas gingivalis. Mol. Microbiol. 79, 1380–1401. 10.1111/j.1365-2958.2010.07530.x [DOI] [PubMed] [Google Scholar]
- Cobessi D., Celia H., Pattus F. (2005). Crystal structure at high resolution of ferric-pyochelin and its membrane receptor FptA from Pseudomonas aeruginosa. J. Mol. Biol. 352, 893–904. 10.1016/j.jmb.2005.08.004 [DOI] [PubMed] [Google Scholar]
- Costa T. R., Felisberto-Rodrigues C., Meir A., Prevost M. S., Redzej A., Trokter M., et al. (2015). Secretion systems in Gram-negative bacteria: structural and mechanistic insights. Nat. Rev. Microbiol. 13, 343–359. 10.1038/nrmicro3456 [DOI] [PubMed] [Google Scholar]
- Coyne M. J., Fletcher C. M., Chatzidaki-Livanis M., Posch G., Schaffer C., Comstock L. E. (2013). Phylum-wide general protein O-glycosylation system of the Bacteroidetes. Mol. Microbiol. 88, 772–783. 10.1111/mmi.12220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis M. A., Kuramitsu H. K., Lantz M., Macrina F. L., Nakayama K., Potempa J., et al. (1999). Molecular genetics and nomenclature of proteases of Porphyromonas gingivalis. J. Periodont. Res. 34, 464–472. 10.1111/j.1600-0765.1999.tb02282.x [DOI] [PubMed] [Google Scholar]
- Dam P., Olman V., Harris K., Su Z., Xu Y. (2007). Operon prediction using both genome-specific and general genomic information. Nucleic Acids Res. 35, 288–298. 10.1093/nar/gkl1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Diego I., Ksiazek M., Mizgalska D., Koneru L., Golik P., Szmigielski B., et al. (2016). The outer-membrane export signal of Porphyromonas gingivalis type IX secretion system (T9SS) is a conserved C-terminal β-sandwich domain. Sci. Rep. 6:23123. 10.1038/srep23123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denks K., Vogt A., Sachelaru I., Petriman N. A., Kudva R., Koch H. G. (2014). The Sec translocon mediated protein transport in prokaryotes and eukaryotes. Mol. Membr. Biol. 31, 58–84. 10.3109/09687688.2014.907455 [DOI] [PubMed] [Google Scholar]
- Desvaux M., Hebraud M., Talon R., Henderson I. R. (2009). Secretion and subcellular localizations of bacterial proteins: a semantic awareness issue. Trends Microbiol. 17, 139–145. 10.1016/j.tim.2009.01.004 [DOI] [PubMed] [Google Scholar]
- Friedrich V., Gruber C., Nimeth I., Pabinger S., Sekot G., Posch G., et al. (2015). Outer membrane vesicles of Tannerella forsythia: biogenesis, composition, and virulence. Mol. Oral Microbiol. 30, 451–473. 10.1111/omi.12104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabarrini G., de Smit M., Westra J., Brouwer E., Vissink A., Zhou K., et al. (2015). The peptidylarginine deiminase gene is a conserved feature of Porphyromonas gingivalis. Sci. Rep. 5:13936. 10.1038/srep13936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S., Li S., Ma Z., Liang S., Shan T., Zhang M., et al. (2016). Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect. Agents Cancer 11, 3. 10.1186/s13027-016-0049-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaytan M. O., Martinez-Santos V. I., Soto E., Gonzalez-Pedrajo B. (2016). Type three secretion system in attaching and effacing pathogens. Front. Cell. Infect. Microbiol. 6:129. 10.3389/fcimb.2016.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genco C. A., Simpson W., Forng R. Y., Egal M., Odusanya B. M. (1995). Characterization of a Tn4351-generated hemin uptake mutant of Porphyromonas gingivalis: evidence for the coordinate regulation of virulence factors by hemin. Infect. Immun. 63, 2459–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach R. G., Hensel M. (2007). Protein secretion systems and adhesins: the molecular armory of Gram-negative pathogens. Int. J. Med. Microbiol. 297, 401–415. 10.1016/j.ijmm.2007.03.017 [DOI] [PubMed] [Google Scholar]
- Glew M. D., Veith P. D., Chen D., Seers C. A., Chen Y. Y., Reynolds E. C. (2014). Blue native-PAGE analysis of membrane protein complexes in Porphyromonas gingivalis. J. Proteomics 110, 72–92. 10.1016/j.jprot.2014.07.033 [DOI] [PubMed] [Google Scholar]
- Glew M. D., Veith P. D., Peng B., Chen Y. Y., Gorasia D. G., Yang Q., et al. (2012). PG0026 is the C-terminal signal peptidase of a novel secretion system of Porphyromonas gingivalis. J. Biol. Chem. 287, 24605–24617. 10.1074/jbc.M112.369223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel W., Hedgpeth J. (1982). Cloning and functional characterization of the plasmid-encoded hemolysin determinant of Escherichia coli. J. Bacteriol. 151, 1290–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorasia D. G., Veith P. D., Chen D., Seers C. A., Mitchell H. A., Chen Y. Y., et al. (2015). Porphyromonas gingivalis Type IX Secretion substrates are cleaved and modified by a sortase-like mechanism. PLoS Pathog. 11:e1005152. 10.1371/journal.ppat.1005152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorasia D. G., Veith P. D., Hanssen E. G., Glew M. D., Sato K., Yukitake H., et al. (2016). Structural insights into the PorK and PorN components of the Porphyromonas gingivalis Type IX secretion system. PLoS Pathog. 12:e1005820. 10.1371/journal.ppat.1005820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulas T., Mizgalska D., Garcia-Ferrer I., Kantyka T., Guevara T., Szmigielski B., et al. (2015). Structure and mechanism of a bacterial host-protein citrullinating virulence factor, Porphyromonas gingivalis peptidylarginine deiminase. Sci. Rep. 5:11969. 10.1038/srep11969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulet V., Britigan B., Nakayama K., Grenier D. (2004). Cleavage of human transferrin by Porphyromonas gingivalis gingipains promotes growth and formation of hydroxyl radicals. Infect. Immun. 72, 4351–4356. 10.1128/IAI.72.8.4351-4356.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal P., Krasteva P. V., Van Gerven N., Gubellini F., Van den Broeck I., Troupiotis-Tsailaki A., et al. (2014). Structural and mechanistic insights into the bacterial amyloid secretion channel CsgG. Nature 516, 250–253. 10.1038/nature13768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmann N. S., Hansen N. U., Jensen A. C., Karsdal M. A., Siebuhr A. S. (2015). Biological relevance of citrullinations: diagnostic, prognostic and therapeutic options. Autoimmunity 48, 73–79. 10.3109/08916934.2014.962024 [DOI] [PubMed] [Google Scholar]
- Guo Y., Nguyen K. A., Potempa J. (2010). Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon's knife to a meat chopper-like brutal degradation of proteins. Periodontol. 2000 54, 15–44. 10.1111/j.1600-0757.2010.00377.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorgy B., Toth E., Tarcsa E., Falus A., Buzas E. I. (2006). Citrullination: a posttranslational modification in health and disease. Int. J. Biochem. Cell Biol. 38, 1662–1677. 10.1016/j.biocel.2006.03.008 [DOI] [PubMed] [Google Scholar]
- Hachani A., Wood T. E., Filloux A. (2016). Type VI secretion and anti-host effectors. Curr. Opin. Microbiol. 29, 81–93. 10.1016/j.mib.2015.11.006 [DOI] [PubMed] [Google Scholar]
- Hajishengallis G. (2015). Periodontitis: from microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 15, 30–44. 10.1038/nri3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y., Iijima Y., Persson K., Nagano K., Yoshida Y., Lamont R. J., et al. (2016). Role of Mfa5 in expression of Mfa1 fimbriae in Porphyromonas gingivalis. J. Dent. Res. 95, 1291–1297. 10.1177/0022034516655083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath J. E., Seers C. A., Veith P. D., Butler C. A., Nor Muhammad N. A., Chen Y. Y., et al. (2016). PG1058 is a novel multidomain protein component of the bacterial Type IX secretion system. PLoS ONE 11:e0164313. 10.1371/journal.pone.0164313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke J. M., Bassler B. L. (2004). Quorum sensing regulates type III secretion in Vibrio harveyi and Vibrio parahaemolyticus. J. Bacteriol. 186, 3794–3805. 10.1128/JB.186.12.3794-3805.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover C. I., Yoshimura F. (1994). Transposon-induced pigment-deficient mutants of Porphyromonas gingivalis. FEMS Microbiol. Lett. 124, 43–48. 10.1111/j.1574-6968.1994.tb07259.x [DOI] [PubMed] [Google Scholar]
- Hunt L. T., Barker W. C., Chen H. R. (1987). A domain structure common to hemopexin, vitronectin, interstitial collagenase, and a collagenase homolog. Protein Seq. Data Anal. 1, 21–26. [PubMed] [Google Scholar]
- Hutcheon G. W., Bolhuis A. (2003). The archaeal twin-arginine translocation pathway. Biochem. Soc. Trans. 31(Pt 3), 686–689. 10.1042/bst0310686 [DOI] [PubMed] [Google Scholar]
- Ibrahim M., Subramanian A., Anishetty S. (2017). Comparative pan genome analysis of oral Prevotella species implicated in periodontitis. Funct. Integr. Genomics. [Epub ahead of print] 10.1007/s10142-017-0550-3 Available online at: http://link.springer.com/journal/10142/onlineFirst/page/1 [DOI] [PubMed]
- Inaba H., Tagashira M., Kanda T., Murakami Y., Amano A., Matsumoto-Nakano M. (2016). Apple- and Hop-polyphenols inhibit Porphyromonas gingivalis-mediated precursor of matrix metalloproteinase-9 activation and invasion of oral squamous cell carcinoma cells. J. Periodontol. 87, 1103–1111. 10.1902/jop.2016.160047 [DOI] [PubMed] [Google Scholar]
- Ishiguro I., Saiki K., Konishi K. (2009). PG27 is a novel membrane protein essential for a Porphyromonas gingivalis protease secretion system. FEMS Microbiol. Lett. 292, 261–267. 10.1111/j.1574-6968.2009.01489.x [DOI] [PubMed] [Google Scholar]
- Jarrell K. F., McBride M. J. (2008). The surprisingly diverse ways that prokaryotes move. Nat. Rev. Microbiol. 6, 466–476. 10.1038/nrmicro1900 [DOI] [PubMed] [Google Scholar]
- Jusko M., Potempa J., Mizgalska D., Bielecka E., Ksiazek M., Riesbeck K., et al. (2015). A metalloproteinase mirolysin of tannerella forsythia inhibits all pathways of the complement system. J. Immunol. 195, 2231–2240. 10.4049/jimmunol.1402892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T., Yukitake H., Naito M., Sato K., Kikuchi Y., Kondo Y., et al. (2016). A two-component system regulates gene expression of the type IX secretion component proteins via an ECF sigma factor. Sci. Rep. 6:23288. 10.1038/srep23288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallberg M., Wang H., Wang S., Peng J., Wang Z., Lu H., et al. (2012). Template-based protein structure modeling using the RaptorX web server. Nat. Protoc. 7, 1511–1522. 10.1038/nprot.2012.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanonenberg K., Schwarz C. K., Schmitt L. (2013). Type I secretion systems - a story of appendices. Res. Microbiol. 164, 596–604. 10.1016/j.resmic.2013.03.011 [DOI] [PubMed] [Google Scholar]
- Karim A. Y., Kulczycka M., Kantyka T., Dubin G., Jabaiah A., Daugherty P. S., et al. (2010). A novel matrix metalloprotease-like enzyme (karilysin) of the periodontal pathogen Tannerella forsythia ATCC 43037. Biol. Chem. 391, 105–117. 10.1515/bc.2010.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebschull M., Demmer R. T., Papapanou P. N. (2010). “Gum bug, leave my heart alone!”–epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J. Dent. Res. 89, 879–902. 10.1177/0022034510375281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharade S. S., McBride M. J. (2014). Flavobacterium johnsoniae chitinase ChiA is required for chitin utilization and is secreted by the type IX secretion system. J. Bacteriol. 196, 961–970. 10.1128/JB.01170-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharade S. S., McBride M. J. (2015). Flavobacterium johnsoniae PorV is required for secretion of a subset of proteins targeted to the type IX secretion system. J. Bacteriol. 197, 147–158. 10.1128/JB.02085-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y., Ohara N., Sato K., Yoshimura M., Yukitake H., Naito M., et al. (2010). Tetratricopeptide repeat protein-associated proteins contribute to the virulence of Porphyromonas gingivalis. Infect. Immun. 78, 2846–2856. 10.1128/IAI.01448-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koneru L., Ksiazek M., Waligorska I., Straczek A., Lukasik M., Madej M., et al. (2017). Mirolysin, a LysargiNase from Tannerella forsythia, proteolytically inactivates the human cathelicidin, LL-37. Biol Chem. 398, 395–409. 10.1515/hsz-2016-0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziel J., Mydel P., Potempa J. (2014). The link between periodontal disease and rheumatoid arthritis: an updated review. Curr. Rheumatol. Rep. 16, 408. 10.1007/s11926-014-0408-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss J. L., Potempa J., Lambris J. D., Hajishengallis G. (2010). Complementary Tolls in the periodontium: how periodontal bacteria modify complement and Toll-like receptor responses to prevail in the host. Periodontol. 2000 52, 141–162. 10.1111/j.1600-0757.2009.00324.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek M., Karim A. Y., Bryzek D., Enghild J. J., Thogersen I. B., Koziel J., et al. (2015a). Mirolase, a novel subtilisin-like serine protease from the periodontopathogen Tannerella forsythia. Biol. Chem. 396, 261–275. 10.1515/hsz-2014-0256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek M., Mizgalska D., Eick S., Thogersen I. B., Enghild J. J., Potempa J. (2015b). KLIKK proteases of Tannerella forsythia: putative virulence factors with a unique domain structure. Front. Microbiol. 6:312. 10.3389/fmicb.2015.00312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFrentz B. R., LaPatra S. E., Call D. R., Wiens G. D., Cain K. D. (2009). Proteomic analysis of Flavobacterium psychrophilum cultured in vivo and in iron-limited media. Dis. Aquat. Org. 87, 171–182. 10.3354/dao02122 [DOI] [PubMed] [Google Scholar]
- Lasica A. M., Goulas T., Mizgalska D., Zhou X., de Diego I., Ksiazek M., et al. (2016). Structural and functional probing of PorZ, an essential bacterial surface component of the type-IX secretion system of human oral-microbiomic Porphyromonas gingivalis. Sci. Rep. 6:37708. 10.1038/srep37708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugisch O., Wong A., Sroka A., Kantyka T., Koziel J., Neuhaus K., et al. (2016). Citrullination in the periodontium–a possible link between periodontitis and rheumatoid arthritis. Clin. Oral Investig. 20, 675–683. 10.1007/s00784-015-1556-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letoffe S., Ghigo J. M., Wandersman C. (1994). Secretion of the Serratia marcescens HasA protein by an ABC transporter. J. Bacteriol. 176, 5372–5377. 10.1128/jb.176.17.5372-5377.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Pelegrin M., Ksiazek M., Karim A. Y., Guevara T., Arolas J. L., Potempa J., et al. (2015). A novel mechanism of latency in matrix metalloproteinases. J. Biol. Chem. 290, 4728–4740. 10.1074/jbc.M114.605956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor A. J., Snieder H., Rigby A. S., Koskenvuo M., Kaprio J., Aho K., et al. (2000). Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum 43, 30–37. [DOI] [PubMed] [Google Scholar]
- Madden T. E., Clark V. L., Kuramitsu H. K. (1995). Revised sequence of the Porphyromonas gingivalis prtT cysteine protease/hemagglutinin gene: homology with streptococcal pyrogenic exotoxin B/streptococcal proteinase. Infect. Immun. 63, 238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerczyk C., Schneider E., Greenberg E. P. (2016). Quorum sensing control of Type VI secretion factors restricts the proliferation of quorum-sensing mutants. Elife 5:e14712. 10.7554/eLife.14712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao F., Dam P., Chou J., Olman V., Xu Y. (2009). DOOR: a database for prokaryotic operons. Nucleic Acids Res. 37, D459–D463. 10.1093/nar/gkn757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marraffini L. A., Ton-That H., Zong Y., Narayana S. V., Schneewind O. (2004). Anchoring of surface proteins to the cell wall of Staphylococcus aureus. A conserved arginine residue is required for efficient catalysis of sortase A. J. Biol. Chem. 279, 37763–37770. 10.1074/jbc.M405282200 [DOI] [PubMed] [Google Scholar]
- McBride M. J., Nakane D. (2015). Flavobacterium gliding motility and the type IX secretion system. Curr. Opin. Microbiol. 28, 72–77. 10.1016/j.mib.2015.07.016 [DOI] [PubMed] [Google Scholar]
- McBride M. J., Zhu Y. (2013). Gliding motility and Por secretion system genes are widespread among members of the phylum bacteroidetes. J. Bacteriol. 195, 270–278. 10.1128/JB.01962-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw W. T., Potempa J., Farley D., Travis J. (1999). Purification, characterization, and sequence analysis of a potential virulence factor from Porphyromonas gingivalis, peptidylarginine deiminase. Infect. Immun. 67, 3248–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes I. B., Schett G. (2011). The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219. 10.1056/NEJMra1004965 [DOI] [PubMed] [Google Scholar]
- McKee A. S., McDermid A. S., Wait R., Baskerville A., Marsh P. D. (1988). Isolation of colonial variants of Bacteroides gingivalis W50 with a reduced virulence. J. Med. Microbiol. 27, 59–64. 10.1099/00222615-27-1-59 [DOI] [PubMed] [Google Scholar]
- Messner P., Schaffer C., Egelseer E. M., Sleytr U. B. (2010). Occurrence, structure, chemistry, genetics, morphogenesis, and functions of s-layers, in Prokaryotic Cell Wall Compounds, Structure and Biochemistry, eds König H., Claus H., Varma A. (Berlin: Springer-Verlag; ), 53–109. [Google Scholar]
- Mikolajczyk J., Boatright K. M., Stennicke H. R., Nazif T., Potempa J., Bogyo M., et al. (2003). Sequential autolytic processing activates the zymogen of Arg-gingipain. J. Biol. Chem. 278, 10458–10464. 10.1074/jbc.M210564200 [DOI] [PubMed] [Google Scholar]
- Montgomery A. B., Kopec J., Shrestha L., Thezenas M. L., Burgess-Brown N. A., Fischer R., et al. (2016). Crystal structure of Porphyromonas gingivalis peptidylarginine deiminase: implications for autoimmunity in rheumatoid arthritis. Ann. Rheum. Dis. 75, 1255–1261. 10.1136/annrheumdis-2015-207656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane D., Sato K., Wada H., McBride M. J., Nakayama K. (2013). Helical flow of surface protein required for bacterial gliding motility. Proc. Natl. Acad. Sci. U.S.A. 110, 11145–11150. 10.1073/pnas.1219753110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K. (2015). Porphyromonas gingivalis and related bacteria: from colonial pigmentation to the type IX secretion system and gliding motility. J. Periodont. Res. 50, 1–8. 10.1111/jre.12255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K., Yoshimura F., Kadowaki T., Yamamoto K. (1996). Involvement of arginine-specific cysteine proteinase (Arg-gingipain) in fimbriation of Porphyromonas gingivalis. J. Bacteriol. 178, 2818–2824. 10.1128/jb.178.10.2818-2824.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita Y., Sato K., Yukitake H., Shoji M., Nakane D., Nagano K., et al. (2014). Lack of a surface layer in Tannerella forsythia mutants deficient in the type IX secretion system. Microbiology 160(Pt 10), 2295–2303. 10.1099/mic.0.080192-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D., Potempa J., Kordula T., Travis J. (1999). Purification and characterization of a novel cysteine proteinase (periodontain) from Porphyromonas gingivalis. Evidence for a role in the inactivation of human alpha1-proteinase inhibitor. J. Biol. Chem. 274, 12245–12251. 10.1074/jbc.274.18.12245 [DOI] [PubMed] [Google Scholar]
- Nelson K. E., Fleischmann R. D., DeBoy R. T., Paulsen I. T., Fouts D. E., Eisen J. A., et al. (2003). Complete genome sequence of the oral pathogenic Bacterium porphyromonas gingivalis strain W83. J. Bacteriol. 185, 5591–5601. 10.1128/JB.185.18.5591-5601.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen K. A., Travis J., Potempa J. (2007). Does the importance of the C-terminal residues in the maturation of RgpB from Porphyromonas gingivalis reveal a novel mechanism for protein export in a subgroup of Gram-Negative bacteria? J. Bacteriol. 189, 833–843. 10.1128/JB.01530-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen K. A., Zylicz J., Szczesny P., Sroka A., Hunter N., Potempa J. (2009). Verification of a topology model of PorT as an integral outer-membrane protein in Porphyromonas gingivalis. Microbiology 155(Pt 2), 328–337. 10.1099/mic.0.024323-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka M., Shoji M., Kadowaki T., Sato K., Yukitake H., Naito M., et al. (2014). Analysis of a Lys-specific serine endopeptidase secreted via the type IX secretion system in Porphyromonas gingivalis. FEMS Microbiol. Lett. 354, 60–68. 10.1111/1574-6968.12426 [DOI] [PubMed] [Google Scholar]
- Okamoto K., Nakayama K., Kadowaki T., Abe N., Ratnayake D. B., Yamamoto K. (1998). Involvement of a lysine-specific cysteine proteinase in hemoglobin adsorption and heme accumulation by Porphyromonas gingivalis. J. Biol. Chem. 273, 21225–21231. 10.1074/jbc.273.33.21225 [DOI] [PubMed] [Google Scholar]
- Olsen I., Potempa J. (2014). Strategies for the inhibition of gingipains for the potential treatment of periodontitis and associated systemic diseases. J. Oral Microbiol. 6:24800. 10.3402/jom.v6.24800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramonov N., Rangarajan M., Hashim A., Gallagher A., Aduse-Opoku J., Slaney J. M., et al. (2005). Structural analysis of a novel anionic polysaccharide from Porphyromonas gingivalis strain W50 related to Arg-gingipain glycans. Mol. Microbiol. 58, 847–863. 10.1111/j.1365-2958.2005.04871.x [DOI] [PubMed] [Google Scholar]
- Park Y., Yilmaz O., Jung I. Y., Lamont R. J. (2004). Identification of Porphyromonas gingivalis genes specifically expressed in human gingival epithelial cells by using differential display reverse transcription-PCR. Infect. Immun. 72, 3752–3758. 10.1128/IAI.72.7.3752-3758.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavloff N., Potempa J., Pike R. N., Prochazka V., Kiefer M. C., Travis J., et al. (1995). Molecular cloning and structural characterization of the Arg-gingipain proteinase of Porphyromonas gingivalis. Biosynthesis as a proteinase-adhesin polyprotein. J. Biol. Chem. 270, 1007–1010. 10.1074/jbc.270.3.1007 [DOI] [PubMed] [Google Scholar]
- Pertea M., Ayanbule K., Smedinghoff M., Salzberg S. L. (2009). OperonDB: a comprehensive database of predicted operons in microbial genomes. Nucleic Acids Res. 37, D479–D482. 10.1093/nar/gkn784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike R., McGraw W., Potempa J., Travis J. (1994). Lysine- and arginine-specific proteinases from Porphyromonas gingivalis. Isolation, characterization, and evidence for the existence of complexes with hemagglutinins. J. Biol. Chem. 269, 406–411. [PubMed] [Google Scholar]
- Pomowski A., Uson I., Nowakowska Z. M., Veillard F., Sztukowska M. N., Guevara T., et al. (2017). Structural insights unravel the zymogenic mechanism of the virulence factor gingipain K from Porphyromonas gingivalis, a causative agent of gum disease from the human oral microbiome. J. Biol. Chem. 292, 5724–5735. 10.1074/jbc.M117.776724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posch G., Andrukhov O., Vinogradov E., Lindner B., Messner P., Holst O., et al. (2013). Structure and immunogenicity of the rough-type lipopolysaccharide from the periodontal pathogen Tannerella forsythia. Clin. Vaccine Immunol. 20, 945–953. 10.1128/CVI.00139-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa J., Banbula A., Travis J. (2000). Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontol. 2000 24, 153–192. 10.1034/j.1600-0757.2000.2240108.x [DOI] [PubMed] [Google Scholar]
- Potempa J., Pike R., Travis J. (1997). Titration and mapping of the active site of cysteine proteinases from Porphyromonas gingivalis (gingipains) using peptidyl chloromethanes. Biol. Chem. 378, 223–230. 10.1515/bchm.1997.378.3-4.223 [DOI] [PubMed] [Google Scholar]
- Potempa J., Sroka A., Imamura T., Travis J. (2003). Gingipains, the major cysteine proteinases and virulence factors of Porphyromonas gingivalis: structure, function and assembly of multidomain protein complexes. Curr. Protein Pept. Sci. 4, 397–407. 10.2174/1389203033487036 [DOI] [PubMed] [Google Scholar]
- Quirke A. M., Lundberg K., Potempa J., Mikuls T. R., Venables P. J. (2015). PPAD remains a credible candidate for inducing autoimmunity in rheumatoid arthritis: comment on the article by Konig et al. Ann. Rheum. Dis. 74, e7. 10.1136/annrheumdis-2014-206665 [DOI] [PubMed] [Google Scholar]
- Rangarajan M., Aduse-Opoku J., Paramonov N., Hashim A., Bostanci N., Fraser O. P., et al. (2008). Identification of a second lipopolysaccharide in Porphyromonas gingivalis W50. J. Bacteriol. 190, 2920–2932. 10.1128/JB.01868-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan M., Smith S. J., Sally U., Curtis M. A. (1997). Biochemical characterization of the arginine-specific proteases of Porphyromonas gingivalis W50 suggests a common precursor. Biochem. J. 323(Pt 3), 701–709. 10.1042/bj3230701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut H., Tang C., Henderson N. S., Pinkner J. S., Wang T., Hultgren S. J., et al. (2008). Fiber formation across the bacterial outer membrane by the chaperone/usher pathway. Cell 133, 640–652. 10.1016/j.cell.2008.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes R. G., Samarasam M. N., Shrivastava A., van Baaren J. M., Pochiraju S., Bollampalli S., et al. (2010). Flavobacterium johnsoniae gldN and gldO are partially redundant genes required for gliding motility and surface localization of SprB. J. Bacteriol. 192, 1201–1211. 10.1128/JB.01495-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes R. G., Samarasam M. N., Van Groll E. J., McBride M. J. (2011). Mutations in Flavobacterium johnsoniae sprE result in defects in gliding motility and protein secretion. J. Bacteriol. 193, 5322–5327. 10.1128/JB.05480-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman S. J., Meyers M., Volz K., Matsumura P. (1992). A chemotactic signaling surface on CheY defined by suppressors of flagellar switch mutations. J. Bacteriol. 174, 6247–6255. 10.1128/jb.174.19.6247-6255.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondelet A., Condemine G. (2013). Type II secretion: the substrates that won't go away. Res. Microbiol. 164, 556–561. 10.1016/j.resmic.2013.03.005 [DOI] [PubMed] [Google Scholar]
- Russell A. B., Wexler A. G., Harding B. N., Whitney J. C., Bohn A. J., Goo Y. A., et al. (2014). A type VI secretion-related pathway in Bacteroidetes mediates interbacterial antagonism. Cell Host Microbe 16, 227–236. 10.1016/j.chom.2014.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi Y., Khan S., Eisenbach M. (2003). Binding of the chemotaxis response regulator CheY to the isolated, intact switch complex of the bacterial flagellar motor: lack of cooperativity. J. Biol. Chem. 278, 25867–25871. 10.1074/jbc.M303201200 [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr., Reddy B. L. (2015). Holins in bacteria, eukaryotes, and archaea: multifunctional xenologues with potential biotechnological and biomedical applications. J. Bacteriol. 197, 7–17. 10.1128/JB.02046-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki K., Konishi K. (2007). Identification of a Porphyromonas gingivalis novel protein sov required for the secretion of gingipains. Microbiol. Immunol. 51, 483–491. 10.1111/j.1348-0421.2007.tb03936.x [DOI] [PubMed] [Google Scholar]
- Saiki K., Konishi K. (2010a). Identification of a novel Porphyromonas gingivalis outer membrane protein, PG534, required for the production of active gingipains. FEMS Microbiol. Lett. 310, 168–174. 10.1111/j.1574-6968.2010.02059.x [DOI] [PubMed] [Google Scholar]
- Saiki K., Konishi K. (2010b). The role of Sov protein in the secretion of gingipain protease virulence factors of Porphyromonas gingivalis. FEMS Microbiol. Lett. 302, 166–174. 10.1111/j.1574-6968.2009.01848.x [DOI] [PubMed] [Google Scholar]
- Saiki K., Konishi K. (2014). Porphyromonas gingivalis C-terminal signal peptidase PG0026 and HagA interact with outer membrane protein PG27/LptO. Mol. Oral Microbiol. 29, 32–44. 10.1111/omi.12043 [DOI] [PubMed] [Google Scholar]
- Sakakibara J., Nagano K., Murakami Y., Higuchi N., Nakamura H., Shimozato K., et al. (2007). Loss of adherence ability to human gingival epithelial cells in S-layer protein-deficient mutants of Tannerella forsythensis. Microbiology 153(Pt 3), 866–876. 10.1099/mic.0.29275-0 [DOI] [PubMed] [Google Scholar]
- Sato K., Naito M., Yukitake H., Hirakawa H., Shoji M., McBride M. J., et al. (2010). A protein secretion system linked to bacteroidete gliding motility and pathogenesis. Proc. Natl. Acad. Sci. U.S.A. 107, 276–281. 10.1073/pnas.0912010107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Sakai E., Veith P. D., Shoji M., Kikuchi Y., Yukitake H., et al. (2005). Identification of a new membrane-associated protein that influences transport/maturation of gingipains and adhesins of Porphyromonas gingivalis. J. Biol. Chem. 280, 8668–8677. 10.1074/jbc.M413544200 [DOI] [PubMed] [Google Scholar]
- Sato K., Yukitake H., Narita Y., Shoji M., Naito M., Nakayama K. (2013). Identification of Porphyromonas gingivalis proteins secreted by the Por secretion system. FEMS Microbiol. Lett. 338, 68–76. 10.1111/1574-6968.12028 [DOI] [PubMed] [Google Scholar]
- Schneewind O., Missiakas D. M. (2012). Protein secretion and surface display in Gram-positive bacteria. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 1123–1139. 10.1098/rstb.2011.0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seers C. A., Slakeski N., Veith P. D., Nikolof T., Chen Y. Y., Dashper S. G., et al. (2006). The RgpB C-terminal domain has a role in attachment of RgpB to the outer membrane and belongs to a novel C-terminal-domain family found in Porphyromonas gingivalis. J. Bacteriol. 188, 6376–6386. 10.1128/JB.00731-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekot G., Posch G., Oh Y. J., Zayni S., Mayer H. F., Pum D., et al. (2012). Analysis of the cell surface layer ultrastructure of the oral pathogen Tannerella forsythia. Arch. Microbiol. 194, 525–539. 10.1007/s00203-012-0792-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah H. N., Seddon S. V., Gharbia S. E. (1989). Studies on the virulence properties and metabolism of pleiotropic mutants of Porphyromonas gingivalis (Bacteroides gingivalis) W50. Oral Microbiol. Immunol. 4, 19–23. 10.1111/j.1399-302X.1989.tb00401.x [DOI] [PubMed] [Google Scholar]
- Shi Y., Ratnayake D. B., Okamoto K., Abe N., Yamamoto K., Nakayama K. (1999). Genetic analyses of proteolysis, hemoglobin binding, and hemagglutination of Porphyromonas gingivalis. Construction of mutants with a combination of rgpA, rgpB, kgp, and hagA. J. Biol. Chem. 274, 17955–17960. 10.1074/jbc.274.25.17955 [DOI] [PubMed] [Google Scholar]
- Shoji M., Nakayama K. (2016). Glycobiology of the oral pathogen Porphyromonas gingivalis and related species. Microb. Pathog. 94, 35–41. 10.1016/j.micpath.2015.09.012 [DOI] [PubMed] [Google Scholar]
- Shoji M., Ratnayake D. B., Shi Y., Kadowaki T., Yamamoto K., Yoshimura F., et al. (2002). Construction and characterization of a nonpigmented mutant of Porphyromonas gingivalis: cell surface polysaccharide as an anchorage for gingipains. Microbiology 148(Pt 4), 1183–1191. 10.1099/00221287-148-4-1183 [DOI] [PubMed] [Google Scholar]
- Shoji M., Sato K., Yukitake H., Kondo Y., Narita Y., Kadowaki T., et al. (2011). Por secretion system-dependent secretion and glycosylation of Porphyromonas gingivalis hemin-binding protein 35. PLoS ONE 6:e21372. 10.1371/journal.pone.0021372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M., Sato K., Yukitake H., Naito M., Nakayama K. (2014). Involvement of the Wbp pathway in the biosynthesis of Porphyromonas gingivalis lipopolysaccharide with anionic polysaccharide. Sci. Rep. 4:5056. 10.1038/srep05056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M., Shibata Y., Shiroza T., Yukitake H., Peng B., Chen Y. Y., et al. (2010). Characterization of hemin-binding protein 35 (HBP35) in Porphyromonas gingivalis: its cellular distribution, thioredoxin activity and role in heme utilization. BMC Microbiol. 10:152. 10.1186/1471-2180-10-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava A., Johnston J. J., van Baaren J. M., McBride M. J. (2013). Flavobacterium johnsoniae GldK, GldL, GldM, and SprA are required for secretion of the cell surface gliding motility adhesins SprB and RemA. J. Bacteriol. 195, 3201–3212. 10.1128/JB.00333-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava A., Lele P. P., Berg H. C. (2015). A rotary motor drives Flavobacterium gliding. Curr. Biol. 25, 338–341. 10.1016/j.cub.2014.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava A., Rhodes R. G., Pochiraju S., Nakane D., McBride M. J. (2012). Flavobacterium johnsoniae RemA is a mobile cell surface lectin involved in gliding. J. Bacteriol. 194, 3678–3688. 10.1128/JB.00588-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui H., Yoder-Himes D. R., Mizgalska D., Nguyen K. A., Potempa J., Olsen I. (2014). Genome sequence of Porphyromonas gingivalis strain HG66 (DSM 28984). Genome Announc. 2:e00947-14. 10.1128/genomeA.00947-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W., Wang C. Y., Mikolajczyk-Pawlinska J., Potempa J., Travis J., Bond V. C., et al. (1999). Transposition of the endogenous insertion sequence element IS1126 modulates gingipain expression in Porphyromonas gingivalis. Infect. Immun. 67, 5012–5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slakeski N., Seers C. A., Ng K., Moore C., Cleal S. M., Veith P. D., et al. (2011). C-terminal domain residues important for secretion and attachment of RgpB in Porphyromonas gingivalis. J. Bacteriol. 193, 132–142. 10.1128/JB.00773-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B., Beveridge T. J. (1999). Bacterial S-layers. Trends Microbiol. 7, 253–260. 10.1016/S0966-842X(99)01513-9 [DOI] [PubMed] [Google Scholar]
- Smalley J. W., Silver J., Marsh P. J., Birss A. J. (1998). The periodontopathogen Porphyromonas gingivalis binds iron protoporphyrin IX in the mu-oxo dimeric form: an oxidative buffer and possible pathogenic mechanism. Biochem. J. 331(Pt 3), 681–685. 10.1042/bj3310681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snel B., Lehmann G., Bork P., Huynen M. A. (2000). STRING: a web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res. 28, 3442–3444. 10.1093/nar/28.18.3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroka A., Sztukowska M., Potempa J., Travis J., Genco C. A. (2001). Degradation of host heme proteins by lysine- and arginine-specific cysteine proteinases (gingipains) of Porphyromonas gingivalis. J. Bacteriol. 183, 5609–5616. 10.1128/JB.183.19.5609-5616.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y. (1942). The cytophaga group: a contribution to the biology of myxobacteria. Bacteriol. Rev. 6, 143–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y. (1947). Studies on nonfruiting myxobacteria; Cytophaga johnsonae, n. sp., a chitindecomposing myxobacterium. J. Bacteriol. 53, 297–315. [DOI] [PubMed] [Google Scholar]
- Stathopulos J., Cambillau C., Cascales E., Roussel A., Leone P. (2015). Crystallization and preliminary X-ray analysis of the C-terminal fragment of PorM, a subunit of the Porphyromonas gingivalis type IX secretion system. Acta Crystallogr. F. Struct. Biol. Commun. 71(Pt 1), 71–74. 10.1107/S2053230X1402559X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztukowska M., Veillard F., Potempa B., Bogyo M., Enghild J. J., Thogersen I. B., et al. (2012). Disruption of gingipain oligomerization into non-covalent cell-surface attached complexes. Biol. Chem. 393, 971–977. 10.1515/hsz-2012-0175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taboada B., Ciria R., Martinez-Guerrero C. E., Merino E. (2012). ProOpDB: prokaryotic operon database. Nucleic Acids Res. 40, D627–D631. 10.1093/nar/gkr1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi Y., Sato K., Yukitake H., Inoue T., Nakayama M., Naito M., et al. (2015). Involvement of an Skp-Like Protein, PGN_0300, in the type IX secretion system of Porphyromonas gingivalis. Infect. Immun. 84, 230–240. 10.1128/IAI.01308-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahara H., Okamoto H., Sugawara K. (1986). Calcium-dependent properties of peptidylarginine deiminase from rabbit skeletal muscle Agric. Biol. Chem. 50, 2899–2904. [Google Scholar]
- Tomek M. B., Neumann L., Nimeth I., Koerdt A., Andesner P., Messner P., et al. (2014). The S-layer proteins of Tannerella forsythia are secreted via a type IX secretion system that is decoupled from protein O-glycosylation. Mol. Oral Microbiol. 29, 307–320. 10.1111/omi.12062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veith P. D., Chen Y. Y., Chen D., O'Brien-Simpson N. M., Cecil J. D., Holden J. A., et al. (2015). Tannerella forsythia outer membrane vesicles are enriched with substrates of the type IX secretion system and tonb-dependent receptors. J. Proteome Res. 14, 5355–5366. 10.1021/acs.jproteome.5b00878 [DOI] [PubMed] [Google Scholar]
- Veith P. D., Chen Y. Y., Gorasia D. G., Chen D., Glew M. D., O'Brien-Simpson N. M., et al. (2014). Porphyromonas gingivalis outer membrane vesicles exclusively contain outer membrane and periplasmic proteins and carry a cargo enriched with virulence factors. J. Proteome Res. 13, 2420–2432. 10.1021/pr401227e [DOI] [PubMed] [Google Scholar]
- Veith P. D., Chen Y. Y., Reynolds E. C. (2004). Porphyromonas gingivalis RgpA and Kgp proteinases and adhesins are C terminally processed by the carboxypeptidase CPG70. Infect. Immun. 72, 3655–3657. 10.1128/IAI.72.6.3655-3657.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veith P. D., Nor Muhammad N. A., Dashper S. G., Likic V. A., Gorasia D. G., Chen D., et al. (2013). Protein substrates of a novel secretion system are numerous in the Bacteroidetes phylum and have in common a cleavable C-terminal secretion signal, extensive post-translational modification, and cell-surface attachment. J. Proteome Res. 12, 4449–4461. 10.1021/pr400487b [DOI] [PubMed] [Google Scholar]
- Veith P. D., O'Brien-Simpson N. M., Tan Y., Djatmiko D. C., Dashper S. G., Reynolds E. C. (2009). Outer membrane proteome and antigens of Tannerella forsythia. J. Proteome Res. 8, 4279–4292. 10.1021/pr900372c [DOI] [PubMed] [Google Scholar]
- Veith P. D., Talbo G. H., Slakeski N., Dashper S. G., Moore C., Paolini R. A., et al. (2002). Major outer membrane proteins and proteolytic processing of RgpA and Kgp of Porphyromonas gingivalis W50. Biochem. J. 363(Pt 1), 105–115. 10.1042/bj3630105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M. S., Canestrari M. J., Leone P., Stathopoulos J., Ize B., Zoued A., et al. (2017). Characterization of the Porphyromonas gingivalis type IX secretion trans-envelope PorKLMNP core complex. J. Biol. Chem. 292, 3252–3261. 10.1074/jbc.M116.765081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M. S., Durand E., Cascales E. (2016). The PorX response regulator of the Porphyromonas gingivalis PorXY two-component system does not directly regulate the type IX secretion genes but binds the PorL subunit. Front. Cell. Infect. Microbiol. 6:96 10.3389/fcimb.2016.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossenaar E. R., Zendman A. J., van Venrooij W. J., Pruijn G. J. (2003). PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays 25, 1106–1118. 10.1002/bies.10357 [DOI] [PubMed] [Google Scholar]
- Wegner N., Wait R., Sroka A., Eick S., Nguyen K. A., Lundberg K., et al. (2010). Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and alpha-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 62, 2662–2672. 10.1002/art.27552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler A. G., Bao Y., Whitney J. C., Bobay L. M., Xavier J. B., Schofield W. B., et al. (2016). Human symbionts inject and neutralize antibacterial toxins to persist in the gut. Proc. Natl. Acad. Sci. U.S.A. 113, 3639–3644. 10.1073/pnas.1525637113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore S. E., Lamont R. J. (2014). Oral bacteria and cancer. PLoS Pathog. 10:e1003933. 10.1371/journal.ppat.1003933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky A., Potempa J., Houri-Haddad Y., Shapira L. (2016). Vaccination with recombinant RgpA peptide protects against Porphyromonas gingivalis-induced bone loss. J. Periodontal. Res. 52, 285–291. 10.1111/jre.12393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. M., Anderson D. E., Bernstein H. D. (2015). Analysis of the outer membrane proteome and secretome of Bacteroides fragilis reveals a multiplicity of secretion mechanisms. PLoS ONE 10:e0117732. 10.1371/journal.pone.0117732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Shoji M., Shibata S., Naito M., Sato K., Elsliger M. A., et al. (2016). A distinct type of pilus from the human microbiome. Cell 165, 690–703. 10.1016/j.cell.2016.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Bu X., Han Q., Wang X., Zhou H., Chen G., et al. (2016). A small periplasmic protein essential for Cytophaga hutchinsonii cellulose digestion. Appl. Microbiol. Biotechnol. 100, 1935–1944. 10.1007/s00253-015-7204-y [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Ohara N., Kondo Y., Shoji M., Okano S., Nakano Y., et al. (2008). Proteome analysis of Porphyromonas gingivalis cells placed in a subcutaneous chamber of mice. Oral Microbiol. Immunol. 23, 413–418. 10.1111/j.1399-302X.2008.00444.x [DOI] [PubMed] [Google Scholar]
- Zhang Z., Liu Q., Hendrickson W. A. (2014). Crystal structures of apparent saccharide sensors from histidine kinase receptors prevalent in a human gut symbiont. FEBS J. 281, 4263–4279. 10.1111/febs.12904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. Y., Gao J. L., Hunter N., Potempa J., Nguyen K. A. (2013). Sequence-independent processing site of the C-terminal domain (CTD) influences maturation of the RgpB protease from Porphyromonas gingivalis. Mol. Microbiol. 89, 903–917. 10.1111/mmi.12319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., McBride M. J. (2014). Deletion of the Cytophaga hutchinsonii type IX secretion system gene sprP results in defects in gliding motility and cellulose utilization. Appl. Microbiol. Biotechnol. 98, 763–775. 10.1007/s00253-013-5355-2 [DOI] [PubMed] [Google Scholar]