Abstract
Cholesterol-core nanoparticles (LDE) have been shown to be recognized by low-density lipoprotein receptors (LDLR) after administration; therefore, LDE is an ideal vehicle to deliver drug with targeting property. Paclitaxel, when incorporated into LDE, promotes atherosclerosis regression with reduced drug toxicity in rabbits through LDLR. Here, we tested whether LDE-paclitaxel could still be effective in reducing diet-induced atherosclerosis in a mouse model without LDLR. Nineteen LDLR knockout male mice were fed 1% cholesterol for 12 weeks. Then, 12 animals received 4-weekly intraperitoneal LDE-paclitaxel (4 mg/kg) while 7 controls received saline solution. On week 12 and 16, in vivo MRI of the aortic roots was performed. Aorta macroscopy was made after euthanasia. Reduction of atherosclerotic lesions was observed. LDE-paclitaxel treatment resulted in reduction of wall area (14%) and stenosis (22%) by MRI and 33% by macroscopy. Thus, LDE-paclitaxel may produce pharmacological effects through LDE uptake by mechanisms other than LDLR.
Keywords: atherosclerosis treatment, lipid solid nanoparticles, emulsions, paclitaxel, MRI, drug targeting
Introduction
Atherosclerosis is a proliferative and inflammatory disease characterized by the formation of foam cells, atheromatous plaques consisting of lipids and inflammatory and smooth muscle cells in the intimal layer of arteries[ 1- 2]. At the present time, the treatment of the disease is limited mainly to the control of risk factors, such as the use of statins, anti-hypertensive and anticoagulant drugs. Novel anti-inflammatory and anti-proliferative approaches to treat atherosclerosis have been studied[ 3- 4] such as the chemotherapeutic agents used in cancer treatment. Those agents are the most potent anti-proliferative compounds in current use[ 5]. In previous studies, we showed that association of drugs such as carmustine, paclitaxel, and etoposide to lipid core nanoparticles can decrease the toxicity of the drugs, which was demonstrated in experimental studies and clinical trials enrolling patients with advanced cancer[ 6 11].
LDE is a cholesterol-rich nanoparticle which resembles the structure of native LDL, except for the absence of apolipoprotein (apo) B100, the main LDL apo. However, when in contact with plasma, LDE acquires apoE and other native apolipoproteins. Through acquired apolipoproteins, LDE can be recognized by LDL receptors (LDLR) or potentially by other LDL related protein receptors (LRP)[ 12]. These receptors are overexpressed in several neoplastic diseases and also in inflammatory sites such as atherosclerotic lesions. Thus, lipophilic drugs delivered through the nanoparticle structure can concentrate in the targeted sites[ 13- 14]. In rabbits with atherosclerosis induced by cholesterol feeding, LDE-paclitaxel treatment reduced 60% of the atherosclerotic lesion, and led to impaired invasion of macrophages and smooth muscle cells into the intima[ 6].
LDLR knockout mice, a model of familial hypercholesterolemia, are one of the common models of study in atherosclerosis. These mice are diet-responsive and may present large atherosclerotic lesions[ 15]. In vivo magnetic resonance imaging (MRI) has been utilized as a non-invasive method to assess atherosclerotic plaques. The method can be extended from the usual single time point to serial imaging to monitoring both disease progression and regression by anti-atherosclerotic therapies[ 16]. LDE is a promising way to targeted drug delivery. Therefore, the aim of this study was to apply serial in vivo MRI to test whether LDE-paclitaxel treatment is effective in attenuating atherosclerotic plaque regression in the aortic root of cholesterol-fed LDLR knockout mice.
Materials and methods
Experimental protocols and treatment
This study was approved by Boston University Institutional Animal Care and Use Committee procedures. Nineteen male LDLR knockout mice entered the study at eight-week-old, and received a high fat diet containing 20% fat and 1.25% cholesterol for 12 weeks. At the end of this period, the animals were fed on normal chow for additional 4 weeks, and separated in two groups randomly for pharmacological treatment. One group received LDE-paclitaxel (4 mg/kg, IP) weekly for 4 weeks (n = 12). The control group was treated with an equivalent volume of saline. MRI data were acquired at week 12 and 16. At the end of week 16, all mice were sacrificed and aortas were extracted for histological analysis. All mice were weighed weekly and the heart weights were measured after sacrifice.
Preparation of LDE containing paclitaxel oleate
LDE was prepared according to the method described by Ginsburg et al.[ 17] and modified by Maranhão et al.[ 18]. Briefly, a mixture was composed of 135 mg cholesteryl oleate (Alfa Aesar, Harverhill, USA), 333 mg egg phosphatidylcholine (Lipoid, Ludwigshafen, Germany), 132 mg miglyol 812N (Sasol, Hamburg, Germany), 6 mg cholesterol (Fabrichem, Trumbull, USA) and 60 mg paclitaxel oleate (Pharmaceuticals, Shangai, China), with the aqueous phase comprised 100 mg of polysorbate 80 (Merck, Darmstadt, Germany) and 10 mL Tris-HCl buffer, pH 8.05. A pre-emulsion was obtained by ultrasonic radiation until complete drug dissolution. Emulsification of all lipids, the drug and the aqueous phase was obtained by high-pressure homogenization using an Emulsiflex C5 homogenizer (Avestin, Ottawa, Canada). After homogenization, the formed emulsion was centrifuged and the nanoparticle sterilized by passage through 0.22-mm pore polycarbonate filter (Millipore, Billerica, MA, USA). The quantification rate of paclitaxel oleate associated with LDE was determined by high pressure liquid chromatography (HPLC) (Shimadzu, Kyoto, Japan). The final concentration of paclitaxel oleate incorporated to LDE was calculated using a calibration curve (1 mg/mL to 1 mg/mL).
In vivo mouse magnetic resonance angiography (MRA) and MRI
On week 12 and 16, in vivo imaging of the aortic root was performed on all mice using a vertical-bore Bruker 11.7-T Avance spectometer (Bruker; Billerica, USA) and a 30 mm probe (Micro 2.5). Mice were anesthetized with 0.5%-2% inhaled isoflurane, and placed in a holder with a bite bar and wrapped with parafilm to reduce motion. Respiration was monitored with a respiration pillow placed on the abdomen using a small animal monitoring and gating system (SA Instruments, Wahkesha, WI). The gated 2D gradient echo MRA was acquired as scout images. Respiration-gated T1-weighted (T1W) black-blood (T1BB) Magnetic Resonance (MR) images were acquired with a 2D axial spin echo sequence 15 minutes after gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA) injection (0.1 mmol/kg Ⅳ) (Magnevist, Germany). The slice thickness in aortic arch was 1.25 mm. Two 7 mm saturation bands were placed both inferior and superior to the imaging plane to further suppress the blood signal. The parameters used were: matrix size= 512×512, field of view= 2.50 cm, repetition time= 1,000 milliseconds, echo time= 15.69 milliseconds. Measurements were performed using ImageJ (National Institutes of Health, USA). Manual drawn contours were to delineate the whole vessel area (VA) and lumen area (LA) slice by slice. Wall area (WA) was calculated as VA-LA, and percentage of stenosis as WA/VA. The mean intensity of the vessel wall after contrast-enhancement was normalized to the mean signal from the external Gd standard from the same level to facilitate cross-sectional comparison. The above data from each slice were averaged to obtain the corresponding mean values for each mouse.
Plasma lipids
Blood samples were taken at 12 and 16 weeks after the beginning of a cholesterol-rich diet from the submandibular facial vein after overnight fasting for determination of total cholesterol and triglycerides level by commercial kits (Wako Diagnostics and Thermo Scientific, OH, USA).
Macroscopic analysis
The aorta was excised from the aortic arch to the abdominal artery, opened longitudinally, washed with saline solution and placed in 10% formalin. After fixation, the aorta was stained with Sudan Ⅳ and photographed immediately to measure the lesions. The macroscopic analysis was shown by total area, lesion area and atherosclerotic lesion area/total area of aorta. All measurements were performed using ImageJ (National Institutes of Health, USA).
Statistical analysis
Analyses were performed using Graph Pad Prism 5 (Graph Pad Software Inc., San Diego, CA, USA). Student's t-test was performed to comparison between the groups and between baseline and final treatment. Paired t-test, unpaired t-test, MannWhitney test and Wilcoxon test were applied when appropriate. The data were presented as mean±standard error of mean (SEM). Probability values of P<0.05 were considered significant.
Results
Blood cholesterol level and body and heart weight
Table 1 shows the comparison of plasma lipid profile at week 12 and 16 for control and LDE-paclitaxel groups. There were no significant differences in the total cholesterol and triglycerides concentrations between the two experimental groups at week 12 and 16.
Tab.1.
Plasma lipid profile of mice measured at 12 and 16 weeks after the beginning of a cholesterol-rich diet subjected to treatment with saline solution (control) or LDE-paclitaxel, 4 mg/(kg bodyweight week).
| Control group (n = 7) | LDE-paclitaxel (n = 12) | ||||
|---|---|---|---|---|---|
| 12th week | 16th week | 12th week | 16th week | ||
| Total cholesterol (mg/dL) | 335±52 | 325±73 | 366±41 | 301±45 | |
| Triglycerides (mg/dL) | 571±52 | 543±59 | 660±47 | 645±52 | |
Data are mean±SEM.
The total body weight at baseline (right before the first MRI at the 12th week) (control 28.6±2.1 g, LDE-paclitaxel 27.4±1.3 g, P = 0.33) and 16th week (control 29.5±1.8 g, LDE-paclitaxel 29.8±0.9 g, P = 0.65) demonstrated no significant difference between the groups. The heart weights at the sacrifice point also demonstrated no significant differences between the studied groups (control 0.14±0.02 g, LDE-paclitaxel 0.15±0.02 g, P = 0.92).
Atherosclerotic lesion measurement by MRI
Fig. 1A illustrates the representative scout image used to identify the anatomy and define the imaging planes. Fig. 1B shows the MRI image after LDE-paclitaxel treatment.
Fig.1.
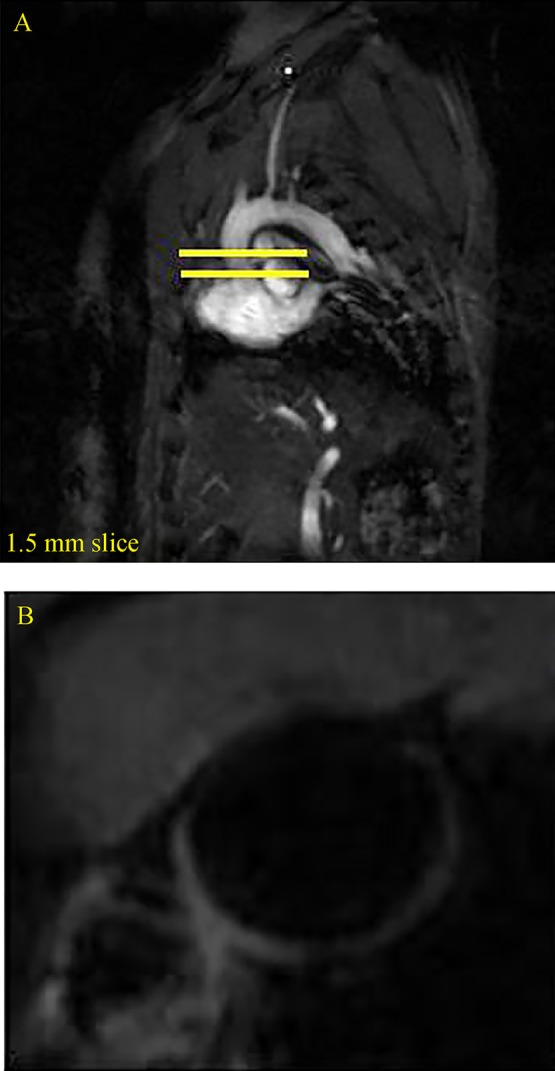
MRI images in sagittal view of the vasculature that were used to identify the anatomy and define the imaging places (A) and MRI images of aortic arch after treatment with LDE-paclitaxel (B).
As shown in Table 2, significant decreases were observed in wall area and percentage of stenosis in the LDE-paclitaxel group compared to control group, at week 12 and 16. However, there is no difference of mean intensity of vessel wall between the groups.
Tab.2.
MRI analysis in aortic root of mice at 12 and 16 weeks after the beginning of a cholesterol-rich diet subjected to treatment with saline solution (control) or LDE-paclitaxel, 4 mg/kg, weekly.
| Control group (n = 7) | LDE-paclitaxel (n = 12) | ||||
|---|---|---|---|---|---|
| 12th week | 16th week | 12th week | 16th week | ||
| Wall area (mm2) | 0.43±0.04 | 0.37±0.06 | 0.39±0.02 | 0.32±0.01*# | |
| Percentage of stenosis (mm2) | 0.43±0.04 | 0.41±0.07 | 0.39±0.02 | 0.32±0.02*†# | |
| Contrast-enhanced vessel wall area | 3072±333 | 3409±511 | 3333±224 | 3606±252 | |
Data are means±SEM. *P<0.05 vs. control week 12. †P<0.05 vs. control week 16. #P<0.05 vs. LDE-paclitaxel week 12.
Atherosclerotic lesion measurement by macroscopic analysis
The images of dissected aortas (Fig. 2) show that, compared to the control group, mice treated with LDE-paclitaxel exhibited reduction (P = 0,04), as shown in Table 3.
Fig.2.
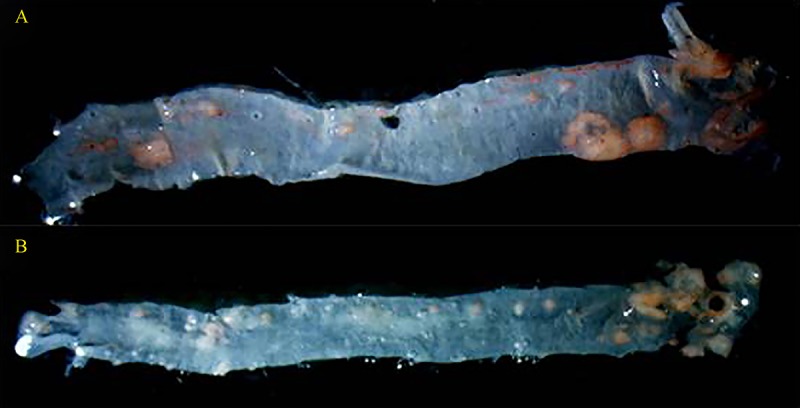
Mouse aortas after treatments with saline solution (A) and with LDE-paclitaxel (B).
Tab.3.
Macroscopic morphometry of aorta in mice treated with saline solution or LDE-paclitaxel, 4 mg/kg, weekly.
| Control group (n=7) | LDE-paclitaxel (n=12) | |
|---|---|---|
| Total area (pixel2 × 106) | 0.85±0.04 | 0.80±0.05 |
| Lesion area (pixel2× 106) | 0.09±0.01 | 0.06±0.01* |
| Atherosclerotic lesion/total area | 0.10±0.01 | 0.08±0.01* |
Data are means±SEM. *P<0.05 vs. control.
Discussion
Although atherosclerosis has long been recognized as a worldwide major public health, it is a complex, multifactorial disease. Specific therapies directed against the proliferative-inflammatory components of atherogenesis have not yet been incorporated to the clinical practice. Therefore, some novel therapeutic strategies are currently under consideration. In this study, it was shown that LDE-paclitaxel treatment could effectively reduce atherosclerotic lesions in LDLR knockout mice under high fat challenge. Paclitaxel is a chemotherapeutic drug, classified as taxane derivative with antiproliferative properties that make it effective in treating coronary restenosis[ 19]. Paclitaxel has been used in most vascular fields, and several trials using paclitaxel coated stents for local delivery of the drug for the treatment of lower-extremity peripheral arterial disease have been reported[ 20 22]. In our previous studies, rabbits with atherosclerosis induced by cholesterol feeding, treatment of paclitaxel, etoposide or methotrexate associated to lipid nanoparticles resulted in reduction of the lesion extension in the range of 60-85%[ 6, 14, 23]. The results of the present study are consistent with previous findings: LDE-paclitaxel decreased wall area and percentage of stenosis as observed by MRI, and reduced lesioned area by macroscopic morphometry.
The body weight in all groups was unchanged, which supports lack of important toxicity in the LDE-paclitaxel group. The results are similar to our previous studies, demonstrating further indication that the LDE-paclitaxel treatment has the ability to reduce the toxicity of chemotherapeutics[ 6, 14, 23].
In our study, we did not observe significant difference of Gd-enhancement between the LDE-paclitaxel and the control groups. The origin of Gd contrast enhancement of atherosclerotic plaque appears complex as several factors associated with inflammation, such as endothelium permeability, neovascularization, and interstitial pressure, contribute to Gd uptake and retention[ 24]. These factors may be not necessarily present in early stages of atherosclerotic lesions as induced in our study. Therefore, it is difficult to discuss the effect of LDE-paclitaxel on transient hyperpermeability of Gd at early stages of atherosclerosis based on our results.
Currently, mouse models are the most frequently used species for atherosclerosis studies[ 25]. Mice with targeted deletion of the gene for the LDL receptor have been widely used as a model to mimic human atherosclerosis and spontaneously develop atherosclerotic lesions at many sites in the arterial tree. The phenomenon of LDL receptor overexpression is not restricted to neoplasias and is possibly present whenever a greater cell input of cholesterol is required by accelerated mitosis. Therefore, other sources of lipids for membrane building are required for the increased mitosis rates in proliferative processes[ 26]. We showed that in patients with thalassemia minor, in whom a non-neoplastic proliferation, there is overexpression of LDL receptors and increased tissue uptake of LDE[ 27]. Moreover, it is remarkable that precisely in aortic tissue the uptake of LDE more than doubled after the induction of atherosclerosis by cholesterol feeding[ 6]. This demonstrates that the atherosclerotic lesion regions, in which inflammatory processes prevail, there is present overexpression of the receptors that take up native LDL and LDE. It was assumed that LDE, which structurally resembles LDL, is recognized mostly by the LDL receptors[ 28]. However, in our model with LDLR knockout suggests that LDE could be uptaken by other LDL receptors, such as LRP-1. However, further studies are needed to clarify the accrual proteins involved. This finding may help better understand the mechanism of LDE uptake and help future studies of LDE-associated drugs in atherosclerosis in mouse model.
In conclusion, our results obtained in LDLR knockout mice showed that the association of LDE-paclitaxel was effective in reducing the wall area, percentage of stenosis and diminishing lesion area. Data obtained from MRI fairly converged with those from aorta macroscopy with Sudan Ⅳ staining, which is also of methodological interest. These findings suggest that LDE can be recognized not exclusively by LDLR but also by other receptor mechanisms. In this setting, scavenger receptors and LRP-1 may thus offer promising strategies for the treatment of atherosclerotic cardiovascular disease.
Acknowledgments
Research funding was provided by a grant from Boston University, United States, Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), São Paulo, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brasília, Brazil. Lima had a scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior CAPES and express her appreciation.
References
- [1].Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions[J]. Nat Rev Cardiol, 2010, 7(2): 7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Libby P, Bornfeldt KE, Tall AR. Atherosclerosis: successes, surprises, and future challenges[J]. Circ Res, 2016, 118(4): 531534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Charo IF, Taub R. Anti-inflammatory therapeutics for the treatment of atherosclerosis[J]. Nat Rev Drug Discov, 2011, 10(5): 365376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Maranhão RC, Leite AC Jr. Development of anti-atherosclerosis therapy based on the inflammatory and proliferative aspects of the disease[J]. Curr Pharm Des, 2015, 21(9): 11961204. [DOI] [PubMed] [Google Scholar]
- [5].Shurin MR, Naiditch H, Gutkin DW, et al. ChemoImmunoModulation: immune regulation by the antineoplastic chemotherapeutic agents[J]. Curr Med Chem, 2012, 19(12): 17921803. [DOI] [PubMed] [Google Scholar]
- [6].Maranhão RC, Tavares ER, Padoveze AF, et al. Paclitaxel associated with cholesterol-rich nanoemulsions promotes atherosclerosis regression in the rabbit[J]. Atherosclerosis, 2008, 197(2): 959966. [DOI] [PubMed] [Google Scholar]
- [7].Rodrigues DG, Covolan CC, Coradi ST, et al. Use of a cholesterol-rich emulsion that binds to low-density lipoprotein receptors as a vehicle for paclitaxel[J]. J Pharm Pharmacol, 2002, 54(6): 765772. [DOI] [PubMed] [Google Scholar]
- [8].Dias ML, Carvalho JP, Rodrigues DG, et al. Pharmacokinetics and tumor uptake of a derivatized form of paclitaxel associated to a cholesterol-rich nanoemulsion (LDE) in patients with gynecologic cancers[J]. Cancer Chemother Pharmacol, 2007, 59(1): 105111. [DOI] [PubMed] [Google Scholar]
- [9].Maranhão RC, Graziani SR, Yamaguchi N, et al. Association of carmustine with a lipid emulsion: in vitro, in vivo and preliminary studies in cancer patients[J]. Cancer Chemother Pharmacol, 2002, 49(6): 487498. [DOI] [PubMed] [Google Scholar]
- [10].Kretzer IF, Maria DA, Maranhão RC. Drug-targeting in combined cancer chemotherapy: tumor growth inhibition in mice by association of paclitaxel and etoposide with a cholesterol-rich nanoemulsion[J]. Cell Oncol (Dordr), 2012, 35(6): 451460. [DOI] [PubMed] [Google Scholar]
- [11].Pinheiro KV, Hungria VT, Ficker ES, et al. Plasma kinetics of a cholesterol-rich microemulsion (LDE) in patients with Hodgkin’s and non-Hodgkin’s lymphoma and a preliminary study on the toxicity of etoposide associated with LDE[J]. Cancer Chemother Pharmacol, 2006, 57(5): 624630. [DOI] [PubMed] [Google Scholar]
- [12].Ruiz J, Kouiavskaia D, Migliorini M, et al. The apoE isoform binding properties of the VLDL receptor reveal marked differences from LRP and the LDL receptor[J]. J Lipid Res, 2005, 46(8): 17211731. [DOI] [PubMed] [Google Scholar]
- [13].Goldstein JL, Brown MS. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia[J]. J Biol Chem, 1974, 249(16): 51535162. [PubMed] [Google Scholar]
- [14].Bulgarelli A, Leite AC Jr, Dias AA, et al. Anti-atherogenic effects of methotrexate carried by a lipid nanoemulsion that binds to LDL receptors in cholesterol-fed rabbits[J]. Cardiovasc Drugs Ther, 2013, 27(6): 531539. [DOI] [PubMed] [Google Scholar]
- [15].Jawień J, Nastałek P, Korbut R. Mouse models of experimental atherosclerosis[J]. J Physiol Pharmacol, 2004, 55(3): 503517. [PubMed] [Google Scholar]
- [16].Hockings PD, Roberts T, Galloway GJ, et al. Repeated three-dimensional magnetic resonance imaging of atherosclerosis development in innominate arteries of low-density lipoprotein receptor-knockout mice[J]. Circulation, 2002, 24;106(13):171621. [DOI] [PubMed] [Google Scholar]
- [17].Ginsburg GS, Small DM, Atkinson D. Microemulsions of phospholipids and cholesterol esters. Protein-free models of low density lipoprotein[J]. J Biol Chem, 1982, 257(14): 82168227. [PubMed] [Google Scholar]
- [18].Maranhão RC, Cesar TB, Pedroso-Mariani SR, et al. Metabolic behavior in rats of a nonprotein microemulsion resembling low-density lipoprotein[J]. Lipids, 1993, 28(8): 691696. [DOI] [PubMed] [Google Scholar]
- [19].Kamath KR, Barry JJ, Miller KM. The Taxus drug-eluting stent: a new paradigm in controlled drug delivery[J]. Adv Drug Deliv Rev, 2006, 58(3): 412436. [DOI] [PubMed] [Google Scholar]
- [20].Dake MD, Ansel GM, Jaff MR, et al. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results[J]. Circ Cardiovasc Interv, 2011, 1;4(5):495504. [DOI] [PubMed] [Google Scholar]
- [21].Tepe G, Zeller T, Albrecht T, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg[J]. N Engl J Med, 2008, 358(7): 689699. [DOI] [PubMed] [Google Scholar]
- [22].Werk M, Langner S, Reinkensmeier B, et al. Inhibition of restenosis in femoropopliteal arteries: paclitaxel-coated versus uncoated balloon: femoral paclitaxel randomized pilot trial[J]. Circulation, 2008, 118(13): 13581365. [DOI] [PubMed] [Google Scholar]
- [23].Tavares ER, Freitas FR, Diament J, et al. Reduction of atherosclerotic lesions in rabbits treated with etoposide associated with cholesterol-rich nanoemulsions[J]. Int J Nanomedicine, 2011, 6: 22972304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chaabane L, Pellet N, Bourdillon MC, et al. Contrast enhancement in atherosclerosis development in a mouse model: in vivo results at 2 Tesla[J]. MAGMA, 2004, 17(3-6): 188195. [DOI] [PubMed] [Google Scholar]
- [25].Getz GS, Reardon CA. Animal models of atherosclerosis[J]. Arterioscler Thromb Vasc Biol, 2012, 32(5): 11041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ishibashi S, Goldstein JL, Brown MS, et al. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice[J]. J Clin Invest, 1994, 93(5): 18851893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Naoum FA, Gualandro SF, Latrilha MdaC, et al. Plasma kinetics of a cholesterol-rich microemulsion in subjects with heterozygous beta-thalassemia[J]. Am J Hematol, 2004, 77(4): 340345. [DOI] [PubMed] [Google Scholar]
- [28].Maranhão RC, Roland IA, Toffoletto O, et al. Plasma kinetic behavior in hyperlipidemic subjects of a lipidic microemulsion that binds to low density lipoprotein receptors[J]. Lipids, 1997, 32: 627633. [DOI] [PubMed] [Google Scholar]


