Abstract
The International Agency for Research on Cancer and the World Health Organization have designated airborne particulates, including particulates of median aerodynamic diameter ≤ 2.5 μm (PM2.5), as Group 1 carcinogens. It has not been determined, however, whether exposure to ambient PM2.5 is associated with an increase in respiratory related diseases. This meta-analysis assessed the association between exposure to ambient fine particulate matter (PM2.5) and the risk of respiratory tract diseases, using relevant articles extracted from PubMed, Web of Science, and Embase. In results, of the 1,126 articles originally identified, 35 (3.1%) were included in this meta-analysis. PM2.5 was found to be associated with respiratory tract diseases. After subdivision by age group, respiratory tract disease, and continent, PM2.5 was strongly associated with respiratory tract diseases in children, in persons with cough, lower respiratory illness, and wheezing, and in individuals from North America, Europe, and Asia. The risk of respiratory tract diseases was greater for exposure to traffic-related than non-traffic-related air pollution. In children, the pooled relative risk (RR) represented significant increases in wheezing (8.2%), cough (7.5%), and lower respiratory illness (15.3%). The pooled RRs in children were 1.091 (95%CI: 1.049, 1.135) for exposure to <25 μg/m3 PM2.5, and 1.126 (95%CI: 1.067, 1.190) for exposure to ≥ 25 μg/m3 PM2.5. In conclusion, exposure to ambient PM2.5 was significantly associated with the development of respiratory tract diseases, especially in children exposed to high concentrations of PM2.5.
Keywords: particulate matter, PM2.5, respiratory tract disease, meta-analysis, cohort study
Introduction
Air pollution is a complicated process involving the spread of distinct pollutants throughout the atmosphere. Air pollution has been found to induce diseases in humans and disorders in other living organisms, as well as destruction of the natural environment[ 1 2]. One type of pollutant, particulate matter (PM)[ 3], has been associated with serious public health problems[ 4], as has combinations of PM and other air pollutants[ 5]. PM is classified according to its aerodynamic diameter, and the gold standard used to evaluate its transport capacity in the atmosphere and inhalation capacity through the respiratory tract[ 6]. PM is primarily categorized as coarse (PM10), of median aerodynamic diameter≤10 μm, and fine (PM2.5), of median aerodynamic diameter≤2.5 μm[ 6].
PM originates from a wide range of sources, including road dust, agricultural dust, industrial emissions, construction sites, mining operations, river beds, crustal materials, and combustion, or as secondary aerosols from distant sources[ 7 9]. Due to the diversity of sources, human exposure is high. Entry of PM into the respiratory tract depends on the physical characteristics, breathing mode and rate, and size of an individual[ 10]. Moreover, PM size has been significantly related to the etiology of pertinent diseases. Frequently, smaller PM such as PM2.5 penetrates the respiratory tract more deeply at a higher rate, and is deposited in the respiratory bronchioles and alveoli or enters the bloodstream, influencing lung function and eventually causing other disorders[ 11]. Exposure to PM has been shown to be harmful to public health, increasing the incidence of respiratory symptoms, reducing lung function, and aggravating respiratory and cardiovascular diseases[ 12].
PM2.5 can also act as a carrier of other harmful constituents, such as heavy metal ions, which add to the deleterious effects of "inert" material[ 13]. Studies analyzing the induction of respiratory diseases by exposure to PM2.5 have yielded different outcomes. To quantitatively and accurately assess the effects of exposure to PM2.5 on respiratory tract diseases, we performed a meta-analysis that included all relevant cohort studies published to date. This meta-analysis showed that exposure to PM2.5 increased the risk of respiratory tract disease.
Materials and methods
Systematic ascertainment of correlative studies
The online databases, including PubMed (National Library of Medicine, Bethesda, MD, USA), Web of Science (Thompson Scientific, Philadelphia, PA, USA), and Embase (Excerpta Medica Database, the Netherlands) were searched for cohort studies published and indexed through May 4, 2016 on the epidemiology of respiratory tract diseases associated with PM2.5 air pollution. Search strings, both free text and medical subject headings (MeSH), included (PM2.5 OR "particulate matter") AND (wheezing OR bronchitis OR cough OR asthma OR pneumonia OR COPD OR "lung cancer" OR "respiratory infections" OR "respiratory tract diseases"). Based on article titles, abstracts, and full texts, these cohort studies were screened for those fulfilling our inclusion criteria. Cohort-specific results reported in previous meta-analyses alone were also considered in the current analysis.
Inclusion criteria
All epidemiological studies involving the impact on human health of exposure to PM2.5 were screened. Health outcomes of interest were morbidities of respiratory tract diseases, according to ICD9 or ICD10, including pneumonia, asthma, bronchitis, upper respiratory tract infections, lower respiratory infections (LRI), wheezing, cough, chronic obstructive pulmonary disease, and lung cancer (Supplementary Table 1). Studies were included if they had cohort designs for respiratory diseases and if they reported relative risk (RR) or odds ratio (OR) with 95% confidence intervals (CIs).
If multiple studies reported an association between respiratory tract disease and PM2.5 in the same study cohort at different times, the latest one was chosen. However, if these studies reported different outcomes in the same cohort, each was included. Only studies published in English and exclusively involving human subjects were included. Cohort-specific results reported in previous meta-analyses were considered. If that cohort was included in a meta-analysis that included several other cohorts, the latest publication by this cohort was included.
Exclusion criteria
Case-control studies, case series, and case reports were excluded, as were studies lacking appropriate data (e.g. useful RRs or ORs and 95% CIs in related cohort studies). These criteria were used to maximize sensitivity and ensure non-omission of any relevant study.
Data extraction
All selected publications were screened independently by two investigators (Q.L. and C.X.), and data extracted using a standardized form. Conflicts were resolved by discussions between the two investigators.
Meta-analysis
Effect estimates for all data collected from the selected studies were summarized using STATA software (version 11; Stata Corp, College Station, TX, USA). The unadjusted RRs and ORs with their 95% CIs were integrated to analyze the strength of the risk of respiratory tract diseases in participants exposed to PM2.5. Heterogeneity among studies was examined using a chi-square-based Q-statistic test and the standard I2 test. The Q-statistic test can only determine the presence or absence of heterogeneity, not the degree of heterogeneity. As the I2 test may quantify the extent of heterogeneity in a meta-analysis[ 14], two methods were chosen to simultaneously assess heterogeneity. Between-study heterogeneity reflected variations in study outcomes among different studies due to inherent differences in study design/populations/exposures, not to chance alone. All pooled RRs were calculated using a random effects model. I2 showed that heterogeneity accounts for a percentage of the overall variability in random error. I2<40%, 30%-60%, 50%-90%, and 75%-100% represented unimportant, medium, substantial, and high degree of heterogeneity, respectively[ 15].
Subjects were stratified into subgroups based on heterogeneity, age, region, PM2.5 exposure level and source, and differences between groups and risk factors were calculated[ 16]. Moreover, sensitivity analyses were performed to estimate the stability of the outcomes. That is, one study at a time was iteratively removed and the results of the remaining studies were determined[ 17]. Begg funnel plots and the Egger test of asymmetry were performed to statistically evaluate publication bias; a P value<0.05 was considered indicative of publication bias.
Results
Characteristics of the eligible studies assessed by meta-analysis
A review of the PubMed, Web of Science and Embase identified 1,126 eligible studies published in English. Based on the inclusion/exclusion criteria for the effects of exposure to PM2.5 pollution on respiratory tract diseases, 35 related studies were retrieved[ 18 52]. A flow diagram of the literature search and selection procedure is shown in Fig. 1. Table 1 shows the details of each of the 35 studies; in these articles, there were 12 outcomes related to wheezing, 5 to bronchitis, 12 to cough, 14 to asthma, 1 to pneumonia, 4 to lower respiratory tract illness (LRI), 0 to upper respiratory tract illness (URI), 4 to lung cancer, 0 to COPD, and 2 to respiratory infections. These 35 articles included a total of 1,135,203 subjects with different respiratory tract diseases. Primary outcomes in our meta-analysis included the incidence of newly developed or exacerbated respiratory tract diseases. These participants resided in a variety of countries or regions and included subjects in different age groups. The mean, median and 50% interquartile range (IQR) for PM2.5 ranged from 3.60 to 100 μg/m3. There were seven articles on traffic-related air pollution and two meta-analyses that included unpublished data of some birth cohort studies (Table 1).
Tab.1.
Characteristics of cohort studies with PM2.5 exposure
| Author (published year) | Study year | Cohort / Study | Outcomes of included studies | Sample number | Age (year) (group) | Country (Continent) | PM2.5 (μg/m3) |
|---|---|---|---|---|---|---|---|
| Neas et al. 1994 [18] | 1983-1988 | - | wheezing, cough, bronchitis, asthma, LRI | 1,237 | 7 to 11 (children) | United States (North America) | 31.1 |
| Romieu et al. 1996 [19] | 1991-1992 | - | wheezing, cough, LRI | 71 | 5 to 13 (children) | Mexico (North America) | 85.7 |
| Tiitanen et al. 1999 [20] | 1995 | the PEACE study | cough | 76 | 8-13 (children) | Finland (Europe) | 15 a |
| Schwartz et al. 2000 [21] | 1990-1991 | the Harvard Six City Study | cough, LRI | 1,844 | school-aged (children) | United States (North America) | 15 |
| Gehring et al. 2002 [22] | 1997-1999 | the PIAMA birth cohort | respiratory infections | 1,606 | Infants (children) | German (Europe) | 13.4 c |
| Gent et al. 2003 [23] | 2001 | - | wheezing, cough | 271 | <12 (children) | New England (Europe) | 13.1 |
| Mar et al. 2004 [24] | 19971999 | - | wheezing, cough | 25 | children and adults | United States (North America) | 10 |
| Millstein et al. 2004 [25] | 1994-1995 | - | wheezing | 2,034 | 9.6 (0.4) (children) | United States (North America) | 5.24 |
| Pino et al. 2004 [26] | 1995-1996 | - | bronchitis | 504 | infants (children) | Chile (South America) | 52 |
| Johnston et al. 2006 [27] | 2004 | - | asthma | 235 | children and adults | Australian (Oceania) | 11.1 |
| Bennett et al. 2007 [28] | 1998-2005 | - | wheezing, cough, asthma | 1,446 | 37.2 (7.2) (adult) | Australian (Oceania) | 6.8 |
| Brauer et al. 2007 [29] | 1999-2003 | the PIAMA birth cohort | bronchitis, cough | 4,146 | 4 (children) | Netherlands (Europe) | 16.9 c |
| Morgenstern et al. 2007 [30] | 1999-2000 | GINI and LISA birth cohort | wheezing, bronchitis, cough, respiratory infections | 2,908 | children (children) | Germany (Europe) | 12.8 c |
| Picciotto et al. 2007 [31] | 1994-2003 | - | bronchitis | 1,492 | 3 to 4.5 (children) | United States (North America) | >25 |
| Rodriguez et al. 2007 [32] | 1996-2003 | - | wheezing, cough | 263 | 5 (children) | Australian (Oceania) | 8.534 |
| Beelen et al. 2008 [52] | 1986-1997 | - | lung cancer | 1,940 | 55 to 69 (adult) | Netherlands (Europe) | 28.2 c |
| Nuñez et al. 2008 [33] | 2003-2005 | the EVA cohort | wheezing, cough | 197 | 6 to 14 (children) | Mexico (North America) | 27.8 c |
| Clark et al. 2010 [34] | 1999-2000 | - | asthma | 3,484 | 3 to 4 (children) | United States (North America) | 4.67 |
| Gehring et al. 2010 [35] | 1996-2006 | the PIAMA birth cohort | wheezing | 3,863 | 8 (children) | Netherlands (Europe) | 16.9 c |
| Gurley et al. 2013 [36] | 2008-2011 | - | LRI | 257 | 2 (children) | Bangladesh (Asia) | 100 |
| Li et al. 2013 [37] | 2006-2009 | - | asthma | 412,832 | >18 (adult) | United States (North America) | 11.6 |
| Nielsen et al. 2013 [51] | - | the ESCAPE study | lung cancer | 2,095 | 43 to 73 (adult) | (Europe) | 5 c |
| Evans et al. 2014 [38] | 2002-2007 | - | asthma | 530 | 3 to10 (children) | United States (North America) | 8.6 |
| Loftus et al. 2014 [39] | 2010-2012 | - | wheezing | 58 | school-aged (children) | United States (North America) | 6.9 b |
| MacIntyre et al. 2014 d[46] | - | 10 European birth cohorts | pneumonia | 14,009 | 36 month (children) | (Europe) | 5 |
| MÖlter et al. 2014 d [40] | - | 6 birth cohort | asthma | 10,377 | 8 / 10 (children) | (Europe) | 5 |
| Puett et al. 2014 [50] | 1994-2010 | - | lung cancer | 2,155 | 67±8.3 (women) | United States (North America) | 10 |
| Wendt et al. 2014 [41] | 2005-2007 | - | asthma | 18,289 | 1to17 (children) | United States (North America) | 14.97 |
| Young et al. 2014 [42] | 2003-2009 | - | wheezing, cough, asthma | 50,884 | 55±9 (adult) | United States (North America) | 3.6 b |
| Jacquemin et al. 2015 d [43] | - | the ESCAPE study | asthma | 23,704 | adult (adult) | (Europe) | 5 |
| Rice et al. 2015 [44] | 1998-2011 | - | wheezing, bronchitis, cough, asthma | 4,444 | 50.4 (12.4) (adult) | United States (North America) | 10.8 a |
| Teresa et al. 2015 [45] | 1998-2006 | the CNBSS study | asthma | 29,549 | 40 to 59 (adult) | Canada (North America) | 12.57 |
| Gehring et al. 2016 [47] | 1996-2010 | 4 European birth cohorts | asthma | 6,864 | 14 to 16 (children) | (Europe) | 10 |
| Guo et al. 2016 [49] | 1990-2009 | - | lung cancer | 368,762 | >30 (adult) | China (Asia) | 10 |
| Tétreault et al. 2016 [48] | 1996-2011 | QICDSS | asthma | 162,752 | 13 (children) | Canada (North America) | 6.5 b |
LRI : Lower respiratory illness ; PM2.5 : mean, a median or b 50% IQR( Interquartile Range ) ; Traffic-related air pollution : c ; meta : d 6 birth cohorts: MAAS, BAMSE, PIAMA, GINI and LISA birth cohort; 10 European birth cohorts: BAMSE, GASPII, GINI and LISA, MAAS, PIAMA and four INMA cohorts; 4 European birth cohorts: BAMSE, PIAMA, GINI and LISA birth cohort
Fig.1.
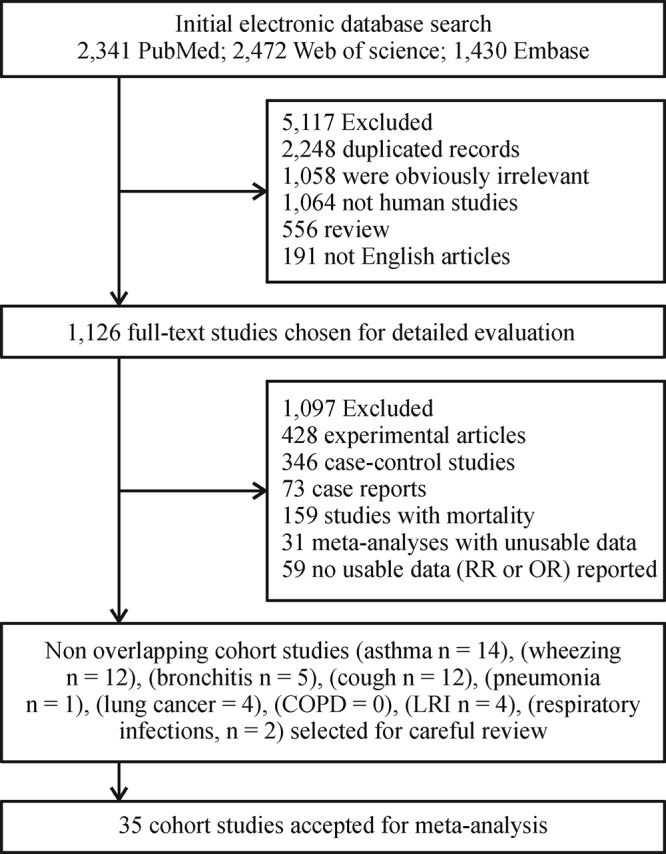
Literature search and article selection protocol used in the present meta-analysis.
Evidence synthesis
Pooled analysis of the 35 included articles showed that exposure to PM2.5 pollution significantly increased the RR for respiratory tract diseases for (RR= 1.076, 95% CI: 1.050, 1.103; Pheterogeneity<0.001, I2 = 93.6%). To assess differences among subgroups, studies were divided by the age of the participants (children or adults), geographic areas (North America, Europe, Oceania, or Asia), types of diseases, and sources of PM2.5 (traffic-related or non-traffic related air pollution) (Figs. 2-5). Studies in children were stratified by types of disease, and extent of exposure concentrations (Table 2, Fig. 6). The pooled RRs in the random-effects model were 1.104 (Table 2 and Fig. 2) for children, 1.099 for Europe, 1.090 for North America, 1.064 (Fig. 3) for Asia, 1.073 for wheezing, 1.153 for LRI, and 1.048 (Fig. 4) for cough. In addition, the pooled RRs exposed to traffic-related and non-traffic related air pollution were 1.085 and 1.076 (Fig. 5), respectively. The pooled RRs in children showed that the rates of wheezing (8.2%), cough (7.5%), and LRI (15.3%) were significantly increased. In adults, however, no positive association was found (Table 2). The pooled RRs in children exposed to PM2.5 concentrations<25 μg/m3 and≥25 μg/m3 were 1.091 and 1.126 (Fig. 6), respectively. However, other subgroups showed no association between RR and exposure to PM2.5.
Fig.2.
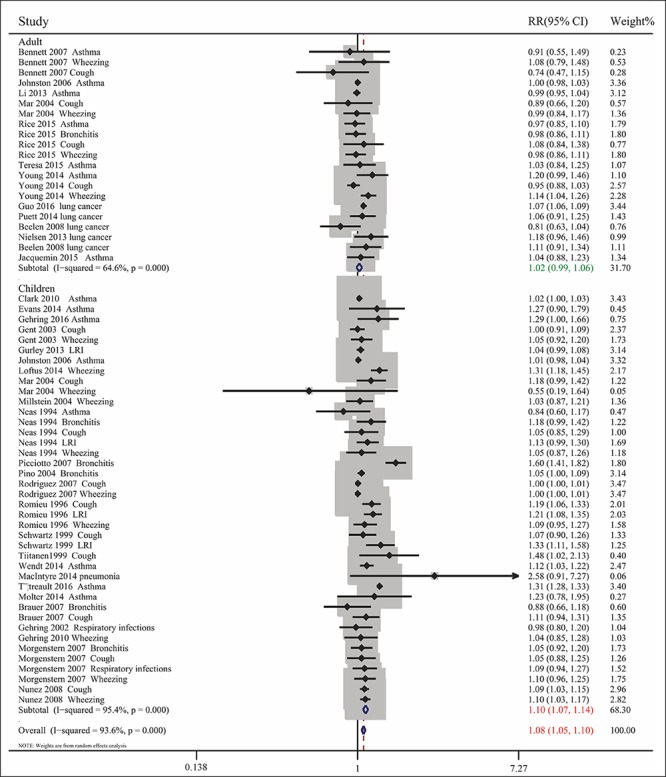
Combined RRs with 95% CIs for the association between PM2.5 exposure and respiratory tract diseases in all subjects and in subpopulations of children and adults.
Fig.3.
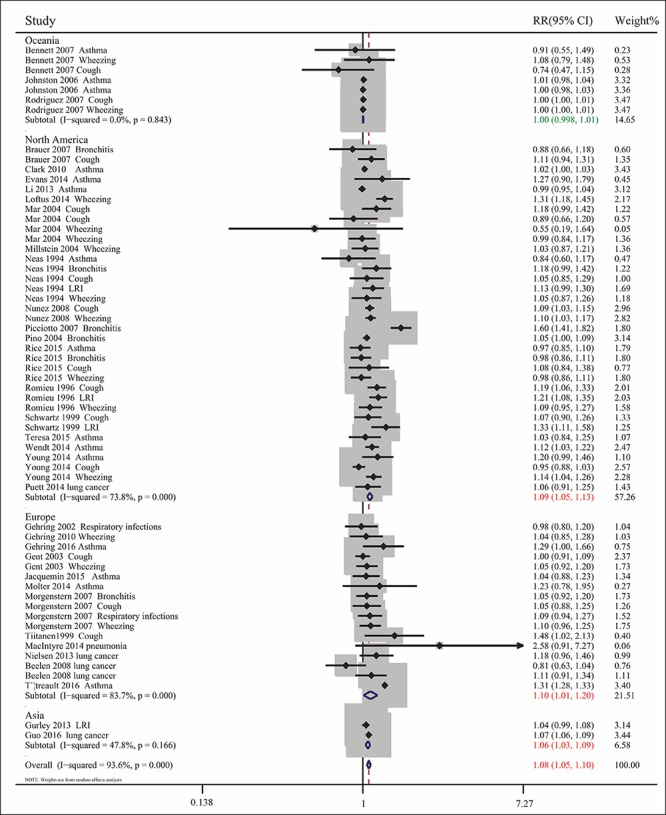
Combined RRs with 95% CIs for the association between PM2.5 exposure and respiratory tract diseases by geographic region (North America, Asia, Europe, and Oceania).
Fig.4.
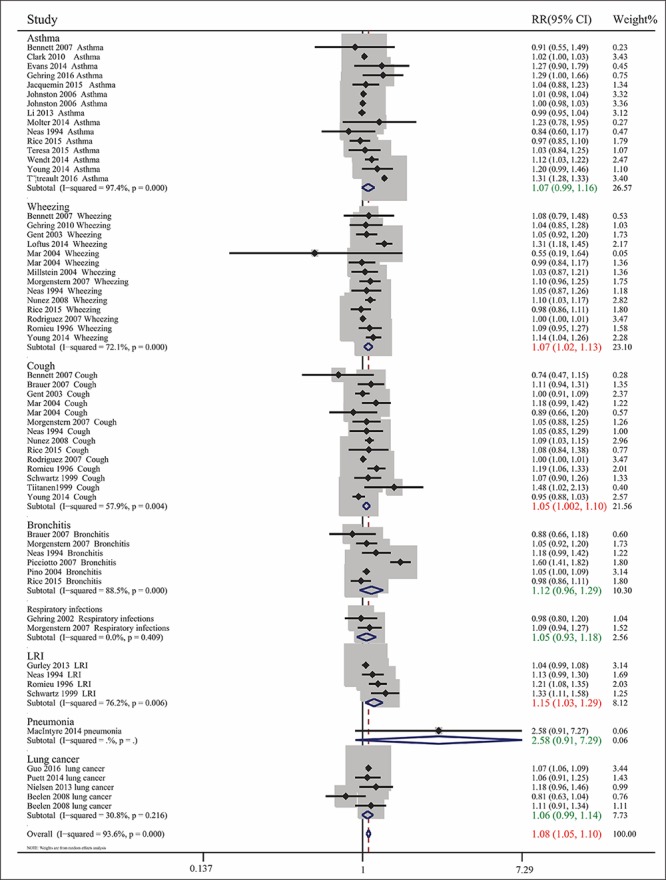
Combined RRs with 95% CIs for the association between PM2.5 exposure and respiratory tract diseases by type of disease (asthma, bronchitis, cough, LRI and wheezing).
Fig.5.
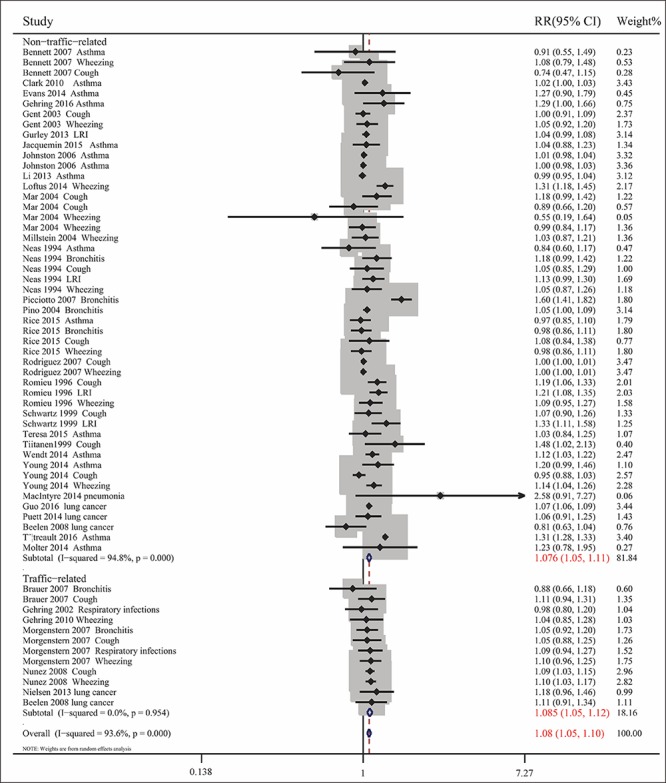
Combined RRs with 95% CIs for the association between exposure to PM2.5 and respiratory tract diseases exposed to traffic-related and non-traffic-related air pollution.
Fig.6.
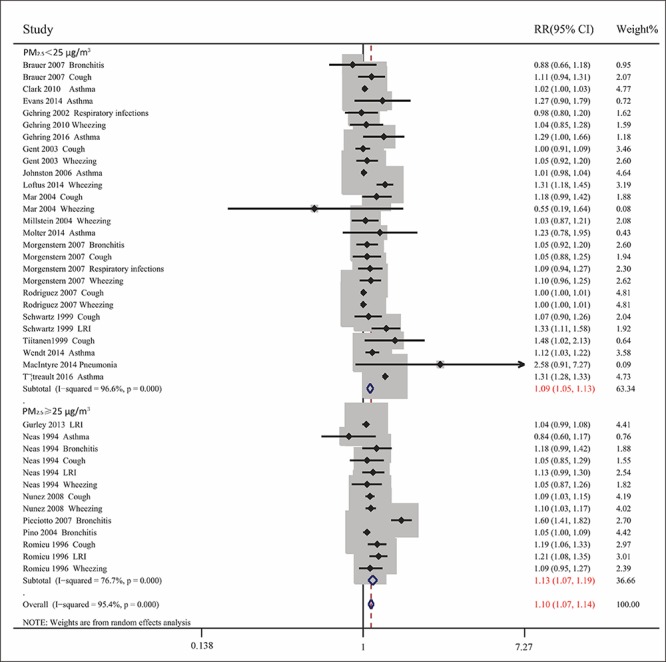
Combined RRs with 95% CIs for the association between exposure to low and high concentrations of PM2.5 and respiratory tract diseases in children.
Tab.2.
Association of PM2.5 exposure with episodes of respiratory tract diseases in children and adult group.
| N | RR (95%CI) Random-effects | P (I2) | P for Egger / Begg bias test | |
|---|---|---|---|---|
| Children Symptom | ||||
| Wheezing | 10 [18,19,23-25,30,32,33,35,39] | 1.082 (1.011, 1.158) | 0.000 (77.2%) | 0.079 / 0.107 |
| Bronchitis | 5 [18,26,29-31] | 1.145 (0.957, 1.370) | 0.000 (90.2%) | 0.593 / 1.000 |
| Cough | 10 [18-21,23,24,29,30,32,33] | 1.075 (1.019, 1.134) | 0.002 (65.4%) | 0.007 / 0.721 |
| Asthma | 8 [18,27,34,38,40,41,47,48] | 1.119 (0.989, 1.266) | 0.000 (98.5%) | 0.910 / 0.536 |
| Lower respiratory illness | 4 [18,19,21,36] | 1.153 (1.033, 1.287) | 0.006 (76.2%) | 0.064 / 0.308 |
| Respiratory infections | 2 [22,30] | 1.050 (0.930,1.184) | 0.409 (0.00%) | N/A / 1.000 |
| Pneumonia | 1 [46] | 2.580 (0.910,7.270) | - | - |
| Total | 40 | 1.104 (1.069, 1.139) | 0.000 (95.4%) | 0.025 / 0.408 |
| Adult Symptom | ||||
| Wheezing | 4 [24,28,42,44] | 1.053 (0.964, 1.150) | 0.229 (30.5%) | 0.593 / 1.000 |
| Bronchitis | 1 [44] | 0.980 (0.860, 1.110) | - | - |
| Cough | 4 [24,28,42,44] | 0.951 (0.885, 1.021) | 0.493 (0.00%) | 0.713 / 0.308 |
| Asthma | 7 [27,28,37,42-45] | 0.999 (0.978, 1.021) | 0.553 (0.00%) | 0.581 / 1.000 |
| Lung cancer | 4 [49,50,51,52] | 1.064 (0.991, 1.142) | 0.216 (30.8%) | 0.691 / 0.806 |
| Total | 20 | 1.022 (0.986, 1.058) | 0.000 (64.6%) | 0.132 / 0.546 |
P (I2): P-value for test of heterogeneity, I2 of Higgins and Thompson reflecting the proportion of total variation in the estimate that is due to heterogeneity between studies. LRI: lower respiratory illness. Bold: significant results.
Sensitivity analysis
Sensitivity analysis was performed by re-analyzing RR after removing one study at a time. The correlation between exposure to PM2.5 pollution and the RR of respiratory tract disease was not driven by any individual study, with no alterations in the significance of the pooled RRs, suggesting that the combined RR remained stable and reliable. Sensitivity analysis indicated that the omission of any one study resulted in RRs between 1.057 (95% CI: 1.038, 1.075) and 1.080 (95% CI: 1.049, 1.112).
Publication bias
Table 2 shows no funnel plot asymmetry, while P values of the Begg and Egger tests were greater than 0.05 in both the global and stratified analyses, respectively. These findings indicated a lack of publication bias.
Discussion
Previous epidemiological and experimental studies have been unable to definitively determine the specific mechanisms by which exposure to PM has adverse effects on human health. However, accumulated evidence suggests that the most deleterious effects of PM are dependent on particle size, with PM2.5 being especially harmful[ 53 54]. Exposure to PM2.5 has been found to increase health risks, particularly with regard to respiratory tract diseases[ 55]. Mortality has been regarded as the one important indicator of the effects of PM2.5 pollution on health outcomes[ 56]. Several case-control studies have also assessed daily hospital admissions or emergency department visits[ 57]. However, these variables are limited by the lack of persistent observation, restriction of end points, and recall bias. Therefore, this meta-analysis was performed to clarify the relationship between exposure to PM2.5 and the incidence or aggravation rate of respiratory tract diseases in cohort studies. In addition, studies were stratified by age, geographic location, and the source and concentration of PM2.5.
This systematic review found that exposure to PM2.5 was positively correlated with risk of respiratory tract disease, especially in children; in subjects with wheezing, cough, and LRI; and in populations in Europe, North America, and Asia; The pooled RRs were greater for traffic-related than non-traffic-related air pollution. Ultimately, we found that PM2.5 in children was significantly associated with cough, wheezing, and LRI. Furthermore, in children, pooled RRs were greater for high (≥25 μg/m3) than low (<25 μg/m3) PM2.5 concentrations. These findings suggested that traffic-related PM2.5 did greater harm to the human body, and that exposure to PM2.5 pollution may pose an increased risk for respiratory tract disease, especially in children exposed to high concentrations of PM2.5. These results are consistent with those of previous reports[ 58 59], as well as being more accurate than the results of a previous meta-analysis of case-control studies[ 60].
Above all, this meta-analysis included many more articles with a larger population, especially birth cohorts, than previous meta-analyses. Second, longitudinal cohort studies with complete and reliable information are considered less likely to be influenced by confounding and better able to address the temporal sequence of events. Finally, this study not only revealed a strong association between PM2.5 and respiratory tract diseases in general, but also strong correlations between PM2.5 and specific types of respiratory tract diseases (wheezing, cough and LRI). Therefore, the results of this meta-analysis could amplify and complete those of earlier meta-analyses.
However, the pathological mechanisms underlying the effects of PM2.5 exposure on the respiratory tract are not fully understood. PM size may be affected by chemical, biologic, and physical properties, resulting in various pathological consequences[ 6]. There are several plausible biomedical explanations for associations between exposure to PM2.5 and respiratory tract diseases. Owing to its fine consistency, PM2.5 can be deposited more deeply into the lungs. Moreover, these particles may contain toxic components or contaminants, such as nitrates, sulfates, acids, and metals, originating from combustion processes or similar activities[ 61]. These particles can therefore lead to stress, inflammation, and allergy. Our results showed that PM2.5 exposure resulted in a greater incidence of cough, wheezing, and LRI in children than in adults, which may be related to differences in the structure of the respiratory tract in adults and children. Specifically, the immature respiratory system in children may be more sensitive to PM2.5. PMc of aerodynamic diameter 2.5-10 μm mainly derives from the soil and abrasive mechanical processes. These particles may transport biologic materials, such as bacteria, molds, and pollens, which may have harmful effects on the respiratory system[ 62 63]. Therefore, some infectious bacterial respiratory diseases or hay fever are more likely to manifest after lengthy exposure to PMc rather than PM2.5. Our results are consistent with these findings.
We also found that the correlation between PM2.5 exposure and respiratory diseases was stronger in European and North American populations, probably due to more studies of PM2.5 were conducted in these populations. The risk of respiratory diseases was also higher following exposure to high than low quantities of PM2.5, suggesting that the concentration of PM2.5 is also a risk factor for respiratory diseases.
This meta-analysis had several limitations. First, some of the outcomes demonstrated heterogeneity. We utilized a random-effects model, with stratified analysis performed to make up for this shortcoming. Second, non-English publications and unpublished results were excluded. Some of these studies may have included negative outcomes, which could have influenced our results. Third, we could not exclude residual confounders, which may have influenced our results. Fourth, the limitations of data collection from all studies prevented a comparison of results from different climate zones, with different temperatures and humidity, which may have influenced the correlation between PM2.5 exposure and respiratory diseases.
In conclusion, we found that PM2.5 may play an important role in respiratory tract diseases, especially in children exposed to high concentrations of PM2.5. Additional studies are needed to assess the quantification and identification of other, as yet undetermined, harmful compounds in ambient air particles, and to determine the underlying mechanisms that cause these particles to affect human health.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (Grant No.81473013 and No. 81673210), Jiangsu Province Blue Project of University, and Innovation of Graduate Student Training Project in Jiangsu Province (KYLX15_0976).
References
- 1.Brauer M, Amann M, Burnett RT, et al. Exposure assessment for estimation of the global burden of disease attributable to outdoor air pollution[J]. Environ Sci Technol, 2012, 46(2): 652660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim KH, Jahan SA, Kabir E. A review on human health perspective of air pollution with respect to allergies and asthma[J]. Environ Int, 2013, 59: 4152. [DOI] [PubMed] [Google Scholar]
- 3.Lu F, Xu D, Cheng Y, et al. Systematic review and meta-analysis of the adverse health effects of ambient PM2.5 and PM10 pollution in the Chinese population[J]. Environ Res, 2015, 136: 196204. [DOI] [PubMed] [Google Scholar]
- 4.Pope CA 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect[J]. J Air Waste Manag Assoc, 2006, 56(6): 709742. [DOI] [PubMed] [Google Scholar]
- 5.RÜckerl R, Schneider A, Breitner S, et al. Health effects of particulate air pollution: A review of epidemiological evidence[J]. Inhal Toxicol, 2011, 23(10): 555592. [DOI] [PubMed] [Google Scholar]
- 6.Kim KH, Kabir E, Kabir S. A review on the human health impact of airborne particulate matter[J]. Environ Int, 2015, 74: 136143. [DOI] [PubMed] [Google Scholar]
- 7.Eeftens M, Hoek G, Gruzieva O, et al. Elemental composition of particulate matter and the association with lung function[J]. Epidemiology, 2014, 25(5): 648657. [DOI] [PubMed] [Google Scholar]
- 8.Kim E, Hopke PK, Pinto JP, et al. Spatial variability of fine particle mass, components, and source contributions during the regional air pollution study in St. Louis[J]. Environ Sci Technol, 2005, 39(11): 41724179. [DOI] [PubMed] [Google Scholar]
- 9.Schwarze PE, Ovrevik J, Låg M, et al. Particulate matter properties and health effects: consistency of epidemiological and toxicological studies[J]. Hum Exp Toxicol, 2006, 25(10): 559579. [DOI] [PubMed] [Google Scholar]
- 10.Brown JS, Gordon T, Price O, et al. Thoracic and respirable particle definitions for human health risk assessment[J]. Part Fibre Toxicol, 2013, 10: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LÖndahl J, Massling A, Pagels J, et al. Size-resolved respiratory-tract deposition of fine and ultrafine hydrophobic and hygroscopic aerosol particles during rest and exercise[J]. Inhal Toxicol, 2007, 19(2): 109116. [DOI] [PubMed] [Google Scholar]
- 12.Bernard SM, Samet JM, Grambsch A, et al. The potential impacts of climate variability and change on air pollution-related health effects in the United States[J]. Environ Health Perspect, 2001, 109(Suppl 2): 199209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinmayr G, Romeo E, De Sario M, et al. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis[J]. Environ Health Perspect, 2010, 118(4): 449457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, et al. Assessing heterogeneity in meta-analysis: Q statistic or I2 index[J]? Psychol Methods, 2006, 11(2): 193206. [DOI] [PubMed] [Google Scholar]
- 15.Deeks JJ, Higgins JP, Altman DG Cochrane Handbook for Systematic Reviews of Interventions[J]. 2011. [Google Scholar]
- 16.Anonymous WHO Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide[J]. Global update 2005. 2006. [Google Scholar]
- 17.Borenstein M, Hedges LV, Higgins JP, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis[J]. Res Synth Methods, 2010, 1(2): 97111. [DOI] [PubMed] [Google Scholar]
- 18.Beelen R, Hoek G, van den Brandt PA, et al. Long-term exposure to traffic-related air pollution and lung cancer risk[J]. Epidemiology, 2008, 19(5): 702710. [DOI] [PubMed] [Google Scholar]
- 19.Bennett CM, Simpson P, Raven J, et al. Associations between ambient PM2.5 concentrations and respiratory symptoms in Melbourne, 1998-2005[J]. J Toxicol Environ Health A, 2007, 70(19): 16131618. [DOI] [PubMed] [Google Scholar]
- 20.Brauer M, Hoek G, Smit HA, et al. Air pollution and development of asthma, allergy and infections in a birth cohort[J]. Eur Respir J, 2007, 29(5): 879888. [DOI] [PubMed] [Google Scholar]
- 21.Clark NA, Demers PA, Karr CJ, et al. Effect of early life exposure to air pollution on development of childhood asthma[J]. Environ Health Perspect, 2010, 118(2): 284290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Escamilla-Nuñez MC, Barraza-Villarreal A, Hernandez-Cadena L, et al. Traffic-related air pollution and respiratory symptoms among asthmatic children, resident in Mexico City: the EVA cohort study[J]. Respir Res, 2008, 9: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans KA, Halterman JS, Hopke PK, et al. Increased ultrafine particles and carbon monoxide concentrations are associated with asthma exacerbation among urban children[J]. Environ Res, 2014, 129: 1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gehring U, Cyrys J, Sedlmeir G, et al. Traffic-related air pollution and respiratory health during the first 2 yrs of life[J]. Eur Respir J, 2002, 19(4): 690698. [DOI] [PubMed] [Google Scholar]
- 25.Gehring U, Wijga AH, Brauer M, et al. Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life[J]. Am J Respir Crit Care Med, 2010, 181(6): 596603. [DOI] [PubMed] [Google Scholar]
- 26.Gehring U, Wijga AH, Hoek G, et al. Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: a population-based birth cohort study[J]. Lancet Respir Med, 2015, 3(12): 933942. [DOI] [PubMed] [Google Scholar]
- 27.Gent JF, Triche EW, Holford TR, et al. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma[J]. JAMA, 2003, 290(14): 18591867. [DOI] [PubMed] [Google Scholar]
- 28.Guo Y, Zeng H, Zheng R, et al. The association between lung cancer incidence and ambient air pollution in China: A spatiotemporal analysis[J]. Environ Res, 2016, 144(Pt A): 6065. [DOI] [PubMed] [Google Scholar]
- 29.Gurley ES, Homaira N, Salje H, et al. Indoor exposure to particulate matter and the incidence of acute lower respiratory infections among children: a birth cohort study in urban Bangladesh[J]. Indoor Air, 2013, 23(5): 379386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hertz-Picciotto I, Baker RJ, Yap PS, et al. Early childhood lower respiratory illness and air pollution[J]. Environ Health Perspect, 2007, 115(10): 15101518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacquemin B, Siroux V, Sanchez M, et al. Ambient air pollution and adult asthma incidence in six European cohorts (ESCAPE)[J]. Environ Health Perspect, 2015, 123(6): 613621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnston FH, Webby RJ, Pilotto LS, et al. Vegetation fires, particulate air pollution and asthma: a panel study in the Australian monsoon tropics[J]. Int J Environ Health Res, 2006, 16(6): 391404. [DOI] [PubMed] [Google Scholar]
- 33.Li T, Lin G. Examining the role of location-specific associations between ambient air pollutants and adult asthma in the United States[J]. Health Place, 2014, 25: 2633. [DOI] [PubMed] [Google Scholar]
- 34.Loftus C, Yost M, Sampson P, et al. Regional PM2.5 and asthma morbidity in an agricultural community: a panel study[J]. Environ Res, 2015, 136: 505512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacIntyre EA, Gehring U, MÖlter A, et al. Air pollution and respiratory infections during early childhood: an analysis of 10 European birth cohorts within the ESCAPE Project[J]. Environ Health Perspect, 2014, 122(1): 107113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mar TF, Larson TV, Stier RA, et al. An analysis of the association between respiratory symptoms in subjects with asthma and daily air pollution in Spokane, Washington[J]. Inhal Toxicol, 2004, 16(13): 809815. [DOI] [PubMed] [Google Scholar]
- 37.Millstein J, Gilliland F, Berhane K, et al. Effects of ambient air pollutants on asthma medication use and wheezing among fourth-grade school children from 12 Southern California communities enrolled in The Children’s Health Study[J]. Arch Environ Health, 2004, 59(10): 505514. [DOI] [PubMed] [Google Scholar]
- 38.MÖlter A, Simpson A, Berdel D, et al. A multicentre study of air pollution exposure and childhood asthma prevalence: the ESCAPE project[J]. Eur Respir J, 2015, 45(3): 610624. [DOI] [PubMed] [Google Scholar]
- 39.Morgenstern V, Zutavern A, Cyrys J, et al. Respiratory health and individual estimated exposure to traffic-related air pollutants in a cohort of young children. Occup Environ Med, 2007, 64(1): 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neas LM, Dockery DW, Ware JH, et al. Concentration of indoor particulate matter as a determinant of respiratory health in children[J]. Am J Epidemiol, 1994, 139(11): 10881099. [DOI] [PubMed] [Google Scholar]
- 41.Pino P, Walter T, Oyarzun M, et al. Fine particulate matter and wheezing illnesses in the first year of life[J]. Epidemiology, 2004, 15(6): 702708. [DOI] [PubMed] [Google Scholar]
- 42.Puett RC, Hart JE, Yanosky JD, et al. Particulate matter air pollution exposure, distance to road, and incident lung cancer in the nurses’ health study cohort[J]. Environ Health Perspect, 2014, 122(9): 926932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raaschou-Nielsen O, Andersen ZJ, Beelen R, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE)[J]. Lancet Oncol, 2013, 14(9): 813822. [DOI] [PubMed] [Google Scholar]
- 44.Rice MB, Ljungman PL, Wilker EH, et al. Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham heart study[J]. Am J Respir Crit Care Med, 2015, 191(6): 656664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez C, Tonkin R, Heyworth J, et al. The relationship between outdoor air quality and respiratory symptoms in young children[J]. Int J Environ Health Res, 2007, 17(5): 351360. [DOI] [PubMed] [Google Scholar]
- 46.Romieu I, Meneses F, Ruiz S, et al. Effects of air pollution on the respiratory health of asthmatic children living in Mexico City[J]. Am J Respir Crit Care Med, 1996, 154(2 Pt 1): 300307. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz J, Neas LM. Fine particles are more strongly associated than coarse particles with acute respiratory health effects in schoolchildren[J]. Epidemiology, 2000, 11(1): 610. [DOI] [PubMed] [Google Scholar]
- 48.Tétreault LF, Doucet M, Gamache P, et al. Childhood exposure to ambient air pollutants and the onset of asthma: an administrative cohort study in Québec[J]. Environ Health Perspect, 2016, 124(8): 12761282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tiittanen P, Timonen KL, Ruuskanen J, et al. Fine particulate air pollution, resuspended road dust and respiratory health among symptomatic children[J]. Eur Respir J, 1999, 13(2): 266273. [DOI] [PubMed] [Google Scholar]
- 50.To T, Zhu J, Villeneuve PJ, et al. Chronic disease prevalence in women and air pollution--A 30-year longitudinal cohort study[J]. Environ Int, 2015, 80: 2632. [DOI] [PubMed] [Google Scholar]
- 51.Wendt JK, Symanski E, Stock TH, et al. Association of short-term increases in ambient air pollution and timing of initial asthma diagnosis among Medicaid-enrolled children in a metropolitan area[J]. Environ Res, 2014, 131: 5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Young MT, Sandler DP, DeRoo LA, et al. Ambient air pollution exposure and incident adult asthma in a nationwide cohort of U.S. women[J]. Am J Respir Crit Care Med, 2014, 190(8): 914921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pope CA 3rd, Ezzati M, Dockery DW. Fine-particulate air pollution and life expectancy in the United States[J]. N Engl J Med, 2009, 360(4): 376386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stieb DM, Szyszkowicz M, Rowe BH, et al. Air pollution and emergency department visits for cardiac and respiratory conditions: a multi-city time-series analysis[J]. Environ Health, 2009, 8: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pope CA 3rd, Burnett RT, Krewski D, et al. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship[J]. Circulation, 2009, 120(11): 941948. [DOI] [PubMed] [Google Scholar]
- 56.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010 [J]. Lancet, 2012, 380(9859): 22242260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atkinson RW, Kang S, Anderson HR, et al. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: a systematic review and meta-analysis[J]. Thorax, 2014, 69(7): 660665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bell ML, Ebisu K, Peng RD, et al. Seasonal and regional short-term effects of fine particles on hospital admissions in 202 US counties, 1999-2005[J]. Am J Epidemiol, 2008, 168(11): 13011310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zanobetti A, Franklin M, Koutrakis P, et al. Fine particulate air pollution and its components in association with cause-specific emergency admissions[J]. Environ Health, 2009, 8: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan J, Li S, Fan C, et al. The impact of PM2.5 on asthma emergency department visits: a systematic review and meta-analysis[J]. Environ Sci Pollut Res Int, 2015. [DOI] [PubMed] [Google Scholar]
- 61.Qiu H, Yu IT, Tian L, et al. Effects of coarse particulate matter on emergency hospital admissions for respiratory diseases: a time-series analysis in Hong Kong[J]. Environ Health Perspect, 2012, 120(4): 572576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Almeida SM, Pio CA, Freitas MC, et al. Approaching PM(2.5) and PM(2.5-10) source apportionment by mass balance analysis, principal component analysis and particle size distribution[J]. Sci Total Environ, 2006, 368(2-3): 663674. [DOI] [PubMed] [Google Scholar]
- 63.Peng RD, Chang HH, Bell ML, et al. Coarse particulate matter air pollution and hospital admissions for cardiovascular and respiratory diseases among Medicare patients[J]. JAMA, 2008, 299(18): 21722179. [DOI] [PMC free article] [PubMed] [Google Scholar]


