Abstract
Background
To date, it remains unsettled whether aortic arch calcification (AAC) has prognostic value in patients with acute coronary syndrome.
Methods
From January 1 to December 31, 2013, a total of 225 patients with acute coronary syndrome (mean age 72 ± 26 years, 75% male) were enrolled in this study. Patients admitted to the coronary care unit of a tertiary referral medical center under the preliminary diagnosis of acute coronary syndrome were retrospectively investigated. The primary endpoint was composite of long-term major adverse cardiovascular events. The secondary endpoints were 30-day and long-term all-cause mortality.
Results
Of the 225 patients enrolled in this study, 143 had detectable AAC. Those who had AAC were older, with higher Killip classification and thrombolysis in myocardial infarction (TIMI) score with a lower probability of single vessel disease. Acute coronary syndrome patients with AAC had significantly higher 30-day mortality (17.3% vs. 7.1%, log-rank p = 0.02). During a mean follow-up period of 165 ± 140 days (maximum 492 days), the calcification group had significantly increased cardiovascular deaths (27.6% vs. 11.2%, log-rank p = 0.002), all-cause mortality (28.3% vs. 11.2%, log-rank p = 0.001) and composite endpoint of major adverse cardiovascular events (39.4% vs. 24.6%, log-rank p = 0.01). After adjusting for age, gender, diabetes mellitus and hypertension, AAC was an independent risk factor for primary and secondary endpoints among patients with acute coronary syndrome.
Conclusions
AAC provided valuable prognostic information on clinical outcomes in patients with acute coronary syndrome. However, different treatment strategies would be warranted for optimal risk reduction in such a population.
Keywords: Acute coronary syndrome, Aortic arch, Critical care, Thoracic radiography, Vascular calcification
INTRODUCTION
Acute coronary syndrome (ACS), including ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI) and unstable angina (UA), is an urgent condition that places patients in critical circumstances, necessitating diagnostic coronary angiography and mostly mandating coronary intervention, either percutaneously or surgically. Due to the elevated frequency of cardiac events in such a population, risk stratification in ACS patients in regards to clinical outcomes was of notable importance. Predictive factors such as diabetes mellitus1 for long term outcomes of ACS patients had been widely surveyed.
Aortic arch calcification (AAC) can easily be detected by chest x-ray examination, and was first advocated foruse in risk stratification of cardiac events among middle-aged patient populations in the 1990s.2,3 Subsequent study had reported that thoracic aortic calcification was also linked with a higher incidence of coronary heart disease.4
Coronary calcification was a good predictor of coronary event,5 and aortic calcification was shown to be related to coronary artery calcium score.6 Thus, it made sense that aortic calcification might be a good predictor of coronary event. In certain patient populations, such as those under hemodialysis,7 peritoneal dialysis8 and those who had undergone renal transplantation,9 aortic calcification also served as an independent risk factor for cardiovascular events and mortality. Progression of aortic calcification predicted unfavorable outcomes for patients undergoing peritoneal dialysis.10 Also, vascular calcification was independently associated with intradialysis hypotension and increased cardiac events in patients with regular hemodialysis.11 Even in patients with rheumatoid arthritis, aortic calcification correlated with enhanced cardiovascular risk.12
The relationship between aortic calcification and hard outcomes suggests that chest x-ray examination may be a good candidate for risk stratification for ACS patients due to its widespread availability, ready feasibility and easy interpretability. Furthermore, AAC is more reliably detected than aorta in the thoracic or abdominal portion in chest x-ray examination, which were often obscured by other intra-thoracic and intra-abdominal organs. The connection between AAC and clinical outcomes in ACS patients was not fully investigated. Our study aimed to examine the epidemiology, coronary characteristics as well as clinical outcomes of ACS patients with AAC and clarify whether AAC plays a prognostic role in ACS patients.
MATERIALS AND METHODS
Study population
Patients admitted to the coronary care unit of Taipei Veterans General Hospital under the impression of acute coronary syndrome, including STEMI, NSTEMI and UA, were recruited retrospectively between January 1 and December 31, 2013. The definitions of STEMI, NSTEMI and UA followed the American college of cardiology foundation/American heart association (ACCF/ AHA) guidelines.13,14 The data collection, processing, analysis and interpretation were approved by the committee of the Institutional Review Board of Taipei Veterans General Hospital (IRB number 2014-11-003AC). The underlying systemic disorders, anginal symptoms, electrocardiography, chest plain film, laboratory investigations, coronary artery angiography, course of hospitalization, in-hospital events and discharge follow-up (if available) of each patient were thoroughly scrutinized. The image interpretation, including electrocardiography, chest plain film, coronary artery angiography, wqs separately performed twice by two experienced cardiologists blinded to clinical conditions. If a discrepancy existed between the two cardiologists involving the same patient, a third experienced cardiologist would join reviewing examinations.
Chest plain film preparation and interpretation
Every study patient received posterior-anterior chest roentgenography plain film (KXO-50R/DST-100A, TOSHIBA, Japan) or portable x-ray examination (FCR-MB 201, FUJI, Japan), following the manufacturer’s instructions. Chest x-ray plain films giving rise to suspicion of aortic dissection were excluded from enrollment. The degree of AAC was divided into 4 levels from AAC grade 0 to grade 3, defined as follows: grade 0, no visible calcification; grade 1, small spots of calcification or a single thin area of calcification; grade 2, one or more areas of thick calcification; grade 3, circular calcification of the aortic knob (Figure 1).15 Patients with AAC grades 1 to 3 were assigned to the calcification group, while those without detectable calcification (grade 0) were assigned to the non-calcification group.
Figure 1.
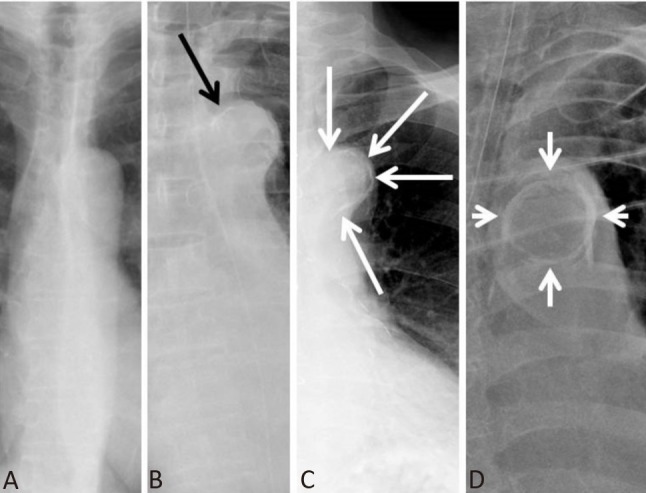
Grades of aortic arch calcification (AAC). Chest x-ray illustration of AAC (A) grade 0, (B) grade 1, (C) grade 2, and (D) grade 3.
Clinical outcomes
The primary outcome was the composite endpoint of long-term major adverse cardiovascular events (MACE) comprising non-fatal myocardial infarction (MI), non-fatal stroke and cardiovascular death, the definition of which followed the universal agreement of consensus.16 The secondary outcomes were 30-day and long-term all-cause mortality.
Statistical analysis
Numerical and nominal variables were expressed as mean ± standard deviation (SD) and frequency percentage, respectively. Analysis of variance (ANOVA) and paired Student’s t-test were used for parametric evaluation procedures. Kolmogorov-Smirnov and Shapiro-Wilk tests were used for determination of skewness and kurtosis. The categorical data between the 2 groups were compared with chi-square test and Yates’ correlation or Fisher’s exact test, as appropriate. All-cause mortality and major adverse cardiovascular events were analyzed with Kaplan-Meier analysis using the log-rank test. The hazard ratio of mortality and major adverse cardiovascular events were evaluated by Cox regression models with adjustment for age, gender, type 2 diabetes mellitus and hypertension. Statistical analyses were performed with the SPSS system 21.0 (SPSS Inc., Chicago, IL, USA). A p-value < 0.05 was considered statistically significant.
RESULTS
A total of 225 patients were enrolled (75% male, 72 ± 26 years of age) with 34.7% STEMI, 58.7% NSTEMI and 6.6% UA. Among the 225 ACS patients, 127 [46 (20%) AAC grade 1, 57 (25%) AAC grade 2 and 24 (11%) AAC grade 3)] had AAC and 98 (44%) (AAC grade 0) did not. The distributions of STEMI, NSTEMI and UA in both groups were not statistically different, though the non-calcification group had numerically higher STEMI and lower NSTEMI.
Patients in the calcification group were older, with a higher percentage having underlying hypertension, peripheral artery disease and calcium-channel blocker use. The body characteristics of the calcification group included lower body weight, body mass index and waist circumference. Regarding lipid profiles, patients with detectable AAC had significantly lower serum levels of total cholesterol and triglycerides as compared to those without AAC. There were significantly elevated baseline serum creatinine and uric acid levels in the calcification group. The baseline characteristics of all patients and their regular medication use before each index ACS visitare shown in Table 1.
Table 1. Baseline characteristics of all patients and between groups.
| All patients (n = 225) | AAC (+) (n = 127) | AAC (–) (n = 98) | p-value | |
| Age (years) | 72 ± 26 | 81 ± 29 | 61 ± 14 | < 0.001 |
| Male | 169 (75) | 90 (71) | 77 (79) | 0.17 |
| Weight (kg) | 66 ± 14 | 62 ± 11 | 72 ± 14 | < 0.001 |
| BMI (kg/m2) | 24.6 ± 4.4 | 23.7 ± 3.9 | 26.3 ± 4.7 | < 0.001 |
| Waist (cm) | 90 ± 11 | 87 ± 11 | 95 ± 10 | 0.002 |
| SBP (mmHg) | 134 ± 32 | 131 ± 34 | 138 ± 29 | 0.08 |
| DBP (mmHg) | 76 ± 19 | 72 ± 19 | 81 ± 17 | < 0.001 |
| HR (1/min) | 85 ± 21 | 85 ± 22 | 85 ± 19 | 0.87 |
| Active smoker | 47 (21) | 24 (19) | 23 (24) | 0.64 |
| Hypertension | 153 (68) | 94 (74) | 58 (60) | 0.03 |
| Diabetes | 99 (44) | 59 (47) | 39 (40) | 0.32 |
| Prior MI | 38 (17) | 19 (15) | 18 (19) | 0.41 |
| Prior PTCA | 47 (21) | 26 (21) | 19 (20) | 0.77 |
| Prior CHF | 20 (9) | 14 (11) | 6 (6) | 0.19 |
| Prior PAOD | 13 (6) | 11 (9) | 2 (2) | 0.03 |
| BUN (mg/dl) | 33 ± 24 | 37 ± 24 | 27 ± 23 | 0.004 |
| Cr (mg/dl) | 2.0 ± 2.1 | 2.4 ± 2.3 | 1.6 ± 1.7 | 0.004 |
| Uric acid (mg/dl) | 7.2 ± 2.5 | 7.6 ± 2.9 | 6.5 ± 1.6 | 0.01 |
| Total cholesterol (mg/dl) | 153 ± 39 | 148 ± 40 | 160 ± 37 | 0.02 |
| HDL (mg/dl) | 37 ± 12 | 37 ± 14 | 37 ± 9 | 0.72 |
| LDL (mg/dl) | 96 ± 35 | 92 ± 35 | 101 ± 37 | 0.11 |
| TG (mg/dl) | 123 ± 70 | 110 ± 60 | 139 ± 77 | 0.003 |
| Fasting blood glucose (mg/dl) | 152 ± 66 | 153 ± 73 | 151 ± 56 | 0.86 |
| Medications | ||||
| Aspirin | 63 (28) | 33 (26) | 29 (30) | 0.51 |
| Clopidogrel | 36 (16) | 20 (16) | 16 (17) | 0.83 |
| ACEI | 29 (13) | 16 (13) | 11 (12) | 0.73 |
| ARB | 52 (23) | 32 (25) | 21 (21) | 0.53 |
| BB | 52 (23) | 24 (19) | 27 (28) | 0.13 |
| CCB | 45 (20) | 35 (28) | 10 (11) | 0.002 |
| Nitrates | 36 (16) | 23 (18) | 12 (13) | 0.32 |
| Statin | 47 (21) | 21 (17) | 26 (27) | 0.08 |
Data are represented as mean ± standard deviation or n (%).
AAC, aortic arch calcification; ACEI, angiotensinogen-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta-adrenergic blocker; BMI, body mass index; BUN, blood urea nitrogen; CCB, calcium channel blocker; CHF, congestive heart failure; Cr, creatinine; DBP, diastolic blood pressure; HDL, high density lipoprotein; HR, heart rate; LDL, low density lipoprotein; MI, myocardial infarction; PAOD, peripheral arterial obstructive disease; PTCA, percutaneous coronary angioplasty; SBP, systolic blood pressure; TG, triglyceride.
Compared with the non-calcification group, patients in the calcification group had significantly higher TIMI score, Killip classification and more left main coronary artery disease. The composition of ACS type, peak level of cardiac enzymes and left ventricular ejection fraction did not differ significantly between groups (Table 2).
Table 2. Clinical and angiographic characteristics between patients with or without AAC.
| All patients (n = 225) | AAC (+) (n = 127) | AAC (–) (n = 98) | p-value | |
| ACS type | ||||
| STEMI | 78 (35) | 40 (32) | 38 (39) | 0.26 |
| NSTEMI | 132 (59) | 80 (63) | 52 (53) | 0.13 |
| UA | 15 (7) | 7 (5) | 8 (8) | 0.43 |
| TIMI scores | 4.2 ± 2.3 | 4.9 ± 2.4 | 3.3 ± 1.7 | < 0.001 |
| Peak | ||||
| CK (U/L) | 1094 ± 1883 | 986 ± 1996 | 1235 ± 1727 | 0.33 |
| CK-MB (U/L) | 70.9 ± 88.7 | 62.6 ± 80.2 | 81.9 ± 98.1 | 0.12 |
| Troponin-I (ng/ml) | 34.9 ± 100.1 | 24.4 ± 45.3 | 48.9 ± 143.2 | 0.12 |
| CAD | ||||
| SVD | 42 (19) | 19 (15) | 23 (24) | 0.03 |
| DVD | 54 (24) | 33 (26) | 21 (22) | 0.96 |
| TVD | 115 (51) | 66 (52) | 49 (50) | 0.5 |
| Insignificant | 9 (4) | 8 (6) | 1 (1) | 0.11 |
| Normal CAG | 5 (2) | 2 (1) | 2 (2) | 0.42 |
| LM disease | 27 (12) | 20 (16) | 6 (7) | 0.04 |
| LVEF | 44 ± 16 | 43 ± 17 | 45 ± 15 | 0.66 |
| Killip class | 2.1 ± 1.1 | 2.4 ± 1.1 | 1.7 ± 1.0 | < 0.001 |
| Revascularization | 190 (84) | 105 (83) | 85 (87) | 0.46 |
Data are represented as mean ± standard deviation or n (%).
AAC, aortic arch calcification; CAD, coronary artery disease; CAG, coronary artery angiography; CK, creatine kinase; DVD, double vessel disease; LM, left main; LVEF, left ventricular ejection fraction; NSTEMI, non-ST elevation myocardial infarction; STEMI, ST elevation myocardial infarction; SVD, single vessel disease; TIMI, thrombolysis in myocardial infarction; TVD, triple vessel disease; UA, unstable angina.
During the mean follow-up of 165 ± 140 days (maximally 492 days), there were 47 all-cause deaths, 46 cardiovascular deaths, 37 non-fatal MI and 5 non-fatal strokes. There was no statistical difference between calcification and non-calcification groups in non-fatal MI (15.0% vs. 18.4%, log-rank, p = 0.47) and non-fatal strokes (3% vs. 1%, log-rank p = 0.28). The calcification group had significantly higher 30-day mortality (17.3% vs. 7.1%, log-rank p = 0.02, Figure 2A). ACS patients with AAC had significantly higher incidence of cardiovascular death (27.6% vs. 11.2%, log-rank p = 0.002, Figure 2B) and all-cause death (28.3% vs. 11.2%, log-rank, p = 0.001, Figure 2C) as compared to those without AAC. As regarding the long-term composite endpoint of non-fatal MI, non-fatal stroke and cardiovascular death, the calcification group had a significantly higher risk as compared to the non-calcification group (39.4% vs. 24.6%, log-rank, p = 0.01, Figure 2D).
Figure 2.
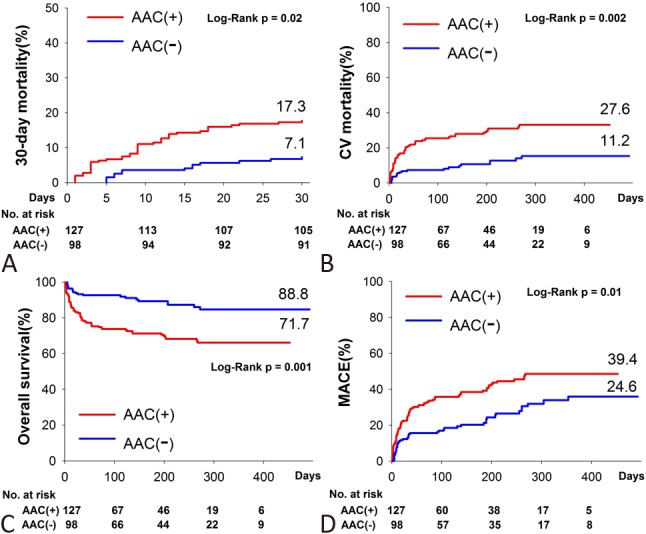
Outcomes analysis according to presence of AAC. Kaplan-Meier analysis of (A) 30-day mortality, (B) cardiovascular mortality, (C) overall survival, and (D) major adverse cardiovascular events (MACE) between those with and without AAC.
Subgroup analysis focusing on primary endpoints demonstrated clinical outcomes in favor of patients without AAC, as compared with those with AAC, in every aspect of grouping, especially in those with hypertension, without diabetes mellitus, and male (Figure 3).
Figure 3.
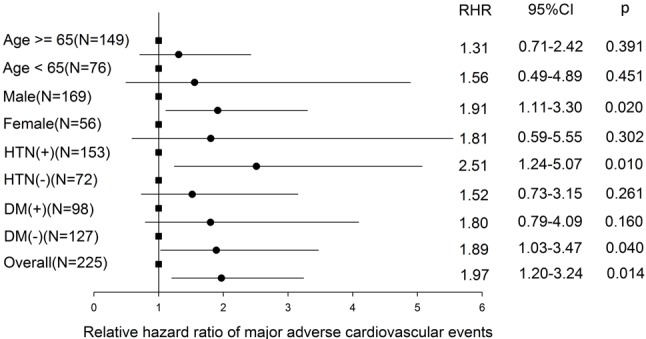
Subgroup analysis of major adverse cardiovascular events between groups. Square dot: AAC negative group (reference group). Circle dot: AAC positive group. CI, confidence interval; DM, diabetes mellitus; HTN, hypertension; RHR, relative hazard ratio to reference group.
All-cause mortality rate during follow-up escalated dramatically with the AAC grade, though the survival differences did not reach statistical significance between grade 0 and 1, and between grade 2 and 3 (Figure 4A). Thirty-six (28.3%) mortalities occurred among the AAC (+) group and 11 (11.2%) among AAC (–) group. Among AAC (+) mortalities, 35 (97.2%) were cardiovascular-related deaths, including fatal MI, heart failure and sudden cardiac death. The only one non-CV death (2.8%) was cancer-related, which occurred on the 27th day of index ACS episode. All 11 AAC (–) mortalities were cardiovascular-related death. Overall, the major adverse cardiovascular event rate significantly escalated with AAC grade (Figure 4B) (p for trend < 0.001).
Figure 4.
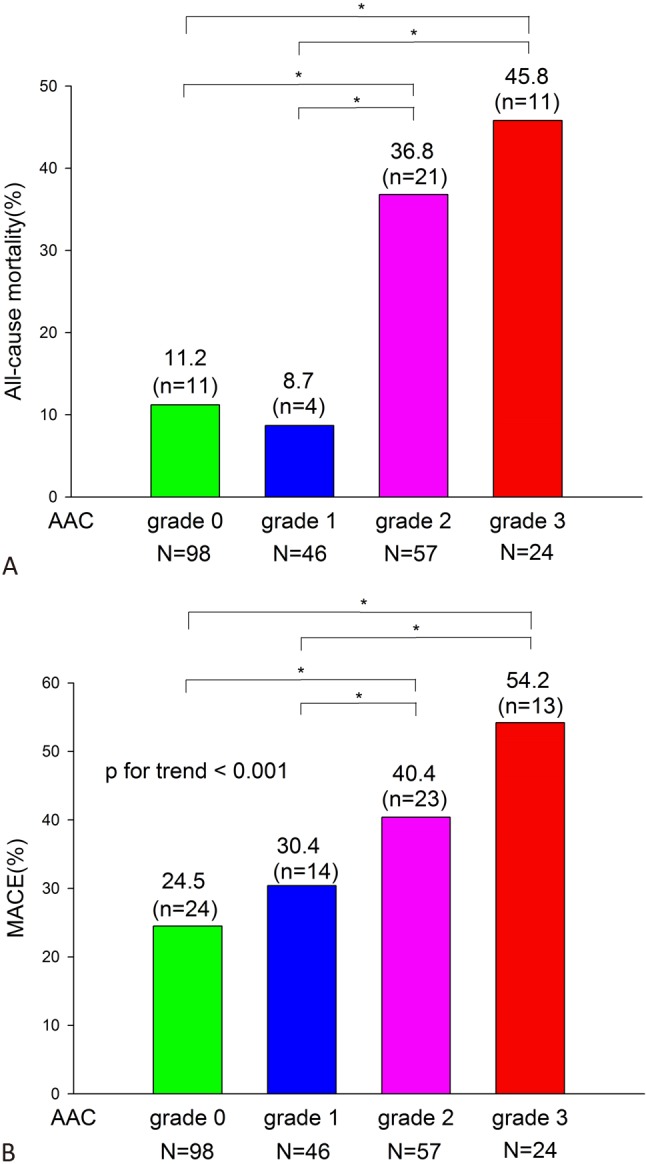
Outcomes analysis according to AAC grade. Survival and MACE rate for each grade of AAC. * p < 0.05.
In multivariate analysis, the presence of AAC was associated with a statistically elevated risk of long-term MACE, all-cause mortality, cardiovascular death and 30-day mortality. After adjustment for age, gender, type 2 diabetes mellitus and hypertension, the presence of AAC still conferred a statistically significant increase of risks (Table 3).
Table 3. Multivariate analysis for hazard ratio of AAC.
| Outcomes | Cumulative incidence estimates | Unadjusted, HR* (95% CI) | Adjusted HR* (95% CI) | |
| AAC (+) | AAC (–) | |||
| Mortality | ||||
| 30-day | 17.3% | 7.1% | 2.61 (1.11-6.11) | 3.38 (1.31-8.72) |
| CV death | 27.6% | 11.2% | 2.80 (1.42-5.51) | 2.94 (1.40-6.17) |
| Mortality# | 28.3% | 11.2% | 2.88 (1.47-5.66) | 3.10 (1.48-6.49) |
| MACE | 39.4% | 24.6% | 1.97 (1.20-3.24) | 2.20 (1.29-3.74) |
AAC, aortic arch calcification; CI, confidence interval; HR, hazard ratio; MACE, major adverse cardiovascular event.
* Hazard ratio of the outcomes between acute coronary syndrome patients with calcified and non-calcified aortic arch (reference group). Adjusted for age, gender, type 2 diabetes mellitus and hypertension. # Maximum follow-up for 1.35 years.
DISCUSSION
The pathophysiology of vascular calcification is affected by multiple factors, including aging, diabetes, renal insufficiency and dyslipidemia.17 Vascular calcification has been shown to be related to arterial stiffness18 and congestive heart failure,19 and is notorious for causing organ damage in ACS patients. Vessel calcification, frequently noted in the elderly and those with other underlying systemic diseases, is a chronically active process in which vascular smooth muscle cells adopt osteoblastic phenotype and deposit calcified crystals.20 Diabetes and hypertension have a synergistic effect on vascular calcification.21 Aortic calcification had been shown to be strongly associated with oxidative stress,22 and it is generally accepted that atherosclerosis is a systemic inflammatory disease of the arterial wall, initiated by endothelial damage.
Previous studies discussing the identification of AAC in different patient populations reported different prevalence ranges between 30-63%.7-9,23-26 In our cohort, AAC was present in 56% of all ACS patients, mostly with AAC grade 2. ACS patients with AAC had similar distributions of STEMI, NSTEMI and UA as those without AAC, though our ACS patients had a lower percentage of UA as compared to other ACS cohorts.27,28 The location of MI for ACS patients with AAC was mostly (> 60%) in the anterior portion of the heart. Interestingly, we found that ACS patients with AAC had significantly more left main coronary artery disease as compared with those without AAC. This observation may partially explain why patients with AAC had higher TIMI score, higher Killip classification and poorer clinical outcomes. Our observations were consistent with another study where patients with detectable AAC were prone to have more complicated coronary artery disease and higher TIMI scores.29 Levent and colleagues also reported that aortic knob calcification on chest x-ray was an independent predictor of complex coronary artery lesion for patients with NSTEMI.30
This article is the first study to elucidate the relationship between AAC and cardiovascular outcomes in patients with ACS, and that the major adverse cardiovascular event rate escalates as each calcification grade point increases. The survival difference between grade 0 and grade 1, and between grade 2 and grade 3, had not reached statistical significance. A possible explanation would be that tiny calcified spot in the aortic arch on chest x-ray may hint a certain but small degree of derangement on cardiovascular system, but may not be sufficient for translating into survival difference.
Our study showed that the presence of AAC was an independent risk factor for 30-day, cardiovascular, all-cause mortality as well as a composite endpoint of MACE. The poor prognosis for those with AAC was consistent through all subgroups without conflicting results, i.e.: all hazard ratio > 1 (and actually all > 1.3). These results suggest that valuable prognostic information about clinical outcomes of ACS patients could be easily obtained with just a simple routine chest x-ray examination in the emergency room.
AAC had been reported to be common in the elderly,3,31 in which overall survival was also influenced by age itself. After adjustment for age, gender and status of type 2 diabetes mellitus and hypertension, we found that AAC demonstrated a consistent risk for 30-day as well as all-cause mortality, cardiovascular death and composite endpoint of MACE. Similar phenomena were observed in another study focusing on elderly females.32
Witteman and colleagues first suggested that aortic calcification was associated with a six-fold increased risk of cardiovascular death in men 45 years of age, and independent of major cardiovascular death risk factors.3 Rodondi et al. reported that during a 16-year follow-up, abdominal aortic calcification in older women was associated with a 37% increase of all-cause mortality after adjustment for age and cardiovascular risk factors.32 These findings clearly demonstrated that the presence of aortic calcification was associated with increased clinical events, particularly cardiovascular mortality. In comparison, our study cohort had a significantly higher mortality rate as compared with previous studies, owing to the nature of ACS. From a maximum follow-up of nearly 1.5 years, ACS patients with AAC had a nearly 30% all-cause mortality, while 10% of those were without AAC. Importantly, more than 90% of mortality in ACS patients with AAC was cardiovascular-related, implicating the important correlation between ACS and cardiovascular death.
Recent study has demonstrated that AAC represented generalized vascular stiffness and enhanced brachial-ankle pulse wave velocity.33 Our study is the first to show that AAC has a strong prognostic correlation in ACS patients. AAC is easily and readily detectable by routine chest x-ray examination, providing practical prognostic information on clinical outcomes when applied to patients with ACS; it is reasonable to pay more attention to these extremely high-risk ACS patients. Further studies focusing on different treatment strategies tailored for optimal risk reduction would be needed in ACS patients with AAC.
Study limitations
The study was based on a retrospective observational ACS registry. The relatively small size of our cohort would be a concern, though the clinical outcomes between the compared groups had statistical significance. Further long-term and larger studies would be needed to confirm our observations and clarify underlying mechanisms.
CONCLUSIONS
In conclusion, AAC from chest x-ray examination in patients with ACS provides valuable prognostic information about future clinical outcomes. However, studies with larger patient numbers would be needed to confirm this observation and delineate the detailed picture of clinical outcomes in 4 AAC grade groups. Different medical management principles for ACS patients with AAC might also be needed and tested in subsequent studies.
Acknowledgments
We sincerely thank Dr. Tsung-Lin Yang for project planning and design. Also, study assistants Yu-Chen Ku and Hui-Yun Yu generously facilitated data collection and analysis. All authors took the responsibility of manuscript writing.
FUNDING
None.
DISCLOSURE STATEMENT
The authors report no conflict of interest.
REFERENCES
- 1.Wei CC, Shyu KG, Cheng JJ, et al. Diabetes and adverse cardiovascular outcomes in patients with acute coronary syndrome - data from Taiwan’s acute coronary syndrome full spectrum data registry. Acta Cardiol Sin. 2016;32:31–38. doi: 10.6515/ACS20150322A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witteman JC, Kannel WB, Wolf PA, et al. Aortic calcified plaques and cardiovascular disease (the framingham study). Am J Cardiol. 1990;66:1060–1064. doi: 10.1016/0002-9149(90)90505-u. [DOI] [PubMed] [Google Scholar]
- 3.Witteman JC, Kok FJ, van Saase JL, et al. Aortic calcification as a predictor of cardiovascular mortality. Lancet. 1986;2:1120–1122. doi: 10.1016/s0140-6736(86)90530-1. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto H, Shavelle D, Takasu J, et al. Valvular and thoracic aortic calcium as a marker of the extent and severity of angiographic coronary artery disease. Am Heart J. 2003;146:153–159. doi: 10.1016/S0002-8703(03)00105-4. [DOI] [PubMed] [Google Scholar]
- 5.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 6.Bannas P, Jung C, Blanke P, et al. Severe aortic arch calcification depicted on chest radiography strongly suggests coronary artery calcification. Eur Radiol. 2013;23:2652–2657. doi: 10.1007/s00330-013-2877-z. [DOI] [PubMed] [Google Scholar]
- 7.Adragao T, Pires A, Lucas C, et al. A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1480–1488. doi: 10.1093/ndt/gfh217. [DOI] [PubMed] [Google Scholar]
- 8.Yoon HE, Park BG, Hwang HS, et al. The prognostic value of abdominal aortic calcification in peritoneal dialysis patients. Int J Med Sci. 2013;10:617–623. doi: 10.7150/ijms.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLoach SS, Joffe MM, Mai X, et al. Aortic calcification predicts cardiovascular events and all-cause mortality in renal transplantation. Nephrol Dial Transplant. 2009;24:1314–1319. doi: 10.1093/ndt/gfn753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee MJ, Shin DH, Kim SJ, et al. Progression of aortic arch calcification over 1 year is an independent predictor of mortality in incident peritoneal dialysis patients. PLoS One. 2012;7:e48793. doi: 10.1371/journal.pone.0048793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SY, Hong YA, Yoon HE, et al. Vascular calcification and intradialytic hypotension in hemodialysis patients: clinical relevance and impact on morbidity and mortality. Int J Cardiol. 2016;217:156–160. doi: 10.1016/j.ijcard.2016.04.183. [DOI] [PubMed] [Google Scholar]
- 12.Mohammad A, Lohan D, Bergin D, et al. Vertebral fracture assessment-detected abdominal aortic calcification and cardiovascular disease in rheumatoid arthritis. Semin Arthritis Rheum. 2014;43:632–637. doi: 10.1016/j.semarthrit.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 13.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American college of cardiology foundation/American heart association task force on practice guidelines: developed in collaboration with the American college of emergency physicians and society for cardiovascular angiography and interventions. Catheter Cardiovasc Interv. 2013;82:E1–E27. doi: 10.1002/ccd.24776. [DOI] [PubMed] [Google Scholar]
- 14.Anderson JL, Adams CD, Antman EM, et al. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2011;123:e426–e579. doi: 10.1161/CIR.0b013e318212bb8b. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto H, Iijima K, Hashimoto M, et al. Validity and usefulness of aortic arch calcification in chest x-ray. J Atheroscler Thromb. 2009;16:256–264. doi: 10.5551/jat.e570. [DOI] [PubMed] [Google Scholar]
- 16.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 17.Toussaint ND, Lau KK, Strauss BJ, et al. Associations between vascular calcification, arterial stiffness and bone mineral density in chronic kidney disease. Nephrol Dial Transplant. 2008;23:586–593. doi: 10.1093/ndt/gfm660. [DOI] [PubMed] [Google Scholar]
- 18.Cecelja M, Jiang B, Bevan L, et al. Arterial stiffening relates to arterial calcification but not to noncalcified atheroma in women. A twin study. J Am Coll Cardiol. 2011;57:1480–1486. doi: 10.1016/j.jacc.2010.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh CR, Cupples LA, Levy D, et al. Abdominal aortic calcific deposits are associated with increased risk for congestive heart failure: the framingham heart study. Am Heart J. 2002;144:733–739. doi: 10.1067/mhj.2002.124404. [DOI] [PubMed] [Google Scholar]
- 20.McCarty MF, DiNicolantonio JJ. The molecular biology and pathophysiology of vascular calcification. Postgrad Med. 2014;126:54–64. doi: 10.3810/pgm.2014.03.2740. [DOI] [PubMed] [Google Scholar]
- 21.Yamada S, Oshima M, Watanabe Y, et al. Arterial location-specific calcification at the carotid artery and aortic arch for chronic kidney disease, diabetes mellitus, hypertension, and dyslipidemia. Calcif Tissue Int. 2014;95:267–274. doi: 10.1007/s00223-014-9891-2. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe K, Ohara M, Suzuki T, et al. Aortic arch calcification detectable on chest x-ray films is associated with plasma diacron-reactive oxygen metabolites in patients with type 2 diabetes but without cardiovascular disease. J Nippon Med Sch. 2013;80:410–419. doi: 10.1272/jnms.80.410. [DOI] [PubMed] [Google Scholar]
- 23.Qureshi AI, Rahman HA, Adil MM, et al. Aortic arch calcification,procedural times, and outcomes of endovascular treatment in patients with acute ischemic stroke. J Vasc Interv Neurol. 2014;7:1–6. [PMC free article] [PubMed] [Google Scholar]
- 24.Lee CT, Huang CC, Hsu CY, et al. Calcification of the aortic arch predicts cardiovascular and all-cause mortality in chronic hemodialysis patients. Cardiorenal Med. 2014;4:34–42. doi: 10.1159/000360230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nitta K, Ogawa T. Aortic arch calcification and clinical outcome in patients with end-stage renal disease. Tohoku J Exp Med. 2011;223:79–84. doi: 10.1620/tjem.223.79. [DOI] [PubMed] [Google Scholar]
- 26.Nitta K, Ogawa T. Aortic arch calcification and mortality in chronic hemodialysis patients. Rev Recent Clin Trials. 2010;5:133–137. doi: 10.2174/157488710792007266. [DOI] [PubMed] [Google Scholar]
- 27.Trzeciak P, Gierlotka M, Gasior M, et al. In-hospital and 12-month outcomes after acute coronary syndrome treatment in patients aged < 40 years of age (from the polish registry of acute coronary syndromes). Am J Cardiol. 2014;114:175–180. doi: 10.1016/j.amjcard.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 28.Singh SM, Fitz Gerald G, Yan AT, et al. High-grade atrioventricular block in acute coronary syndromes: insights from the global registry of acute coronary events. Eur Heart J. 2014. doi: 10.1093/eurheartj/ehu357. [DOI] [PubMed] [Google Scholar]
- 29.Parthenakis F, Skalidis E, Simantirakis E, et al. Absence of atherosclerotic lesions in the thoracic aorta indicates absence of significant coronary artery disease. Am J Cardiol. 1996;77:1118–1121. doi: 10.1016/s0002-9149(96)00146-4. [DOI] [PubMed] [Google Scholar]
- 30.Korkmaz L, Adar A, Ata Korkmaz A, et al. Aortic knob calcification and coronary artery lesion complexity in non-ST-segment elevation acute coronary syndrome patients. Turk Kardiyol Dern Ars. 2012;40:606–611. doi: 10.5543/tkda.2012.38963. [DOI] [PubMed] [Google Scholar]
- 31.Tobler HG, Edwards JE. Frequency and location of atherosclerotic plaques in the ascending aorta. J Thorac Cardiovasc Surg. 1988;96:304–306. [PubMed] [Google Scholar]
- 32.Rodondi N, Taylor BC, Bauer DC, et al. Association between aortic calcification and total and cardiovascular mortality in older women. J Intern Med. 2007;261:238–244. doi: 10.1111/j.1365-2796.2007.01769.x. [DOI] [PubMed] [Google Scholar]
- 33.Shin MC, Lee MY, Huang JC, et al. Association of brachial-ankle pulse wave velocity and cardiomegaly with aortic arch calcification in patients on hemodialysis. Medicine (Baltimore) 2016;95:e3643. doi: 10.1097/MD.0000000000003643. [DOI] [PMC free article] [PubMed] [Google Scholar]


