Abstract
Background
Drug-eluting stents are widely used in coronary artery intervention. However, vessel caging and very late thrombotic events are of persistent and substantial concern. Bioresorbable vascular scaffolds (BVS) were developed to deliver vascular reparative therapy, by eliminating permanent mechanical restraint. However, data regarding its clinical performance is lacking.
Methods
After the BVS implantation procedure received national approval in May 2014, patients receiving BVS implantation until November 2014 in National Taiwan University Hospital (NTUH) were enrolled. Clinical variables, angiographic data, procedural details, and follow-up information were collected and compared with those receiving BVS at NTUH as part of the global ABSORB EXTEND trial.
Results
A total of 35 patients (38 target vessels) with 48 BVS implanted after approval were enrolled, as the "real-world practice" group. Data of the 34 patients (34 target vessels) with 37 BVS implanted in the ABSORB EXTEND trial were also obtained. Differences in lesion complexity (0% type B2/C lesion in ABSORB EXTEND, versus 23.7% in real-world, p = 0.007) and lesion length (20.9 ± 6.1 mm in ABSORB EXTEND, versus 29.5 ± 15.9 mm in real-world, p = 0.008) were noted. The ischemia-driven target vessel revascularization after an average of 732 days follow-up was 11.8% in the ABSORB EXTEND trial. However, there was no ischemia-driven target lesion revascularization (TLR), no scaffold thrombosis, no myocardial infarction (MI), and no patients passed during the follow-up period. In real-world patients, there is 5.3% of MI, 2.6% ischemia-driven TLR, and 2.6% of non-fatal probable scaffold thrombosis.
Conclusions
The use of BVS in real-world practice is feasible, with clinical outcomes comparable to those in the ABSORB EXTEND trial.
Keywords: Coronary artery disease, Coronary stenting
INTRODUCTION
Since the advent of percutaneous coronary intervention (PCI), enormous advances have been made in the treatment of coronary artery disease. Bare metal stents (BMS) effectively prevented dissection and acute closure caused by balloon angioplasty,1 with restenosis rates ranging from 11-40%.2 Overall, use of Drug-eluting stent (DES) significantly reduced restenosis compared with BMS.3,4 Among a high-risk population undergoing unprotected left main PCI, the use of DES appeared to be beneficial in reducing the risk of long-term cardiovascular death.5 DES may provide advantages over BMS to patients with acute coronary syndrome.6 However, permanent vessel caging and prolonged antiplatelet medication have generated concerns and continuing medical issues. Bioresorbable vascular scaffold (BVS) was subsequently developed, based on the premise that scaffolding and drug delivery to the diseased coronary vessel are only required on a temporary basis following coronary interventions. Absorb Bioresorbable Vascular Scaffold (Abbott Vascular, Santa Clara, CA, USA) is the world’s first commercially available drug-eluting coronary BVS. Its poly-L-lactide (PLLA) struts are coated with everolimus-containing poly-D, L-lactide (PDLLA) polymer, with the whole scaffold designed to be resorbed in approximately 2-3 years.7 Although the safety and performance of Absorb BVS has been previously established in clinical trials,7 the data in real-world practice, especially in a Taiwanese cohort, is lacking. The National Taiwan University Hospital is 1 of the 100 global enrolling sites of the ABSORB EXTEND trial, and has also continued with BVS implantation in real-world patients following the national approval of BVS in May 2014. We hereafter have presented a retrospective analysis to evaluate and compare the clinical performances of Absorb BVS in both the ABSORB EXTEND trial and in real-world patients.
METHODS
Patients
We reviewed patients who received percutaneous coronary intervention with implant of Absorb BVS in real-world practice and also from the ABSORB EXTEND trial.8 Clinical variables including epidemiology, symptomatology, angiographic results, interventional details, and post procedure follow-up were collected for each patient. The Institutional Review Board at National Taiwan University Hospital approved the study.
Patients from ABSORB EXTEND trial
The trial is a single-arm prospective non-US global registry of Absorb BVS in the treatment of de-novo native coronary artery (clinicaltrials.gov, unique identifier NCT01023789). Patients over 18 years of age with 1 or 2 de-novo lesions located in different native coronary arteries were enrolled. Target lesions were to be located in a major epicardial vessel or side branch, with estimated stenosis of ≥ 50% and < 100%, with a thrombolysis in myocardial infarction (TIMI) flow grade of ≥ 1. The diameter of target lesions should be 2.0-3.3 mm, and length ≤ 28 mm by online quantitative coronary angiography. Aorto-ostial lesions, left main coronary artery lesions, total occlusions, lesions with visible thrombus, heavily calcified lesions, and bifurcation lesions involving a side branch ≥ 2 mm in diameter with ostial stenosis > 40% requiring pre-dilation were excluded.8 During the trial period from July 2012 through September 2013, a total of 34 patients were enrolled into the ABSORB EXTEND trial at National Taiwan University Hospital.
Patients from real-world practice
A total of 35 consecutive patients receiving percutaneous coronary intervention with implant of Absorb BVS during the period May-November 2014 in National Taiwan University Hospital were retrospectively reviewed. The indications were at the operators’ discretion according to individual clinical situation, but basically followed the manufacturer’s recommendations. The data were further compared with those of patients enrolled in National Taiwan University Hospital for the ABSORB EXTEND trial.8
Outcomes
Clinical outcomes were collected via review of clinical notes both with and without telephone interview. The major adverse cardiovascular event (MACE) was defined as the composite endpoint of cardiac death, myocardial infarction (MI), coronary artery bypass graft surgery (CABG), ischemia-driven target lesion revascularization (TLR), ischemia-driven target vessel revascularization (TVR), and scaffold thrombosis. MI was defined as the presence of a rise and/or fall of cardiac biomarker values with either clinical symptoms of ischemia, new relevant electrocardiographic change, imaging evidence or pathology evidence. PCI related MI is defined by an elevation of cardiac troponin values [> 5 × 99th percentile upper reference limit (URL)] in patients with normal baseline, in addition to other supporting clinical evidence.9 TLR is defined as any repeat PCI or CABG of the target vessel performed for restenosis or other complication of the target lesion. TVR is defined as any repeat PCI or CABG of any segment of the target vessel. The occurrence of scaffold thrombosis was defined on the basis of the Academic Research Consortium definition,10 and lesions were classified according to the modified American College of Cardiology/American Heart Association (ACC/AHA) grading system.11 A chronic total occlusion (CTO) was defined as a lesion with a TIMI grade 0 flow within the occluded segment and angiographic or clinical evidence or high likelihood of an occlusion of a duration ≥ 3 months.12 The follow-up duration is at least 30 days after the index BVS implantation procedure.
Statistical analysis
All continuous variables were expressed as mean ± standard deviation, and categorical variables in numbers and percentage. The chi-square test or Fisher’s exact test (if the group’s number is 5 or less) was used to compare groups of categorical data. A two-sided p value of 0.05 was considered statistically significant. Stata/SE 11.0 for Windows (StataCorp LP, College Station, TX, USA) was used for statistical analyses.
RESULTS
Demographics
During the ABSORB EXTEND trial period between July 2012 and September 2013, a total of 34 patients (34 target vessels) receiving 37 BVS were enrolled at National Taiwan University Hospital. In real-world practice during May 2014 and November 2014, a total of 913 patients received percutaneous coronary interventions at National Taiwan University Hospital. Patient demographics were summarized in Table 1. Among them, 35 (3.8%) patients receiving 48 BVS in 38 target vessels. The baseline characteristics were similar between the ABSORB EXTEND trial and real-world patients, but the incidence of hypertension was higher in the ABSORB EXTEND trial (94.1% versus 60.0% in real-world, p = 0.001). The clinical presentation differed between patients from the ABSORB EXTEND trial and real-world practice. While 94.1% of patients in ABSORB EXTEND trial presented with asymptomatic or stable angina and 5.9% with unstable angina, 80.0% of real-world patients were asymptomatic or had stable angina status, 2.9% with unstable angina and 17.1% with acute myocardial infarction (p = 0.04). The target vessel distribution was similar in both groups.
Table 1. Patient demographic data.
| ABSORB EXTEND (n = 34) | BVS in real world (n = 35) | p value | |
| Age, years | 62.5 ± 11.8 | 60.8 ± 10.7 | 0.66 |
| Male gender, n (%) | 28 (82.3) | 29 (82.9) | 0.96 |
| Diabetes mellitus, n (%) | 16 (47.1) | 10 (28.6) | 0.11 |
| Hypertension, n (%) | 32 (94.1) | 21 (60.0) | 0.001 |
| Smoker and ex-smoker, n (%) | 23 (67.6) | 21 (60.0) | 0.51 |
| eGFRa, ml/min | 82.6 ± 23.2 | 83.6 ± 18.1 | 0.82 |
| Total cholesterol, mg/dL | 153 ± 35 | 167 ± 39 | 0.12 |
| Previous myocardial infarction, n (%) | 6 (17.6) | 3 (8.5) | 0.26 |
| Clinical feature, n (%) | 0.04 | ||
| Asymptomatic or stable angina | 32 (94.1) | 28 (80.0) | |
| Unstable angina | 2 (5.9) | 1 (2.9) | |
| Myocardial infarction | 0 | 6 (17.1) | |
| Coronary artery disease status, n (%) | 0.50 | ||
| 1-vessel-disease | 12 (35.2) | 9 (25.7) | |
| 2-vessel-disease | 11 (32.4) | 10 (28.6) | |
| 3-vessel-disease | 11 (32.4) | 16 (45.7) |
a eGFR, estimated glomerular filtration rate, was calculated using the simplified Modification of Diet in Renal Disease (MDRD) formula.
Lesion characteristics
None of the lesion from the ABSORB EXTEND trial was a AHA-type B2/C lesion, but 23.7% of lesions in real-world group were. There was a trend of more BVS/BVS overlapping (real-world: 21.1% vs. ABSORB EXTEND trial: 8.8%, p = 0.15) and significantly longer BVS length (real-world: 30.3 ± 19.2 mm vs. ABSORB EXTEND trial: 20.9 ± 6.1 mm, p = 0.008) in the real-world group. Also, 47.4% of BVS implanted in real-world group were 3.5 mm in diameter, where only 17.6% of BVS implanted in ABSORB EXTEND trial were 3.5 mm in diameter (p = 0.02). Table 2 summarizes the angiographic and procedure characteristics. There were 13.2% of lesions treated with metallic stent/BVS overlapping in the real-world group, 23.7% of target lesions were evaluated by intravascular ultrasound (IUVS), and 7.9% of target lesions were chronic total occlusion. These characteristics did not exist in the ABSORB EXTEND trial, as protocol dictates. During the procedure, the pre-dilatation percentage, pre-dilatation pressures, BVS implantation pressures, balloon-scaffold ratio, post-dilatation percentage, and post-dilatation pressures were all similar in both groups.
Table 2. Lesion and procedural characteristics.
| ABSORB EXTEND (n = 34) | BVS in real world (n = 38) | p value | |
| Vessels treated, n (%) | 0.57 | ||
| Left anterior descending | 12 (35.3) | 18 (47.4) | |
| Left circumflex | 9 (26.5) | 9 (23.7) | |
| Ramus | 1 (2.9) | 0 | |
| Right coronary artery | 12 (35.3) | 11 (28.9) | |
| AHA lesion typesa | 0.007 | ||
| A | 16 (47.1) | 20 (52.6) | |
| B1 | 18 (52.9) | 9 (23.7) | |
| B2 | 0 | 2 (5.3) | |
| C | 0 | 7 (18.4) | |
| Number of scaffold use | 37 | 49 | 0.14 |
| Scaffold diameter | 0.02 | ||
| 2.5 mm | 6 (17.6) | 6 (15.8) | |
| 3.0 mm | 22 (64.7) | 14 (36.8) | |
| 3.5 mm | 6 (17.6) | 18 (47.4) | |
| Total scaffold length per lesion, mm | 20.9 ± 6.1 | 30.3 ± 19.2 | 0.008 |
| Scaffold-scaffold overlap | 3 (8.8) | 8 (21.1) | 0.15 |
| Stent-scaffold overlap | 0 | 5 (13.2) | 0.03 |
| Chronic total occlusion lesion | 0 | 3 (7.9) | 0.09 |
| Pre-dilatation | 34 (100) | 37 (97.7) | 0.34 |
| Pre-dilatation max balloon size, mm | 2.88 ± 0.34 | 3.05 ± 0.39 | 0.96 |
| Pre-dilatation max inflation pressure, atm | 10.9 ± 2.1 | 11.3 ± 3.5 | 0.74 |
| BVS deployment pressure, atm | 11.8 ± 1.6 | 11.3 ± 2.1 | 0.14 |
| Post-dilatation | 34 (100) | 37 (97.7) | 0.34 |
| Post-dilatation max balloon size, mm | 3.02 ± 0.30 | 3.16 ± 0.43 | 0.94 |
| Post-dilatation max inflation pressure, atm | 17.9 ± 3.0 | 17.2 ± 4.2 | 0.22 |
| Balloon-scaffold ratio | 0.96 ± 0.07 | 0.97 ± 0.09 | 0.69 |
| Intravascular ultrasound | 0 | 9 (23.7) | 0.002 |
a Lesions were classified according to the modified American College of Cardiology/American Heart Association (ACC/AHA) grading system. BVS, bioresorbable vascular scaffold.
Outcomes
The average follow-up duration of the ABSORB EXTEND trial patients were 732 days. Follow-up outcomes were summarized in Table 3. A total of 4 ischemia-driven TVR occurred in the ABSORB EXTEND group, including a case with non-target lesion DES restenosis treated by coronary artery bypass surgery. No TLR, cardiac death, MI or scaffold thrombosis was observed.The overall MACE rate in the ABSORB EXTEND trial was 11.8%.
Table 3. Clinical outcomes by absorb extend trials and BVS in real-world.
| ABSORB EXTEND (n = 34) | BVS in real-world (n=38) | |
| Follow up period, days | 732 ± 172 | 300 ± 78 |
| DAPT duration, months | 14.7 ± 7.5 | 11.1 ± 3.1 |
| Cardiac death | 0 | 0 |
| Myocardial Infarction | 0 | 2 (5.3) |
| CABG | 1 (2.9) | 0 |
| Ischemia driven TLR | 0 | 1 (2.6) |
| Ischemia driven TVR* | 4 (11.8) | 0 |
| Scaffold thrombosisa | 0 | 1 (2.6) |
| MACEb | 4 (11.8) | 3 (7.9) |
CABG, coronary artery bypass grafting surgery; DAPT, dual antiplatelet therapy; MACE, major adverse cardiac event; TLR, target lesion revascularization; TVR, target vessel revascularization.
a Scaffold thrombosis includes definite/probable/possible stent thrombosis by Academic Research Consortium; b MACE was defined as the composite endpoint of cardiac death, myocardial infarction, CABG, ischemia-driven TLR, ischemia-driven TVR and scaffold thrombosis.
* p = 0.04.
The average follow-up duration of the real-world patients was 300 days. Additionally, 2 cases (5.3%) of myocardial infarction occurred after BVS implantation. The first case involved a 38-year-old man receiving 5 overlapping BVSs for right coronary artery chronic total occlusion. Slow flow and ST segment elevation developed at the end of the procedure, with creatinine kinase peaking at 1245 U/L (URL for creatinine kinase: 223 UI/L) and troponin I at 22.7 ng/mL (URL for troponin I: 0.12 ng/mL). The second case is a 90-year-old woman who was receiving 1 BVS in the left circumflex artery. Acute myocardial infarction with precordial leads ST segment depression developed 30 days after the procedure, with creatinine kinase peaking at 281 U/L and troponin I at 7.24 ng/mL. The patient was treated medically without further angiography, and therefore was designated as a probable scaffold thrombosis. One patient receiving overlapping BVS/DES developed ischemia-driven TLR 10 months after procedure. The overall MACE rate in the real-world group was 7.9%.
AHA lesion types
Further analysis on the outcomes according to AHA lesion types are shown in Table 4 and Figure 1. There has been a tendency of elevated overall MACE in type B and C lesions (13.9% vs. 5.6% in type A lesions), with a hazard ratio of 2.35 (0.46-12.15, p = 0.31).
Table 4. Clinical outcomes by the lesion types.
| Type A (n = 36) | Type B and C (n = 36) | |
| Follow up period, days | 509 ± 254 | 496 ± 257 |
| DAPT duration, months | 13.2 ± 4.9 | 12.9 ± 7.2 |
| Cardiac death | 0 | 0 |
| Myocardial infarction | 0 | 2 (5.6) |
| CABG | 1 (2.8) | 0 |
| Ischemia driven TLR | 1 (2.8) | 0 |
| Ischemia driven TVR | 1 (2.8) | 3 (8.3) |
| Scaffold thrombosisa | 0 | 1 (2.8) |
| MACEb | 2 (5.6) | 5 (13.9) |
CABG, coronary artery bypass grafting surgery; DAPT, dual antiplatelet therapy; MACE, major adverse cardiac event; TLR, target lesion revascularization; TVR, target vessel revascularization.
a Scaffold thrombosis includes definite/probable/possible stent thrombosis by Academic Research Consortium; b MACE was defined as the composite endpoint of cardiac death, myocardial infarction, CABG, ischemia-driven TLR, ischemia-driven TVR and scaffold thrombosis.
Figure 1.
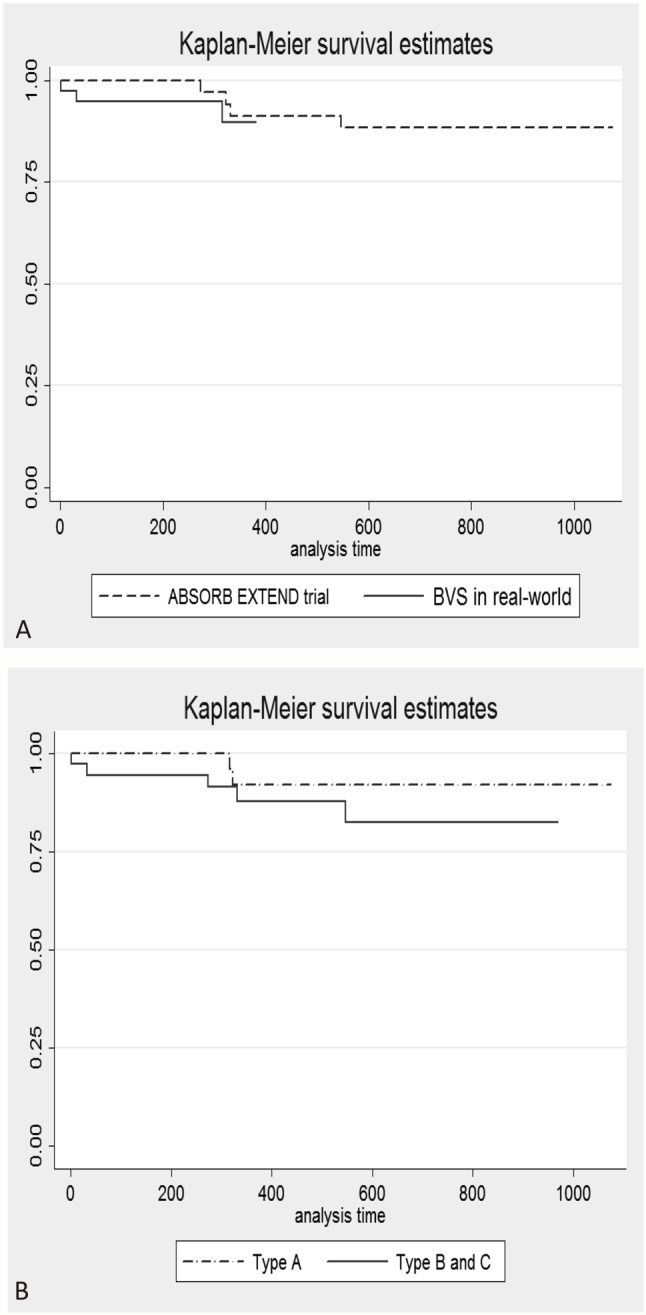
Major advance cardiovascular event-free survival curve by ABSORB EXTEND trial and real-world practice (A), and no significant difference was observed (p = 0.65). Major advance cardiovascular event free survival curve by lesion types (B) revealed slight increased events in lesion type B and C (p = 0.31).
DISCUSSION
We performed a retrospective analysis in consecutive patients who received BVS implants in real-world practice, comparing our results with the results of selected study patients from the ABSORB EXTEND trial cohort. The analysis explored the very early experience of BVS use in a single Taiwan center, with thorough follow-up. Our study revealed a high procedure success rate, with acceptable outcomes in a wide range of diseases.
In comparison to current use of metallic stents, the major advantage of BVS would be dismantling and complete resorption of the bioresorbable scaffold after 2 to 3 years.13,14 This will provide the possibility of late positive vessel remodeling and late lumen gain,14,15 maintain vasomotor function,16,17 and reduce the limitation for future intervention or imaging. Earlier BVS trials including ABSORB cohort A,7 cohort B,18-21 ABSORB EXTEND,8 and ABSORB II22 all demonstrated the safety and performance of the Absorb BVS. However, extensive lesion selection and meticulous procedural requirements in these trials preclude the generalization of trial results to real-world lesions and practice.
Comparing the baseline characteristics of patients from real-world practice and the ABSORB EXTEND trial, a significant difference was found regarding the clinical presentation. Most of patients in ABSORB EXTEND trial (94.1%) presented as either asymptomatic or having stable angina, and only 5.9% with unstable angina. In contrast, 80% of real-world patients were in asymptomatic or stable angina status, 2.9% with unstable angina and 17.1% with acute myocardial infarction (p = 0.04). This important difference was attributed to the ABSORB EXTEND trial design per se. During the ABSORB EXTEND trial, patients presenting with acute myocardial infarction were excluded, and none of the patients from the ABSORB EXTEND trial had acute myocardial infarction, compared to 17.1% with acute myocardial infarction in real-world patients.
In the present analysis, lesions in the real-world BVS patients were significantly more complex than those in the ABSORB EXTEND cohort, with more type B2/C lesions, longer lesion length and BVS coverage, and more BVS/BVS overlapping. Similar to other real-world registry results from Australia and Europe,23-25 our analysis showed low overall MACE rates to 7.9%, including 5.3% of MI, 2.6% of ischemia-driven TLR and 2.6% of scaffold thrombosis. The early and midterm outcomes from European multicenter GHOST-EU registry revealed a 99.7% technical success rate, with 2.5% of TLR, 4.0% of TVR, 2.1% of definite/probable scaffold thrombosis and 1.0% of cardiac death at 6 months.24 The ASSURE registry from six German centers revealed 100% technical success rate with 2.8% of TLR at the one-year period.25 Robaei et al. reported procedural and 30-day outcomes at two Australian centers showed a 95.3% procedural success rate and 4% of peri-procedure MI.23 Costopoulos et al. further compared the 6-month clinical outcomes between BVS and cobalt chromium everolimus-eluting stents (EES) in real-world patients with mostly complex disease (77.4-83.9% with B2/C lesion type). The major adverse cardiac events (defined as the composite of TVR, follow-up myocardial infraction and all-cause death) were 3.3% in the BVS group vs. 7.6% in the EES group.26
Most of reports of real-world practice, including our analysis, consistently showed high rates of procedural success with minimal complications with use of the Absorb BVS. However, there was a tendency of more ischemia-driven MACE in a complex lesion subset, with a hazard ratio of 2.35 in our analysis, including 1 probable scaffold thrombosis. Although the stent thrombosis rate is low in the BVS trials, alarming cases of BVS thrombosis have been reported in real-world registries.24,27-29 Similar to metallic stents, suboptimal implantation with incomplete lesion coverage, under-expansion, mal-apposition, and early dual antiplatelet therapy discontinuation comprise the main mechanisms for BVS thrombosis.27 With more complex lesions being treated with BVS, adherence to the adequate lesion selection, deployment techniques, and medication compliance should be strongly emphasized.
In our real-world cases, 13.2% of the lesions were treated with metallic stent/BVS overlapping. The reasons for this practice may be varied: reduction in overall cost, avoidance of major side branch jailing by BVS, precise ostial coverage, sizing discrepancy, or preservation of a "window" for future bypass surgery. These concepts may be legitimate and sound, but need to be proven in future studies.29 In addition, optimal deployment sequence and techniques in metallic stent/BVS overlaps need to be reliably defined both in animal and clinical studies.
Limitation
The study is a single center experience comparing results of cases from both the ABSORB EXTEND trial and a real-world population; thus, the cohorts were not concurrent and matched. As BVS is not reimbursed in Taiwan, the use of BVS in the real-world cohort is not only influenced by lesion complexity but also by the patients’ financial capacity to pay.
CONCLUSIONS
The use of the Absorb BVS in real-world practice is feasible, with acceptable clinical outcomes in Taiwan. A tendency towards higher MACE in more complex lesions was observed, which highlighted the importance of case selection and deployment techniques for BVS usage in real-world practice.
CONFLICTS OF INTEREST
The authors had no conflicts of interest to declare in relation to this article.
REFERENCES
- 1.Fischman DL, Leon MB, Baim DS, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994;331:496–501. doi: 10.1056/NEJM199408253310802. [DOI] [PubMed] [Google Scholar]
- 2.Katz G, Harchandani B, Shah B. Drug-eluting stents: the past, present, and future. Curr Atheroscler Rep. 2015;17:485. doi: 10.1007/s11883-014-0485-2. [DOI] [PubMed] [Google Scholar]
- 3.Kastrati A, Mehilli J, Pache J, et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med. 2007;356:1030–1039. doi: 10.1056/NEJMoa067484. [DOI] [PubMed] [Google Scholar]
- 4.Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 5.Lai CH, Lee WL, Sung SH, et al. Comparison of bare-metal stent and drug-eluting stent for the treatment of patients undergoing percutaneous coronary intervention for unprotected left main coronary artery disease – long-term result from a single center experience. Acta Cardiol Sin. 2015;31:381–389. doi: 10.6515/ACS20140630G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai CC, Yip HK, Lin TH, et al. Drug-eluting stents versus bare-metal stents in Taiwanese patients with acute coronary syndrome: an outcome report of a multicenter registry. Acta Cardiol Sin. 2014;30:553–564. doi: 10.6515/ACS20140421B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ormiston JA, Serruys PW, Regar E, et al. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet. 2008;371:899–907. doi: 10.1016/S0140-6736(08)60415-8. [DOI] [PubMed] [Google Scholar]
- 8.Abizaid A, Ribamar Costa J, Jr., Bartorelli AL, et al. The ABSORB EXTEND study: preliminary report of the twelve-month clinical outcomes in the first 512 patients enrolled. EuroIntervention. 2015;10:1396–1401. doi: 10.4244/EIJV10I12A243. [DOI] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1598. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 11.Ellis SG, Vandormael MG, Cowley MJ, et al. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Multivessel Angioplasty Prognosis Study Group. Circulation. 1990;82:1193–1202. doi: 10.1161/01.cir.82.4.1193. [DOI] [PubMed] [Google Scholar]
- 12.Di Mario C, Werner GS, Sianos G, et al. European perspective in the recanalisation of chronic total occlusions (CTO): consensus document from the EuroCTO Club. EuroIntervention. 2007;3:30–43. [PubMed] [Google Scholar]
- 13.Serruys PW, Ormiston JA, Onuma Y, et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet. 2009;373:897–910. doi: 10.1016/S0140-6736(09)60325-1. [DOI] [PubMed] [Google Scholar]
- 14.Serruys PW, Onuma Y, Garcia-Garcia HM, et al. Dynamics of vessel wall changes following the implantation of the absorb everolimus-eluting bioresorbable vascular scaffold: a multi-imaging modality study at 6, 12, 24 and 36 months. EuroIntervention. 2014;9:1271–1284. doi: 10.4244/EIJV9I11A217. [DOI] [PubMed] [Google Scholar]
- 15.Strandberg E, Zeltinger J, Schulz DG, Kaluza GL. Late positive remodeling and late lumen gain contribute to vascular restoration by a non-drug eluting bioresorbable scaffold: a four-year intravascular ultrasound study in normal porcine coronary arteries. Circ Cardiovasc Interv. 2012;5:39–46. doi: 10.1161/CIRCINTERVENTIONS.111.964270. [DOI] [PubMed] [Google Scholar]
- 16.Lane JP, Perkins LE, Sheehy AJ, et al. Lumen gain and restoration of pulsatility after implantation of a bioresorbable vascular scaffold in porcine coronary arteries. JACC Cardiovasc Interv. 2014;7:688–695. doi: 10.1016/j.jcin.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 17.Brugaletta S, Heo JH, Garcia-Garcia HM, et al. Endothelial-dependent vasomotion in a coronary segment treated by ABSORB everolimus-eluting bioresorbable vascular scaffold system is related to plaque composition at the time of bioresorption of the polymer: indirect finding of vascular reparative therapy? Eur Heart J. 2012;33:1325–1333. doi: 10.1093/eurheartj/ehr466. [DOI] [PubMed] [Google Scholar]
- 18.Serruys PW, Onuma Y, Ormiston JA, et al. Evaluation of the second generation of a bioresorbable everolimus drug-eluting vascular scaffold for treatment of de novo coronary artery stenosis: six-month clinical and imaging outcomes. Circulation. 2010;122:2301–2312. doi: 10.1161/CIRCULATIONAHA.110.970772. [DOI] [PubMed] [Google Scholar]
- 19.Serruys PW, Onuma Y, Dudek D, et al. Evaluation of the second generation of a bioresorbable everolimus-eluting vascular scaffold for the treatment of de novo coronary artery stenosis: 12-month clinical and imaging outcomes. J Am Coll Cardiol. 2011;58:1578–1588. doi: 10.1016/j.jacc.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 20.Onuma Y, Serruys PW, Ormiston JA, et al. Three-year results of clinical follow-up after a bioresorbable everolimus-eluting scaffold in patients with de novo coronary artery disease: the ABSORB trial. EuroIntervention. 2010;6:447–453. doi: 10.4244/EIJ30V6I4A76. [DOI] [PubMed] [Google Scholar]
- 21.Dudek D, Onuma Y, Ormiston JA, et al. Four-year clinical follow-up of the ABSORB everolimus-eluting bioresorbable vascular scaffold in patients with de novo coronary artery disease: the ABSORB trial. EuroIntervention. 2012;7:1060–1061. doi: 10.4244/EIJV7I9A168. [DOI] [PubMed] [Google Scholar]
- 22.Serruys PW, Chevalier B, Dudek D, et al. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet. 2015;385:43–54. doi: 10.1016/S0140-6736(14)61455-0. [DOI] [PubMed] [Google Scholar]
- 23.Robaei D, Back LM, Ooi SY, et al. Everolimus-eluting bioresorbable vascular scaffold implantation in real world and complex coronary disease: procedural and 30-day outcomes at two Australian centres. Heart Lung Circ. 2015 doi: 10.1016/j.hlc.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Capodanno D, Gori T, Nef H, et al. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention. 2015;10:1144–1153. doi: 10.4244/EIJY14M07_11. [DOI] [PubMed] [Google Scholar]
- 25.Wohrle J, Naber C, Schmitz T, et al. Beyond the early stages:insights from the ASSURE registry on bioresorbable vascular scaffolds. EuroIntervention. 2015;11:149–156. doi: 10.4244/EIJY14M12_10. [DOI] [PubMed] [Google Scholar]
- 26.Costopoulos C, Latib A, Naganuma T, et al. Comparison of early clinical outcomes between ABSORB bioresorbable vascular scaffold and everolimus-eluting stent implantation in a real-world population. Catheter Cardiovasc Interv. 2015;85:E10–E15. doi: 10.1002/ccd.25569. [DOI] [PubMed] [Google Scholar]
- 27.Karanasos A, Van Mieghem N, van Ditzhuijzen N, et al. Angiographic and optical coherence tomography insights into bioresorbable scaffold thrombosis: single-center experience. Circ Cardiovasc Interv. 2015;8:e002369. doi: 10.1161/CIRCINTERVENTIONS.114.002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraak RP, Hassell ME, Grundeken MJ, et al. Initial experience and clinical evaluation of the Absorb bioresorbable vascular scaffold (BVS) in real-world practice: the AMC Single Centre Real World PCI Registry. EuroIntervention. 2015;10:1160–1168. doi: 10.4244/EIJY14M08_08. [DOI] [PubMed] [Google Scholar]
- 29.Ishibashi Y, Onuma Y, Muramatsu T, et al. Lessons learned from acute and late scaffold failures in the ABSORB EXTEND trial. EuroIntervention. 2014;10:449–457. doi: 10.4244/EIJV10I4A78. [DOI] [PubMed] [Google Scholar]


