Abstract
Background
It is well-known that cardiovascular risk and all-cause mortality is increased in hemodialysis patients. Epicardial fat thickness (EFT), which reflects visceral adiposity, has been suggested as a new cardiometabolic risk factor. The purpose of this study was to investigate EFT in hemodialysis patients.
Methods
A total of 144 consecutive patients (60 hemodialysis patients and 84 controls) were enrolled into the study, and patients with diabetes mellitus and cardiovascular diseases (CVD) were excluded. EFT was measured on the free wall of the right ventricle at end-diastole from the parasternal long-axis view by standard transthorasic 2D echocardiography.
Results
The groups were similar in terms of sex distribution, age, blood pressure, heart rate and frequencies of CAD risk factors including smoking status, family history of CAD and hypertension. There were no significant differences between the hemodialysis patients and controls in 2D echocardiographic parameters, including ejection fraction and biochemical parameters except low-density lipoprotein, high-density lipoprotein and c- reactive protein. Despite having lower body mass index, EFT levels were significantly higher in hemodialysis patients compared to the controls (8.0 ± 2.2 mm vs. 5.8 ± 1.9 mm; p < 0.01). In multivariate linear regression analysis we determined that hemodialysis patient status was found to be an independent predictor for both EFT (β = 0. 700, p = 0.014) and carotid intima-media thickness (CIMT, β = 0. 614, p = 0.047).
Conclusions
Hemodialysis patients are independently associated with high EFT and CIMT.
Keywords: Atherosclerosis, End-stage renal disease, Risk factors
INTRODUCTION
Chronic renal failure (CRF) and cardiovascular disease (CVD) are closely related clinical entities. It is generally understood that cardiovascular risk and all-cause mortality is increased in hemodialysis patients.1 Additionally, atherosclerosis and coronary artery disease (CAD) are more common in CRF.2 Therefore, clinical predictors of premature atherosclerosis are crucial. Epicardial fat thickness (EFT) which reflects cardiac and visceral adiposity is suggested to be a new cardiometabolic risk factor.3-5 The relationship between CVD and visceral adiposity rather than subcutaneous fat accumulation, and the correlation between increased EFT and insulin resistance or metabolic syndrome have previously been reported.6,7 Studies about the association between EFT and CRF patient are controversial.8-11 Carotid intima-media thickness (CIMT) is another parameter that shows atherosclerosis and coronary artery disease.12-14 It is useful for the prediction of cardiovascular events in patients with CRF.13 The aim of this study was to investigate EFT and CIMT in our hemodialysis patients without CVD.
METHODS
Study population
In all, 144 consecutive patients (60 with dialysis and 84 controls) were prospectively enrolled into the study between September 2013 and September 2014. Demographic data, risk factors for CVD, medications, anthropometric and biochemical findings were recorded. Body mass index (BMI) was defined as weight (kg)/height (m)2. Patients with diabetes mellitus, CVD, systolic heart failure, severe valvular disease, hypertrophic cardiomyopathy, chronic obstructive pulmonary disease, sepsis, chronic liver disease, peripheral artery arterial disease, and patients with inadequate echogenicity were excluded. CVD was considered if angina pectoris, ST-T waves changes, Q waves, left bundle branch block on electrocardiogram, regional wall motion abnormalities on echocardiogram, ischemia detected by non invasive stress tests, history of myocardial infarction, coronary artery stenosis ≥ 50% on coronary angiography or a history of coronary revascularization existed.
Data acquisition and analysis
Routine two dimensional (2D), conventional spectral Doppler and epicardial fat thickness (EFT) data
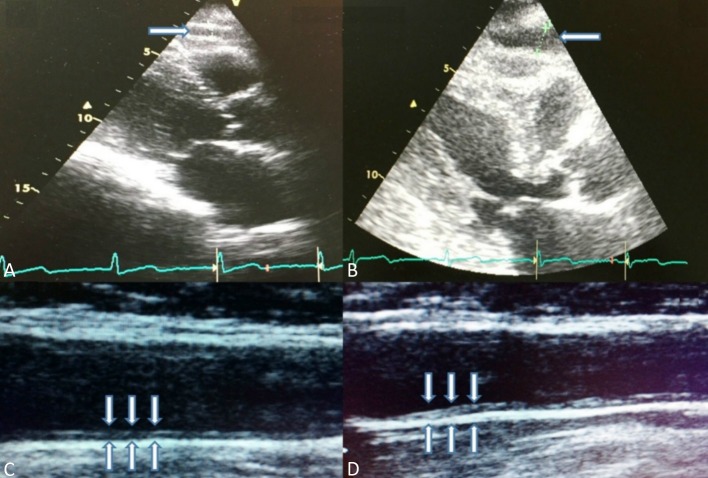
All patients underwent standard 2D and Doppler echocardiography conforming to the American Society of Echocardiography/European Association of Echocardiography recommendations.15 A Vivid S5 ultrasound machine (GE Healthcare, Horten, Norway), equipped with a 3SRS broadband transducer was used. Ejection fraction (EF) was calculated by modified Simpson’s method. EFT was identified as the echo-free space between the outer wall of the myocardium and visceral layer of pericardium.3-6 It was measured on the free wall of the right ventricle perpendicularly at end-diastole from the parasternal long-axis views of 3 cardiac cycles by standard transthorasic 2D echocardiography (Figure 1).
Figure 1.
(A-B) Epicardial fat tissue measurement on the free wall of the right ventricle at end-diastole from the parasternal long-axis. (C-D) Measurement of the carotid intima-media thickness was performed 2 cm below the carotid bifurcation in a plaque-free arterial segment and an average of four measurements used for both side (A: Control, B: Hemodialysis patient).
Overall, 144 consecutive patients (60 dialysis and 84 controls) were examined by ultrasonography (Hitachi EUB 7000, Japan), with 13.5 mHz high-resolution linear probe, which was performed bilaterally by two radiologists for each examination who were blinded to the clinical and biochemical data. CIMT was defined as the distance between the leading edge of the first and second echogenic lines. Measurements were performed 2 cm below the carotid bifurcation in a plaque-free arterial segment, and each measurement represented an average of four measurements for both sides.
Statistical analysis
Variables were tested for normal distribution by using the Kolmogorov-Smirnov test. Differences between the groups were assessed by using unpaired t test, and p < 0.05 was accepted as statistically significant. The mean values of CIMT and EFT between patients and matched controls were compared statistically by using the Student’s t-test. SPSS 16.0 for Windows (Statistical Program for the Social Services Inc, Chicago, IL, USA) program was used for statistical analysis. Multivariate linear regression analysis was used to define independent predictors of CIMT and EFT among well-known confounding variables such as age, blood pressure, BMI, EF, and levels of fasting glucose, lipids, creatinine and C-reactive protein (CRP). Interclass correlation coefficient (ICC) was calculated with a 95% confidence interval (CI) to evaluate the reliability of the EFT and CIMT measurement. ICC had adopted the same interpretation as the kappa statistics: ICC > 0.75 was considered as excellent, 0.4 to 0.75 as good, and ICC < 0.4 as poor.16
RESULTS
Groups were similar in terms of sex distribution, age, blood pressure, heart rate and frequencies of CAD risk factors including smoking status, family history of CAD and hypertension. There were no significant differences in biochemical parameters between the CRF patients and controls except serum creatinine, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and CRP levels. Baseline clinical, anthropometric and biochemical findings of our study group were provided in Table 1. Serum LDL and HDL levels were significantly lower in patients with CRF (104.5 ± 32.2 vs. 152.6 ± 39.3 and 33.6 ± 8.5 vs. 45.2 ± 12.8; p < 0.01), whereas serum CRP level was higher in this group (0.9 ± 0.7 vs. 0.5 ± 0.6; p < 0.01) (Table 1). There were also no significant differences in two dimensional echocardiographic parameters including ejection fraction. EFT and CIMT measurement of hemodialysis patients was compared with control group, whose members were the same age, gender and without any history of coronary artery disease. Despite having lower BMI (24.3 ± 5.0 vs. 27.8 ± 3.5; p < 0.01) EFT was significantly higher in CRF group compared to the controls (8.0 ± 2.2 vs. 5.8 ± 1.9 mm; p < 0.01). CIMT was also significantly higher in hemodialysis patients than in the control group (0.84 ± 0.23 to 0.78 ± 0.2 mm, p < 0.01) (Figure 2-3).
Table 1. Mean EFT values, demographic features and biochemical data in our study groups.
| Control | HDP | p value | |
| Number | 84 | 60 | |
| Male sex (%) | 50 (59.5) | 33 (55) | NS |
| Age (years) | 58.83 ± 10.5 | 56.7 ± 16.2 | NS |
| Smoking (%) | 24 (28.6) | 18 (30) | NS |
| Family history of CAD (%) | 33 (39.3) | 23 (38.3) | NS |
| SBP (mmHg) | 132.1 ± 13.0 | 128.3 ± 17.0 | NS |
| DBP (mmHg) | 82.1 ± 8.5 | 79.8 ± 8.2 | NS |
| Heart Rate (beat/min) | 73.8 ± 10.7 | 75.7 ± 11.7 | NS |
| BMI (kg/m2) | 27.8 ± 3.5 | 24.3 ± 5.0 | < 0.01 |
| FPG (mg/dl) | 98.1 ± 8.6 | 97.3 ± 15.4 | NS |
| LDL (mg/dl) | 152.6 ± 39.3 | 104.5 ± 32.2 | < 0.01 |
| HDL (mg/dl) | 45.2 ± 12.8 | 33.6 ± 8.5 | < 0.01 |
| Triglyceride (mg/dl) | 162.8 ± 80.2 | 166.8 ± 81.4 | NS |
| Creatinine (mg/dl) | 0.85 ± 0.1 | 8.5 ± 2.3 | < 0.01 |
| ALT (U/L) | 17.6 ± 7.2 | 15.2 ± 7.9 | NS |
| CRP (mg/dl) | 0.5 ± 0.6 | 0.9 ± 0.7 | < 0.01 |
| EF (%) | 58.4 ± 4.1 | 57.3 ± 4.2 | NS |
| EFT (mm) | 5.8 ± 1.9 | 8.0 ± 2.2 | < 0.01 |
| CIMT (mm) | 0.78 ± 0.2 | 0.84 ± 0.23 | < 0.01 |
ALT, alanine transaminase; BMI, body mass index; CAD, coronary artery disease; CIMT, carotid intima media thickness; CRP, C-reactive protein; DBP, diastolic blood pressure; EF, ejection fraction; EFT, epicardial fat thickness; FPG, fasting plasma glucose; HDL, high-density lipoprotein; HDP, hemodialysis patient; LDL, low-density lipoprotein; SBP, systolic blood pressure.
Figure 2.
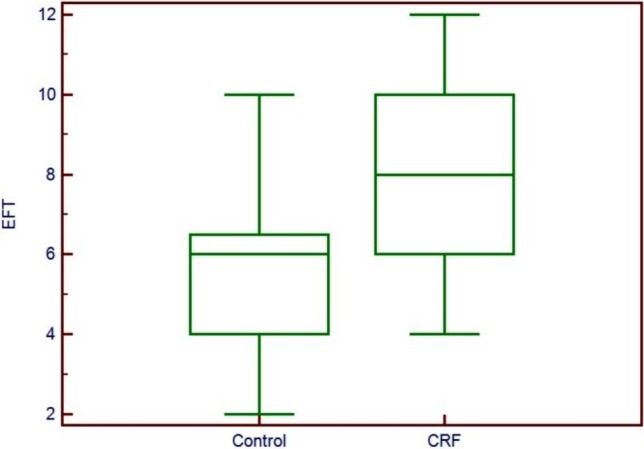
Comparison of epicardial fat tissue measurements between control group and hemodialysis patients.
Figure 3.
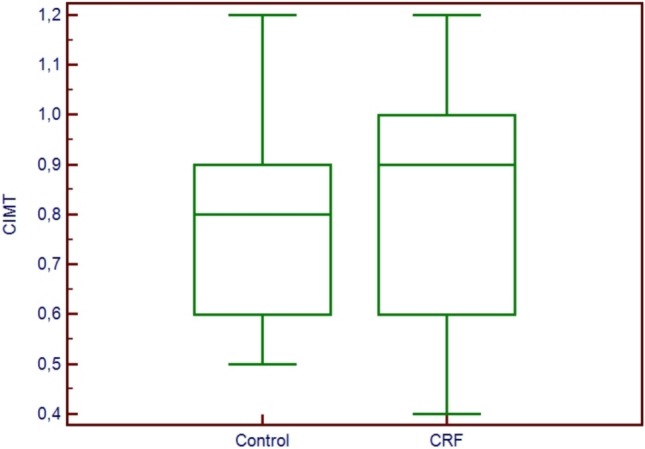
Comparison of carotid intima-media thickness measurements between control group and hemodialysis patients.
In multivariate linear regression analysis to define independent predictors of carotid IMT and EFT, among variables including age, BMI, blood pressure, EF and certain biochemical parameters including fasting plasma glucose, LDL, HDL, triglyceride, hematocrit, creatinine and CRP; we found that being a hemodialysis patient was the independent predictor for both EFT (β = 0.700, p = 0.014) and CIMT (β = 0.614, p = 0.047) (Table 2). ICC was used to assess interobserver reproducibility of EFT and CIMT in 18 consecutive patients. ICC agreement on measurements were 0.91 (95% CI: 0.76-0.97) for CIMT and 0.90 (95% CI: 0.75-0.96) for EFT which suggests excellent reproducibility.
Table 2. Multiple linear regression analysis of epicardial fat thickness and carotid intima media thickness.
| Independent variables | EFT | CIMT | ||
| Correlation coefficients β | p value | Correlation coefficients β | p value | |
| HDP | 0.700 | 0.014 | 0.614 | 0.047 |
| BMI | 0.114 | 0.219 | 0.138 | 0.170 |
| Age | -0.012 | 0.890 | -0.042 | 0.661 |
| SBP | -0.027 | 0.788 | -0.020 | 0.860 |
| DBP | -0.014 | 0.892 | -0.011 | 0.917 |
| EF | 0.020 | 0.808 | 0.134 | 0.144 |
| Hematocrit | 0.107 | 0.335 | 0.006 | 0.962 |
| FPG | 0.055 | 0.501 | 0.163 | 0.069 |
| Creatinine | 0.169 | 0.487 | 0.381 | 0.151 |
| CRP | 0.005 | 0.952 | 0.080 | 0.422 |
| HDL-C | -0.047 | 0.641 | -0.029 | 0.789 |
| LDL-C | 0.021 | 0.833 | 0.085 | 0.444 |
| Triglyceride | -0.161 | 0.090 | -0.005 | 0.958 |
BMI, body mass index; CIMT, carotid intima media thickness; CRP, C-reactive protein; DBP, diastolic blood pressure; EF, ejection fraction; EFT, epicardial fat thickness; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; HDP, hemodialysis patients; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure.
DISCUSSION
CRF patients have high morbidity and mortality rates because of the atherosclerotic vascular disease.1,2 CRF can cause hemodynamic overload, anemia, malnutrition, increased oxidative stress, and hyperhomocystinemia, which are the factors that increase atherosclerosis and vascular disease.12,17 It is crucial to detect premature atherosclerosis for prevention of CVD in CRF patients.
Epicardial fat is a metabolic active organ that produces several proatherogenic cytokines and which may have a role in the pathogenesis of CVD. It shares the same microcirculation with myocardium. In terms of greater capacity of releasing free fatty acids, EFT differs from other visceral fat tissues. Elevated free fatty acid concentration may stimulate autonomic nervous system activity at the same time.18,19
BMI and waist circumference (WC) are poor indicators for cardiovascular risk. They reflect generalized adiposity rather than visceral adiposity and have some limitations especially in older patients. In addition to low sensitivity and specificity, the inter/intraobserver variability is another disadvantage of WC.20,21
In our study; patients with CRF had lower BMI and LDL, which are independent indicators for CVD. Despite having high cardiovascular risk, BMI and WC are frequently lower due to nutritional disorders in CRF.1,17 Therefore BMI and WC may not be preciously valid in CRF.
Magnetic resonance imaging (MRI) and computed tomography (CT) are gold standard methods for quantifying visceral adiposity and EFT. EFT can be measured in the right ventricular free wall and around the main coronary arteries or the inter- and atrioventricular grooves. Due to the difficulty in standardizing measurement locations, EFT reference value by CT is unclear.22-24 EFT measurements have excellent reproducibility however, it is technically more difficult. It was found that coefficient of variability was 5.9% for the volumetric method and 13.6% for EFT at the long axis.24 The measurement of maximum EFT is more feasible, without significant accuracy decrease.24 Flutcher et al.25 evaluated EFT by MRI using the mean of maximum EFT at several points of the right ventricular free wall and found mean values comparable to those found by Schejbal et al.26 in 200 autopsies (mean thickness: 4.12 ± 1.4 mm). EFT studies up to date have evaluated small samples of patients. Therefore, reference values are unclear. When comparing ultrasound and cardiac MRI for detection of total fat and EFT, two techniques provided comparable results.27 In addition to EFT, some studies suggested that increased cardiac fat in the pericardial adipose tissue is strongly associated with features of metabolic syndrome and pericardial fat, which is a better cardiometabolic risk marker than epicardial fat tissue.27
The high cost, necessity of more clinical experience, the longer time to perform and radiation (for CT) are important disadvantages for these imaging modalities. Nevertheless the measurement of EFT by echocardiography is simple, safe, cheap, rapid method and well-correlated to gold standard modalities.28,29
Data so far indicated that, measurement of EFT is independently associated with hypertension, insulin resistance, metabolic syndrome, prediabetes, hyperlipidemia, endothelial dysfunction, the presence and severity of CAD, and subclinical atherosclerosis.30-35 We considered EFT is not only a non-invasive, but also a practical method to screen CVD in hemodialysis patients.
CIMT is another marker that shows atherosclerosis and also coronary artery disease.12-14 Benedetto et al.36 found in their study that CIMT and concentric left ventricular hypertrophy is related with cardiovascular death. Kawagishi et al.37 indicated that CIMT was significantly higher in hemodialysis patients than in age- and gender-matched control subjects.Pascazio et al.38 showed that the frequency of atherosclerotic plaques is larger in dialysis patients than in healthy subjects and patients who have traditional cardiovascular risk factors. A 0.1 mm increase in CIMT causes a 24% higher risk for cardiovascular death.36
Ultrasonography is a noninvasive and easy method for CIMT measurement to detect premature vascular atherosclerotic disease. Other modalities such as MRI and CT can be also used for measurement of CIMT. There is good correlation between wall area, wall thickness, and plaque index measured by MRI and CIMT measurements obtained by ultrasound, but carotid MRI has higher reproducibility compared with ultrasound. However, our results indicate an excellent reproducibility in measurements of CIMT. Compared with carotid ultrasound, MRI is more expensive and also involves longer scan times and sensitivity to motion which restricts its use.39 CT is not a preferred modality for measuring carotid wall or plaque due to its limitations associated with dense calcification, poor contrast between lipid and fibrotic components, and exposure to radiation. Nevertheless, good agreement in measurement of CIMT between multi detector-row CT angiography and ultrasound was reported in a study.40
Kiykim et al.41 stated that there is a positive correlation between CIMT and the following parameters: age, left ventricular mass, serum homocystein level, CRP, ESR, albumin and mean hematocrit in CRF patients. Hakan et al.42 found higher values of CIMT in CRF children comparing with the control group, as revealed in our study. Moreover, Delucchie et al.43 determined there was a significant correlation between CIMT and duration of dialysis. Interestingly, Hakan et al.41 found the same results in the pediatric group.
CIMT and EFT are useful for risk stratification in hemodialysis population and they have positive correlation with cardiovascular death. One of the most important limitations of our study is its cross-sectional design. It may affect the outcome and causal-relationship. Long term prospective analyses are required to support this hypothesis.
CONCLUSIONS
Hemodialysis patients are independently associated with high EFT and CIMT. These modalities are simple, inexpensive, easily accessible, non-invasive and objective method for screening of subclinical CVD to reduce morbidity and mortality in hemodialysis patients.
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community based studies. J Am Soc Nephrol. 2004;15:1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 2.Pascazio L, Bianco F, Giorgini A, et al. Echo color Doppler imaging of carotid vessels in hemodialysis patients: evidence of high levels of atherosclerotic lesions. Am J Kidney Dis. 1996;28:713–720. doi: 10.1016/s0272-6386(96)90253-x. [DOI] [PubMed] [Google Scholar]
- 3.Gastaldelli A, Basta G. Ectopic fat and cardiovascular disease:what is the link? Nutr Metab Cardiovasc Dis. 2010;20:481–490. doi: 10.1016/j.numecd.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro-Filho FF, Faria AN, Azjen S, et al. Methods of estimation of visceral fat: advantages of ultrasonography. Obes Res. 2003;11:1488–1494. doi: 10.1038/oby.2003.199. [DOI] [PubMed] [Google Scholar]
- 5.Iacobellis G, Leonetti F, Di Mario U. Images in cardiology: massive epicardial adipose tissue indicating severe visceral obesity. Clin Cardiol. 2003;26:237. doi: 10.1002/clc.4960260508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iacobellis G, Assael F, Ribaudo MC, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 2003;11:304–310. doi: 10.1038/oby.2003.45. [DOI] [PubMed] [Google Scholar]
- 7.Iacobellis G, Leonetti F. Epicardial adipose tissue and insulin resistance in obese subjects. J Clin Endocrinol Metab. 2005;90:6300–6302. doi: 10.1210/jc.2005-1087. [DOI] [PubMed] [Google Scholar]
- 8.Turkmen K, Ozbek O, Kayikcioglu H, et al. The relationship between epicardial adipose tissue and coronary artery calcification in peritoneal dialysis patients. Cardiorenal Med. 2012;2:43–51. doi: 10.1159/000335495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turan MN, Gungor O, Asci G, et al. Epicardial adipose tissue volume and cardiovascular disease in hemodialysis patients. Atherosclerosis. 2013;226:129–133. doi: 10.1016/j.atherosclerosis.2012.10.061. [DOI] [PubMed] [Google Scholar]
- 10.Ozkurt S, Karavelioglu Y, Musmul A. Echocardiographic evaluation of epicardial adipose tissue in non-diabetic, non-hypertensive hemodialysis patients. Ren Fail. 2013;35:891–895. doi: 10.3109/0886022X.2013.794682. [DOI] [PubMed] [Google Scholar]
- 11.Altun B, Tasolar H, Eren N, et al. Epicardial adipose tissue thickness in hemodialysis patients. Echocardiography. 2014;31:941–946. doi: 10.1111/echo.12498. [DOI] [PubMed] [Google Scholar]
- 12.Kumar KS, Lakshmi AY, Srinivasa Rao PVLN, et al. Carotid intima-media thickness in patients with end-stage renal disease. Indian J Nephrol. 2009;19:13–14. doi: 10.4103/0971-4065.50674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Preston E, Ellis MR, Kulinskaya E, et al. Association between carotid artery intima-media thickness and cardiovascular risk factors in CKD. Am J Kidney Dis. 2005;46:56–62. doi: 10.1053/j.ajkd.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 14.Hansa G, Bhargava K, Bansal M, et al. Carotid intima-media thickness and coronary artery disease: an Indian perspective. Asian Cardiovasc Thorac Ann. 2003;11:217–221. doi: 10.1177/021849230301100308. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 17.Papagianni A, Kokolina E, Kalovoulos M, et al. Carotid atherosclerosis is associated with inflammation, malnutrition and intercellular adhesion molecule-1 in patients on continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant. 2004;19:1258–1263. doi: 10.1093/ndt/gfh078. [DOI] [PubMed] [Google Scholar]
- 18.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 19.Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 20.Natale F, Tedesco MA, Mocerino R, et al. Visceral adiposity and arterial stiffness: echocardiographic epicardial fat thickness reflects, better than waist circumference, carotid arterial stiffness in a large population of hypertensives. Eur J Echocardiogr. 2009;10:549–555. doi: 10.1093/ejechocard/jep002. [DOI] [PubMed] [Google Scholar]
- 21.Yasuda T, Matsuhisa M, Fujiki N, et al. Is central obesity a good predictor of carotid atherosclerosis in Japanese type 2 diabetes with metabolic syndrome? Endocr J. 2007;54:695–702. doi: 10.1507/endocrj.k06-210. [DOI] [PubMed] [Google Scholar]
- 22.Wang CP, Hsu HL, Hung WC, et al. Increased epicardial adipose tissue (EAT) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin Endocrinol. 2009;70:876–882. doi: 10.1111/j.1365-2265.2008.03411.x. [DOI] [PubMed] [Google Scholar]
- 23.Gorter PM, de Vos AM, van der Graaf Y, et al. Relation of epicardial and pericoronary fat to coronary atherosclerosis and coronary artery calcium in patients undergoing coronary angiography. Am J Cardiol. 2008;102:380–385. doi: 10.1016/j.amjcard.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Bertaso AG, Bertol D, Duncan BB, Foppa M. Epicardial fat: definition, measurements and systematic review of main outcomes. Arquivosbrasileiros de Cardiologia. 2013;101:e18–e28. doi: 10.5935/abc.20130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flüchter S, Haghi D, Dinter D, et al. Volumetric assessment of epicardial adipose tissue with cardiovascular magnetic resonance imaging. Obesity. 2007;15:870–878. doi: 10.1038/oby.2007.591. [DOI] [PubMed] [Google Scholar]
- 26.Schejbal V. Epicardial fatty tissue of the right ventricle-morphology, morphometry and functional significance. Pneumologie. 1989;43:490–499. [PubMed] [Google Scholar]
- 27.Sicari R, Sironi AM, Petz R, et al. Pericardial rather than epicardial fat is a cardiometabolic risk marker: an MRI vs echo study. J Am Soc Echocardiogr. 2011;24:1156–1162. doi: 10.1016/j.echo.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Kessels K, Cramer MJ, Veldhuis B. Epicardial adipose tissue imaged by magnetic resonance imaging: an important risk marker of cardiovascular disease. Heart. 2006;92:262. doi: 10.1136/hrt.2005.074872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iacobellis G. Imaging of visceral adipose tissue: an emerging diagnostic tool and therapeutic target. Curr Drug Targets Cardiovasc Hematol Dis. 2005;5:345–353. doi: 10.2174/1568006054553408. [DOI] [PubMed] [Google Scholar]
- 30.Nabati M, Saffar N, Yazdani J, et al. Relationship between epicardial fat measured by echocardiography and coronary atherosclerosis: a single-blind historical cohort study. Echocardiography. 2013;30:505–511. doi: 10.1111/echo.12083. [DOI] [PubMed] [Google Scholar]
- 31.Sengul C, Cevik C, Ozveren O, et al. Epicardial fat thickness is associated with non-dipper blood pressure pattern in patients with essential hypertension. Clin Exp Hypertens. 2012;34:165–170. doi: 10.3109/10641963.2011.577488. [DOI] [PubMed] [Google Scholar]
- 32.Sengul C, Cevik C, Ozveren O, et al. Echocardiographic epicardial fat thickness is associated with carotid intima-media thickness in patients with metabolic syndrome. Echocardiography. 2011;28:853–858. doi: 10.1111/j.1540-8175.2011.01471.x. [DOI] [PubMed] [Google Scholar]
- 33.Altin C, Sade LE, Gezmis E, et al. Assessment of subclinical atherosclerosis by carotid intima-media thickness and epicardial adipose tissue thickness in prediabetes. Angiology. 2016;67:961–969. doi: 10.1177/0003319716643669. [DOI] [PubMed] [Google Scholar]
- 34.Nelson MR, Mookadam F, Thota V, et al. Epicardial fat: an additional measurement for subclinical atherosclerosis and cardiovascular risk stratifcation? J Am Soc Echocardiogr. 2011;24:339–345. doi: 10.1016/j.echo.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Aydin H, Toprak A, Deyneli O, et al. Epicardial fat tissue thickness correlates with endothelial dysfunction and other cardiovascular risk factors in patients with metabolic syndrome. Metab Syndr Relat Disord. 2010;8:229–234. doi: 10.1089/met.2009.0080. [DOI] [PubMed] [Google Scholar]
- 36.Benedetto FA, Mallamaci F, Tripepi G, et al. Prognostic value of ultrasonographic measurement of carotid intima media thickness in dialysis patients. J Am Soc Nephrol. 2001;12:2458–2464. doi: 10.1681/ASN.V12112458. [DOI] [PubMed] [Google Scholar]
- 37.Kawagishi T, Nishizawa Y, Konishi T, et al. High-resolution B-mode ultrasonography in evaluation of atherosclerosis in uremia. Kidney Int. 1995;48:820–826. doi: 10.1038/ki.1995.356. [DOI] [PubMed] [Google Scholar]
- 38.Pascazio L, Bianco F, Giorgini A, et al. Echo color doppler imaging of carotid vessels in hemodialysis patients: evidence of high levels of atherosclerotic 14 lesions. Am J Kidney Dis. 1996;28:713–720. doi: 10.1016/s0272-6386(96)90253-x. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Guallar E, Qiao Y, Wasserman BA. Is carotid intima-media thickness as predictive as other noninvasive techniques for the detection of coronary artery disease? Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34:1341–1345. doi: 10.1161/ATVBAHA.113.302075. [DOI] [PubMed] [Google Scholar]
- 40.Saba L, Sanfilippo R, Montisci R, Mallarini G. Carotid artery wall thickness: comparison between sonography and multi-detector row CT angiography. Neuroradiology. 2010;52:75–82. doi: 10.1007/s00234-009-0589-5. [DOI] [PubMed] [Google Scholar]
- 41.Kiykim AA, Camsari A, Kahraman S, et al. Increased incidence of carotid artery wall changes and associated variables in hemodialysis patients without symptomatic cardiovascular disease. Yonsei Med J. 2004;45:247–254. doi: 10.3349/ymj.2004.45.2.247. [DOI] [PubMed] [Google Scholar]
- 42.Hakan M, Poyrazoğlu MH, Düşünsel R, et al. Carotid artery thickness in children and young adults with end stage renal disease. Pediatr Nephrol. 2007;22:109–116. doi: 10.1007/s00467-006-0268-2. [DOI] [PubMed] [Google Scholar]
- 43.Delucchi A, Dinamarca H, Gainza H, et al. Carotid intima-media thickness as a cardiovascular risk marker in pediatric end-stage renal disease patients on dialysis and in renal transplantation. Transplant Proc. 2008;40:3244–3246. doi: 10.1016/j.transproceed.2008.03.126. [DOI] [PubMed] [Google Scholar]