Abstract
For idiopathic pulmonary artery hypertension (PAH) patients with end-stage right heart failure who received maximal medical therapy, balloon atrial septostomy (BAS) is recommended by most guidelines as a palliative therapy or a bridging treatment before lung transplantation. In this report, we described a 32-year-old woman with idiopathic PAH, who received maximal PAH-specific medical treatment, including intravenous prostacyclin, but still suffered from refractory right heart failure. The markedly enlarged right atrium (RA), high mean RA pressure of 23 mmHg, low systemic arterial oxygen saturation of 86% and concomitant pancytopenia all increased the patient’s risk for BAS. We used intracardiac echocardiography (ICE) guidance to facilitate trans-septal puncture, and performed graded BAS four times within 7 months to stabilize the patient. Our case showed that with dedicated PAH treatment, an experienced structural heart interventionist and ICE guidance, BAS could be done safely even in a patient in unfavorable clinical and hemodynamic condition.
Keywords: Balloon atrial septostomy (BAS), Intracardiac echocardiography (ICE), Pulmonary artery hypertension (PAH)
INTRODUCTION
Treatment of idiopathic pulmonary arterial hypertension (PAH) is challenging. Recently, the medical options available to address this condition improved dramatically after development of several novel drugs approved for PAH. However, some patients with idiopathic PAH respond poorly even under optimal medical treatment. For these patients, balloon atrial septostomy (BAS) can create an iatrogenic right-to-left inter-atrial shunt, which decreases right heart filling pressure, increases left heart filling pressure, increases systemic cardiac output and oxygen delivery at the expense of systemic arterial desaturation.1 The procedure has been validated in several case series for improvement of clinical symptoms, functional class and cardiac index.1 In addition, BAS is recommended by most current guidelines as a palliative therapy or bridge method to lung transplantation.2-4 However, selecting appropriate PAH candidates for BAS and successfully performing the risky procedure remain a challenge to cardiologists.
Herein, we reported a 32-year-old woman with idiopathic PAH, who received maximal PAH-specific medical treatment including intravenous prostacyclin but still suffered from refractory right side heart failure. The markedly enlarged right atrium (RA), mean RA pressure of 23 mmHg, low systemic arterial oxygen saturation of 86% and concomitant pancytopenia all increased the patient’s risk for BAS. Under intracardiac echocardiography (ICE) guidance to facilitate trans-septal puncture, we performed graded BAS safely four times over a period of 7 months.
CASE REPORT
The 32-year-old woman had idiopathic PAH for 11 years. She took endothelin receptor antagonist, phosphodiesterase type-5 inhibitor and inhaled prostacyclin regularly. She had frequent admissions to the hospital because of decompensated right side heart failure, which included peripheral edema, increased abdominal girth and shortness of breath while at rest in the past one year. The symptoms progressed despite transitioning PAH medication from inhaled to subcutaneous, and then to intravenous prostacyclin. For intractable right heart failure, she received intravenous inotropics, intravenous diuretics, and abdominal paracentesis for ascites. In addition, she was put on the waiting list for lung transplantation after a series of pre-surgical surveys.
The laboratory tests showed pancytopenia (white blood cell count 3000/mm3, hemoglobin 8.7 g/dL, platelet count 34,000/mm3), jaundice (total bilirubin 3.7 mg/dL) and acute renal function deterioration (serum creatinine up to 3 mg/dL). The echocardiogram showed severely dilated right atrium and ventricle, and severe tricuspid valve regurgitation (Figure 1A). She received blood transfusion for correcting anemia and thrombocytopenia. Because the patient suffered from a functional class IV end-stage right heart failure without anticipated lung transplantation in the near future, BAS was the last available treatment for this case.
Figure 1.
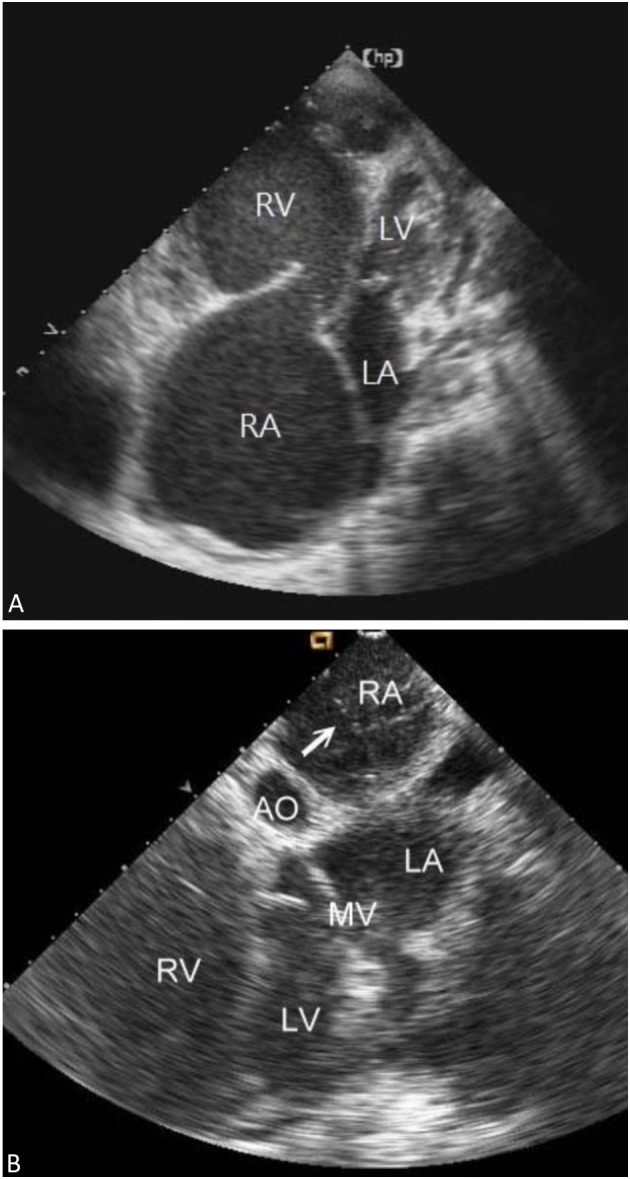
(A) The baseline transthoracic echocardiogram revealed markedly dilated right atrium and ventricle. (B) Under the real-time intracardiac echocardiography guidance, the borders of inter-atrial septum was delineated clearly. We used a Brockenbrough needle and a Mullins sheath to probe the inter-atrial septum. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle. Arrow: Brockenbrough needle and Mullins sheath.
The patient was on oxygen inhalation via mask and had intravenous inotropics and prostacyclin. We performed standard right and left heart catheterization via bilateral femoral vessels. An ICE catheter (AcuNac catheter, Siemens, Mountain View, CA, USA) was introduced via the left femoral vein into RA and the image was displayed on a Cypress Echocardiography System (Siemens, Mountain View, CA, USA). Using real-time ICE guidance, the inter-atrial septum and fossa ovalis could be delineated. We used a Brockenbrough needle and a Mullins sheath (Medtronic, Minneapolis, MN, USA) to probe the inter-atrial septum and entered the left atrial cavity (Figure 1B). Then, the atrial septum was dilated with a 14 F Inoue dilator (Toray Medical, Tokyo, Japan) (Figure 2A). The pre-procedure hemodynamic parameters showed a mean RA pressure of 23 mmHg, left ventricle end-diastolic pressure (LVEDP) of 12 mmHg, mean pulmonary artery pressure of 55 mmHg and pulmonary vascular resistance of 15 Wood unit. After the procedure, systemic arterial oxygen saturation decreased from 86% to 78% in room air. The saturation elevated to 88% by applying a 75% oxygen mask. LVEDP increased from 12 to 16 mmHg and cardiac output increased from 2.9 L/min to 3.3 L/min after BAS (Table 1). We completed the procedure without further balloon dilatation because the patient had poor systemic arterial oxygen saturation and a high RA pressure.
Figure 2.
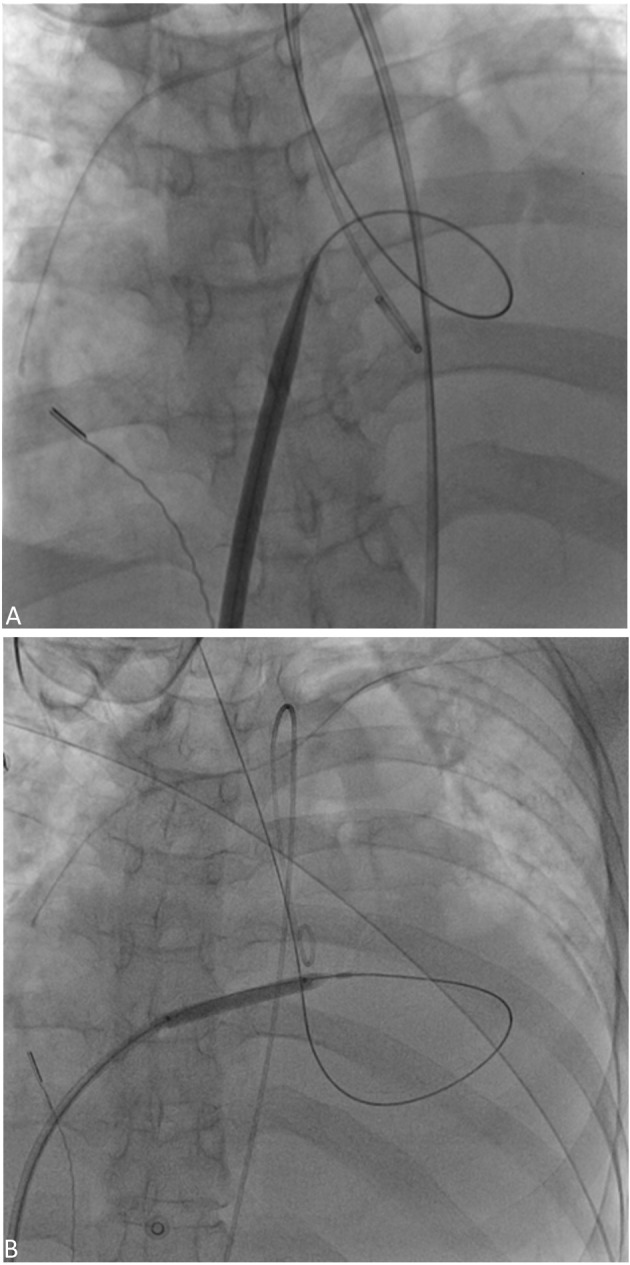
(A) The inter-atrial septum was dilated with a 14 F Inoue dilator. (B) The inter-atrial septum was dilated with a 5 mm × 4 cm balloon.
Table 1. Hemodynamic data before and after 1st balloon atrial septostomy.
| Baseline | Post-BAS | |
| Arterial O2 saturation (%) in room air | 86% | 78% |
| PA pressure (mean, mmHg) | 55 | 52 |
| PVR (Wood unit) | 15 | 13 |
| Systemic cardiac output (L/min) | 2.9 | 3.3 |
| Systemic cardiac index (L/min∙m2) | 2.0 | 2.2 |
| RA pressure (mean, mmHg) | 23 | 21 |
| LVEDP (mmHg) | 12 | 16 |
BAS, balloon atrial septostomy; LVEDP, left ventricle end-diastolic pressure; PA, pulmonary artery; PVR, pulmonary vascular resistance; RA, right atrium.
Initially, the patient responded well with a decrease in body weight and relief of dyspnea for the first three days after the procedure. Nevertheless, the patient was still dependent on intravenous inotropics and diuretics, which could not be titrated down. One week after the first ICE-guided BAS, we used the same protocol for the second BAS. This time, we further dilated the inter-atrial shunt with a 5 mm × 4 cm balloon (CONQUEST, Bard Peripheral Vascular, Inc. Tempe, AZ, USA) (Figure 2B). We transitioned the intravenous high dose diuretics to oral diuretics after the 2nd BAS. Because the patient had a high natriuretic peptide level (22600 pg/ml) and was still partially dependent on intravenous inotropics, we carried out the third BAS one month after the second BAS and dilated the shunt with 5 mm × 4 cm balloon, which was the same size as the one used in the second BAS. After 3 consecutive ICE-guided BAS procedures within 5 weeks in the same admission, the patient was gradually weaned from intravenous inotropics and diuretics. She was discharged uneventfully two weeks after the last BAS and remained in functional class III for 6 months. Unfortunately, her symptoms progressed to functional class IV right heart failure with intractable ascites again 7 months after the third BAS. Thereafter, transthoracic echocardiogram revealed closure of the BAS shunt. Mean RA pressure was 26 mmHg, LVEDP 16 mmHg, and platelet count was approximately 35,000/ mm3. We did the 4th BAS with 5 mm balloon dilatation again, and the patient’s symptoms improved to functional class III following the procedure. On the 7th day post 4th BAS, she was informed that a donor lung was available, and underwent double lung transplantation. But unfortunately, she passed away on the 2nd day post lung transplantation due to bi-ventricular failure with low cardiac output and massive intraoperative bleeding, despite extra-corporeal membrane oxygenation support.
DISCUSSION
Rich and Lam5 first introduced BAS for idiopathic PAH patient with refractory right side heart failure in 1983. BAS creates iatrogenic right-to-left shunting, which decompresses the right heart, increases the left heart preload, increases systemic cardiac output and oxygen delivery but decreases systemic arterial oxygen saturation.1 The use of BAS has been limited by high procedure-related mortality, which was 16% and 23% in two respective case series.1,6
Concerning the timing and the indication for BAS, the latest guidelines all have recommended BAS in cases of idiopathic PAH patients with functional class III or IV symptoms, who have received maximal medical therapy, including combination therapy and intravenous prostacyclin.3,4 As in our case, the patient had received intravenous prostacyclin, full combination PAH target medication, as well as intravenous inotropics and diuretics. BAS is the last resort and bridging treatment before lung transplantation.
In our case, the patient had a markedly enlarged RA and a small compressed left atrium (Figure 1A), which increased the risk and difficulty of performing the trans-septal procedure. Besides, the patient had pancytopenia, which can cause a catastrophic outcome if there are repeated false punctures. ICE has been used for facilitating trans-septal puncture in some cardiac catheterization procedures, such as ablation for atrial fibrillation and percutaneous transvenous mitral commissurotomy. Moscucci et al.7 first reported ICE guidance for BAS in 2001. In our case, with ICE guidance, we could visualize the atrial septum clearly and were able to perform BAS safely. The advantage of ICE in guiding BAS in our patient was corroborated in the recent report by Kuhn et al., who used ICE guidance as part of the protocol for 23 BAS procedures in 16 patients.8
Patients with high mean RA pressure over 20 mmHg have had high BAS procedure-related mortality, and in most guidelines BAS is contraindicated for such patients.1,3,6 Law et al.1 reported that mean RA pressure was the best predictor of procedural mortality; a mean RA pressure of 18 mm Hg yielded optimal sensitivity and specificity for predicting early mortality. Moreover, different guidelines and studies set different recommended thresholds for systemic arterial oxygen saturation before BAS, ranging from 80-90% in room air use.1,3,6 Our case had a baseline mean RA pressure of 23 mmHg, systemic arterial oxygen saturation of 86%, indicating she was at high risk for the BAS procedure. However, since the patient suffered from a functional class IV end-stage right heart failure without anticipated lung transplantation in the near future, BAS was her last resort treatment. Many studies and guidelines suggest that BAS should be carried out by an experienced structural heart interventionist in PAH referral center to reduce procedure-related risks.3,8 In our case, the BAS was performed by an interventional cardiologist experienced in trans-septal puncture and ICE manipulation. In addition, the patient was under adequate medical treatment, including intravenous prostanoids, inotropics and diuretics, and she received skilled care at our intensive care unit. In our case, a dedicated PAH medical care team, an experienced structural heart interventionist, and ICE guidance increased safety and improved outcome for BAS.
Stepwise or graded BAS is the current procedure of choice.3 Nevertheless, There is no consensus about the optimal size of iatrogenic right-to-left shunt.8,9 The maximal diameter of shunts in several case series ranged from 5 mm to 18 mm.1,8-10 In our patient, we performed BAS four times but only dilated the shunt with 5 mm balloon, which was relatively small compared with the ones used in previous studies. Overall, the patient’s high mean RA pressure, low systemic arterial blood pressure and oxygen saturation accounted for the conservative strategy we chose.
In conclusion, our case showed that with dedicated PAH treatment, an experienced structural heart interventionist and ICE guidance, BAS could be performed safely even in a patient in an unfavorable clinical and hemodynamic condition.
CONFLICT OF INTEREST
None.
REFERENCES
- 1.Law MA, Grifka RG, Mullins CE, et al. Atrial septostomy improves survival in select patients with pulmonary hypertension. Am Heart J. 2007;153:779–784. doi: 10.1016/j.ahj.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society,Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Galie N, Corris PA, Frost A, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D60–D72. doi: 10.1016/j.jacc.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 4.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 5.Rich S, Lam W. Atrial septostomy as palliative therapy for refractory primary pulmonary hypertension. Am J Cardiol. 1983;51:1560–1561. doi: 10.1016/0002-9149(83)90678-1. [DOI] [PubMed] [Google Scholar]
- 6.Sandoval J, Rothman A, Pulido T. Atrial septostomy for pulmonary hypertension. Clin Chest Med. 2001;22:547–560. doi: 10.1016/s0272-5231(05)70291-4. [DOI] [PubMed] [Google Scholar]
- 7.Moscucci M, Dairywala IT, Chetcuti S, et al. Balloon atrial septostomy in end-stage pulmonary hypertension guided by a novel intracardiac echocardiographic transducer. Catheter Cardiovasc Interv. 2001;52:530–534. doi: 10.1002/ccd.1116. [DOI] [PubMed] [Google Scholar]
- 8.Kuhn BT, Javed U, Armstrong EJ, et al. Balloon dilation atrial septostomy for advanced pulmonary hypertension in patients on prostanoid therapy. Catheter Cardiovasc Interv. 2014 doi: 10.1002/ccd.25751. [DOI] [PubMed] [Google Scholar]
- 9.Kurzyna M, Dabrowski M, Bielecki D, et al. Atrial septostomy in treatment of end-stage right heart failure in patients with pulmonary hypertension. Chest. 2007;131:977–983. doi: 10.1378/chest.06-1227. [DOI] [PubMed] [Google Scholar]
- 10.Sandoval J, Gaspar J, Pena H, et al. Effect of atrial septostomy on the survival of patients with severe pulmonary arterial hypertension. Eur Respir J. 2011;38:1343–1348. doi: 10.1183/09031936.00072210. [DOI] [PubMed] [Google Scholar]


