Abstract
Background
Relevance of diminished mental capacity in anorexia nervosa (AN) to course of disorder is unknown.
Aims
To examine prognostic relevance of diminished mental capacity in AN.
Method
A longitudinal study was conducted in 70 adult female patients with severe AN. At baseline, mental capacity was assessed by psychiatrists, and clinical and neuropsychological data (decision-making) were collected. After 1 and 2 years, clinical and neuropsychological assessments were repeated, and remission and admission rates were calculated.
Results
People with AN with diminished mental capacity had a less favourable outcome with regard to remission and were admitted more frequently. Their appreciation of illness remained hampered. Decision-making did not improve, in contrast to people with full mental capacity.
Conclusions
People with AN with diminished mental capacity seem to do less well in treatment and display decision-making deficiencies that do not ameliorate with weight improvement.
Declaration of interest
None.
Copyright and usage
© The Royal College of Psychiatrists 2017. This is an open access article distributed under the terms of the Creative Commons Non-Commercial, No Derivatives (CC BY-NC-ND) license.
Anorexia nervosa (AN) is a severe psychiatric illness usually beginning in adolescence with a lifetime prevalence among 1 to 4% of females in Europe.1,2 Intense fear of weight gain, body image disturbance and disturbed cognitive and emotional functioning are central features of the disorder. These features lead to weight loss behaviours such as dietary restriction, purging or excessive physical activity.3 Acute and chronic effects of starvation complicate treatment of people with AN, and the standardised mortality ratio is around 5.5.4,5 In older adolescents and adults, AN commonly has a relapsing or protracted course,6 and only 50% of the adults recover completely.7 Psychiatric comorbidity is the rule with mood disorders (nearly 75% of people with AN) and a range of anxiety disorders (between 25 and 75%) being most common.8,9 Mean duration of illness is nearly 7 years,6,10 and as this disorder usually starts in adolescence, transition to adult life is fraught with difficulties finishing education, starting a job or engaging in relationships.
Patients are typically reluctant to engage in treatment, especially when it has a clear focus on weight gain, and this tendency to avoid treatment is reflected in the finding that only a minority is treated within the mental healthcare system.11,12 Where onset of the disorder is triggered by genetic, psychosocial and interpersonal factors, changes in neural networks sustain the illness. Altered reward processing to modulate emotional distress,13,14 increased compulsivity because of being severely underweight15 and neuropsychological difficulties16–21 might lead to the typical persistence of people with AN in their self-destructive behaviour. Final factors of importance are the altered values towards life and death in people who are currently ill with AN as opposed to recovered people with AN and the sense that the AN is part of the personal identity instead of an acquired illness.22,23
These findings together with the obvious severity of the disorder lead to doubts regarding decision-making abilities or, in other words, mental capacity to consent to treatment. These are relevant doubts, as only a person with full mental capacity with regard to the medical issue at hand can refuse or consent to necessary treatment according to most international health legislations. Assessment of mental capacity generally considers four abilities:24 the ability to understand information provided regarding illness and treatment; the ability to appreciate this information as relevant to one’s own situation; the ability to reason with this information, weighing the consequence of decisions; and the ability to express a choice about treatment. A semi-structured interview has been developed to aid clinicians in the assessment of mental capacity to consent to treatment judging these four abilities (MacCAT-T25). Although in general psychiatry a number of studies have been done (for a review see Okai et al26), in AN studies have been scarce. Two small studies have been done in adolescents, with inconsistent results.27,28 In the study by Tan et al, mental capacity of people with AN was excellent, whereas in the study by Turrell et al impairments in reasoning were shown.
It is tempting to assume that the presence of diminished mental capacity to consent to treatment is relevant to treatment outcome, but this has not yet been studied. Therefore, a longitudinal study was conducted in our treatment centre. Baseline results from this sample of 70 adult people who are severely ill with AN were reported on previously.29 One-third of participants had diminished mental capacity at baseline (as assessed by psychiatrists). Diminished mental capacity was associated with a lower body mass index (BMI), more previous treatment for AN and a lower appreciation of disorder and treatment; duration of illness did not differ between the groups.
Although BMI was significantly different between the two mental capacity groups, still 43% of participants with a BMI below 15 kg/m2 was judged to have full mental capacity, highlighting the importance of looking further than BMI alone in the assessment of mental capacity to consent to treatment in AN. The picture that emerged from these baseline data was that a significant group of participants had diminished mental capacity and that not only BMI was of importance (as yet not clear in what way) but also the ability to use one’s emotions to come to adaptive decision-making. What also became clear was that almost all participants (92%) did follow the treatment advice (whether this was in-patient or out-patient treatment), suggesting that agreement with the treatment advice does not necessarily mean possessing full mental capacity.
More insight in possible differences between the two mental capacity groups in the course of the disorder is relevant to clinical practice as treatment may need to be tailored more to specific needs of the group with diminished mental capacity. The initial group of 70 participants was divided into two groups on the basis of clinical judgment, one with diminished mental capacity and one with full mental capacity to consent to treatment. In this longitudinal part of the study, we aim to answer the following questions:
(1) Is the course of disorder different between the two mental capacity groups on clinical variables (BMI, percentage of in-patient treatment, duration of in-patient stay, remission rate and eating disorder pathology)?
(2) Is the course of disorder different on psychological variables (symptoms of depression, anxiety and alexithymia)?
(3) Is the course of disorder different on decision-making (appreciation of illness and treatment and more general assessment of decision-making ability)?
Method
The study design has been described in detail elsewhere.29 A longitudinal cohort study was conducted in our national specialist centre for the treatment of eating disorders and at baseline 70 consecutively referred female adults with AN were included. Males (5% of referrals) were excluded to increase homogeneity of the sample. Treatment offered in our centre follows guidelines for eating disorders and entails a range of individual and group therapies, psychomotor therapy and attention to rehabilitation on an out-patient, day patient or in-patient basis. For a description of baseline characteristics, see Table 1. The study was conducted in accordance with the ethical standards described by the Medical Research Involving Human Subjects Act (WMO) and was approved by the institutional review board. After complete description of the study to the participants, written informed consent was obtained. One and 2 years after inclusion, participants were contacted by phone and asked for their cooperation with the follow-up measurements. Some of the required data were collected from patient files (e.g. current BMI, medication, days of in-patient treatment), other were collected during assessments by psychiatrists and psychologists (e.g. interviews and the decision-making task). When patients were no longer in treatment at our centre, data on current treatment, BMI and medication were asked from them.
Table 1. Baseline characteristics of participants.
| Total group (n=70) | Full MC (n=46) | Diminished MC (n=24) | P | |
|---|---|---|---|---|
| Age, years: mean (s.d.) | 27.3 (9.7) | 26.2 (9.3) | 29.4 (10.3) | 0.19 |
| Age at illness onset (years): mean (s.d.) | 17.8 (4.9) | 17.7 (4.6) | 18.1 (5.7) | 0.82 |
| Duration of illness (years): mean (s.d.) | 8.6 (8.1) | 8.5 (8.2) | 8.9 (7.9) | 0.84 |
| BMI: mean (s.d.) | 15.5 (1.9) | 16.1 (1.6) | 14.2 (1.9) | <0.001 |
| EDE: mean (s.d.) | 3.6 (1.3) | 3.7 (1.4) | 3.5 (1.2) | 0.59 |
| ANR/ANP, % | 49/51 | 47/53 | 55/45 | 0.55 |
| Previous ED treatment, % | 74 | 65 | 91 | 0.02 |
| Previous hospitalisation, % | 46 | 36 | 65 | 0.02 |
| Medication, % | 58 | 52 | 68 | 0.22 |
| Relationship, % | 37 | 43 | 24 | 0.13 |
| Welfare, % | 25 | 11 | 53 | 0.001 |
| BDI: mean (s.d.) | 29.9 (13.8) | 28.8 (13.2) | 32.1 (14.9) | 0.35 |
| STAI trait: mean (s.d.) | 59 (10.7) | 59.3 (10.7) | 58.4 (10.9) | 0.73 |
| STAI state: mean (s.d.) | 56.7 (12.8) | 56.6 (12.7) | 56.9 (13.3) | 0.94 |
| TAS: mean (s.d.) | 61.3 (9.2) | 61.4 (9.2) | 61.2 (9.4) | 0.94 |
| MacCAT-T appreciation: mean (s.d.) | 3.71 (0.62) | 3.82 (0.44) | 3.48 (0.85) | 0.03 |
| Depressive disorder, % | 48.4 | 45.6 | 55.6 | 0.47 |
| PTSD, % | 23.3 | 23.8 | 22.2 | 0.89 |
MC, mental capacity; BMI, body mass index; EDE, Eating Disorder Examination; ANR, anorexia nervosa restrictive type; ANP, anorexia nervosa purging type; ED, eating disorder; BDI, Beck Depression Inventory; STAI, Spielberger Trait State Anxiety Inventory; TAS, Toronto Alexithymia Scale; MacCAT-T, MacArthur Competence Assessment Tool-Treatment; PTSD, post-traumatic stress disorder.
Clinical measures
Severity of eating disorder symptoms was rated with the Eating Disorder Examination Questionnaire (EDEQ30,31) and the BMI (in kg/m2) as measured by the Digital Tanita scale (Tanita Cooperation of America, Inc., Arlington Heights, IL). Eating disorder treatments and admissions were collected from the participants and from their files (when available), social functioning from the social history. Remission rates were assessed. Full remission was defined as having a weight in the normal range (BMI 18.5–25 kg/m2), having resumed menses (or likely to when contraception was used and weight was in the normal range) and having no more disabling anorectic cognitions (assessed in an interview). Partial remission was defined as having two out of three of these criteria. The presence of legal measures regarding their AN was asked for. Depression and anxiety levels were measured with the Beck Depression Inventory (BDI-II32) and Spielberger Stait Trait Anxiety Inventory (STAI33), respectively. Furthermore, levels of alexithymia were assessed with the Toronto Alexithymia Scale (TAS34,35).
Mental capacity
At baseline, mental capacity to consent to treatment was assessed by psychiatrists experienced in the treatment of severe eating disorders. Based on this assessment, two groups were distinguished: a group with full mental capacity to consent to treatment and a group with diminished mental capacity to do so. At baseline, the MacArthur Competence Assessment Tool-Treatment (MacCAT-T25) was used. The MacCAT-T is a semi-structured interview designed to aid clinicians in determining the level of mental capacity to consent to treatment. It has good interrater reliability and construct validity and has been used in mental capacity studies in several psychiatric populations.26 As previously reported,29 appreciation of disorder and treatment was significantly lower in the group with diminished mental capacity. Therefore, we repeated this MacCAT-T measurement at follow-up. This was done in a face-to-face interview, when this was not feasible it was done by phone.
Decision-making
The Iowa Gambling Task (IGT36) was used to assess decision-making ability. The task requires participants to choose a card from four different decks and with each choice they win and sometimes also lose money. Two decks are more advantageous, whereas the other two decks are disadvantageous in the long run and participants have to find this out by relying on their ‘gut feeling’. Decision-making is determined by calculating the net score for all 100 trials as the difference in number of choices between the advantageous and disadvantageous decks.
Statistical analysis
A fully direct Bayes approach (FDB) was used to optimally account for the sample size and missing data attributable to attrition.37 Each outcome of interest was fitted as a separate univariate model. There was no a priori hypothesis for the growth trajectory over time, so latent basis models were fitted, allowing the data to determine linear or non-linear growth. BMI is included as a control variable in all models to satisfy the assumption that missing values are missing at random. Prior distributions were formed using the method of McNeish38, and models were fit in Mplus 7.1 with a Bayesian MCMC (Markow chain Monte Carlo) algorithm with a Gibbs Sampler and two chains with 50 000 MCMC iterations per chain. Bayesian estimation was utilised, so frequentist P-values are unavailable. To keep reporting of results succinct and familiar to readers unfamiliar with Bayesian statistics, we report Bayesian P-values (PB). These P-values are based on the quantiles of the posterior distribution rather than area beyond a test statistic under a null distribution (as with frequentist P-values). Thus, ‘significance’ in our results refers to a value of 0 being highly unlikely in the posterior distribution rather than rejecting the null hypothesis in a frequentist setting, which are conceptually related but not identical.39
Results
Sample characteristics
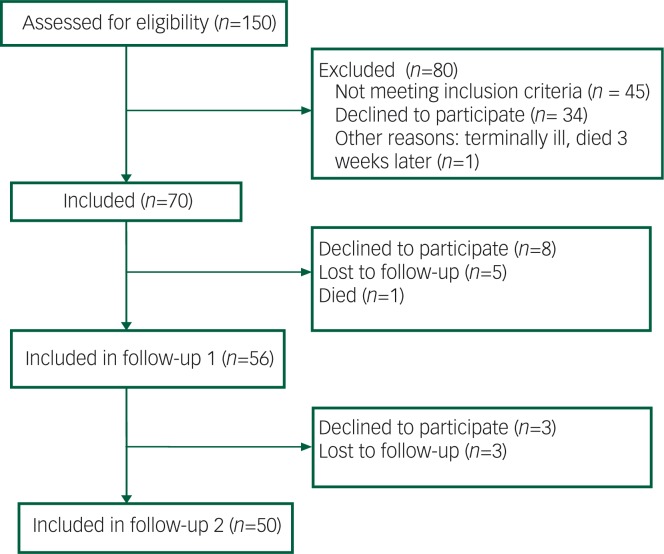
After 1 year, 56 people (80%) agreed to cooperate again and after 2 years 50 people (71%) of the original 70 participants (see Fig. 1). After 1 year, 57 of the people (82%) were still in care for their eating disorder and after 2 years 48 (69%); this was not significantly different between the two groups (P=0.39 and P=0.91, respectively). Only one participant in our study was held under the Mental Health Act (MHA) at baseline, not because of his or her eating disorder, but because of comorbid alcohol dependence. No one was held under a section of the MHA during follow-up. In the first year of follow-up, one patient died of AN-related complications. We know that after the end of the study (after the second follow-up), another patient died. Both were judged to have diminished mental capacity to consent to treatment at baseline and had a BMI below 15 kg/m2.
Fig. 1. Flow diagram of inclusion and follow-up process.
Is the course of disorder different with respect to clinical variables?
Body mass index
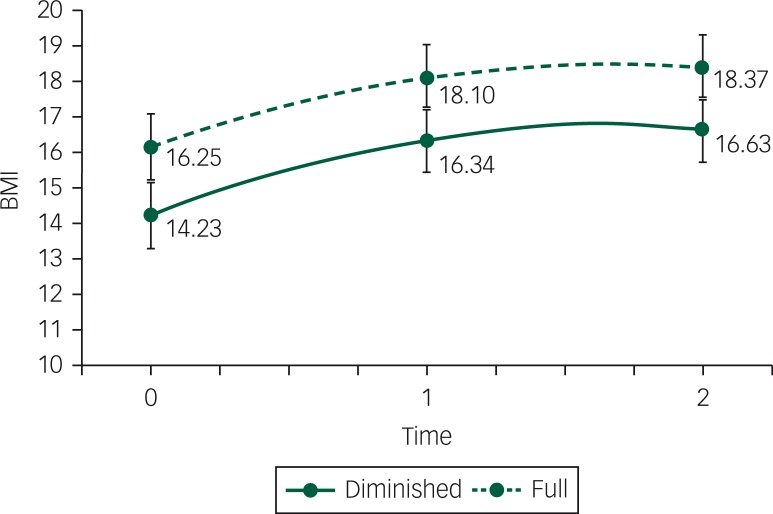
Figure 2 shows results from an unconditional growth model for BMI. Both groups show a significant increase in BMI from baseline to the Year 1 (PB<0.001) but a non-significant change from Year 1 to Year 2 (PB=0.38). The growth over time was not significantly different between the groups (PB=0.70). The diminished mental capacity group had a significantly lower BMI at baseline than the full mental capacity group (14.2 kg/m2, PB<0.001), and this significant difference persisted over time. In DSM-5 terminology, this represents on average a change in category from moderate to mild for the group with full mental capacity and from extreme to moderate for the group with diminished mental capacity. The fit of the model was quite good with a posterior predicted P (PPP) value of 0.542 (PPP ranges from 0 to 1 and 0.50 is ideal).
Fig. 2. BMI change (in kg/m2) between baseline, first follow-up (after 1 year) and second follow-up (after 2 years) between the group with full mental capacity (full) and the group with diminished mental capacity (diminished).
In-patient treatment
Difficulties with sample size and missing data were too great to fit a growth model with this outcome, so we fit models separately at each time point. At both follow-up time points, the group with diminished mental capacity had a borderline significant increase in the probability of having received in-patient treatment after controlling for BMI at baseline (after 1 year 63% v. 47%, PB=0.053; after 2 years, 37% v. 23% PB=0.057). However, after controlling for previous admissions, this difference was no longer significant at 1 year follow-up (PB=0.096), but the difference became more significant after 2 years follow-up (PB=0.034). Patients with previous admissions were more likely to have been admitted in the course of 1 year (PB=0.029) and between the first and second year of follow-up (PB=0.022), regardless of group. After 1 year follow-up, previous admission is largely responsible for the differences between the groups. Between the first and second year follow-up, the difference between the groups was not fully explained by BMI and previous admissions.
Remission rate
Unlike all other models, the remission outcome includes BMI as part of the criteria. We considered the conceptual appropriateness of including BMI as a covariate and whether the partial redundancy would hinder interpretation or estimation of the model. We ran the model two different ways, one with BMI as a covariate and one without. The results were quite similar, and inferential conclusion did not differ between the models. We therefore report only the model with BMI as a covariate in Table 2. Similar to the model for in-patient treatment, estimation difficulties required that we fit a separate model to each time point rather than a single longitudinal model. Remission rate is also a binary variable, so we used logistic models to determine predicted probabilities of remission. As shown in Table 2, after 2 years the group with diminished mental capacity was more likely to show no remission (53.9% compared with 26.6% in the full mental capacity group, PB=0.026). Full remission rates were still low (35.8 and 38.9%, respectively) in both groups at 2 year follow-up.
Table 2. Percentage of each mental capacity group in each remission category.
| No remission | Partial remission | Full remission | ||
|---|---|---|---|---|
| After 1 year | Diminished MC | 69.8 | 16.3 | 14.0 |
| Full MC | 44.8 | 40.7 | 13.7 | |
| PB | 0.03 | 0.05 | 0.49 | |
| BMI PB | 0.42 | 0.16 | 0.16 | |
| After 2 years | Diminished MC | 53.9 | 10.8 | 35.8 |
| Full MC | 26.6 | 33.7 | 38.9 | |
| PB | 0.03 | 0.05 | 0.48 | |
| BMI PB | 0.45 | 0.32 | 0.27 |
MC, mental capacity; BMI, body mass index.
The P-value is the comparison between the diminished and full groups in each column. For example, the first PB-value of 0.03 shows that the difference between 69.6 and 45.0% is significant.
Eating disorder pathology
At the two follow-up measurements, the EDE-Q was measured. In the analysis BMI was controlled for. The group with full mental capacity went from an EDEQ global score of 3.08 at follow-up 1 to 2.76 at follow-up 2, which is a non-significant change (PB=0.15). The group with diminished mental capacity went from 2.59 to 2.27, which is also non-significant (PB=0.27). Eating disorder pathology as measured by the EDE-Q did not differ between the groups after 1 year or after 2 years (PB=0.21).
Is the course of disorder different with respect to psychological variables?
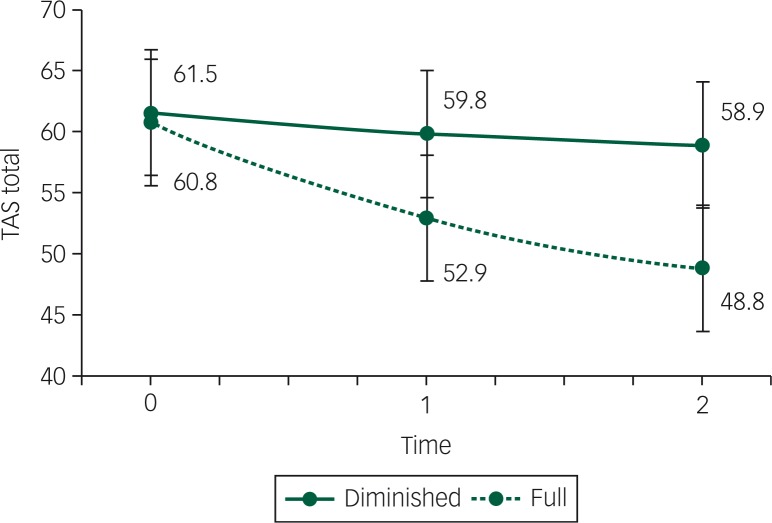
Separate latent growth models were fit to each outcome all controlling for BMI. Both the full and the diminished mental capacity group improved significantly after 2 years in depression score (BDI improved from both 30 to both 20, PB<0.001) and anxiety score (STAI state improved from 57–54 to 44–42, respectively, PB<0.001), there are no significant differences between groups at any time-point (BDI: PB=0.44, STAI: PB=0.42). As shown in Fig. 3, the full mental capacity groups showed a significant improvement in alexithymia score (60.8– 48.8, PB<0.001), which was below the clinical cut-off of 52 for possible alexithymia.34,35 The diminished mental capacity group did not improve (PB=0.10) and stayed around the clinical cut-off of 61 for alexithymia. The difference over time between groups was significant (PB=0.006). The PPP values of the alexithymia, depression and anxiety score models were 0.41, 0.48 and 0.59, respectively, all indicating acceptable fit.
Fig. 3. Alexithymia scores between baseline, first follow-up (after 1 year) and second follow-up (after 2 years) between the group with full mental capacity (full) and the group with diminished mental capacity (diminished), controlled for BMI (in kg/m2).
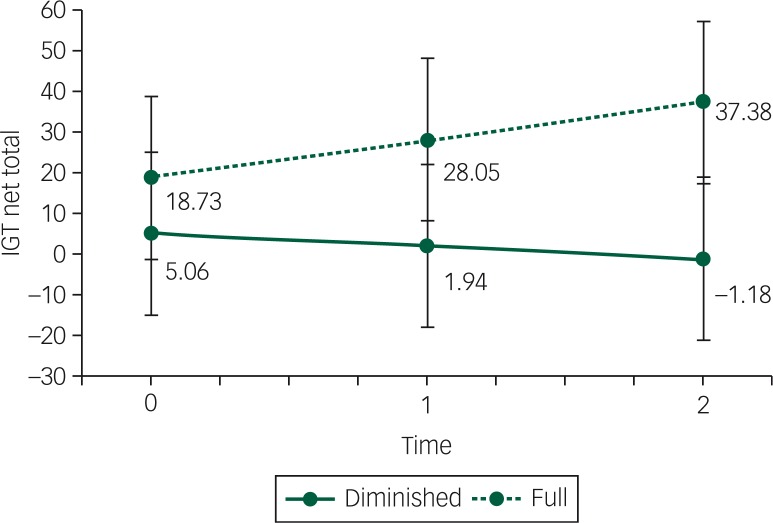
Fig. 4. Iowa Gambling Task (IGT) scores between baseline, first follow-up (after 1 year) and second follow-up (after 2 years) between the group with full mental capacity (full) and the group with diminished mental capacity (diminished), controlled for BMI (in kg/m2), depression and alexithymia scores.
Is the course of disorder different with respect to decision-making?
Decision-making task
Changes in decision-making were modelled with a latent growth model. Because symptoms of depression and alexithymia may theoretically have an influence on decision-making ability,40,41 a model was tested including BDI and TAS next to BMI as control variables. The group difference at baseline was not significant in this model (19.0 v. 5.9; PB=0.11). BMI (PB=0.43), TAS (PB=0.32) and BDI (PB=0.30) were not significant predictors of the IGT score at baseline nor were they significant predictors of the change over time (BMI PB=0.16, TAS PB=0.41 and BDI PB=0.38). The two groups differed in their change over time, with the full mental capacity group doing better than the diminished mental capacity group (PB=0.040; see Fig. 4). The PPP value for this model was 0.514.
Appreciation
The appreciation scores of the MacCAT-T were difficult to analyse over time as the distribution was highly negatively skewed (most participants scored 3 or higher). Therefore, we conducted a mean comparison test in the Bayesian framework which allows the normality assumption to be relaxed. The descriptive mean scores, Bayesian P-values and standardised effect differences are reported for each time point in Table 3. The group with full mental capacity did well at baseline and kept appreciating well. The group with diminished mental capacity differed noticeably at baseline and after 2 years.
Table 3. Appreciation scores over time between groups.
| Full mental capacity mean (s.d.) | Diminished mental capacity mean (s.d.) | Group difference PB | Standardised effect | |
|---|---|---|---|---|
| Baseline | 3.8 (0.4) | 3.5 (0.8) | 0.02 | 0.25 |
| One year follow-up | 3.8 (0.5) | 3.6 (0.5) | 0.12 | 0.16 |
| Two year follow-up | 3.8 (0.5) | 2.9 (1.4) | <0.01 | 0.40 |
Discussion
This is the first large study into mental capacity to consent to treatment in people who are severely ill with AN using a longitudinal design. The results of this follow-up study have two important implications. First, prognosis seems more unfavourable for the group with diminished mental capacity to consent to treatment. Participants with full mental capacity had a mild AN at follow-up, whereas those with diminished mental capacity still fell within the moderately ill category (DSM-541). And as the rise in BMI mainly occurred in the first year after starting treatment and the percentage of no and partial remission is significantly higher in the group with diminished mental capacity, one would not expect the diminished mental capacity group to reach full remission in the near future. In the long run, diminished mental capacity to consent to treatment means a higher likelihood of in-patient treatment. These findings suggest a longer duration of care for this particular group. Although the diminished mental capacity group had a lower BMI at baseline, other parameters of prognostic relevance such as duration of illness, percentage of participants with the purging subtype of AN or comorbidity were equal between the groups. Diminished mental capacity therefore seems a factor of relevance to prognosis, next to the more obvious factor of BMI. Both groups improved on BMI at 1 year follow-up and maintained this improvement 1 year later at 2 year follow-up, which is reassuring. Also, anxiety and depression levels lower equally in the course of treatment, and remission rates after 2 years were similar.
A second important finding in this study supports the decision-making difficulties clinicians so clearly observe in everyday practice on a more fundamental level. Using the IGT, it has become clear that decision-making is more maladaptive in people with diminished mental capacity, independent of BMI. The IGT is based upon the theoretical model by Damasio (somatic marker hypothesis42,43), which states that adaptive decision-making is not a merely rational process but relies heavily on emotional factors and ‘gut feeling’. Emotional dysregulation has been found to be an important maintaining factor in AN.23,41,44–46 In this study, participants also displayed high levels of emotional problems, such as severe depressive symptoms, a high level of anxiety and moderate to high levels of alexithymia. Although depression scores decreased significantly during treatment, levels indicative of moderate depression still existed after 2 years. In addition, in the diminished mental capacity group, alexithymia levels remained at a clinical level even after weight improvement. However, even after controlling for BMI, depression and alexithymia, the diminished mental capacity group still performed significantly worse on decision-making over time. This suggests that the difference between groups cannot be fully explained by the difference in emotional problems as measured in this study (BDI, TAS). Appreciation of the diminished mental capacity group over time remained less than that of the full mental capacity group, linking the concept of more fundamental decision-making (and ‘gut feeling’) to the concept of appreciation in AN. Future research should therefore be focused on the interplay between emotional dysregulation (as measured with more direct markers) so often found in AN and the diminished appreciation of illness and treatment.
The high level of emotional problems in our participants support the emphasis laid on emotion recognition and processing in recently developed cognitive–affective treatments for AN, such as the Maudsley Anorexia Nervosa Treatment for Adults (MANTRA47) and enhanced form of cognitive–behavioural therapy (CBT-E48). In recent years, several randomised clinical trials have been done that have shown that both MANTRA, CBT-E and Focal Psychodynamic Therapy (FPT) have a positive effect on outcome in AN.49–51 It is as yet not clear what works for whom. Perhaps, MANTRA is of more value to people with AN with a higher level of affective dysregulation, but this is speculative. Also motivational stage of change might be an important factor, although a recent study could only show a relation between the stage of contemplation and the strength of the therapeutic alliance, but not outcome.52,53
Strengths and limitations
Major strengths in this study are the longitudinal design, the high participation after 1 and 2 year follow-up (80 and 70%, respectively) and the generalisability of the results, as this was a naturalistic study. Also the assessment of decision-making on a clinical as well as on a more fundamental level provided the opportunity to bring together more basic science and bedside, a strategy very fruitful to move forward in the body of knowledge regarding an issue so complex as decision-making.
This study being a naturalistic study with hardly any exclusion criteria, there were missing data in the course of follow-up. For a clinical study with people with AN, who are usually highly avoidant of treatment,11,12 we consider the loss to follow-up of 20 and 29% after 1 and 2 years as acceptable. Also, in the statistical analyses missing data were accounted for. Nevertheless, outcome might have been influenced by these missing data.
Although clinicians doing the clinical judgment were masked to the outcome of the MacCAT-T, for obvious reasons, they could not be masked to their own assessment of mental capacity. We do not think this resulted in treatment bias on the part of the clinicians as this assessment is part of routine clinical practice, especially in complex cases and therefore has not had a major influence on treatment advised.
In our previously reported study,29 a substantial group was found to have diminished capacity to consent to treatment. Contrary to what might be expected, follow-up results show that this particular group improves on both weight, eating disorder and more general psychopathology, which is encouraging. On the down side, after 2 years BMI is still in the moderately severe range and longer duration of care is expected. Decision-making on a more fundamental level is maladaptive proposed to be caused by an interaction between emotional dysregulation and lack of appreciation of illness and treatment. Therefore, a key component in treatment should be to improve precisely this hampered emotional functioning, a feature central to more recently developed AN treatments like MANTRA and CBT-E. Whether or not the heightened focus on these emotional difficulties will improve adaptive decision-making is an issue that should be assessed in future research.
Funding
This research was supported by a grant from the Nuts Ohra Foundation.
References
- 1.Smink FRE, van Hoeken D, Hoek HW. Epidemiology, course, and outcome of eating disorders. Curr Opin Psychiatry 2013; 26: 543–8. [DOI] [PubMed] [Google Scholar]
- 2.Keski-Rahkonen A, Mustelin L. Epidemiology of eating disorders in Europe: Prevalence, incidence, comorbidity, course, consequences and risk factors. Curr Opin Psychiatry 2016; 29: 340–5. [DOI] [PubMed] [Google Scholar]
- 3.Zipfel S, Giel KE, Bulik C, Hay P, Schmidt U. Anorexia nervosa: aetiology, assessment and treatment. Lancet Psychiatry 2015; 2: 1099–111. [DOI] [PubMed] [Google Scholar]
- 4.Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 36 studies. Arch Gen Psychiatry 2011; 68: 724–31. [DOI] [PubMed] [Google Scholar]
- 5.Fichter MM, Quadflieg N. Mortality in eating disorders – results of a large prospective clinical longitudinal study. Int J Eat Disord 2016; 49: 391–401. [DOI] [PubMed] [Google Scholar]
- 6.Herzog W, Deter H, Fiehn W, Petzold E. Medical findings and predictors of long-term physical outcome in anorexia nervosa: a prospective, 12-year follow-up study. Psychol Med 1997; 27: 269–79. [DOI] [PubMed] [Google Scholar]
- 7.Keel PK, Brown TA. Update on course and outcome in eating disorders. Int J Eat Disord 2010; 43: 195–204. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Aranda F, Pinheiro AP, Tozzi F, La Via M, Thornton LM, Plotnicov KH, et al. Symptom profile of major depressive disorder in women with eating disorders. Aust N Z J Psychiatry 2007; 41: 24–31. [DOI] [PubMed] [Google Scholar]
- 9.Raney TJ, Thornton LM, Berrettini W, Brandt H, Crawford S, Fichter MM, et al. Influence of overanxious disorder of childhood on the expression of anorexia nervosa. Int J Eat Disord 2008; 41: 326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strober M, Freeman R, Morrell W. The long-term course of severe anorexia nervosa in adolescents: survival analysis of recovery, relapse, and outcome predictors over 10–15 years in a prospective study. Int J Eat Disord 1997; 22: 339–60. [DOI] [PubMed] [Google Scholar]
- 11.Hoek HW. Incidence, prevalence and mortality of anorexia nervosa and other eating disorders. Curr Opin Psychiatry 2006; 19: 389–94. [DOI] [PubMed] [Google Scholar]
- 12.Keski-Rahkonen A, Hoek HW, Susser ES, Linna MS, Sihvola E, Raevuori A, et al. Epidemiology and course of anorexia nervosa in the community. Am J Psychiatry 2007; 164: 1259–65. [DOI] [PubMed] [Google Scholar]
- 13.Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Bischoff-Grethe A. Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends Neurosci 2013; 36: 110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinglass JE, Walsh TB. Neurobiological model of the persistence of anorexia nervosa. J Eating Disord 2016; 4: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh BT. The enigmatic persistence of anorexia nervosa. Am J Psychiatry 2013; 170: 477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez C, Tchanturia K, Stahl D, Treasure J. Central coherence in eating disorders: a systematic review. Psychol Med 2008; 38: 1393–404. [DOI] [PubMed] [Google Scholar]
- 17.Tchanturia K, Davies H, Roberts M, Harrison A, Nakazato M, Schmidt U, et al. Poor cognitive flexibility in eating disorders: examining the evidence using the Wisconsin Card Sorting Task. PLoS One 2012; 7: e28331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tchanturia K, Liao PC, Uher R, Lawrence N, Treasure J, Campbell IC. An investigation of decision making in anorexia nervosa using the Iowa Gambling Task and skin conductance measurements. J Int Neuropsychol Soc 2007; 13: 635–41. [DOI] [PubMed] [Google Scholar]
- 19.Danner UN, Sanders N, Smeets PAM, van Meer F, Adan RAH, Hoek HW, et al. Neuropsychological weaknesses in anorexia nervosa: set-shifting, central coherence, and decision making in currently ill and recovered women. Int J Eat Disord 2012; 45: 685–94. [DOI] [PubMed] [Google Scholar]
- 20.Galimberti E, Fadda E, Cavallini MC, Martoni RM, Erzegovesi S, Bellodi L. Executive functioning in anorexia nervosa patients and their unaffected relatives. Psychiatry Res 2013; 208: 238–44. [DOI] [PubMed] [Google Scholar]
- 21.Chan TWS, Ahn WY, Bates JE, Busemeyer JR, Guillaume S, Redgrave GW, et al. Differential impairments underlying decision making in anorexia nervosa and bulimia nervosa: a cognitive modeling analysis. Int J Eat Disord 2014; 47: 157–67. [DOI] [PubMed] [Google Scholar]
- 22.Tan JOA, Hope T, Stewart A. Anorexia nervosa and personal identity: the accounts of patients and their parents. Int J Law Psychiatry 2003; 26: 533–48. [DOI] [PubMed] [Google Scholar]
- 23.Treasure J, Schmidt U. The cognitive-interpersonal maintenance model of anorexia nervosa revisited: a summary of the evidence for cognitive, socio-emotional and interpersonal predisposing and perpetuating factors. J Eat Disord 2013; 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grisso T, Appelbaum PS. Assessing Competence to Consent to Treatment: A Guide for Physicians and Other Health Professionals. Oxford University Press, 1998. [Google Scholar]
- 25.Grisso T, Appelbaum PS, Hill-Fotouhi C. The MacCAT-T: a clinical tool to assess patients’ capacities to make treatment decisions. Psychiatr Serv 1997; 48: 1415–9. [DOI] [PubMed] [Google Scholar]
- 26.Okai D, Owen G, McGuire H, Singh S, Churchill R, Hotopf M. Mental capacity in psychiatric patients: systematic review. Br J Psychiatry 2007; 191: 291–7. [DOI] [PubMed] [Google Scholar]
- 27.Tan J, Hope T, Stewart A. Competence to refuse treatment in anorexia nervosa. Int J Law Psychiatry 2003; 26: 697–707. [DOI] [PubMed] [Google Scholar]
- 28.Turrell SL, Peterson-Badali M, Katzman DK. Consent to treatment in adolescents with anorexia nervosa. Int J Eat Disord 2011; 44: 703–7. [DOI] [PubMed] [Google Scholar]
- 29.Elzakkers IFFM, Danner UN, Hoek HW, van Elburg AA. Mental capacity to consent to treatment in anorexia nervosa: explorative study. BJPsych Open 2016; 2: 147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire? Int J Eat Disord 1994; 16: 363–70. [PubMed] [Google Scholar]
- 31.Aardoom JJ, Dingemans AE, Slof Op’t Landt MC, Van Furth EF. Norms and discriminative validity of the Eating Disorder Examination Questionnaire (EDE-Q). Eat Behav 2012; 13: 305–9. [DOI] [PubMed] [Google Scholar]
- 32.Beck AT, Steer RA, Brouwn GK. Manual for the Beck Depression Inventory-II. Psychological Corporation, 1996. [Google Scholar]
- 33.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press, 1983. [Google Scholar]
- 34.Bagby RM, Parker JDA, Taylor GJ. The twenty-item Toronto Alexithymia scale – I. Item selection and cross-validation of the factor structure. J Psychosom Res 1994; 38: 23–32. [DOI] [PubMed] [Google Scholar]
- 35.Bagby RM, Taylor GJ, Parker JDA. The twenty-item Toronto Alexithymia scale – II. Convergent, discriminant, and concurrent validity. J Psychosom Res 1994; 38: 33–40. [DOI] [PubMed] [Google Scholar]
- 36.Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 1994; 50: 7–15. [DOI] [PubMed] [Google Scholar]
- 37.Barnes SA, Lindborg SR, Seaman JW. Multiple imputation techniques in small sample clinical trials. Stat Med 2006; 25: 233–45. [DOI] [PubMed] [Google Scholar]
- 38.McNeish DM. Using data-dependent priors to mitigate small sample bias in latent growth models: a discussion and illustration using Mplus. J Educ Behav Stat 2016; 41: 27–56. [Google Scholar]
- 39.Muthen B. Bayesian Analysis in Mplus: A Brief Introduction. 2010. (http://www.statmodel.com/download/IntroBayesVersion%203.pdf). [Google Scholar]
- 40.Danner UN, Sternheim L, Bijsterbosch JM, Dingemans AE, van Elburg AA. The influence of negative emotions on decision making in women with restrictive and binge-purge type anorexia nervosa. Psychiatry Res 2016; 239: 39–46. [DOI] [PubMed] [Google Scholar]
- 41.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). APA, 2013. [Google Scholar]
- 42.Damasio AR. Descartes’ error and the future of human life. Sci Am 1994; 271: 144. [DOI] [PubMed] [Google Scholar]
- 43.Bechara A, Damasio H, Tranel D, Damasio AR. The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends Cogn Sci 2005; 9: 159–62; discussion 162–4. [DOI] [PubMed] [Google Scholar]
- 44.Fairburn CG, Cooper Z, Doll HA, O’Connor ME, Bohn K, Hawker DM, et al. Transdiagnostic cognitive-behavioral therapy for patients with eating disorders: a two-site trial with 60-week follow-up. Am J Psychiatry 2009; 166: 311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrison A, Sullivan S, Tchanturia K, Treasure J. Emotional functioning in eating disorders: attentional bias, emotion recognition and emotion regulation. Psychol Med 2010; 40: 1887–97. [DOI] [PubMed] [Google Scholar]
- 46.Wildes JE, Marcus MD, Cheng Y, McCabe EB, Gaskill JA. Emotion acceptance behavior therapy for anorexia nervosa: a pilot study. Int J Eat Disord 2014; 47: 870–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt U, Wade TD, Treasure J. The Maudsley model of anorexia nervosa treatment for adults (MANTRA): development, key features, and preliminary evidence. J Cogn Psychother 2014; 28: 48–71. [DOI] [PubMed] [Google Scholar]
- 48.Fairburn CG. Cognitive Behavioral Therapy for Eating Disorders. Guilford Press, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt U, Oldershaw A, Jichi F, Sternheim L, Startup H, McIntosh V, et al. Out-patient psychological therapies for adults with anorexia nervosa: randomised controlled trial. Br J Psychiatry 2012; 201: 392–9. [DOI] [PubMed] [Google Scholar]
- 50.Zipfel S, Wild B, Groß G, Friederich H, Teufel M, Schellberg D, et al. Focal psychodynamic therapy, cognitive behaviour therapy, and optimised treatment as usual in outpatients with anorexia nervosa (ANTOP study): randomised controlled trial. Lancet 2014; 383: 127–37. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt U, Magill N, Renwick B, Keyes A, Kenyon M, Dejong H, et al. The Maudsley Outpatient Study of Treatments for Anorexia Nervosa and Related Conditions (MOSAIC): Comparison of the Maudsley Model of Anorexia Nervosa Treatment for Adults (MANTRA) with Specialist Supportive Clinical Management (SSCM) in outpatients with broadly defined anorexia nervosa: a randomized controlled trial. J Consult Clin Psychol 2015; 83: 796–807. [DOI] [PubMed] [Google Scholar]
- 52.Mander J, Keifenheim K, Zipfel S, Giel KE. Stages of change, treatment outcome and therapeutic alliance in adult inpatients with chronic anorexia nervosa. BMC Psychiatry 2013; 13: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mander JV, Jacob GA, Götz L, Sammet I, Zipfel S, Teufel M. Associations between Grawe’s general mechanisms of change and Young’s early maladaptive schemas in psychotherapy research: a comparative study of change processes. Psychother Res 2015; 25: 249–62. [DOI] [PubMed] [Google Scholar]