Abstract
Prevalence of dementia is expected to increase three- to four-fold in the next 50 years. In 1986, New York State established the Alzheimer's Disease and Other Dementias Registry, one of a few such registries in the United States. We identify surveillance challenges within the Registry.
Data quality — specifically, the attributes of completeness and accuracy — is the primary challenge to the New York State dementias registry. Completeness may be undermined when hospitals and nursing homes fail to report data, and hospital charts do not record dementia diagnoses. Failure to record diagnoses may occur because of diagnosis uncertainty, perceived stigma, clinical attention on the primary reason for hospitalization, and financial disincentives. Dementia is well recorded in nursing home data because care planning requires frequent resident evaluations. The accuracy of recording specific forms of dementia is limited because coding terminology has not kept pace with physicians' perspectives on dementias. Hospitals and nursing homes document dementia and comorbidities more effectively among frail individuals and those with advanced dementias than among individuals who appear to be relatively healthy.
One way to overcome challenges of data quality is to form partnerships with organizations that have expertise in managing medical records and coding dementias. As medical advances make early diagnoses more possible outside the hospital or nursing home setting, we will need to redesign the current surveillance system to capture this additional dementia data and ensure a representative system.
Introduction
The purpose of this article is to provide a simple description of the New York State Department of Health (NYSDOH) Alzheimer's Disease and Other Dementias Registry (the Registry) and outline several challenges. We do not intend to offer a formal evaluation of the Registry, although a thorough set of guidelines for conducting a formal evaluation is available, and we use these guidelines as framework for our article (1). A formal evaluation addresses many topics and concerns in depth, but we focus here primarily on only 2: describing the surveillance system itself as well as the health-related event under surveillance (dementia), and gathering evidence on the performance of the surveillance system.
Purpose and Operation of Surveillance System
The increasing prevalence of dementia among the aging population in New York State during the 1980s prompted concern about the ability to meet future service demands. With encouragement from the New York City Chapter of the Alzheimer's Association, advocates, concerned physicians, and other stakeholders, a bill establishing the Alzheimer's Disease and Other Dementias Registry became law in 1986. The state legislature introduced several bills between 1987 and 1992 to fund research on Alzheimer's disease, but these bills were never enacted. The NYSDOH funded the Registry, and the Registry began to collect reports in 1988.
Article 20 of the New York State Public Health Law states that the purpose of the Registry is to collect information necessary to "identify, locate and investigate the occurrence, frequency, incidence, cause, effect and prognosis of Alzheimer's disease" and maintain this information for research purposes (2). Reporting is mandated for physicians and facilities including "any governmental or private agency, department, institution, clinic, laboratory, hospital, nursing care facility, health maintenance organization, association of other similar entity that provides medical care" (2).
The Registry requires a confidential case report to the Department of Health each time a new case of Alzheimer's disease or other dementia is diagnosed or confirmed in New York State. In practice, the Registry receives dementia reports primarily from the 400 hospitals and 700 nursing homes throughout the state. The state public health law only includes Alzheimer's disease, but state regulations (also in 1987) broadened reporting requirements to include reports of other related dementias in addition to Alzheimer's disease. By 1997, the state reporting form included 47 codes for diseases and disorders related to dementia based on the International Classification of Diseases, 9th Revision, Clinical Modification coding system (ICD-9-CM). Dementia can result from Alzheimer's disease, stroke, Parkinson's disease, AIDS, and a number of other less common illnesses.
The Registry collects data on types of dementia diagnosed as well as socio-demographic data such as age, race, ethnicity, gender, and address. For identification purposes, the Registry uses an individual's actual name or a unique identifier formed from elements of the name and social security number. The Registry gathers information on the type of facility reporting data (hospital, nursing home, physician's office, specialized Alzheimer's disease program). The Registry also collects data on hospitalization (length of stay and patient's destination at discharge).
The Registry staff has ranged from 2 to 4 professional or clerical staff. Today, one full-time professional serves as director of the Registry. Additional NYSDOH staff are available as other duties permit. Limited staffing requires that the operation of the Registry take advantage of information collected by other existing data systems.
Public Health Importance of Alzheimer's Disease and Other Dementias
The Centers for Disease Control and Prevention lists 7 parameters for assessing the public health importance of a health-related event under surveillance. These include indices of frequency and severity of disease, disparities or inequities, costs, preventability, potential clinical course in absence of intervention, and public interest. Let us first examine dementia as a public health issue from the perspective of each of these parameters (with the exception of potential clinical course in absence of intervention).
Frequency
The national mortality rate for Alzheimer's disease has increased dramatically. The age-adjusted mortality rate in 1995 was 15 times the rate in 1979 (3). Mortality data alone do not completely reflect the number of individuals who have been affected by Alzheimer's disease. Nonetheless, Alzheimer's disease has been added to the List of 72 Selected Causes of Death, diseases selected by the National Center for Health Statistics for their public health importance (3). Although Alzheimer's disease is not usually viewed as contributing to premature mortality, individuals with Alzheimer's or other dementias are at increased risk of death compared to individuals without dementia who have other comparable health conditions (4).
From study to study, estimates of the prevalence of Alzheimer's disease vary by two- to ten-fold; when disease severity is specified, variation is reduced (5). Using data on white individuals pooled from 20 studies, Hy and Keller estimated that 1.7 million Americans were affected with mild to severe Alzheimer's disease in 1996 (5). Other researchers estimated the prevalence of Alzheimer's disease in the United States to be between 1.1 and 4.6 million in 1997 (6). Despite this range, public health practitioners agree that the number of individuals with Alzheimer's disease could triple or quadruple in the next 50 years as a result of the aging of the U.S. population (6, 7). If new treatments that would both delay onset and progression of disease are developed, the number of affected individuals would double by 2050 rather than quadruple (7). Dementia can also occur as a result of vascular disease, HIV infection, Parkinson's disease, and other diseases.
Individuals with dementia account for nearly 58,000 hospitalizations each year in New York State. In 43% of the hospitalizations among individuals with dementia, the individual is discharged or returned to a skilled nursing facility (P.P.L., unpublished data, 2003).
Severity
Dementia can result in a diminished quality of life not only for the affected individual but also for the family. Individuals with Alzheimer's disease experience problems with memory and may also have other cognitive impairments such as difficulties with language, inability to recognize and name objects or people, and loss of judgment and problem-solving skills (8). Behavioral symptoms such as agitation, wandering, and inappropriate behavior and psychological problems including delusions or hallucinations may also occur (9). Individuals with dementia are 5 times more likely to be admitted to a nursing home than others of the same age (4). One fourth of individuals with mild Alzheimer's disease progress to severe Alzheimer's within 5 years; another quarter die in this time period (10).
In a recent study of hospitalizations in New York State, the majority of hospitalizations for individuals with a diagnosis of dementia occurred on an emergency basis (86%). Many of the hospitalizations were due to serious illness such as pneumonia and pneumonitis (13%), septicemia (4%), and heart failure (4%) (P.P.L., unpublished data, 2003).
Disparities
In the United States African American and Caribbean Hispanic individuals have been found to be at twice the risk of Alzheimer's disease as white individuals (11). Data from Medicare claims supports high risk of other dementias in African American individuals as well (12).
In hospitalizations in New York State, the number of hospitalizations among women with Alzheimer's disease was twice the number among men. Nearly half of New York hospitalizations that included a diagnosis of dementia among women occurred for women over the age of 84; for men, a third of New York hospitalizations that included a diagnosis of dementia occurred among men over the age of 84. African American individuals accounted for 10% of all hospitalizations among New York State's hospitalized individuals over the age of 65, but were 14% of the New York hospitalizations that included a diagnosis of dementia (P.P.L., unpublished data, 2003).
Costs
Estimates of the cost of Alzheimer's disease vary widely — but all are high (13). Medicare and Medicaid expenditures are 50% to 100% higher among recipients with Alzheimer's disease compared with other recipients (14, 15). Medicare beneficiaries with Alzheimer's disease are hospitalized more frequently and stay in the hospital longer than other Medicare beneficiaries but receive fewer diagnostic and therapeutic procedures (16). Care for individuals with vascular dementia is even more costly (17). Alzheimer's disease and other dementias are suggested as major hidden contributors to the high costs of treating other chronic diseases such as diabetes and cancer (17). The value of lost productivity among caregivers substantially exceeds the direct medical costs among community-dwelling individuals with Alzheimer's disease (18).
Preventability
Medical advances are offering the opportunity to slow the progress of Alzheimer's disease. The U.S. Food and Drug Administration has approved a number of pharmacological interventions that improve cognitive function in Alzheimer's patients; more are being investigated (8). Preventing or controlling the underlying disease can prevent dementias such as vascular dementia or dementia resulting from HIV.
Public Interest
Dementia does not appear in the popular news media to the degree that other health issues, such as breast cancer, do. This could be due to misconceptions about the inevitability of cognitive decline with aging.
Challenges in Surveillance of Dementias
Level of Usefulness
The legislative intent of the law establishing the Registry was to create a registry as a resource for describing the magnitude of dementia in New York State and supporting research on dementia.
The Registry has contributed to its research mission in a number of ways:
A study of physician diagnosis of dementia established baseline data for Healthy People 2000: National Health Promotion and Disease Prevention Objectives and was used to target educational interventions and teleconferences;
Creutzfeldt-Jakob Disease (CJD) surveillance linking Registry, death records, and hospital data is conducted as part of a national effort to more rapidly identify potential instances of CJD and variant CJD in New York State; and
Registry data was used in a study of caregivers' need for health and human services conducted by the Institute of Gerontology, University at Albany, State University of New York (19, 20).
Many of the challenges described in this paper have limited the usefulness of the Registry in the past and are currently being addressed.
System Attributes
The CDC guidelines list 9 attributes for evaluating a surveillance system (1). These include simplicity, flexibility, data quality, acceptability, sensitivity, positive predictive value, representativeness, timeliness, and stability. A brief review of these attributes as they apply to the Registry follows. A focus on data quality will serve to clarify the challenges faced in conducting surveillance of dementia.
Simplicity
Simplicity refers to structure and ease of operation of the surveillance system (1). Simplicity is enhanced by use of other existing sources of reporting data. Figure 1 illustrates the anticipated flow of information to the Registry from nursing homes, and Figure 2 shows the flow from hospitals.
Figure 1.
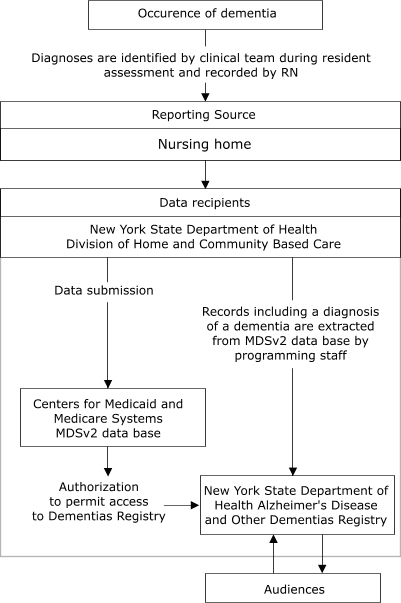
Proposed flow of data from nursing homes to New York State Department of Health Alzheimer's Disease and Other Dementias Registry, 2003. MDSv2 indicates Minimum Data Set 2.0, Centers for Medicare and Medicaid Services, U.S. Department of Health and Human Services. Permission to use MDSv2 data for Dementias reporting is being sought by the Registry.
Figure 2.
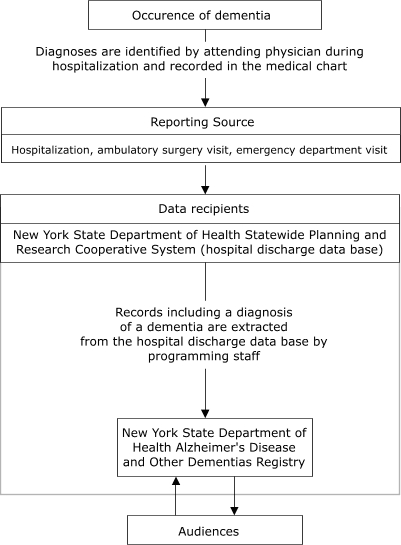
Flow of data from hospitals to New York State Department of Health Alzheimer's Disease and Other Dementias Registry, 2003.
Flexibility
Flexibility refers to how quickly a system can adapt to changing information needs or operating conditions (1). Because dementia is a chronic disease, information needs and operating conditions change slowly, and flexibility is not a critical issue. Nevertheless, the Registry seeks to maximize flexibility by obtaining data from existing resources, rather than collecting its own data in isolation. The process of procuring data from other sources has not been difficult, but the process of obtaining permissions and developing programs to extract data has been time-consuming. Technology permits extraction of high-quality data from other systems, facilitating operation of the Registry with little staffing, but the Registry is only as flexible as its existing data sources.
Data quality
Data quality reflects the completeness and validity of data recorded (1). Data quality — specifically, completeness and coding accuracy — is the primary challenge to the Registry. The Registry relies on 2 primary data sources for completeness of information: hospitals and nursing homes. Between 1986 and early 2003, hospitals reported data on paper forms sent directly to the Registry. During this time, hospital reporting via paper forms yielded approximately 20,000 reports each year. In comparison, New York State's hospital discharge database received more than 50,000 medical records that include a diagnosis of dementia each year. The hospital discharge database collects information on up to 15 ICD-9-CM diagnoses from over 2.4 million hospitalizations statewide each year. In spring 2003, the Registry eliminated paper reporting from hospitals, converting to computerized extraction of hospitalizations with a dementia diagnosis from the hospital discharge database. This eliminated the need for hospital staff to make separate reports to the Registry, and it also eliminated the need for the Registry to spend resources on data entry. The hospital discharge database locates additional reports on individuals with dementia, and it maintains quality control mechanisms and important hospital relationships.
Use of the hospital discharge database does not eliminate all concerns about completeness of hospital reporting. Completeness also depends on dementia diagnoses being recorded in medical records in the first place. Dementia diagnoses may be underreported for several reasons. The first reason is diagnostic uncertainty. Unlike a cancer diagnosis, which is based on laboratory pathology, Alzheimer's disease and most other dementias are diagnosed clinically. Although it is commonly believed that Alzheimer's disease can only be definitively diagnosed at autopsy, practice parameters developed by the American Academy of Neurology indicate that Alzheimer's disease can be diagnosed clinically with good reliability (21). National Center for Health Statistics guidelines call for coding discharge diagnoses that are suspected, possible, or probable as if the condition "existed or was established" (22). Because of uncertainties in assigning a diagnosis, physicians may hesitate to record a clinical diagnosis or possible diagnosis of dementia in the medical record. Second, general practitioners have been found to delay diagnoses of dementia because of embarrassment about communicating the diagnosis (23). The degree to which doctors hesitate is unknown. Third, financial disincentives for reporting dementia have also existed. Insurers have denied payment for services such as physical, occupational, or speech therapy to patients with Alzheimer's disease in the belief that patients could not benefit from therapy (23, 24). Automatic denial based solely on a diagnosis of dementia has been prohibited since September 2001 (24).
Fourth, most individuals with dementia are hospitalized for illnesses other than dementia, such as cardiovascular disease or respiratory disease (P.P.L., unpublished data, 2003). Failure to record diagnoses may occur because of clinical attention to the primary reason for hospitalization. The degree to which hospitals vary in recording secondary diagnoses is unknown.
Nursing homes provide another source of information on patients with dementia. A diagnosis of dementia is likely to be well recorded in nursing home data because nursing homes are mandated to assess residents' abilities and limitations frequently to provide a responsive plan of care. Higher reimbursement rates for residents who require more care may also encourage complete reporting of dementia by nursing homes. Approximately 200,000 assessments are performed each year for nursing home residents described as having dementia; the evaluations are recorded in the Centers for Medicare and Medicaid Services' Minimum Data Set. A registered nurse coordinates the assessment and certifies that the assessment form is complete. Other licensed health professionals, such as the attending physician, social workers, dietitians, and physical therapists may be assigned to complete relevant sections of the assessment instrument.
The Registry is now pilot-testing the ability of this computerized database to replace paper reporting from nursing homes. Preliminary data show that only one third of nursing homes that reported using the Minimum Data Set also completed paper Registry reporting forms. Use of the Minimum Data Set to retrieve information on nursing home residents with dementia would eliminate the need for nursing home staff to report data to 2 systems, and Registry staff would not need to enter data separately. Administrators of the nursing home data system monitor quality control, provide training on filling out forms, and maintain relationships with nursing homes.
Accuracy in coding the dementia diagnosis is another important component of data quality. Diagnoses in medical records are currently coded and reported under the ICD-9-CM coding system. Conceptualization of Alzheimer's disease and other dementias has evolved over time (25), and coding reflects this evolution. Under the ICD-9-CM system, dementias are grouped with psychotic mental disorders, while Alzheimer's disease is classified as a nervous system disorder. Consequently, reporting 2 codes is necessary for some dementias: one to document dementia symptoms and another to document the disease responsible for the dementia, such as Alzheimer's or Parkinson's disease. ICD-9-CM terminology further complicates coding accuracy because it has not kept pace with terms used by physicians and neurologists in discussing dementias. Recent practice parameters from the American Academy of Neurology discuss criteria for diagnosing Alzheimer's disease, vascular dementia, dementia with Lewy Bodies, and frontotemporal dementia (21). Several ICD-9-CM codes document Alzheimer's disease. In contrast, no ICD-9-CM codes existed prior to October 2003 for frontotemporal dementia or dementia with Lewy Bodies (26). As a result, the consistency of coding dementias may vary from facility to facility, given differences between how physicians describe dementia and how the coding system offers options. A study of hospitalizations that included a diagnosis of dementia found various dementia diagnoses recorded for the same patient both over time and within the same hospital stay (P.P.L., unpublished data, 2003). The Medical Economics and Management Subcommittee of the American Academy of Neurology is working to develop an ICD-9-CM Dementia coding index to address some of these issues (written communication, Gina Gjorvad, American Academy of Neurology, 31 July 2003).
Acceptability
Acceptability indicates the willingness of persons and organizations to participate in the surveillance system (1). Use of existing sources of data from hospitals and nursing homes clearly enhances acceptability of the Registry. Uncertainty about diagnosing dementia and a possible perceived stigma or embarrassment among physicians may pose a significant obstacle to acceptability, but the extent of significance is unknown. A recent review of hospitalizations found one third of men and nearly half of women hospitalized with a recorded dementia diagnosis were over age 85, suggesting that physicians may be more willing to record a dementia diagnosis for older patients (P.P.L, unpublished data, 2003).
Sensitivity
Sensitivity refers to the proportion of disease cases detected by the surveillance system as well as the ability to detect outbreaks, including the ability to monitor changes in the number of cases over time (1).
Historically, the majority of Registry reports have come from hospitals and nursing homes. The mandate to report Alzheimer's disease and other dementias is not contained in the same section of New York State public health law as communicable diseases. Direct reporting of communicable diseases is a traditional public health activity that is familiar to physicians and county health departments. Reporting of dementia is less familiar to physicians than reporting communicable diseases, particularly since county health departments are not used as a conduit of dementia data to the state. Resources have not permitted the degree of outreach and education necessary to inform physicians of their duty to report or to monitor completeness and accuracy of their reporting.
As a result, the surveillance system is sensitive enough to monitor changes over time, but is most sensitive to individuals with dementia who are frail or have comorbid conditions and least sensitive to individuals in relatively good health or those diagnosed in the early stages of dementia. The idea of representativeness is discussed further below.
Predictive Value Positive
Predictive value positive is the proportion of individuals reported with diesease that actually have disease (1). Factors that limit the recording of dementia diagnoses in medical charts also make false-positive reports of dementia unlikely.
Representativeness
Representativeness is a measure of how accurately the surveillance system describes the occurrence and distribution of a health-related event over time (1). The Registry represents individuals with dementia who are frail or who have comorbid conditions better than it represents individuals in relatively good health or individuals diagnosed at early stages of disease, mainly because reports are made primarily from hospitals and nursing homes. Medical advances are making early diagnoses more possible. As more individuals receive early diagnoses outside the hospital or nursing home setting, the current system of surveillance will not be able to capture these diagnoses, and the system will become less representative of the population with dementia.
Timeliness
Timeliness reflects the speed between surveillance system steps (1). Timeliness is less important to surveillance of chronic diseases such as dementia compared to surveillance of communicable diseases, which demands a rapid public health response (1). Long-range planning of support services is enhanced by information on statistical trends in Alzheimer's disease and other dementias. The NYSDOH provides small grants to Alzheimer's Disease Assistance Centers and Alzheimer's Disease Community Service Programs in New York State. Assistance Centers provide diagnosis, assessment and treatment for patients, educational/training services for health care professionals, care planning for the patient and family and serve as a clearinghouse for information on dementia. Community Service Programs provide respite care, caregiver training, support group services, and information and referral to patients and families. Information on trends in Alzheimer's disease and other dementias will enhance long-range planning of services by these and other groups. Staff members from the Registry have participated in annual meetings of these organizations.
Stability
Stability refers to the reliability (i.e., the ability to collect, manage, and provide data without failure) and availability (i.e., the ability to be operational when needed) of a surveillance system (1). Because timeliness is not crucial to dementia surveillance, the Registry is considered to be a stable system — that is, reliable and available with given resources.
Conclusions and Recommendations
This article has highlighted data quality as the main challenge to surveillance of dementias in New York State. Data quality is likely to be the primary challenge to other surveillance systems focusing on dementias. A system that obtains reports from existing hospital and nursing home databases can improve completeness of reporting, but such a system may be biased toward documenting illness in frail individuals with other health conditions as well as those living in nursing homes. Terminology reflected in ICD-9-CM coding has not kept pace with terms used by physicians and neurologists in discussing dementias. The accuracy of coding dementias in medical records from facility to facility is unknown; differences exist between how dementias are described in medical charts and how they are translated into coding choices.
A number of additional activities may improve completeness and accuracy of dementia reporting from nursing homes and hospitals. These recommended activities include:
Educating physicians on how to diagnose and code for dementia accurately once American Academy of Neurology guidelines are finalized.
Examining patterns of dementia diagnoses reported longitudinally for a sample of patients from different sources to assess the consistency of dementia diagnoses.
Enlisting the state's professional society for medical information management as a partner to highlight the importance of completely recording diagnoses of dementia and to help make informed recommendations about best practices in coding.
As medical advances lead to earlier diagnoses of dementia in individuals without other health conditions, we will need to develop better mechanisms for capturing data from organizations other than hospitals and nursing homes. A number of approaches could be tested:
Conduct a capture-recapture study using existing data sources to estimate the proportion of individuals with dementias missed in current surveillance. Such a study was recently undertaken by the South Carolina Alzheimer's Disease Registry; use of capture-recapture methodology was shown to identify 25% more individuals with dementia in South Carolina for an overall prevalence of 14% among individuals 65 years of age and older (27).
Partner with neurology practices and community-based organizations providing support services to individuals with Alzheimer's disease to study the proportion of individuals with dementia who go unreported.
Explore the utility of other databases, such as Medicare encounter data.
Despite the limitations of dementia surveillance, surveillance data are useful to individuals and agencies involved in monitoring trends, conducting research, or planning future services for an aging population. To that end, the Registry will publish its first report on hospitalizations that include a diagnosis of dementia in New York State. The report is scheduled for release at the end of 2003. Its intended audience includes public health practitioners and planners, clinicians, Alzheimer's Disease Assistance Centers, Alzheimer's Disease Community Service Programs, and reporting facilities. By studying hospitalizations, the Registry can begin to characterize the experience of New York's frailest residents coping with dementias and other illnesses.
Footnotes
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the U.S. Department of Health and Human Services, the Public Health Service, Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
Suggested citation for this article: Lillquist PP, Challenges in surveillance of dementias in New York State. Preventing Chronic Disease [serial online] 2004 Jan [date cited]. Available from: URL: http://www.cdc.gov/pcd/issues/2004/jan/03_0011.htm
References
- 1.Updated guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group. Centers for Disease Control. MMWR Morb Mortal Wkly Rep. 2001;50(No. RR-13):1–35. [PubMed] [Google Scholar]
- 2.NY Pub Health, Article 20. §2000-2004.
- 3.Hoyert DL, Rosenberg HM. Alzheimer's disease as a cause of death in the United States. Public Health Rep. 1997;112:497–505. [PMC free article] [PubMed] [Google Scholar]
- 4.Eaker ED, Vierkant RA, Mickel SF. Predictors of nursing home admission and/or death in incident Alzheimer's disease and other dementia cases compared to controls: a population-based study. J Clin Epidemiol. 2002;55:462–468. doi: 10.1016/s0895-4356(01)00498-x. [DOI] [PubMed] [Google Scholar]
- 5.Hy LX, Keller DM. Prevalence of AD among whites. Neurology. 2000;55:198–204. doi: 10.1212/wnl.55.2.198. [DOI] [PubMed] [Google Scholar]
- 6.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sloane PD, Zimmerman S, Suchindran C, Reed P, Wang L, Boustani M, et al. The public health impact of Alzheimer's disease, 2000-2050: potential implication of treatment advances. Annual Rev Public Health. 2002;23:213–231. doi: 10.1146/annurev.publhealth.23.100901.140525. [DOI] [PubMed] [Google Scholar]
- 8.Dugué M, Neugroschl J, Sewell M, Marin D. Review of dementia. Mt Sinai J Med. 2003;70(1):45–53. [PubMed] [Google Scholar]
- 9.Parnetti L, Amici S, Lanari A, Gallai V. Pharmacological treatment of non-cognitive disturbances in dementia disorders. Mech Ageing Dev. 2001;122:2063–2069. doi: 10.1016/s0047-6374(01)00316-5. [DOI] [PubMed] [Google Scholar]
- 10.Neumann PJ, Araki SS, Arcelus A, Longo A, Papadopoulos G, Kosik KS, et al. Measuring Alzheimer's disease progression with transition probabilities: estimates from CERAD. Neurology. 2001;57:957–964. doi: 10.1212/wnl.57.6.957. [DOI] [PubMed] [Google Scholar]
- 11.Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001 Jan 9;56(1):49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 12.Husaini BA, Sherkat DE, Moonis M, Levine R, Holzer C, Cain VA. Racial differences in the diagnosis of dementia and in its effects on the use and costs of health care services. Psychiatr Serv. 2003 Jan;54(1):92–96. doi: 10.1176/appi.ps.54.1.92. [DOI] [PubMed] [Google Scholar]
- 13.Bloom BS, de Pouvourville N, Straus WL. Cost of illness of Alzheimer's disease: how useful are current estimates? Gerontologist. 2003 Apr;43(2):158–164. doi: 10.1093/geront/43.2.158. [DOI] [PubMed] [Google Scholar]
- 14.Sloan FA, Taylor DH., Jr. Effect of Alzheimer disease on the cost of treating other diseases. Alzheimer Dis Assoc Disord. 2002 Jul–Sep;16(3):137–143. doi: 10.1097/00002093-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Martin BC, Ricci JF, Kotzan JA, Lang K, Menzin J. The net cost of Alzheimer disease and related dementia: a population-based study of Georgia Medicaid recipients. Alzheimer Dis Assoc Disord. 2000 Jul–Sep;14(3):151–159. doi: 10.1097/00002093-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Fillenbaum G, Heyman A, Peterson P, Pieper C, Weiman AL. Frequency and duration of hospitalization of patients with AD based on Medicare data: CERAD XX. Neurology. 2000;54:740–743. doi: 10.1212/wnl.54.3.740. [DOI] [PubMed] [Google Scholar]
- 17.Fillit H, Hill J. The costs of vascular dementia: a comparison with Alzheimer's disease. J Neurol Sc. 2002;203-204:35–39. doi: 10.1016/s0022-510x(02)00257-5. [DOI] [PubMed] [Google Scholar]
- 18.Small GW, McDonnell DD, Brooks RL, Papadopoulos G. The impact of symptom severity on the cost of Alzheimer's disease. J Am Geriatr Soc . 2002;50:321–327. doi: 10.1046/j.1532-5415.2002.50065.x. [DOI] [PubMed] [Google Scholar]
- 19.Toseland RW, McCallion P, Gerber T, Dawson C, Cieryic C, Guilamo-Ramos V. Use of health and human services by community-residing people with dementia. Soc Work. 1999 Nov;44(6):535–548. doi: 10.1093/sw/44.6.535. [DOI] [PubMed] [Google Scholar]
- 20.Toseland RW, McCallion P, Gerber T, Banks S. Predictors of health and human services use by persons with dementia and their family caregivers. Soc Sci Med. 2002 Oct;55(7):1255–1266. doi: 10.1016/s0277-9536(01)00240-4. [DOI] [PubMed] [Google Scholar]
- 21.Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, et al. Practice parameter: diagnosis of dementia (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 22.National Center for Health Statistics ICD-9-CM Official Guidelines for Coding and Reporting, October 2002. http://www.eicd.com/Guidelines/Default.htm
- 23.van Hout H, Vernooij-Dassen M, Bakker K, Blom M, Grol R. General practitioners on dementia: tasks, practices and obstacles. Patient Educ Couns. 2000;39(2-3):219–225. doi: 10.1016/s0738-3991(99)00034-8. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Medicare & Medicaid Services Program Memorandum Intermediaries/Carriers Transmittal AB-01-135, CMS-Pub 60AB, September 25, 2001. http://www.cms.gov/manuals/pm_trans/AB01135.pdf
- 25.White L. Alzheimer's Disease: The evolution of a diagnosis. Public Health Rep. 1997;112(6):495–496. [PMC free article] [PubMed] [Google Scholar]
- 26.ICD-9-CM Coordination and Maintenance Committee Meeting, Volumes 1 and 2, Diagnostic Presentations, December 6, 2002 http://www.cdc.gov/nchs/data/icd9/icdm1100.pdf
- 27.Sanderson M, Benjamin JT, Lane MJ, Cornman CB, Davis DR. Application of capture-recapture methodology to estimate the prevalence of dementia in South Carolina. Ann Epidemiol. 2003 Aug;13(7):518–524. doi: 10.1016/s1047-2797(03)00036-x. [DOI] [PubMed] [Google Scholar]


