Abstract
Hepatitis E virus (HEV) is the causative agent of hepatitis E in humans, an emerging zoonosis mainly transmitted via food in developed countries and for which domestic pigs are recognised as the main reservoir. It therefore appears important to understand the features and drivers of HEV infection dynamics on pig farms in order to implement HEV surveillance programmes and to assess and manage public health risks. The authors have reviewed the international scientific literature on the epidemiological characteristics of HEV in swine populations. Although prevalence estimates differed greatly from one study to another, all consistently reported high variability between farms, suggesting the existence of multifactorial conditions related to infection and within-farm transmission of the virus. Longitudinal studies and experimental trials have provided estimates of epidemiological parameters governing the transmission process (e.g. age at infection, transmission parameters, shedding period duration or lag time before the onset of an immune response). Farming practices, passive immunity and co-infection with immunosuppressive agents were identified as the main factors influencing HEV infection dynamics, but further investigations are needed to clarify the different HEV infection patterns observed in pig herds as well as HEV transmission between farms. Relevant surveillance programmes and control measures from farm to fork also have to be fostered to reduce the prevalence of contaminated pork products entering the food chain.
Electronic supplementary material
The online version of this article (doi:10.1186/s13567-017-0436-3) contains supplementary material, which is available to authorized users.
Introduction
Hepatitis E virus (HEV) is a non-enveloped single-stranded RNA virus. It is transmitted via the faecal-oral route and causes acute hepatitis in humans, clinical signs being similar to hepatitis A infection but usually more severe [1]. Chronic cases have also been described in immunocompromised patients [2]. There are four HEV genotypes: genotypes 1 and 2 are specific to humans and are currently circulating in Asia, Africa and Central America in epidemic waves linked to the consumption of contaminated water [3]. Genotypes 3 and 4 are responsible for sporadic autochthonous human cases in developed countries and are common to humans and other animal species [3, 4]. Genotype 3 in particular is highly prevalent in wild and domestic pigs, but the infection does not lead to a clinical disease [5]. Swine and human HEV strains are genetically very close, and cross-species transmission has been proved [6]. Moreover, a number of sporadic autochthonous cases have been related to the consumption of raw or undercooked pork products, especially liver-based products [7–9]. Thus, hepatitis E is considered to be an emerging zoonosis, domestic pigs being recognised as its main reservoir in industrialised countries [4, 10]. It is crucial to fully understand the conditions related to pig farm infection and HEV transmission dynamics within the swine population in order to limit the risk of introducing contaminated products into the food chain.
Several prevalence studies have been carried out in pig herds, either on a farm or individual scale. Prevalence estimates derived from either virological or serological analyses have evidenced wide differences depending on the country and year of study. However, the available data are difficult to compare since the pigs’ age and production stage vary according to studies, as do the HEV detection methods and biological matrix used for analyses. Moreover, the precision of the different estimates varies greatly between studies owing to huge differences in sample sizes. Even within the same study, the individual and farm-scale prevalences observed are also highly heterogeneous. This wide dispersion suggests the existence of various infection dynamics linked to farm-specific risk factors which have only been sporadically investigated to date. Observational studies mainly report the implication of farming practices in terms of hygiene, biosecurity and rearing conditions. Complementary to this approach, mathematical modelling studies, based either on experimental trials or longitudinal studies on infected farms have helped reveal new insights on HEV infection dynamics.
It is important to explore the epidemiological characteristics of HEV on pig farms for several purposes, e.g. to set up a surveillance programme, or identify control measures to manage the risk of HEV infection and transmission with the ultimate aim of reducing the prevalence of HEV-containing livers at the slaughterhouse. Information available from published papers has therefore been comprehensively gathered to identify key patterns of HEV infection as well as knowledge gaps and research needs. We have specifically focused our study on the epidemiological characteristics of the virus in domestic pigs and their products, since other aspects of this zoonosis have already been reviewed in various papers. The scope of this review thus covers prevalence, risk factors, transmission routes and infection dynamics on pig farms, surveillance and control strategies throughout the pork chain.
HEV prevalence in farmed pigs
It is crucial to know the prevalence of HEV on pig farms so as to be able to assess the health situation of the pig population and thus the risk to public health. We counted 86 studies (from 43 different countries) addressing HEV prevalence in farmed pigs. These studies are summarised in Additional file 1 [5, 11–21, 24, 47, 57, 66, 70–73, 79–139]. Various methods were used for data collection: samples were collected from slaughterhouses, randomly-selected or specifically-selected farms, or from serum/faeces/organ banks. Some studies were conducted at a given point in time, leading to an instantaneous prevalence estimate, whereas others were retrospective and estimated the prevalence from sera collected over a given period of time. The number of farms varied from 1 to 2 001; the number of samples from 40 to 6 565. Pigs included in the studies differed in age and rearing conditions (family-scale or large-scale farms, organic or industrial production, for example). Little information was available on the swine breed or strain. Prevalence was investigated either on a farm or individual level. The virus was sought in several different biological materials, including various organs (e.g. the intestines or liver), serum, faeces, bile and caecal content. Individual or pooled samples were processed using RT-PCR, nested RT-PCR, real-time RT-PCR or antigen detection. The serological response to HEV infection was assessed by detection of anti-HEV antibodies (IgG, IgM or IgA) using ELISA tests with specificity ranging from 85 to 100% and sensitivity from 50 to 100%. The viral strains detected belonged either to genotype 3 or genotype 4.
Farm-scale prevalence
Farm-scale seroprevalence
Farm-scale seroprevalence reported in 15 studies ranged from 30 to 98% (Additional file 1). For instance, in a study conducted in France in 2011, 65% of the 186 randomly-selected farms were found to have at least one seropositive animal (95% confidence interval 57–74) [5]. The serological prevalence was even higher in a retrospective study conducted in Spain, 204 out of 208 farms (98%, 95% CI 96.1–99.9) having at least one anti-HEV IgG-positive pig [11]. Similarly, in a retrospective study recently carried out in Norway, anti-HEV IgG were detected in 90% (137/153) of the herds [12].
Farm-scale virological prevalence
Farm-scale virological prevalence reported in 25 studies ranged from 10 to 100% (Additional file 1). Widen et al. detected HEV-RNA in swine faeces from 17 out of 22 randomly-selected farms in Sweden (72.7%) [13]. Virological prevalence has also been estimated from HEV-RNA detection in sera: in 72 herds selected in Spain, at least one slaughtered pig tested positive for HEV-RNA in serum on 47.2% of farms [14]. Regarding the presence of HEV RNA in liver, 24% (95% CI 17–31) of 186 randomly-selected pig farms had at least one positive liver in the French national prevalence study conducted by Rose et al. in 2011 [5].
Individual prevalence
Individual seroprevalence
Individual seroprevalence ranged from 8 to 93% in the 45 studies analysed (Additional file 1). In France, 31% (95% CI 24–38) of the slaughter-aged pigs in 2011 were found HEV seropositive [5]. Similarly, Jinshan et al. detected 52% of sampled pigs positive for anti-HEV antibodies in Mongolia [15]. Crossan et al. separately tested the presence of the different types of anti-HEV antibodies in Scotland and reported that, of 176 serum samples tested, 29% (n = 51) were anti-HEV IgG-positive, 36.9% (n = 65) anti-HEV IgA-positive and 29% (n = 51) anti-HEV IgM-positive. Overall seroprevalence (anti-HEV IgG+ and/or IgA+ and/or IgM+) was 61.4% (n = 108) [16]. In the same region and period, individual HEV seroprevalence was found by Grierson et al. to be even higher; they reported that 584 out of 629 pigs (92.8%) had anti-HEV antibodies at the time of slaughter [17].
Individual virological prevalence
Individual virological prevalence ranged from 1 to 89% in the 69 reported studies (Additional file 1). For instance, the HEV genome was detected in the faeces of 42% of 274 randomly-selected pigs from six different swine farms in northern Italy [18]. HEV RNA was also detected in serum: Crossan et al. reported a virological prevalence of 44.4% in serum (72/162) [16], whereas Grierson et al. detected HEV RNA in only 3% of plasma samples (22/629) in pigs at slaughter age [17]. In the same study, 15% of caecal contents (93/629) were found positive to HEV RNA [17]. Regarding the detection of HEV in liver, Rose et al. reported an individual prevalence of HEV RNA-positive livers of 4% (95% CI 2–6) at slaughter age [5].
Both at farm and individual levels, studies carried out in a given country at different times or retrospectively did not show any significant change in prevalence estimates over time, suggesting that HEV was constantly circulating in pig farms. The marked variability in individual prevalence estimates between farms is noteworthy: from 12.8 to 72.5% in Italy [18], from 4 to 58% in Argentina [19], and from 5 to 90% in France [5]. This may reflect different infection dynamics related to farm-specific risk factors.
Factors influencing HEV prevalence estimates
To date, few studies have reported the risk factors associated with high HEV prevalence on pig farms. We identified 12 studies addressing HEV risk factors, but only six of them quantified the impact of risk factors on HEV seroprevalence or on the prevalence of shedding pigs through odds ratio estimates (Table 1). The risk factors for a high HEV seroprevalence were mainly related to (1) farm characteristics and (2) farming practices. The farming scale (medium-size and family-scale farms, linked to the number of pigs and sows) was identified as a risk factor related to HEV seroprevalence [15, 18, 20, 21]. It was also shown that HEV seroprevalence was significantly higher in organic farms than in conventional ones [22]. Several high-risk rearing practices were reported, the main ones being late weaning, mingling practices at the nursery stage and poor hygiene [23]. Biosecurity measures such as requiring a shower upon entry were also found to be protective factors with respect to the prevalence of faecal HEV RNA shedding [24]. A seasonal influence on the prevalence of HEV RNA among swine was also reported, with a major peak in March–April followed by a smaller peak in September–October [25].
Table 1.
Quantified risk factors associated with a high HEV seroprevalence in pig farms
| Di Bartolo et al. [18] | Number of sows > 1000: HEV seroprevalence = 54.2 vs 18.9% |
| Li et al. [21] | HEV seroprevalence on family-scale farms = 90 vs 76% in large-scale farms (p < 0.01) |
| Jinshan et al. [15] | Number of pigs > 600: HEV seroprevalence ranged from 78 to 100%, vs 0 to 29% |
| Hinjoy et al. [20] | Medium-sized farms compared with large farms: OR 4.95 (1.79–13.70) |
| Presence of bird faeces inside the pig house: OR 2.87 (1.07–7.71) | |
| Walachowski et al. [23] | Duration of the nursery down period < 4 days: OR 1.7 (1.04–2.9) |
| Distance between pit manure and slatted floor in fattening premises < 80 cm: OR 1.9 (1.1–3.5) | |
| Mingling of pigs from different premises between farrowing and nursery stages: OR 1.8 (1.1–2.9) | |
| Pen size in nursery rooms > 26 pigs/pen: OR 2.4 (1.2–4.8) | |
| Rutjes et al. [22] | HEV seroprevalence on organic farms = 89 vs 72% on conventional farms (p = 0.04) |
| HEV seroprevalence on free-range farms = 76 vs 72% on conventional farms (p = 0.06) |
OR odds ratio.
European wild boars are recognized as a potential reservoir of HEV [26, 27]. Moreover, some experimental studies evidenced that HEV strains could be transmitted from European wild boars (Sus scrofa) to domestic pigs [26, 28, 29]. Though no study directly related HEV prevalence in pig farms to contact with wild boars, they may play a potential role in the swine HEV epidemiology in free-ranged pig production units. The role of wild boars as HEV risk exposure for domestic pigs would deserve further investigation.
HEV infection characteristics and dynamics on pig farms
HEV infection features in pigs
Age at HEV infection and shedding
The age at infection was only sporadically reported in the literature, with only three studies inferring from serological results the window within which infection took place. Almost all the studies conducted on pig farms only reported the age at shedding, and not the age at infection. Based on a large-scale seroprevalence survey conducted in Japan, the average age at infection was estimated to range from 59.0 to 67.3 days with more than 80% of infections occurring between the ages of 30 and 90 days [30]. The results of a longitudinal study on three French farms were quite different, most HEV infections occurring between 105 and 140 days of age [31]. Using Spanish data, Andraud et al. estimated the age at infection between 60.9 and 96.6 days [32]. Based on serological data from longitudinal studies in six pig herds, passive immunity was shown to delay early HEV infection of piglets by about 6 weeks in all but one farm on which the dynamics of infection were similar, whatever the animals’ initial serological status. Although the protective role of passive immunity cannot be denied, the latter case highlighted the strong interaction between farm-specific husbandry and hygiene practices and the HEV transmission process [32].
HEV infection dynamics have in the majority of studies been described through the monitoring of shedding pigs. These studies showed that the prevalence of HEV RNA in swine faeces and serum depend on the production stage, i.e. the pig’s age (Additional file 2 [14, 15, 18, 21, 57, 66, 86–89, 91–93, 95, 96, 104, 108, 109, 113, 114, 118–120, 133, 135, 136, 140–150]). A broad shedding period from 1.5 to 5 months of age was globally reported at farm scale. In most cases, the faecal shedding peak was described in 3-month-old to 4-month-old pigs, and few animals had PCR-positive faecal samples after 6 months of age. We performed a meta-regression analysis using data from 31 studies published between 2002 and 2016 which reported the prevalence of faecal HEV shedding or presence of HEV in livers depending on pig age. A weighted generalised linear mixed-effect model with the publication as a random effect, using intra and inter-study variances for a given age category as weight for individual studies, was fitted to age-specific prevalence data. Despite marked variability between studies, the model showed that the probability of faecal shedding peaked around 90 days of age (Figure 1). The shedding prevalence estimate at 185 days (a common slaughter age) was 6.1% [1.2–15.4].
Figure 1.
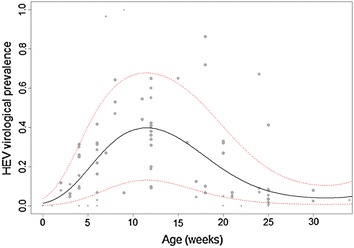
Predicted HEV prevalence in faeces according to animal age. The virological prevalence data (faecal shedding or presence in livers) depending on age (obtained from 31 published studies) were used to construct a meta-regression (generalised linear mixed-effect model) taking into account the respective weights of publications calculated using the inverse of the sum of inter-study and intra-study variance for a given age category. The mean predicted response of the model (black line) and its confidence interval (red dashed lines) are presented in this figure. The size of the points is proportional to the weight of the study.
Few studies have explored the factors influencing variations in age at shedding. A longitudinal study conducted on three swine farms showed that HEV shedding in pigs which had been previously infected by Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) occurred later than in pigs that were PRRSV-negative or which had been infected by PRRSV after HEV infection (hazard ratio = 0.49, p < 0.01) [33]. Hence, the latency period (delay between infection and shedding) may be affected by different circumstances, modifying the age at shedding. When pigs were infected intravenously, the latency period measured by Bouwknegt et al. was 3 days, compared to 6.9 days [5.82–7.9] when inoculated orally [34, 35]. According to the results of a co-infection trial, the HEV latency period was extended by a factor of 1.9 in the event of PRRSV co-infection (12.9 days [12.8–14.4]) [36]. In a recent longitudinal study on two Finnish farms identified as HEV-positive, piglets started to shed HEV between 7 and 12 weeks of age. Of these, 62.5% only began shedding HEV between the ages of 10 and 12 weeks [37]. The authors suspected that the difference in age of the onset of infection or shedding may have resulted from the quantity and/or quality of colostrum providing the piglets with maternal antibodies. The quantity of virus particles ingested may also modify the course of infection at the individual level. The minimal per os infectious dose is still not well known. However, it has been estimated that oral infection would require about 20 mg of faeces per day containing on average 108 genome equivalent (GE) per gram over three consecutive days to achieve a 50% probability of infection [38]. These results were then confirmed by testing different infection doses for inoculation by the oral route: a minimal viral load of 106 GE was necessary for pigs to be orally infected and to shed and transmit the virus [35]. Below that level, only sporadic shedding was detected, with no transmission to sentinel piglets.
Shedding duration and quantity of virus particles shed
Shedding duration is not easy to measure on farms as it requires an individual follow-up of pigs. The HEV shedding period was estimated at around 27 days in two studies carried out on commercial pig farms in Europe [33, 39]. Data from three pig herds evidenced a huge variation in the infection dynamics according to the farms, with some batches exhibiting late and short-term infections, while others had early and long-term shedding periods [31]. When pigs were experimentally infected by the intravenous route, shedding lasted from 13 to 49 days depending on the viral dose inoculated [34], whereas an HEV infection trial described a shedding duration of 9.7 days [8.2–11.2] when pigs were orally infected [35]. This experimental estimate was lower than shedding durations observed on farms, suggesting the existence of factors influencing duration of the shedding period. One of them may be other pathogens co-infecting pigs. For instance, one trial showed that a PRRSV/HEV co-infection dramatically extended the shedding period by a factor of 5 to 48.6 vs 9.7 days [36].
Few data are available on the quantity of virus particles shed by infected animals. In field conditions, one study carried out in two pig herds in Japan reported an HEV load in faeces of between 103.8 and 106 GE/g throughout the pigs’ life [40]. Similarly, the quantity of HEV particles shed was evaluated between 104 and 106 GE/g of faeces in an experimental trial involving 18 pigs that had been orally infected [35]. When pigs were co-infected with PRRSV, the viral load shed increased to between 105 and 108 GE/g of faeces, and the accumulation of HEV in the environment was significantly higher too [36].
Humoral immune response
Fourteen studies investigated the humoral response of pigs following HEV infection (Additional file 2). In a longitudinal study carried out on six Spanish farms [41], IgM antibodies were first detected at 7 weeks of age in five farms and at 13 weeks of age in only one farm, whereas IgG antibodies were firstly observed at 13 weeks of age in four farms and at 18 weeks of age in the two other farms. At slaughter age (26 weeks), IgG antibodies were detected in 50 to 100% of pigs on five out of the six farms. In the study conducted by de Deus et al. [42], IgG antibodies were detected later (around 15 weeks), whereas IgA and IgM appeared at around 12 weeks. Similarly to the previous study, IgG antibodies were detected up to the slaughter age (22 weeks), whereas IgA and IgM only remained for 4–7 weeks.
Thanks to field data collected in Japan, Satou and Nishiura estimated the time required for seroconversion at 25 days (95% CI 20.9, 31.3) [30]. Similarly, the time to HEV seroconversion was estimated at 26.3 days in an experimental study and it was shown that co-infection with PRRSV delayed the time to seroconversion to 43.1 days, increasing the lag to seroconversion by a factor of 1.6 [36]. The presence of maternal antibodies was also found to delay seroconversion in piglets [40, 43]. The time taken for maternally-derived antibodies to wane depends on the quantity of the initial intake of colostral antibodies [41], which is itself related to the sow’s age [44] and HEV immune status. Passively acquired IgG remained detectable up to 9 weeks of age in piglets born to highly HEV-seropositive sows, compared to 1–3 weeks in piglets born to sows with weak anti-HEV immunity [42].
HEV viraemia
The natural course of infection in pigs involves infection at around 8–12 weeks of age coinciding with declining maternal antibodies, with a generally short viraemia lasting from 1–2 weeks followed by a more prolonged period of viral shedding in the faeces (Additional file 2). However, longer viraemia periods have also been reported, especially in the case of late HEV infections in pigs, possibly lasting up to slaughter age (Additional file 2). In an experimental infection study, Sanford et al. observed more prolonged periods of viraemia in some pigs, and one pig was continuously viraemic for 12 weeks post-infection [45]. A study in Scotland showed that 44.4% of pigs tested were viraemic at slaughter age [16], whereas another study conducted in the UK only reported 3% of viraemic pigs at slaughter age [17]. Maternally-derived antibodies were found to delay the onset of viraemia [40]. Furthermore, the amount of HEV RNA in the serum was found to be lower than that in the faeces, the highest serum HEV RNA titre being on day 90 in a pig from a litter with passive immunity (104.2 copies/mL) and on day 60 in a pig from a litter without passive immunity (105.6 copies/mL).
Routes of HEV transmission between pigs
It has been proved that the virus is mainly shed by the faecal route, leading to an accumulation of HEV in the pigs’ environment at all production stages on infected farms, as well as in manure pits [46]. Depending on the type of floor (litter or slatted floor), the animals are constantly in contact (more or less direct) with the environmental HEV reservoir. The virus has also been detected in the urine of HEV-infected pigs [38, 47, 48], making urine a potential transmission route, especially given the considerable volume produced per day and the potentially longer viral shedding in this medium [48]. Given the urinary and faecal shedding routes, drinking water and/or feed may also be indirect vectors of HEV transmission, especially if feeding and drinking equipment can be easily contaminated by faeces and urine [46]. Finally, daily repeated contacts between pigs kept in the same pen and housed in a confined environment, as well as the mingling of pigs at different production stages may increase the propagation of HEV on farms [23, 42, 49, 50]. These findings confirm that the faecal-oral route is the major transmission route of HEV in pigs [48, 49], even if several trials have highlighted the difficulty in inoculating pigs per os [51, 52]. Indirect transmission from one pen to another (without any pig mingling) was found to be low [35].
Three-month old and older pigs were recognised as the major shedding sources in farm conditions (Figure 1). Fernandez-Barredo et al. showed that weaning and early fattening stages were critical periods for HEV shedding with respectively 45 and 60% of shedding animals [46]. Breeding animals also play an important role in the spread and persistence of HEV within pig production units in two ways: (1) by providing maternally-derived antibodies that protect their piglets from early-life infection, (2) by possibly transmitting the virus via farrowing crates during lactation periods. Indeed, investigations into faecal shedding in sows around the farrowing period revealed prevalences ranging from 16 to 21% [41, 42, 46]. A high proportion of multiparous sows were found to shed the virus, as well as gilts and young sows but to a lesser extent [18]. A study recently carried out in China showed that farrowing sows had an approximately 2.5-fold higher risk of infection (OR 2.46, p < 0.01) than pre-farrowing sows [53]. Another study on Göttingen Minipigs in the context of xenotransplantation safety detected HEV in the sera of three sows 6 days after delivery and in their offspring [54]. Finally, in a longitudinal study on three pig farms, piglets from two farms shed the virus as early as the lactation phase in farrowing facilities [31]. Thus, horizontal transmission between sows and their piglets may occur in the early stage of a piglet’s life. Moreover, sows may transmit the virus to the foetus by the transplacentary route should viraemia occur during gestation, viral RNA having been detected in the livers of aborted fœtuses [55]. However, these results are still controversial, since one experimental study did not show any vertical transmission after intravenous inoculation of HEV to pregnant gilts [56]. Nevertheless, it cannot be excluded that breeding animals may constitute an HEV reservoir on infected farms, periodically shedding the virus according to changes in their immune status due to physiological conditions (pregnancy, farrowing). Sows may thus maintain viral propagation in swine herds.
Quantitative data on HEV transmission
The persistence of a virus on farms is linked to (1) the intrinsic ability of the virus to remain in the animals’ environment, (2) the possibility of regular reintroductions of the virus onto farms and (3) the ability of the virus to survive and spread in the population. This last criterion can be studied through the basic reproduction number (R0) of the virus, which measures the number of secondary infections due to one infectious pig during its entire shedding period in a fully susceptible population. The higher the basic reproduction number, the easier it is for the virus to spread and the greater its ability to stay within the population. Using a large-scale seroprevalence survey dataset from Japanese pig farms, Satou and Nishiura estimated the HEV R0 between 4.02 and 5.17, meaning that one infectious animal could theoretically infect four to five other pigs during its infectious period [30]. Based on an experimental trial carried out in the Netherlands, this ratio was estimated at 8.8 [34]. However, this assessment relied on one-to-one HEV transmission experiments, accounting for horizontal transmission by direct contact only. The trial by Andraud et al. investigated the transmission of HEV from pigs inoculated by the oral route to pigs in direct contact (in the same pen) or indirect contact (in an adjacent pen) with the inoculated pigs, assuming both environmental and direct transmission routes [35]. Although much lower than previous estimates with a partial reproduction number of 1.41 [0.21–3.02], direct transmission alone could be considered as a factor fostering the infection’s persistence within a population. The quantity of virus present in the environment was found to play a pivotal role in the transmission process, strongly influencing the probability of infection, with a within-pen transmission rate estimated at 2.10−6 g/GE/day [1.10−7–7.10−6]. Between-pen environmental transmission occurred to a lesser extent (transmission rate: 7.10−8 g/GE/day [5.10−9– 3.10−7]) but could further generate a within-group infection process. The combination of these transmission routes could explain the persistence and high prevalence of HEV in pig populations. Moreover, the transmission of HEV was found even enhanced in the presence of co-infections. Indeed, based on a similar experimental design with pigs co-infected with PRRSV, the transmission of HEV by direct contact was estimated to be 4.7 times higher in pigs co-infected with PRRSV (direct transmission rate = 0.70 [1.18.10−3–3.67]). Direct transmission therefore plays a more important role in HEV transmission when animals were co-infected and reflecting the increased quantity of virus particles shed [36]. The indirect transmission rate, considered to be the average number of animals that could be infected by a single genome equivalent present in the pen environment, was estimated at 6.59.10−6 g/GE/day [1.43.10−10–1.27.10−4], i.e. 3.3 times higher with co-infection than without. In other words, 3.3 times fewer virus particles were required to infect a co-infected animal than an HEV-only infected animal. The impact of maternally-derived antibodies on HEV transmission was also assessed by modelling field-based longitudinal data on HEV dynamics of infection [32]. In this study, HEV transmission among piglets with passive immunity was estimated to be 13 times lower than in fully susceptible animals, with a relatively marked variability between herds (range: 5–21).
Consequences of HEV infection dynamics on the prevalence of contaminated livers and pork products
Prevalence of HEV-containing livers at the slaughterhouse
In the ten studies investigating the prevalence of HEV-containing livers in pigs of slaughter age (Table 2), all but one reported prevalences ranging between 0.8 and 10% of liver samples, but the prevalence reported in Italy was over 20% [57]. Two conditions are required for a high prevalence of HEV-containing livers at slaughter age: (1) the virus has to spread massively on farms; (2) the later the infection occurred, the higher the risk that pigs are still infectious at slaughter. One study on French pig farms reported several risk factors, such as the slaughter age, genetic background, lack of hygiene measures and origin of drinking water [23]. An experimental trial also showed that the co-infection of pigs with HEV and PRRSV increased the likelihood of HEV-containing livers at slaughter time [36]. Satou and Nishiura built a model from field data and using a sensitivity analysis, they showed that a decline in the force of infection would postpone the infectious process to a later age, which would in turn heighten the risk of pork-to-human transmission through the consumption of infected products [30].
Table 2.
Prevalence of HEV RNA in livers collected at slaughterhouses reported in ten studies
| References | Country | No. of samples | Prevalence of RNA-positive livers (%) [95% CI] |
|---|---|---|---|
| Bouwknegt et al. [51] | Netherlands | 62 | 6.5 [1.8–15.7] |
| Rose et al. [5] | France | 3 715 | 4 [2–6] |
| Di Bartolo et al. [57] | Italy | 48 | 20.8 |
| Di Bartolo et al. [68] | Spain | 39 | 3 |
| Italy | 33 | 6 | |
| Czech Republic | 40 | 5 | |
| Berto et al. [67] | UK | 40 | 3 |
| Gardinali et al. [70] | Brazil | 118 | 1.7 |
| de Souza et al. [71] | Brazil | 453 | 1.3 |
| Temmam et al. [72] | Madagascar | 250 | 1.2 |
| de Paula et al. [73] | Cameroon | 345 | 0.8 |
| Mykytczuk et al. [74] | Canada | 19 | 10.5 |
Consequences on the safety of pork products entering the food chain
Nine prevalence studies were conducted on marketed pork products (Table 3). Different kinds of pork products were tested, such as raw livers, sausages, figatelli, pâté, etc. The prevalence of contaminated pork products varied from less than 1% to more than 50% depending on the country and the product. The highest prevalences were observed in products prepared with raw pork liver [7, 58]. No study was led on meat but, given the late viraemia at slaughter age that was observed in several studies (see above), there may be a potential risk to public health linked to the consumption of raw or undercooked pork meat.
Table 3.
Prevalence of HEV-positive marketed pork products reported in nine studies
| References | Country | No. of samples | Prevalence of RNA-positive pork products (%) |
|---|---|---|---|
| Yazaki et al. [75] | Japan | 363 | 1.9% of livers sold in local grocery stores |
| Feagins et al. [76] | USA | 127 | 11% of livers sold in local grocery stores |
| Colson et al. [7] | France | 12 | 58% of marketed figatelli |
| Wenzel et al. [77] | Germany | 200 | 4% of livers sold in butcher’s shops and grocery stores |
| Berto et al. [67] | UK | 63 | 10% of marketed sausages |
| Di Bartolo et al. [68] | Spain | 93 | 6% of marketed sausages |
| Czech Republic | 92 | 0% of sausages | |
| Italy | 128 | 0% of sausages | |
| Pavio et al. [58] | France | 394 | 30% of figatelli, 29% of liver sausages, 25% of quenelles, 3% of dried salted livers |
| Heldt et al. [78] | Brazil | 50 | 36% of marketed pâté and blood sausages |
| Mykytczuk et al. [74] | Canada | 111 | 47% of pork pâté, 0% of raw pork sausages |
The presence of HEV in food products consumed raw or undercooked raises the question of the thermal stability of HEV, which was addressed in three studies. The first one was based on heating faecal suspensions of HEV genotypes 1 and 2 to temperatures between 45 and 70 °C and inoculation in a cell culture permissive to HEV [59]. The second study used pigs inoculated with pork liver homogenates containing infectious genotype 3 HEV heated to 56 °C for 1 h, fried for 5 min (71 °C internal temperature) or boiled for 5 min [60]. Both studies showed that HEV was more likely to resist when heated to only 56 °C and was inactivated at temperatures higher than 71 °C. The third study was conducted on more complex foodstuffs prepared according to industrial recipes (liver pâté) and showed that it was necessary to heat the food to an internal temperature of 71 °C for 20 min to fully inactivate HEV [61]. To date, no information is available about the efficacy of drying on HEV persistence.
Improving HEV surveillance and control in the swine reservoir: from farm-targeted actions to pork product control
Options for control measures on pig farms
HEV vaccination on farms
No commercial vaccine is currently available against HEV in pigs. Some theoretical work has been carried out to evaluate the benefits of vaccination against this zoonosis, which does not have any consequences on pig health or the economic performance of swine herds. Using a modelling approach, Backer et al. tested three effects of vaccination: a decrease in the virus transmission rate, in animal susceptibility to HEV infection, and in the duration of the infectious period [39]. As previously shown by Satou and Nishiura [30], a reduced transmission rate and susceptibility, which induces a decrease in the force of infection, led to an increase in the number of infectious animals at the slaughterhouse. When the vaccine affected the duration of the infectious period, the proportion of pigs still infectious at slaughter age was lower. Further work would be needed to evaluate the required efficacy for a vaccine to eradicate the infection and to develop the corresponding efficient vaccine, without forgetting considerations on interference with passive immunity, co-infecting pathogens and rearing practices. A cost-benefit analysis of vaccine development would also be necessary, including public health consequences in the event of widespread consumer exposure to contaminated pork products, and the economic consequences linked to a potential loss of consumer confidence in pork safety.
Control of risk factors and co-infecting pathogens
As previously mentioned, a lack of hygiene measures and several farming practices (such as late weaning or mingling practices at the nursery stage) were reported as risky for HEV transmission and persistence. Biosecurity and farming practices should therefore be enhanced to reduce HEV risks [23].
As reported previously, co-infections with immunosuppressive swine viruses — frequently observed in pig herds — could lead to chronic HEV infection, which may dramatically increase the risk of pig livers containing HEV at slaughter time. For instance, a PRRSV/HEV co-infection or a PRRSV infection prior to HEV infection delayed HEV shedding and the onset of the anti-HEV humoral immune response, increased the quantity of virus particles shed and extended the shedding period, increased the direct transmission rate and HEV infection susceptibility, and increased the proportion of HEV-positive livers at slaughter time [33, 36]. Thus, controlling intercurrent swine diseases (e.g. through PRRSV vaccination programmes) could be a major lever in the control of hepatitis E. Further research is needed in this domain to better understand the interactions between HEV and immunosuppressive pathogens, including an evaluation of the effect of other immunosuppressive co-infections frequently encountered in the field as well as non-biotic components such as mycotoxins which are likely to interfere with the immune response.
Organisation of the pig production network
To prevent the spread of infectious agents, it is necessary to consider the pyramidal structure of the pig production sector and the way contacts between pig farms are organised [62]. Few data are available yet. A recent study reported the presence of HEV inside and outside farm buildings, on trucks and in the slaughterhouse yard, thus suggesting viral transmission between farms and throughout the production network [63]. However, further research is needed to (1) model the pig production network; (2) explain, assess and quantify the risk of HEV transmission between pig farms through animal introductions (replacement) or indirect vectors.
Surveillance throughout the pork chain
To our knowledge, no uninterrupted surveillance programme of the swine reservoir has ever been implemented in any country. Surveillance actions could be implemented at different steps: on pigs at the farming stage or at the slaughterhouse, or on pig livers and pork products.
Monitoring of pigs on farms or at the slaughterhouse
Pig monitoring could be either serological or virological. (1) Serological monitoring could be a feasible large-scale approach. Data are available on the intrinsic features of the serological tests that could be used [64, 65], but further comparative analysis is still needed. Indeed, although a single HEV serotype exists, test performance varies depending on the HEV genotype [65]. However, more and more commercial ELISA tests are available and geared to HEV genotype 3, which is the main one circulating on pig farms in Europe and the US (e.g. HEV ELISA 4.0 V, MP Biomedicals). Moreover, some tests only detect IgM whereas others detect all immunoglobulin classes. Regarding the relevance of using serological tests, studies revealed a significant relationship between within-farm seroprevalence and the probability of detecting HEV-positive livers on that farm [5]. Indeed, Rose et al. observed that the probability of viral presence in the liver was significantly higher on farms where seroprevalence at the finishing stage was greater than 25%: OR 6.7 [2.1–21.6]. This result suggests that farms at risk are those in which the virus circulates intensely and spreads to more than 25% of fattening pigs [66]. However, at an individual level, some HEV RNA-positive pigs (detected in the liver) are seronegative because infection occurs late, not long before slaughter. This is why it appears that serological tests on fattening pigs from farrow-to-finish farms should be supplemented by tests on sows in order to clearly determine the HEV status of the farm. (2) The virus could also be detected in faeces as it appears that the virus’ presence in the liver and viral shedding are well correlated [31]. This surveillance action could be performed on farms, e.g. for a pre-slaughter check by sampling several animals. It could also be done at the slaughterhouse, in ante mortem waiting areas.
Surveillance of pig livers and pork products
Many human cases in industrialised countries are related to the consumption of so-called “high-risk” products, i.e. pork products consumed raw or not well cooked and containing a high proportion of pork liver. Surveillance could therefore target those specific products (liver sausages, liver pâté, figatelli, etc.). To date, few detection tests have been developed [61, 67, 68] and only one method for HEV detection in food has been marketed (HepatitisE@CeeramTools™, quantitative RT-PCR Kits for food & environmental samples). The viral concentration in food is often low. Moreover, these complex matrices are composed of liver, fat, salt and spices that make detection difficult. The analysis of meat matrices requires a rotary mill that is not frequently employed in non-specialised laboratories. Fat removal is essential, but tedious and mostly manual. Analysing food products is more complex than analysing livers, so livers could be tested after mixing and before adding other ingredients. As the transformation steps do not affect HEV stability (see above), the contamination of livers may be a relevant indicator of the risk of human exposure to HEV.
Applications and research needs
HEV monitoring activities in the pork production chain are needed for several purposes: (1) to acquire an uninterrupted series of prevalence data and monitor changes in prevalence and the virus itself (e.g. evolution of the prevalence of the different HEV subtypes and emergence of genotype 4, which is still only sporadically detected in Europe and the US); (2) to investigate more precisely HEV infection dynamics and factors influencing their variation; (3) to prevent contaminated livers from entering the food chain. For that purpose, the qualification of farms and/or animals and/or livers with regard to their HEV status could be considered. A French expert appraisal suggested three options that could be jointly implemented in order to prevent HEV-positive liver being used for the preparation of products containing raw liver [69]:
Qualification of HEV-free farms The farm could be qualified following serological testing on sampled animals (see above). This would enable the identification of farms eligible to market raw livers. However, this approach would be costly and would require constant testing, since the HEV status is unlikely to remain stable over time. Moreover, the logistics in slaughterhouses would then be complex, requiring an additional means of keeping HEV-free animals completely separate from HEV-positive ones.
Real-time qualification of HEV-free batches at the slaughterhouse Faecal samples could be taken from a determined number of pigs per batch, either in the ante mortem waiting area or the post mortem chain. The batches would be released after test results on a just-on-time basis. The HEV status of batches would be precisely known and only HEV-free batches would be used for the preparation of products containing raw liver. However, the logistics for the slaughterhouse would be both complicated and costly.
Qualification of liver homogenates RT-PCR could be performed on livers or liver homogenates to determine their HEV status. This approach would be less expensive yet would still enable an immediate risk management procedure to be followed depending on the result of the analysis. However, in the light of the HEV prevalence in livers, there would be a risk of detecting and rejecting many liver mixes.
These three options could lead to the creation of a separate sector dedicated to the fabrication of foodstuffs containing raw liver. Such a certification procedure requires regular food control capabilities relying on effective analytical tools for routine use, particularly on farms, at processing facilities and points of sale. The effectiveness of the certification system also relies on the traceability of pork livers, and requires a reference on the product label for all items containing pork liver. The label should provide consumers with information on the possible hazards related to consumption of these products. The coexistence of these two sectors may pose problems both in terms of logistics for the slaughterhouses and processing plants, and a risk of confusion for the consumer between products with different food safety statuses.
Whatever the qualification method, further studies are needed to compare the current tests, develop a reference method and establish a sampling plan geared to the sector’s situation. It is also necessary to investigate more precisely the risk linked to pork meat in order to assess the need for a meat surveillance and control plan. Figure 2 summarises the options for control measures throughout the food chain, and identifies knowledge gaps and challenges.
Figure 2.

HEV surveillance and control of the swine reservoir: from farm-targeted actions to pork product control (adapted from [ 69 ]). The left side of the diagram presents a number of measures to mitigate the risk of human exposure to swine HEV, with actions applying to both farms and foodstuffs. A certification process (green and red squares) could be implemented throughout the food chain to guarantee the absence of HEV in products derived from raw pork liver. The right side lists several knowledge gaps and research needs (black squares) in addition to the challenges involved in implementing these measures (in red).
Conclusion
The epidemiology of HEV in the swine reservoir is far from being fully elucidated. Though many prevalence studies have been carried out in numerous countries in the last decade, there remain knowledge gaps that still have to be addressed. Research needs to focus on the factors that could explain the huge between-herd variation in infection dynamics, HEV transmission between farms and throughout the pig production network, and finally the mechanisms of action and impact of intercurrent swine diseases. Further work also needs to be carried out to harmonise diagnostic tests and develop a standard reference method to detect HEV in complex foodstuffs. Surveillance plans and control programmes have to be carefully considered to mitigate the risk of human exposure to HEV through the consumption of pork products.
Additional files
Additional file 1. HEV serological and virological prevalence in the pig population at farm and individual levels. Farm-scale prevalence figures were reported in 34 studies. Farm-scale seroprevalence ranged from 30 to 98%, while farm-scale virological prevalence ranged from 10 to 100%. Individual prevalence figures were reported in 82 studies. Individual seroprevalence ranged from 8 to 93%, whereas individual virological prevalence ranged from 1 to 89%.
Additional file 2. Evolution of HEV RNA prevalence and anti-HEV antibody prevalence according to pig age. Thirty-seven studies explored the variation of HEV virological and serological prevalence with the pig age. m: months; w: weeks; d: days.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MS reviewed literature and drafted the manuscript. MA and NR supervised the project. All co-authors revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors are grateful to the French Ministry for Agriculture, Food and Forestry and to INAPORC for their financial support.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13567-017-0436-3) contains supplementary material, which is available to authorized users.
Contributor Information
Morgane Salines, Email: morgane.salines@anses.fr.
Mathieu Andraud, Email: mathieu.andraud@anses.fr.
Nicolas Rose, Email: nicolas.rose@anses.fr.
References
- 1.Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145–154. doi: 10.1002/rmv.384. [DOI] [PubMed] [Google Scholar]
- 2.Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, Dumortier J, Cannesson A, Cassuto-Viguier E, Thervet E, Conti F, Lebray P, Dalton HR, Santella R, Kanaan N, Essig M, Mousson C, Radenne S, Roque-Afonso AM, Izopet J, Rostaing L. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140:1481–1489. doi: 10.1053/j.gastro.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 3.Purcell RH, Emerson SU. Hepatitis E: an emerging awareness of an old disease. J Hepatol. 2008;48:494–503. doi: 10.1016/j.jhep.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Dalton HR, Bendall R, Ijaz S, Banks M. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis. 2008;8:698–709. doi: 10.1016/S1473-3099(08)70255-X. [DOI] [PubMed] [Google Scholar]
- 5.Rose N, Lunazzi A, Dorenlor V, Merbah T, Eono F, Eloit M, Madec F, Pavio N. High prevalence of hepatitis E virus in French domestic pigs. Comp Immunol Microbiol Infect Dis. 2011;34:419–427. doi: 10.1016/j.cimid.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Meng XJ, Halbur PG, Shapiro MS, Govindarajan S, Bruna JD, Mushahwar IK, Purcell RH, Emerson SU. Genetic and experimental evidence for cross-species infection by swine hepatitis E virus. J Virol. 1998;72:9714–9721. doi: 10.1128/jvi.72.12.9714-9721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colson P, Borentain P, Queyriaux B, Kaba M, Moal V, Gallian P, Heyries L, Raoult D, Gerolami R. Pig liver sausage as a source of hepatitis E virus transmission to humans. J Infect Dis. 2010;202:825–834. doi: 10.1086/655898. [DOI] [PubMed] [Google Scholar]
- 8.Moal V, Gerolami R, Colson P. First human case of co-infection with two different subtypes of hepatitis E virus. Intervirology. 2012;55:484–487. doi: 10.1159/000335664. [DOI] [PubMed] [Google Scholar]
- 9.Motte A, Roquelaure B, Galambrun C, Bernard F, Zandotti C, Colson P. Hepatitis E in three immunocompromized children in southeastern France. J Clin Virol. 2012;53:162–166. doi: 10.1016/j.jcv.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Pavio N, Meng XJ, Renou C. Zoonotic hepatitis E: animal reservoirs and emerging risks. Vet Res. 2010;41:46. doi: 10.1051/vetres/2010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casas M, Pujols J, Rosell R, de Deus N, Peralta B, Pina S, Casal J, Martin M. Retrospective serological study on hepatitis E infection in pigs from 1985 to 1997 in Spain. Vet Microbiol. 2009;135:248–252. doi: 10.1016/j.vetmic.2008.09.075. [DOI] [PubMed] [Google Scholar]
- 12.Lange H, Øverbø J, Borgen K, Dudman S, Hoddevik G, Urdahl AM, Vold L, Sjurseth SK. Hepatitis E in Norway: seroprevalence in humans and swine. Epidemiol Infect. 2017;145:181–186. doi: 10.1017/S0950268816002144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Widen F, Sundqvist L, Matyi-Toth A, Metreveli G, Belak S, Hallgren G, Norder H. Molecular epidemiology of hepatitis E virus in humans, pigs and wild boars in Sweden. Epidemiol Infect. 2011;139:361–371. doi: 10.1017/S0950268810001342. [DOI] [PubMed] [Google Scholar]
- 14.Jiménez de Oya N, de Blas I, Blázquez AB, Martin-Acebes MA, Halaihel N, Gironés O, Saiz JC, Escribano-Romero E. Widespread distribution of hepatitis E virus in Spanish pig herds. BMC Res Notes. 2011;4:412. doi: 10.1186/1756-0500-4-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jinshan Jirintai, Manglai D, Takahashi M, Nagashima S, Okamoto H. Molecular and serological survey of hepatitis E virus infection among domestic pigs in Inner Mongolia, China. Arch Virol. 2010;155:1217–1226. doi: 10.1007/s00705-010-0706-0. [DOI] [PubMed] [Google Scholar]
- 16.Crossan C, Grierson S, Thomson J, Ward A, Nunez-Garcia J, Banks M, Scobie L. Prevalence of hepatitis E virus in slaughter-age pigs in Scotland. Epidemiol Infect. 2015;143:2237–2240. doi: 10.1017/S0950268814003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grierson S, Heaney J, Cheney T, Morgan D, Wyllie S, Powell L, Smith D, Ijaz S, Steinbach F, Choudhury B, Tedder RS. Prevalence of hepatitis E virus infection in pigs at the time of slaughter, United Kingdom, 2013. Emerg Infect Dis. 2015;21:1396–1401. doi: 10.3201/eid2108.141995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Bartolo I, Martelli F, Inglese N, Pourshaban M, Caprioli A, Ostanello F, Ruggeri FM. Widespread diffusion of genotype 3 hepatitis E virus among farming swine in Northern Italy. Vet Microbiol. 2008;132:47–55. doi: 10.1016/j.vetmic.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 19.Munne MS, Vladimirsky S, Otegui L, Castro R, Brajterman L, Soto S, Guarnera E, Molina V, Monfellano M, Schlauder GG, González JE. Identification of the first strain of swine hepatitis E virus in South America and prevalence of anti-HEV antibodies in swine in Argentina. J Med Virol. 2006;78:1579–1583. doi: 10.1002/jmv.20741. [DOI] [PubMed] [Google Scholar]
- 20.Hinjoy S, Nelson KE, Gibbons RV, Jarman RG, Chinnawirotpisan P, Fernandez S, Tablerk P, Labrique AB, Patchanee P. A cross-sectional study of hepatitis E virus infection in pigs in different-sized farms in northern Thailand. Foodborne Pathog Dis. 2013;10:698–704. doi: 10.1089/fpd.2012.1369. [DOI] [PubMed] [Google Scholar]
- 21.Li W, She R, Wei H, Zhao J, Wang Y, Sun Q, Zhang Y, Wang D, Li R. Prevalence of hepatitis E virus in swine under different breeding environment and abattoir in Beijing, China. Vet Microbiol. 2009;133:75–83. doi: 10.1016/j.vetmic.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 22.Rutjes SA, Bouwknegt M, van der Giessen JW, de Roda Husman AM, Reusken CB. Seroprevalence of hepatitis E virus in pigs from different farming systems in The Netherlands. J Food Prot. 2014;77:640–642. doi: 10.4315/0362-028X.JFP-13-302. [DOI] [PubMed] [Google Scholar]
- 23.Walachowski S, Dorenlor V, Lefevre J, Lunazzi A, Eono F, Merbah T, Eveno E, Pavio N, Rose N. Risk factors associated with the presence of hepatitis E virus in livers and seroprevalence in slaughter-age pigs: a retrospective study of 90 swine farms in France. Epidemiol Infect. 2014;142:1934–1944. doi: 10.1017/S0950268813003063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilhelm B, Leblanc D, Leger D, Gow S, Deckert A, Pearl DL, Friendship R, Rajić A, Houde A, McEwen S. Farm-level prevalence and risk factors for detection of hepatitis E virus, porcine enteric calicivirus, and rotavirus in Canadian finisher pigs. Can J Vet Res. 2016;80:95–105. [PMC free article] [PubMed] [Google Scholar]
- 25.Lu YH, Qian HZ, Hu AQ, Qin X, Jiang QW, Zheng YJ. Seasonal pattern of hepatitis E virus prevalence in swine in two different geographical areas of China. Epidemiol Infect. 2013;141:2403–2409. doi: 10.1017/S0950268813000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jori F, Laval M, Maestrini O, Casabianca F, Charrier F, Pavio N. Assessment of domestic pigs, wild boars and feral hybrid pigs as reservoirs of hepatitis E virus in Corsica, France. Viruses. 2016 doi: 10.3390/v8080236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thiry D, Mauroy A, Saegerman C, Licoppe A, Fett T, Thomas I, Brochier B, Thiry E, Linden A. Belgian wildlife as potential zoonotic reservoir of hepatitis E virus. Transbound Emerg Dis. 2015 doi: 10.1111/tbed.12435. [DOI] [PubMed] [Google Scholar]
- 28.Thiry D, Rose N, Mauroy A, Paboeuf F, Dams L, Roels S, Pavio N, Thiry E. Susceptibility of pigs to zoonotic hepatitis E virus genotype 3 isolated from a wild boar. Transbound Emerg Dis. 2016 doi: 10.1111/tbed.12550. [DOI] [PubMed] [Google Scholar]
- 29.Schlosser J, Vina-Rodriguez A, Fast C, Groschup MH, Eiden M. Chronically infected wild boar can transmit genotype 3 hepatitis E virus to domestic pigs. Vet Microbiol. 2015;180:15–21. doi: 10.1016/j.vetmic.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 30.Satou K, Nishiura H. Transmission dynamics of hepatitis E among swine: potential impact upon human infection. BMC Vet Res. 2007;3:9. doi: 10.1186/1746-6148-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose N, Pavio N. Epidemiology of hepatitis E virus in pigs: the way forward to limit consumer exposure (in french) Journées Recherche Porcine. 2014;46:159–168. [Google Scholar]
- 32.Andraud M, Casas M, Pavio N, Rose N. Early-life hepatitis E infection in pigs: the importance of maternally-derived antibodies. PLoS One. 2014;9:e105527. doi: 10.1371/journal.pone.0105527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rose N, Salines M, Andraud M, Bourry O, Barnaud E, Pavio N (2017) The infection by the Porcine Reproductive and Respiratory Syndrome (PRRS) virus promotes chronic hepatitis E virus infection in pigs (in french). Bulletin Epidemiologique Santé Alimentation (in press). http://bulletinepidemiologique.mag.anses.fr/sites/default/files/M-083_2016-10-19_SRDP-HepE_Pub-Antpdf
- 34.Bouwknegt M, Frankena K, Rutjes SA, Wellenberg GJ, de Roda Husman AM, van der Poel WH, de Jong MC. Estimation of hepatitis E virus transmission among pigs due to contact-exposure. Vet Res. 2008;39:40. doi: 10.1051/vetres:2008017. [DOI] [PubMed] [Google Scholar]
- 35.Andraud M, Dumarest M, Cariolet R, Aylaj B, Barnaud E, Eono F, Pavio N, Rose N. Direct contact and environmental contaminations are responsible for HEV transmission in pigs. Vet Res. 2013;44:102. doi: 10.1186/1297-9716-44-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salines M, Barnaud E, Andraud M, Eono F, Renson P, Bourry O, Pavio N, Rose N. Hepatitis E virus chronic infection of swine co-infected with porcine reproductive and respiratory syndrome virus. Vet Res. 2015;46:55. doi: 10.1186/s13567-015-0207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kantala T, Heinonen M, Oristo S, von Bonsdorff CH, Maunula L. Hepatitis E virus in young pigs in Finland and characterization of the isolated partial genomic sequences of genotype 3 HEV. Foodborne Pathog Dis. 2015;12:253–260. doi: 10.1089/fpd.2014.1841. [DOI] [PubMed] [Google Scholar]
- 38.Bouwknegt M, Teunis PF, Frankena K, de Jong MC, de Roda Husman AM. Estimation of the likelihood of fecal-oral HEV transmission among pigs. Risk Anal. 2011;31:940–950. doi: 10.1111/j.1539-6924.2010.01546.x. [DOI] [PubMed] [Google Scholar]
- 39.Backer JA, Berto A, McCreary C, Martelli F, van der Poel WH. Transmission dynamics of hepatitis E virus in pigs: estimation from field data and effect of vaccination. Epidemics. 2012;4:86–92. doi: 10.1016/j.epidem.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Kanai Y, Tsujikawa M, Yunoki M, Nishiyama S, Ikuta K, Hagiwara K. Long-term shedding of hepatitis E virus in the feces of pigs infected naturally, born to sows with and without maternal antibodies. J Med Virol. 2010;82:69–76. doi: 10.1002/jmv.21647. [DOI] [PubMed] [Google Scholar]
- 41.Casas M, Cortés R, Pina S, Peralta B, Allepuz A, Cortey M, Casal J, Martín M. Longitudinal study of hepatitis E virus infection in Spanish farrow-to-finish swine herds. Vet Microbiol. 2011;148:27–34. doi: 10.1016/j.vetmic.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 42.de Deus N, Casas M, Peralta B, Nofrarias M, Pina S, Martin M, Segales J. Hepatitis E virus infection dynamics and organic distribution in naturally infected pigs in a farrow-to-finish farm. Vet Microbiol. 2008;132:19–28. doi: 10.1016/j.vetmic.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 43.dos Santos DR, Vitral CL, de Paula VS, Marchevsky RS, Lopes JF, Gaspar AM, Saddi TM, Júnior NC, Guimarães Fde R, Júnior JG, Ximenes LL, Souto FJ, Pinto MA. Serological and molecular evidence of hepatitis E virus in swine in Brazil. Vet J. 2009;182:474–480. doi: 10.1016/j.tvjl.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Klobasa F, Butler JE. Absolute and relative concentrations of immunoglobulins G, M, and A, and albumin in the lacteal secretion of sows of different lactation numbers. Am J Vet Res. 1987;48:176–182. [PubMed] [Google Scholar]
- 45.Sanford BJ, Dryman BA, Huang YW, Feagins AR, LeRoith T, Meng XJ. Prior infection of pigs with a genotype 3 swine hepatitis E virus (HEV) protects against subsequent challenges with homologous and heterologous genotypes 3 and 4 human HEV. Virus Res. 2011;159:17–22. doi: 10.1016/j.virusres.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernández-Barredo S, Galiana C, García A, Vega S, Gómez MT, Pérez-Gracia MT. Detection of hepatitis E virus shedding in feces of pigs at different stages of production using reverse transcription-polymerase chain reaction. J Vet Diagn Invest. 2006;18:462–465. doi: 10.1177/104063870601800506. [DOI] [PubMed] [Google Scholar]
- 47.Banks M, Heath GS, Grierson SS, King DP, Gresham A, Girones R, Widen F, Harrison TJ. Evidence for the presence of hepatitis E virus in pigs in the United Kingdom. Vet Rec. 2004;154:223–227. doi: 10.1136/vr.154.8.223. [DOI] [PubMed] [Google Scholar]
- 48.Bouwknegt M, Rutjes SA, Reusken CB, Stockhofe-Zurwieden N, Frankena K, de Jong MC, de Roda Husman AM, Poel WH. The course of hepatitis E virus infection in pigs after contact-infection and intravenous inoculation. BMC Vet Res. 2009;5:7. doi: 10.1186/1746-6148-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kasorndorkbua C, Guenette DK, Huang FF, Thomas PJ, Meng XJ, Halbur PG. Routes of transmission of swine hepatitis E virus in pigs. J Clin Microbiol. 2004;42:5047–5052. doi: 10.1128/JCM.42.11.5047-5052.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bouwknegt M, Engel B, Herremans MM, Widdowson MA, Worm HC, Koopmans MP, Frankena K, de Roda Husman AM, De Jong MC, Van Der Poel WH. Bayesian estimation of hepatitis E virus seroprevalence for populations with different exposure levels to swine in The Netherlands. Epidemiol Infect. 2008;136:567–576. doi: 10.1017/S0950268807008941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bouwknegt M, Lodder-Verschoor F, van der Poel WH, Rutjes SA, de Roda Husman AM. Hepatitis E virus RNA in commercial porcine livers in The Netherlands. J Food Prot. 2007;70:2889–2895. doi: 10.4315/0362-028X-70.12.2889. [DOI] [PubMed] [Google Scholar]
- 52.Casas M, Pina S, de Deus N, Peralta B, Martín M, Segalés J. Pigs orally inoculated with swine hepatitis E virus are able to infect contact sentinels. Vet Microbiol. 2009;138:78–84. doi: 10.1016/j.vetmic.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 53.Liang H, Su S, Deng S, Gu H, Ji F, Wang L, Liang C, Wang H, Zhang G. The prevalence of hepatitis E virus infections among swine, swine farmers and the general population in Guangdong province, China. PLoS One. 2014;9:e88106. doi: 10.1371/journal.pone.0088106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morozov VA, Morozov AV, Rotem A, Barkai U, Bornstein S, Denner J. Extended microbiological characterization of Göttingen minipigs in the context of xenotransplantation: detection and vertical transmission of hepatitis E virus. PLoS One. 2015;10:e0139893. doi: 10.1371/journal.pone.0139893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hosmillo M, Jeong YJ, Kim HJ, Park JG, Nayak MK, Alfajaro MM, Collantes TM, Park SJ, Ikuta K, Yunoki M, Kang MI, Park SI, Cho KO. Molecular detection of genotype 3 porcine hepatitis E virus in aborted fetuses and their sows. Arch Virol. 2010;155:1157–1161. doi: 10.1007/s00705-010-0679-z. [DOI] [PubMed] [Google Scholar]
- 56.Kasorndorkbua C, Thacker BJ, Halbur PG, Guenette DK, Buitenwerf RM, Royer RL, Meng XJ. Experimental infection of pregnant gilts with swine hepatitis E virus. Can J Vet Res. 2003;67:303–306. [PMC free article] [PubMed] [Google Scholar]
- 57.Di Bartolo I, Ponterio E, Castellini L, Ostanello F, Ruggeri FM. Viral and antibody HEV prevalence in swine at slaughterhouse in Italy. Vet Microbiol. 2011;149:330–338. doi: 10.1016/j.vetmic.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 58.Pavio N, Merbah T, Thébault A. Frequent hepatitis E virus contamination in food containing raw pork liver, France. Emerg Infect Dis. 2014;20:1925–1927. doi: 10.3201/eid2011.140891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Emerson SU, Arankalle VA, Purcell RH. Thermal stability of hepatitis E virus. J Infect Dis. 2005;192:930–933. doi: 10.1086/432488. [DOI] [PubMed] [Google Scholar]
- 60.Feagins AR, Opriessnig T, Guenette DK, Halbur PG, Meng XJ. Inactivation of infectious hepatitis E virus present in commercial pig livers sold in local grocery stores in the United States. Int J Food Microbiol. 2008;123:32–37. doi: 10.1016/j.ijfoodmicro.2007.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barnaud E, Rogée S, Garry P, Rose N, Pavio N. Thermal inactivation of infectious hepatitis E virus in experimentally contaminated food. Appl Environ Microbiol. 2012;78:5153–5159. doi: 10.1128/AEM.00436-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dorjee S, Revie CW, Poljak Z, McNab WB, Sanchez J. Network analysis of swine shipments in Ontario, Canada, to support disease spread modelling and risk-based disease management. Prev Vet Med. 2013;112:118–127. doi: 10.1016/j.prevetmed.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 63.Nantel-Fortier N, Letellier A, Lachapelle V, Fravalo P, L’Homme Y, Brassard J. Detection and phylogenetic analysis of the hepatitis E virus in a canadian swine production network. Food Environ Virol. 2016;8:296–304. doi: 10.1007/s12560-016-9252-6. [DOI] [PubMed] [Google Scholar]
- 64.Casas M, Pina S, Peralta B, Mateu E, Casal J, Martín M. Comparison of muscle fluid and serum for detection of antibodies against hepatitis E virus in slaughter pigs. Vet J. 2011;190:179–180. doi: 10.1016/j.tvjl.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 65.Rose N, Boutrouille A, Fablet C, Madec F, Eloit M, Pavio N. The use of Bayesian methods for evaluating the performance of a virus-like particles-based ELISA for serology of hepatitis E virus infection in swine. J Virol Methods. 2010;163:329–335. doi: 10.1016/j.jviromet.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 66.Gardinali NR, Barry AF, da Silva PF, de Souza C, Alfieri AF, Alfieri AA. Molecular detection and characterization of hepatitis E virus in naturally infected pigs from Brazilian herds. Res Vet Sci. 2012;93:1515–1519. doi: 10.1016/j.rvsc.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Berto A, Martelli F, Grierson S, Banks M. Hepatitis E virus in pork food chain, United Kingdom, 2009–2010. Emerg Infect Dis. 2012;18(8):1358–1360. doi: 10.3201/eid1808.111647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Di Bartolo I, Diez-Valcarce M, Vasickova P, Kralik P, Hernandez M, Angeloni G, Ostanello F, Bouwknegt M, Rodríguez-Lázaro D, Pavlik I, Ruggeri FM. Hepatitis E virus in pork production chain in Czech Republic, Italy, and Spain, 2010. Emerg Infect Dis. 2012;18:1282–1289. doi: 10.3201/eid1808.111783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.ANSES (2013) Opinion of the French Agency for Food, Environmental and Occupational Health & Safety concerning the “Request to assess the risks related to contamination of delicatessen meats products derived from raw pork liver with hepatitis E virus (HEV)”. ANSES Opinion, Request No. 2012-SA-0012 https://www.anses.fr/fr/system/files/BIORISK2012sa0012EN.pdf
- 70.Gardinali NR, Barry AF, Otonel RA, Alfieri AF, Alfieri AA. Hepatitis E virus in liver and bile samples from slaughtered pigs of Brazil. Mem Inst Oswaldo Cruz. 2012;107:935–939. doi: 10.1590/S0074-02762012000700016. [DOI] [PubMed] [Google Scholar]
- 71.de Souza AJ, Gomes-Gouvêa MS, Soares Mdo C, Pinho JR, Malheiros AP, Carneiro LA, dos Santos DR, Pereira WL. HEV infection in swine from Eastern Brazilian Amazon: evidence of co-infection by different subtypes. Comp Immunol Microbiol Infect Dis. 2012;35:477–485. doi: 10.1016/j.cimid.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 72.Temmam S, Besnard L, Andriamandimby SF, Foray C, Rasamoelina-Andriamanivo H, Héraud JM, Cardinale E, Dellagi K, Pavio N, Pascalis H, Porphyre V. High prevalence of hepatitis E in humans and pigs and evidence of genotype-3 virus in swine, Madagascar. Am J Trop Med Hyg. 2013;88:329–338. doi: 10.4269/ajtmh.2012.12-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Paula S, Wiele M, Mbunkah AH, Daniel AM, Kingsley MT, Schmidt-Chanasit J. Hepatitis E virus genotype 3 strains in domestic pigs, Cameroon. Emerg Infect Dis. 2013;19:666–668. doi: 10.3201/eid1904.121634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mykytczuk O, Harlow J, Bidawid S, Corneau N, Nasheri N. Prevalence and molecular characterization of the hepatitis E virus in retail pork products marketed in Canada. Food Environ Virol. 2017 doi: 10.1007/s12560-017-9281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yazaki Y, Mizuo H, Takahashi M, Nishizawa T, Sasaki N, Gotanda Y, Okamoto H. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J Gen Virol. 2003;84:2351–2357. doi: 10.1099/vir.0.19242-0. [DOI] [PubMed] [Google Scholar]
- 76.Feagins AR, Opriessnig T, Guenette DK, Halbur PG, Meng XJ. Detection and characterization of infectious hepatitis E virus from commercial pig livers sold in local grocery stores in the USA. J Gen Virol. 2007;88:912–917. doi: 10.1099/vir.0.82613-0. [DOI] [PubMed] [Google Scholar]
- 77.Wenzel JJ, Preiss J, Schemmerer M, Huber B, Plentz A, Jilg W. Detection of hepatitis E virus (HEV) from porcine livers in Southeastern Germany and high sequence homology to human HEV isolates. J Clin Virol. 2011;52:50–54. doi: 10.1016/j.jcv.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 78.Heldt FH, Staggmeier R, Gularte JS, Demoliner M, Henzel A, Spilki FR. Hepatitis E virus in surface water, sediments, and pork products marketed in Southern Brazil. Food Environ Virol. 2016;8:200–205. doi: 10.1007/s12560-016-9243-7. [DOI] [PubMed] [Google Scholar]
- 79.Clayson ET, Innis BL, Myint KS, Narupiti S, Vaughn DW, Giri S, Ranabhat P, Shrestha MP. Detection of hepatitis E virus infections among domestic swine in the Kathmandu Valley of Nepal. Am J Trop Med Hyg. 1995;53:228–232. doi: 10.4269/ajtmh.1995.53.228. [DOI] [PubMed] [Google Scholar]
- 80.Wu JC, Chen CM, Chiang TY, Sheen IJ, Chen JY, Tsai WH, Huang YH, Lee SD. Clinical and epidemiological implications of swine hepatitis E virus infection. J Med Virol. 2000;60:166–171. doi: 10.1002/(SICI)1096-9071(200002)60:2<166::AID-JMV10>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 81.Garkavenko O, Obriadina A, Meng J, Anderson DA, Benard HJ, Schroeder BA, Khudyakov YE, Fields HA, Croxson MC. Detection and characterisation of swine hepatitis E virus in New Zealand. J Med Virol. 2001;65:525–529. doi: 10.1002/jmv.2067. [DOI] [PubMed] [Google Scholar]
- 82.Arankalle VA, Joshi MV, Kulkarni AM, Gandhe SS, Chobe LP, Rautmare SS, Mishra AC, Padbidri VS. Prevalence of anti-hepatitis E virus antibodies in different Indian animal species. J Viral Hepat. 2001;8:223–227. doi: 10.1046/j.1365-2893.2001.00290.x. [DOI] [PubMed] [Google Scholar]
- 83.Yoo D, Willson P, Pei Y, Hayes MA, Deckert A, Dewey CE, Friendship RM, Yoon Y, Gottschalk M, Yason C, Giulivi A. Prevalence of hepatitis E virus antibodies in Canadian swine herds and identification of a novel variant of swine hepatitis E virus. Clin Diagn Lab Immunol. 2001;8:1213–1219. doi: 10.1128/CDLI.8.6.1213-1219.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Withers MR, Correa MT, Morrow M, Stebbins ME, Seriwatana J, Webster WD, Boak MB, Vaughn DW. Antibody levels to hepatitis E virus in North Carolina swine workers, non-swine workers, swine, and murids. Am J Trop Med Hyg. 2002;66:384–388. doi: 10.4269/ajtmh.2002.66.384. [DOI] [PubMed] [Google Scholar]
- 85.Huang FF, Haqshenas G, Guenette DK, Halbur PG, Schommer SK, Pierson FW, Toth TE, Meng XJ. Detection by reverse transcription-PCR and genetic characterization of field isolates of swine hepatitis E virus from pigs in different geographic regions of the United States. J Clin Microbiol. 2002;40:1326–1332. doi: 10.1128/JCM.40.4.1326-1332.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Choi IS, Kwon HJ, Shin NR, Yoo HS. Identification of swine hepatitis E virus (HEV) and prevalence of anti-HEV antibodies in swine and human populations in Korea. J Clin Microbiol. 2003;41:3602–3608. doi: 10.1128/JCM.41.8.3602-3608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wibawa ID, Muljono DH, Mulyanto Suryadarma IG, Tsuda F, Takahashi M, Nishizawa T, Okamoto H. Prevalence of antibodies to hepatitis E virus among apparently healthy humans and pigs in Bali, Indonesia: identification of a pig infected with a genotype 4 hepatitis E virus. J Med Virol. 2004;73:38–44. doi: 10.1002/jmv.20059. [DOI] [PubMed] [Google Scholar]
- 88.Vitral CL, Pinto MA, Lewis-Ximenez LL, Khudyakov YE, dos Santos DR, Gaspar AM. Serological evidence of hepatitis E virus infection in different animal species from the Southeast of Brazil. Mem Inst Oswaldo Cruz. 2005;100:117–122. doi: 10.1590/S0074-02762005000200003. [DOI] [PubMed] [Google Scholar]
- 89.Fernández-Barredo S, Galiana C, García A, Gomez-Muñoz MT, Vega S, Rodríguez-Iglesias MA, Pérez-Gracia MT. Prevalence and genetic characterization of hepatitis E virus in paired samples of feces and serum from naturally infected pigs. Can J Vet Res. 2007;71:236–240. [PMC free article] [PubMed] [Google Scholar]
- 90.Jung K, Kang B, Song DS, Chae C. Prevalence and genotyping of hepatitis E virus in swine population in Korea between 1995 and 2004: a retrospective study. Vet J. 2007;173:683–687. doi: 10.1016/j.tvjl.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 91.Kim SE, Kim MY, Kim DG, Song YJ, Jeong HJ, Lee SW, Lee JB, Park SY, Song CS, Oh SJ, Yoo HS, Choi IS. Determination of fecal shedding rates and genotypes of swine hepatitis E virus (HEV) in Korea. J Vet Med Sci. 2008;70:1367–1371. doi: 10.1292/jvms.70.1367. [DOI] [PubMed] [Google Scholar]
- 92.Seminati C, Mateu E, Peralta B, de Deus N, Martin M. Distribution of hepatitis E virus infection and its prevalence in pigs on commercial farms in Spain. Vet J. 2008;175:130–132. doi: 10.1016/j.tvjl.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 93.McCreary C, Martelli F, Grierson S, Ostanello F, Nevel A, Banks M. Excretion of hepatitis E virus by pigs of different ages and its presence in slurry stores in the United Kingdom. Vet Rec. 2008;163:261–265. doi: 10.1136/vr.163.9.261. [DOI] [PubMed] [Google Scholar]
- 94.Ning H, Yu S, Zhu Y, Dong S, Yu R, Shen S, Niu Z, Li Z. Genotype 3 hepatitis E has been widespread in pig farms of Shanghai suburbs. Vet Microbiol. 2008;126:257–263. doi: 10.1016/j.vetmic.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 95.Zhang W, Shen Q, Mou J, Gong G, Yang Z, Cui L, Zhu J, Ju G, Hua X. Hepatitis E virus infection among domestic animals in eastern China. Zoonoses Public Health. 2008;55:291–298. doi: 10.1111/j.1863-2378.2008.01136.x. [DOI] [PubMed] [Google Scholar]
- 96.Li X, Zhao C, Harrison TJ, Song A, Fan J, Zhang J, Wang Y. Investigation of hepatitis E virus infection in swine from Hunan province, China. J Med Virol. 2008;80:1391–1396. doi: 10.1002/jmv.21246. [DOI] [PubMed] [Google Scholar]
- 97.Ward P, Muller P, Letellier A, Quessy S, Simard C, Trottier YL, Houde A, Brassard J. Molecular characterization of hepatitis E virus detected in swine farms in the province of Quebec. Can J Vet Res. 2008;72:27–31. [PMC free article] [PubMed] [Google Scholar]
- 98.Yan Y, Zhang W, Shen Q, Cui L, Hua X. Prevalence of four different subgenotypes of genotype 4 hepatitis E virus among swine in the Shanghai area of China. Acta Vet Scand. 2008;50:12. doi: 10.1186/1751-0147-50-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu JN, Kim MY, Kim DG, Kim SE, Lee JB, Park SY, Song CS, Shin HC, Seo KH, Choi IS. Prevalence of hepatitis E virus and sapovirus in post-weaning pigs and identification of their genetic diversity. Arch Virol. 2008;153:739–742. doi: 10.1007/s00705-008-0046-5. [DOI] [PubMed] [Google Scholar]
- 100.Reuter G, Fodor D, Forgách P, Kátai A, Szucs G. Characterization and zoonotic potential of endemic hepatitis E virus (HEV) strains in humans and animals in Hungary. J Clin Virol. 2009;44:277–281. doi: 10.1016/j.jcv.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 101.Shao ZJ, Li JH, Zheng YJ, Zhang JX, Ma YH, Ma WT, Jiang QW, Dang RL. Epidemiological screening for hepatitis E virus in bile specimens from livestock in northwest China. J Clin Microbiol. 2009;47:814–816. doi: 10.1128/JCM.01309-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Masia G, Orru G, Liciardi M, Desogus G, Coppola RC, Murru V, Argiolas M, Orru G. Evidence of hepatitis E virus (HEV) infection in human and pigs in Sardinia, Italy. J Prev Med Hyg. 2009;50:227–231. [PubMed] [Google Scholar]
- 103.Sakano C, Morita Y, Shiono M, Yokota Y, Mokudai T, Sato-Motoi Y, Noda A, Nobusawa T, Sakaniwa H, Nagai A, Kabeya H, Maruyama S, Yamamoto S, Sato H, Kimura H. Prevalence of hepatitis E virus (HEV) infection in wild boars (Sus scrofa leucomystax) and pigs in Gunma Prefecture, Japan. J Vet Med Sci. 2009;71:21–25. doi: 10.1292/jvms.71.21. [DOI] [PubMed] [Google Scholar]
- 104.Forgách P, Nowotny N, Erdélyi K, Boncz A, Zentai J, Szucs G, Reuter G, Bakonyi T. Detection of hepatitis E virus in samples of animal origin collected in Hungary. Vet Microbiol. 2010;143:106–116. doi: 10.1016/j.vetmic.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 105.Di Martino B, Di Profio F, Martella V, Di Felice E, Di Francesco CE, Ceci C, Marsilio F. Detection of hepatitis E virus in slaughtered pigs in Italy. Arch Virol. 2010;155:103–106. doi: 10.1007/s00705-009-0544-0. [DOI] [PubMed] [Google Scholar]
- 106.Kaba M, Colson P, Musongela JP, Tshilolo L, Davoust B. Detection of hepatitis E virus of genotype 3 in a farm pig in Kinshasa (Democratic Republic of the Congo) Infect Genet Evol. 2010;10:154–157. doi: 10.1016/j.meegid.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 107.Geng JB, Fu HW, Wang L, Wang XJ, Guan JM, Chang YB, Li LJ, Zhu YH, Zhuang H, Liu QH, Peng XC. Hepatitis E virus (HEV) genotype and the prevalence of anti-HEV in 8 species of animals in the suburbs of Beijing. Zhonghua Liu Xing Bing Xue Za Zhi. 2010;31:47–50. [PubMed] [Google Scholar]
- 108.Breum SØ, Hjulsager CK, de Deus N, Segales J, Larsen LE. Hepatitis E virus is highly prevalent in the Danish pig population. Vet Microbiol. 2010;146:144–149. doi: 10.1016/j.vetmic.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 109.Geng Y, Wang C, Zhao C, Yu X, Harrison TJ, Tian K, Wang Y. Serological prevalence of hepatitis E virus in domestic animals and diversity of genotype 4 hepatitis E virus in China. Vector Borne Zoonotic Dis. 2010;10:765–770. doi: 10.1089/vbz.2009.0168. [DOI] [PubMed] [Google Scholar]
- 110.Xia YG, Hu AQ, Lu YH, Liu HY, Zheng YJ. Molecular-epidemiological research on hepatitis E virus among commercial swine herds in Anqing, China. Fudan Univ J Med Sci. 2010;37:676–679. [Google Scholar]
- 111.Hakze-van der Honing RW, van Coillie E, Antonis AF, van der Poel WH. First isolation of hepatitis E virus genotype 4 in Europe through swine surveillance in the Netherlands and Belgium. PLoS One. 2011;6:e22673. doi: 10.1371/journal.pone.0022673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dell’Amico MC, Cavallo A, Gonzales JL, Bonelli SI, Valda Y, Pieri A, Segund H, Ibanez R, Mantella A, Bartalesi F, Tolari F, Bartoloni A. Hepatitis E virus genotype 3 in humans and swine, Bolivia. Emerg Infect Dis. 2011;17:1488–1490. doi: 10.3201/eid1708.100769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaba M, Davoust B, Cabre O, Colson P. Hepatitis E virus genotype 3f in pigs in New Caledonia. Aust Vet J. 2011;89:496–499. doi: 10.1111/j.1751-0813.2011.00848.x. [DOI] [PubMed] [Google Scholar]
- 114.Steyer A, Naglic T, Mocilnik T, Poljsak-Prijatelj M, Poljak M. Hepatitis E virus in domestic pigs and surface waters in Slovenia: prevalence and molecular characterization of a novel genotype 3 lineage. Infect Genet Evol. 2011;11:1732–1737. doi: 10.1016/j.meegid.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 115.dos Santos DR, de Paula VS, de Oliveira JM, Marchevsky RS, Pinto MA. Hepatitis E virus in swine and effluent samples from slaughterhouses in Brazil. Vet Microbiol. 2011;149:236–241. doi: 10.1016/j.vetmic.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 116.Vivek R, Kang G. Hepatitis E virus infections in swine and swine handlers in Vellore, Southern India. Am J Trop Med Hyg. 2011;84:647–649. doi: 10.4269/ajtmh.2011.10-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Conlan JV, Jarman RG, Vongxay K, Chinnawirotpisan P, Melendrez MC, Fenwick S, Thompson RC, Blacksell SD. Hepatitis E virus is prevalent in the pig population of Lao People’s Democratic Republic and evidence exists for homogeneity with chinese genotype 4 human isolates. Infect Genet Evol. 2011;11:1306–1311. doi: 10.1016/j.meegid.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 118.Shen Q, Ren R, Zhang W, Yang Z, Yang S, Chen Y, Cui L, Hua X. Prevalence of hepatitis E virus and porcine caliciviruses in pig farms of Guizhou province, China. Hepat Mon. 2011;11:459–463. [PMC free article] [PubMed] [Google Scholar]
- 119.Martinelli N, Luppi A, Cordioli P, Lombardi G, Lavazza A. Prevalence of hepatitis E virus antibodies in pigs in Northern Italy. Infect Ecol Epidemiol. 2011 doi: 10.3402/iee.v1i0.7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Berto A, Mesquita JR, Hakze-van der Honing R, Nascimento MS, van der Poel WH. Detection and characterization of hepatitis E virus in domestic pigs of different ages in Portugal. Zoonoses Public Health. 2012;59:477–481. doi: 10.1111/j.1863-2378.2012.01488.x. [DOI] [PubMed] [Google Scholar]
- 121.Wacheck S, Werres C, Mohn U, Dorn S, Soutschek E, Fredriksson-Ahomaa M, Martlbauer E. Detection of IgM and IgG against hepatitis E virus in serum and meat juice samples from pigs at slaughter in Bavaria, Germany. Foodborne Pathog Dis. 2012;9:655–660. doi: 10.1089/fpd.2012.1141. [DOI] [PubMed] [Google Scholar]
- 122.Lipej Z, Novosel D, Vojta L, Roic B, Simpraga M, Vojta A. Detection and characterisation of hepatitis E virus in naturally infected swine in Croatia. Acta Vet Hung. 2013;61:517–528. doi: 10.1556/AVet.2013.031. [DOI] [PubMed] [Google Scholar]
- 123.de la Caridad Montalvo Villalba M, Owot JC, Benedito EC, Corredor MB, Flaquet PP, Frometa SS, Wong MS, Rodriguez Lay Lde L. Hepatitis E virus genotype 3 in humans and swine, Cuba. Infect Genet Evol. 2013;14:335–339. doi: 10.1016/j.meegid.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 124.Kantala T, Oristo S, Heinonen M, von Bonsdorff CH, Maunula L. A longitudinal study revealing hepatitis E virus infection and transmission at a swine test station. Res Vet Sci. 2013;95:1255–1261. doi: 10.1016/j.rvsc.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 125.Machnowska P, Ellerbroek L, Johne R. Detection and characterization of potentially zoonotic viruses in faeces of pigs at slaughter in Germany. Vet Microbiol. 2014;168:60–68. doi: 10.1016/j.vetmic.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 126.Thiry D, Mauroy A, Saegerman C, Thomas I, Wautier M, Miry C, Czaplicki G, Berkvens D, Praet N, van der Poel W, Cariolet R, Brochier B, Thiry E. Estimation of hepatitis E virus (HEV) pig seroprevalence using ELISA and Western blot and comparison between human and pig HEV sequences in Belgium. Vet Microbiol. 2014;172:407–414. doi: 10.1016/j.vetmic.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 127.da Costa Lana MV, Gardinali NR, da Cruz RA, Lopes LL, Silva GS, Caramori Junior JG, de Oliveira AC, de Almeida Souza M, Colodel EM, Alfieri AA, Pescador CA. Evaluation of hepatitis E virus infection between different production systems of pigs in Brazil. Trop Anim Health Prod. 2014;46:399–404. doi: 10.1007/s11250-013-0503-3. [DOI] [PubMed] [Google Scholar]
- 128.Aniţă A, Gorgan L, Aniţă D, Oşlobanu L, Pavio N, Savuţa G. Evidence of hepatitis E infection in swine and humans in the East Region of Romania. Int J Infect Dis. 2014;29:232–237. doi: 10.1016/j.ijid.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 129.Owolodun OA, Gerber PF, Giménez-Lirola LG, Kwaga JK, Opriessnig T. First report of hepatitis E virus circulation in domestic pigs in Nigeria. Am J Trop Med Hyg. 2014;91:699–704. doi: 10.4269/ajtmh.14-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang XJ, Zhao Q, Jiang FL, Liu BY, Zhao JN, Dang L, Sun YN, Mu Y, Xiao SQ, Wang CB, Hsu WH, Liu L, Widén F, Zhou EM. Genetic characterization and serological prevalence of swine hepatitis E virus in Shandong province, China. Vet Microbiol. 2014;172:415–424. doi: 10.1016/j.vetmic.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 131.Shu X, Duan X, Song C, Li J, Jiang L, Yin G, Li W. Genetic heterogeneity of swine hepatitis E virus isolates from Yunnan province, China in 2011–2012. Virol J. 2014;11:162. doi: 10.1186/1743-422X-11-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Burri C, Vial F, Ryser-Degiorgis MP, Schwermer H, Darling K, Reist M, Wu N, Beerli O, Schöning J, Cavassini M, Waldvogel A. Seroprevalence of hepatitis E virus in domestic pigs and wild boars in Switzerland. Zoonoses Public Health. 2014;61:537–544. doi: 10.1111/zph.12103. [DOI] [PubMed] [Google Scholar]
- 133.Monini M, Di Bartolo I, Ianiro G, Angeloni G, Magistrali CF, Ostanello F, Ruggeri FM. Detection and molecular characterization of zoonotic viruses in swine fecal samples in Italian pig herds. Arch Virol. 2015;160:2547–2556. doi: 10.1007/s00705-015-2538-4. [DOI] [PubMed] [Google Scholar]
- 134.Ivanova A, Tefanova V, Reshetnjak I, Kuznetsova T, Geller J, Lundkvist A, Janson M, Neare K, Velström K, Jokelainen P, Lassen B, Hütt P, Saar T, Viltrop A, Golovljova I. Hepatitis E virus in domestic pigs, wild boars, pig farm workers, and hunters in Estonia. Food Environ Virol. 2015;7:403–412. doi: 10.1007/s12560-015-9210-8. [DOI] [PubMed] [Google Scholar]
- 135.Costanzo N, Sarno E, Peretti V, Ciambrone L, Casalinuovo F, Santoro A. Serological and molecular investigation of swine hepatitis E virus in pigs raised in Southern Italy. J Food Prot. 2015;78:2099–2102. doi: 10.4315/0362-028X.JFP-15-159. [DOI] [PubMed] [Google Scholar]
- 136.Liu X, Saito M, Sayama Y, Suzuki E, Malbas FF, Jr, Galang HO, Furuse Y, Saito M, Li T, Suzuki A, Oshitani H. Seroprevalence and molecular characteristics of hepatitis E virus in household-raised pig population in the Philippines. BMC Vet Res. 2015;11:11. doi: 10.1186/s12917-015-0322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.O’Connor M, Roche SJ, Sammin D. Seroprevalence of hepatitis E virus infection in the Irish pig population. Ir Vet J. 2015;68:8. doi: 10.1186/s13620-015-0036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Caruso C, Peletto S, Rosamilia A, Modesto P, Chiavacci L, Sona B, Balsamelli F, Ghisetti V, Acutis PL, Pezzoni G, Brocchi E, Vitale N, Masoero L. Hepatitis E virus: a cross-sectional serological and virological study in pigs and humans at zoonotic risk within a high-density pig farming area. Transbound Emerg Dis. 2016 doi: 10.1111/tbed.12533. [DOI] [PubMed] [Google Scholar]
- 139.Merino-Ramos T, Martín-Acebes MA, Casal J, Saiz JC, Loza-Rubio E. Prevalence of hepatitis E virus (HEV) antibodies in mexican pigs. Food Environ Virol. 2016;8:156–159. doi: 10.1007/s12560-016-9231-y. [DOI] [PubMed] [Google Scholar]
- 140.Wu JC, Chen CM, Chiang TY, Tsai WH, Jeng WJ, Sheen IJ, Lin CC, Meng XJ. Spread of hepatitis E virus among different-aged pigs: 2-year survey in Taiwan. J Med Virol. 2002;66:488–492. doi: 10.1002/jmv.2170. [DOI] [PubMed] [Google Scholar]
- 141.Cooper K, Huang FF, Batista L, Rayo CD, Bezanilla JC, Toth TE, Meng XJ. Identification of genotype 3 hepatitis E virus (HEV) in serum and fecal samples from pigs in Thailand and Mexico, where genotype 1 and 2 HEV strains are prevalent in the respective human populations. J Clin Microbiol. 2005;43:1684–1688. doi: 10.1128/JCM.43.4.1684-1688.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nakai I, Kato K, Miyazaki A, Yoshii M, Li TC, Takeda N, Tsunemitsu H, Ikeda H. Different fecal shedding patterns of two common strains of hepatitis E virus at three Japanese swine farms. Am J Trop Med Hyg. 2006;75:1171–1177. [PubMed] [Google Scholar]
- 143.Leblanc D, Ward P, Gagné MJ, Poitras E, Müller P, Trottier YL, Simard C, Houde A. Presence of hepatitis E virus in a naturally infected swine herd from nursery to slaughter. Int J Food Microbiol. 2007;117:160–166. doi: 10.1016/j.ijfoodmicro.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 144.de Deus N, Seminati C, Pina S, Mateu E, Martín M, Segalés J. Detection of hepatitis E virus in liver, mesenteric lymph node, serum, bile and faeces of naturally infected pigs affected by different pathological conditions. Vet Microbiol. 2007;119:105–114. doi: 10.1016/j.vetmic.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 145.Zhang W, Yang S, Ren L, Shen Q, Cui L, Fan K, Huang F, Kang Y, Shan T, Wei J, Xiu H, Lou Y, Liu J, Yang Z, Zhu J, Hua X. Hepatitis E virus infection in central China reveals no evidence of cross-species transmission between human and swine in this area. PLoS One. 2009;4:e8156. doi: 10.1371/journal.pone.0008156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kaba M, Davoust B, Marié JL, Barthet M, Henry M, Tamalet C, Raoult D, Colson P. Frequent transmission of hepatitis E virus among piglets in farms in Southern France. J Med Virol. 2009;81:1750–1759. doi: 10.1002/jmv.21553. [DOI] [PubMed] [Google Scholar]
- 147.Martelli F, Toma S, Di Bartolo I, Caprioli A, Ruggeri FM, Lelli D, Bonci M, Ostanello F. Detection of hepatitis E Virus (HEV) in italian pigs displaying different pathological lesions. Res Vet Sci. 2010;88:492–496. doi: 10.1016/j.rvsc.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 148.Feng R, Zhao C, Li M, Harrison TJ, Qiao Z, Feng Y, Ma Z, Wang Y. Infection dynamics of hepatitis E virus in naturally infected pigs in a chinese farrow-to-finish farm. Infect Genet Evol. 2011;11:1727–1731. doi: 10.1016/j.meegid.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 149.Utsumi T, Hayashi Y, Lusida MI, Amin M, Soetjipto Hendra A, Soetjiningsih Yano Y, Hotta H. Prevalence of hepatitis E virus among swine and humans in two different ethnic communities in Indonesia. Arch Virol. 2011;156:689–693. doi: 10.1007/s00705-010-0883-x. [DOI] [PubMed] [Google Scholar]
- 150.Thiry D, Mauroy A, Brochier B, Thomas I, Miry C, Czaplicki G, Thiry E (2012) Hepatitis E virus infection in domestic swine in Belgium. XIIIe congress of the International Society for Veterinary Epidemiology and Economics (ISVEE XIII), Maastricht, Netherlands, 2012
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. HEV serological and virological prevalence in the pig population at farm and individual levels. Farm-scale prevalence figures were reported in 34 studies. Farm-scale seroprevalence ranged from 30 to 98%, while farm-scale virological prevalence ranged from 10 to 100%. Individual prevalence figures were reported in 82 studies. Individual seroprevalence ranged from 8 to 93%, whereas individual virological prevalence ranged from 1 to 89%.
Additional file 2. Evolution of HEV RNA prevalence and anti-HEV antibody prevalence according to pig age. Thirty-seven studies explored the variation of HEV virological and serological prevalence with the pig age. m: months; w: weeks; d: days.


