Abstract
Background
Critically ill patients in intensive care face hazardous conditions. Among these, acute kidney injury (AKI) is frequently seen as a result of sepsis. Early diagnosis of kidney injury is of the utmost importance in the guidance of interventions or avoidance of treatment-induced kidney injury. On these grounds, we searched for markers that could indicate proximal tubular cell injury.
Methods
Urine samples of 90 patients admitted to the intensive or intermediate care unit were collected over 2 to 5 days. The biomarker neprilysin (NEP) was investigated in urine using several methods such as dot blot, ELISA and immunofluorescence of urinary casts. Fifty-five healthy donors acted as controls.
Results
NEP was highly significantly elevated in the urine of patients who suffered AKI according to the KDIGO criteria in comparison to healthy controls. It was also found to be elevated in ICU patients without overt signs of AKI according to serum creatinine changes, however they were suffering from potential nephrotoxic insults. According to our findings, urinary NEP is indicative of epithelial cell alterations at the proximal tubule. This was elaborated in ICU patients when ghost fragments and NEP+ microvesicles were observed in urinary sediment cytopreparations. Furthermore, NEP+ immunofluorescence of healthy kidney tissue showed staining at the proximal tubules.
Conclusions
NEP, a potential marker for proximal tubular epithelia, can be measured in urine. This does not originate from leakage of elevated serum levels, but indicates proximal tubular cell alterations such as brush border severing, which can heal in most cases.
Keywords: Acute renal injury, Biomarkers, Proximal tubular injury
Background
Acute kidney injury (AKI) is a common complication of serious infections such as sepsis. About 20–50% of patients suffering from the disease suffer AKI in various stages depending on the severity of the septic symptoms [1, 2]. In a minor group of these patients it may lead to end stage kidney disease with the necessity of renal replacement therapy (RRT). However, in most cases patients regain kidney function when antimicrobial treatment is initiated and hemodynamic parameters are stabilized rapidly.
The predominant targets of cellular injury are proximal epithelial cells, although glomerular lesions have also been described [3]. Loss of brush border and disintegration of tubular cells have been demonstrated [4]. The clinical observation that a phase of oliguria is followed by a polyuric episode [5] preceding recovery of kidney function is reflective of a process of cellular repair mechanisms, especially at the tubular compartment [6–8].
Major kidney insults result in changes of fractional excretion of sodium (FeNa), a decrease in urine output and finally a rise in serum creatinine (sCr). These parameters are still the most widely used diagnostic indicators of acute kidney injury [9]. Nevertheless, laboratory findings such as serum creatinine indicate kidney damage due to an injury that occurred 12–24 h earlier. It represents loss of more than half of the glomerular filtration rate (GFR) [10]. The lack of diagnostic indicators for early phases of tubular cell injury, ideally detectable in urine, represents a challenge for biomarker development. A huge array of urine proteomic data has been provided in the past years [11–15]. KIM-1 [16], NGAL [17] and a MALDI MS-based platform was tested for early detection for AKI [15]. Furthermore IGFBP7 [18] and TIMP-2 [19] are simply examples which investigators had been testing for their specificity and clinical value in detecting the extent of kidney injuries. However, some of them are associated with drawbacks. The NephroCheck measuring TIMP2 and IGFBP7 has been developed very recently as a rapid bedside diagnostic tool. This yielded promising results in validation studies [19, 20].
Inspired by the rapid pace of research and the development of specific inhibitors [21, 22], we selected neprilysin, also called neutral endopeptidase (NEP) representing a single-pass membrane glycoprotein with zinc-dependent endopeptidase activity and a short cytosolic tail [23], as a promising diagnostic marker. The expression in the brush border of proximal tubular cells, the most vulnerable stretch of epithelial cells, was one of the main motives to start testing its occurrence in urine [24]. In addition this protein was found among many others in urinary exosomes [25].
Furthermore, its involvement in physiological processes such as blood pressure regulation and the development of inhibitors has brought this protein to the forefront of medical interest [26, 27]. As a marker for kidney function that is measured in urine in patients undergoing cardiac surgery it has been shown to significantly increase 3–36 h post-surgical intervention when tubular damage was thought to occur. It was thus hypothesized that NEP constitutes an indicator of early tubular injury. However, the study had been performed in the specific clinical setting of cardiac surgery [28].
Following this line of thought, we collected urine from critically ill and sepsis patients over a time period of 2–5 days in order to evaluate whether this diagnostic marker can be detected in urine under these conditions. In addition, urinary sediment has been investigated using immunofluorescence for NEP positive tubular cells and cellular fragments.
Methods
Study population
Critically ill patients (n = 90) fulfilling sepsis criteria [29] at the Intensive Care Unit (ICU) Department of Medicine III and the Department of Nephrology, Medical University of Vienna have been continuously enrolled in this study between 2009 and 2013. Informed consent was obtained from the participants. Inclusion criteria were defined as age above 18 years and admission to intensive or intermediate care of the Department of Medicine III. End Stage Renal Disease (ESRD) with the need for renal replacement therapy constituted an exclusion criterion, whereas patients who had had kidney transplantation with or without delayed graft function were included (n = 6). Participation in another pharmacotherapeutic study requiring blood or other sampling was an exclusion criterion. The date and time of study enrollment was defined as 1, with the first sample being taken at this point in time.
Plasma aliquots of 21 patients enrolled between November 2011 and November 2012 were kept frozen at −80 °C on account of the continuous research project regarding AKI-specific biomarkers. Plasma samples of these patients were thawed and analyzed with the sole aim of answering the question of whether urine NEP originates from leakage via the glomerular barrier or from tubular compartments. A time-matched plasma/urine ratio was established for these patients, however, the main goal of this study was to investigate urinary neprilysin in critically ill patients.
Patients’ clinical data, demographics, medical history, medication and laboratory data were recorded from hospital files and databases of the general hospital of Vienna. Patients were subdivided into two groups (AKI vs. No AKI) and AKI was graded according to the KDIGO guidelines [30]. Fifty-five healthy donors served as controls. Their urine was obtained without the presence of a catheter. The study was approved by the local Ethics Committee of the Medical University of Vienna.
Sample collection
All patients had an indwelling Foley catheter and urine specimens were collected from the chamber which represented fluid no older than 2 h. Samples were obtained at an interval of 24 h over 2 to 5 consecutive days, with the exception of the second sample which was obtained 8–12 h following the first sample. Serum creatinine was evaluated at exactly the same time point when NEP was measured. Within 1 h of collection, samples were centrifuged at 3000 RPM and the resultant supernatant was immediately stored frozen at −80 °C for further analysis in several aliquots.
Selecting proteins to be analysed
Initial analyses comprised the gene product of C20orf3 (chromosome 20 open reading frame 3), C6orf62 (chromosome 6 open reading frame 62) and CD133 (prominin). NEP could be verified by searching the human protein atlas for proteins expressed in the proximal kidney tubule [31]. Similarly it has been previously demonstrated that NEP antibodies could specifically select proximal epithelial tubular cells (PTC) [32].
Cytospin preparation
For cytospin preparations, the urinary sediment was re-suspended in tissue culture medium RPMI 1640 containing 10% fetal calf serum (GIBCO, Grand Island, N.Y). 200 μl of the resultant cell suspension was applied to slides through a funnel of a cyto-centrifuge (Cytospin 3 Shandon, England). Following this, samples were loaded and spun at 1200 RPM for 4 min. Cytoslides were air-dried and either wrapped in aluminum foil and stored at −20 °C or used immediately for immunofluorescence staining.
Immunofluorescence
Immunofluorescence staining was performed by fixing the slides in acetone for 5 min.
The primary antibody (NCL-L-CD10–270) diluted 1:300 in phosphate-buffered saline (PBS) and blocking solution (BSA), was added to each sample. After incubation in a moist chamber at 4 °C overnight the slides were washed twice with PBS. The secondary antibody (Alexa fluor 488, goat anti-mouse, Invitrogen), diluted 1:300 in PBS and BSA blocking solution, was applied and incubated for 2 h at 4 °C. Then 40 μl of DAPI was applied for the last 5 min of incubation followed by washing with Tween PBS (TPBS) and subsequently PBS for 10 min each. As a final step, slides were mounted in Vectashield mounting medium (Vector Laboratories), and investigated by confocal microscopy (Zeiss Axiovert). Images were captured via Zen 12 software and further edited using Adobe Photoshop CS6.
Kidney tissue staining was similarly performed as described above for cytopreparations.
Immunoblotting
For investigative purposes, we established a NEP dot blotting test as described in brief: 35 μl of urine was applied to individual wells of a dot blotting device (Schleicher & Schüller, Minifold I). The fluid was filtered through the inserted nitrocellulose membrane by applying vacuum to the bottom chamber. The resultant blot was then immersed in PBS after 10 min of air drying and blocked in one blocking solution (KPL, Gaitersburg, MD, USA) for 30 min. The blocked membrane was then incubated in 10 ml of anti-NEP antibody (NCL-L-CD10–270) overnight at 4 °C followed by the second incubation with the detection antibody (HRP-conjugated goat anti-mouse, Dako, P0447). Each incubation period was followed by a 10-min TPBS wash twice. Finally, the site of antibody binding was visualized using the chemiluminescence reagent (BM Chemiluminescence Blotting Substrate POD, Roche) and developed under the imager system Lumi-Imager F1 (Roche) using Fusion FX (Vilber Lourmat) software for recording pictures and evaluating dot intensity.
ELISA
Quantitative measurements of urine NEP of 90 patients with sepsis and 55 controls were taken with a 96-well plate human NEP ELISA (RayBiotech, Inc.) as described in the company’s ELISA Kit protocol. The standard dilution series as well as urine samples were applied to the wells and incubated at 4 °C overnight. The next morning, the plate was washed three times with the washing buffer provided using an automated ELISA plate washing machine (ELx50 Auto Strip Washer, BIO-TEK INSTRUMENTS, INC). A detection antibody for human NEP was added to each well and incubated for 1 h at room temperature with constant shaking. After a washing step, HRP-streptavidin was incubated for 45 min at room temperature. Following the final washing step, the TMB substrate/chromogen solution was added for developing (15 min of incubation at room temperature under light protection). Subsequently, the stop solution was pipetted into each well and the plate was read at 450 nm by the ELISA reader (Synergy H1 Hybrid Reader, BioTek).
Statistical analysis
NEP data are presented, using scatter dot plots, wherein the middle line represents the median. Different groups were compared with the Mann Whitney test using GraphPad Prism5 software. A p-level of <0.05 was considered statistically significant. Sensitivity, specificity and ROC curve analyses, correlation of plasma and urine NEP according to Pearson were all calculated using the GraphPad Prism.
Results
Protein analysis
In an initial experiment, we observed during the analysis of urinary NEP levels with dot blot measurements that this protein might be an early indicator of kidney injury. In order to follow up on this, we performed dot blot measurements on 18 arbitrarily chosen patients (Fig. 1a ). In half of the patients, NEP already showed its peak levels 24 h before serum creatinine (sCr) rise. However, this was not the case in all of the patients as shown in Fig. 1b.
Fig. 1.
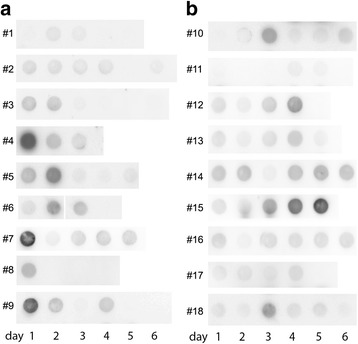
NEP Dot blot analyses of 18 patients. a Dot blot of 9 patients over 3–6 days of follow-up, presenting with elevated levels of NEP in the dot blot method within the first 24 h of admission to ICU. b Dot blot analyses for course of NEP secretion of other 9 patients in follow-up, presenting an increase during ICU stay
We performed immunofluorescence staining on healthy kidney tissue obtained from tumor nephrectomies using the same immunoreagents. This staining confirmed NEP expression in proximal tubules in all four samples (Fig. 2).
Fig. 2.
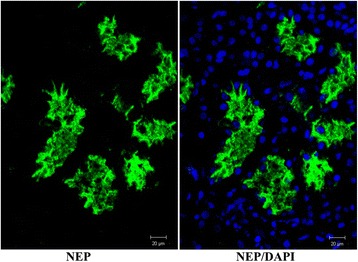
NEP staining of normal healthy kidney tissue obtained from tumor nephrectomy. An area showing proximal kidney tubules with prominent apical staining of epithelial cells is depicted. Magnification indicated at the bottom of each picture
As a second step, cytospins had been prepared from urinary cells and casts obtained from 8 arbitrarily chosen sepsis patients, which were similarly stained for NEP expression. Interestingly, NEP-positive tubular epithelial cells were rarely detected. In contrast, small microvesicles were demonstrated in 5 out of 8 cases. In addition, urinary sediment from kidney allograft recipients was taken to evaluate proximal tubular cells in accordance with this method using confocal microscopy. As depicted in Fig. 3, cell ghosts and fragmented proximal tubular cells were visualized.
Fig. 3.
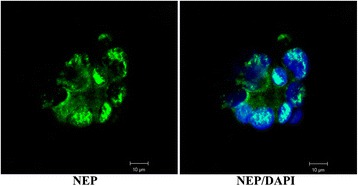
NEP staining of urinary cells. Urinary cells from a patient having undergone renal transplantation 5 days beforehand. Proximal tubular cell ghosts with ruptured cell membranes and spreading DNA due to nuclear membrane damage are depicted. Magnification indicated at the bottom of each picture
Quantitative measurement of urinary NEP by ELISA
A commercially available ELISA test was employed for the quantitative measurement of NEP.
Ninety patients (32 female and 58 male), of whom 24 (26.67%) had undergone cardiopulmonary resuscitation, were included and tracked for at least 48 h (Table 1). The mean age of patients was 60 ± 16 years. Sixty-five (72.22%) of them were diagnosed with pneumonia as the predominant focus of infection. Urinary tract infection was verified as the source of infection in 11 patients (12.22%). In 17 patients (18.89%), sepsis origin varied from spontaneous bacterial peritonitis (SBP), septic shock and wound infections to gastrointestinal infections.
Table 1.
Demographics of the study cohort. Demographic table of the study cohort showing patients characteristics including age, sex, sepsis origin, sedoanalgesia, intubation, need for catecholamines and stage of kidney injury according to the KDIGO criteria
| No AKI | Stage 1 | Stage 2 | Stage 3 | Controls | Total | |
|---|---|---|---|---|---|---|
| Number of patients | 22 | 42 | 12 | 14 | 55 | 90 |
| 24.44% | 46.67% | 13.33% | 15.56% | |||
| Age, mean ± SD | 60 ± 16 | 60 ± 16 | 59 ± 17 | 60 ± 16 | 60 ± 16 | |
| Female / Male | 9 / 13 | 17 / 25 | 4 / 8 | 2 / 12 | 40 / 15 | 32 / 58 |
| Sepsis originated pneumonia | 19 | 30 | 9 | 7 | 65 | |
| 86.36% | 71.43% | 75.00% | 50.00% | 72.22% | ||
| Sepsis originated urinary tract | 0 | 5 | 1 | 5 | 11 | |
| 0.00% | 11.90% | 8.33% | 35.71% | 12.22% | ||
| Sepsis origin others | 3 | 8 | 2 | 4 | 17 | |
| 13.64% | 19.05% | 16.67% | 28.57% | 18.89% | ||
| CPR | 7 | 12 | 4 | 1 | 24 | |
| 31.82% | 28.57% | 33.33% | 7.14% | 26.67% | ||
| Sedoanalgesia | 17 | 29 | 7 | 7 | 60 | |
| 77.27% | 69.05% | 58.33% | 50.00% | 66.67% | ||
| Intubation | 18 | 28 | 7 | 6 | 59 | |
| 81.82% | 66.67% | 58.33% | 42.86% | 65.56% | ||
| Catecholamines | 15 | 23 | 7 | 7 | 52 | |
| 68.18% | 54.76% | 58.33% | 50.00% | 57.78% | ||
| NEP positive | 9 | 32 | 10 | 9 | 5 | 60 |
| NEP positive % | 40.91% | 76.19% | 83.33% | 64.29% | 9.09% | 66.67% |
| NEP negative | 13 | 10 | 2 | 5 | 50 | 30 |
| NEP negative % | 59.09% | 23.81% | 16.67% | 35.71% | 90.91% | 33.33% |
Based on serum creatinine, patients were subdivided into 2 categories: patients without the presence of AKI including patients with a history of chronic kidney disease CKD (n = 22) and patients with AKI (n = 68), shown in Fig. 4. Patients suffering AKI had been further subdivided and graded according to the KDIGO criteria (stage 1, n = 42; stage 2, n = 12; stage 3, n = 14), depicted in Fig. 5. This was established by medical history data. As a control, healthy urine donors were chosen (n = 55). Surprisingly, five healthy male donors presented elevated NEP values which had not been described before. By further investigating the human protein atlas, it proved to be clear that the prostate epithelium exhibits high expression of NEP. Despite this fact, male and female control donors were included for statistical analyses. In this study all ICU and intermediate care patients had an indwelling urinary catheter, whereas control donors delivered spot urine.
Fig. 4.
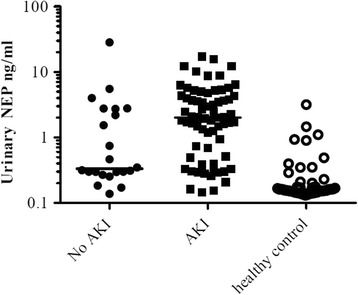
Column scatter graph comparing NEP in patients without AKI, with AKI and healthy control. The scatter plot of NEP (highest ELISA values detected during the observation period) of 90 sepsis patients is depicted, and the patients were divided into 2 groups: no AKI including patients with a history of CKD (n = 22), patients with AKI (n = 68) dynamics reaching one of the defined KDIGO stages and healthy individuals (n = 55)
Fig. 5.
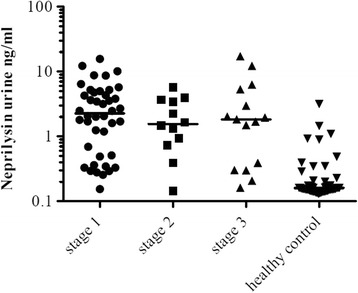
Column scatter graph of patients with AKI and healthy control. Scatter plot analysis of patients suffering AKI, divided into stage 1 (n = 42), stage 2 (n = 12) and stage 3 (n = 14) by KDIGO in comparison to healthy controls. The highest level detected during the observation period is depicted
Of the 90 critically ill patients enrolled in this study, 68 patients had an acute kidney injury and 22 patients showed stable kidney function (75.56% versus 24.44%).
Using a cut-off value of 0.5 ng/ml for NEP, sensitivity of this urinary test was 75.00% (CI 95%, 0.63–0.84), with a specificity of 59.09% (CI 95%, 0.36–0.79). A positive predictive value was calculated at 85.00% (CI 95%, 0.73–0.93) and a negative predictive value at 43.33% (CI 95%, 0.25–0.62).
As demonstrated in Fig. 4, there was highly elevated NEP in patients suffering AKI at various AKI stages in comparison to the healthy control. In the performed ROC curve analysis shown in Fig. 6 the AUC value was 0.93 (95% CI 0.88–0.97, SE = 0.02, p < 0.0001). To evaluate the discriminative power of NEP to distinguish between clinically ill AKI and critically ill no-AKI patients we calculated ROC curve analysis also shown in Fig. 6 (hatched line). This resulted with an AUC of 0,68 (95% CI 0.54–0.82, SE = 0.07, p < 0.01) and thereby showing that NEP has little power to discriminate between these two groups.
Fig. 6.
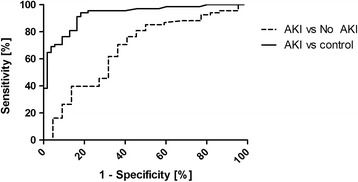
ROC curve of maximum NEP in critically ill AKI patients versus healthy controls (solid line). ROC curve of maximum NEP in critically ill AKI patients versus no-AKI patients (hatched line). Receiver-operating characteristics curve (ROC)
In contrast to patients with elevated urinary NEP, 17 patients with AKI did not show NEP increase during the observation period (false negative rate of 24.64%). This might be due to the fact that the underlying pathology for AKI is not located at the proximal tubular site. These patients presented with dehydration (n = 2), ascending urinary tract infection (n = 2), cardiogenic shock (n = 4), multi-organ failure due to septic shock (n = 5) and AKI after respiratory failure (n = 2). There was one case of spontaneous bacterial peritonitis and one patient with AKI after chemotherapy.
In addition, 9 out of 22 patients (40.91%) in the non-AKI group showed higher NEP levels (false positive rate of 40.91%). In 4 of the 9 patients, contrast media application due to cardiac catheterization or computed tomography could be delineated shortly before NEP increase as a possible underlying cause. One patient suffered from severe gastrointestinal chemical burn with normal renal parameters. Whether these conditions indeed caused high NEP levels could not be proven within the framework of this study. Most importantly, a technical term for describing this entity has recently been established, which describes a KDIGO/RIFLE negative but biomarker positive, so called “non-creatinine-increased AKI” (NCRIAKI) [33]. Other authors refer to this entity as subclinical AKI [34].
Moreover, we evaluated time-matched plasma samples (n = 57) obtained from 21 patients and found no correlation of plasma levels with those in urine (Pearson r − 0.2, 95% CI, −0.46-0.03). Interestingly, in 5 patients presenting with NEP of more than 25 ng/ml and up to 80 ng/ml in plasma due to pulmonary infections, the urine level was below 1 ng/ml (Fig. 7).
Fig. 7.
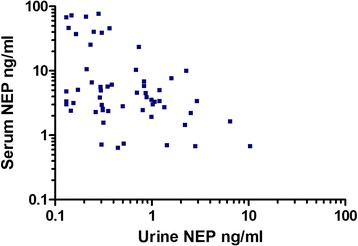
Correlation analysis of plasma and urine NEP. Scatter plot of correlation of plasma and urine NEP in 57 time-matched samples obtained from 21 patients
Discussion
In this study we investigated urinary NEP in critically ill patients admitted to the ICU at the Medical University of Vienna.
Two major facts could be elaborated within the framework of this study; on one hand, this study investigated sequential urinary NEP levels in sepsis patients and acquired new information regarding the course of its excretion. It demonstrated high levels of NEP in AKI patients. However, no correlation with KDIGO stages of AKI and urinary NEP was found. An underlying cause might be that the extent of NEP expression at the proximal tubule varies among individuals depending on genetic markers and various premedication [35]. On the other hand, this study demonstrates that not all AKI patients (n = 17) present with elevated NEP. This can be explained by the fact that the pathology occurs in specific cases at sites other than the proximal tubular epithelium. The peritubular capillary endothelia represent a vulnerable cell type [36–38]. This confirms well-known facts that AKI is a complex and widely heterogeneous disorder, even among critically ill and sepsis patients. It has been demonstrated that septic AKI seems to affect the medullary thick ascending limbs and medullary collection ducts to a higher extend than proximal tubular regions [39]. In order to exclude any relation between plasma and urine NEP concentration, we elaborated plasma/urine ratios of NEP in 57 samples. There was no correlation, which was especially well documented in 4 patients who had plasma levels between 20 and 80 ng/ml, whereas their urinary NEP was below 1 ng/ml in time-matched samples.
A second important observation was that NEP levels in general were higher among ICU patients not suffering AKI according to the KDIGO criteria in comparison to healthy controls. It is conceivable that tubular cell lesions with loss of brush border proteins are common in ICU patients with the absence of overt signs of kidney dysfunction, such as those measured in terms of serum creatinine. Experts in this field attribute the delayed diagnosis in sepsis patients to reduced production of creatinine, increased distribution volume and fluid overload [40]. It is important to note that these very minor changes in serum creatinine seem to be relevant to the medical history of patients [41, 42]. Such forms of subclinical AKI have been termed “non-creatinine-increased AKI” (NCRIAKI), analogous to the non-ST elevation myocardial infarction (NSTEMI) in cardiological sub-classification of myocardial ischemia [33]. In this respect, it is noteworthy that urinary biomarkers are gaining more importance in early diagnosis of AKI at various clinical settings [43, 44]. Since AKI does not have any specific symptoms, it is even more important to improve early marker diagnostics beyond serum creatinine and urine output as these only represent late-outcome markers. Furthermore, multiple “kidney attacks” can occur in intensive care settings, which can be detected with urinary biomarkers but not using the KDIGO/RIFLE criteria. Progress in this direction can improve the outcome of AKI, which has been absent in recent years.
With the aim of explaining the high rate of recovery in our study group, we have to mention that tubular regeneration processes play a part in modulating the outcome. These regenerative processes are thought to repair such small cellular impairments [45, 46]. In this respect, during our study all patient records were looked up again in retrospect and in most patient data a potential toxic insult could be attributed to the point in time when a NEP increase occurred.
It has been clearly demonstrated in experimental models that NEP is specifically expressed at the proximal tubular cells [32]. This protein has been previously measured in patients undergoing open heart surgery and was thereby presented as an early detection marker for AKI with its peak level 3–36 h after surgery [28]. In addition, it has very recently become a promising therapeutic target in cardiovascular medicine following the development of specific inhibitors [21]. Whether this might be relevant to AKI has not been investigated.
Although NEP seems to be a good indicator of tubular alterations, we have made a striking observation that the normal urinary excretion rate of NEP is gender-dependent. Higher levels in male donors were found which is due to the fact that the prostate secrets some of this enzyme [47]. In female healthy controls the secreted NEP level is below the detection limit of this ELISA test method.
NEP represents a new urine marker protein indicative of proximal kidney tubule cell injury. A limitation of the study is that no conclusion can be drawn on the effect or clinical relevance beyond the advice to apply supportive measures to prevent progression of renal failure in patients with elevated NEP, as they are at high risk. This pilot study performed with a specific patient group will gain importance when a validation study is performed on a wider disease range and a higher patient number. Further studies are warranted in order to elaborate on whether patients undergoing NEP+ urine tests will develop chronic kidney disease during their further life span.
In recent months it has proved to be clear that a single biomarker would not suffice for the diagnosis of acute kidney injury, instead, it has been elaborated that a consortium of peptides such as KIM-1, NGAL, IL18 and IGFB7, TIMP-2 will be indicative for injuries of the nephron. In this respect, they might be more predictive when considered individually in specific clinical settings, as it has been shown with the NephroCheck – being the method of choice at the ICU or in the case of sudden deterioration of a critically ill patient [48].
Conclusions
Elevated urinary NEP is indicative of proximal tubular cell stress or injury. As not all AKI patients show this urinary protein, various injury sites at the nephron have to be suggested depending on the type and cause of AKI. Moreover, extended tubular damage cannot be assessed by urinary NEP levels. This study confirms the previous work of others that a “non-creatinine-increased AKI” (NCRIAKI) or in other words “subclinical AKI” has to be introduced, which was used for patients suffering episodes with elevated AKI markers.
Acknowledgments
We would like to thank Heinz Regele for providing renal tissue from tumor nephrectomy.
Funding
No funding was obtained. Laboratory expenses were funded by the department’s research budget.
Availability of data and materials
Data that support the findings of this study are available upon request from the corresponding author.
Authors’ contributions
SP: analyses of samples and writing the manuscript. KM: analyses of proteins. LW: support for analyses and manuscript editing. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The study was approved by the local Ethics Committee of the Medical University of Vienna. Informed consent was obtained from the participants. Regarding tissue staining informed consent was obtained from each patient in order to use small parts of the surgically removed tissue for research in accordance with the ethical committee of the Medical University of Vienna.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AKI
Acute kidney injury
- AUC
Area under curve
- BSA
Blocking solution
- CKD
Chronic kidney disease
- ESRD
End stage renal disease
- FeNa
Fractional excretion of sodium
- GFR
Glomerular filtration rate
- ICU
Intensive care unit
- IGFBP 7
Insulin like growth factor 7
- KDIGO
Kidney disease improving global outcomes
- KIM-1
Kidney injury molecule-1
- NCRIAKI
Non-creatinine increase AKI
- NEP
Neprilysin
- NGAL
Neutrophil gelatinase-associated lipocalin
- NSTEMI
Non-ST elevation myocardial infarction
- PBS
Phosphate-buffered saline
- PTC
Proximal tubular cells
- ROC
Receiver operating characteristic
- RPM
Revolution per minute
- RRT
Renal replacement therapy
- SBP
Spontaneous bacterial peritonitis
- sCr
Serum creatinine
- TIMP-2
Tissue inhibitor of metalloproteinase-2
- TPBS
Tween PBS
Contributor Information
Sahra Pajenda, Phone: +43 (1) 40400 43910, Email: sahra.pajenda@meduniwien.ac.at.
Karl Mechtler, Phone: +43 (1) 79044 4280, Email: mechtler@imp.ac.at.
Ludwig Wagner, Phone: +43 (1) 40400 43910, Email: ludiwg.wagner@meduniwien.ac.at.
References
- 1.Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351(2):159–169. doi: 10.1056/NEJMra032401. [DOI] [PubMed] [Google Scholar]
- 2.Bagshaw SM, George C, Bellomo R. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12(2):R47. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lerolle N, Nochy D, Guerot E, Bruneval P, Fagon JY, Diehl JL, et al. Histopathology of septic shock induced acute kidney injury: apoptosis and leukocytic infiltration. Intensive Care Med. 2010;36(3):471–478. doi: 10.1007/s00134-009-1723-x. [DOI] [PubMed] [Google Scholar]
- 4.Langenberg C, Bagshaw SM, May CN, Bellomo R. The histopathology of septic acute kidney injury: a systematic review. Crit Care. 2008;12(2):R38. doi: 10.1186/cc6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prowle JR, Liu YL, Licari E, Bagshaw SM, Egi M, Haase M, et al. Oliguria as predictive biomarker of acute kidney injury in critically ill patients. Crit Care. 2011;15(4):R172. doi: 10.1186/cc10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindgren D, Bostrom AK, Nilsson K, Hansson J, Sjolund J, Moller C, et al. Isolation and characterization of progenitor-like cells from human renal proximal tubules. Am J Pathol. 2011;178(2):828–837. doi: 10.1016/j.ajpath.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta S, Verfaillie C, Chmielewski D, Kren S, Eidman K, Connaire J, et al. Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol. 2006;17(11):3028–3040. doi: 10.1681/ASN.2006030275. [DOI] [PubMed] [Google Scholar]
- 8.Romagnani P. Of mice and men: the riddle of tubular regeneration. J Pathol. 2013;229(5):641–644. doi: 10.1002/path.4162. [DOI] [PubMed] [Google Scholar]
- 9.Vanmassenhove J, Glorieux G, Hoste E, Dhondt A, Vanholder R, Van Biesen W. Urinary output and fractional excretion of sodium and urea as indicators of transient versus intrinsic acute kidney injury during early sepsis. Crit Care. 2013;17(5):R234. doi: 10.1186/cc13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365(9457):417–430. doi: 10.1016/S0140-6736(05)70238-5. [DOI] [PubMed] [Google Scholar]
- 11.Kim SC, Page EK, Knechtle SJ. Urine proteomics in kidney transplantation. Transplant Rev. 2014;28(1):15–20. doi: 10.1016/j.trre.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Marimuthu A, O'Meally RN, Chaerkady R, Subbannayya Y, Nanjappa V, Kumar P, et al. A comprehensive map of the human urinary proteome. J Proteome Res. 2011;10(6):2734–2743. doi: 10.1021/pr2003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Shao C, Wei L, Duan J, Wu S, Li X, et al. An individual urinary proteome analysis in normal human beings to define the minimal sample number to represent the normal urinary proteome. Proteome Sci. 2012;10(1):70. doi: 10.1186/1477-5956-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goligorsky MS, Addabbo F, O'Riordan E. Diagnostic potential of urine proteome: a broken mirror of renal diseases. J Am Soc Nephrol. 2007;18(8):2233–2239. doi: 10.1681/ASN.2006121399. [DOI] [PubMed] [Google Scholar]
- 15.Carrick E, Vanmassenhove J, Glorieux G, Metzger J, Dakna M, Pejchinovski M, et al. Development of a MALDI MS-based platform for early detection of acute kidney injury. Proteomics Clin Appl. 2016;10(7):732–742. doi: 10.1002/prca.201500117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 17.Parikh CR, Jani A, Mishra J, Ma Q, Kelly C, Barasch J, et al. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant. 2006;6(7):1639–1645. doi: 10.1111/j.1600-6143.2006.01352.x. [DOI] [PubMed] [Google Scholar]
- 18.Aregger F, Uehlinger DE, Witowski J, Brunisholz RA, Hunziker P, Frey FJ, et al. Identification of IGFBP-7 by urinary proteomics as a novel prognostic marker in early acute kidney injury. Kidney Int. 2014;85(4):909–919. doi: 10.1038/ki.2013.363. [DOI] [PubMed] [Google Scholar]
- 19.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17(1):R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruz DN, Mehta RL. Acute kidney injury in 2013: breaking barriers for biomarkers in AKI--progress at last. Nat Rev Nephrol. 2014;10(2):74–76. doi: 10.1038/nrneph.2013.268. [DOI] [PubMed] [Google Scholar]
- 21.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 22.Jessup M. Neprilysin inhibition--a novel therapy for heart failure. N Engl J Med. 2014;371(11):1062–1064. doi: 10.1056/NEJMe1409898. [DOI] [PubMed] [Google Scholar]
- 23.Oefner C, D'Arcy A, Hennig M, Winkler FK, Dale GE. Structure of human neutral endopeptidase (Neprilysin) complexed with phosphoramidon. J Mol Biol. 2000;296(2):341–349. doi: 10.1006/jmbi.1999.3492. [DOI] [PubMed] [Google Scholar]
- 24.Truong LD, Shen SS. Immunohistochemical diagnosis of renal neoplasms. Arch Pathol Lab Med. 2011;135(1):92–109. doi: 10.5858/2010-0478-RAR.1. [DOI] [PubMed] [Google Scholar]
- 25.Gonzales PA, Pisitkun T, Hoffert JD, Tchapyjnikov D, Star RA, Kleta R, et al. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol. 2009;20(2):363–379. doi: 10.1681/ASN.2008040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skidgel RA, Erdos EG. Angiotensin converting enzyme (ACE) and neprilysin hydrolyze neuropeptides: a brief history, the beginning and follow-ups to early studies. Peptides. 2004;25(3):521–525. doi: 10.1016/j.peptides.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Judge P, Haynes R, Landray MJ, Baigent C. Neprilysin inhibition in chronic kidney disease. Nephrol Dial Transplant. 2015;30(5):738–743. doi: 10.1093/ndt/gfu269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blaikley J, Sutton P, Walter M, Lapsley M, Norden A, Pugsley W, et al. Tubular proteinuria and enzymuria following open heart surgery. Intensive Care Med. 2003;29(8):1364–1367. doi: 10.1007/s00134-003-1876-y. [DOI] [PubMed] [Google Scholar]
- 29.Funk D, Sebat F, Kumar A. A systems approach to the early recognition and rapid administration of best practice therapy in sepsis and septic shock. Curr Opin Crit Care. 2009;15(4):301–307. doi: 10.1097/MCC.0b013e32832e3825. [DOI] [PubMed] [Google Scholar]
- 30.KDIGO Acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 31.The Human Protein Atlas. [http://www.proteinatlas.org/ENSG00000196549-MME/tissue/kidney].
- 32.Van der Hauwaert C, Savary G, Gnemmi V, Glowacki F, Pottier N, Bouillez A, et al. Isolation and characterization of a primary proximal tubular epithelial cell model from human kidney by CD10/CD13 double labeling. PLoS One. 2013;8(6):e66750. doi: 10.1371/journal.pone.0066750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ronco C, McCullough PA, Chawla LS. Kidney attack versus heart attack: evolution of classification and diagnostic criteria. Lancet. 2013;382(9896):939–940. doi: 10.1016/S0140-6736(13)61932-7. [DOI] [PubMed] [Google Scholar]
- 34.Vanmassenhove J, Glorieux G, Lameire N, Hoste E, Dhondt A, Vanholder R, et al. Influence of severity of illness on neutrophil gelatinase-associated lipocalin performance as a marker of acute kidney injury: a prospective cohort study of patients with sepsis. BMC Nephrol. 2015;16:18. doi: 10.1186/s12882-015-0003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang R, Wang S, Malter JS, Wang DS. Effects of HNE-modification induced by Abeta on neprilysin expression and activity in SH-SY5Y cells. J Neurochem. 2009;108(4):1072–1082. doi: 10.1111/j.1471-4159.2008.05855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121(11):4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto T, Tada T, Brodsky SV, Tanaka H, Noiri E, Kajiya F, et al. Intravital videomicroscopy of peritubular capillaries in renal ischemia. Am J Physiol Renal Physiol. 2002;282(6):F1150–F1155. doi: 10.1152/ajprenal.00310.2001. [DOI] [PubMed] [Google Scholar]
- 38.Sutton TA, Mang HE, Campos SB, Sandoval RM, Yoder MC, Molitoris BA. Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol Renal Physiol. 2003;285(2):F191–F198. doi: 10.1152/ajprenal.00042.2003. [DOI] [PubMed] [Google Scholar]
- 39.Heyman SN, Rosenberger C, Rosen S. Experimental ischemia-reperfusion: biases and myths-the proximal vs. distal hypoxic tubular injury debate revisited. Kidney Int. 2010;77(1):9–16. doi: 10.1038/ki.2009.347. [DOI] [PubMed] [Google Scholar]
- 40.Doi K, Yuen PS, Eisner C, Hu X, Leelahavanichkul A, Schnermann J, et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol. 2009;20(6):1217–1221. doi: 10.1681/ASN.2008060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lassnigg A, Schmid ER, Hiesmayr M, Falk C, Druml W, Bauer P, et al. Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: do we have to revise current definitions of acute renal failure? Crit Care Med. 2008;36(4):1129–1137. doi: 10.1097/CCM.0b013e318169181a. [DOI] [PubMed] [Google Scholar]
- 42.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15(6):1597–1605. doi: 10.1097/01.ASN.0000130340.93930.DD. [DOI] [PubMed] [Google Scholar]
- 43.Dusse F, Edayadiyil-Dudasova M, Thielmann M, Wendt D, Kahlert P, Demircioglu E, et al. Early prediction of acute kidney injury after transapical and transaortic aortic valve implantation with urinary G1 cell cycle arrest biomarkers. BMC Anesthesiol. 2016;16:76. doi: 10.1186/s12871-016-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pajenda S, Ilhan-Mutlu A, Preusser M, Roka S, Druml W, Wagner L. Nephro check data compared to serum creatinine in various clinical settings. BMC Nephrol. 2015;16(1):206. doi: 10.1186/s12882-015-0203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smeets B, Boor P, Dijkman H, Sharma SV, Jirak P, Mooren F, et al. Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J Pathol. 2013;229(5):645–659. doi: 10.1002/path.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol. 2003;14(Suppl 1):S55–S61. doi: 10.1097/01.ASN.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- 47.Song J, Aumuller G, Xiao F, Wilhelm B, Albrecht M. Cell specific expression of CD10/neutral endopeptidase 24.11 gene in human prostatic tissue and cells. Prostate. 2004;58(4):394–405. doi: 10.1002/pros.10345. [DOI] [PubMed] [Google Scholar]
- 48.Vijayan A, Faubel S, Askenazi DJ, Cerda J, Fissell WH, Heung M, et al. Clinical use of the urine biomarker [TIMP-2] x [IGFBP7] for acute kidney injury risk assessment. Am J Kidney Dis. 2016;68:19. doi: 10.1053/j.ajkd.2015.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data that support the findings of this study are available upon request from the corresponding author.


