Abstract
Background
The diagnosis of myeloma, a plasma dyscrasia, often results from the workup of unexplained renal disease. Persistent renal failure in myeloma is commonly caused by tubular nephropathy due to circulating immunoglobulins and free light chains. Myeloma cast nephropathy is characterized by crystalline precipitates of monoclonal light chains within distal tubules. Immunoglobulin crystallization rarely occurs intracellularly, within proximal tubular cells (light chain proximal tubulopathy) and interstitial histiocytes (crystal-storing histiocytosis). We present a case report of a rare simultaneous occurrence of light chain proximal tubulopathy, crystal-storing histiocytosis, and myeloma cast nephropathy in a patient with κ light chain multiple myeloma.
Case presentation
A 48-years-old man presented with uremia and anemia. Laboratory examination revealed low levels of serum IgG, IgA, and IgM. Serum and urine immunofixation electrophoresis showed a free κ monoclonal band. Bone marrow aspiration and biopsy revealed hypercellularity with marked plasmacytosis. Light microscopy revealed eosinophilic cuboid- and rhomboid-shaped crystals in the cytoplasm of proximal tubular epithelial cells, diffuse large mononuclear and multinuclear cells in the interstitium, and obstructed distal tubules with cast and giant cell reaction. Immunohistochemical examination indicated intense staining for κ light chains within casts, histiocytes, and tubular epithelial cells. Electron microscopy revealed electro-dense cuboid-, rhomboid-, or needle-shaped crystalline inclusions in proximal tubular epithelial cells and interstitial histiocytes. According to these results, we confirmed that this patient with myeloma exhibited simultaneous light chain proximal tubulopathy, crystal-storing histiocytosis, and myeloma cast nephropathy, which were attributed to monoclonal κ light chains. In addition to dialysis, the patient received induction chemotherapy with a combination of bortezomib, cyclophosphamide, and dexamethasone, followed by maintenance therapy with thalidomide. However, the patient did not regain renal function even when less than 5% plasma cells were detected in the bone marrow.
Conclusion
To the best of our knowledge, this is the first report of simultaneous light chain proximal tubulopathy, crystal-storing histiocytosis, and myeloma cast nephropathy in κ light chain multiple myeloma.
Keywords: Multiple myeloma, Light chain proximal tubulopathy, Crystal-storing histiocytosis, Myeloma cast nephropathy
Background
Multiple myeloma (MM) is a malignant neoplasm arising from clonal proliferation of plasma cells in the bone marrow; MM can cause renal dysfunction through various mechanisms, including paraprotein- or nonparaprotein-associated renal complications [1]. Persistent renal failure in MM commonly results from tubular nephropathy due to circulating paraproteins of various types secreted by plasma cell clones, most commonly immunoglobulins(Igs) and free light chains (LCs) [2]. Myeloma cast nephropathy (MCN), the most common Ig-related crystalline nephropathy, is characterized by crystalline precipitates of monoclonal LC (either κ or λ) within distal tubules [1]. On rare occasions, Ig crystallization occurs intracellularly within proximal tubular cells (LC proximal tubulopathy [LCPT]) [3, 4] and interstitial histiocytes (crystal-storing histiocytosis [CSH]) [5, 6]. In LCPT and CSH, these reabsorbed LCs are typically of κ type and possess innate physicochemical properties that resist proteolysis and promote self-aggregation and crystal formation [7–9]. Recently, the pathologic spectrum of LCPT has been expanded to include noncrystalline morphology [10–12].
Here, we report a rare case of myeloma with combined LCPT, CSH, and MCN attributable to free κ LC that presented clinically as uremia and anemia.
Case presentation
A 48-years-old man with urolithiasis history experienced dizziness, anorexia, and shortness of breath for 2 weeks. During this period, he also exhibited nausea and vomiting. Physical examination revealed a pale and ill-looking patient with blood pressure of 135/71 mmHg, blood temperature of 36.8 °C, and a pulse rate of 86 beats/min. The hemogram revealed hematocrit of 16.3%, a leukocyte count of 1.24 × 104/μL, and a platelet count of 9.8 × 104/μL. The biochemical assay results were as follows: blood urea nitrogen, 94 mg/dL; serum creatinine, 12.6 mg/dL; uric acid, 11.5 mg/dL; sodium, 135 mmol/L; potassium, 4.2 mmol/L; ionized calcium, 5.72 mg/dL; phosphate, 6.1 mg/dL; serum iron, 110 μg/dL; total iron binding capacity, 305 μg/dL; and ferritin, 647 ng/mL. Urinalysis results were 1+ for occult blood and 1+ for protein. The urine microalbumin-to-creatinine ratio and urine total protein-to-creatinine ratio were 36.25 mg/g and 3.53, respectively.
The patient was initially treated with hemodialysis and blood transfusion. Examination of serum Ig revealed low IgG, IgA and IgM levels, which were 302 mg/dL (751–1560 mg/dL), 10 mg/dL (82–453 mg/dL), and 9.3 mg/dL (46–304 mg/dL), respectively. Examination of serum complement (C) revealed normal C3 and high C4 levels, which were 135 mg/dL and 53.6 mg/dL, respectively. Serum and urine immunofixation electrophoresis showed a free κ monoclonal band. Beta-2 microglobulin was higher than 5 × 104 ng/mL (609–2366 ng/mL). Long bone and skull radiography revealed osteolytic foci in the inferior ramus of the bilateral pubic bones and no punched out lucencies in the skull. Bone marrow aspiration revealed a monotonous pattern with marked plasmacytosis and mononuclear cell distribution of 57%. Bone marrow biopsy revealed hypercellularity with diffuse infiltration of plasmacytoid cells, and more than 50% of the cells exhibited positive immunohistochemical staining for CD138 and κ chains. Additional laboratory data revealed negative serum antibodies against HIV, hepatitis B and C, syphilis, and negative antinuclear antibody.
Renal sonography showed a normal size for both kidneys. Examination of all material from percutaneous ultrasound-guided renal biopsy revealed a total of 30 glomeruli per level section, of which 12 were globally sclerosed and 4 were segmentally sclerosed (all of “no otherwise specified” variant) (Fig. 1a). Renal tubules demonstrated more than 50% tubular atrophy. Eosinophilic crystals in the cytoplasm of proximal tubular epithelial cells (Fig. 1b) and obstructed tubules with fractured dense cast and giant cell reaction (Fig. 1c) were also observed. More than 50% of the renal interstitium exhibited interstitial fibrosis and inflammatory change. Diffuse mononuclear cells, plasma cells, and histiocytes (Figs. 1d, 2a) were observed in the renal interstitium. Immunohistochemical studies of κ and λ LCs revealed intense staining for κ LCs within casts, histiocytes, and tubular epithelial cells (Fig. 2b, c) but negative staining for λ LCs (Fig. 2d). Electron microscopy showed irregular effacement of podocyte foot process and electro-dense cuboid- and rhomboid-shaped crystals in proximal tubular epithelial cells (Fig. 3a) and electro-dense rhomboid- or needle-shaped crystalline inclusions in interstitial histiocytes (Fig. 3b).
Fig. 1.
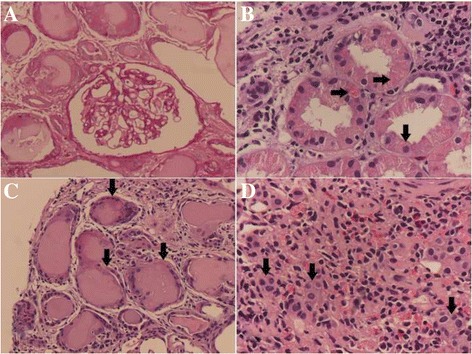
Light microscopic features of proximal tubulopathy, histiocytosis, and cast nephropathy. a Segmental sclerosis of glomerulus (Periodic acid-Schiff). b Eosinophilic cuboid and rhomboid crystals (arrows) in the cytoplasm of proximal tubular epithelial cells. c Giant cell reaction around the casts and obstructed distal tubule with fractured dense cast (arrows). d Many mononuclear and multinuclear cells (arrows) in the renal interstitium (hematoxylin and eosin)
Fig. 2.
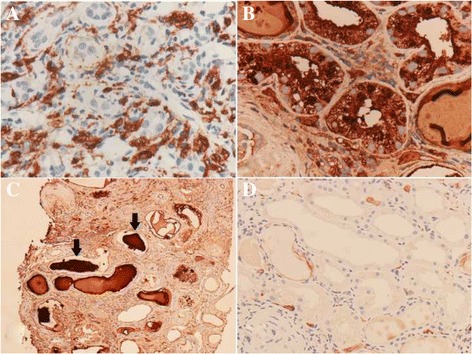
Immunohistochemical staining for renal involvement of monoclonal k light chain gammopathy. a CD68 immunostaining showed reactivity in interstitial mononuclear and multinucleated cells. b κ light chain staining in tubular epithelial cells. c κ light chain staining in casts (arrows). d λ light chains were not detected
Fig. 3.
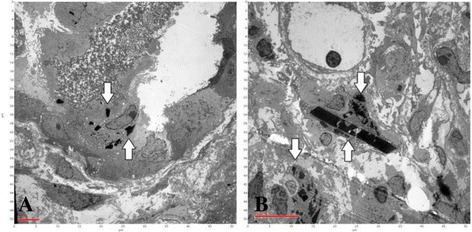
Electron microscopy showing crystalline inclusions in proximal tubular epithelial cells and histiocytes. a Cuboid- and rhomboid-shaped crystalline inclusions (arrows) in proximal tubular epithelial cells. b Needle- and rhomboid-shaped crystalline inclusions (arrows) in histiocytes
The patient was treated using induction chemotherapy with a combination of bortezomib, cyclophosphamide, and dexamethasone (VAD) for eight cycles. After induction therapy, the beta-2 microglobulin level decreased to 9212 ng/mL, and serum IgG increased to 950 mg/dL. Bone marrow biopsy revealed less than 5% plasma cells. Subsequently, the patient received maintenance therapy with thalidomide. Nevertheless, his renal function did not recover, and he continues to undergo hemodialysis.
Discussion
MM, a malignant plasma cell disorder, is defined by the presence of a serum monoclonal spike (M-spike) of more than 3 g/dL or more than 10% clonal plasma cells in the bone marrow and at least one myeloma-defining event, such as hypercalcemia, renal impairment, anemia, or bone lesions [13]. The kidney is a major target organ, and renal impairment is frequently the first manifestation of the disease. Renal impairment occurs in up to 40% of patients and 10–20% will require dialysis [14]. In our patient, more than 50% of the cells exhibited positive staining for CD138 and κ chain in the bone marrow. Moreover, the patient presented with hypercalcemia, renal failure, anemia, and pubic bone lesions. Hence, a diagnosis of MM was made.
Monoclonal Ig LCs are the major causes of renal complications in MM. Renal disease in most patients with myeloma is caused by MCN [15]. Most cases of MCN occur in patients with serum free LCs (FLCs) above 100 mg/dL, and FLCs less than 70 mg/dL are rarely observed [16, 17]. These high FLC concentrations overwhelm the reabsorption capacity of proximal tubules; thus, FLCs pass into the loop of Henle, where they bind to the Tamm-Horsfall protein and subsequently aggregate to form casts [18]. Histologically, MCN is characterized by the presence of intratubular LC casts in the distal tubules and collecting ducts. The casts often have a “hard” and “fractured” appearance. Giant cell reaction is commonly observed around the casts, because mononuclear cells are recruited in an attempt to remove these casts. Pathologically, our patient exhibited typical hard and fractured myeloma casts in the distal tubules with giant cell reaction.
Myeloma-associated renal Fanconi syndrome is a rare disorder characterized by proximal tubular dysfunction due to reabsorption of monoclonal Ig LCs, nearly invariably of the κ type [19]. These LCs possess innate physicochemical properties that resist proteolysis and promote self-aggregation and crystal formation [3, 8, 20]. The pathologic spectrum of LCPT has been expanded to include noncrystalline morphology. Patients with noncrystalline LCPT may exhibit droplets, granules, or vacuoles in the cytoplasm of proximal tubular cells [21]. Clinically, our patient was diagnosed with κ LC MM. Pathologically, proximal tubular cells were distended with intracytoplasmic cuboidal or rhomboidal inclusions with the focal loss of brush border.
CSH, an uncommon phenomenon in disorders associated with the expression of monoclonal Ig, is defined as the accumulation of histiocytes in the bone marrow and the presence of large histiocytes containing numerous Ig crystals at extramedullary sites [9]. It is presumed to be an intralysosomal accumulation of secreted paraproteins or Ig, which aggregate in crystals. In CSH, κ LCs of Ig are almost exclusively involved without a consistent association of a particular heavy chain [9]. The bone marrow is the most common site of histiocytes accumulation, whereas histiocytes containing crystals in the kidney have rarely been reported. Patients with CSH-associated renal disorder exhibit tubule-interstitial lesions and proximal tubular lesions [22–24]. Our patient predominately exhibited interstitial lesions with infiltration of large mononuclear cells and histiocytes containing rhomboid- and needle-shaped crystalline inclusions accompanied by interstitial fibrosis and tubular atrophy.
The main goal of therapy in MM is to reduce light chain production through chemotherapy alone or followed by autologous stem cell transplant (ASCT). For newly diagnosed MM, the preferred combinations for induction therapy include bortezomib, lenalidomide, and dexamethasone (VRD); carfilzomib, lenalidomide, and dexamethasone (KRD); and bortezomib, cyclophosphamide, and dexamethasone (VCD). The drug choice is determined by the risk classification of MM and the patient’s clinical status [25]. Because LCPT and CSH are relatively rare forms of renal disease in MM according to medical literature, no preferred chemotherapy regimen has been mentioned, and ASCT results in stable or improved renal function in patients with crystalline LCPT [21]. Our patient received induction therapy with bortezomib, cyclophosphamide, and dexamethasone (VCD) for eight cycles because of his poor renal function.
Patients with cast nephropathy who present with advanced renal failure are most likely to exhibit irreversible diseases, with >80% requiring dialysis at presentation and only 15% regaining renal function [26]. The prognosis of patients on dialysis depends on the response to chemotherapy, with responders surviving 37 months compared with 12 months for nonresponders [27]. Prognosis and optimal therapy for LCPT remain largely unknown. Although the majority of patients exhibit indolent kidney dysfunction, more precipitous development of acute renal failure infrequently occurs. Median renal survival from the time of renal biopsy is shorter for noncrystalline LCPT (64 ± 17.8 months) than for crystalline LCPT (135 ± 5.5 months) [21]. In the absence of renal failure, many MM patients with CSH exhibit higher survival rates after diagnosis than do patients with MM alone [9]. However, renal involvement in CSH is uncommon; the prognosis of CSH with renal involvement remains unclear. Our patient exhibited advanced renal failure accompanied by monoclonal LC-related renal disorders, including MCN, crystalline proximal tubulopathy, and CSH. After induction chemotherapy, the patient’s renal function still did not recover even when his beta-2 microglobulin level and serum IgG improved and when less than 5% plasma cells were detected in the bone marrow.
Conclusion
In summary, we report a rare simultaneous occurrence of LCPT, CSH, and MCN in a patient with MM. This patient exhibited a wide spectrum of renal lesions caused by monoclonal κ LC proliferations, and renal prognosis was as poor as that for MCN.
Acknowledgements
Not applicable.
Funding
No funding was obtained for this study.
Availability of data and materials
All the data supporting our findings is contained within the manuscript.
Authors’ contributions
CW wrote the manuscript and was a treating physician for the patient. AY interpreted the histopathological data and helped draft the manuscript. HL was a treating physician for the patient and assisted drafting the manuscript. BL was a treating physician for the patient and assisted drafting the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor of this journal.
Ethics approval and consent to participate
Not applicable.
Abbreviations
- ASCT
Autologous stem cell transplant
- CSH
Crystal-storing histiocytosis
- FLC
Free light chain
- Ig
Immunoglobulin
- LC
Light chain
- LCPT
Light chain proximal tubulopathy
- MCN
Myeloma cast nephropathy
- MM
Multiple myeloma
Contributor Information
Chung-Kuan Wu, Email: chungkuan.wu@gmail.com.
An-Hang Yang, Email: ahyang@vghtpe.gov.tw.
Hung-Chih Lai, Email: ctpetlai@gmail.com.
Bing-Shi Lin, Phone: (886)-2-2833-2221-422386, Email: m004278@ms.skh.org.tw.
References
- 1.Nasr SH, Valeri AM, Sethi S, Fidler ME, Cornell LD, Gertz MA, Lacy M, Dispenzieri A, Rajkumar SV, Kyle RA, et al. Clinicopathologic correlations in multiple myeloma: a case series of 190 patients with kidney biopsies. Am J Kidney Dis. 2012;59(6):786–794. doi: 10.1053/j.ajkd.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 2.Bridoux F, Fermand JP. Optimizing treatment strategies in myeloma cast nephropathy: rationale for a randomized prospective trial. Adv Chronic Kidney Dis. 2012;19(5):333–341. doi: 10.1053/j.ackd.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Messiaen T, Deret S, Mougenot B, Bridoux F, Dequiedt P, Dion JJ, Makdassi R, Meeus F, Pourrat J, Touchard G, et al. Adult Fanconi syndrome secondary to light chain gammopathy. Clinicopathologic heterogeneity and unusual features in 11 patients. Medicine (Baltimore) 2000;79(3):135–154. doi: 10.1097/00005792-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Ma CX, Lacy MQ, Rompala JF, Dispenzieri A, Rajkumar SV, Greipp PR, Fonseca R, Kyle RA, Gertz MA. Acquired Fanconi syndrome is an indolent disorder in the absence of overt multiple myeloma. Blood. 2004;104(1):40–42. doi: 10.1182/blood-2003-10-3400. [DOI] [PubMed] [Google Scholar]
- 5.Stokes MB, Aronoff B, Siegel D, D'Agati VD. Dysproteinemia-related nephropathy associated with crystal-storing histiocytosis. Kidney Int. 2006;70(3):597–602. doi: 10.1038/sj.ki.5001524. [DOI] [PubMed] [Google Scholar]
- 6.El Hamel C, Thierry A, Trouillas P, Bridoux F, Carrion C, Quellard N, Goujon JM, Aldigier JC, Gombert JM, Cogne M, et al. Crystal-storing histiocytosis with renal Fanconi syndrome: pathological and molecular characteristics compared with classical myeloma-associated Fanconi syndrome. Nephrol Dial Transplant. 2010;25(9):2982–2990. doi: 10.1093/ndt/gfq129. [DOI] [PubMed] [Google Scholar]
- 7.Rocca A, Khamlichi AA, Touchard G, Mougenot B, Ronco P, Denoroy L, Deret S, Preud'homme JL, Aucouturier P, Cogne M. Sequences of V kappa L subgroup light chains in Fanconi's syndrome. Light chain V region gene usage restriction and peculiarities in myeloma-associated Fanconi's syndrome. J Immunol. 1995;155(6):3245–3252. [PubMed] [Google Scholar]
- 8.Leboulleux M, Lelongt B, Mougenot B, Touchard G, Makdassi R, Rocca A, Noel LH, Ronco PM, Aucouturier P. Protease resistance and binding of Ig light chains in myeloma-associated tubulopathies. Kidney Int. 1995;48(1):72–79. doi: 10.1038/ki.1995.269. [DOI] [PubMed] [Google Scholar]
- 9.Jones D, Bhatia VK, Krausz T, Pinkus GS. Crystal-storing histiocytosis: a disorder occurring in plasmacytic tumors expressing immunoglobulin kappa light chain. Hum Pathol. 1999;30(12):1441–1448. doi: 10.1016/S0046-8177(99)90166-1. [DOI] [PubMed] [Google Scholar]
- 10.Larsen CP, Bell JM, Harris AA, Messias NC, Wang YH, Walker PD. The morphologic spectrum and clinical significance of light chain proximal tubulopathy with and without crystal formation. Mod Pathol. 2011;24(11):1462–1469. doi: 10.1038/modpathol.2011.104. [DOI] [PubMed] [Google Scholar]
- 11.Kapur U, Barton K, Fresco R, Leehey DJ, Picken MM. Expanding the pathologic spectrum of immunoglobulin light chain proximal tubulopathy. Arch Pathol Lab Med. 2007;131(9):1368–1372. doi: 10.5858/2007-131-1368-ETPSOI. [DOI] [PubMed] [Google Scholar]
- 12.Herrera GA. Proximal tubulopathies associated with monoclonal light chains: the spectrum of clinicopathologic manifestations and molecular pathogenesis. Arch Pathol Lab Med. 2014;138(10):1365–1380. doi: 10.5858/arpa.2013-0493-OA. [DOI] [PubMed] [Google Scholar]
- 13.Landgren O, Kyle RA, Rajkumar SV. From myeloma precursor disease to multiple myeloma: new diagnostic concepts and opportunities for early intervention. Clin Cancer Res. 2011;17(6):1243–1252. doi: 10.1158/1078-0432.CCR-10-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blade J, Fernandez-Llama P, Bosch F, Montoliu J, Lens XM, Montoto S, Cases A, Darnell A, Rozman C, Montserrat E. Renal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institution. Arch Intern Med. 1998;158(17):1889–1893. doi: 10.1001/archinte.158.17.1889. [DOI] [PubMed] [Google Scholar]
- 15.Kyle RA. Monoclonal gammopathies and the kidney. Annu Rev Med. 1989;40:53–60. doi: 10.1146/annurev.me.40.020189.000413. [DOI] [PubMed] [Google Scholar]
- 16.Hutchison CA, Bradwell AR, Cook M, Basnayake K, Basu S, Harding S, Hattersley J, Evans ND, Chappel MJ, Sampson P, et al. Treatment of acute renal failure secondary to multiple myeloma with chemotherapy and extended high cut-off hemodialysis. Clin J Am Soc Nephrol. 2009;4(4):745–754. doi: 10.2215/CJN.04590908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winearls CG. Acute myeloma kidney. Kidney Int. 1995;48(4):1347–1361. doi: 10.1038/ki.1995.421. [DOI] [PubMed] [Google Scholar]
- 18.Batuman V, Verroust PJ, Navar GL, Kaysen JH, Goda FO, Campbell WC, Simon E, Pontillon F, Lyles M, Bruno J, et al. Myeloma light chains are ligands for cubilin (gp280) Am J Physiol. 1998;275(2 Pt 2):F246–F254. doi: 10.1152/ajprenal.1998.275.2.F246. [DOI] [PubMed] [Google Scholar]
- 19.Lee DB, Drinkard JP, Rosen VJ, Gonick HC. The adult Fanconi syndrome: observations on etiology, morphology, renal function and mineral metabolism in three patients. Medicine (Baltimore) 1972;51(2):107–138. doi: 10.1097/00005792-197203000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Aucouturier P, Bauwens M, Khamlichi AA, Denoroy L, Spinelli S, Touchard G, Preud'homme JL, Cogne M. Monoclonal Ig L chain and L chain V domain fragment crystallization in myeloma-associated Fanconi's syndrome. J Immunol. 1993;150(8 Pt 1):3561–3568. [PubMed] [Google Scholar]
- 21.Stokes MB, Valeri AM, Herlitz L, Khan AM, Siegel DS, Markowitz GS, D'Agati VD. Light chain proximal Tubulopathy: clinical and pathologic characteristics in the modern treatment era. J Am Soc Nephrol. 2016;27(5):1555–1565. doi: 10.1681/ASN.2015020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sethi S, Cuiffo BP, Pinkus GS, Rennke HG. Crystal-storing histiocytosis involving the kidney in a low-grade B-cell lymphoproliferative disorder. Am J Kidney Dis. 2002;39(1):183–188. doi: 10.1053/ajkd.2002.29914. [DOI] [PubMed] [Google Scholar]
- 23.Pitman SD, Wang J, Serros ER, Zuppan C. A 70-years-old woman with acute renal failure. Crystal-storing histiocytosis. Arch Pathol Lab Med. 2006;130(7):1077–1078. doi: 10.5858/2006-130-1077-AYWWAR. [DOI] [PubMed] [Google Scholar]
- 24.Gu X, Barrios R, Cartwright J, Font RL, Truong L, Herrera GA. Light chain crystal deposition as a manifestation of plasma cell dyscrasias: the role of immunoelectron microscopy. Hum Pathol. 2003;34(3):270–277. doi: 10.1053/hupa.2003.27. [DOI] [PubMed] [Google Scholar]
- 25.Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91(7):719–734. doi: 10.1002/ajh.24402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irish AB, Winearls CG, Littlewood T. Presentation and survival of patients with severe renal failure and myeloma. QJM. 1997;90(12):773–780. doi: 10.1093/qjmed/90.12.773. [DOI] [PubMed] [Google Scholar]
- 27.Abbott KC, Agodoa LY. Multiple myeloma and light chain-associated nephropathy at end-stage renal disease in the United States: patient characteristics and survival. Clin Nephrol. 2001;56(3):207–210. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data supporting our findings is contained within the manuscript.


