Key Points
Atherogenic conditions promote platelet CD36 signaling by generating specific reactive oxygen species.
Redox sensor ERK5 MAP kinase activation by reactive oxygen species potentiates thrombus formation in hyperlipidemic conditions.
Abstract
Atherothrombosis is a process mediated by dysregulated platelet activation that can cause life-threatening complications and is the leading cause of death by cardiovascular disease. Platelet reactivity in hyperlipidemic conditions is enhanced when platelet scavenger receptor CD36 recognizes oxidized lipids in oxidized low-density lipoprotein (oxLDL) particles, a process that induces an overt prothrombotic phenotype. The mechanisms by which CD36 promotes platelet activation and thrombosis remain incompletely defined. In this study, we identify a mechanism for CD36 to promote thrombosis by increasing activation of MAPK extracellular signal-regulated kinase 5 (ERK5), a protein kinase known to be exquisitely sensitive to redox stress, through a signaling pathway requiring Src kinases, NADPH oxidase, superoxide radical anion, and hydrogen peroxide. Pharmacologic inhibitors of ERK5 blunted platelet activation and aggregation in response to oxLDL and targeted genetic deletion of ERK5 in murine platelets prevented oxLDL-induced platelet deposition on immobilized collagen in response to arterial shear. Importantly, in vivo thrombosis experiments after bone marrow transplantation from platelet-specific ERK5 null mice into hyperlipidemic apolipoprotein E null mice showed decreased platelet accumulation and increased thrombosis times compared with mice transplanted with ERK5 expressing control bone marrows. These findings suggest that atherogenic conditions critically regulate platelet CD36 signaling by increasing superoxide radical anion and hydrogen peroxide through a mechanism that promotes activation of MAPK ERK5.
Introduction
Atherothrombosis is the most common underlying cause of death from cardiovascular disease and is initiated by dysregulated platelet activation following atherosclerotic plaque rupture. Platelet activation in this condition promotes thrombus formation and occlusion of the vessel, which will ultimately manifest as a thrombotic emergency such as heart attack or stroke.1 Studies have linked elevated cholesterol in the form of low-density lipoprotein (LDL) as a risk factor for atherothrombosis.2 Prothrombotic properties of LDL particles are generated by phospholipid oxidation during the inflammatory and oxidative processes of plaque formation. Importantly, hyperlipidemic mice and humans with high LDL cholesterol have detectable levels of circulating oxidized lipids,3 and atherosclerotic plaque isolated from humans and mice contain abundant epitopes reactive with antibodies to oxidized lipids in oxidized LDL (oxLDL).4 The oxidized lipids in oxLDL particles present a “danger” signal that is recognized by specific scavenger receptors of the innate immune system present on platelets and monocyte/macrophages.3,5
CD36 is a scavenger receptor expressed at 20 000 copies per platelet5 that recognizes specific oxidized lipid motifs commonly referred to as oxPCcd36 found in oxLDL particles.3,6 Although circulating levels of oxLDL are not well defined, studies mixing “normal” platelets with plasma isolated from hyperlipidemic humans or mice (which contain detectable oxidized lipids) show platelet activation in a CD36-dependent manner.3 These oxidized lipids trigger multiple signaling pathways consisting of immediate recruitment and activation of Src family kinases Fyn and Lyn,7 activation of Vav family guanine nucleotide exchange factors,8 MAPK JNK,7 cytoskeleton rearrangement via the Rho/Rock signaling module,9 and assembly of the reduced NADPH oxidase 2 (NOX2) complex,10 all of which lower the threshold for platelet activation. However, the downstream signaling mechanisms that link CD36 to classic platelet activation pathways are unclear. The potential importance of the CD36 signaling pathway in atherothrombosis is supported by genetic studies showing protection from diet-induced atherosclerosis and thrombosis in mice lacking CD363 and human studies linking polymorphisms in the CD36 gene to levels of platelet CD36 expression and to risk of myocardial infarction (MI).5
A role for reactive oxygen species (ROS) as regulators of signaling pathways has been demonstrated in a variety of cells by acting on redox-sensitive motifs in effector proteins.11 ROS generation is characteristic of CD36 signaling in vascular and blood cells including platelets.10,12-14 Assembly of critical subunits for NOX activation was proposed to be the major event increasing ROS generated by platelet CD36 signaling, which is linked to signaling pathways requiring Src kinases and protein kinase C.10 However, the downstream effectors and the target of ROS for CD36 to promote thrombosis are incompletely defined.
MAPK family members are sensitive to cellular oxidative stress15 and are essential components of platelet signaling pathways.16 Importantly, the MAPK family member extracellular signal-regulated kinase 5 (ERK5) was recently identified in platelets and shown to function as a redox switch to promote maladaptive platelet signaling during MI, a condition with greatly elevated ROS.17 A role for ERK5 in platelet activation induced by hyperlipidemia is logical, but has never been investigated. ERK5 could represent an intermediate step in platelet CD36 signaling to integrate into classic agonist-induced signaling pathways for platelet activation. Furthermore, because the absence of CD36 in mice and humans is not associated with increased risk of bleeding, determining downstream signaling networks for platelet CD36 could identify novel therapeutic targets for individuals at high risk for atherothrombosis while simultaneously protecting them from bleeding diatheses, which are common to antiplatelet medications in clinical use. In this study, we hypothesized that CD36 promotes thrombosis in atherogenic conditions by generating a redox-regulated signaling pathway requiring ERK5. We found that ERK5 was activated in platelets by oxLDL in a CD36-dependent manner, that activation requires generation of specific ROS, and that pharmacologic inhibition or targeted genetic interruption of CD36/ROS/ERK5 signaling pathway decreased platelet activation by oxLDL ex vivo and ameliorated arterial thrombosis in a hyperlipidemic mouse model.
Methods
Superoxide radical anion measurements
Washed human platelets (600 × 103/μL) were incubated with 10 μM hydroethidine for 30 minutes at room temperature in the dark. Platelets were treated with 1 μg/mL FA6 blocking anti-CD36 antibody or nonimmune immunoglobulin G (IgG) for 15 minutes before addition of 2 mM CaCl2, 1 mM MgCl2, and either 50 μg/mL of LDL or oxLDL. After 45 minutes, platelets were pelleted by centrifugation and the pellets were frozen at −80°C. Thawed samples were analyzed by high-performance liquid chromatography (HPLC) as previously described.18
Platelet aggregometry and flow cytometry
Washed human platelets (300 × 103/μL) in Tyrode buffer with 0.3% bovine serum albumin and 0.1% glucose were treated with inhibitors for 10 minutes at room temperature before adding phosphate-buffered saline (PBS), LDL, or oxLDL (50 μg/mL) for 15 minutes. A total of 1 mM CaCl2/MgCl2 was added before initiating aggregation in a Chrono-Log Aggregometer Model 540-VS. Within 30 seconds, 200 μg/mL fibrinogen was added. For mouse studies, platelets were purified by gel filtration and analyzed using 0.2 μM adenosine 5′-diphosphate (ADP) to induce aggregation.
For detection of integrin activation and granule secretion, AlexaFluor 647-conjugated fibrinogen or anti-CD62P-phycoerythrin (PE) IgG were added to platelets for 15 minutes after incubation with LDL or oxLDL, as previously. Platelets were fixed in 2% paraformaldehyde and analyzed by flow cytometry using an ACCURI C6 cytometer (BD Bioscience).
Microfluidic ex vivo thrombosis under shear
Platelets in mouse whole blood were labeled with mepacrine (200 μg/mL) for 30 minutes at room temperature. The blood was then incubated with PBS or oxLDL (50 μg/mL) for 10 minutes in the dark. A total of 1 mM CaCl2/MgCl2 was added before perfusing the blood through collagen-coated Vena8 Fluoro+ Biochip microchannels (Cellix Ltd). Platelet accumulation was detected by fluorescence microscopy. The rate of platelet accumulation was determined by the percentage of area covered over time.
Bone marrow transplantation
Apolipoprotein E (apoE) null mice between 8 and 12 weeks were γ-irradiated at 1100 rads. Bone marrow cells from ERK5flox/flox and ERK5flox/PF4-cre+ donor mice were isolated from the femur and tibia. Irradiated recipient apoE null mice were anesthetized and 4 × 106 bone marrow cells from donor mice were injected retro-orbitally. Platelet counts were not different between chimeras (P = .46 apoE:ERKflox/flox vs apoE:ERK5flox/PF4-cre+ mice) or between the wild-type (WT) and 2 types of chimeras (P values of .89 for apoE:ERK5flox/flox and .27 apoE:ERK5flox/PF4-cre+ mice). Similarly, red cell counts were not statistically different among the strains. White blood cell counts (specifically lymphocyte counts) were lower in the apoE:ERK5flox/PF4-cre+ compared with the apoE:ERKflox/flox controls (1.1 × 103/mm3 vs 1.6 × 103/mm3; P < .05 in both parameters), but it is very unlikely that these small differences affect thrombosis because lymphocytes are not known to play a role in arterial thrombosis. A table of blood cell parameters is shown in supplemental Table 1, available on the Blood Web site.
In vivo thrombosis
Ferric chloride–induced carotid artery thrombosis was performed as previously described.19,20 If blood flow continued for more than 30 minutes, the experiment was terminated, and 30 minutes was assigned for statistical analysis. In addition, a carotid artery thrombosis model induced by transplanting a segment of the epigastric artery into the carotid lumen was performed as previously described.21 In brief, mice were anesthetized and the epigastric artery was carefully dissected and inserted into the carotid artery with the adventitial surface subsequently exposed to circulating blood. Platelet accumulation was monitored and quantified via intravital fluorescence imaging with time-lapse video capture as previously described.21
Animals
WT C57Bl/6 and apoE null mice were from The Jackson Laboratory. CD36 null mice were generated as previously described.22 ERK5flox/flox mice were created by the laboratory of Cathy Tournier (University of Manchester, United Kingdom)23 and the ERK5flox/PF4-cre+ mice were generated as previously described.17 Animals were fed standard chow diet unless indicated.
Study approval
Written informed consent was received before enrolling healthy human subjects to the study. All studies involving donor samples were in accordance with the Medical College of Wisconsin’s Institutional Review Board. All animal studies were in accordance to the Institutional Animal Care and Use Committee of Medical College of Wisconsin, University of North Carolina, and Cleveland Clinic Lerner Research Institute.
Results
OxLDL increases accumulation of platelet O2•− and hydrogen peroxide to promote platelet activation and aggregation through CD36
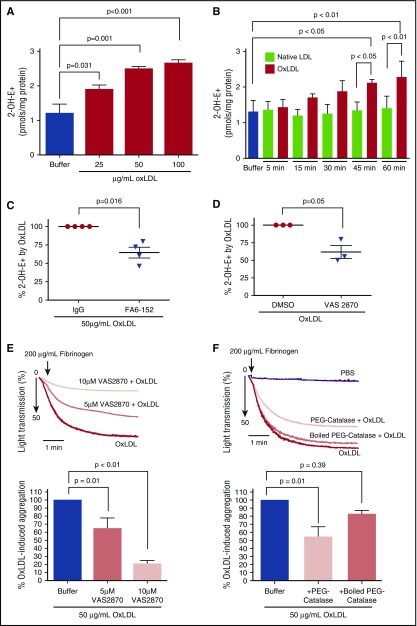
Studies using fluorescent probes have suggested that generation of intracellular ROS is characteristic of platelet CD36 signaling.10 To confirm this and determine the effector reactive species important in platelet CD36 signaling, washed human platelets were loaded with the fluorescent probe hydroethidine (HE) and then stimulated with oxLDL or control native LDL. In the presence of superoxide radical anion (O2•−) HE generates the O2•−-specific fluorescent product 2-hydroxyethidium (2-OH-E+) or a fluorescent nonspecific oxidation product ethidium (E+).24 2-OH-E+ and E+ have overlapping fluorescence spectra; therefore, to quantify O2•−, an HPLC system equipped with a sensitive fluorescence detector that can distinguish 2-OH-E+ from E+ was used.18 As shown in Figure 1A-B, oxLDL induced a time- and dose-dependent accumulation of 2-OH-E+. Maximal 2-OH-E+ accumulation was seen after treatment with 50 μg/mL of oxLDL, which is consistent with previous studies showing saturable binding of oxLDL to CD36 at this concentration.3 To determine whether 2-OH-E+ accumulation by oxLDL was mediated through CD36 signaling, a monoclonal antibody that masks the receptor-ligand binding site (FA6)25 was added along with oxLDL; levels of 2-OH-E+ by oxLDL decreased significantly. Increasing the concentration of FA6 does not further inhibit oxLDL-induced 2-OH-E+ accumulation (data not shown). An isotype-matched control mouse IgG had no effect (Figure 1C), indicating that oxLDL specifically stimulates O2•− formation via CD36 signaling.
Figure 1.
OxLDL increases platelet intracellular O2•−and H2O2 to promote platelet aggregation. 2-OH-E+ was detected by HPLC after washed human platelets (6 × 108/mL) were stimulated with varying concentrations of oxLDL for 45 minutes (A) (n = 3) over the course of time with buffer, native LDL (50 µg/mL), or oxLDL (50 µg/mL) (B) (n = 3), and after oxLDL stimulation in the presence of CD36 blocking antibody FA6-152 or control IgG antibody (1 µg/mL) (C) (n = 4). Platelet aggregation by oxLDL was measured over time after washed human platelets were treated with NOX inhibitor VAS2870 or DMSO control (5 or 10 µM) (n = 3) (D-E) in the presence or absence of buffer, PEG-catalase (1000 U/mL), or boiled PEG-catalase (1000 U/mL) (F) (n = 4). Data represented as mean ± standard error of the mean (SEM). P value determined by 1-way analysis of variance (ANOVA) with Dunnett post hoc multiple comparisons analysis (A), 2-tailed Student t test (C-D), and 1-way ANOVA with Tukey post hoc multiple comparisons analysis (B,E-F).
Previous studies suggested that NOX2 is the major source of oxidant species generated through CD36 signaling in platelets.10 2-OH-E+ accumulation was measured in the presence of VAS2870, a pharmacologic small molecule inhibitor that prevents the assembly of critical subunits required for NOX activation,26 and showed that oxLDL-induced 2-OH-E+ was decreased significantly (Figure 1D). Furthermore, Figure 1E shows that oxLDL-induced platelet aggregation was decreased dose dependently in the presence of VAS2870. These studies confirm the functional role of NOX in platelet CD36 signaling.
Given that O2•− spontaneously or catalytically converts to hydrogen peroxide (H2O2) by superoxide dismutase,27 we tested the hypothesis that H2O2 is important for oxLDL-mediated platelet activation. Polyethylene glycol–conjugated catalase (PEG-catalase) has been used to catalyze the degradation of intracellular H2O2 to water28 and was recently used to show the functional relevance of H2O2 in the prothrombotic activity of aged platelets.29 Figure 1F shows that PEG-catalase reduced oxLDL-induced platelet aggregation by 46 ± 13%. Denaturing PEG-catalase by boiling abrogated its ability to prevent oxLDL-induced platelet aggregation, demonstrating that the PEG tag did not influence platelet function or impact the nature of O2•− and H2O2 (supplemental Figure 2). Together, these data indicate that O2•− and H2O2 are important effector species in the mechanisms of oxLDL-CD36 mediation of platelet hyperactivation and aggregation.
Redox-sensitive MAPK ERK5 is activated by oxLDL-CD36 signaling
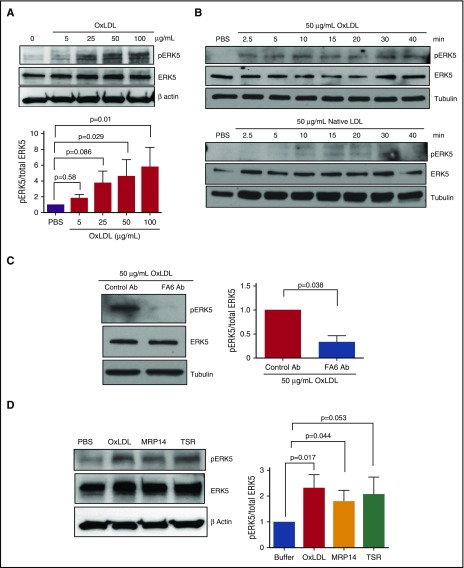
MAPK ERK5, also known as Big MAPK 1, is a platelet redox-sensitive kinase that is activated in MI, a condition with elevated cellular ROS, and promotes MI expansion.17 To determine the mechanism by which O2•− and H2O2 promote platelet activation by oxLDL, we tested the hypothesis that these reactive species modulate the ERK5 signaling pathway. Washed human platelets were stimulated with increasing concentrations of oxLDL (Figure 2A), which induced ERK5 phosphorylation at Thr218/Tyr220, a molecular signature for ERK5 activation. A time course study showed that oxLDL induced a rapid and sustained phosphorylation of ERK5 with phosphorylation seen at 2.5 minutes and maintained for at least 40 minutes. Native LDL induced minimal ERK5 phosphorylation (Figure 2B). To determine whether activation of ERK5 was dependent on CD36 stimulation, human platelets were pretreated with the FA6 blocking antibody before stimulating with oxLDL. In this case, phosphorylation of ERK5 was decreased by 67 ± 13% compared with a nonimmune control antibody, suggesting that ERK5 activation by oxLDL is mediated predominantly through CD36 (Figure 2C). Because CD36 recognizes multiple ligands that are important in pathophysiologic conditions, we also tested the hypothesis that CD36 ligands that do not contain oxidized lipids, such as the S100A protein family member MRP1430 and recombinant thrombospondin type I domain (TSR),31 can also induce ERK5 activation and found that both MRP14 and TSR induced ERK5 phosphorylation (Figure 2D).
Figure 2.
OxLDL induces activation of redox sensor ERK5 in a CD36-dependent manner. Platelet ERK5 activation was detected in washed human platelets (3 × 108/mL) (A) after stimulation with varying doses of oxLDL for 15 minutes, (B) after the time course stimulation with oxLDL (top) or native LDL (bottom) (50 µg/mL), (C) after the treatment with CD36 blocking antibody FA6 or control IgG antibody (1 µg/mL) (n = 3), and (D) after stimulation with other CD36 ligands, the prothrombotic S100A protein family member MRP14 (1 µg/mL), and TSR (0.35 µg/mL) (n > 4). Significance was determined by 2-tailed Student t test (C) and 1-way ANOVA with Dunnett post hoc analysis (A,D). Data represented as mean ± SEM.
ERK5 activation by CD36 signaling is mediated by Src kinases, NOX, and O2•−/H2O2
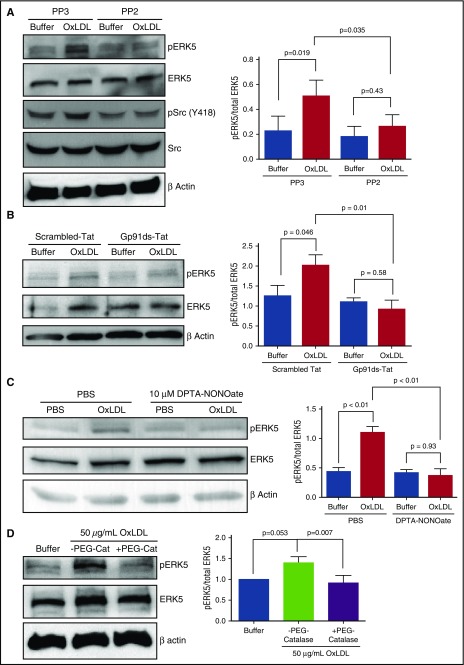
We postulated that Src kinases Fyn and Lyn are important for CD36-mediated ERK5 activation because they are known to be activated and recruited to platelet CD36 upon oxLDL ligation.7 As shown in Figure 3A, oxLDL induced phosphorylation of Src kinases detected by immunoblot with an antibody to phosphorylated Tyr418. As expected, the pan-Src kinase inhibitor, PP2, but not a control analog, PP3, prevented Src phosphorylation. PP2 also inhibited ERK5 phosphorylation in response to oxLDL. We next tested the hypothesis that NOX, the key ROS generator in platelet CD36 signaling,10 is upstream of ERK5. The small peptide inhibitor gp91ds-Tat, which prevents the assembly of the NOX complex, blocked phosphorylation of ERK5 by oxLDL, whereas a control peptide had no effect (P = .01) (Figure 3B), indicating that NOX activation and ROS generation are events upstream of ERK5. To determine whether O2•− and H2O2 are important for oxLDL-CD36–mediated ERK5 activation, the nitric oxide donor DPTA-NONOate, which competitively scavenges O2•− and therefore prevents dismutation to H2O2,32 was added to platelets in the presence of oxLDL, and as shown in Figure 3C, phosphorylation of ERK5 was prevented (P < .01). PEG-catalase, which degrades H2O2, also prevented ERK5 phosphorylation by oxLDL-CD36 signaling (Figure 3D). Altogether, these data suggest that CD36 induces ERK5 activation through a Src kinase-NOX-O2•−/H2O2 signaling pathway.
Figure 3.
Src kinase, NOX, and O2•−/H2O2 are important for platelet ERK5 activation by oxLDL-CD36 signaling. ERK5 activation by 15-minute stimulation with oxLDL was detected in human washed platelets (3 × 108/mL) after the pretreatment of (A) control Src inhibitor PP3 and Src inhibitor PP2 (10 µM) (n = 4), (B) NOX peptide inhibitor gp91ds-Tat or control scrambled-Tat (2 µM) (n = 3), (C) nitric oxide donor DPTA-NONOate (10 µM) to scavenge O2•− (n = 3), and (D) PEG-catalase (1000 U/mL) (n = 5). Data represented as mean ± SEM. P value determined by 1-way ANOVA with Tukey post hoc analysis.
CD36-mediated ERK5 activation promotes platelet integrin activation, aggregation, and accumulation on immobilized collagen under shear
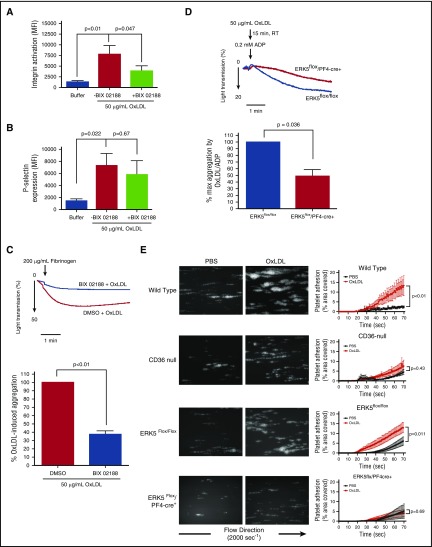
OxLDL promotes platelet integrin activation, aggregation, and thrombus formation by CD36 through multiple signaling pathways involving Src kinases Fyn/Lyn7 and NOX.10 In addition, ERK5 was shown to be important for platelet activation and secretion by certain classic agonists.17 The rapid and sustained phosphorylation of ERK5 by a CD36-Src kinase-NOX–O2•−/H2O2-dependent mechanism suggests a role for ERK5 in platelet activation by oxLDL. To test this hypothesis, we measured activated integrin αIIbβ3 and surface expression of P-selectin as indicators of platelet activation and α granule secretion, respectively. BIX 02188, a pharmacological inhibitor of ERK5 and the upstream ERK5 protein kinase activator MEK5,33 decreased platelet integrin activation by oxLDL compared with dimethyl sulfoxide (DMSO) control, as shown in Figure 4A. Only a slight decrease in P-selectin expression was observed in the presence of BIX 02188 (Figure 4B). Recognition of oxLDL by CD36 promotes platelet aggregation in both human and murine platelets.3,5 Using light transmittance platelet aggregometry with washed human platelets, we found that BIX 02188 decreased aggregation induced by oxLDL compared with vehicle control (Figure 4C). Additionally, we used a mouse model with targeted genetic deletion of ERK5 in platelets (ERK5flox/PF4-cre+) and performed aggregometry on gel-filtered platelets. Because murine platelets are typically less responsive to oxLDL than human platelets, we stimulated mouse platelets with a low dose of ADP (0.2 μM) in the presence of oxLDL to observe potentiation of platelet aggregation by CD36. In the absence of ERK5, potentiation of platelet aggregation by oxLDL was decreased when compared with platelets from ERK5flox/flox control mice (Figure 4D). Furthermore, using an ex vivo microfluidics thrombosis model that measures platelet deposition from whole blood on collagen-coated microchannels under arterial shear, we found an increase in the rate of platelet adhesion and accumulation in oxLDL-stimulated whole blood from WT C57Bl/6 (Figure 4E; supplemental Figure 3A-B; supplemental Videos 1 and 2). Whole blood from CD36 null mice did not respond to oxLDL (supplemental Videos 4 and 5). Notably, whole blood from mice with targeted genetic deletion of ERK5 in platelets did not show enhanced rate of platelet adhesion and accumulation in response to oxLDL compared with the ERK5flox/flox controls (supplemental Videos 5-8). These data suggest that ERK5 is important for platelet adhesion, activation, and accumulation by oxLDL-CD36 signaling.
Figure 4.
Platelet ERK5 promotes integrin activation, aggregation, and accumulation under shear by oxLDL-CD36 signaling. Washed human platelets (3 × 108/mL) were pretreated with DMSO or BIX 02188 (10 µM) before stimulation with oxLDL (50 µg/mL). Integrin activation was detected with Alexa Fluor 647-labeled fibrinogen (15 µg/mL) (n = 7) (A), and α granule secretion was detected by PE-labeled P-selectin antibody (n = 7) (B) using flow cytometry. (C) Platelet aggregation over time was measured after washed human platelets (3 × 108/mL) were preincubated with DMSO or BIX 02188 (10 µM) before stimulating with oxLDL (50 µg/mL) (n = 3). (D) Platelet aggregation was measured after gel-filtered mouse platelets (3 × 108/mL) were pretreated with oxLDL (50 µg/mL) at room temperature (RT), before stimulating with 0.2 µM ADP (n = 3). (E) Platelet adhesion over time on immobilized collagen was measured after whole blood from WT C57Bl/6 (n = 5), CD36 null (n = 5), ERK5flox/flox (n = 6), and ERK5flox/PF4-cre+ (n = 6) mice were stimulated with PBS or oxLDL (50 µg/mL). Data represented as mean ± SEM. P value determined by 2-tailed Student t test (C-D) and 1-way ANOVA with Tukey’s post hoc analysis (A-B), between WT and CD36-null (E), and between ERK5flox/flox and ERK5flox/PF4-cre+ (E).
ERK5 deficiency decreases platelet hyperactivity in hyperlipidemic and oxidant stress conditions
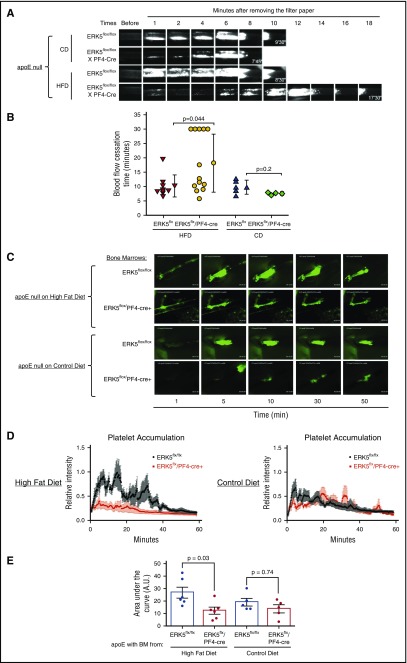
To demonstrate the functional relevance of ERK5 activation on thrombosis in vivo under conditions of hyperlipidemia and oxidant stress, we took advantage of the apoE null strain of mice that is widely used as a model to study diet-induced hyperlipidemia and atherosclerosis. These mice develop detectable levels of circulating oxidized lipids by 5 weeks of high-fat diet feeding.3,34 Bone marrow from ERK5flox/flox control mice or from platelet-specific ERK5 null mice (ERK5flox/PF4-cre+) were transplanted into lethally irradiated recipient apoE null mice, which were then placed on control or high-fat diets for 5 weeks before performing carotid artery thrombosis experiments. ApoE null chimeric mice fed a high-fat diet were hyperlipidemic, as determined by total plasma cholesterol levels (supplemental Figure 4A); standard chow diet did not increase levels of total plasma cholesterol. Platelets from chimeric mice transplanted with ERK5flox/PF4-cre+ bone marrow were indeed ERK5-deficient (supplemental Figure 4B). Tail transection bleeding time assays showed comparable time to hemostasis and comparable blood loss in the 2 groups of chimeric mice (supplemental Figure 4C-D). These data indicate the suitability of these chimeric mice for in vivo thrombosis experiments.
Using the standard FeCl3-induced arterial injury thrombosis model, we found previously that genetic deletion of CD36 in high-fat diet–fed apoE null mice restored the time to cessation of blood flow to that seen in chow-fed apoE null or WT mice.3 Similarly, high-fat diet–fed apoE null mice transplanted with bone marrow from platelet-specific ERK5 null mice had significantly delayed time to cessation of arterial blood flow compared with mice transplanted with control ERK5flox/flox bone marrow (18.15 vs 10.2 minutes) (Figure 5A-B). Thrombosis times between animals with ERK5flox/flox bone marrow and ERK5flox/PF4-cre+ bone marrow were similar in control chow diet–fed mice (Figure 5A-B).
Figure 5.
ERK5 deficiency in platelets decreases platelet accumulation in hyperlipidemic and oxidant stress conditions. Bone marrow from ERK5flox/flox or ERK5flox/PF4-cre+ mice was transplanted to 8- to 12-week-old apoE null recipient mice. Recipient mice were fed a Western HFD or CD for 5 weeks before performing in vivo thrombosis experiments. (A) Representative video images of carotid artery thrombi formation after 7.5% FeCl3 treatment. Platelets were labeled by direct jugular vein injection of rhodamine 6G (0.5 mg/mL, 100 μL) and arteries were observed in real time using fluorescent intravital microscopy. The last image in each row indicates time to flow cessation. (B) Accumulating data showing blood flow cessation time. CD, chow diet; HFD, high-fat diet. (C) Representative video images of platelet accumulation in collagen-mediated thrombosis. (D-E) Quantification of platelet accumulation. For FeCl3-mediated thrombosis experiments, P value was determined by Mann-Whitney rank sum test. For collagen-mediated thrombosis experiments, P value was determined by 2-way ANOVA with Tukey post hoc analysis. Data represented as mean ± SEM.
Platelet ERK5 can function as a sensor of extracellular ROS.17,35 Therefore, to address potential confounding effects of exogenous oxidant stress on in vivo thrombosis, we used a novel model that relies on autologous transplantation of a segment of epigastric artery into the lumen of the carotid artery. Unlike the commonly used FeCl3 and Rose Bengal arterial injury thrombosis models, this procedure does not generate a chemical oxidant injury,21,36 but rather initiates thrombus formation in a consistent and reproducible manner by exposing flowing blood to the collagen-rich outer adventitial layer of the implanted epigastric artery.21 We found that high-fat diet–fed apoE null mice transplanted with control ERK5flox/flox bone marrow developed rapid platelet accumulation, whereas similar mice fed a control chow diet showed markedly diminished platelet accumulation, confirming that this model can detect the diet-induced prothrombotic state (Figure 5C-D; supplemental Videos 9 and 11). After initial thrombus formation, embolization of platelet-rich thrombi was observed with rapid re-formation of the thrombus over a 60-minute time frame. High-fat diet–fed mice transplanted with ERK5flox/PF4-cre+ bone marrow (Figure 5C-D; supplemental Video 10) did not show enhanced platelet accumulation (P = .03) and were indistinguishable from the chow-fed animals (supplemental Video 12). Figure 5E shows the area under the curve for total platelet accumulation. Thromboemboli were still observed in control diet studies, but no potentiation of thrombus formation was seen. These data are thus consistent with the hypothesis that platelet ERK5 participates in hyperlipidemia-induced thrombosis.
Discussion
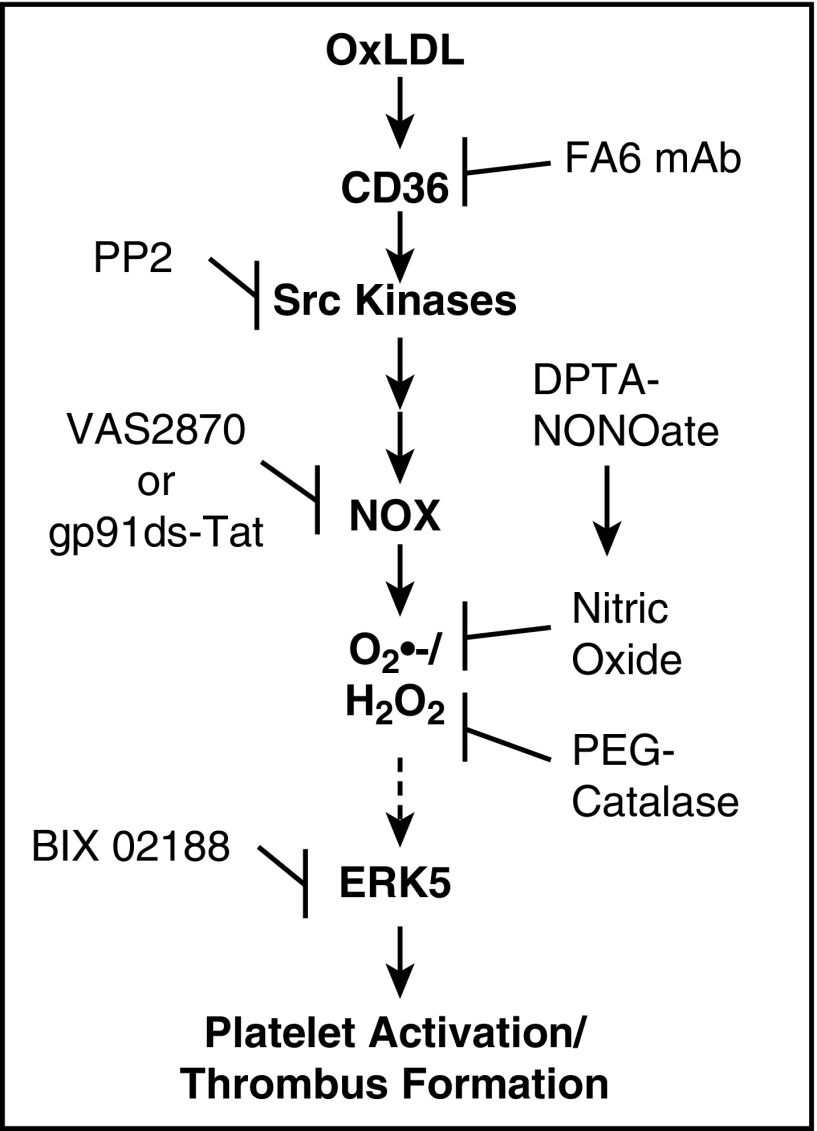
Hyperlipidemia and oxidant injury associated with the process of atherosclerosis promotes platelet hyperactivity and increases the risk for MI and stroke.3 This condition is mediated by the presence of circulating oxLDL that can be recognized by platelet CD36, leading to a decrease in the threshold for platelet activation.3,8 In this study, we have identified a CD36 signaling pathway in platelets for oxLDL to promote thrombosis that is dependent on the generation of ROS (Figure 6). This signaling pathway requires CD36, Src kinases, NOX, and the formation of O2•−/H2O2 to promote ERK5 activation. A pharmacologic inhibitor of the MEK5-ERK5 signaling pathway decreased platelet activation and aggregation, and specific genetic deletion of ERK5 in megakaryocytes and platelets decreased platelet adhesion, activation, and accumulation in vitro. Using 2 models of in vivo arterial thrombosis, ERK5 deficiency in platelets abrogated the enhanced thrombosis seen in hyperlipidemic conditions.
Figure 6.
Model for redox-regulated platelet CD36 prothrombotic signaling. Recognition of oxLDL by platelet CD36 induces a signaling pathway that involves the activation of Src family kinases, NOX, and accumulation of O2•− and H2O2. Generation of these ROS lead to the activation of redox sensitive MAPK ERK5 through a mechanism that is not yet clear. ERK5 activation promotes platelet integrin activation, aggregation, and thrombus formation in conditions of hyperlipidemia and oxidant stress.
Current antiplatelet agents that target activation pathways are effective at preventing platelet activation37; however, they compromise the ability of platelets to maintain hemostasis and are associated with increased risk of bleeding complications.38 CD36 is an attractive target to prevent platelets from acquiring a “hypersensitive” phenotype because its absence does not compromise the normal physiologic hemostasis response.3,5 However, CD36 is present on multiple cell types of the vasculature, which represents a degree of complexity if targeting platelet CD36. Therefore, understanding the CD36 signaling pathway is critical to determining how CD36 promotes a prothrombotic phenotype.
Advances in free radical detection by fluorescent probes using HPLC allows for sensitive and quantitative detection of O2•− generated from within the cell.18 Specific accumulation of O2•− by oxLDL-CD36 signaling was observed by HPLC, which is consistent with published data that CD36 promotes O2•− accumulation in platelets.10 The accumulation of 2-OH-E+ is affected by the rate-limiting steps of HE oxidation to HE-radical cation, which is hydroxylated by O2•−.18,39 Thus, kinetics of 2-OH-E+ accumulation may not be an accurate reflection of the kinetics of intracellular ROS generation. The FA6 anti-CD36 blocking monoclonal antibody partially prevented O2•− accumulation by oxLDL signaling. The remaining O2•− that accumulated may be derived from known sources of ROS in platelets (eg, mitochondria, xanthine/xanthine oxidase, uncoupled platelet endothelial nitric oxide synthase), which warrants further investigation. It is also known that oxLDL particles carry lipid peroxides or reactive aldehydes40 that could influence the oxidation of HE to 2-OH-E+, but this possibility was excluded based on the specific mechanism for HE to be oxidized by O2•− to 2-OH-E+.18,39 Furthermore, the ability of nonlipid CD36 ligands to induce ERK5 activation strongly supports a specific receptor-mediated mechanism (Figure 2D). Although accumulation of 2-OH-E+ was observed time and dose dependently, the accumulation of E+, the nonspecific oxidation product of HE, also occurred time and dose dependently (supplemental Figure 1A-B). It is plausible that 2-electron oxidants such as H2O2 accumulate by oxLDL-CD36 signaling and may oxidize HE to E+, therefore causing an increase in E+. This assumption is supported by blunting oxLDL-induced platelet aggregation with PEG-catalase (Figure 1F). The slight inhibition of oxLDL-induced platelet aggregation by boiled PEG-catalase is consistent with the level of residual catalase activity present after boiling (supplemental Figure 2).
Conditions of hyperlipidemia and plaque progression were shown to have impaired redox metabolism12,13 that can maintain activation of specific MAPKs. The MAPK activation profiles in platelets typically proceed in 2 stages: (1) agonist-mediated transient activation leading to platelet inside out signaling and (2) delayed activation by platelet outside-in signaling that facilitates clot retraction.16 ERK5 activation by oxLDL-CD36 is rapidly turned on and is sustained for 40 minutes after stimulation. This activation profile is atypical for the 2-stage platelet MAPK activation seen with classic surface agonists, suggesting an important role for ROS-regulated MAPK activation with CD36 ligands.
The hypothesis that ERK5 activation is mediated by an increase in ROS was based on published data suggesting that exogenous addition of the oxidant donor H2O2 activates ERK5.17,35 Preventing ERK5 activation by using a NOX inhibitor, scavenging O2•− with nitric oxide donor DPTA-NONOate, or degrading H2O2 with catalase further supports a role for ROS in promoting ERK5 activation. Although ERK5 activation can be blunted by DPTA-NONOate, the possibility that peroxynitrite is generated cannot be excluded and requires further investigation. Additionally, inhibiting ERK5 activation with PP2 is consistent with previous reports that Src kinases are upstream of ERK5.41 NOX activation by platelet CD36 signaling is mediated through a Src-dependent pathway10; therefore, inhibiting Src kinases or NOX would prevent the generation of ROS that would subsequently limit ERK5 activation. OxLDL-induced ERK5 phosphorylation by CD36 in the presence of indomethacin and apyrase was not definitively affected (data not shown). However, this does not exclude the possibility of ERK5 activation by CD36 through platelet autocrine signaling because it has been shown that thrombin and ADP have differential activation of ERK5.17
Thrombosis is mediated by a multistep process involving platelet adhesion, integrin activation, granule secretion, and platelet accumulation.42 The available MEK5/ERK5 pharmacologic inhibitor BIX 02188 was used as a tool to study the impact of ERK5 on integrin activation, granule secretion, and platelet accumulation without affecting activation of MAPKs ERK1/2.33 Although our studies indicate that BIX 02188 prevented integrin activation and platelet aggregation, the inhibitor did not significantly impact granule secretion; however, we do not exclude the role of ERK5 in granule secretion. The impact of ERK5 on granule secretion would require further investigation. Targeted deletion of ERK5 in megakaryocytes and platelets17 further supported the inhibitor studies that ERK5 is critical to potentiate platelet activation and aggregation. These studies indicate the essential role of ERK5 in platelet activation by CD36 and are consistent with previously published data that ERK5 is required for optimal platelet activation.17 The in vivo data in this study suggest that ERK5 is functionally important for platelet CD36 to potentiate thrombus formation in hyperlipidemic and oxidant stress conditions.
In conclusion, our study identified an O2•−/H2O2-mediated ERK5 pathway as a critical modulator of thrombosis in hyperlipidemic conditions that could be exploited therapeutically because ERK5 is an important effector to integrate the nonclassic activation pathway of CD36 signaling into known platelet activation pathways.
Acknowledgments
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute grants 5R01HL111614, 3R01HL111614-S1, and R01 HL126645 (R.L.S.), coinvestigator grants R01HL130090 and R01HL129179 (W.L.), grants K08HL128856 and T32HL066988 (S.J.C.), and grant R01HL124018 (C.N.M.), the American Heart Association Established Investigator Award (13EIA14250023) (C.N.M.), and the National Institutes of Health, National Institute of Neurological Disorders and Stroke grant R01NS081936 (J.V.-V.).
M.Y. is a PhD candidate at the Medical College of Wisconsin; this work is submitted in partial fulfillment of the requirement for the doctorate.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.Y. designed and conducted most of the experiments, acquired the data, and wrote the manuscript. B.C.C. conducted the collagen-mediated in vivo thrombosis experiments and acquired the data. W.L. conducted the ferric chloride in vivo thrombosis experiments and acquired the data. J.V.-V. provided reagents and helped measure reactive oxygen species. Y.C. helped with designing the experiments and assisted with the bone marrow transplantation. N.O.S. conducted the experiments. S.J.C. and C.N.M. provided the mice, reagents, and protocols, and helped design the experiments and wrote parts of the manuscript. R.L.S. designed and supervised all the experiments and wrote the manuscript. All the authors analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Roy L. Silverstein, Medical College of Wisconsin, Clinical Cancer Center, Suite C-5038, 9200 West Wisconsin Ave, Milwaukee, WI 53226; e-mail: rsilverstein@mcw.edu.
References
- 1.Stalker TJ, Welsh JD, Brass LF. Shaping the platelet response to vascular injury. Curr Opin Hematol. 2014;21(5):410-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ouweneel AB, Van Eck M. Lipoproteins as modulators of atherothrombosis: From endothelial function to primary and secondary coagulation. Vascul Pharmacol. 2016;82:1-10. [DOI] [PubMed] [Google Scholar]
- 3.Podrez EA, Byzova TV, Febbraio M, et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat Med. 2007;13(9):1086-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briley-Saebo K, Yeang C, Witztum JL, Tsimikas S. Imaging of oxidation-specific epitopes with targeted nanoparticles to detect high-risk atherosclerotic lesions: progress and future directions. J Cardiovasc Transl Res. 2014;7(8):719-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosh A, Murugesan G, Chen K, et al. Platelet CD36 surface expression levels affect functional responses to oxidized LDL and are associated with inheritance of specific genetic polymorphisms. Blood. 2011;117(23):6355-6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Podrez EA, Poliakov E, Shen Z, et al. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J Biol Chem. 2002;277(41):38503-38516. [DOI] [PubMed] [Google Scholar]
- 7.Chen K, Febbraio M, Li W, Silverstein RL. A specific CD36-dependent signaling pathway is required for platelet activation by oxidized low-density lipoprotein. Circ Res. 2008;102(12):1512-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen K, Li W, Major J, Rahaman SO, Febbraio M, Silverstein RL. Vav guanine nucleotide exchange factors link hyperlipidemia and a prothrombotic state. Blood. 2011;117(21):5744-5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wraith KS, Magwenzi S, Aburima A, Wen Y, Leake D, Naseem KM. Oxidized low-density lipoproteins induce rapid platelet activation and shape change through tyrosine kinase and Rho kinase-signaling pathways. Blood. 2013;122(4):580-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magwenzi S, Woodward C, Wraith KS, et al. Oxidized LDL activates blood platelets through CD36/NOX2-mediated inhibition of the cGMP/protein kinase G signaling cascade. Blood. 2015;125(17):2693-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Truong TH, Carroll KS. Redox regulation of protein kinases. Crit Rev Biochem Mol Biol. 2013;48(4):332-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Febbraio M, Reddy SP, Yu DY, Yamamoto M, Silverstein RL. CD36 participates in a signaling pathway that regulates ROS formation in murine VSMCs. J Clin Invest. 2010;120(11):3996-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest. 2009;119(1):136-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramakrishnan DP, Hajj-Ali RA, Chen Y, Silverstein RL. Extracellular vesicles activate a CD36-dependent signaling pathway to inhibit microvascular endothelial cell migration and tube formation. Arterioscler Thromb Vasc Biol. 2016;36(3):534-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Runchel C, Matsuzawa A, Ichijo H. Mitogen-activated protein kinases in mammalian oxidative stress responses. Antioxid Redox Signal. 2011;15(1):205-218. [DOI] [PubMed] [Google Scholar]
- 16.Flevaris P, Li Z, Zhang G, Zheng Y, Liu J, Du X. Two distinct roles of mitogen-activated protein kinases in platelets and a novel Rac1-MAPK-dependent integrin outside-in retractile signaling pathway. Blood. 2009;113(4):893-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cameron SJ, Ture SK, Mickelsen D, et al. Platelet extracellular regulated protein kinase 5 is a redox switch and triggers maladaptive platelet responses and myocardial infarct expansion. Circulation. 2015;132(1):47-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zielonka J, Vasquez-Vivar J, Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc. 2008;3(1):8-21. [DOI] [PubMed] [Google Scholar]
- 19.Li W, McIntyre TM, Silverstein RL. Ferric chloride-induced murine carotid arterial injury: a model of redox pathology. Redox Biol. 2013;1:50-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Nieman M, Sen Gupta A. Ferric chloride-induced murine thrombosis models. J Vis Exp. 2016;115(115). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooley BC. Collagen-induced thrombosis in murine arteries and veins. Thromb Res. 2013;131(1):49-54. [DOI] [PubMed] [Google Scholar]
- 22.Febbraio M, Podrez EA, Smith JD, et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105(8):1049-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Finegan KG, Robinson AC, et al. Activation of extracellular signal-regulated protein kinase 5 downregulates FasL upon osmotic stress. Cell Death Differ. 2006;13(12):2099-2108. [DOI] [PubMed] [Google Scholar]
- 24.Zhao H, Kalivendi S, Zhang H, et al. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34(11):1359-1368. [DOI] [PubMed] [Google Scholar]
- 25.Legrand C, Pidard D, Beiso P, Tenza D, Edelman L. Interaction of a monoclonal antibody to glycoprotein IV (CD36) with human platelets and its effect on platelet function. Platelets. 1991;2(2):99-105. [DOI] [PubMed] [Google Scholar]
- 26.Altenhöfer S, Kleikers PW, Radermacher KA, et al. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci. 2012;69(14):2327-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15(6):1583-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beckman JS, Minor RL Jr, White CW, Repine JE, Rosen GM, Freeman BA. Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J Biol Chem. 1988;263(14):6884-6892. [PubMed] [Google Scholar]
- 29.Dayal S, Wilson KM, Motto DG, Miller FJ Jr, Chauhan AK, Lentz SR. Hydrogen peroxide promotes aging-related platelet hyperactivation and thrombosis. Circulation. 2013;127(12):1308-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Fang C, Gao H, et al. Platelet-derived S100 family member myeloid-related protein-14 regulates thrombosis. J Clin Invest. 2014;124(5):2160-2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klenotic PA, Page RC, Li W, Amick J, Misra S, Silverstein RL. Molecular basis of antiangiogenic thrombospondin-1 type 1 repeat domain interactions with CD36. Arterioscler Thromb Vasc Biol. 2013;33(7):1655-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zielonka J, Zielonka M, Sikora A, et al. Global profiling of reactive oxygen and nitrogen species in biological systems: high-throughput real-time analyses. J Biol Chem. 2012;287(5):2984-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tatake RJ, O’Neill MM, Kennedy CA, et al. Identification of pharmacological inhibitors of the MEK5/ERK5 pathway. Biochem Biophys Res Commun. 2008;377(1):120-125. [DOI] [PubMed] [Google Scholar]
- 34.Eitzman DT, Westrick RJ, Xu Z, Tyson J, Ginsburg D. Hyperlipidemia promotes thrombosis after injury to atherosclerotic vessels in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20(7):1831-1834. [DOI] [PubMed] [Google Scholar]
- 35.Abe J, Kusuhara M, Ulevitch RJ, Berk BC, Lee JD. Big mitogen-activated protein kinase 1 (BMK1) is a redox-sensitive kinase. J Biol Chem. 1996;271(28):16586-16590. [DOI] [PubMed] [Google Scholar]
- 36.Cooley BC. Murine arterial thrombus induction mechanism influences subsequent thrombodynamics. Thromb Res. 2015;135(5):939-943. [DOI] [PubMed] [Google Scholar]
- 37.Yousuf O, Bhatt DL. The evolution of antiplatelet therapy in cardiovascular disease. Nat Rev Cardiol. 2011;8(10):547-559. [DOI] [PubMed] [Google Scholar]
- 38.McFadyen JD, Jackson SP. Differentiating haemostasis from thrombosis for therapeutic benefit. Thromb Haemost. 2013;110(5):859-867. [DOI] [PubMed] [Google Scholar]
- 39.Zielonka J, Sarna T, Roberts JE, Wishart JF, Kalyanaraman B. Pulse radiolysis and steady-state analyses of the reaction between hydroethidine and superoxide and other oxidants. Arch Biochem Biophys. 2006;456(1):39-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levitan I, Volkov S, Subbaiah PV. Oxidized LDL: diversity, patterns of recognition, and pathophysiology. Antioxid Redox Signal. 2010;13(1):39-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abe J, Takahashi M, Ishida M, Lee JD, Berk BC. c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase 1. J Biol Chem. 1997;272(33):20389-20394. [DOI] [PubMed] [Google Scholar]
- 42.Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. 2013;93(1):327-358. [DOI] [PubMed] [Google Scholar]